Introduction
Diabetic retinopathy (DR) is a microvascular
complication of diabetes mellitus (DM) and remains the most common
cause of vision loss worldwide (1).
It is estimated that an increasing number of individuals will
suffer from DR and its complications in the future. Almost all
patients with Type I and 60% of patients with Type II diabetes have
developed a certain degree of retinopathy after 20 years (2). Proliferative DR (PDR) is a type of DR
that severely threatens vision and features vitreous hemorrhage and
neovascularization of the eye fundus and iris (3). In China, the major method of treating
PDR is laser photocoagulation, but this technique may damage
retinal function, which makes it an imperfect strategy. Usually,
pars plana vitrectomy (PPV) is used to cure patients with PDR and
is a useful tool that avoids different kinds of advanced
complications. However, during or after PPV, vitreous hemorrhage
may lead to severe consequences including retinal detachment
(4).
With deeper recognition of the pathologic mechanisms
of DR, medical therapy has made significant progress, including the
use of glucocorticoids, anti-vascular endothelial growth factor
(VEGF) drugs, anti-platelet-derived growth factor drugs,
nonsteroidal anti-inflammatory drugs and drugs that improve the
function of the optic nerve. Anti-VEGF agents have gained
popularity in the treatment of diabetic macular edema. VEGF
destroys the tight junction between retinal vascular endothelial
cells and leads to the destruction of the blood-retinal barrier,
leading to intravascular fluid leakage and macular edema (5). Ostensibly, VEGF is recognized as a
crucial mediator in diabetic macular edema and vitreous injection
of anti-VEGF agents has become a promising therapy for PDR.
Intravitreal injection of VEGF inhibitors reduces the leakage of
diabetic neovascular lesions and is suitable for vitreous
hemorrhage (6,7); it improves the visual acuity and
prognosis of patients with PDR and reduces surgical complications
in patients with PDR. Furthermore, intravitreal injection of VEGF
inhibitors is less likely to cause cataracts and high intraocular
pressure than vitreous injection of glucocorticoids (5). In addition, the vision prognosis of
patients receiving continuous injection of VEGF inhibitors within
two years is better than that of patients treated by panretinal
photocoagulation (8).
In PDR-associated retinal hypoxia, leukocytes adhere
to retinal capillaries under hyperglycemic conditions, leading to
augmentation of vascular permeability and capillary occlusion. As a
consequence, VEGF is stimulated to induce retinal ischemia, which
is closely correlated with neovascularization, vitreous hemorrhage
and severe vision loss (9,10). Therefore, angiogenesis is closely
related to the occurrence and development of PDR, and VEGF has a
pivotal role in PDR by promoting angiogenesis and vascular
hyperpermeability. It is well known that VEGF-A activates both VEGF
receptor (VEGFR)-1 and VEGFR-2, while VEGF-B binds selectively to
VEGFR-1(11). The VEGF-A protein
targets endothelial cells and has multiple effects, including cell
growth and migration, angiogenesis and increased vascular
permeability (12). The function of
VEGF-B in ophthalmology remains controversial, although it has been
investigated in cardiovascular disease, diabetes and cancers
(13). In the present study,
changes in the concentration of VEGF-A and VEGF-B after
intravitreal injection of conbercept were investigated.
Conbercept is a soluble VEGFR protein and is
composed of the extracellular domain 2 of VEGFR-1 and extracellular
domains 3 and 4 of VEGFR-2. It has been suggested that conbercept
pretreatment accelerates postoperative vitreous clear-up and
maintains stable visual acuity restoration in patients with PDR
(11). However, changes in VEGF-A
and VEGF-B levels in the aqueous and vitreous humors of patients
with PDR after intravitreal injection of conbercept have been
insufficiently reported in previous studies and require to be
determined. In the present study, whether injection of a VEGF
inhibitor would decrease VEGF levels was assessed and the efficacy
of conbercept for the treatment of PDR was also evaluated.
Patients and methods
Patients
A total of 60 patients diagnosed with diabetic
retinopathy induced by type 2 diabetes at Shanxi Eye Hospital
(Taiyuan, China) between January 2016 and January 2018 were
examined. The cohort included 34 males and 26 females with an age
of 32-76 years, a mean age of 55.67±8.21 and a mean proportion of
glycosylated hemoglobin of 7.45±1.23%. All of the samples were
monocular and patients had been treated by surgery first. All
patients underwent fundus photography, mydriatic indirect
ophthalmoscopy and B-ultrasound, and certain patients underwent
fundus fluorescein angiography. The examination results were in
accordance with the PDR staging criteria of the fundus disease
group of the Chinese Ophthalmological Society from 1985(11) and patients in phase V and VI were
included. Patients with other ocular diseases, including
nondiabetic retinopathy of retinal arteriovenous obstruction, high
myopia and polypoid choroidal neovascularization, were excluded.
Furthermore, patients who underwent anti-VEGF therapy in the past
12 months and vitrectomy, patients with severe organic diseases
(such as severe heart disease, liver and kidney failure and
cancer), patients during a menstrual period or who were taking
aspirin or other anticoagulants were also excluded. The grading for
preoperative vitreous hemorrhage was performed as in a previous
study (14). Grade 0, no vitreous
hemorrhage; grade 1, visible fundus details, but the retinal nerve
fiber layer or small blood vessels cannot be assessed; grade 2,
more bleeding and large blood vessels of the optic disc compared to
grade 1; grade 3, only a slight reflex of the fundus and only the
disc is visible; grade 4, no red light reaction and the fundus
cannot be seen. This study was approved by the research ethics
board of Shanxi Eye Hospital (Taiyuan, China; no. 2019B01). This
study was a retrospective analysis summarizing our previous cases
and analyzing their data.
Interventions
Patients were divided into the surgical group and
the preoperative intravitreal conbercept group (the drug group).
Patients in the surgical group received PPV directly and their
aqueous and vitreous humors were extracted during PPV surgery. The
aqueous humor of patients in the preoperative intravitreal
conbercept group was extracted prior to treatment and seven days
after intravitreal conbercept injection before PPV. The vitreous
humor of patients in the preoperative intravitreal conbercept group
was extracted during PPV surgery. All of the patients received an
explanation of the off-label use of conbercept in PDR and its
potential benefits and risks. Prophylactic topical antibiotics
(levofloxacin; Selleck Chemicals) were instilled for 3 days prior
to treatment. After topical anesthesia and sterilizing the
operating field, 0.5 mg (0.05 ml) conbercept (Chengdu Kanghong
Biotechnologies Co., Ltd.) was injected intravitreally at the
superior temporal pars plana (4 mm posterior to the limbus). After
the injection, the intraocular pressure was measured and
prophylactic topical antibiotic drops were instilled for another 3
days. Standard 25-gauge three-port PPV was performed one week after
the intravitreal injection by the same experienced ocular surgeon.
The intraoperative bleeding grades were assigned as follows
(15): Grade 0, no bleeding or
small bleeding spots with no requirement for hemostasis; grade 1,
self-stopping small bleeding or bleeding that may be stopped
through elevated perfusion pressure or glass-cutting head
compression; grade 2, electrocoagulation is required to stop the
bleeding due to the high bleeding volume; grade 3, the blood clot
formed exceeds the posterior pole or affects the surgical field of
view.
Aqueous humor extraction
A 1-ml syringe was used to enter the anterior
chamber at the edge of the horn-sclera. The fluid was extracted
from the anterior chamber and rapidly filled in an Eppendorf tube,
followed by centrifugation in a low-temperature ultracentrifuge at
2,000 x g for 20 min at 4˚C. The supernatant was obtained and
stored in the freezer at -80˚C. Aqueous humor was collected via
anterior paracentesis prior to conbercept injection and at the
beginning of vitrectomy in the operation room. The aqueous humor
sample was collected in a tuberculin syringe, filled in a sterile
Eppendorf tube and stored at -80˚C prior to use.
Vitreous humor extraction
A 25G vitrectomy head was used to cut 0.5 ml
vitreous humor in the center of the vitreous cavity, and then the
vitreous humor was quickly added to an Eppendorf tube. The sample
was placed into a low-temperature ultracentrifuge, centrifuged at
2,000 x g for 20 min at 4˚C and stored in a -80˚C freezer.
Proliferative membrane extraction and
histopathological examination
The neovascular proliferative membrane of patients
with PDR was removed during the operation and its size was
~2.0x2.0x0.5 mm3. The membrane was fixed in a 10%
formalin solution and then stored in a wax block. The paraffin
specimen was cut into 3-µm slices. The number of blood vessels in
the fibrovascular proliferative membrane was determined by H&E
staining. Paraffin sections were dewaxed, hydrated, stained with
hematoxylin, acidified with hydrochloric acid and observed under a
light microscope (magnification, x200). Five fields were observed
for each specimen to obtain the average number of blood
vessels.
Semiquantitative determination of
VEGF-A and VEGF-B in fibrovascular proliferative membranes by
immunohistochemical staining
Fibrovascular proliferative membrane sections were
deparaffinized in water and washed with PBS three times for 10 min.
Subsequent to hot antigen retrieval (citrate buffer, pH 6.0, 100˚C
for 25 min), 5% bovine serum albumin (Beijing Solarbio Science
& Technology Co., Ltd.) blocking solution was added, followed
by incubation at room temperature for 20 min. The sample was then
incubated with primary antibody (anti-VEGF-A, cat. no. ab51745;
anti-VEGF-B, cat. no. ab185696; 1:200 dilution, Abcam) at 4˚C
overnight, followed by incubation with the secondary antibody (cat.
no. ab205718, 1:5,000 dilution, Abcam) at 37˚C for 20 min. After
washing with PBS three times, the sample was incubated with strept
avidin-biotin complex (Beijing Solarbio Science & Technology
Co., Ltd.) at 37˚C for 20 min. Diaminobenzidine staining was then
performed prior to washing three times with distilled water.
Hematoxylin stain was applied for 2 min followed by washing with
water three times, acidification for 2 sec, washing three times,
dehydration and sealing with neutral gum. For the negative control,
PBS was used instead of the primary antibody. Images of the
immunohistochemically stained tissue sections were obtained under a
light microscope using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.) (16,17). The images were analyzed with this
software and the average optical density (AOD) value of the
positive region was calculated. A total of three regions were
randomly selected for each specimen and the average AOD value was
calculated.
ELISA
The concentrations of VEGF-A and VEGF-B in aqueous
and vitreous humor samples were determined by using an ELISA kit
(VEGF-A kit, cat. no. F3255; VEGF-B kit, cat. no. F3254; Shanghai
Westang Bio-Tech Co., Ltd.) according to the manufacturer's
protocol. The plates were incubated with capture antibodies and
were then covered with 100 µl standard, controls or samples for 40
min at room temperature. Subsequently, the plates were washed and
incubated with the detection antibody for 20 min at room
temperature. The plates were washed again and the substrate was
added. The reaction was stopped after 15 min of incubation at room
temperature in the dark, the stop buffer was added and the plates
were read at 450 nm.
Statistical analysis
SPSS 20.0 software (IBM Corp.) was applied for data
analysis. Measurement data were expressed as the mean ± standard
deviation. Student's t-test was used to compare the mean difference
of numerical variables between groups, the Chi-square test was used
for analyzing count data, and the Mann-Whitney U-test was performed
for non-parametric data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Preoperative vitreous hemorrhage and
vitreoretinal adhesion are similar in the surgery group and the
drug group
The study included 34 males and 26 females with an
age range of 32-76 years, a mean age of 55.67±8.21 and a mean
proportion of glycosylated hemoglobin of 7.45±1.23%. According to a
previous study, the classification criteria for vitreoretinal
adhesion are as follows (18):
Grade 0, no adhesion; grade 1, <3 point-level adhesions; grade
2, >1 extensive adhesion or adhesion at the optic disc, macula
lutea or vascular arch; grade 3, vitreoretinal adhesion beyond the
peripheral part. As presented in Table
I, the P-values for the grades of preoperative vitreous
hemorrhage and retinal adhesion between the surgery and drug groups
were 0.940 and 0.816, respectively. The P-values were both
>0.05, indicating that the changes between the two groups were
not significant. There were no significant changes observed in
preoperative vitreous hemorrhage and vitreoretinal adhesion between
the surgery and drug groups.
 | Table IPreoperative vitreous hemorrhage and
adhesion grades between the neovascular proliferation membrane and
retina in patients with proliferative diabetic retinopathy. |
Table I
Preoperative vitreous hemorrhage and
adhesion grades between the neovascular proliferation membrane and
retina in patients with proliferative diabetic retinopathy.
| | Preoperative vitreous
hemorrhage grade (n) | Retinal adhesion
grade (n) |
|---|
| Group | Eyes (n) | 1 | 2 | 3 | 4 | 1 | 2 | 3 |
|---|
| Drug | 30 | 2 | 11 | 14 | 3 | 14 | 18 | 8 |
| Surgery | 30 | 4 | 13 | 11 | 2 | 11 | 23 | 6 |
| U | | -0.075 | -0.232 |
| P-value | | 0.940 | 0.816 |
Reduced intraoperative bleeding in the
drug group compared with that in the surgery group
As presented in Table
II, the surgery time in the drug group was shorter than that in
the surgery group and the difference was statistically significant
(P=0.01). Intraoperative bleeding in the surgery group was more
severe than that in the drug group and the difference was
statistically significant (P=0.04).
 | Table IIComparison of surgery time and
intraoperative bleeding between the drug group and the surgery
group. |
Table II
Comparison of surgery time and
intraoperative bleeding between the drug group and the surgery
group.
| Item | Drug group
(n=30) | Surgery group
(n=30) |
t/χ2 | P-value |
|---|
| Surgery time
(min) | 54.54±10.93 | 64.97±20.01 | 2.51 | 0.01 |
| Intraoperative
bleeding grade | | | 6.37 | 0.04 |
|
0 | 18 | 9 | | |
|
1 | 12 | 20 | | |
|
2 | 0 | 1 | | |
VEGF-A and VEGF-B levels in aqueous
humor prior to and after conbercept treatment in the drug
group
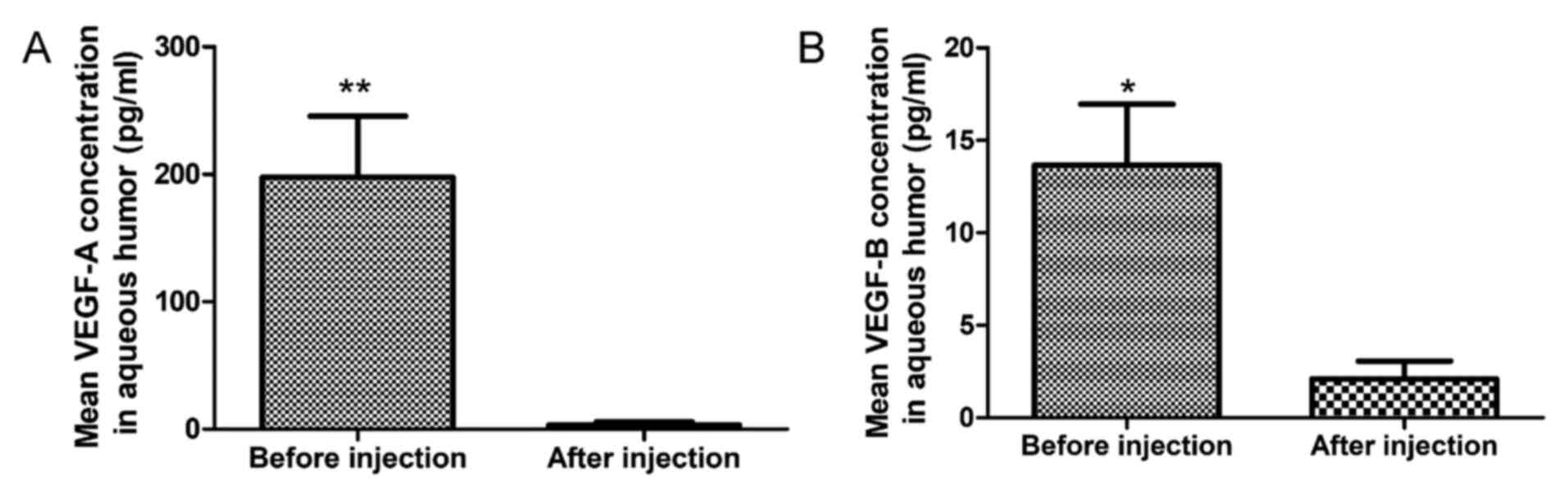
As presented in Fig.
1, the mean VEGF-A concentration in aqueous humor was
197.66±48.00 pg/ml prior to conbercept treatment, which was higher
than the concentration after conbercept treatment (3.39±2.54 pg/ml)
and the difference was statistically significant (P=0.001). The
mean VEGF-B concentration in aqueous humor prior to conbercept
treatment (13.66±3.30 pg/ml) was higher than that after conbercept
treatment (2.17±0.94 pg/ml) and the difference was statistically
significant (P=0.022).
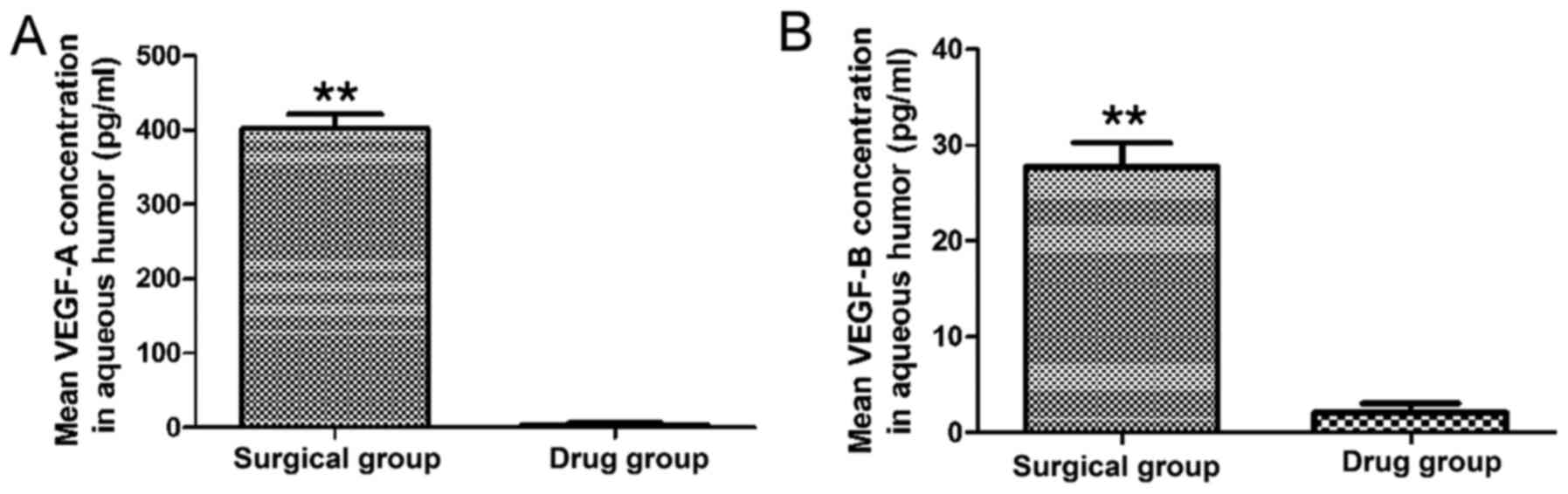
Comparison of VEGF-A and VEGF-B levels
in aqueous humor between the surgical group and the preoperative
intravitreal conbercept group
As presented in Fig.
2, the mean VEGF-A concentration in the aqueous humor of
patients in the surgical group (402.18±19.37 pg/ml) was higher than
that in the drug group (3.39±2.54 pg/ml) and the difference was
statistically significant (P=0.001). Furthermore, the mean VEGF-B
concentration in the aqueous humor of patients in the drug group
was 2.17±0.94 pg/ml, which was lower than that of patients in the
surgical group (27.77±2.52 pg/ml) and the difference was
statistically significant (P=0.001).
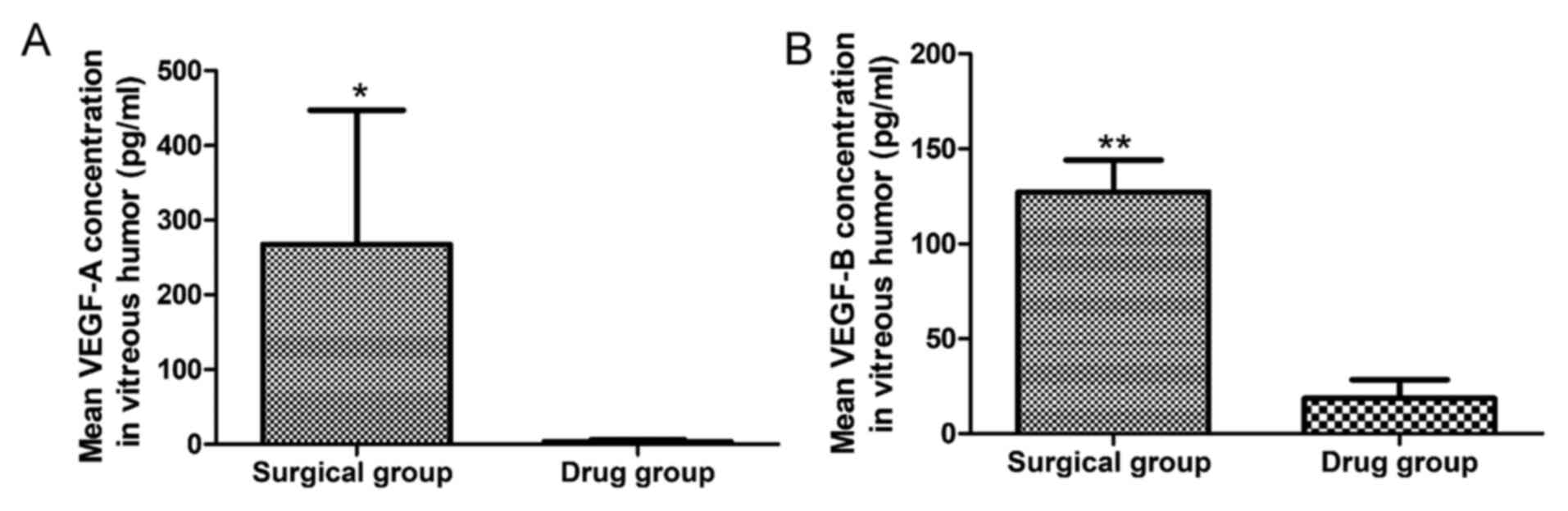
Comparison of VEGF-A levels and VEGF-B
levels in vitreous humor between the surgical group and the
preoperative intravitreal conbercept group
As indicated in Fig.
3, the mean VEGF-A concentration in the vitreous humor of
patients in the surgical group (267.53±179.60 pg/ml) was higher
compared with that of patients in the drug group (21.43±5.81 pg/ml)
and the difference was statistically significant (P=0.037).
Similarly, the mean VEGF-B level was higher in the vitreous humor
of patients in the surgical group (127.36±16.72 pg/ml) compared
with that of patients in the drug group (18.56±9.82 pg/ml) and the
difference was statistically significant (P=0.001).
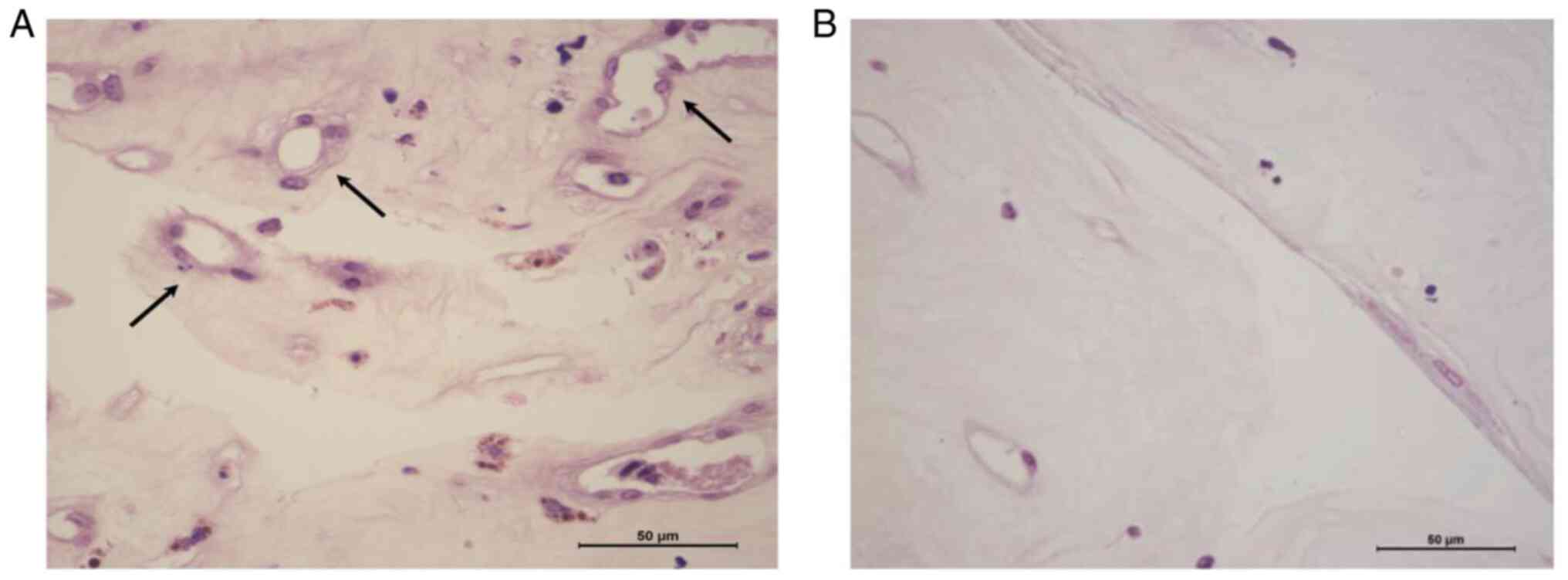
Reduced number of blood vessels in the
proliferative membrane in the drug vs. surgery group
As presented in Fig.
4 and Table III, the number
of blood vessels in the proliferative membrane in the drug
treatment group was 17.83±3.90, which was significantly lower than
that in the simple surgery group (32.17±5.80) and the difference
was statistically significant (t=11.23, P<0.001).
 | Table IIINumber of blood vessels in the
proliferative membrane. |
Table III
Number of blood vessels in the
proliferative membrane.
| Item | Surgery group
(n=30) | Drug group
(n=30) | t value | P-value |
|---|
| Number of
vessels | 32.17±5.80 | 17.83±3.90 | 11.23 | <0.001 |
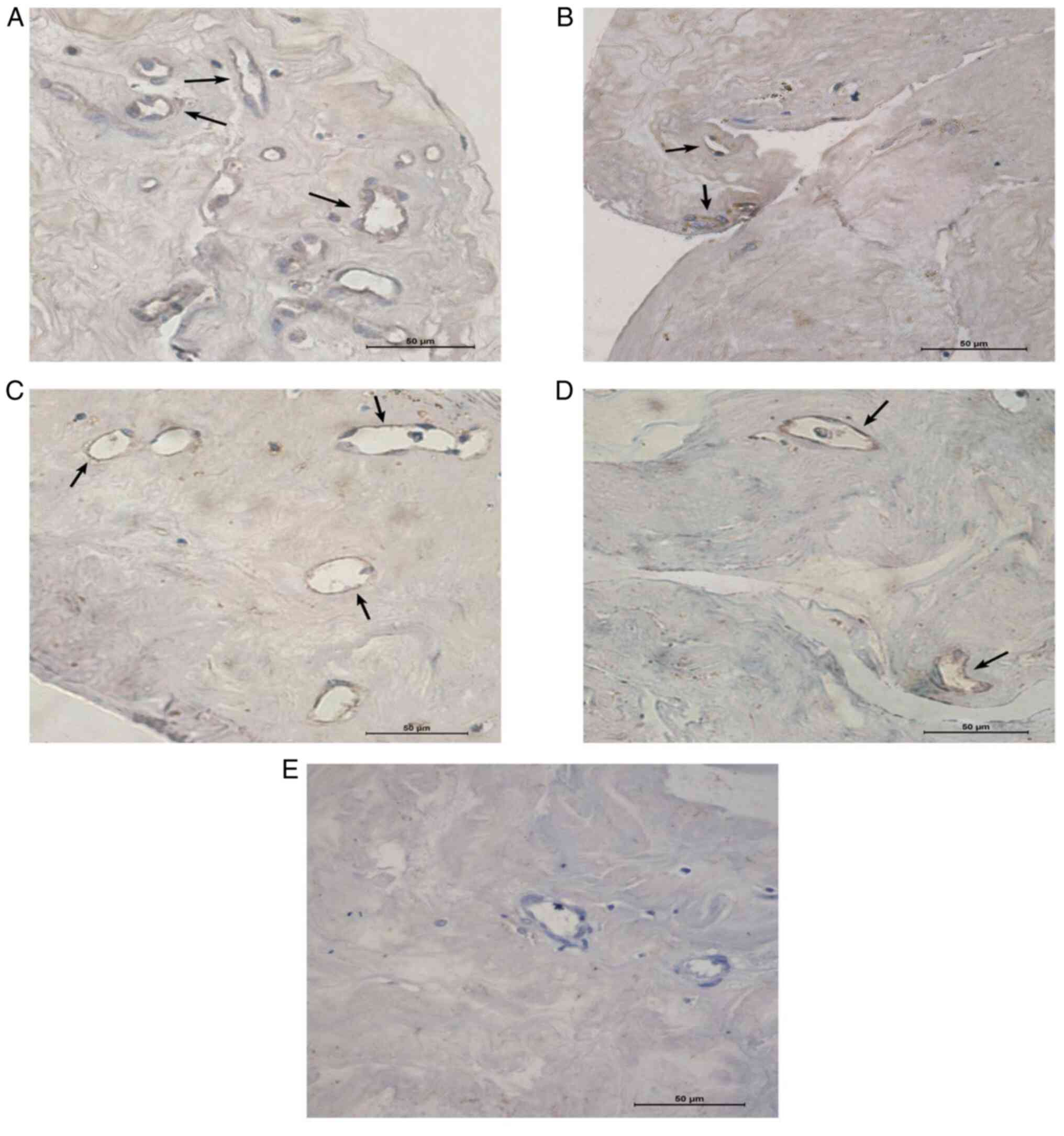
Immunohistochemical staining for
VEGF-A and VEGF-B in the fibrovascular proliferative membrane
As presented in Fig.
5 and Table IV, the levels of
VEGF-A and VEGF-B in the neovascular proliferation membrane of the
drug group were 0.005±0.003 and 0.001±0.003, respectively, which
were significantly lower than those of the surgery group
(0.023±0.008 and 0.009±0.005, respectively). Of note, the
difference was statistically significant (P<0.001).
 | Table IVOptical density value of immunostain
for each factor in the proliferative membrane. |
Table IV
Optical density value of immunostain
for each factor in the proliferative membrane.
| Protein | Surgery group
(n=30) | Drug group
(n=30) | t value | P-value |
|---|
| VEGF-A | 0.023±0.008 | 0.005±0.003 | 7.51 | <0.001 |
| VEGF-B | 0.009±0.005 | 0.001±0.003 | 11.54 | <0.001 |
Discussion
VEGF family members, particularly VEGF-A, have
crucial roles in neovascularization and are the major promoters of
pathological angiogenesis (19).
VEGF family members are capable of changing vascular morphology by
increasing the number of endothelial cells and stromal cells
(19). VEGF levels in patients with
PDR are obviously increased and are closely correlated with the
severity of DR (20). Decreasing
VEGF-A levels prevents VEGF-A from inducing vascular leakage and
promoting neovascularization, reducing the thickness of the central
fovea of the macula in patients with diabetic macular edema
(21) and improving the safety of
surgery (22). The results of the
present study suggested that the level of VEGF-A in the aqueous
humor of patients with PDR in the combined drug-treated group was
significantly lower than that of patients in the surgery group.
The occurrence and development of neovascularization
in patients with PDR is a clinical problem, as hemorrhage in
neovascularization leads to vitreous opacity, retinal proliferative
membrane occurrence, retinal traction detachment and impaired
visual function (19). Furthermore,
intraoperative bleeding affects the surgical field and prolongs the
operation time (23). The present
results demonstrated that intraoperative bleeding was reduced in
the combined drug treatment group during the PPV operation compared
with that in the simple surgery group due to the reduced number of
new blood vessels in the neovascular proliferation membrane of
patients. Thus, the surgical field was clear, the operation time
was shortened, the safety of the operation was improved and
suffering was reduced for patients, which was similar to the
results of previous studies (18-22).
Accordingly, it is suggested that the widespread use of conbercept
in clinical practice is able to improve the safety of PPV surgery
in patients with PDR.
Previously, the concentrations of VEGF-A and VEGF-B
after conbercept injection have not been determined in human eyes.
The present study reported that the levels of both VEGF-A and
VEGF-B obviously dropped after intravitreal injection of conbercept
in patients with PDR. Furthermore, the levels of both VEGF-A and
VEGF-B declined in the drug group compared with those in the PPV
surgical group and the differences were statistically significant.
Patients with PDR in the drug group received conbercept injection
and the aqueous humor of patients was extracted 7 days after
treatment but prior to surgery. However, VEGF-A and VEGF-B levels
in the vitreous humor of patients with PDR were not measured prior
to conbercept injection due to ethical considerations and
consequently, no data for comparison were obtained. Furthermore,
the drug treatment time was not prolonged to identify any changes
in cytokines out of safety considerations after surgery. Due to the
small number of cases in the present study, it is imperative to
expand the sample size in future studies to increase the
reliability of the results.
The function of VEGF-B in eye diseases remains
controversial and requires further study. The present study
suggested that the concentration of VEGF-B in the aqueous humor of
patients with PDR decreased significantly after conbercept
injection. Furthermore, the VEGF-B level declined significantly in
vitreous humor after conbercept injection compared with that of
patients directly receiving PPV. In most tissue types, VEGF-B has a
minor role in inducing angiogenesis. Usually, a lack of VEGF-B does
not affect the normal development of the retinal vasculature.
However, VEGF-B may promote the survival of novel blood vessels by
affecting endothelial cells, pericytes, smooth muscle cells and
endothelial progenitor cells (24).
When the level of VEGF-B is dropped, pathological
neovascularization cannot exert pathological effects after
formation because the vessels cannot stably survive, which was
consistent with a previous study (25). Taken together, these results suggest
that VEGF-B has a supporting role in the development of
pathological neovascularization. Previous studies concerning the
addition of ranibizumab and VEGF-B changes have provided
conflicting results (26,27) and it is controversial whether VEGF-B
stimulates angiogenesis (28,29).
Therefore, it is essential to further identify the inhibitory
effect and mechanism of VEGF-B on angiogenesis and visual acuity of
patients. Furthermore, it was reported that the aqueous humor
levels of placenta growth factor (PIGF) were correlated with
VEGF-B, and the levels of VEGF-A, VEGF-B and PlGF decreased after
intravitreal conbercept injection in patients with active PDR
(30). The patient samples analyzed
were mainly from the Beijing region and the samples were mainly
aqueous humor. However, the samples of the present study included
aqueous humor and vitreous humor. In the present study, the patient
samples were analyzed from 60 patients diagnosed with DR induced by
type 2 diabetes at Shanxi Eye Hospital (Taiyuan, China) between
January 2016 and January 2018.
Although the outlook is promising, vitreous
injection of VEGF inhibitors is not without risk. Adverse effects
of anti-VEGF agents include traction retinal detachment (TRD) and
endophthalmitis. It was previously reported that bevacizumab may
cause TRD in patients with severe PDR (31). Fortunately, the incidence of
endophthalmitis is not high. On the other hand, long-term vitreous
injection of VEGF inhibitors may lead to neurodegeneration of
residual healthy retina and increase the risk of choroidal
circulatory disorders, which has been confirmed in mouse
experiments (32). Furthermore,
VEGF produced by retinal pigment epithelial cells is conducive to
maintaining choroidal capillaries (33), and whether anti-VEGF treatment
potentially causes pathological atrophy of the diabetic retina
requires further investigation. Furthermore, the grading for
preoperative vitreous hemorrhage and the intraoperative bleeding
grades used in this study were established by some medical
organizations (14,15) rather than scales postulated in small
clinical studies, this is a limitation of this study. Finally,
whether the reduced capillaries in the VEGF inhibitor group are
reversible, and how long it takes to normalize, and whether it
affects the healing after surgery, these unsolved problems will be
investigated in the future.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Shanxi Province
Science Foundation for Youths (grant no. 201801D221014).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG conceived and designed the experiments. YZ and ZG
performed the experiments. XZ and ZY analyzed the data. TM and GL
contributed reagents/materials/analytical tools. YZ wrote the
manuscript. All authors read and approved the final version of the
manuscript. YZ and XG checked and confirmed the authenticity of
data.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Board of Shanxi Eye Hospital (Taiyuan, China; approval no.
2019B01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dehdashtian E, Mehrzadi S, Yousefi B,
Hosseinzadeh A, Reiter RJ, Safa M, Ghaznavi H and Naseripour M:
Diabetic retinopathy pathogenesis and the ameliorating effects of
melatonin; involvement of autophagy, inflammation and oxidative
stress. Life Sci. 193:20–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yau JW, Rogers SL, Kawasaki R, Lamoureux
EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund
J, et al: Global prevalence and major risk factors of diabetic
retinopathy. Diabetes Care. 35:556–564. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wilkinson CP, Ferris FL III, Klein RE, Lee
PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R,
Verdaguer JT, et al: Proposed international clinical diabetic
retinopathy and diabetic macular edema disease severity scales.
Ophthalmology. 110:1677–1682. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hershberger VS, Augsburger JJ, Hutchins
RK, Raymond LA and Krug S: Fibrovascular ingrowth at sclerotomy
sites in vitrectomized diabetic eyes with recurrent vitreous
hemorrhage: Ultrasound biomicroscopy findings. Ophthalmology.
111:1215–1221. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lang GE: Diabetic macular edema.
Ophthalmologica. 227 (Suppl 1):S21–S29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Waisbourd M, Goldstein M and Loewenstein
A: Treatment of diabetic retinopathy with anti-VEGF drugs. Acta
Ophthalmol. 89:203–207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nicholson BP and Schachat AP: A review of
clinical trials of anti-VEGF agents for diabetic retinopathy.
Graefes Arch Clin Exp Ophthalmol. 248:915–930. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brown DM, Schmidt-Erfurth U, Do DV, Holz
FG, Boyer DS, Midena E, Heier JS, Terasaki H, Kaiser PK, Marcus DM,
et al: Intravitreal aflibercept for diabetic macular edema:
100-week results from the VISTA and VIVID studies. Ophthalmology.
122:2044–2052. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ciulla TA, Amador AG and Zinman B:
Diabetic retinopathy and diabetic macular edema: Pathophysiology,
screening, and novel therapies. Diabetes Care. 26:2653–2664.
2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grauslund J, Green A and Sjolie AK:
Prevalence and 25 year incidence of proliferative retinopathy among
Danish type 1 diabetic patients. Diabetologia. 52:1829–1835.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang X, Xu J, Wang R, Mei Y, Lei H, Liu J,
Zhang T and Zhao H: A randomized controlled trial of conbercept
pretreatment before vitrectomy in proliferative diabetic
retinopathy. J Ophthalmol. 2016(2473234)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tetikoglu M, Yuksel Z, Aktas S, Sagdik HM
and Ozcura F: VEGF-A gene polymorphisms and responses to
intravitreal ranibizumab treatment in patients with diabetic
macular edema. Int Ophthalmol. 38:2381–2388. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mesquita J, Castro de Sousa J, Vaz-Pereira
S, Neves A, Tavares-Ratado P, M Santos F, A Passarinha L and T
Tomaz C: VEGF-B levels in the vitreous of diabetic and non-diabetic
patients with ocular diseases and its correlation with structural
parameters. Med Sci (Basel). 5(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ahn J, Woo SJ, Chung H and Park KH: The
effect of adjunctive intravitreal bevacizumab for preventing
postvitrectomy hemorrhage in proliferative diabetic retinopathy.
Ophthalmology. 118:2218–2226. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yeh PT, Yang CM, Lin YC, Chen MS and Yang
CH: Bevacizumab pretreatment in vitrectomy with silicone oil for
severe diabetic retinopathy. Retina. 29:768–774. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pan GD, Yang JQ, Yan LN, Chu GP, Liu Q,
Xiao Y and Yuan L: Reversal of multi-drug resistance by
pSUPER-shRNA-mdr1 in vivo and in vitro. World J Gastroenterol.
15:431–440. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Takahashi S: Vascular endothelial growth
factor (VEGF), VEGF receptors and their inhibitors for
antiangiogenic tumor therapy. Biol Pharm Bull. 34:1785–1788.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang CM, Yeh PT, Yang CH and Chen MS:
Bevacizumab pretreatment and long-acting gas infusion on vitreous
clear-up after diabetic vitrectomy. Am J Ophthalmol. 146:211–217.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Tolentino MJ, Miller JW, Gragoudas ES,
Chatzistefanou K, Ferrara N and Adamis AP: Vascular endothelial
growth factor is sufficient to produce iris neovascularization and
neovascular glaucoma in a nonhuman primate. Arch Ophthalmol.
114:964–970. 1996.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ferrone PJ and Jonisch J: Comparison of
ranibizumab 0.5 mg versus 1.0 mg for the treatment of patients with
clinically significant diabetic macular edema: A randomized,
clinical trial. Ophthalmic Surg Lasers Imaging Retina. 47:536–543.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dong F, Yu C, Ding H, Shen L and Lou D:
Evaluation of intravitreal ranibizumab on the surgical outcome for
diabetic retinopathy with tractional retinal detachment. Medicine
(Baltimore). 95(e2731)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Arevalo JF, Lasave AF, Kozak I, Al Rashaed
S, Al Kahtani E, Maia M, Farah ME, Cutolo C, Brito M, Osorio C, et
al: Preoperative bevacizumab for tractional retinal detachment in
proliferative diabetic retinopathy: A prospective randomized
clinical trial. Am J Ophthalmol. 207:279–287. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang F, Tang Z, Hou X, Lennartsson J, Li
Y, Koch AW, Scotney P, Lee C, Arjunan P, Dong L, et al: VEGF-B is
dispensable for blood vessel growth but critical for their
survival, and VEGF-B targeting inhibits pathological angiogenesis.
Proc Natl Acad Sci USA. 106:6152–6157. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhong X, Huang H, Shen J, Zacchigna S,
Zentilin L, Giacca M and Vinores SA: Vascular endothelial growth
factor-B gene transfer exacerbates retinal and choroidal
neovascularization and vasopermeability without promoting
inflammation. Mol Vis. 17:492–507. 2011.PubMed/NCBI
|
|
26
|
Puddu A, Sanguineti R, Traverso CE,
Viviani GL and Nicolo M: Response to anti-VEGF-A treatment of
retinal pigment epithelial cells in vitro. Eur J Ophthalmol.
26:425–430. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Puddu A, Sanguineti R, Traverso CE,
Viviani GL and Nicolo M: Response to anti-VEGF-A treatment of
endothelial cells in vitro. Exp Eye Res. 146:128–136.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wafai R, Tudor EM, Angus JA and Wright CE:
Vascular effects of FGF-2 and VEGF-B in rabbits with bilateral hind
limb ischemia. J Vasc Res. 46:45–54. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rissanen TT, Markkanen JE, Gruchala M,
Heikura T, Puranen A, Kettunen MI, Kholová I, Kauppinen RA, Achen
MG, Stacker SA, et al: VEGF-D is the strongest angiogenic and
lymphangiogenic effector among VEGFs delivered into skeletal muscle
via adenoviruses. Circ Res. 92:1098–1106. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang X, Wu J, Wu C, Bian AL, Geng S and
Dai RP: Comparison of aqueous humor levels of PlGF and VEGF in
proliferative diabetic retinopathy before and after intravitreal
conbercept injection. Diabetes Res Clin Pract.
162(108083)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Torres-Soriano ME, Reyna-Castelan E,
Herna´ndez-Rojas M, Garcıa-Aguirre G, Kon-Jara V, Diaz-Rubio JL,
Guerrero-Naranjo JL, Jimenez-Sierra JM and Quiroz-Mercado H:
Tractional retinal detachment after intravitreal injection of
bevacizumab in proliferative diabetic retinopathy. Retin Cases
Brief Rep. 3:70–73. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Simo R, Sundstrom JM and Antonetti DA:
Ocular Anti-VEGF therapy for diabetic retinopathy: The role of VEGF
in the pathogenesis of diabetic retinopathy. Diabetes Care.
37:893–899. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Spilsbury K, Garrett KL, Shen WY,
Constable IJ and Rakoczy PE: Overexpression of vascular endothelial
growth factor (VEGF) in the retinal pigment epithelium leads to the
development of choroidal neovascularization. Am J Pathol.
157:135–144. 2000.PubMed/NCBI View Article : Google Scholar
|