Introduction
Diabetes is a chronic progressive disease associated
with endocrine and metabolic disorders, and common clinical
characteristics include proteinuria, progressive renal damage,
hypertension and oedema (1).
Diabetes is considered a serious threat to human health, and is the
most prevalent disease, apart from cardiovascular disease and
cancer (2). There are a number of
complications associated with diabetes, such as an increased risk
of a cerebrovascular accident, coronary heart disease and
retinopathy (3). Diabetic
nephropathy (DN) with extracellular matrix (ECM) accumulation is a
common characteristic of diabetes (1). Physiologically, ECM synthesis and
degradation is balanced; however, under several pathophysiological
conditions, the balance is disrupted, which results in ECM
accumulation, glomerular structure damage and glomerular sclerosis,
ultimately causing DN (4).
Tissue transglutaminase (tTG) is a member of the
Ca2+-dependent TG family, which catalyzes the formation
of the γ-glutamyl-ε-lysine isopeptide bond and introduces a
covalent crosslink between the protein and peptide, thus inducing
resistance to enzymatic degradation (5). tTG is predominantly located in the
cytoplasm, while small amounts exist in the nucleus and nuclear
membrane under normal physiological conditions (6). Under high levels of glucose, tTG
expression increases, the protein translocates to the outside of
the cell and crosslinks with type IV collagen (Col IV) and
fibronectin (FN), which are major constituents of the ECM (7). The crosslinked product is highly
stable and difficult to degrade (8). In a previous study, the administration
of specific inhibitors of tTG to streptozotocin (STZ)-induced DN
rats was found to significantly decrease the abundance of the
extracellular crosslinked product (9). Therefore, tTG is considered a
regulator of the ECM and is involved in the development of DN.
Transforming growth factor (TGF)-β is a peptide that
reacts with different types of cells and is involved in numerous
different biological functions, such as promoting cell hypertrophy,
accelerating apoptosis and improving the content of the ECM
(10). TGF-β mediates the synthesis
of matrix proteins, such as Col IV and FN, under high glucose
conditions and promotes the adhesion between cells and the ECM by
increasing the expression of the ECM receptor (11). A previous study have demonstrated
that TGF-β expression was notably increased in STZ-induced diabetic
kidney tissues of rats, which was positively associated with the
degree of renal fibrosis (12).
Connective tissue growth factor (CTGF) is a downstream target of
TGF-β, and the biological effect of TGF-β is partially mediated by
CTGF (13). Another previous study
demonstrated that CTGF may be associated with the pathogenesis of
DN, as patients with DN had increased concentrations of CTCF in
peripheral blood and urine. CTCF was also found to be associated
with the albuminuria excretion index (14). Furthermore, CTGF is expressed at
significantly high levels in the mesangial area of patients and
animals with DN compared with normal tissues, and in high
glucose-cultured mesangial cells (15). It has also been reported that the
concentrations of FN and collagen are significantly increased in
CTGF-treated mesangial cells (16).
Taken together, these results suggest that TGF-β and CTGF serve
important roles in the process of ECM accumulation. However, to the
best of our knowledge, whether there is an association between tTG
and TGF-β or CTGF in DN remains unclear.
Ginkgo biloba leaf extract (GBE) has been
widely used to prevent and treat cardiovascular diseases due to its
reported ability to induce vasodilation, inhibit the development of
atherosclerosis and inflammation, and repress free radicals
(17). Analogous effects have also
been observed in mesangial cells cultured in high glucose medium,
where GBE was demonstrated to decrease the expression levels of
TGF-β and CTGF, as well as the expression levels of Col IV
(18). Our previous study indicated
that GBE can be used to prevent and treat renal fibrosis in rats,
which may inhibit the Angiotensin (Ang) II-induced upregulation of
the mRNA expression levels of TGF-β and CTGF (19). However, to the best of our
knowledge, the effects of GBE on tTG have not yet been elucidated.
Thus, the present study aimed to investigate the effects of GBE on
tTG expression in the diabetic kidneys of rats and high
glucose-treated mesangial cells to determine whether GBE exerts
protective mechanisms.
Materials and methods
GBE extract
GBE was purchased from JiangSu Xuzhou Huakang
Biological Products Co., Ltd. The extract was obtained through
ethanol extraction method and the contents of total Ginkgo
flavonol glycosides and terpene lactones in GBE were detected with
high-performance liquid chromatography by the aforementioned
company. According to the Chinese Pharmacopoeia (2015) (20), the total Ginkgo flavonol
glycosides content in GBE is 25.3% (recommended, >24.0%) and the
terpene lactones content in GBE is 6.37% (recommended, >6.0%),
its moisture content is 4.6% (recommended, ≤5.0%).
Chemical reagents
Streptozotocin (STZ) was purchased from
MilliporeSigma. Ginaton (GBE injection) was from Dr Willmar Schwabe
GmbH & Co. KG. Rabbit anti-tTG polyclonal antibody (cat. no.
121495) was from Abcam. Rabbit anti-CTGF polyclonal antibody (cat.
no. 323092), anti-TGF-β polyclonal antibody (cat. no. 324045),
HRP-anti-rabbit IgG and 3,3'-diaminobenzidine (DAB) were from
OriGene Technologies, Inc. TRIzol® reagent, molecular
weight protein marker, enhanced chemiluminescence (ECL),
Lipofectamine® 2000 and SuperScriptⅡ were all from
Thermo Fisher Scientific, Inc. Primers were purchased from RuiJie
Biological. PVDF membranes were from EMD Millipore. Rabbit anti-FN
(cat. no. 0666R) and anti-Col IV (cat. no. 0553R) polyclonal
antibodies were from BIOSS. Mouse anti-GAPDH polyclonal antibody
(cat. no. 365062) and peroxidase-conjugate secondary antibody (cat.
no. sc2004) were from Santa Cruz Biotechnology, Inc. Rat mesangial
cells (HBZY-1) were purchased from Wuxi BioHermes Biological Co.,
Ltd. DMEM was supplied by Gibco; Thermo Fisher Scientific, Inc.
TGF-β ELISA kit (cat. no. BMS623-3) was from Thermo Fisher
Scientific, Inc., FN ELISA kit (cat. no. EK0350) was from Wuhan
Boster Biological Technology, Ltd., and Col IV ELISA kit (cat. no.
H145) and BCA kit were from Nanjing Jiancheng Bioengineering
Institute. CTGF and tTG ELISA kits (cat. nos E90010Ra and E90053Ra)
were from YouerSheng Technology Co., Ltd. Mayer's hematoxylin
solution and periodic acid-schiff (PAS) staining were from Beijing
Dingguo Changsheng Biotechnology Co., Ltd.
DN animal model protocol
Healthy male Wistar rats (weight, 180-220 g; age, 6
weeks) were provided by the Laboratory Animal Center of Jilin
University. All the rats were housed in a pathogen-free facility
with free access to a standard dried chow diet and water throughout
the period of study. During the present study, the animals were
housed in a temperature of 22-26˚C and a humidity of 50-65% in a
controlled environment with a 12-h light/dark cycle. The padding
was changed twice a week and the health status was observed with no
mortalities. All the animal experiments were conducted following
internationally recognized guidelines proposed by the World
Association for the Protection of Animals on animal welfare and the
regulations on the administration of laboratory animals in China.
All the in vivo experiments were approved by the Animal
Experimental Ethical Inspection Committee of Jilin University,
School of Pharmaceutical Sciences (ethical permission code
20190014; Changchun, China).
A single intraperitoneal injection of 50 mg/kg STZ
was used to induce diabetes in the rats. The blood and urine
glucose levels of each rat were tested 3 days later. The DN animal
model standard was as follows: Blood glucose level ≥16.7 mmol/l and
24-h urinary albumin excretion >20-200 µg/min (21). The DN rats were randomly divided
into two groups, ten for DN group and ten for GBE group. Ten
healthy rats for the control group. The GBE group rats were
intragastrically administered with 100 mg/kg GBE once a day
(dissolved in 0.5% carboxymethylcellulose sodium; Beijing Dingguo
Changsheng Biotechnology Co., Ltd.), an equal volume of vehicle was
given in the control and DN groups for 12 weeks. The rats were
subsequently euthanized using pentobarbital sodium (150 mg/kg body
weight; Beijing Dingguo Changsheng Biotechnology Co., Ltd.) through
the intraperitoneal route, followed by cervical dislocation.
Measurement of blood glucose and renal
function
Blood and urine of all the rats were collected
before the animals were sacrificed at the end of study, and the
24-h urinary albumin excretion and blood glucose were examined
using biuret (Beijing Dingguo Changsheng Biotechnology Co., Ltd.)
and 7150 Automatic Biochemical Analyzer (Hitachi, Ltd.),
respectively.
PAS staining for mesangial matrix
expansion
Rats were sacrificed following GBE administration as
aforedescribed. Then the whole kidneys were removed completely and
fixed in 10% buffered formalin at room temperature for 24 h, kidney
tissues were cut longitudinally in the same position and embedded
in paraffin for a light microscopic study (Olympus Corporation).
The 5 µm kidney tissue sections were all stained with PAS reagent.
The paraffin sections were incubated in periodate alcohol solution
(0.088 mol/l periodic acid, 0.05 mol/l sodium acetate and 4.30
mol/l ethyl alcohol) at 17-20˚C for 10 min. Afterwards, they were
washed with 70% ethanol and transferred into reductant (0.125 mol/l
sodium thiosulfate, 10.187 mol/l ethyl alcohol, 0.02 mol/l HCl and
0.12 mol/l KI) at 17-20˚C for a 10-min incubation. Subsequently,
the sections were washed with 70% alcohol again and soaked in the
PAS solution for 1-1.5 h at room temperature. The sections were
rinsed with running water for 10 min. The nuclei of cells were
stained with Mayer's hematoxylin for 3-5 min at room temperature
followed by 1% hydrochloric acid alcohol (1% concentrated
hydrochloric acid and 99% ethyl alcohol) wash. Finally, the
sections were washed with running water for 3 min, and then
dehydrated to reinforce the diaphaneity and sealing. Glycogen and
mucin in the glomerulus were stained purple by PAS, whereas the
nuclei were stained blue by Mayer's hematoxylin. PAS-positive
staining areas were analyzed using Image-Pro Plus 6.0 (Media
Cybernetics, Inc.) analysis software, and total glomerular tuft
areas were analyzed using ImageJ software (version 1.51d; National
Institutes of Health). The glomerulosclerosis area (%) was
calculated using the following formula: (PAS-positive staining
area/total glomerular tuft area) x100%.
Immunohistochemical analysis of tTG
expression
The kidney paraffin sections, prepared as described
in the previous section, were dewaxed at 60˚C for 30 min, and
washed with boiled 0.01 mol/l PBS (pH 7.4, 0.0203 mol/l
Na2HPO4, 0.00167 mol/l
NaH2PO4, 0.1367 mol/l NaCl) three times (3
min each), blocked in 5% bovine serum albumin (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) at room temperature for 1 h.
Afterwards, the samples were incubated with rabbit anti-tTG
antibody for 24 h at 4˚C (1:300 dilution), the bound antibodies
were subsequently detected with HRP-anti-rabbit IgG for 20 min at
room temperature (1:1,000 dilution) and DAB, followed by
counterstaining with Mayer's hematoxylin for 2 min at room
temperature, and negative controls were incubated with PBS.
Finally, images were captured under a light microscope (Nikon
TE-2000U; Nikon Corporation), and analyzed using Image-Pro Plus 6.0
(Media Cybernetics, Inc.) analysis software.
Culture of HBZY-1 cells
HBZY-1 cells were cultured in low glucose (5.5 mM)
DMEM containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 10% newborn calf serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
at 37˚C with 5% CO2 and 95% air. Confluent cells were
used for experiments between passages 3 and 7. HBZY-1 cells at
85-90% confluence were seeded into six-well plates
(1x106 cells/well) and incubated with high glucose (30
mM) DMEM without serum and different concentrations (0, 3.125,
6.25, 12.5, 25 and 50 µg/ml) of Ginaton at 37˚C for 24 h. Control
group cells were cultured with low glucose (5.5 mM) DMEM without
serum at 37˚C for 24 h.
Semi-quantitative reverse
transcription-PCR (RT-PCR)
Total mRNA was extracted with TRIzol following the
manufacturer's protocol. RT-PCR reactions were prepared according
to the manufacturer's instructions of SuperScriptⅡ reverse
transcriptase (10,000 units total, at 200 U/µl; 5 X first-strand
buffer, 250 mol/l Tris-HCl, 375 mol/l KCl, 15 mol/l
MgCl2); 100 mol/l DTT), using 1 µg of total mRNA as the
template. The upstream and downstream primers were designed for rat
tTG mRNA, and GAPDH mRNA was used for sample normalization. The
sequences of the primers used were as follows: tTG forward,
5'-GGCAATGACTTTGACGTGTTTG-3' and reverse,
5'-ATACAGGGAATCAGAAAGTGGGTTC-3'; and GAPDH forward,
5'-ACCACAGTCCATGCCATCAC-3' and reverse, 5'-TCCACCACCCTGTTGCTGTA-3'.
The molecular sizes of the amplification products were 396 and 452
bp, respectively. The following thermocycling conditions were used
for semi-quantitative PCR with DNA polymerase (Thermo Fisher
Scientific, Inc.): Initial denaturation at 94˚C for 5 min; 30
cycles of 94˚C for 30 sec, 55˚C for 30 sec and 72˚C for 30 sec; and
a final extension at 72˚C for 10 min. PCR products (5 µl) were used
for gel electrophoresis (1% agarose gel) and target bands
visualized by ethidium bromide, the results were utilized to
quantify the intensity of nucleic acid bands with ImageJ 1.25
software and calculate the ratio of tTG/GAPDH.
Western blotting
Fresh frozen renal cortical tissues (100 µg) were
homogenized in 100 µl lysis buffer [0.01 mol/l Tris-HCl (pH 7.5),
0.1 mol/l NaCl, 0.001 mol/l EDTA, 100 µg/ml PMSF, 1 µg/ml
Aprotinin] for 30 min and thereafter centrifuged at 12,000 x g for
15 min at 4˚C. The BCA method was used to quantify the level of
protein in each sample to ensure equal protein loading. Proteins
were heated with 2X SDS-PAGE sample buffer [100 mol/l Tris-HCl (pH
6.8), 200 mol/l DTT, 4% SDS, 0.2% bromophenol blue, 20% glycerin]
for 5 min and separated by SDS-PAGE according to their molecular
weight. Briefly, 50 µl proteins, along with a molecular weight
protein marker, were subjected to SDS-PAGE (12% acrylamide gel) and
electroblotted onto PVDF membranes. The membranes were blocked with
5% non-fat milk in TBS containing 0.1% Tween-20 for 1 h at room
temperature and then probed at 4˚C with anti-tTG (1:300 dilution)
or anti-GAPDH antibody (1:500 dilution) overnight. After incubation
with peroxidase-conjugated secondary antibody (1:5,000 dilution;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature,
membranes were developed using ECL. In addition, rat mesangial
cells (HBZY-1) were cultured in vitro for further study,
confluent HBZY-1 cells were harvested from 6-well plates, lysed on
ice with 100 µl lysis buffer (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) per well and centrifuged at 12,000 x g for
15 min at 4˚C. The supernatant was collected and western blotting
was performed as described above. All the band intensities were
analyzed using ImageJ 1.25 software.
MTT assay
HBZY-1 cells in the logarithmic growth phase were
dissociated by trypsinization at 37˚C for 2 min and seeded into
96-well plates at a density of 2x104 cells/ml and 200 µl
cell suspension/well overnight. Cells were treated with different
concentrations of GBE injection, as described previously, under
high glucose (30 mM) DMEM at 37˚C for 72 h in order to estimate
whether this agent induced cell injury. Subsequently, 20 µl MTT (5
mg/ml in PBS) solution was added into each well and the samples
were incubated at 37˚C for 4 h. A total of 150 µl dimethylsulfoxide
was added to each well and the plates were placed on a shaker at
room temperature for 10 min. The absorbance at 570 nm was measured
with a microplate reader (SpectraMax Plus384; Molecular Devices,
LLC). The percentage of surviving cells was calculated as a
fraction of the negative control group cells which were treated
with an equal volume of low glucose (5.5 mM) DMEM.
ELISA for testing the contents of FN,
Col IV, tTG, TGF-β and CTGF
The cells were treated as described in previous
sections, then culture supernatant of each sample was collected and
diluted with a coating buffer (pH 9.6, 0.06 mol/l carbonate) at a
dilution of 1:9. A total of 100 µl diluent was added to each well
in the ELISA plate at 4˚C overnight and the coating buffer without
supernatant fluid was used as the control. The coating buffer was
removed and samples washed three times with PBS with 5% Tween-20 (3
min each). The wells were blocked with PBS containing 2% fetal calf
serum (Gibco; Thermo Fisher Scientific, Inc.) for 1.5 h at 37˚C.
The plate was subsequently washed again and incubated with
antibodies against tTG, TGF-β, CTGF, FN and Col IV (1:1,000
dilution) for 1.5 h at 37˚C. After incubation with a HRP-conjugated
secondary antibody (1:2,000 dilution) for 1 h at 37˚C the
supernatant fluid was disposed of and O-phenylenediamine was added
and incubated in the dark at room temperature for 5 min.
H2SO4 was added to stop the reaction and the
absorbance value was detected at a wavelength of 490 nm with a
DG5033A Automatic Microplate ELISA Analyzer (Nanjing Huadong
Electronics Group Medical Equipment Co., Ltd.).
Transfection with small interfering
RNA (siRNA) against TGF-β and CTGF
HBZY-1 were cultured in 6-well plates to 85-90%
confluence, at which point they were transfected with four separate
gene phosphorylated double-stranded siRNA oligonucleotides
targeting TGF-β (5'-GCAACAAUUCCUGGCGUUA-3',
5'-GCAACAACGCAAUCUAUGA-3', 5'-GGACUACGCCAAAGAA-3' and
5'-GAACCAAGGAGACGGAAUA-3') or CTGF (5'-GAAGACGCGUUUGGCCCUG-3',
5'-GACAAUACCUUCUGCAGGC-3', 5'-GUGAAGACCUACCGGGCUA-3' and
5'-CCAAAGCAGUUGCAAAUAC-3') as well as non-specific pooled duplex
negative control siRNA (5'-AUGAACGUGAAUUGCUCAAUU-3' and
5'-UUGAGCAAUUCACGUUCAUUU-3') using Lipofectamine® 2000,
according to the manufacturer's protocol. HBZY-1 at density of
1x106 cells/well were plated for at least 24 h before
transfection, and transfected with 5 µl siRNA at 37˚C for 6 h. A
total of 6 h after transfection, the HBZY-1 were exposed to 12.5
µg/ml of GBE for 72 h, and cultured with high glucose (30 mM) DMEM,
the control siRNA group were transfected with 5 at 37˚C for 6 h and
exposed only to high glucose DMEM. Finally, tTG protein expression
levels were detected by western blotting, as described above.
Statistical analysis
Statistical analysis was performed using the SPSS
22.0 statistical package (IBM Corp.). The results are presented as
the mean ± SD, and three repeats were performed for each
experiment. Differences among all groups were evaluated using
one-way analysis of variance with Tukey's post hoc test,
correlation analysis were evaluated using Pearson's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
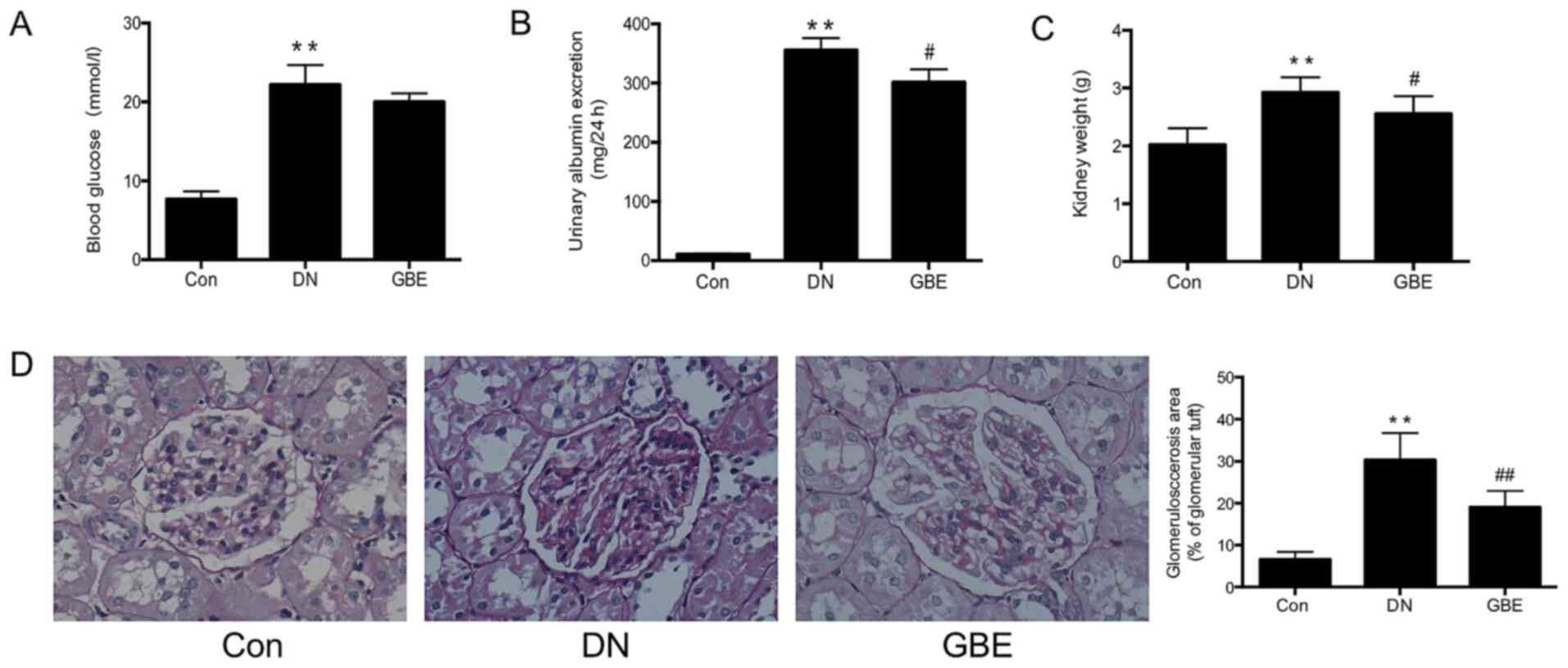
GBE decreases blood glucose levels and
relieves renal injury in a rat model of DN
In the present study, the rat models of DN had blood
glucose levels of ≥13.8 mmol/l and a 24-h urinary albumin excretion
of >20-200 µg/min (28.8-288 mg/24 h), which were significantly
higher compared with those in the control group. In addition, the
kidney weights of rats with DN were significantly elevated compared
with those in the control group (all P<0.01; Fig. 1A-C), and exhibited an increase in
the glomerulosclerosis area (P<0.01; Fig. 1D). These results indicated that the
STZ-induced rat model of DN had been successfully established.
Decreased 24-h urinary albumin excretion levels and lower kidney
weights were observed in the GBE group compared with those in the
DN group (P<0.05), and the glomerulosclerosis area was
significantly decreased (P<0.01), suggesting that GBE may not
significantly reduce glucose levels, while significantly decrease
the renal injury of DN rats.
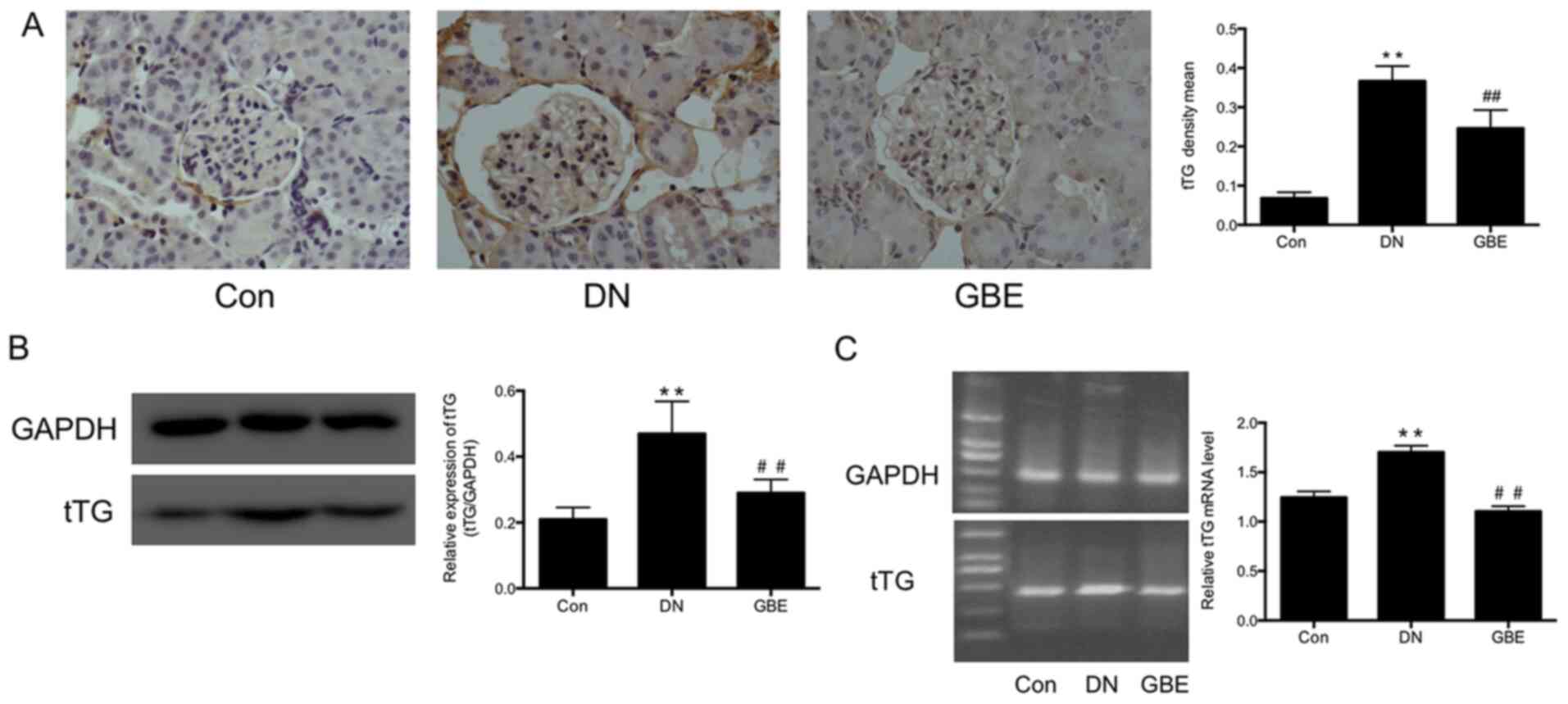
GBE inhibits tTG expression in rat
models of DN
In the present study, histological examination was
performed to detect tTG expression levels in kidney tissues, and
western blot and PCR analyses were further performed to detect tTG
protein and mRNA expression levels, respectively.
Immunohistochemistry analysis demonstrated that tTG expression was
significantly upregulated in the kidney glomeruli and tubules of
rat models of DN compared with that in the control group
(P<0.01; Fig. 2A). Furthermore,
tTG protein (P<0.01; Fig. 2B)
and mRNA levels (P<0.01; Fig.
2C) were significantly higher in DN rats compared with those in
the control rats. Notably, following treatment with GBE, tTG
protein (P<0.01) and mRNA (P<0.01) levels were significantly
decreased compared with those in the DN group.
GBE decreases the expression levels of
FN, Col IV, tTG, TGF-β and CTGF in high glucose-induced HBZY-1
cells
To determine whether GBE exerted protective effects
on HBZY-1 cells, cell viability was assessed using an MTT assay.
HBZY-1 cells were treated with different concentrations of GBE for
72 h. The results demonstrated that GBE failed to significantly
alter the viability of HBZY-1 cells compared with the control group
(Fig. 3A).
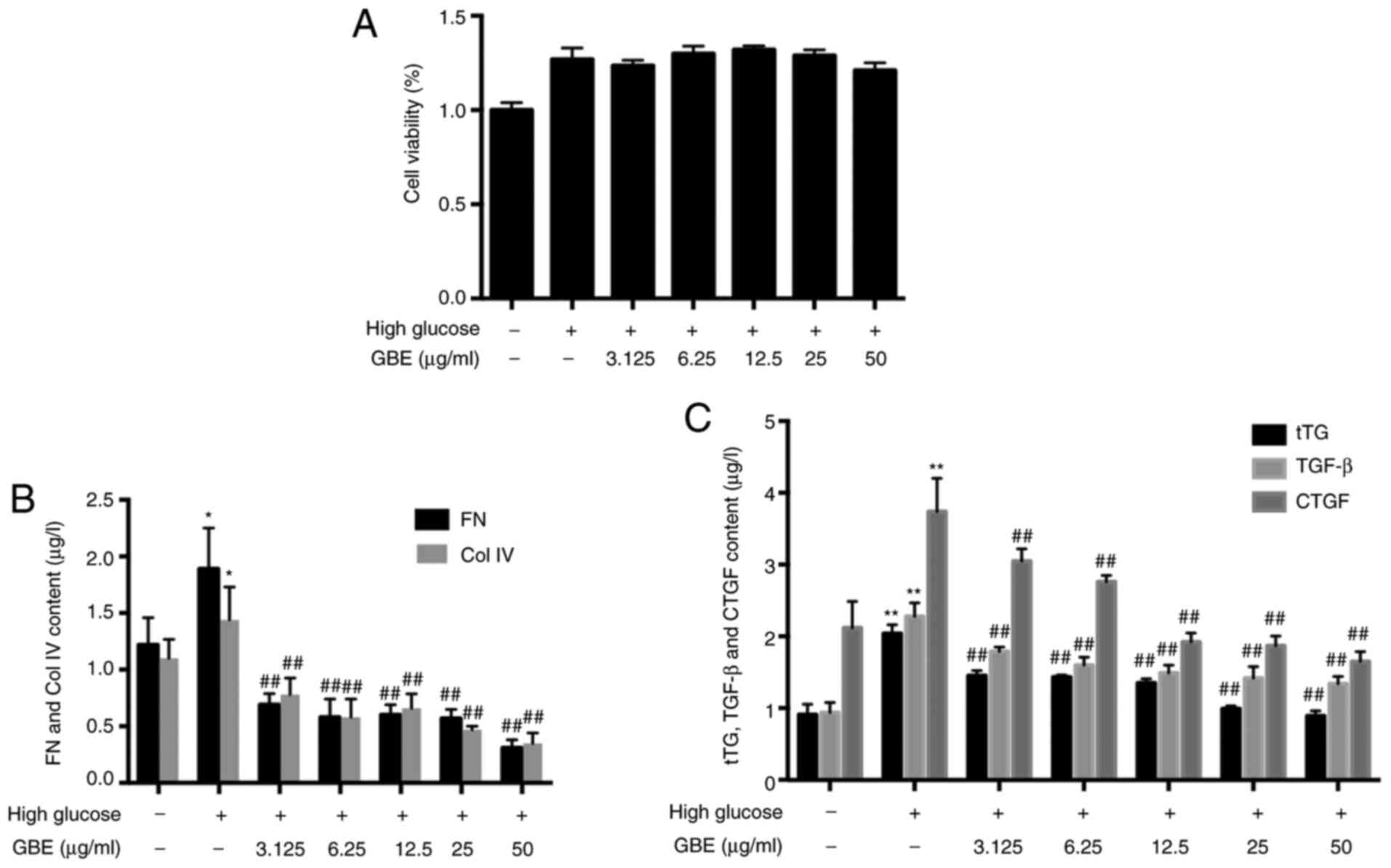 | Figure 3Effects of GBE on secretion of FN,
Col IV, tTG, TGF-β and CTGF. (A) Effect of GBE on cell viability.
(B) Protein levels of FN and Col IV. (C) Protein levels of tTG,
TGF-β and CTGF. Data are presented as the mean ± SD.
*P<0.05 and **P<0.01 vs. low glucose
control group; ##P<0.01 vs. high glucose group. tTG,
tissue transglutaminase; FN, fibronectin; Col IV, type IV collagen;
TGF-β, transforming growth factor-β; CTGF, connective tissue growth
factor; GBE, Ginkgo biloba leaf extract. |
The results of the present study demonstrated that
Col IV and FN protein levels were increased in HBZY-1 cells treated
with high glucose (P<0.05; Fig.
3B). Following treatment with different concentrations of GBE
(3.125, 6.25, 12.5, 25 and 50 µg/ml) the levels of Col IV and FN
were decreased (P<0.01).
The protein expression levels of tTG, TGF-β and CTGF
were increased in high glucose-treated HBZY-1 cells compared with
those in low glucose-treated HBZY-1 cells (P<0.01; Fig. 3C). Furthermore, GBE significantly
downregulated the expression levels of tTG, TGF-β and CTGF
(P<0.01). Correlation between tTG and TGF-β or CTGF was
assessed, and the results demonstrated that tTG expression was
closely positive associated with both TGF-β and CTGF expression
(P<0.0001; Table I).
 | Table ICorrelation analysis between tTG and
TGF-β and CTGF protein expression levels. |
Table I
Correlation analysis between tTG and
TGF-β and CTGF protein expression levels.
| | tTG expression |
|---|
| Parameter | Pearson's
correlation (r values) | n |
|---|
| TGF-β
expression | 0.867a | 48 |
| CTGF
expression | 0.924a | 48 |
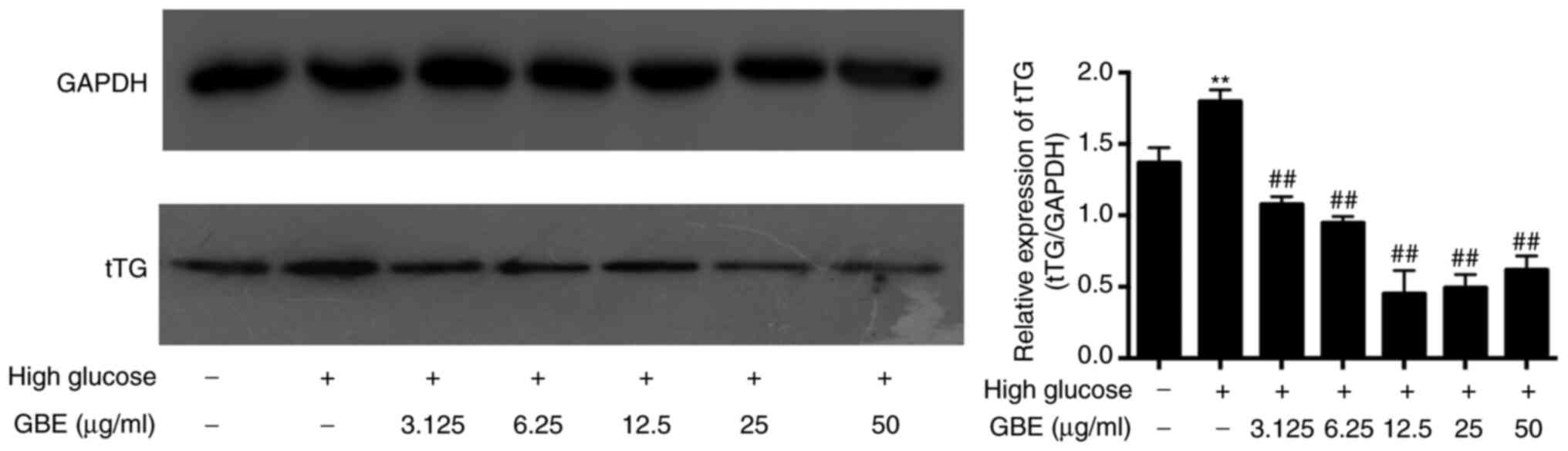
GBE inhibits tTG expression in high
glucose-cultured HBZY-1
Western blot analysis was performed to detect tTG
protein expression in high glucose-treated HBZY-1 cells and in
cells following treatment with different concentrations of GBE. The
results demonstrated that tTG protein expression was significantly
increased in high glucose-treated HBZY-1 cells compared with that
in low glucose-treated control cells (P<0.01; Fig. 4). Notably, tTG expression decreased
following treatment with GBE, in a concentration-dependent manner
(P<0.01). Compared with the high glucose group, tTG expression
levels in the 12.5, 25 and 50 µg/ml GBE-treated groups were reduced
by >50%, and no statistically significant differences were
observed between the three groups. Thus, 12.5 µg/ml GBE was
selected for subsequent experimentation. Collectively, these
results suggest that GBE may decrease tTG expression in DN.
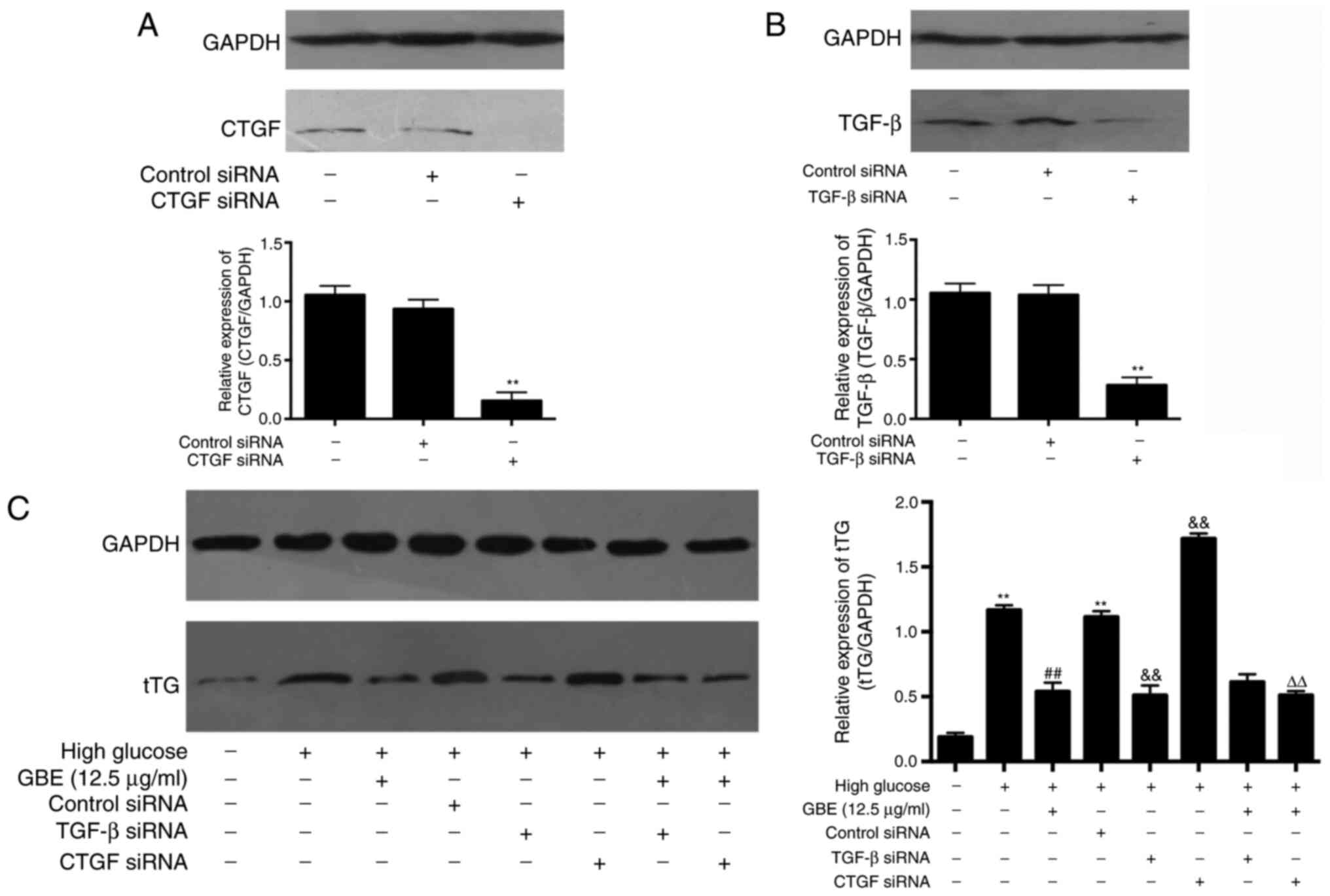
Role of TGF-β and CTGF in the
downregulation of tTG expression levels following GBE
treatment
Subsequent experiments were performed to confirm the
involvement of TGF-β and CTGF in the protective effects of GBE.
Western blot analysis demonstrated that transfection with CTGF or
TGF-β siRNA significantly inhibited the protein expression levels
of CTGF and TGF-β compared with those in the respective control
siRNA groups (Fig. 5A and B). In addition, transfection with CTGF
siRNA failed to significantly decrease tTG protein expression in
HBZY-1 cells, while GBE notably inhibited tTG expression in CTGF
siRNA-transfected HBZY-1 cells compared with the CTGF
siRNA-transfected only group (Fig.
5C), suggesting that increased tTG expression in HBZY-1 cells
under high glucose levels may not be regulated by CTGF, and the
inhibitory effect of GBE on tTG expression may be independent of
CTGF. Transfection with TGF-β siRNA significantly decreased tTG
protein expression compared with the control siRNA group
(P<0.01), while tTG expression was similar in the GBE, GBE +
TGF-β siRNA and GBE + CTGF siRNA groups, indicating that the
GBE-induced inhibition of tTG expression may be associated with
downregulating TGF-β expression.
Discussion
DN is a serious complication of diabetes and is
closely associated with the early mortality of affected patients
(1). Suppression of high blood
glucose levels and high blood pressure is a basic treatment of DN
(22). To a certain extent, these
methods can effectively delay the onset of kidney disease; however,
they fail to inhibit ECM accumulation, resulting in some patients
eventually developing end-stage renal disease (23,24).
Further studies are required to identify and develop novel
treatments to cure DN. Currently, Traditional Chinese Medicine has
become an important research focus, with promising new treatments,
such as ginkgo (25). ECM
accumulation in the glomerular mesangium and tubulointerstitium is
the main structural feature of DN, and the clinical diagnostic
indicators include thickening of the glomerular basement membrane
and broadening of the mesangial matrix, as well as albuminuria
(26,27). Preliminary studies have demonstrated
that GBE affects the protection of kidneys in STZ-induced diabetic
rats. For example, it has been reported that blood glucose, 24-h
urinary albumin excretion and ECM accumulation notably decreased
following treatment with GBE (28).
Earlier studies have demonstrated that extract of Ginkgo
biloba 761 can inhibit the thickening of the basement membrane
and ECM deposition in endotoxaemic rat kidneys, and increase
antioxidant enzyme activity to prevent renal tissue damage
(29). Lasaite et al
(30) treated patients with type 2
diabetes with GBE for 18 months, and revealed that the patients'
glycated hemoglobin levels notably decreased, while their quality
of life significantly improved. GBE is a Traditional Chinese
Medicine that has been previously reported to improve DN (31).
Under continuously high blood glucose levels, tTG
expression increases in the tubules and glomeruli (6), which regulates the aggregation of a
variety of ECM proteins, including FN, collagen and collagen
peptide, thus resulting in the accumulation of the ECM (7). The results of the present study
demonstrated that tTG expression increased in the kidneys of
diabetic rats, while GBE effectively decreased tTG protein and mRNA
levels. TGF-β is an important regulatory factor of tTG, which
functions as an inducer of several cytokines and fibrosis (32). A number of stimulating factors, such
as hyperglycemia, advanced glycation end products and oxidative
stress can stimulate the production of TGF-β (33,34).
In addition, growth factors, such as CTGF, are closely associated
with the promotive effect on fibrosis of TGF-β, and can enhance the
biological function of TGF-β by combining with the TGF-β domain
(35). In the present study, the
secretion of Col IV and FN was increased in HBZY-1 cells cultured
with high glucose, while GBE notably decreased the levels of FN and
Col IV, in a concentration-dependent manner. Furthermore, the
levels of tTG, TGF-β and CTGF were all decreased following
treatment with GBE in high glucose-cultured HBZY-1 cells, and tTG
expression was positively associated with TGF-β and CTGF
expression. The siRNA-mediated knockdown of TGF-β and CTGF was
performed to determine the potential inhibitory mechanism of GBE on
tTG. HBZY-1 cells transfected with TGF-β siRNA had a markedly
impaired ability to upregulate the expression levels of tTG
simulated by high glucose exposure, and tTG expression in these
cells was similar to that in the GBE-treated HBZY-1 cells
transfected with TGF-β siRNA. tTG expression was not significantly
suppressed following the siRNA-mediated knockdown of CTGF, whereas
tTG expression was notably downregulated following treatment with
GBE. Taken together, these results suggested that increased tTG
expression in HBZY-1 cells under high glucose environments may not
be directly mediated by CTGF but by TGF-β, and GBE may repress tTG
expression by inhibiting TGF-β, which is independent of CTGF. Thus,
it may be hypothesized that GBE protects ECM accumulation in DN
mainly by inhibiting tTG expression via regulation of TGF-β.
Cui et al (34) injected human recombinant TGF-β into
isolated perfused rat kidneys and the results revealed that tTG
mRNA expression was upregulated by 8-fold compared with that in
normal rats, resulting in the accumulation of ECM (36). Furthermore, tTG expression is
considered to be closely associated with CTGF expression (37). These findings may suggest potential
targets for the treatment of DN. DNA methylation is an important
regulatory mechanism of CTGF expression, whereby high glucose
levels can induce the demethylation process of the CTGF gene
promoter and increase CTGF expression in human glomerular mesangial
cells or DN model mice, thus altering the expression of its
downstream factor (38). In
addition, several microRNA (miRNAs/miRs) play an important role in
altering TGF-β expression levels; for example, upregulated miR-27a
expression is closely associated with diabetes, and high
glucose-induced upregulation of miR-27a expression activates
TGF-β/Smad3 signaling, which contributes to the upregulated changes
of CTGF in NRK-52E cells (39). In
GBE serum-treated mesangial cells cultured with high glucose
medium, Smad2/3 expression decreased, Smad7 expression increased,
while Col IV, laminin and TGF-β mRNA levels were decreased
(40). TGF-β receptor 1 has been
identified as a target of miR-130b. A previous study demonstrated
that miR-130b expression was significantly downregulated in mouse
glomerular mesangial cells treated with TGF-β. Notably, TGF-β
induced miR-130b suppression via nuclear transcription factor Y
subunit γ, which subsequently upregulated TGF-β receptor 1 to
increase the expression of TGF-β target fibrotic genes (such as
PAI-1), along with upregulating profibrotic genes (Col IV α1 and
CTGF) in the progression of DN (26).
The present experiments were the first to observe
whether GBE treatment has an effect on DN. After obtaining the
present results, screening experiments were performed to identify
possible effective chemical elements, and it was initially revealed
that the total flavonoids in GBE can notably inhibit ECM
accumulation, and a study on its possible mechanism will be
published in future. Meanwhile, the total lactone sof GBE,
including ginkgolide A, B, C, did not significantly inhibit ECM
accumulation, and ECM aggregation represents one of the mechanisms
of DN. Whether total lactone level has other renal protective
mechanisms requires further experiments to elucidate. In future
studies, microarray detection will be performed to identify the
potential miRNAs that are involved in the regulation of TGF-β by
GBE, and to further verify the potential protective mechanism of
GBE on DN, which may help to determine novel therapeutic strategies
for the prevention and treatment of kidney injury-associated
diseases.
In conclusion, the results of the present study
demonstrated that GBE decreased tTG expression both in DN rats and
rat mesangial cells in vitro under high glucose conditions,
and GBE may protect rat mesangial cells by inhibiting tTG via
modulating TGF-β expression, thus providing a novel strategy for
the treatment of DN.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Technology
Development Planning Projects of Jilin, China (grant nos.
20180414026GH and 20190201086JC) and the National Natural Science
Foundation of China (grant no. 30800423).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and QS conceived and designed the study. JG, HL,
YL, JL and YS performed the experiments. XY and QS wrote the
manuscript. YZ and XY analyzed the data. YZ reviewed and edited the
manuscript. YZ and XY confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All the in vivo experiments were approved by
the Animal Experimental Ethical Inspection Committee of Jilin
University School of Pharmaceutical Sciences (ethical permission
code, 20190014; Changchun, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guthrie RA and Guthrie DW: Pathophysiology
of diabetes mellitus. Crit Care Nurs Q. 27:113–125. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Islam MA, Amin MN, Siddiqui SA, Hossain
MP, Sultana F and Kabir MR: Trans fatty acids and lipid profile: A
serious risk factor to cardiovascular disease, cancer and diabetes.
Diabetes Metab Syndr. 13:1643–1647. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schmidt AM: Highlighting diabetes
mellitus: The epidemic continues. Arterioscler Thromb Vasc Biol.
38:e1–e8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tung CW, Hsu YC, Shih YH, Chang PJ and Lin
CL: Glomerular mesangial cell and podocyte injuries in diabetic
nephropathy. Nephrology (Carlton). 23 (Suppl 4):S32–S37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schelling JR: Tissue transglutaminase
inhibition as treatment for diabetic glomerular scarring: It's good
to be glueless. Kidney Int. 76:363–365. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Skill NJ, Johnson TS, Coutts IG, Saint RE,
Fisher M, Huang L, El Nahas AM, Collighan RJ and Griffin M:
Inhibition of transglutaminase activity reduces extracellular
matrix accumulation induced by high glucose levels in proximal
tubular epithelial cells. J Biol Chem. 279:47754–47762.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fleckenstein B, Qiao SW, Larsen MR, Jung
G, Roepstorff P and Sollid LM: Molecular characterization of
covalent complexes between tissue transglutaminase and gliadin
peptides. J Biol Chem. 279:17607–17616. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
El Nahas AM, Abo-Zenah H, Skill NJ, Bex S,
Wild G, Griffin M and Johnson TS: Elevated
epsilon-(gamma-glutamyl)lysine in human diabetic nephropathy
results from increased expression and cellular release of tissue
transglutaminase. Nephron Clin Pract. 97:c108–c117. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Skill NJ, Griffin M, El Nahas AM, Sanai T,
Haylor JL, Fisher M, Jamie MF, Mould NN and Johnson TS: Increases
in renal epsilon-(gamma-glutamyl)-lysine crosslinks result from
compartment-specific changes in tissue transglutaminase in early
experimental diabetic nephropathy: Pathologic implications. Lab
Invest. 81:705–716. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Loeffler I and Wolf G: Transforming growth
factor-β and the progression of renal disease. Nephrol Dial
Transplant. 29 (Suppl 1):i37–i45. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Border WA and Noble NA: Evidence that
TGF-beta should be a therapeutic target in diabetic nephropathy.
Kidney Int. 54:1390–1391. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sutariya B, Jhonsa D and Saraf MN: TGF-β:
The connecting link between nephropathy and fibrosis.
Immunopharmacol Immunotoxicol. 38:39–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee HS: Paracrine role for TGF-β-induced
CTGF and VEGF in mesangial matrix expansion in progressive
glomerular disease. Histol Histopathol. 27:1131–1141.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Roestenberg P, van Nieuwenhoven FA, Wieten
L, Boer P, Diekman T, Tiller AM, Wiersinga WM, Oliver N, Usinger W,
Weitz S, et al: Connective tissue growth factor is increased in
plasma of type 1 diabetic patients with nephropathy. Diabetes Care.
27:1164–1170. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wahab NA, Yevdokimova N, Weston BS,
Roberts T, Li XJ, Brinkman H and Mason RM: Role of connective
tissue growth factor in the pathogenesis of diabetic nephropathy.
Biochem J. 359:77–87. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rosenbloom J, Ren S and Macarak E: New
frontiers in fibrotic disease therapies: The focus of the Joan and
Joel rosenbloom center for fibrotic diseases at thomas jefferson
university. Matrix Biol. 51:14–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu Q, Yin XX, Wang JY, Gao YY and Pan YM:
Effects of Ginkgo biloba on prevention of development of
experimental diabetic nephropathy in rats. Acta Pharmacol Sin.
28:818–828. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ji L, Yin XX, Wu ZM, Wang JY, Lu Q and Gao
YY: Ginkgo biloba extract prevents glucose-induced
accumulation of ECM in rat mesangial cells. Phytother Res.
23:477–485. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Wang D, Li X, Yu X, Shi Y and Yin L:
Expression of tissue transglutaminase on renal interstitial
fibrosis rats and intervention of GBE. Zhongguo Zhong Yao Za Zhi.
34:1133–1136. 2009.PubMed/NCBI(In Chinese).
|
|
20
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the People's Republic of China. Vol. 1. Beijing.
China Medical Science and Technology Press, 2015.
|
|
21
|
Yang M, Kan L, Wu L, Zhu Y and Wang Q:
Effect of baicalin on renal function in patients with diabetic
nephropathy and its therapeutic mechanism. Exp Ther Med.
17:2071–2076. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Afghahi H, Svensson MK, Pirouzifard M,
Eliasson B and Svensson AM: Blood pressure level and risk of major
cardiovascular events and all-cause of mortality in patients with
type 2 diabetes and renal impairment: An observational study from
the swedish national diabetes register. Diabetologia. 58:1203–1211.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rogers NM, Teubner DJ and Coates PT:
Calcific uremic arteriolopathy: Advances in pathogenesis and
treatment. Semin Dial. 20:150–157. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Eberhardt W, Engels C, Müller R and
Pfeilschifter J: Mechanisms of dexamethasone-mediated inhibition of
cAMP-induced tPA expression in rat mesangial cells. Kidney Int.
62:809–821. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fang J, Wang Z, Wang P and Wang M:
Extraction, structure and bioactivities of the polysaccharides from
Ginkgo biloba: A review. Int J Biol Macromol. 162:1897–1905.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Castro NE, Kato M, Park JT and Natarajan
R: Transforming growth factor β1 (TGF-β1) enhances expression of
profibrotic genes through a novel signaling cascade and microRNAs
in renal mesangial cells. J Biol Chem. 289:29001–29013.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Solini A, Manca ML, Penno G, Pugliese G,
Cobb JE and Ferrannini E: Prediction of declining renal function
and albuminuria in patients with type 2 diabetes by metabolomics. J
Clin Endocrinol Metab. 101:696–704. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tian J, Popal MS and Liu Y, Gao R, Lyu S,
Chen K and Liu Y: Ginkgo biloba leaf extract attenuates
atherosclerosis in streptozotocin-induced diabetic
ApoE-/- mice by inhibiting endoplasmic reticulum stress
via restoration of autophagy through the mTOR signaling pathway.
Oxid Med Cell Longev. 2019(8134678)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Coskun O, Armutcu F, Kanter M and Kuzey
GM: Protection of endotoxin-induced oxidative renal tissue damage
of rats by vitamin E or/and EGb 761 treatment. J Appl Toxicol.
25:8–12. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Lasaite L, Spadiene A, Savickiene N,
Skesters A and Silova A: The effect of Ginkgo biloba and
Camellia sinensis extracts on psychological state and
glycemic control in patients with type 2 diabetes mellitus. Nat
Prod Commun. 9:1345–1350. 2014.PubMed/NCBI
|
|
31
|
Cui JF, Yang W, Xie YM, Sun Y, Zhuang Y
and Wang YY: Real-world analysis of concurrent diseases and
medicine use among patients with insomnia. Zhongguo Zhong Yao Za
Zhi. 39:3519–3526. 2014.PubMed/NCBI(In Chinese).
|
|
32
|
Pohlers D, Brenmoehl J, Löffler I, Müller
CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW and Wolf G:
TGF-beta and fibrosis in different organs-molecular pathway
imprints. Biochim Biophys Acta. 1792:746–756. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Serban AI, Stanca L, Geicu OI, Munteanu MC
and Dinischiotu A: RAGE and TGF-β1 cross-talk regulate
extracellular matrix turnover and cytokine synthesis in AGEs
exposed fibroblast cells. PLoS One. 11(e0152376)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cui Y, Robertson J, Maharaj S, Waldhauser
L, Niu J, Wang J, Farkas L, Kolb M and Gauldie J: Oxidative stress
contributes to the induction and persistence of TGF-β1 induced
pulmonary fibrosis. Int J Biochem Cell Biol. 43:1122–1133.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yin Q and Liu H: Connective tissue growth
factor and renal fibrosis. Adv Exp Med Biol. 1165:365–380.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Douthwaite JA, Johnson TS, Haylor JL,
Watson P and El Nahas AM: Effects of transforming growth
factor-beta1 on renal extracellular matrix components and their
regulating proteins. J Am Soc Nephrol. 10:2109–2119.
1999.PubMed/NCBI
|
|
37
|
Akimov SS and Belkin AM: Cell-surface
transglutaminase promotes fibronectin assembly via interaction with
the gelatin-binding domain of fibronectin: A role in
TGFbeta-dependent matrix deposition. J Cell Sci. 114:2989–3000.
2001.PubMed/NCBI
|
|
38
|
Zhang H, Cai X, Yi B, Huang J, Wang J and
Sun J: Correlation of CTGF gene promoter methylation with CTGF
expression in type 2 diabetes mellitus with or without nephropathy.
Mol Med Rep. 9:2138–2144. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hou X, Tian J, Geng J, Li X, Tang X, Zhang
J and Bai X: MicroRNA-27a promotes renal tubulointerstitial
fibrosis via suppressing PPARγ pathway in diabetic nephropathy.
Oncotarget. 7:47760–47776. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tang D, Zhang Z, Gao Y, Wei Y and Han L:
Protective effects of serum containing Ginkgo biloba extract
on glomerulosclerosis in rat mesangial cells. J Ethnopharmacol.
124:26–33. 2009.PubMed/NCBI View Article : Google Scholar
|