1. Introduction
The outbreak and subsequent pandemic of Coronavirus
disease 2019 (COVID-19) is a public health emergency of
international concern (1). As of
December 16, 2020, a total of 71,581,532 confirmed cases and
1,618,374 deaths have been reported by the World Health
Organization (WHO) (2). COVID-19 is
caused by severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2), which was most likely transmitted to humans from wild
bats (3). SARS-CoV-2 closely
resembles SARS-CoV (79% sequence identity) and Middle East
respiratory syndrome coronavirus (MERS)-CoV (51.8% identity)
(4), both of which are also
believed to have originated in bats (5,6). All
three of these viruses are members of the Coronaviridae family.
Coronaviruses often cause a series of diseases in
humans and animals, ranging from the common cold to more severe
illness, such as pneumonia. Zoonotic transmission of coronaviruses,
such as SARS-CoV, SARS-CoV-2 and MERS-CoV, may be associated with
severe lower respiratory tract infections. These related infections
present as pneumonia as the primary clinical feature, sharing
symptoms including fever, cough and shortness of breath (7).
Although viruses within the same family often share
similarities in the pathogenesis of pneumonia, their imaging
results may exhibit distinguishable features. As for the
Coronaviridae family, imaging is an important basis for the
diagnosis and evaluation of the underlying viral infections.
However, to the best of our knowledge, no studies have summarized
the imaging features at different stages of coronavirus pneumonias.
To highlight the differences, the current review presents imaging
features at the early, progressive, severe and recovery phases of
these viruses.
2. Pathogenesis and pathological
manifestations of coronavirus
Coronavirus infections first enter susceptible host
cells by binding to specific receptors (8). Angiotensin converting enzyme 2 (ACE-2)
is a receptor of SARS-CoV and is expressed in tracheobronchial
epithelial cells, alveolar epithelial cells and in monocytes and
macrophages (9). The downregulation
of ACE-2 is considered to be associated with SARS-CoV-induced lung
injury (10). The structure of
SARS-CoV-2 is similar to that of SARS-CoV, suggesting that the
virus may utilize ACE-2 receptors in alveolar type II epithelial
cells for cell invasion, thereby replicating into bronchial
epithelial cells (11). Smoking and
obesity increases the expression of the ACE-2 gene, which explains
why smokers and obese individuals are susceptible to infection
(12,13). Smoking and obesity are also
independent risk factors for the deterioration of COVID-19
infection (12,13). ACE-2 receptors are present in many
animals, which enables inter-species contamination (14). The efficiency of binding depends on
the affinity between the receptor-binding domain of the virus and
the species-specific ACE-2 receptor (14). As such, it is likely that the
clinical characteristics and infectivity of SARS and COVID-19 are
similar, especially in severe cases (15). Dipeptidyl peptidase-4 is a receptor
for MERS-CoV, which is a versatile cell surface protein (16). This virus demonstrates high homology
in its primary and tertiary structure with the receptor-binding
domain of SARS-CoV (16). However,
the simulation of their protein structure exhibits a disparity in
the receptors (ACE-2 and dipeptidyl peptidase-4) between the two
coronaviruses, and the mechanism that causes this phenomenon
remains unclear (17).
Pathological changes of the lung observed in
patients with SARS-CoV infection are usually diffuse, involving
several lung lobes and manifesting as diffuse alveolar damage
(18,19). Histopathological assessment of
MERS-CoV infection has indicated necrotizing pneumonia, pulmonary
diffuse alveolar damage and acute kidney injury (20). On 27 January 2020, a death
attributed to COVID-19 was pathologically dissected for the first
time in China. The pulmonary manifestations were diffuse alveolar
injury and hyaline membranes, which are consistent with acute
respiratory distress syndrome (ARDS) (21). However, another report of five cases
revealed that no viral cytopathic changes were observed in
COVID-19. Moreover, diffuse alveolar injury with hyaline membrane
formation, inflammation and type II pneumocyte hyperplasia were not
prominent (22). Therefore,
microvascular injury alongside thrombosis may serve an important
role when hyaline membrane formation is not prominent in certain
patients. Although the overall pathological manifestations of the
lungs are similar to SARS and MERS, there are also differences.
3. Imaging at different stages of disease
progression
COVID-19, SARS and MERS are novel infectious
diseases with general stages of progression that are consistent
with other infectious diseases, such as influenza. These can
manifest as different clinical types following the natural course
of the disease and during the pathophysiological changes that occur
(18,19,23).
Combined with clinical classification and imaging features
(18,23), the progression of these diseases is
currently classed into four stages: Early, progressive, severe and
recovery.
Early stage
In this stage, clinical symptoms exhibited by
patients with COVID-19 are mild to moderate, although some patients
are asymptomatic. Usually there is no imaging evidence of pneumonia
in patients that are asymptomatic or those with mild symptoms, and
the changes of imaging are often atypical, which may result in
omissions. For example, Zhang et al (24) demonstrated that high-resolution
computed tomography (HRCT) exhibited multiple instances of
ground-glass opacity (GGO) and may be accompanied by consolidation
in patients with early stage COVID-19. The study also revealed that
certain patients did not present imaging results that were
indicative of pneumonia, and others exhibited normal chest
radiographs, but HRCT results revealed pneumonia. Therefore, with
imaging as an important supplement to the screening of COVID-19,
HRCT should be recommended as the initial imaging technique, as
X-rays often result in missed diagnoses in the early stage.
Pulmonary CT manifestations are usually as follows: i) GGO or
consolidation changes, in which multiple lesions on the bilateral
lung are common. The scope of consolidation is small and localized
(25); ii) the density of the
lesions is uneven, and they are distributed in a localized manner.
Generally, only parts of the lung segment are affected, mostly
within the extrapulmonary zone and the lower lung (25); iii) there are no manifestations of
mediastinal or hilar lymphadenopathy, pleural thickening or pleural
effusion (26). Typical HRCT
patterns of patients with early stage COVID-19 are presented in
Fig. 1A and B.
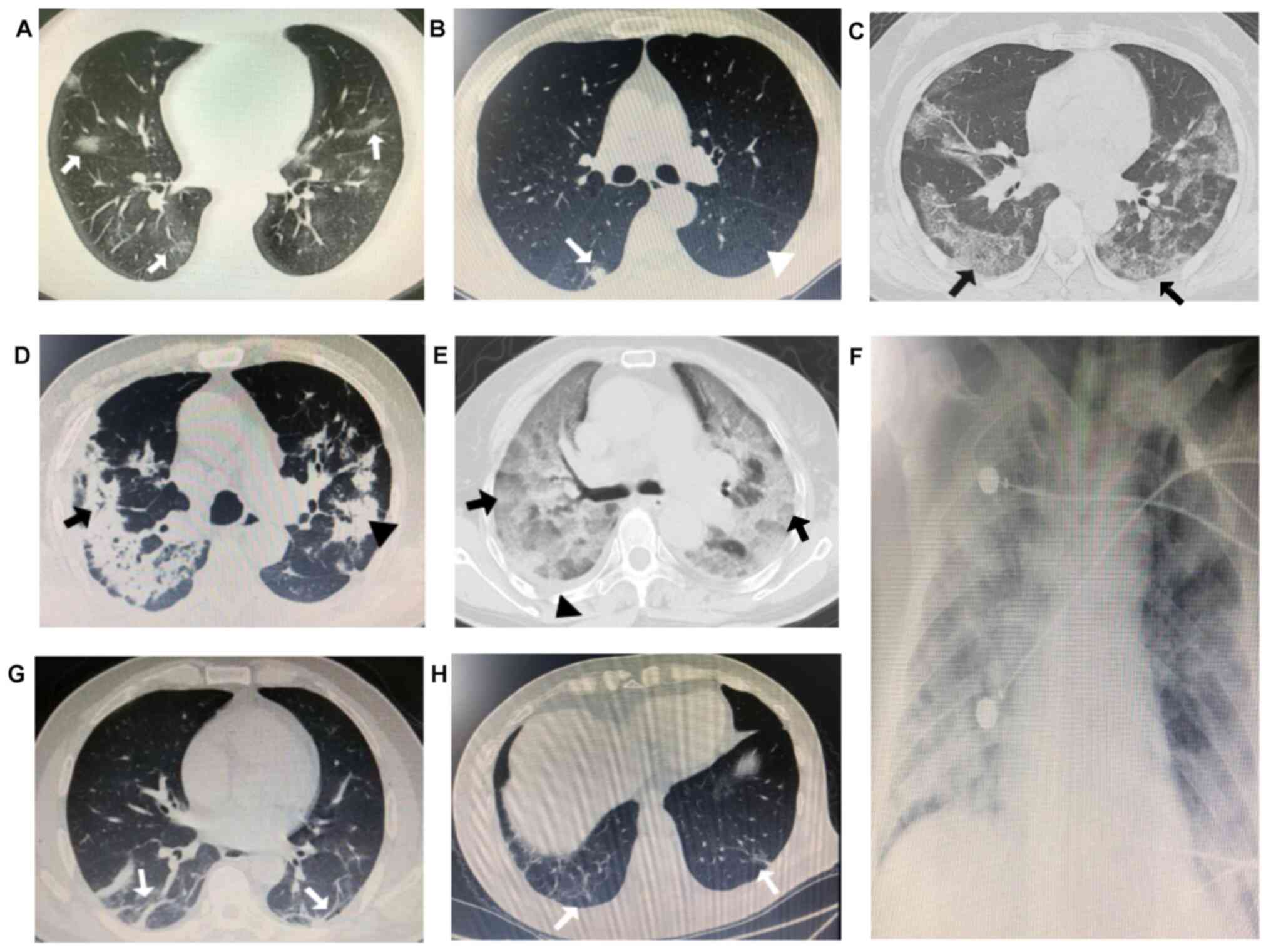 | Figure 1Imaging patterns of COVID-19. (A) A
29-year-old male with early stage COVID-19 exhibited mild fever,
aversion to cold, dry cough and dizziness. Chest CT demonstrated
multiple localized light and thin GGOs in the bilateral lower lung
(white arrows). (B) A 61-year-old male with early stage COVID-19
presented fever and limb weakness. Chest CT indicated localized
round consolidation of the right lower lung (white arrow) and
centrilobular emphysema of the left lower lung with reduced density
and no visible wall was visible in the bilateral lung (white
arrowhead). (C) A 47-year-old female with progressive stage
COVID-19 presented with fever, cough and shortness of breath. Chest
CT revealed multiple GGOs in the subpleural lung, accompanied by
reticular changes, presenting as ‘crazy-paving pattern’ (black
arrows). (D) A 71-year-old male with progressive stage COVID-19
exhibited wheezing, a cough and chest pain. Chest CT indicated
multiple consolidations in the bilateral lung (black arrowhead),
and signs of air bronchogram (black arrow). (E) A 72-year-old
female with severe stage COVID-19 presented with fever, dyspnea,
weakness and fatigue. Chest CT demonstrated large and diffuse GGO
with mixed consolidation in the bilateral lung, presenting as
‘white lung’ coalesced with a thickened interlobular septum (black
arrows) and a small degree of pleural effusion in the right lung
(black arrowhead). (F) A 35-year-old woman with severe stage
COVID-19 exhibited dyspnea and a minimally productive cough. X-rays
revealed an extensive range of lesions, with diffuse and exudative
lesions in the bilateral lungs. (G) A 56-year-old male with
recovery stage of COVID-19 presented with a dry cough only. Chest
CT revealed pulmonary fibrosis, scaring and stripe shadows in the
bilateral lower lung (white arrows). (H) A 71-year-old male with
recovery stage COVID-19 presented with a mild cough. Chest CT
indicated pulmonary fibrosis and stripe shadows in the bilateral
lower lung (white arrows). COVID-19, Coronavirus disease 2019. |
SARS is an acute infectious disease with fever as
the first and primary symptom, often occurring without upper
catarrhal symptoms (18). At the
early stage, the time from clinical symptom presentation to chest
imaging abnormalities is generally only 2-3 days. X-rays and CT
scans of the lungs demonstrate small or round-shaped GGO, with some
patients presenting this alongside lung consolidation. Single
lesions are more common, and those involving the lung segment are
rare. Most of the lesions are distributed in the lower field and
lateral bands of both lungs (18).
The early stage of MERS usually manifests as an
acute respiratory infection. Patients with low immune function or
underlying diseases, including coronary heart disease and diabetes,
may have more severe symptoms, such as dyspnea (27). However, for those without underlying
disease, symptoms are mild or asymptomatic, and some patients do
not exhibit imaging changes (28).
The primary features of the lung that are visible in HRCT images
are GGO changes and occasionally mixed consolidation or small
nodules, most of which are distributed in the subpleural and basal
lung regions (29,30). Some cases may demonstrate varying
degrees of pleural effusion (31,32).
Early imaging features of the three diseases share
several similarities. GGO is the primary symptom, and a small
degree of consolidation may be observed. Lesions generally do not
affect the entire lung segment and are most common in the lower
lung field and lateral bands. However, certain patients with
COVID-19 do not present changes in chest images in the early stage,
whereas patients with SARS demonstrate pneumonia within a short
period of time (2-3 days). The reasons for this difference may be
the duration of the viral incubation period, the method of virus
detection used or the popularity of pulmonary HRCT. The reason of
pulmonary HRCT being not commonly used is attributed to the cost of
the CT examination and the doctors' cognitive level of the
characteristics of different coronavirus diseases. The
aforementioned similarities and differences of early-stage features
of COVID-19, SARS and MERS are summarized in Tables I and II.
 | Table ISimilarities in imaging features of
COVID-19, SARS and MERS at each stage. |
Table I
Similarities in imaging features of
COVID-19, SARS and MERS at each stage.
| Stage | Similarities
between COVID-19, SARS and MERS |
|---|
| Early | GGO is the primary
feature. A small degree of consolidation is visible. Lesions are
typically localized and mostly involve the dorsal or lateral
segments of the middle and lower part of the lungs. Lesions are
more concentrated in the bilateral lower lung and the
extrapulmonary band. |
| Progressive | The scope of GGO is
enlarged and there is a high density of consolidation lesions. GGO
may also be combined with consolidation. The scope of the lesion is
more extensive, involving the bilateral lung or multiple lung
fields. The disease progresses rapidly, with white lung being
visible due to the deterioration of the infection. The possibility
of ARDS should be considered. |
| Severe | Diffuse lesions are
present in the bilateral lung. The changes in the images are
observed over a short period of time and signs of white lung may
appear, indicating that ARDS has developed. |
| Recovery | The scope and
density of the lesions subside or disappear. Pulmonary fibrosis
persists in some patients. Imaging lesions usually disappear after
the improvement of clinical symptoms. |
 | Table IIDifferences in imaging features of
COVID-19, SARS and MERS at each stage. |
Table II
Differences in imaging features of
COVID-19, SARS and MERS at each stage.
| Stage | Imaging features
COVID-19 | SARS | MERS |
|---|
| Early | No evidence of
pneumonia is demonstrated in images obtained from patients with
mild disease. Multiple lesions on the bilateral lung are common.
Pleural effusion is rare. X-rays have a high rate of misdiagnoses,
and HRCT is the first imaging technique used for screening. | Pneumonia
associated imaging changes are common. Single lesions are more
common on the unilateral lung. Pleural effusion is rare. X-rays or
HRCT are recommended. | There is no
evidence of pneumonia on the images of certain patients with mild
or asymptomatic disease. Single or multiple lesions are visible on
the unilateral or bilateral lung. Easily coalesces with pleural
effusion. X-rays or HRCT are recommended. |
| Progressive | There are signs of
air bronchogram in consolidation lesions. There is usually no
pleural effusion. | Signs of air
bronchogram or GGO halos are rare. Pleural effusion is also
rare. | GGO halos are
visible. Easily coalesces by varying degrees with pleural
effusion. |
| Severe | The time-point of
developing severe disease after onset isuncertain. Primarily
manifests with consolidation lesions in combination with GGO. There
is a small degree of pleural effusion in certain patients. | Mostly occurs
within 2-3 weeks after onset. Primarily manifests with GGO in
combination with consolidation lesions. There is a small degree of
pleural effusion. | Mostly occurs
within 1 week after onset. Primarily manifests with GGO in
combination with consolidation lesions. Pleural effusions of
varying degrees are more common. |
| Recovery | Mostly occurs
within 1-2 weeks after onset. The condition can be further
aggravated with increased or new lesions. | Mostly occurs
within 2-3 weeks after onset. The condition is relatively stable,
and recurrence is unusual. | Mostly occurs
within 2 weeks after onset. The condition is relatively stable, and
recurrence is unusual. |
Progressive stage
There are several pulmonary HRCT imaging features of
COVID-19: i) The confluence or expansion of GGO lesions may be
demonstrated, with some being accompanied by certain reticular
changes, such as the ‘crazy-paving pattern’. Sometimes lesions
appear as consolidations, and signs of air bronchogram may be
observed. GGO can also appear around consolidations or other lung
fields (26). ii) The lesion area
may increase due to multiple lesions fusing together or through
diffusion into multiple lung lobes, demonstrating asymmetric
distribution in the lungs. This is most commonly observed feature
in the middle and lateral bands. iii) Enlargement of the
mediastinum and hilar lymph nodes may occur, although this is rare.
The lesions progress rapidly and clear changes in imaging
morphology appears within a short period (several days) (25,26).
Active treatment is required and the possibility of ARDS must be
considered (26). Typical imaging
patterns of progressive stage COVID-19 are presented in Fig. 1C and D.
In the progressive stage of SARS, fever and other
symptoms of infection persist, with imaging demonstrating
progressive deterioration within 3-7 days after onset (18). The features of pulmonary CT imaging
are as follows: i) GGO increases or occurs alongside consolidation,
and the lesions are large or diffuse; and ii) the lesions may
spread from one lung field to multiple lung fields, with lesions of
the unilateral lung progressing into the bilateral lung. Lesions
are distributed in multiple lung lobes, but primarily in the lower
lobe, with a mixed distribution in the inner and outer lung fields
(18,33). However, central distributions are
rare (18). At this stage,
pulmonary lesions proliferate.
From the date of onset, MERS can progress within 2-3
weeks. Additionally, certain patients may progress rapidly from
asymptomatic infection to pneumonia within 4-7 days (34-36),
demonstrating pneumonia-associated clinical symptoms and typical
imaging changes (37). Multifocal
nodular consolidation with rapid progression in the lower lung and
the lateral zone of the lung may be demonstrated in pulmonary HRCT
images. This is often accompanied with a GGO halo, mixed
consolidation (34) and bilateral
interstitial infiltration (38).
Overall, the presentation of COVID-19, SARS and MERS
is similar in the progressive stage. Each demonstrates larger
lesion areas, pronounced consolidation shadows and a wide
distribution of the lesions. During this stage, the disease
progresses rapidly and can lead to ARDS if the condition worsens.
The differences observed between infections include the
presentation of pleural effusions, evidence of an air bronchogram
or evidence of a GGO halo. The similarities and differences in
images at the progressive stage of COVID-19, SARS and MERS are
summarized in Tables I and II.
Severe stage
A retrospective study by Guan et al (39) summarized the clinical
characteristics of 1,099 patients with COVID-19 in 552 hospitals
located in China. The results revealed that 15.7% of patients
developed severe pneumonia. An additional study demonstrated this
value to be 25.5% (40). For
COVID-19 to be classified as severe, patients must meet any of the
following criteria: i) Respiratory distress (respiratory rate, ≥30
breaths'min), ii) oxygenation index ≤300 mmHg, iii) finger oxygen
saturation ≤93% in a resting state and iv) chest images presenting
>50% lesion progression within 24-48 h (23).
Pulmonary HRCT images suggest that as the GGO
density increases, the lesions fuse and progress into multiple,
large and diffuse consolidations on the bilateral lung from the
periphery to the center, involving multiple lobes and presenting as
“white lung”. Additionally, certain patients demonstrate a small
degree of pleural effusion. This phase of treatment is difficult,
and the mortality rate is 49%. Certain patients may exhibit
insignificant changes in imaging, despite worsening clinical
symptoms. This is most common in patients with other underlying
diseases, such as cerebral vascular disease (26). Typical imaging patterns of patients
with severe COVID-19 are presented in Fig. 1E and F.
The majority of patients with SARS enter the very
severe stage 2-3 weeks after onset. Imaging morphology and lesion
range change rapidly at this stage, with some changes in chest
imaging occurring within 1-3 days (33,41).
Patients may demonstrate ‘white lung’ in images, which indicates
that ARDS had occurred (41). ARDS
may develop in 10-15% of patients with SARS (41,42),
which is a life-threatening condition. The presentation of ‘white
lung’ in images may indicate poor prognosis and death, but it can
also disappear after treatment in certain patients (18). In addition, SARS in the severe stage
is prone to relapse. The images of certain patients may indicate
that the lesion has disappeared; however, it may then reappear or
become aggravated in a short period of time (18).
Most severe cases of MERS progress into severe
pneumonia within 1 week. This can lead to ARDS, acute renal
failure, septic shock or multiple organ failure. Patients with MERS
are more prone to acute renal failure than those with SARS
(29,37). The WHO reported that 12.4% of
patients with MERS develop ARDS (43). The primary imaging feature of this
stage is bilateral interstitial infiltration that progresses
rapidly (44). Furthermore, imaging
typically indicates the deterioration of lesions, including those
patients previously presenting with ‘white lung’. The changes in
images are rapid and require attentive monitoring (30).
The differences in imaging features between
COVID-19, SARS and MERS include the progression rate of lesions,
the likelihood of pleural effusion, the main clinical
manifestations of consolidation or GGO and whether the
consolidation or GGO is the primary manifestation. The similarities
and differences in images at the severe stage of these diseases are
summarized in Tables I and II.
Recovery stage
The recovery stage of COVID-19 typically occurs 1-2
weeks after the onset of pneumonia. The imaging features include a
decrease in the scope and density of lesions, a gradual
disappearance in consolidation lesions and the beginning of
organizing pneumonia. The lesions may completely disappear, or part
of the funicular shadow may remain (25,26).
Changes in imaging at the recovery stage generally lag behind the
improvement of clinical symptoms (25). However, the lesions may subsequently
enlarge, or new lesions may appear in certain cases (25). Typical imaging patterns of patients
in recovery stage of COVID-19 are presented in Fig. 1G and H.
The majority of SARS cases proceed to the recovery
stage within 2-3 weeks after onset. The range and density of the
lesions observed in images may exhibit a gradual decrease, or they
may disappear entirely. Pulmonary fibrosis is also a common imaging
feature during recovery (18).
Patients with severe cases are more prone to pulmonary fibrosis
compared with those with ordinary infection, with fibrosis
disappearing at a slower rate (45). The majority of patients recover
within 2-3 months post-discharge (18) and 7-8% of patients demonstrate
pronounced sequelae of pulmonary fibrosis (46).
The imaging features of patients with MERS during
the recovery stage include the scope of lesions decreasing
significantly and certain patients experiencing left pulmonary
fibrosis. The rate of improvement in clinical symptoms is slightly
faster than what is exhibited by images (30,34). A
case study reporting imaging follow-up revealed that abnormalities
in multiple nodules combined with GGO declined after treatment, but
progression in fibrosis was observed (34).
The overall condition of patients with COVID-19,
SARS and MERS during the recovery stage tends to be stable, and
images usually indicate that the lesions have gradually
disappeared. In general, changes in imaging occur later than
improvements in clinical manifestations. Regarding disease
progression, the recovery stage of COVID-19 is earlier than that of
SARS; however, some patients with COVID-19 may exhibit recurrent
conditions that require attention (47). The similarities and differences in
images at the recovery stage of COVID-19, SARS and MERS are
summarized in Tables I and II.
4. Suggestions for viral pneumonia chest
imaging
Chest imaging is important to the diagnosis,
management and prognosis of patients with viral pneumonia. The
benefits of imaging are numerous.
Evaluation of imaging when diagnosing
patients at their first hospital visit
The majority of chest images obtained at the first
hospital visit in patients with SARS or MERS are abnormal, which
aids the successful and early diagnosis of patients (18,31).
However, for patients exhibiting early stage COVID-19, the
interpretation of imaging results requires careful attention. Guan
et al (39) demonstrated
that radiologic abnormalities were not identified in the initial
presentation of 17.9 and 2.9% of non-severe and severe cases,
respectively. Although the detection of viral nucleic acid is the
first method used to diagnose COVID-19, and while chest imaging
should not replace this method, many countries are still facing a
shortage of nucleic acid reagents, particularly in poor and
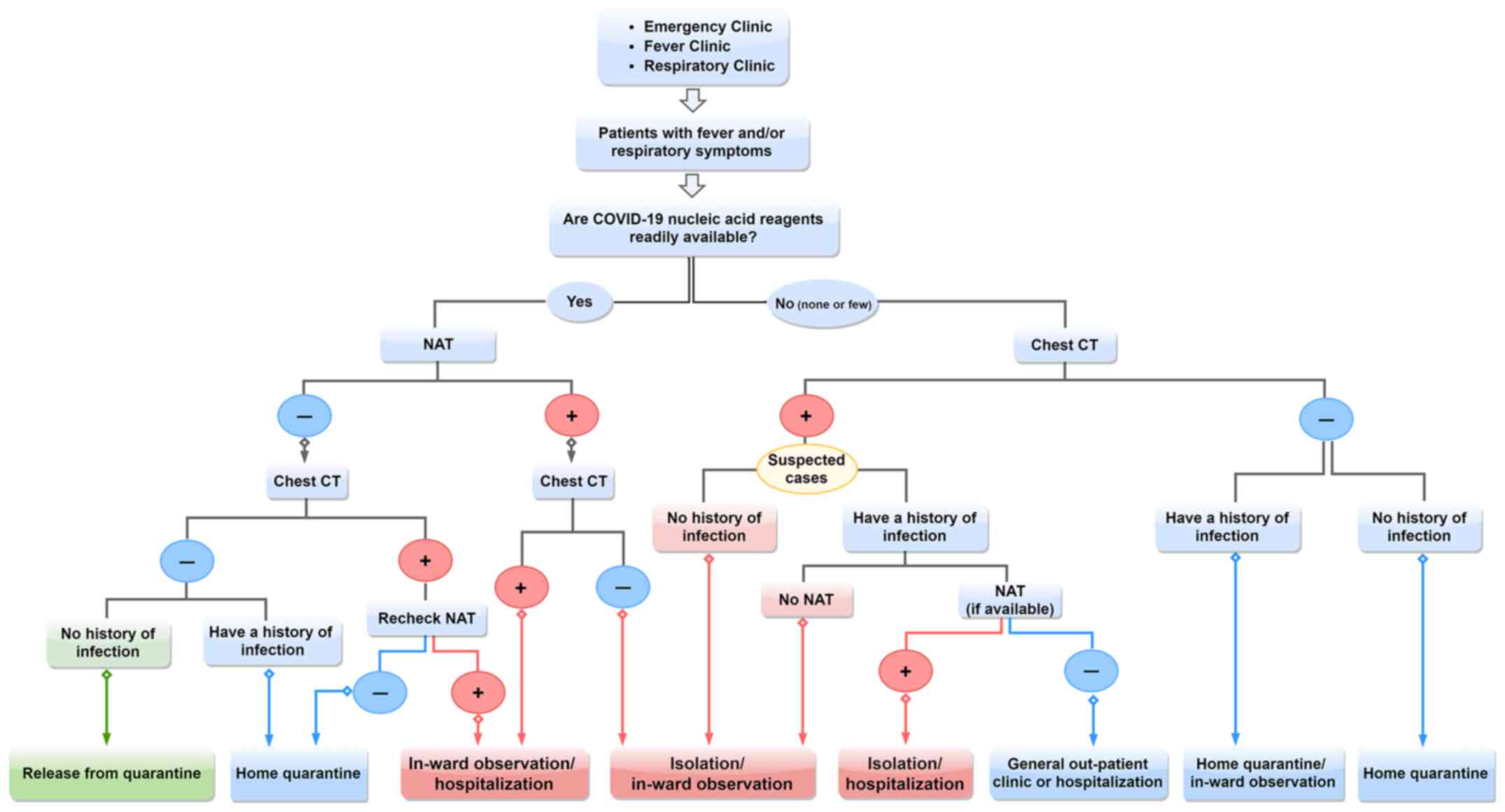
developing countries (48,49). During the early stages of COVID-19
outbreaks or during large-scale outbreaks, the flowchart for
screening COVID-19 presented in Fig.
2, which was constructed based on our previous clinical
experience in Wuhan, may be used as a reference for diagnosis in
the absence of nucleic acid testing kits.
Evaluation of disease progression and
prognosis by imaging
A previous study has indicated that consolidation
lesions could serve as a marker of disease progression or a more
severe disease state following COVID-19 infection (50). Furthermore, the consolidation of
lesions can be indicative of disease progression or deterioration
(51). A second study also
indicated that pleural effusion was identified in 33% of patients
with MERS and was associated with poor prognosis (52). An observational study on the chest
radiographs of 55 patients with MERS revealed higher rates of
pneumothorax and pleural effusion in deceased patients compared
with those who had recovered (53).
Evaluation of lesion scope for
prognosis
A previous study that assessed the clinical outcome
of 70 patients with MERS revealed that the imaging manifestations
of the bilateral lung were a risk factor for intensive care unit
admission (44). Imaging
manifestations of patients with severe COVID-19 include bilateral
lung involvement and interstitial change, which indicates poor
prognosis (39). Patients with SARS
may demonstrate multiple lung lobe lesions. If the range of lesions
usually exceeds one third of the lung lobe, the patient may be at
the severe stage of infection (54).
Evaluation of disease progression
speed for prognosis
Two consecutive contrast HRCT scans of the lungs
that demonstrate rapid lesion progression, mainly consisting of
consolidation combined with GGO and air bronchogram, may indicate
that the patient is at a high risk of COVID-19 progression from a
common type to severe type (55).
X-rays obtained in a previous study demonstrated progression in
>50% of lesions within 48 h, which was indicative of severe SARS
(54).
Effect of early diagnosis on
prognosis
As of December 16, 2020, the mortality rate of
patients with COVID-19 has been reported to be 2.26% worldwide
(2). However, among male patients
aged ≥60, an initial diagnosis of severe pneumonia and a delay in
diagnosis were associated with elevated mortality rates (40). Early diagnosis is an important
measure for the prevention of severe pneumonia or death, and
imaging examination may therefore be helpful. The majority of
patients with severe cases demonstrate imaging abnormalities at the
time of onset, and consolidation generally indicates disease
progression. Pleural effusion, pneumothorax, bilateral lung
involvement and the rapid progression of lesions can be indicative
of severe cases. If necessary, chest X-rays or pulmonary CT scans
should be re-examined within 48 h (25,56).
Chest imaging can be used for early diagnosis, the early
identification of severe cases and for early treatment guidance. It
can also reduce the risk of death (25).
5. Imaging implications for corticosteroid
therapy
The current COVID-19 pandemic has urged the
scientific community internationally to find methods in terms of
therapeutics and vaccines to control SARS-CoV-2. Despite the
rapidly increasing volume of scientific data on the possible
treatments of COVID-19, none have yet demonstrated unequivocal
clinical utility against the virus (57). For COVID-19, the immunization of a
population through vaccination is recognized as a public health
priority (58). WHO and other
national organizations collaborate on the response and tracking of
the COVID-19 pandemic, advising on critical interventions and
attempting to develop safe and effective vaccines (2). As of 12 December 2020, three COVID-19
vaccines (Pfizer, Moderna and AstraZeneca) have been authorized by
certain national regulatory authorities. None have yet received WHO
emergency use listing' prequalification authorization, but an
assessment of the Pfizer vaccine by the end of December and of
other candidates soon thereafter is expected by WHO (59).
As research and clinical trials continue to develop
vaccines and therapies, scientists have gained an increased
understanding of Coronaviridae characteristics. For example, the
acute aggravation of SARS and MERS is considered to be associated
with cytokine storms. Previous studies have suggested that
prolonged and dysregulated cytokine production occurs in SARS
(60), and large increases in
pro-inflammatory cytokines in the serum of patients with SARS have
been associated with extensive inflammatory damage to the lungs
(61). Additionally, Mahallawi
et al (62) analyzed
cytokine responses in plasma samples obtained from patients with
MERS. The results demonstrated a marked pro-inflammatory cytokine
response during the acute phase of MERS-CoV infection. Furthermore,
Liu et al (63) suggested
that a cytokine storm may also be associated with disease severity
and should be considered as an important cause of death in patients
with severe and critical COVID-19.
Corticosteroids are commonly used to treat patients
with severe pneumonia, with the purpose of inhibiting abnormal
pathological immune responses and reducing systemic inflammation.
Chest imaging evaluation may also provide a basis for assessing the
severity of lung injury to guide the use of corticosteroids
(64). The evaluation of lung
images may help to determine whether corticosteroids can be used in
patients with SARS. The imaging features of corticosteroid use
correspond to an X-ray exhibiting large or multiple pulmonary
shadows that progress rapidly, and a lesion area that increases
>50% within 48 h and accounts for over one quarter of the
bilateral lung area. However, previous studies have demonstrated
that corticosteroids may increase the mortality rate of patients
with SARS and delay viral clearance (65,66).
At present, there are conflicting opinions on whether to administer
corticosteroids to patients with MERS. The Chinese expert consensus
recommendation for the use of corticosteroids for COVID-19 suggests
that imaging-confirmed pneumonia and rapid progression are
conditions for which corticosteroid application must be considered
(67). According to China's Novel
Coronavirus Pneumonia Diagnosis and Treatment Plan (Trial Version
7) (3), patients with progressive
deterioration of the oxygenation index, rapid progression that is
visible following imaging and patients exhibiting an increased
inflammatory response may receive a short course of corticosteroids
for 3-5 days as appropriate. An early short course of
methylprednisolone in hospitalized patients with moderate to severe
COVID-19 has been confirmed to reduce escalation of care and length
of hospital stay (68).
It is important to avoid high-dose corticosteroid
shock therapy, as this approach delays the clearance of coronavirus
due to immunosuppression (69). The
dosage and course of treatment should be adjusted based on the
severity of the patient's condition and disease status, with an
overall goal of medium dosage and short course of treatment
(67,70). For example, methylprednisolone is
usually administered at a dosage of 40-160 mg once per day for 5
days, with a maximum course lasting no more than 7-10 days
(70).
Whether corticosteroids can prevent inflammatory
cytokine storms and reduce the mortality of patients with viral
pneumonia remains unclear. It is expected that high-quality
clinical studies (large sample, multicenter, randomized,
double-blind, placebo-controlled trials) will provide more evidence
to guide practice. Once corticosteroid therapy is required, chest
imaging evidence is a crucial factor to consider.
6. Conclusion
Although the imaging results of COVID-19, SARS and
MERS demonstrate clear similarities, there are also differences
that must be considered. The present review has summarized the key
imaging features of coronavirus pneumonia at different stages in
order to aid its diagnosis. The imaging features of SARS and MERS
provide a reference for the better prevention and control of
COVID-19.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported by The Guangdong
Provincial Key Laboratory of Research on Emergency in TCM (grant
no. 2017B030314176).
Availability of data and materials
Not applicable.
Authors' contributions
LL and YGW conceived the project. YBC and LYG
contributed to draft the manuscript. YQJ, FTF and JL contributed to
searching the electronic databases and interpreting the image data.
LL, YGW, MC and HPY revised the contents and polished the language
of the translation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The images in the manuscript came from a clinical
study of Chinese medicine in the treatment of COVID-19, which was
approved by the Ethics Committee of Guangdong Provincial Hospital
of Chinese medicine (Guangzhou, China; approval no. 2020-049-01).
All participants provided informed consent for participation in the
study. In the informed consent form, it was clearly stated that the
participant agreed that their data and biological specimens of this
project may be used for other studies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
World Health Organization: Clinical
Management of Severe Acute Respiratory Infection when Novel
Coronavirus (nCoV) Infection is Suspected: Interim Guidance.
January 11, 2020.
|
|
2
|
World Health Organization: Coronavirus
Disease 2020 (COVID-19) Pandemic. 16 December, 2020. Available
from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
|
|
3
|
National Health Commission of the People's
Republic of China: China's Novel Coronavirus Pneumonia Diagnosis
and Treatment Plan (Trial version 7). March 3, 2020. Available
from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
|
|
4
|
Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L,
Xu T, Jiang YZ, Xiong Y, Li YJ, Li XW, et al: Identification of a
novel coronavirus causing severe pneumonia in human: A descriptive
study. Chin Med J (Engl). 133:1015–1024. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fan Y, Zhao K, Shi ZL and Zhou P: Bat
Coronaviruses in China. Viruses. 11(210)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ye ZW, Yuan SF, Yuen KS, Fung SY, Chan CP
and Jin DY: Zoonotic origins of human coronaviruses. Int J Biol
Sci. 16:1686–1697. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
in-fected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bourgonje AR, Abdulle AE, Timens W,
Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors
AA, Osterhaus AD, et al: Angiotensin-converting enzyme 2 (ACE2),
SARS-CoV-2 and the pathophysiology of corona-virus disease 2019
(COVID-19). J Pathol. 251:228–248. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan
B, Huan Y, Yang P, Zhang Y, Deng W, et al: A crucial role of
angi-otensin converting enzyme 2 (ACE2) in SARS coronavirus-induced
lung injury. Nat Med. 11:875–879. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Rabaan AA, Al-Ahmed SH, Haque S, Sah R,
Tiwari R, Malik YS, Dhama K, Yatoo MI, Bonilla-Aldana DK and
Rodriguez-Morales AJ: SARS-CoV-2, SARS-CoV, and MERS-COV: A
comparative overview. Infez Med. 28:174–184. 2020.PubMed/NCBI
|
|
12
|
Goumenou M, Sarigiannis D, Tsatsakis A,
Anesti O, Docea AO, Petrakis D, Tsoukalas D, Kostoff R, Rakitskii
V, Spandidos DA, et al: COVID 19 in Northern Italy: An integrative
overview of factors possibly influencing the sharp increase of the
outbreak (Review). Mol Med Rep. 22:20–32. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Petrakis D, Margină D, Tsarouhas K, Tekos
F, Stan M, Nikitovic D, Kouretas D, Spandidos DA and Tsatsakis A:
Obesity a risk factor for increased COVID 19 prevalence, severity
and lethality (Review). Mol Med Rep. 22:9–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goumenou M, Spandidos DA and Tsatsakis A:
[Editorial] Possibility of transmission through dogs being a
con-tributing factor to the extreme Covid 19 outbreak in North
Italy. Mol Med Rep. 21:2293–2295. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou P, Yang X, Wang X, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: Discovery of a novel
coronavirus associated with the recent pneumonia outbreak in humans
and its potential bat origin. Nature: Jan 23, 2020 (Epub ahead of
print). doi: 10.1038/s41586-020-2012-7.
|
|
16
|
Meyerholz DK, Lambertz AM and McCray PB
Jr: Dipeptidyl peptidase 4 distribution in the human respiratory
tract: Implications for the Middle East respiratory syndrome. Am J
Pathol. 186:78–86. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tao JL, Wang AX, Guan CL, et al:
Protein-protein docking research on spike protein of three
human-coronavirus and its receptors. J Logis Univ CPAPF.
26:392–396. 2017.
|
|
18
|
Chinese Medical Association, China
Association of Chinese Medicine. Diagnosis and treatment of severe
acute respiratory syndrome (SARS). Chin Med J (Engl). 83:1731–1752.
2003.
|
|
19
|
van den Brand JM, Smits SL and Haagmans
BL: Pathogenesis of Middle East respiratory syndrome coronavirus. J
Pathol. 235:175–184. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alsaad KO, Hajeer AH, Al Balwi M, Al
Moaiqel M, Al Oudah N, Al Ajlan A, AlJohani S, Alsolamy S, Gmati
GE, Balkhy H, et al: Histopathology of Middle East respiratory
syndrome coronovirus (MERS-CoV) infection - clinico-pathological
and ultrastructural study. Histopathology. 72:516–524.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu Z, Shi L, Wang Y, Zhang J, Huang L,
Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al: Pathological findings
of COVID-19 associated with acute respiratory distress syndrome.
Lancet Respir Med. 8:420–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Magro C, Mulvey JJ, Berlin D, Nuovo G,
Salvatore S, Harp J, Baxter-Stoltzfus A and Laurence J: Complement
associated microvascular injury and thrombosis in the pathogenesis
of severe COVID-19 infection: A report of five cases. Transl Res.
220:1–13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wei PF: National Health Commission and
National Administration of Traditional Chinese Medicine. Diagnosis
and treatment of novel coronavirus pneumonia (Trial version 7).
Chin Med J. 133:1087–1095. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang MQ, Wang XH, Chen YL, Zhao KL, Cai
YQ, An CL, Lin MG and Mu XD: Clinical features of 2019 novel
coronavirus pneumonia in the early stage from a fever clinic in
Beijing. Zhonghua Jie He He Hu Xi Za Zhi. 43:215–218.
2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
25
|
Guideline for medical imaging auxiliary
diagnosis of coronavirus disease 2019. Zhongguo Yi Xue Ying Xiang
Ji Shu. 36:321–330. 2020.(In Chinese).
|
|
26
|
Chinese Medical Association, Chinese
Medical Doctor Association, China Research Hospital Association.
Diagnostic guidelines for novel coronavirus pneumonia (2020 Version
1). New Med (Wars). 30:22–34. 2020.
|
|
27
|
de Groot RJ, Baker SC, Baric RS, Brown CS,
Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE,
Memish ZA, et al: Middle East respiratory syndrome coronavirus
(MERS-CoV): Announcement of the Coronavirus Study Group. J Virol.
87:7790–7792. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu FQ and Lee G: Middle East respiratory
syndrome. J Clin Inter Med. 33:91–93. 2016.
|
|
29
|
Meng JG and Lee YQ: Symptoms, imaging and
laboratory diagnosis of Middle East respiratory syndrome. Zhonghua
Linchuang Yishi Zazhi. 43:3–5. 2015.
|
|
30
|
Drosten C, Seilmaier M, Corman VM,
Hartmann W, Scheible G, Sack S, Guggemos W, Kallies R, Muth D,
Jun-glen S, et al: Clinical features and virological analysis of a
case of Middle East respiratory syndrome coronavirus in-fection.
Lancet Infect Dis. 13:745–751. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A
and Madani TA: Middle East respiratory syndrome coronavirus
(MERS-CoV) infection: Chest CT findings. AJR Am J Roentgenol.
203:782–787. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Das KM, Lee EY, Enani MA, AlJawder SE,
Singh R, Bashir S, Al-Nakshbandi N, AlDossari K and Larsson SG: CT
correlation with outcomes in 15 patients with acute Middle East
respiratory syndrome coronavirus. AJR Am J Roentgenol. 204:736–742.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhong NS: Diagnosis and treatment of SARS.
Chin J Med. 83:95–116. 2003.
|
|
34
|
Choi WJ, Lee KN, Kang EJ and Lee H: Middle
East Respiratory Syndrome-Coronavirus Infection: A case report of
serial computed tomographic findings in a young male patient.
Korean J Radiol. 17:166–170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hijawi B, Abdallat M, Sayaydeh A,
Alqasrawi S, Haddadin A, Jaarour N, Alsheikh S and Alsanouri T:
Novel coronavirus infections in Jordan, April 2012: Epidemiological
findings from a retrospective investigation. East Medi-terr Health
J. 19 (Suppl 1):S12–S18. 2013.PubMed/NCBI
|
|
36
|
Chen S and Chen MQ: The recent progress on
clinical control for Middle East respiratory syndrome. J Micro
Infect. 10:208–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
WHO Mers-Cov Research Group: State of
knowledge and data gaps of middle east respiratory syndrome
coronavirus (MERS-CoV) in humans. PLoS Curr: Nov 12,2013 (Epub
ahead of print). doi:
10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8.
|
|
38
|
Nowotny N and Kolodziejek J: Middle East
respiratory syndrome coronavirus (MERS-CoV) in dromedary camels,
Oman, 2013. Euro Surveill. 19(20781)2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DS, et al: Clinical Characteristics
of Coronavirus Disease 2019 in China. N Engl J Med. 382:1708–1720.
2020.
|
|
40
|
Yang Y, Lu QB, Liu MJ, Wang YX, Zhang AR,
Jalali N, Dean NE, Longini I, Halloran ME, Xu B, et al:
Epidemiological and clinical features of the 2019 novel coronavirus
outbreak in China. medRxiv: Feb 11, 2020 (Epub ahead of print).
doi: org/10.1101/2020.02.10.20021675.
|
|
41
|
Peiris JS, Chu CM, Cheng VC, Chan KS, Hung
IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, et al: HKU/UCH SARS
Study Group: Clinical progression and viral load in a community
outbreak of corona-virus-associated SARS pneumonia: A prospective
study. Lancet. 361:1767–1772. 2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lapinsky SE and Hawryluck L: ICU
management of severe acute respiratory syndrome. Intensive Care
Med. 29:870–875. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
World Health Organization: State of
knowledge and data gaps of Middle East respiratory syndrome
coronavirus. Available from: http://www.who.int/csr/disease/coronavirus_infections/en.
|
|
44
|
Saad M, Omrani AS, Baig K, Bahloul A,
Elzein F, Matin MA, Selim MA, Al Mutairi M, Al Nakhli D, Al
Ai-daroos AY, et al: Clinical aspects and outcomes of 70 patients
with Middle East respiratory syndrome coronavirus infection: A
single-center experience in Saudi Arabia. Int J Infect Dis.
29:301–306. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lu PX, Yang GL, Yu WY, et al: Imaging
follow-up of SARS patients complicated with pulmonary fibrosis.
Chin J Med Imaging Tech. 20:1901–1903. 2004.
|
|
46
|
Kong Q and Qin C: Comparative study on
pathogenesis of SARS pulmonary fibrosis. Chin J Comp Med.
15:335–338. 2005.
|
|
47
|
Lei J, Li J, Li X and Qi X: CT Imaging of
the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology.
295(18)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Vandenberg O, Martiny D, Rochas O, van
Belkum A and Kozlakidis Z: Considerations for diagnostic COVID-19
tests. Nat Rev Microbiol. 14:1–13. 2020.
|
|
49
|
Hoque MN, Chaudhury A, Akanda MA, Hossain
MA and Islam MT: Genomic diversity and evolution, diagno-sis,
prevention, and therapeutics of the pandemic COVID-19 disease.
PeerJ. 8(e9689)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Shang L, Zhao J, Hu Y, Du R and Cao B: On
the use of corticosteroids for 2019-nCoV pneumonia. Lancet.
395:683–684. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
World Health Organization: Clinical
management of severe acute respiratory infection when novel
coronavirus (nCoV) infection is suspected. Available from:
https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected.
|
|
52
|
Du B, Qiu HB, Zhan X, Wang YS, Kang HY, Li
XY, Wang F, Sun B and Tong ZH: Pharmacotherapeutics for the new
coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 43:173–176.
2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
53
|
Koo HJ, Lim S, Choe J, Choi SH, Sung H and
Do KH: Radiographic and CT features of viral pneumonia.
Radiographics. 38:719–739. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Guo YB: Clinical classification and early
warning indicators of SARS. Chin Med News. 18:7. 2003.
|
|
55
|
Tongji Hospital Affiliated to Tongji
Medical College of Huazhong University of Science and Technology,
Peking Union Medical College Hospital, China-Japan Friendship
Hospital. Diagnosis, treatment and management of the severe type of
novel coronavirus pneumonia (Novel Coronavirus Pneumonia Treatment
Cooperation Group of Tongji Hospital). J Inter Inten Med. 26:1–5.
2020.
|
|
56
|
Shi F, Yu Q, Huang W and Tan C: 2019 Novel
Coronavirus (COVID-19) pneumonia with hemoptysis as the initial
symptom: CT and clinical features. Korean J Radiol. 21:537–540.
2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Nitulescu GM, Paunescu H, Moschos SA,
Petrakis D, Nitulescu G, Ion GN, Spandidos DA, Nikolouzakis TK,
Drakoulis N and Tsatsakis A: Comprehensive analysis of drugs to
treat SARS CoV 2 infection: Mechanistic insights into current COVID
19 therapies (Review). Int J Mol Med. 46:467–488. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Car-valho F,
Vinceti M, et al: Towards effective COVID 19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
World Health Organization: Coronavirus
Disease 2020 (COVID-19) Pandemic. 16 September, 2020. Available
from: https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines.
|
|
60
|
Jones BM, Ma ES, Peiris JS, Wong PC, Ho
JC, Lam B, Lai KN and Tsang KW: Prolonged disturbances of in vitro
cytokine production in patients with severe acute respiratory
syndrome (SARS) treated with ribavirin and steroids. Clin Exp
Immunol. 135:467–473. 2004.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chien JY, Hsueh PR, Cheng WC, Yu CJ and
Yang PC: Temporal changes in cytokine/chemokine profiles and
pulmonary involvement in severe acute respiratory syndrome.
Respirology. 11:715–722. 2006.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Mahallawi WH, Khabour OF, Zhang Q,
Makhdoum HM and Suliman BA: MERS-CoV infection in humans is
associated with a pro-inflammatory Th1 and Th17 cytokine profile.
Cytokine. 104:8–13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Liu J, Li S, Liu J, Liang B, Wang X, Wang
H, Li W, Tong Q, Yi J, Zhao L, et al: Longitudinal characteristics
of lymphocyte responses and cytokine profiles in the peripheral
blood of SARS-CoV-2 infected patients. EBioMedicine.
55(102763)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Stern A, Skalsky K, Avni T, Carrara E,
Leibovici L and Paul M: Corticosteroids for pneumonia. Cochrane
Data-base Syst Rev. 12(CD007720)2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Auyeung TW, Lee JS, Lai WK, Choi CH, Lee
HK, Lee JS, Li PC, Lok KH, Ng YY and Wong WM: The use of
corticosteroid as treatment in SARS was associated with adverse
outcomes: A retrospective cohort study. J Infect. 51:98–102.
2005.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A,
Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, et al: Effects of early
corticosteroid treatment on plasma SARS-associated Coronavirus RNA
concentrations in adult patients. J Clin Virol. 31:304–309.
2004.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zhao JP, Hu Y, Du RH, Chen ZS, Jin Y, Zhou
M, Zhang J, Qu JM and Cao B: Expert consensus on the use of
corticosteroid in patients with 2019-nCoV pneumonia. Zhonghua Jie
He He Hu Xi Za Zhi. 43:183–184. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
68
|
Fadel R, Morrison AR, Vahia A, Smith ZR,
Chaudhry Z, Bhargava P, Miller J, Kenney RM, Alangaden G, Ramesh
MS, et al: Early short-course corticosteroids in hospitalized
patients with COVID-19. Clin Infect Dis. 71:2114–2120.
2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Schoot TS, Kerckhoffs AP, Hilbrands LB and
van Marum RJ: Immunosuppressive drugs and COVID-19: A Review. Front
Pharmacol. 11(1333)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
She Y: Is corticosteroid used or not used
in the treatment of new coronavirus pneumonia? Chin J Crit Care
Intensive Care Med. 6:53–55. 2020.
|