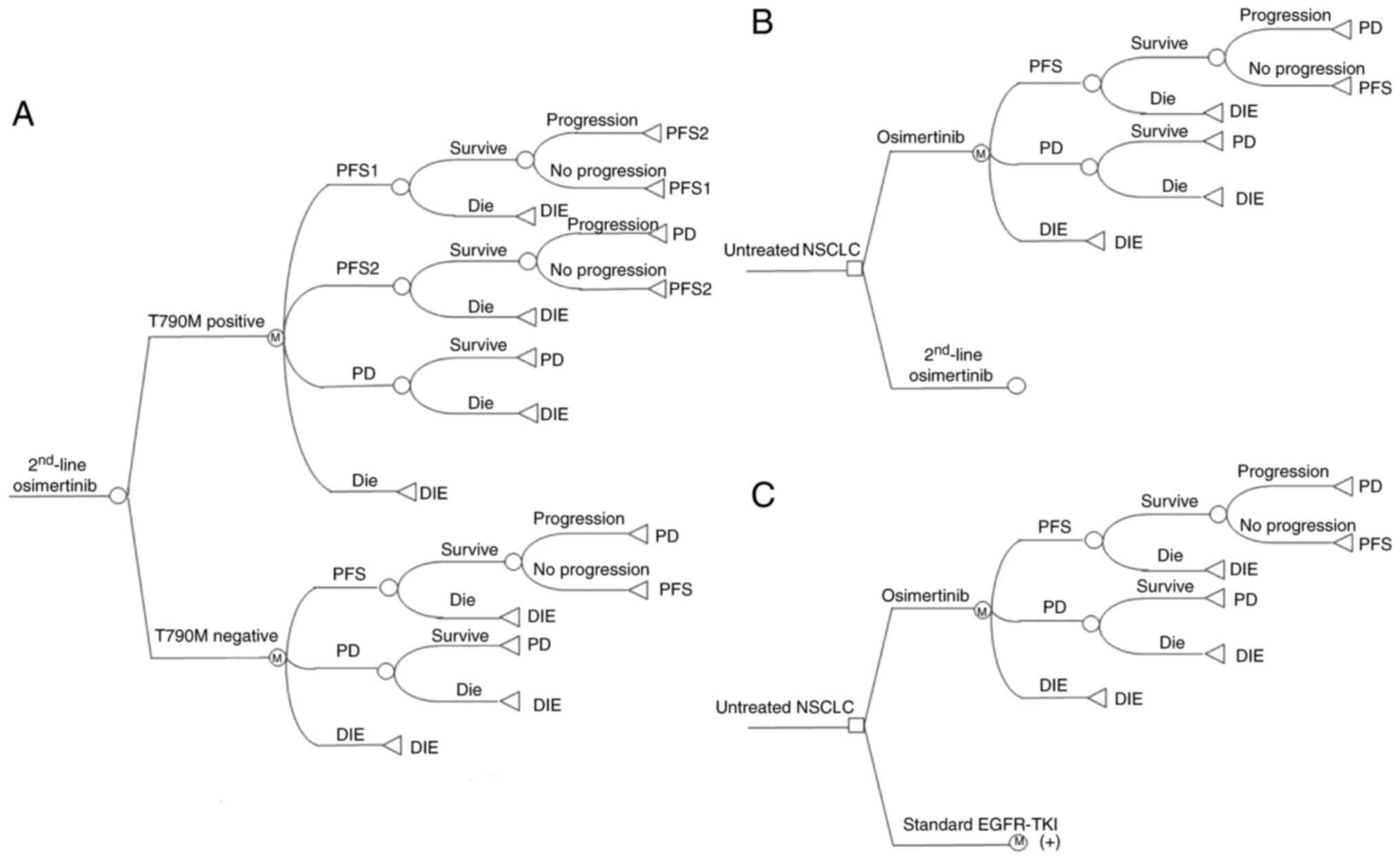
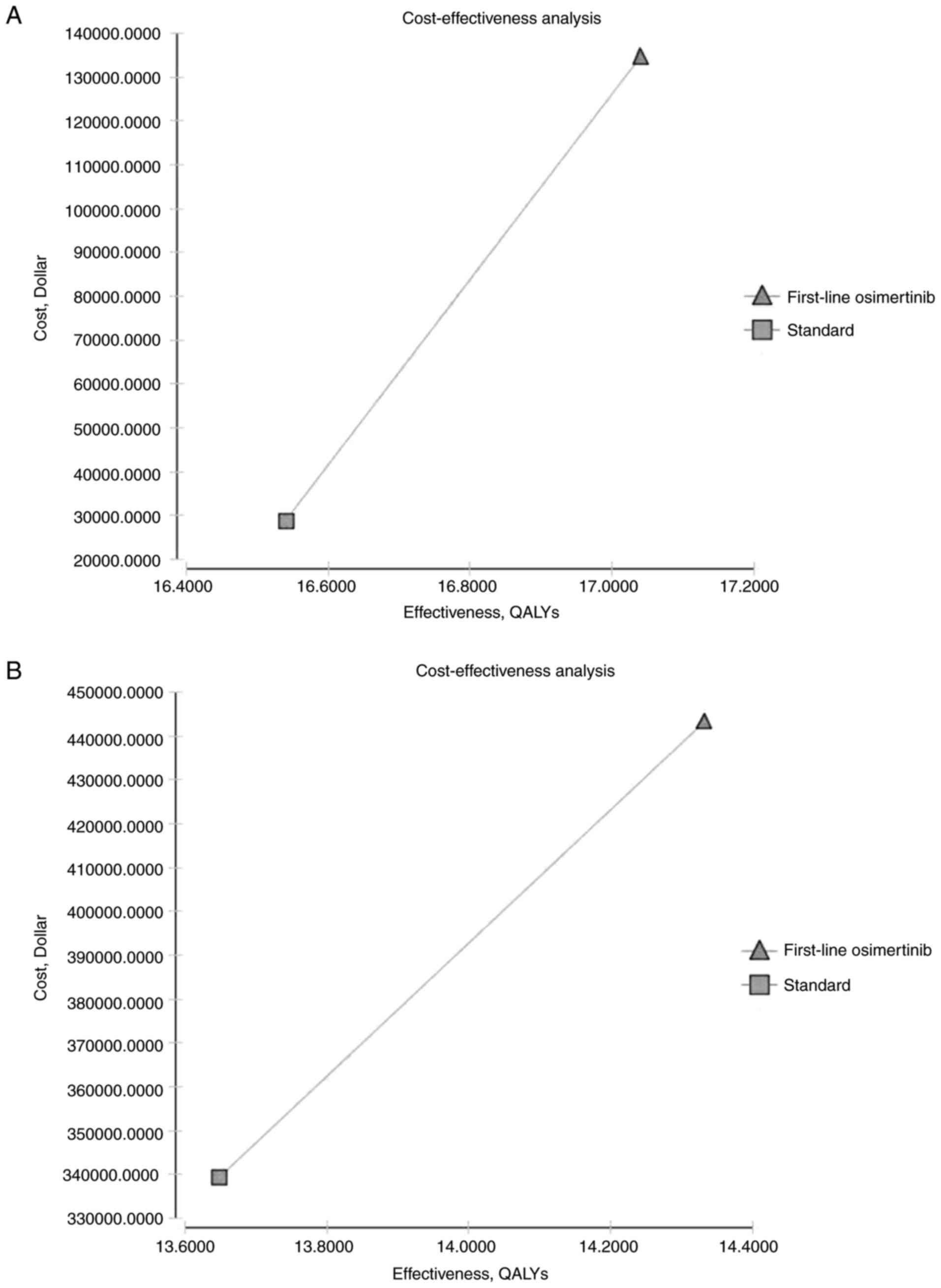
|
1
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000-14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rotow J and Bivona TG: Understanding and
targeting resistance mechanisms in NSCLC. Nat Rev Cancer.
17:637–658. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lategahn J, Keul M and Rauh D: Lessons to
be learned: The molecular basis of kinase-targeted therapies and
drug resistance in non-small cell lung cancer. Angew Chem Int Ed
Engl. 57:2307–2313. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Meza R, Meernik C, Jeon J and Cote ML:
Lung cancer incidence trends by gender, race and histology in the
United States, 1973-2010. PLoS One. 10(e0121323)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lindeman NI, Cagle PT, Aisner DL, Arcila
ME, Beasley MB, Bernicker EH, Colasacco C, Dacic S, Hirsch FR, Kerr
K, et al: Updated molecular testing guideline for the selection of
lung cancer patients for treatment with targeted tyrosine kinase
inhibitors: Guideline from the college of American pathologists,
the international association for the study of lung cancer, and the
association for molecular pathology. Arch Pathol Lab Med.
142:321–346. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee CK, Davies L, Wu YL, Mitsudomi T,
Inoue A, Rosell R, Zhou C, Nakagawa K, Thongprasert S, Fukuoka M,
et al: Gefitinib or erlotinib vs chemotherapy for EGFR
mutation-positive lung cancer: Individual patient data
meta-analysis of overall survival. J Natl Cancer Inst.
109:2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hsu WH, Yang JC, Mok TS and Loong HH:
Overview of current systemic management of EGFR-mutant NSCLC. Ann
Oncol. 29 (Suppl 1):i3–i9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Popat S: Osimertinib as first-line
treatment in EGFR-mutated non-small-cell lung cancer. N Engl J Med.
378:192–193. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lin CC, Shih JY, Yu CJ, Ho CC, Liao WY,
Lee JH, Tsai TH, Su KY, Hseih MS, Chang YL, et al: Outcomes in
patients with non-small-cell lung cancer and acquired Thr790Met
mutation treated with osimertinib: A genomic study. Lancet Respir
Med. 6:107–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ramalingam SS, Yang JC, Lee CK, Kurata T,
Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, et al:
Osimertinibas first-line treatment of EGFR mutation-positive
advanced non-small-cell lung cancer. J Clin Oncol. 36:841–849.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Goss G, Tsai CM, Shepherd FA, Bazhenova L,
Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, et al:
Osimertinib for pretreated EGFR Thr790Met-positive advanced
non-small-cell lung cancer (AURA2): A multicentre, open-label,
single-arm, phase 2 study. Lancet Oncol. 17:1643–1652.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang JC, Ahn MJ, Kim DW, Ramalingam SS,
Sequist LV, Su WC, Kim SW, Kim JH, Planchard D, Felip E, et al:
Osimertinib in pretreated T790M-positive advanced non-small-cell
lung cancer: AURA study phase II extension component. J Clin Oncol.
35:1288–1296. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Osimertinib (TAGRISSO). https://www.fda.gov/default.htm.
|
|
17
|
EMEA-002125-PIP01-17. http://www.ema.europa.eu/ema/.
|
|
18
|
Osimertinib (TAGRISSO). http://www.sfda.gov.cn/WS01/CL0001/.
|
|
19
|
Novello S, Barlesi F, Califano R, Cufer T,
Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al:
Metastatic non-small-cell lung cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
(Suppl 5):v1–v27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ramalingam SS, Yang JC, Lee CK, Kurata T,
Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, et al:
Osimertinib as first-line treatment of EGFR mutation-positive
advanced non-small-cell lung cancer. J Clin Oncol. 36:841–849.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Remon J, Steuer CE, Ramalingam SS and
Felip E: Osimertinib and other third-generation EGFR TKI in
EGFR-mutant NSCLC patients. Ann Oncol. 29 (Suppl 1):i20–i27.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chamberlain MC, Baik CS, Gadi VK, Bhatia S
and Chow LQ: Systemic therapy of brain metastases: Non-small cell
lung cancer, breast cancer, and melanoma. Neuro Oncol. 19:i1–i24.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Planchard D, Brown KH, Kim DW, Kim SW, Ohe
Y, Felip E, Leese P, Cantarini M, Vishwanathan K, Jänne PA, et al:
Osimertinib Western and Asian clinical pharmacokinetics in patients
and healthy volunteers: Implications for formulation, dose, and
dosing frequency in pivotal clinical studies. Cancer Chemother
Pharmacol. 77:767–776. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Goss G, Tsai CM, Shepherd FA, Ahn MJ,
Bazhenova L, Crinò L, de Marinis F, Felip E, Morabito A, Hodge R,
et al: CNS response to osimertinib in patients with T790M-positive
advanced NSCLC: Pooled data from two Phase II trials. Ann Oncol.
29:687–693. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu B, Gu X and Zhang Q: Cost-effectiveness
of osimertinib for EGFR mutation-positive non-small cell lung
cancer after progression following first-line EGFR TKI therapy. J
Thorac Oncol. 13:184–193. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bertranou E, Bodnar C, Dansk V, Greystoke
A, Large S and Dyer M: Cost-effectiveness of osimertinib in the UK
for advanced EGFR-T790M non-small cell lung cancer. J Med Econ.
21:113–121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aguiar PN Jr, Haaland B, Park W, San Tan
P, Del Giglio A and de Lima Lopes G Jr: Cost-effectiveness of
osimertinib in the first-line treatment of patients with
EGFR-mutated advanced non-small cell lung cancer. JAMA Oncol.
4:1080–1084. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu B, Gu X, Zhang Q and Xie F:
Cost-effectiveness of osimertinib in treating newly diagnosed,
advanced EGFR-mutation-positive non-small cell lung cancer.
Oncologist. 24:349–357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang S, Peng L, Li J, Zeng X, Ouyang L,
Tan C and Lu Q: A trial-based cost-effectiveness analysis of
erlotinib alone versus platinum-based doublet chemotherapy as
first-line therapy for eastern asian nonsquamous non-small-cell
lung cancer. PLoS One. 8(e55917)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee VW, Schwander B and Lee VH:
Effectiveness and cost-effectiveness of erlotinib versus geftinib
in frst-line treatment of epidermal growth factor
receptor-activating mutation-positive non-small-cell lung cancer
patients in Hong Kong. Hong Kong Med J. 20:178–86. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
NSCLC Meta-Analyses Collaborative Group.
Chemotherapy in addition to supportive care improves survival in
advanced non-small-cell lung cancer: A systematic review and
meta-analysis of individual patient data from 16 randomized
controlled trials. J ClinOncol. 26:4617–4625. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
National healthcare security
administration. http://www.nhsa.gov.cn/.
|
|
34
|
New York State Registered Pharmacy.
https://www.rxusa.com/wp/.
|
|
35
|
Zeng X, Karnon J, Wang S, Wu B, Wan X and
Peng L: The cost of treating advanced non-small cell lung cancer:
Estimates from the Chinese experience. PLoS One.
7(e48323)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Isla D, De Castro J, Juan O, Grau S,
Orofino J, Gordo R, Rubio-Terrés C and Rubio-Rodríguez D: Costs of
adverse events associated with erlotinib or afatinib in first-line
treatment of advanced EGFR-positive non-small cell lung cancer.
Clinicoecon Outcomes Res. 9:31–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang S, Peng L, Li J, Zeng X, Ouyang L,
Tan C and Lu Q: A trial-based cost-effectiveness analysis of
erlotinib alone versus platinum-based doublet chemotherapy as
first-line therapy for Eastern Asian nonsquamous non-small-cell
lung cancer. PLoS One. 8(e55917)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Klein R, Wielage R, Muehlenbein C, Liepa
AM, Babineaux S, Lawson A and Schwartzberg L: Cost-effectiveness of
pemetrexed as first-line maintenance therapy for advanced
nonsquamous non-small cell lung cancer. J Thorac Oncol.
5:1263–1272. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kumar G, Woods B, Hess LM, Treat J, Boye
ME, Bryden P and Winfree KB: Cost-effectiveness of first-line
induction and maintenance treatment sequences in non-squamous
non-small cell lung cancer (NSCLC) in the U.S. Lung Cancer.
89:294–300. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wu B, Ye M, Chen H and Shen JF: Costs of
trastuzumab in combination with chemotherapy for HER2-positive
advanced gastric or gastroesophageal junction cancer: An economic
evaluation in the Chinese context. Clin Ther. 34:468–479.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhu J, Li T, Wang X, Ye M, Cai J, Xu Y and
Wu B: Gene-guided gefitinib switch maintenance therapy for patients
with advanced EGFR mutation-positive non-small cell lung cancer: An
economic analysis. BMC Cancer. 13(39)2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Nafees B, Lloyd AJ, Dewilde S, Rajan N and
Lorenzo M: Health state utilities in non-small cell lung cancer: An
international study. Asia Pac J Clin Oncol. 13:e195–e203.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Labbé C, Leung Y, Silva Lemes JG, Stewart
E, Brown C, Cosio AP, Doherty M, O'Kane GM, Patel D, Cheng N, et
al: Real-world EQ5D health utility scores for patients with
metastatic lung cancer by molecular alteration and response to
therapy. Clin Lung Cancer. 18:388–395.e4. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang JJ, Zhou C, Huang Y, Feng J, Lu S,
Song Y, Huang C, Wu G, Zhang L, Cheng Y, et al: Icotinib versus
whole-brain irradiation in patients with EGFR-mutant non-small-cell
lung cancer and multiple brain metastases (BRAIN): A multicentre,
phase 3, open-label, parallel, randomised controlled trial. Lancet
Respir Med. 5:707–716. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shi Y, Zhang L, Liu X, Zhou C, Zhang L,
Zhang S, Wang D, Li Q, Qin S, Hu C, et al: Icotinib versus
gefitinib in previously treated advanced non-small-cell lung cancer
(ICOGEN): A randomised, double-blind phase 3 non-inferiority trial.
Lancet Oncol. 14:953–961. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Park K, Tan EH, O'Byrne K, Zhang L, Boyer
M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al: Afatinib versus
gefitinib as first-line treatment of patients with EGFR
mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase
2B, open-label, randomised controlled trial. Lancet Oncol.
17:577–589. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhao Y, Liu J, Cai X, Pan Z, Liu J, Yin W,
Chen H, Xie Z, Liang H, Wang W, et al: Efficacy and safety of first
line treatments for patients with advanced epidermal growth factor
receptor mutated, non-small cell lung cancer: Systematic review and
network meta-analysis. BMJ. 367(l5460)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wu B, Dong B, Xu Y, Zhang Q, Shen J, Chen
H and Xue W: Economic evaluation of first-line treatments for
metastatic renal cell carcinoma: A cost-effectiveness analysis in a
health resource-limited setting. PLoS One. 7(e32530)2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu B, Chen H, Shen J and Ye M:
Cost-effectiveness of adding rh-endostatin to first-line
chemotherapy in patients with advanced non-small-cell lung cancer
in China. Clin Ther. 33:1446–1455. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Eichler HG, Kong SX, Gerth WC, Mavros P
and Jönsson B: Use of cost-effectiveness analysis in health-care
resource allocation decision-making: How are cost-effectiveness
thresholds expected to emerge? Value Health. 7:518–528.
2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Murray CJ, Evans DB, Acharya A and
Baltussen RM: Development of WHO guidelines on generalized
cost-effectiveness analysis. Health Econ. 9:235–251.
2000.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hughes DL: Patent review of manufacturing
routes to oncology drugs: Carfilzomib, osimertinib, and venetoclax.
Org Process Res Dev. 20:2028–2042. 2016.
|
|
53
|
Haspinger ER, Agustoni F, Torri V,
Gelsomino F, Platania M, Zilembo N, Gallucci R, Garassino MC and
Cinquini M: Is there evidence for different effects among
EGFR-TKIs? Systematic review and meta-analysis of EGFR tyrosine
kinase inhibitors (TKIs) versus chemotherapy as first-line
treatment for patients harboring EGFR mutations. Crit Rev Oncol
Hematol. 94:213–227. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Giuliani J and Bonetti A: Pharmacologic
costs of tyrosine kinase inhibitors in first-line therapy for
advanced non-small-cell lung cancer with activating epidermal
growth factor receptor mutations: A review of pivotal phase III
randomized controlled trials. Clin Lung Cancer. 17:91–94.
2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Gelatti ACZ, Drilon A and Santini FC:
Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in
epidermal growth factor receptor (EGFR) mutation-positive non-small
cell lung cancer (NSCLC). Lung Cancer. 137:113–122. 2019.PubMed/NCBI View Article : Google Scholar
|