Introduction
Tongue cancer is one of the most common malignant
tumor types that occurs in the oral and maxillofacial region
(1). Of the pathological types of
tongue cancer, >80% are tongue squamous cell carcinoma (TSCC)
(2). The majority of lesions occur
at the edge of one third of the tongue, but the second most common
area that lesions occur is the root, dorsum and tip of the tongue
(3). TSCC is prone to metastasis
via the lymph and blood circulation (4). Patients with low differentiation are
prone to recurrence after operation (3), and radiotherapy and chemotherapy
treatment strategies are not effective, resulting in a poor
prognosis. Therefore, identifying an effective treatment strategy
is the focus of clinical research on TSCC.
MicroRNAs (miRNAs/miRs) are a group of non-coding
single-stranded RNA molecules that are involved in the regulation
of post-transcriptional gene expression in plants and animals
(5). Previous studies have reported
that a single miRNA can affect the expression of 1,000s of genes
and that several miRNAs can affect the expression of a single gene
at the same time (6-8).
miRNAs are associated with the expression of one third of human
genes. In the study of oncogenesis, it has been revealed that
miRNAs can interfere with the synthesis of proteins by affecting
the initiation of translation, thereby further affecting the
biological effects of oncogenes and tumor suppressor genes
(9).
miR-145, located on human chromosome 5q32, acts as a
tumor suppressor in a variety of tumors, including prostate,
bladder, colon, ovarian and esophageal cancer, and is expressed at
lower levels in tumor tissues compared with normal tissues
(10-14).
However, the role of miR-145 in TSCC and its related mechanisms are
yet to be elucidated. Thus, the present study investigated the role
and mechanism underlying miR-145 in SCC9 and Cal27 cell apoptosis
and oxidative stress, with the aim of providing a theoretical
foundation for the identification of novel molecular markers and
treatments.
Materials and methods
Patients and tissue samples
A total of 43 tumor tissue samples from patients
with TSCC (26 male patients and 17 female patients; age range,
32-74 years; median age, 52 years) who were admitted to the
Department of Oral and Maxillofacial Surgery, the Second Affiliated
Hospital of Jinzhou Medical University were obtained between
January 2017 and December 2018. None of the patients had received
chemotherapy or radiotherapy. The inclusion criteria were as
follows: i) Diagnosed with TSCC by pathology; and ii) had not
received radiation therapy or chemotherapy prior to the biopsy.
Patients with ≥1 of the following conditions were excluded from the
present study: i) Infectious disease; ii) acute cardiovascular and
cerebrovascular diseases; iii) rheumatic disease; iv) diabetes; and
v) other tumors. In addition, the paracancerous tissues (adjacent
non-tumorous tissues, normal tongue tissues) of 43 patients with
TSCC were obtained from The Second Affiliated Hospital of Jinzhou
Medical University as the control group.
The present study was approved by the Ethics
Committees of Jinzhou Medical University on October 26, 2016
(approval no. JZH2016052). Written informed consent was obtained
from all patients included in the present study.
Cell culture
TSCC Cal27 and SCC9 cell lines were purchased from
The Cell Bank of Type Culture Collection of Chinese Academy of
Sciences. Cal27 cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) in a 5% CO2 incubator at 37˚C with
saturated humidity. SCC9 cells were incubated in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS in a 5%
CO2 incubator at 37˚C.
Transfection
miR-145-5p mimics (cat. no. miR30000157-1-2) and
inhibitors (cat. no. miR20000437-1-2) were synthesized along with a
corresponding negative control (NC; Lentiviral vector without gene
sequence) by Shanghai GenePharma Co., Ltd. Plasmid production and
purification were also performed by Shanghai GenePharma Co., Ltd,
following the manufacturer's protocols. miR-145-5p mimic and
inhibitor sequences were cloned into the lentivirus without green
fluorescence (GeneChem, Inc.). Subsequently, 6 µg/ml polybrene
(GeneChem, Inc.) and an appropriate amount of lentivirus were added
and incubated at 37˚C for 24 h. Cells transfected with lentivirus
were screened with puromycin to increase transfection efficiency.
Transfection efficiency was assessed by performing reverse
transcription-quantitative PCR (RT-qPCR).
RT-qPCR
Total RNA was extracted from the tissues or cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol. Total RNA
was reverse transcribed into cDNA using the Prime Script™ RT Master
Mixture (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. The following thermocycling conditions
were used for cDNA: 37˚C for 15 min and 85˚C for 15 sec.
Subsequently, qPCR was performed using the SYBR Prime Script miRNA
RT-PCR kit (Takara Biotechnology Co., Ltd.). The following
thermocycling conditions were used for qPCR: Pre-denaturation at
95˚C for 1 min, followed by 40 cycles of denaturation at 95˚C for
15 sec, annealing at 60˚C for 40 sec and extension at 72˚C for 15
sec. The expression level of miR-145-5p was calculated using the
2-ΔΔCq (15) method and
normalized to the internal reference gene U6. The primers used were
as follows: miR-145-5p forward, 5'-ACAC
TCCAGCTGGGGTCCAGTTTTCCCAGGA-3' and reverse,
5'-ACACTCCAGCTGGGGTCCAGTTTTCCCAGGA-3'; U6 forward,
5'-CGCTTCGGCACATATACTA-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCA-3'.
MTT assay
Cells (8x104 cells/well) in logarithmic
growth were seeded into a 96-well culture plate. Subsequently, 20
µl MTT solution (5 mg/ml) was added to each well and incubated at
37˚C for 4-6 h. After the supernatant was removed, 150 µl DMSO was
added and the 96-well culture plate was gently agitated for 10 min
to dissolve the formazan crystals. The optical density (OD) of each
well was measured at a wavelength of 570 nm using a plate reader.
The rate of proliferation (%) was calculated as follows: (OD
sample - OD sample blank)/(OD
control - OD control blank) x 100.
Transwell assay
SCC9 and Cal27 cells were re-suspended in medium
without serum to a concentration of 1x105 cells/ml.
Subsequently, cells (200 µl) were seeded into the upper chamber of
Matrigel®-coated (Sigma-Aldrich; Merck KGaA) Transwell
inserts (8-µm pore; Sigma-Aldrich; Merck KGaA). RPMI-1640 or DMEM
containing 10% FBS was plated into the lower chamber. Following
incubation at 37˚C for 48 h, cells on the upper surface of the
Transwell membrane were removed using a cotton swab. Subsequently,
cells were fixed using 4% paraformaldehyde solution for 20 min at
room temperature, stained with 0.1% trypan blue for 15 min at room
temperature and observed using a light microscope (magnification,
x100).
Apoptosis staining
SCC9 and Cal27 cells were collected, washed and then
resuspended. Cells were incubated with 5 µl Annexin V (cat. no.
556547; BD Pharmingen; BS Biosciences) and 5 µl PI (cat. no.
556547; BD Pharmingen; BS Biosciences) at room temperature for 20
min in the dark. Subsequently, cells were washed with PBS and
re-suspended in 300 ml PBS. Cell apoptosis was analyzed via Calibur
flow cytometer (BD Biosciences) and quantified using FlowJo
software (v. 10.1.1; FlowJo LLC).
ELISA
The cell supernatants of SCC9 and Cal27 cells were
collected. Levels of malondialdehyde (MDA; cat. no. CEA597Ge; Cloud
Clone Corp.), superoxide dismutase (SOD; cat. no. SES134Hu; Cloud
Clone Corp.) and glutathione peroxidase (GSH-Px; cat. no. CEA294Ge;
Cloud Clone Corp.) were measured using ELISA kits according to the
manufacturer's protocols. The OD of each sample was measured at a
wavelength of 450 nm using a microplate reader. A standard curve
was generated by plotting OD value vs. standard concentration. The
curve equation and r value were calculated and used to determine
concentrations of each sample.
Western blotting
Total protein was extracted from the cells using
RIPA lysis buffer (Thermo Fisher Scientific, Inc.). Total protein
was quantified using a bicinchoninic acid protein assay kit.
Proteins (50 µg/lane) were separated via 10% SDS-PAGE and
transferred onto PVDF membranes. Subsequently, the membranes were
incubated at 4˚C overnight with the following primary antibodies:
Anti-PI3K (1:1,000; ab127617; Abcam), anti-AKT (1:1,000; ab179463;
Abcam), anti-phosphorylated (p)-AKT (1:800; ab131443; Abcam) and
anti-GAPDH (1:2,000; ab181602; Abcam) antibodies. After washing
with PBS three times, the membranes were incubated with a secondary
polyclonal peroxidase-labeled antibody (1:4,000; ab7090, Abcam) for
2 h. Protein bands were visualized using enhanced chemiluminescence
reagent (Thermo Fisher Scientific, Inc.). Protein expression levels
were semi-quantified using ImageJ software (v2.1.4.7; National
Institutes of Health) with GAPDH as the loading control.
Statistical analysis
All experiments were performed 2-3 times. Data are
presented as the mean ± SD. Statistical analyses were performed
using GraphPad Prism software (version 6.0; GraphPad Software,
Inc.). Comparisons between two groups were analyzed using the
unpaired two-tailed Student's t-test. Comparisons between multiple
groups were analyzed using one-way ANOVA or Kruskal-Wallis test
followed by Dunn's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics of patients
with TSCC
The clinical characteristics of the patients with
TSCC are presented in Table I.
According to the TNM classification for TSCC (The American Joint
Committee on Cancer and Union for International Cancer Control
2010; 7th edition) (16), patients
were divided into four stages: i) stage I (n=4); ii) stage II
(n=12); iii) stage III (n=20); and iv) stage IV (n=7).
 | Table ICharacteristic features of study
subjects. |
Table I
Characteristic features of study
subjects.
|
Characteristics | Patients with
TSCC | Controls |
|---|
| Age, years | | |
|
Range | 32-74 | 33-71 |
|
Mean ±
SD | 52±8.58 | 54±10.21 |
| Smoking | 23 | 23 |
| Non-smoking | 20 | 20 |
| Drinking | 17 | 23 |
| Non-drinking | 26 | 20 |
| Tumor location | | - |
|
Tongue
margin | 29 | |
|
Tongue
root | 8 | |
|
Ventral of
tongue | 6 | |
| Tumor size | | - |
|
T1 | 4 | |
|
T2 | 23 | |
|
T3 | 11 | |
|
T4 | 5 | |
| Lymph node
involvement | | - |
|
N0 | 25 | |
|
N+ | 18 | |
| Pathological
classification | | - |
|
Squamous
cell carcinoma | 43 | |
| Histological
classification | | - |
|
Well
differentiated | 11 | |
|
Moderately
differentiated | 27 | |
|
Poorly
differentiated | 5 | |
| Clinical stage | | - |
|
І | 4 | |
|
Ⅱ | 12 | |
|
Ⅲ | 20 | |
|
Ⅳ | 7 | |
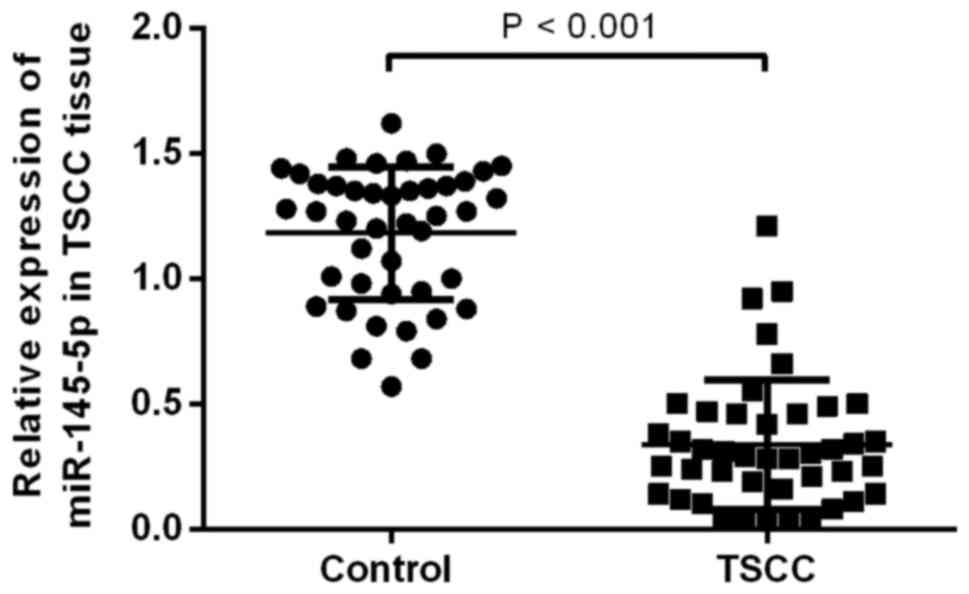
miR-145-5p is downregulated in TSCC
tissues
To determine the level of miR-145-5p in tissues of
patients with TSCC, miR- 145-5p expression was detected by
performing RT-qPCR. The results indicated that the expression level
of miR-145-5p in patients with TSCC was significantly decreased
compared with the control group (P<0.05; Fig. 1).
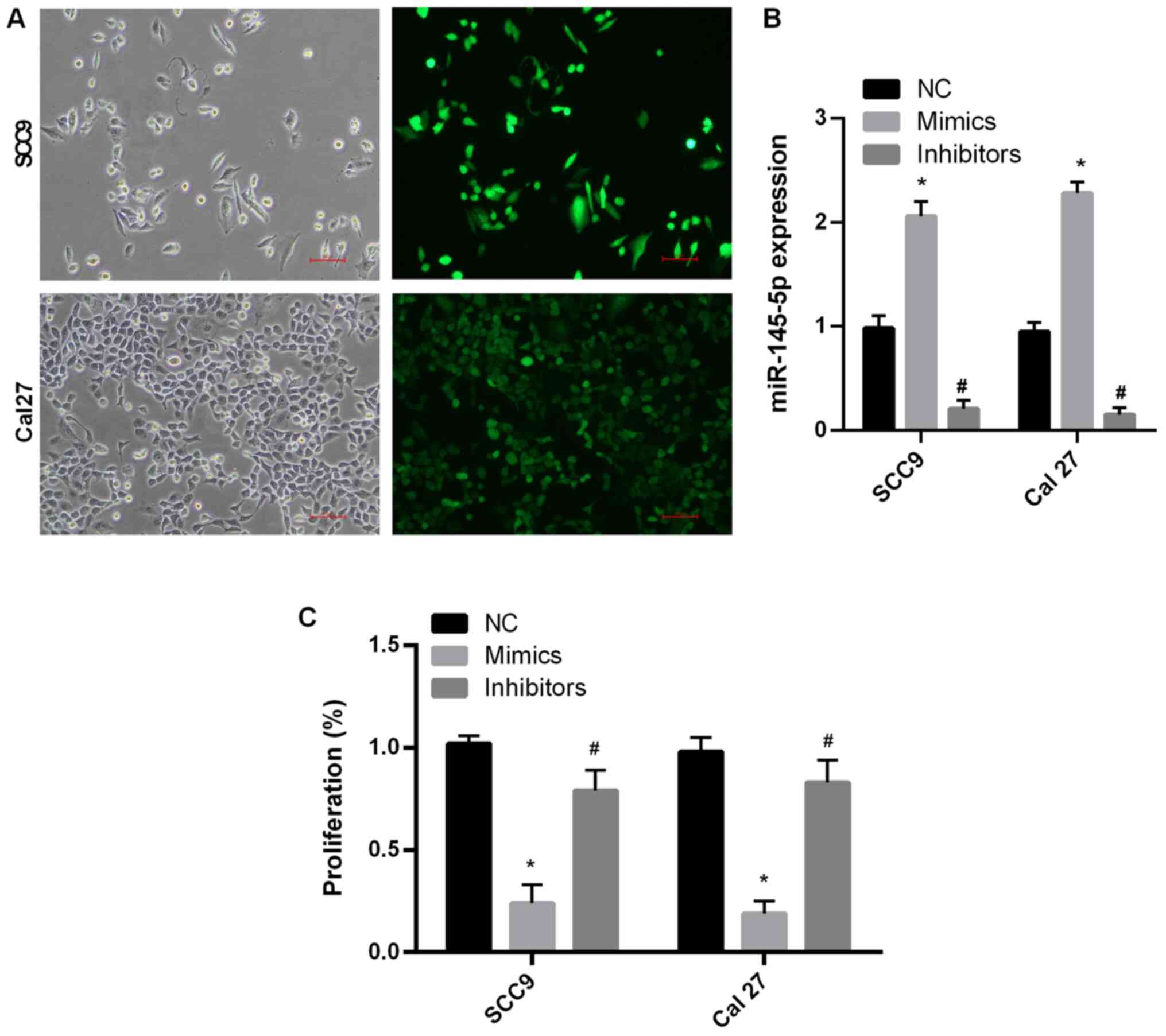
miR-145-5p overexpression attenuates
SCC9 and Cal27 cell proliferation
To determine the function of miR-145-5p, SCC9 and
Cal27 cell lines were used. miR-145-5p expression levels were
detected via RT-qPCR following transfection with NC, miR-145-5p
mimics and miR-145-5p inhibitors. The results indicated that
miR-145 expression was significantly increased following
transfection with miR-145-5p mimic compared with the NC group in
both cell lines (P<0.05; Fig. 2A
and B). Moreover, miR-145
expression was significantly decreased following transfection with
miR-145-5p inhibitor compared with the NC group (P<0.05;
Fig. 2A and B). Cell proliferation following
transfection was also assessed. The results demonstrated that cell
proliferation was significantly decreased following transfection
with miR-145-5p mimics compared with the NC group (P<0.05;
Fig. 2C). These results suggested
that miR-145-5p overexpression suppressed SCC9 and Cal27 cell
proliferation. Moreover, inhibiting the expression of miRNA-145-5p
did not inhibit cell proliferation.
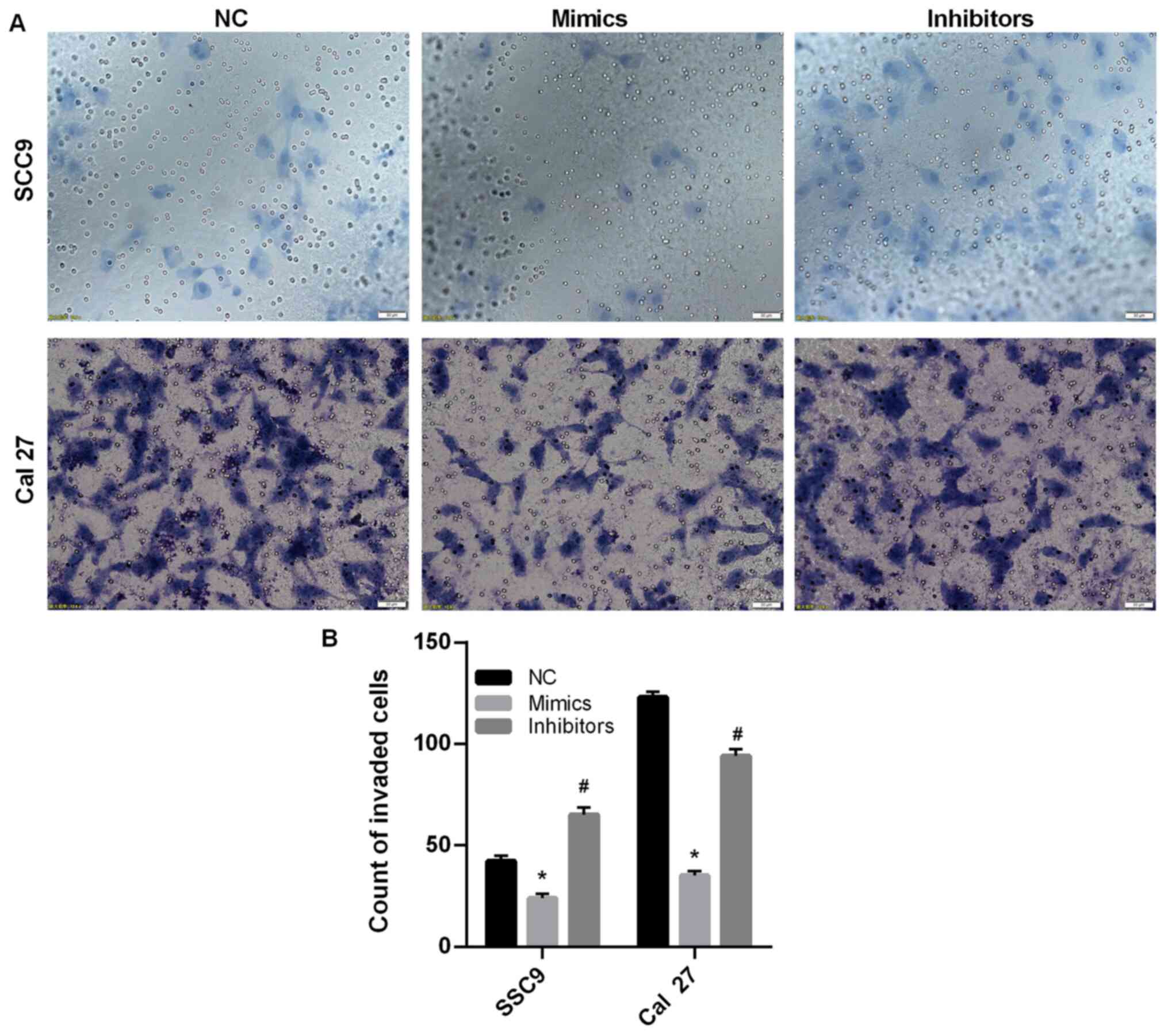
miR-145-5p overexpression mitigates
SCC9 and Cal27 cell invasion
Tumor cells have the ability to metastasize and
invade (5); therefore, a Transwell
assay was conducted to detect SCC9 and Cal27 cell invasion. The
results indicated that SCC9 and Cal27 cell invasion was
significantly decreased following transfection with miR-145-5p
mimics compared with the NC group (P<0.05; Fig. 3A and B). Furthermore, the results suggested that
miR-145-5p knockdown promoted SCC9 and Cal27 cell invasion.
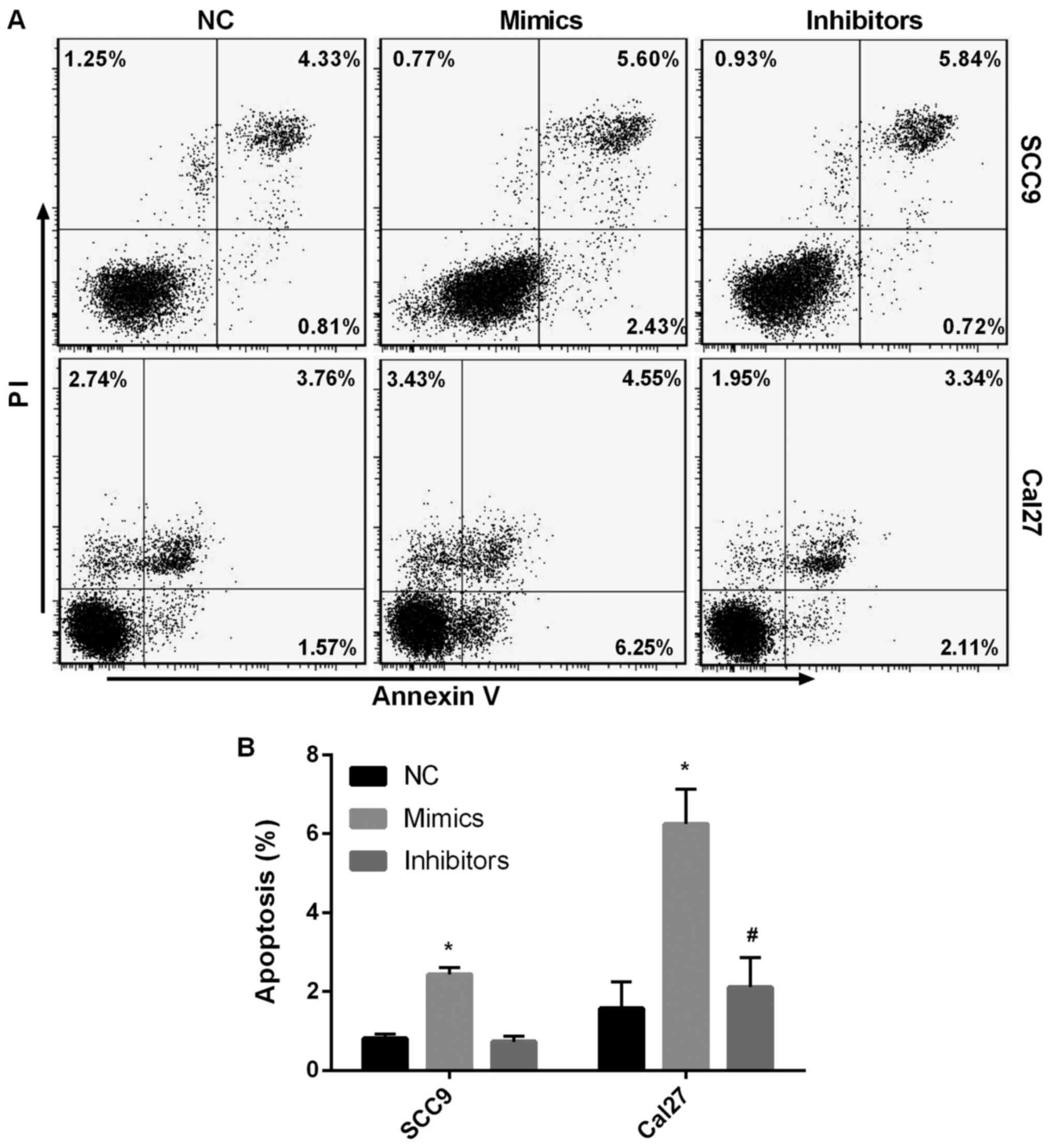
miR-145-5p overexpression aggravates
SCC9 and Cal27 cell apoptosis
PI and Annexin V staining was used to assess early
SCC9 and Cal27 cell apoptosis. It was found that early SCC9 and
Cal27 cell apoptosis was significantly increased following
miR-145-5p overexpression compared with the NC group (P<0.05;
Fig. 4A and B). Following miR-145-5p knockdown, early
SCC9 and Cal27 cell apoptosis was significantly diminished compared
with the NC group (P<0.05). The results indicated that
miR-145-5p overexpression aggravated SCC9 and Cal27 cell
apoptosis.
miR-145-5p overexpression promotes
SCC9 and Cal27 cell oxidative stress
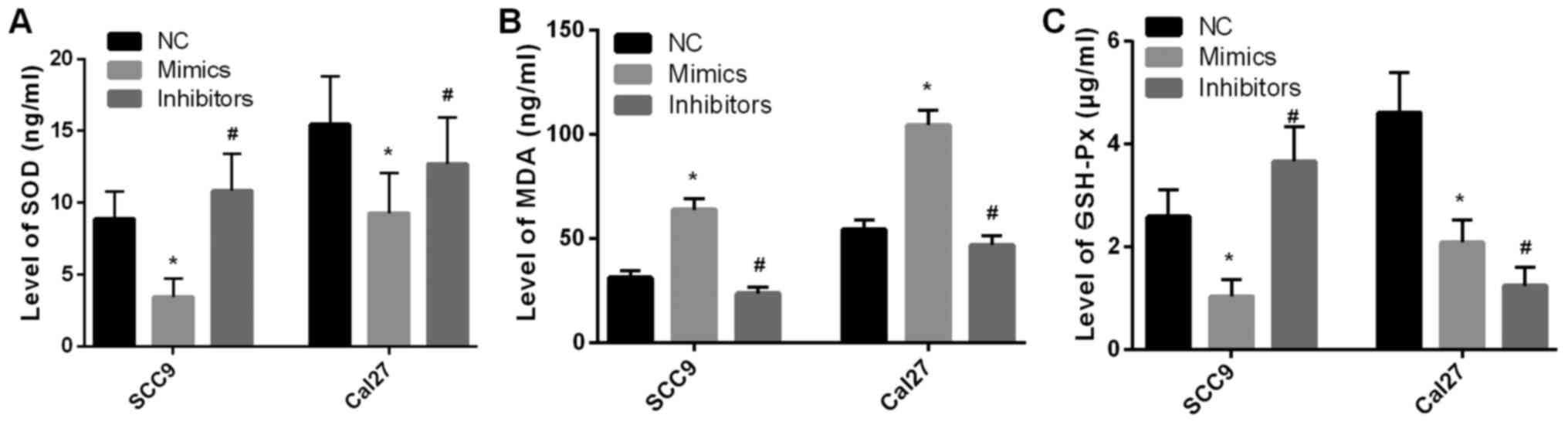
The supernatants of SCC9 and Cal27 cells were
utilized to detect the levels of SOD, MDA and GSH-Px. As presented
in Fig. 5A and C, SOD and GSH-Px levels were significantly
decreased after miR-145-5p overexpression compared with the NC
group (P<0.05). By contrast, MDA levels were significantly
increased after miR-145-5p overexpression compared with the NC
group (P<0.05; Fig. 5B). The
results suggested that miR-145-5p overexpression promoted SCC9 and
Cal27 cell oxidative stress. Inhibition of mirna-145-5p expression
did not result in inhibition oxidative stress.
miR-145-5p regulates the PI3K/AKT
signaling pathway
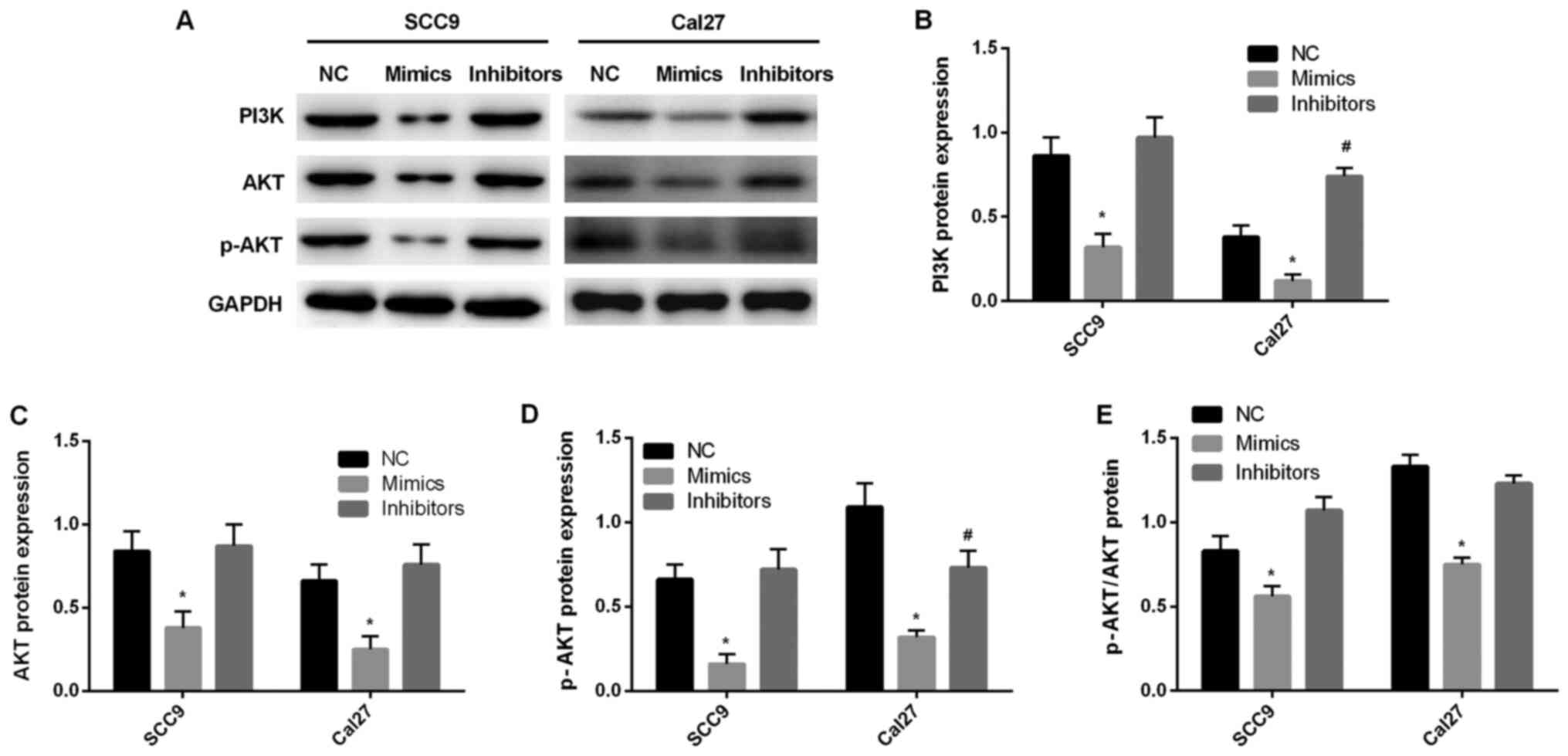
In order to further investigate the mechanism
underlying miR-145-5p, western blotting was performed to detect
PI3K signaling pathway-associated protein expression levels. The
results indicated that the expression levels of PI3K, AKT and p-AKT
were significantly decreased after miR-145-5p overexpression
compared with the NC group (P<0.05; Fig. 6A-E). After inhibiting the expression
of miR-145-5p, the expression levels of PI3K, Akt and p-Akt were
significantly increased. Compared with NC group, there was no
significant difference. Thus, it was suggested that miR-145-5p
regulated the PI3K/AKT signaling pathway.
Discussion
miRNA is a highly conserved endogenous non-coding
RNA that consists of 18-25 nucleotides (5). Since the discovery of the first miRNA
in 1993, an increasing number of studies have demonstrated that
miRNA serves an important role in the growth, differentiation and
proliferation of organisms (17,18).
miR-145-5p is downregulated in a number of different types of
cancer, including cervical (19),
colorectal (20) and non-small cell
lung cancer (NSCLC) (21), as well
as in esophageal squamous cell carcinoma. miR-145-5p functions as a
tumor suppressor in carcinogenesis (13,22-27),
which may be associated with the fragile site of epigenetic
alteration caused by the deletion of the gene encoding miR-145 in
cancer cells (28). miR-145-5p
downregulation in normal tissue cells results in the lack of
protection and monitoring from miR-145-5p, leading to altered cell
functions, including proliferation, apoptosis and migration, and
the induction of carcinogenesis. Jin et al (29) reported that miR-145-5p directly
targeted the 3'-untranslated region of Toll-like receptor 4,
inhibiting the occurrence of malignant melanoma and metastasis.
Furthermore, Liu et al (21)
reported that miR-145-5p knockdown upregulated N-cadherin, vimentin
and E-cadherin protein expression levels and increased
epithelial-mesenchymal transition activity, which led to the
progression of NSCLC. Kliese et al (30) also revealed that miR-145
overexpression in meningioma cells resulted in decreased
proliferation, increased sensitivity to apoptosis and reduced
orthotopic tumor growth in nude mice. miR-145 serves an important
role in the initiation, proliferation, apoptosis and tumorigenesis
of cancer cells (19).
In the present study, the expression of miR-145-5p
was decreased in TSCC tissues compared with normal tissues, and
miR-145-5p overexpression in SCC9 and Cal27 cells inhibited cell
stability and invasion, as well as promoted cell apoptosis.
Furthermore, miR-145-5p overexpression in SCC9 and Cal27 cells
inhibited the levels of SOD and GSH-Px, and promoted the level of
MDA. It was also found that miR-145-5p overexpression promoted
oxidative stress in these TSCC cells. However, the level of GSH-Px
was decreased in miR-145-5p inhibitors group with TSCC Cal27 cells,
and it was suggested that some components of the inhibitors
interfere with the level of GSH-Px. The present results also
demonstrated that miR-145-5p overexpression promoted cell apoptosis
and oxidative stress by suppressing the PI3K/AKT signaling
pathway.
The PI3K/AKT signaling pathway is an important
signal transduction pathway of cell metabolism and antiapoptotic
mechanisms, which is closely associated with the occurrence and
development of malignant tumors (31). PI3K is a phosphatidylinositol kinase
that phosphorylates the third hydroxyl group of the inositol ring.
Its activation is affected by the cytokine-induced activation of
Ras molecule, and the activation signal is transmitted to
phosphatidylinositol-3 dependent kinase and then to AKT (32). Zhu et al (33) reported that curcumin inhibited the
PI3K/AKT/mTOR signaling pathway by upregulating the expression of
miR-145 and attenuated the development of laryngeal squamous cell
carcinoma. Moreover, Liu et al (34) revealed that miR-145-5p suppressed
VMM917 and CHL-1 cell proliferation, invasion and migration, and
induce apoptosis by inhibiting the mitogen activated protein kinase
and PI3K/AKT signaling pathways. Li et al (35) also indicated that miR-145 knockdown
in NSCLC A549 cells increased cell proliferation, but reduced
lactate dehydrogenase expression, apoptosis, caspase-3/-9 levels
and Bax protein expression by regulating the EGFR/PI3K/AKT
signaling pathway (36). In the
present study, miR-145 overexpression inhibited the PI3K/AKT
signaling pathway, which may be the mechanism underlying the
effects of miR-145-5p on SCC9 and Cal27 cell invasion, apoptosis
and oxidative stress.
There are some limitations to the present study. It
remains unknown whether there are differences in the expression of
mir-145-5p with regards to tumor size and node metastasis.
Moreover, the targeting gene of miR-145-5p is yet to be identified,
These questions should be scientifically examined in future
studies.
In conclusion, the present study demonstrated that
miR-145-5p downregulation affected the progression of cancer.
Furthermore, miR-145-5p overexpression increased the antiapoptotic
and antioxidative stress effects of SCC9 and Cal27 cells by
inhibiting the PI3K/AKT signaling pathway. Therefore, the present
study provided a theoretical basis for the use of miR-145 as a
molecular marker of TSCC.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the Nature Science Foundation
of Liaoning Province (grant no. 2015020326).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Study design: ZX and CL. Performance of the study:
ZX. Data collection: ZT. Data interpretation: JT and ZT. Drafting
of the manuscript: ZX and ZT. All authors have read and approved
the final submitted manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of Jinzhou Medical University on 26 October, 2016
(approval no. JZH2016052). Informed consent was obtained from all
patients included in the study. The research was conducted in
accordance with the Helsinki Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weatherspoon DJ, Chattopadhyay A,
Boroumand S and Garcia I: Oral cavity and oropharyngeal cancer
incidence trends and disparities in the United States: 2000-2010.
Cancer Epidemiol. 39:497–504. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rusthoven K, Ballonoff A, Raben D and Chen
C: Poor prognosis in patients with stage I and II oral tongue
squamous cell carcinoma. Cancer. 112:345–351. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
St John MA, Abemayor E and Wong DT: Recent
new approaches to the treatment of head and neck cancer. Anticancer
Drugs. 17:365–375. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yan D, Cai X and Feng Y: miR-183 modulates
cell apoptosis and proliferation in tongue squamous cell carcinoma
SCC25 cell line. Oncol Res. 24:399–404. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Isayeva T, Brandwein-Gensler M, Somarathna
M, Moore-Smith LD and Lee T: Micro-RNA profiling as a predictor of
clinical outcomes for head and neck cancer patients. Curr Pharm
Des. 23:4729–4744. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shi B, Yan W, Liu G and Guo Y:
MicroRNA-488 inhibits tongue squamous carcinoma cell invasion and
EMT by directly targeting ATF3. Cell Mol Biol Lett.
23(28)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pardini B, De Maria D, Francavilla A, Di
Gaetano C, Ronco G and Naccarati A: MicroRNAs as markers of
progression in cervical cancer: A systematic review. BMC Cancer.
18(696)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arndt GM, Dossey L, Cullen LM, Lai A,
Druker R, Eisbacher M, Zhang C, Tran N, Fan H, Retzlaff K, et al:
Characterization of global microRNA expression reveals oncogenic
potential of miR-145 in metastatic colorectal cancer. BMC Cancer.
9(374)2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chiyomaru T, Enokida H, Tatarano S,
Kawahara K, Uchida Y, Nishiyama K, Fujimura L, Kikkawa N, Seki N
and Nakagawa M: miR-145 and miR-133a function as tumour suppressors
and directly regulate FSCN1 expression in bladder cancer. Br J
Cancer. 102:883–891. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zaman MS, Chen Y, Deng G, Shahryari V, Suh
SO, Saini S, Majid S, Liu J, Khatri G, Tanaka Y, et al: The
functional significance of microRNA-145 in prostate cancer. Br J
Cancer. 103:256–264. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ye D, Shen Z and Zhou S: Function of
microRNA-145 and mechanisms underlying its role in malignant tumor
diagnosis and treatment. Cancer Manag Res. 11:969–979.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kano M, Seki N, Kikkawa N, Fujimura L,
Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M and
Matsubara H: miR-145, miR-133a and miR-133b: Tumor-suppressive
miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J
Cancer. 127:2804–2814. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Karatas OF, Oner M, Abay A and Diyapoglu
A: MicroRNAs in human tongue squamous cell carcinoma: From
pathogenesis to therapeutic implications. Oral Oncol. 67:124–130.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Harding RL and Velleman SG: MicroRNA
regulation of myogenic satellite cell proliferation and
differentiation. Mol Cell Biochem. 412:181–195. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou X, Yue Y, Wang R, Gong B and Duan Z:
MicroRNA-145 inhibits tumorigenesis and invasion of cervical cancer
stem cells. Int J Oncol. 50:853–862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shen X, Jiang H, Chen Z, Lu B, Zhu Y, Mao
J, Chai K and Chen W: MicroRNA-145 inhibits cell migration and
invasion in colorectal cancer by targeting TWIST. OncoTargets Ther.
12:10799–10809. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu Q, Chen J, Wang B, Zheng Y, Wan Y,
Wang Y, Zhou L, Liu S, Li G and Yan Y: miR-145 modulates
epithelial-mesenchymal transition and invasion by targeting ZEB2 in
non-small cell lung cancer cell lines. J Cell Biochem.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xia F, Xiong Y and Li Q: Interaction of
lincRNA ROR and p53/miR-145 correlates with lung cancer stem cell
signatures. J Cell Biochem: May 18, 2017. https://doi.org/10.1002/jcb.25960.
|
|
23
|
Wei H, Wen-Ming C and Jun-Bo J: Plasma
miR-145 as a novel biomarker for the diagnosis and radiosensitivity
prediction of human cervical cancer. J Int Med Res. 45:1054–1060.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tabrizi M, Khalili M, Vasei M, Nouraei N,
Mansour Samaei N, Khavanin A, Khajehei M and Mowla SJ: Evaluating
the miR-302b and miR-145 expression in formalin-fixed
paraffin-embedded samples of esophageal squamous cell carcinoma.
Arch Iran Med. 18:173–178. 2015.PubMed/NCBI
|
|
25
|
Sheng N, Tan G, You W, Chen H, Gong J,
Chen D, Zhang H and Wang Z: MiR-145 inhibits human colorectal
cancer cell migration and invasion via PAK4-dependent pathway.
Cancer Med. 6:1331–1340. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J and
Shi ZZ: miR-145-5p suppresses tumor cell migration, invasion and
epithelial to mesenchymal transition by regulating the Sp1/NF-κB
signaling pathway in esophageal squamous cell carcinoma. Int J Mol
Sci. 18(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Han Q, Zhang HY, Zhong BL, Wang XJ, Zhang
B and Chen H: MicroRNA-145 inhibits cell migration and invasion and
regulates epithelial-mesenchymal transition (EMT) by targeting
connective tissue growth factor (CTGF) in esophageal squamous cell
carcinoma. Med Sci Monit. 22:3925–3934. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M,
et al: Human microRNA genes are frequently located at fragile sites
and genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jin C, Wang A, Liu L, Wang G, Li G and Han
Z: miR-145-5p inhibits tumor occurrence and metastasis through the
NF-κB signaling pathway by targeting TLR4 in malignant melanoma. J
Cell Biochem. 120:11115–11126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kliese N, Gobrecht P, Pachow D, Andrae N,
Wilisch-Neumann A, Kirches E, Riek-Burchardt M, Angenstein F,
Reifenberger G, Riemenschneider MJ, et al: miRNA-145 is
downregulated in atypical and anaplastic meningiomas and negatively
regulates motility and proliferation of meningioma cells. Oncogene.
32:4712–4720. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang C, Xia Z, Zhu L, Li Y, Zheng Z, Liang
J and Wu L: MicroRNA-139-5p modulates the growth and metastasis of
malignant melanoma cells via the PI3K/AKT signaling pathway by
binding to IGF1R. Cell Cycle. 18:3513–3524. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shi X, Wang J, Lei Y, Cong C, Tan D and
Zhou X: Research progress on the PI3K/AKT signaling pathway in
gynecological cancer (Review). Mol Med Rep. 19:4529–4535.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhu X and Zhu R: Curcumin suppresses the
progression of laryngeal squamous cell carcinoma through the
upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR
pathway. OncoTargets Ther. 11:3521–3531. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu S, Gao G, Yan D, Chen X, Yao X, Guo S,
Li G and Zhao Y: Effects of miR-145-5p through NRAS on the cell
proliferation, apoptosis, migration, and invasion in melanoma by
inhibiting MAPK and PI3K/AKT pathways. Cancer Med. 6:819–833.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li B, Ding CM, Li YX, Peng JC, Geng N and
Qin WW: MicroRNA-145 inhibits migration and induces apoptosis in
human non-small cell lung cancer cells through regulation of the
EGFR/PI3K/AKT signaling pathway. Oncol Rep. 40:2944–2954.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cheng Z, Dai LL, Wang X, Jia LQ, Jing XG,
Li PF, Liu M, Wang H and An L: MicroRNA-145 down-regulates mucin
5AC to alleviate airway remodeling and targets EGFR to inhibit
cytokine expression. Oncotarget. 8:46312–46325. 2017.PubMed/NCBI View Article : Google Scholar
|