Introduction
Osteoarthritis (OA) is the most common degenerative
joint disorder, which leads to chronic bone and muscle pain
(1). OA is characterized by
degradation of articular cartilage, synovial inflammation,
subchondral bone sclerosis and osteophyte formation (2,3).
Current strategies for treating OA remain limited and did not seem
to improve the management of OA. To date, application of
nonsteroidal anti-inflammatory drugs (NSAIDs) is the primary
therapeutic approach for the treatment of OA (4). However, side-effects, including peptic
ulcers, hemorrhage and perforations, frequently occur during the
therapeutic process (5,6). Therefore, the development of novel
agents for treating OA remain urgently sort after.
OA exhibits several risk factors (7), where the inflammatory mediator
interleukin (IL)-1β has been shown to serve an important role in
its pathogenesis (8). The adult
articular cartilage consists of the extracellular matrix (ECM) and
chondrocytes (9). ECM provides the
necessary tension and strength to the articular cartilage (10). Under physiological conditions, a
subtle balance between ECM synthesis and degradation maintains the
homeostasis of the cartilage (11).
Previous studies have suggested that IL-1β significantly stimulates
chondrocytes to secrete matrix metalloproteinases (MMPs) (12,13)
and promotes the production of inflammatory mediators, including
prostaglandin E2 and nitric oxide (NO) (14). It has been suggested that MMPs are
responsible for the degradation of ECM during the progression of OA
(15). ECM is composed of type II
collagen, proteoglycans and aggrecan (16,17).
In previous studies, IL-1β has been reported to downregulate the
expression of type II collagen and aggrecan in vitro,
thereby leading to the degradation of articular cartilage (18,19).
Therefore, agents targeting the IL-1β-induced inflammatory response
during the pathogenesis of OA can potentially attenuate the
progression of OA.
Oroxylin A (OrA) is a natural mono-flavonoid that
can be extracted from the herb Scutellariae radix (20). Accumulating evidence has
demonstrated that OrA exerts multiple pharmacological effects,
including anti-inflammatory (21,22),
anti-oxidative (23,24) and anti-tumorigenic (25,26)
properties. Therefore, it has been extensively used to treat a
variety of diseases. The anti-inflammatory effects of OrA are
mainly mediated by blocking the phosphorylation and subsequent
activation of the PI3K/AKT signaling pathway (27). By contrast, a previous study has
also reported that OrA reduces lipopolysaccharide-induced
inflammatory reactions by activating the NF-κB signaling pathway
(28). However, the comprehensive
role of OrA in OA progression remains poorly understood.
The present study aimed to perform western blotting,
qPCR and cell immunofluorescence to evaluate the protective effect
of OrA on IL-1β-induced chondrocyte inflammation and its underlying
mechanism.
Materials and methods
Reagents
OrA and the Cell Counting Kit (CCK)-8 were purchased
from MedChemExpress. Primary antibodies against the
unphosphorylated forms of PI3K (cat. no. 4255), AKT (cat. no.
9272), ERK (cat. no. 4695), p38 (cat. no. 14451), JNK (cat. no.
9252), p65 (cat. no. 8242), NF-κB inhibitor α (IκBα; cat. no.
4814), inducible nitric oxide synthase (iNOS; cat. no. 39898),
cyclooxygenase 2 (COX-2; cat. no. 12282) and β-actin (cat. no.
3700), and the phosphorylated (p) forms of AKT (cat. no. 13038),
ERK (cat. no. 4370), p38 (cat. no. 9216), JNK (cat. no. 9251), and
p65 (cat. no. 3039) were obtained from Cell Signaling Technology,
Inc. Primary antibodies against MMP3 (cat. no. 17873-1-AP) and
MMP13 (cat. no. 18165-1-AP) were purchased from Proteintech Group,
Inc., whereas those against aggrecan, disintegrin and
metalloproteinase with thrombospondin motifs ADAMTS-4 (cat. no.
ab185722), ADAMTS-5 (cat. no. ab41037) and type II collagen (cat.
no. ab188570) were from purchased from Abcam. Secondary antibodies
(anti-mouse cat. no. 7076 and anti-rabbit cat. no. 7074) were
obtained from Cell Signaling Technology, Inc and diluted in
secondary antibody diluent (Beyotime Institute of Biotechnology;
cat. no. P0258; 1:100). DMEM/Ham's F12 medium (DMEM/F12) was
obtained from Hyclone, Cytiva. FBS was purchased from Gibco (Thermo
Fisher Scientific, Inc.) and recombinant rat IL-1β (cat. no.
211-11B) was purchased from PeproTech, Inc.
Isolation and culture of primary
chondrocytes
A total of 30 C57BL/6 mice (age, 2-3 days) were
purchased from the Animal Center of Chinese Academy of Sciences and
were decapitated before chondrocytes were isolated from their
articular cartilage. Briefly, the articular cartilages of each
mouse were carefully extracted under aseptic conditions and cut
into ~1-2 mm2 slices, followed by washing with PBS three
times at room temperature. The pieces were then digested using with
DMEM/F12 medium supplemented with 0.1% collagenase II at 37˚C in a
humidified atmosphere containing 5% CO2 for 8 h. The
cells were then collected via centrifugation at 1,000 x g for 3 min
at 25˚C, washed with PBS three times, plated into cell culture
flasks in DMEM/F12 supplemented with 10% FBS, 100 U/ml penicillin
and 100 mg/ml streptomycin and incubated at 37˚C in an atmosphere
of 95% air and 5% CO2. The medium was changed after 24 h
and the cells were harvested when 80-90% confluence was reached.
Only chondrocytes from passages 1-2 were used in the present study
to avoid the loss of phenotype. Light microscopy (upper panel
original magnification, x100; lower panel original magnification,
x200) was performed to observe the cell morphology of chondrocytes.
Cells at passages 1-2 had a rounded or polygonal structure.
CCK-8 assay
The primary chondrocytes were plated at a density of
8x103 cells/well into 96-well plates followed by
treatment with DMEM/F12 medium containing increasing concentrations
of OrA (0, 2, 4, 8, 16, 32, 64 and 128 µM) at 37˚C in a humidified
atmosphere containing 5% CO2 for 24 and 48 h. At the end
of each time point, 10 µl CCK-8 solution was added into each well
and the chondrocytes were incubated further for an additional 4 h
at 37˚C in an atmosphere with 95% air and 5% CO2.
Absorbance was then measured at 450 nm using a
Multiskan™ GO microplate reader (Thermo Fisher
Scientific, Inc.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
The primary chondrocytes were seeded into six-well
plates at a density of 5x105 cells/well. Once they
adhered to the plates, chondrocytes were pre-treated with various
concentrations of OrA (4, 8, and 16 µM) for 2 h before being
stimulated with or without IL-1β (10 ng/ml) at 37˚C in a 5%
CO2 incubator for 24 h. Total RNA was extracted using a
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Subsequently, a total of
1,000 ng RNA was reverse transcribed into cDNA (PrimeScript™
Reverse Transcriptase kit; Takara Biotechnology Co., Ltd.) using
the following temperature protocol: 30˚C for 10 min, 42˚C for 30
min and 70˚C for 15 min, after which the sample was cooled on
ice.
RT-qPCR was conducted using the SYBR green Master
Mix (Takara Biotechnology Co., Ltd.) and performed with the ViiA™ 7
real-time PCR system (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The thermocycling conditions of qPCR
were as follows: 95˚C for 10 min, followed by 50 cycles of 95˚C for
15 sec and 60˚C for 1 min. The cycle threshold (Ct) of
each sample was normalized to the expression levels of β-actin. The
2-ΔΔCq method was used to assess
the relative expression of various target genes (29). The primer sequences of tumor
necrosis factor-α (TNF-α), IL-6, iNOS, MMP-3, MMP-13 and β-actin
are listed in Table I.
 | Table ISequences of primers used in reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used in reverse
transcription-quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|
| TNF-α |
5'-GGAACACGTCGTGGGATAATG-3' |
5'-GGCAGACTTTGGATGCTTCTT-3' |
| IL-6 |
5'-GGCGGATCGGATGTTGTGAT-3' |
5'-GGACCCCAGACAATCGGTTG-3' |
| iNOS |
5'-CAGGGAGAACAGTACATGAACAC3' |
5'-TTGGATACACTGCTACAGGGA-3' |
| MMP-3 |
5'-TTAAAGACAGGCACTTTTGGCG-3' |
5'-CCCTCGTATAGCCCAGAACT-3' |
| MMP-13 |
5'-CTATCCCTTGATGCCATTACCAG-3' |
5'-ATCCACATGGTTGGGAAGTTC-3' |
| β-actin |
5'-AGCCATGTACGTAGCCATCC-3' |
5'-CTCTCAGCAGTGGTGGTGAA-3' |
Immunofluorescence microscopy
Primary chondrocytes (5x104 cells/ml)
were seeded into a 12-well plate and were then stimulated at 37˚C
in a humidified atmosphere containing 5% CO2 for 48 h.
Cells were fixed with 4% paraformaldehyde for 30 min at 25˚C and
washed with PBS. Subsequently, the cell slides were treated with
0.1% Triton X-100 for 10 min at room temperature, blocked with 10%
bovine serum albumin for 1 h at 25˚C and then incubated with
primary antibodies against COX-2 (dilution, 1:500), MMP3 (dilution,
1:500), MMP13 (dilution, 1:500) or type II collagen (dilution,
1:200) at 4˚C overnight. The following day, cells were incubated
with fluorescein-conjugated goat anti-rabbit IgG antibody (Abcam;
cat. nos. ab150077 and ab150115) diluted in immunofluorescence
secondary antibody diluent (Beyotime Institute of Biotechnology;
cat. no. P0265; 1:100) for 1 h at room temperature in the dark.
Subsequently, the cell nuclei were treated with 0.05% DAPI
(Beyotime Institute of Biotechnology; cat. no. C1002) for an
additional 5 min at room temperature in the dark. All images were
captured using a fluorescence microscope (Olympus Corporation).
Fluorescence intensity was measured using ImageJ software (v.
d1.47; National Institutes of Health).
Western blot analysis
To measure the expression levels of iNOS, COX-2,
MMP-3, MMP-13, aggrecan, ADAMTS-4, ADAMTS-5 and type II collagen, a
total of 5x105 chondrocytes/well were seeded into
six-well plates and pre-treated with various concentrations of OrA
(4, 8 and 16 µM) for 2 h, followed by stimulation with or without
IL-1β (10 ng/ml) at 37˚C in a 5% CO2 incubator for 24 h.
To explore the molecular mechanism of OrA in the progression of OA,
a total of 5x105 chondrocytes/well were seeded into
six-well plates, pre-treated with or without 16 µM OrA for 2 h and
then stimulated with or without IL-1β (10 ng/ml) for different time
periods (0, 15, 30 and 60 min) at 37˚C in a 5% CO2
incubator. Following treatment, total proteins were extracted using
a RIPA lysis buffer containing 1% protease and phosphorylase
inhibitors (Beyotime Institute of Biotechnology). Proteins were
then incubated on ice for an additional 30 min and centrifuged at
12,000 x g for 10 min at 4˚C. Total protein concentration was
measured using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). A total of 40 µg of protein from each
group was separated using SDS-PAGE on a 10% gel and then
transferred onto 0.22-µm PVDF membranes. Following blocking with 5%
non-fat dry milk for 2 h at room temperature, membranes were
incubated with primary antibodies against iNOS, COX-2, MMP3,
MMP-13, ADAMTS-4, ADAMTS-5, PI3K, p-AKT, AKT, p-ERK, ERK, p-p38,
p38, p-JNK, JNK, p-p65, p65, IκBα, β-actin (all in dilution
1:1,000) or type II collagen (dilution, 1:500) overnight at 4˚C.
The membranes were then washed three times with TBS-0.1% Tween-20
for 5 min each time. Subsequently, the membranes were incubated
with the secondary antibodies (Cell Signaling Technology, Inc.;
anti-mouse cat. no. 7076 and anti-rabbit cat. no. 7074) diluted in
secondary antibody diluent (Beyotime Institute of Biotechnology;
cat. no. P0258; 1:100) for 2 h at room temperature. The protein
bands were visualized by using electrochemiluminescence (Beyotime
Institute of Biotechnology; cat. no. P0018FS) and captured by a
BioSpectrum imaging system (Thermo Fisher Scientific, Inc.) and
densitometry analysis was performed using the ImageJ software (v.
d1.47; National Institutes of Health).
Animals
Animal experiments were conducted in accordance with
the International Ethical guidelines (30) and the National Institutes of Health
Guide for Care and Use of Laboratory Animals (NIH Pub No 85-23,
revised 1996) (31). The procedures
were approved by the Ethics Committee of Ningbo No. 6 Hospital
(Ningbo, China).
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD) from three experimental repeats. The GraphPad Prism software
(version 7.0; GraphPad Software Inc.) was applied for all
statistical analyses. One-way ANOVA followed by Tukey's post hoc
test was performed to detect significant differences among groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of OrA on murine chondrocyte
viability
Chondrocytes were extracted from the articular
cartilage of each mice and their morphology was examined. The
results revealed that chondrocytes in the cartilage matrix had a
rounded or polygonal structure (Fig.
1A). Chondrocytes were seeded into 96-well plates at a density
of 8x103 cells/well. After adhesion to the dishes, cells
were treated with ascending concentrations of OrA (0, 2, 4, 8, 16,
32, 64, and 128 µM) for 24 and 48 h. The results revealed that OrA
at lower concentrations (0, 2, 4, 8, and 16 µM) was not toxic for
chondrocytes (Fig. 1B), while the
higher concentrations (>32 µM) were toxic for chondrocytes, with
the majority of cells dying after stimulation.
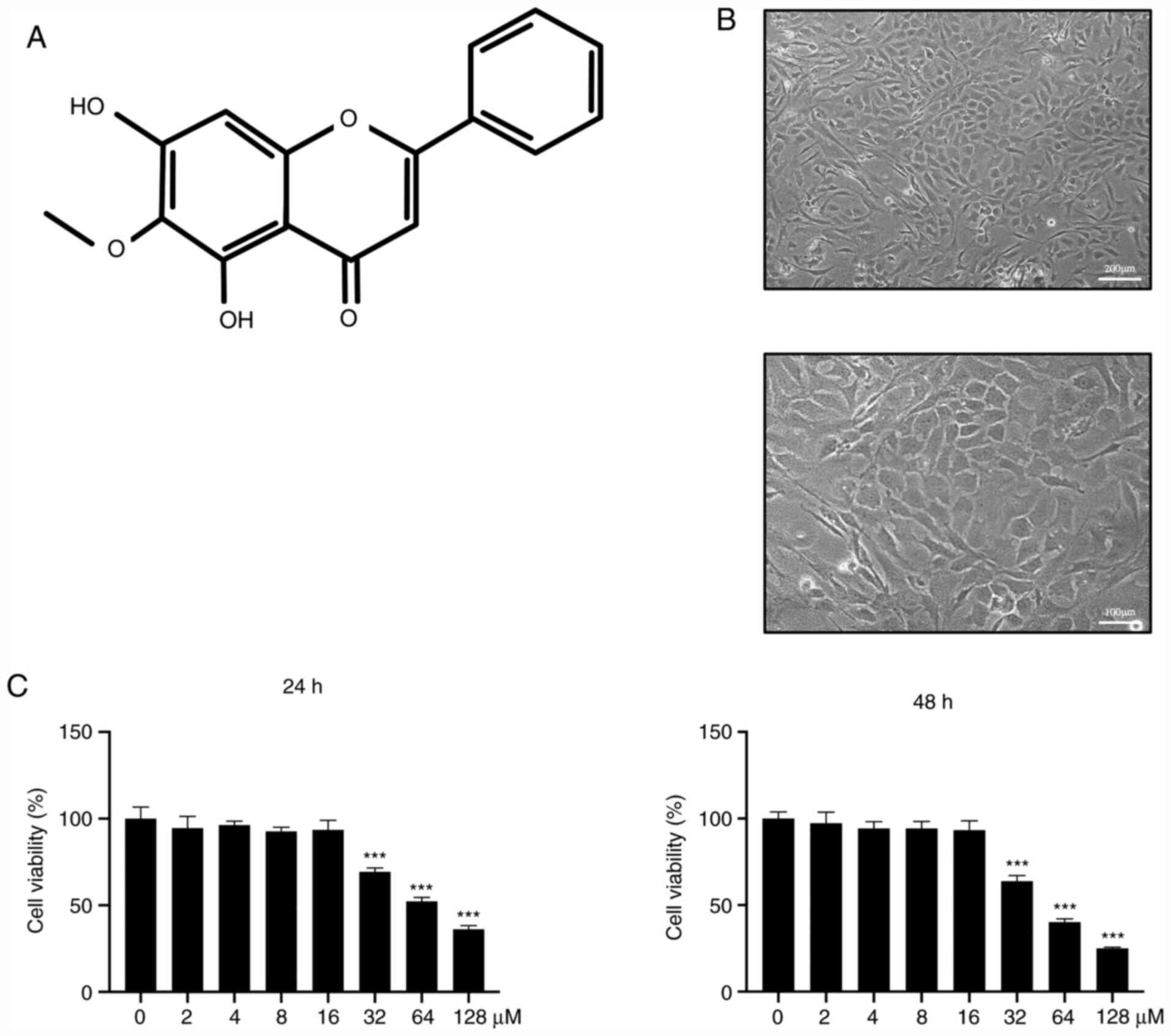 | Figure 1Effect of OrA on murine chondrocyte
viability. (A) Chemical structure of OrA. (B) Morphology of
chondrocytes (upper panel original magnification, x100; lower panel
original magnification, x200). (C) Chondrocytes were treated with
medium supplemented with different concentrations of OrA (0, 2, 4,
8, 16, 32, 64 and 128 µM) for 24 and 48 h before the CCK-8 assay
was performed to assess cell viability. Each experiment was
repeated three times independently. Data are expressed as the mean
± standard deviation.
***P<0.001 vs. untreated. OrA,
oroxylin A; CCK-8, Cell Counting Kit. |
OrA attenuates IL-1β-induced
inflammation
The potentially protective effects of OrA against
IL-1β-induced inflammatory reaction was next determined by RT-qPCR
and western blotting. Although IL-1β significantly promoted the
expression of the inflammatory factors TNF-α, IL-6 and iNOS,
treatment with OrA significantly reversed this effect in a
dose-dependent manner (Fig. 2A).
Furthermore, western blot analysis revealed that 16 µM OrA markedly
inhibited IL-1β-induced upregulation of iNOS and COX-2 (Fig. 2B and C). The protective effects of OrA on
IL-1β-induced inflammatory reaction was also supported by results
from immunofluorescence analysis. Significantly lower expression
levels of COX-2 were observed in cells pre-treated with OrA
compared with those in cells treated with IL-1β alone without OrA
pre-treatment (Fig. 2D and E).
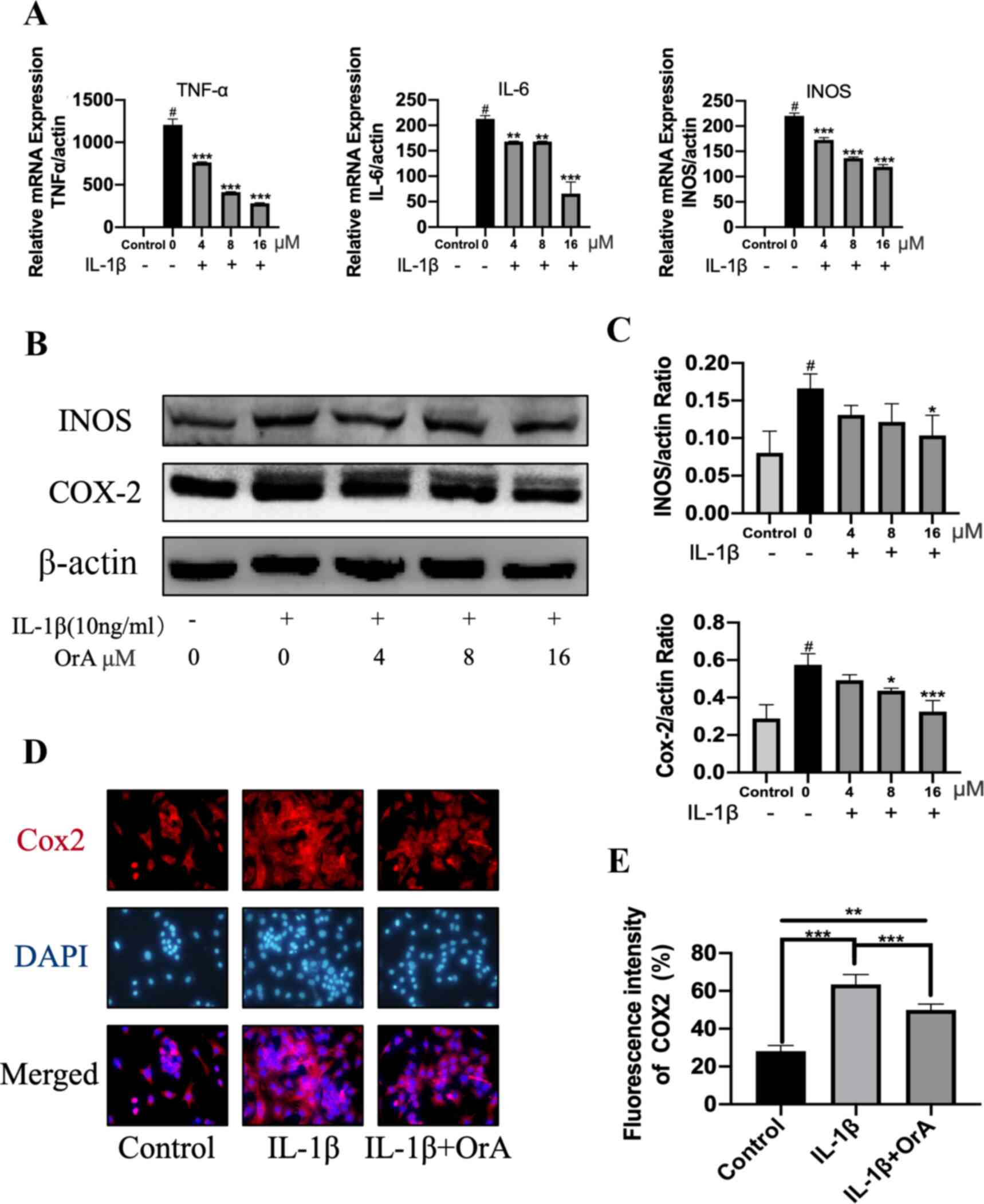 | Figure 2OrA attenuates IL-1β-induced
inflammation. Chondrocytes were treated with various concentrations
of OrA (4, 8, and 16 µM) and stimulated with or without IL-1β (10
ng/ml) for 24 h. (A) Relative mRNA expression levels of TNF-α, IL-6
and iNOS were determined by reverse-transcription-quantitative PCR.
#P<0.01 vs. untreated;
**P<0.01 and
***P<0.001 vs. IL-1β only. (B)
Protein expression levels of iNOS and COX-2 were determined by
western blot analysis. (C) Quantification of iNOS and COX-2
expression. #P<0.01 vs. untreated;
*P<0.05 and
***P<0.001 vs. IL-1β only. (D)
Immunofluorescence analysis of COX-2 expression, (E) which was
quantified. Original magnification, x200. Each experiment was
repeated three times independently. Data are expressed as the mean
± standard deviation. **P<0.01 and
***P<0.001. OrA, oroxylin A;
IL-1β, interleukin-1β; TNF-α, tumor necrosis factor α; iNOS,
inducible nitric oxide synthase; COX-2, cyclooxygenase 2. |
OrA reverses IL-1β-induced degradation
of ECM
To explore the effect of OrA on IL-1β-induced
degradation of ECM, RT-qPCR was first performed to evaluate the
expression levels of the matrix-degrading enzymes MMP-3 and MMP-13.
The results demonstrated that OrA significantly attenuated the
IL-1β-mediated upregulation of MMP-3 and MMP-13 mRNA (Fig. 3A). The 16 µM OrA-mediated protective
effects against IL-1β-induced MMP-3 and MMP-13 upregulation was
also confirmed on protein level using western blot analysis
(Fig. 3B and C). Consistent with the previous findings
of the present study, immunofluorescence results supported the
potentially suppressive effects of OrA on IL-1β-mediated increased
expression of MMP-3 and MMP-13. Significantly lower expression
levels of MMP-3 and MMP-13 were observed in cells pre-treated with
OrA compared with those in cells treated with IL-1β alone without
OrA pre-treatment (Fig. 3D and
E).
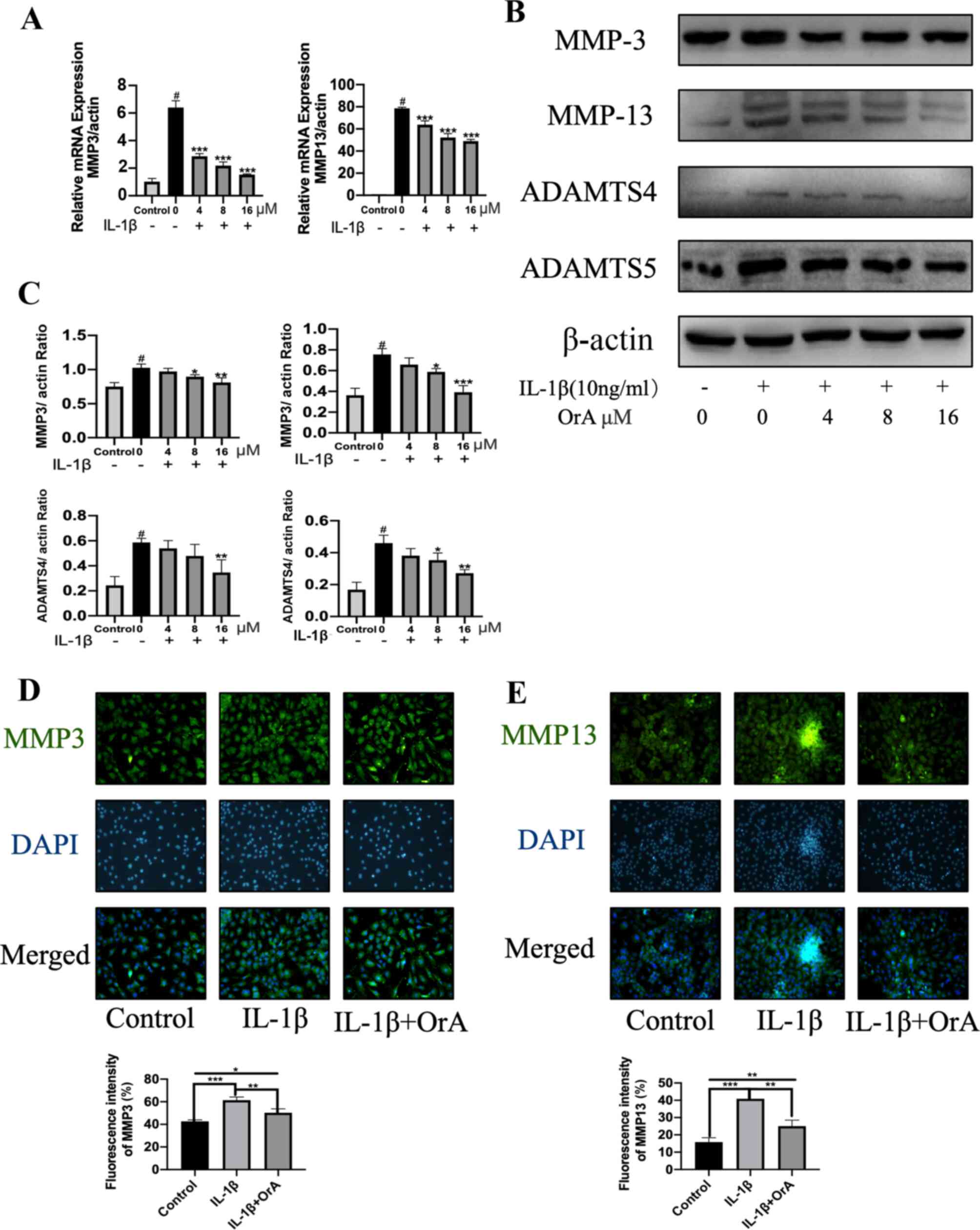 | Figure 3OrA treatment prevents IL-1β-induced
degradation of ECM. Chondrocytes were treated with various
concentrations of OrA (4, 8 and 16 µM) and stimulated with or
without IL-1β (10 ng/ml) for 24 h. (A) Relative mRNA expression
levels of MMP-3 and MMP-13 were determined by reverse
transcrtiption-quantitative PCR. (B) The protein expression levels
of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 were determined by western
blot analysis. (C) Quantification of MMP-3, MMP-13, ADAMTS-4 and
ADAMTS-5 expression. Data are expressed as the mean ± standard
deviation from three experimental repeats. #P<0.01
vs. untreated; *P<0.05,
**P<0.01 and
***P<0.001 vs. IL-1β only.
Immunofluorescence analysis of (D) MMP-3 and (E) MMP-13 expression,
which were quantified (original magnification, x100). Data are
expressed as the mean ± standard deviation from three experimental
repeats. *P<0.05, **P<0.01
and ***P<0.001. OrA, oroxylin
A; ECM, extracellular matrix; IL-1β, interleukin-1β; MMP3, matrix
metalloproteinase; ADAMTS, disintegrin and metalloproteinase with
thrombospondin motifs. |
The ADAMTS family of metalloproteases has been
previously reported to be involved in the cleavage of aggrecan
(32). Therefore, the present study
investigated the effects of OrA on IL-1β-induced expression of
ADAMTS-4 and ADAMTS-5. The results revealed that pre-treatment with
16 µM OrA markedly attenuated IL-1β-mediated upregulation of
ADAMTS-4 and ADAMTS-5 (Fig. 3B and
C).
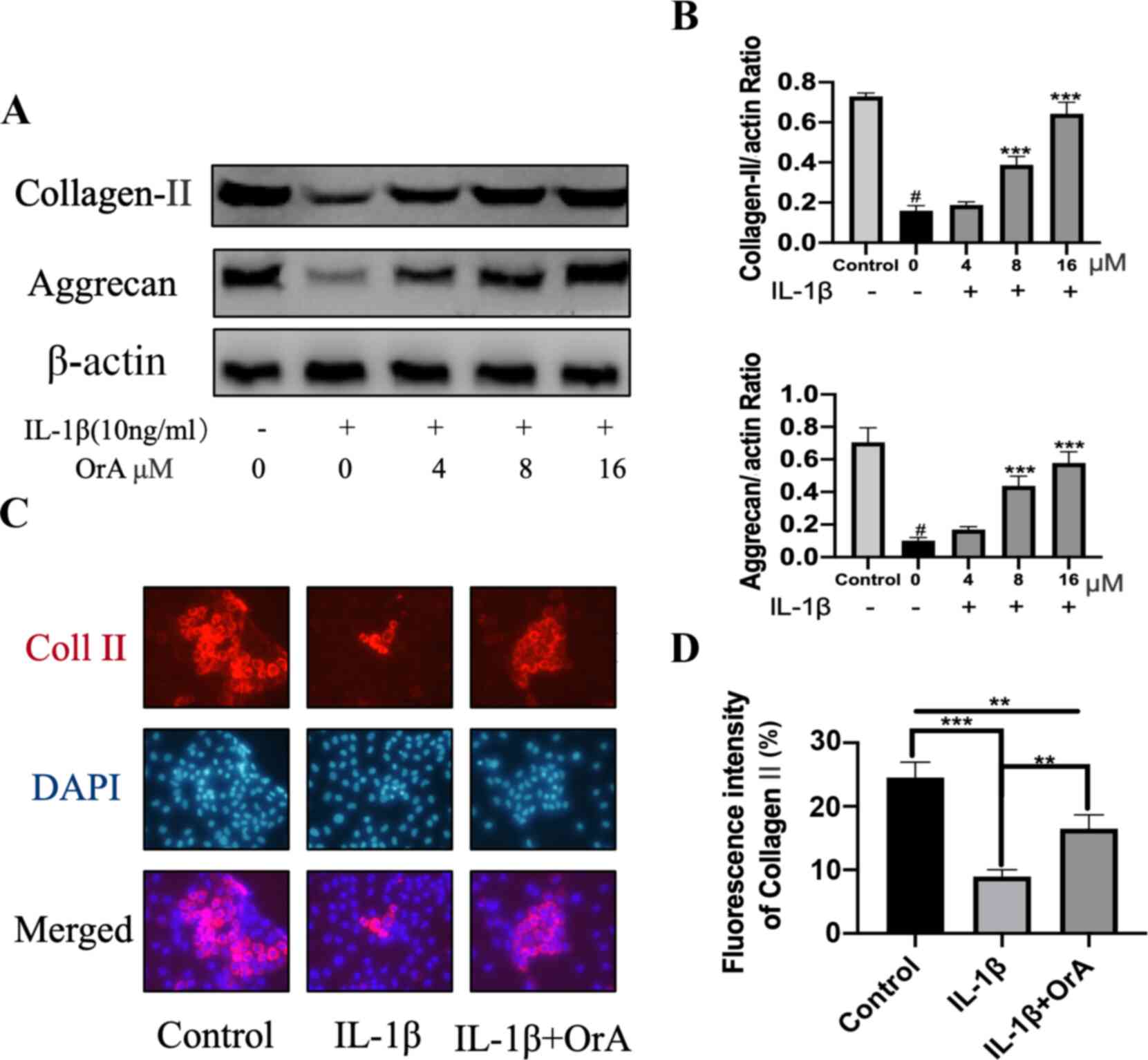
Type II collagen and aggrecan are the main
components of ECM (33,34). Treatment with IL-1β significantly
decreased the expression of both molecules, whilst pre-treatment
with 8 and 16 µM OrA significantly prevented this effect (Fig. 4A and B). Immunofluorescence analysis of type II
collagen also indicated that OrA pre-treatment effectively
protected against IL-1β-mediated downregulation of type II collagen
(Fig. 4C and D).
Effect of OrA on IL-1β-induced NF-κ
and MAPK activation
Western blot analysis indicated that cell
stimulation with IL-1β for 15 min markedly increased the
phosphorylation of p65 and IκBα degradation (Fig. 5A and B). However, pre-treatment with OrA had no
effects on the NF-κB signaling pathway activation (Fig. 5A and B). The effect of OrA on IL-1β-mediated
phosphorylation of ERK, JNK and p38 was subsequently investigated
using western blot analysis. Although OrA exerted no effects on the
activation of JNK and p38, it significantly prevented the
IL-1β-mediated phosphorylation of ERK at 15 min compared with that
cells that were not pre-treated with OrA (Fig. 5C and D).
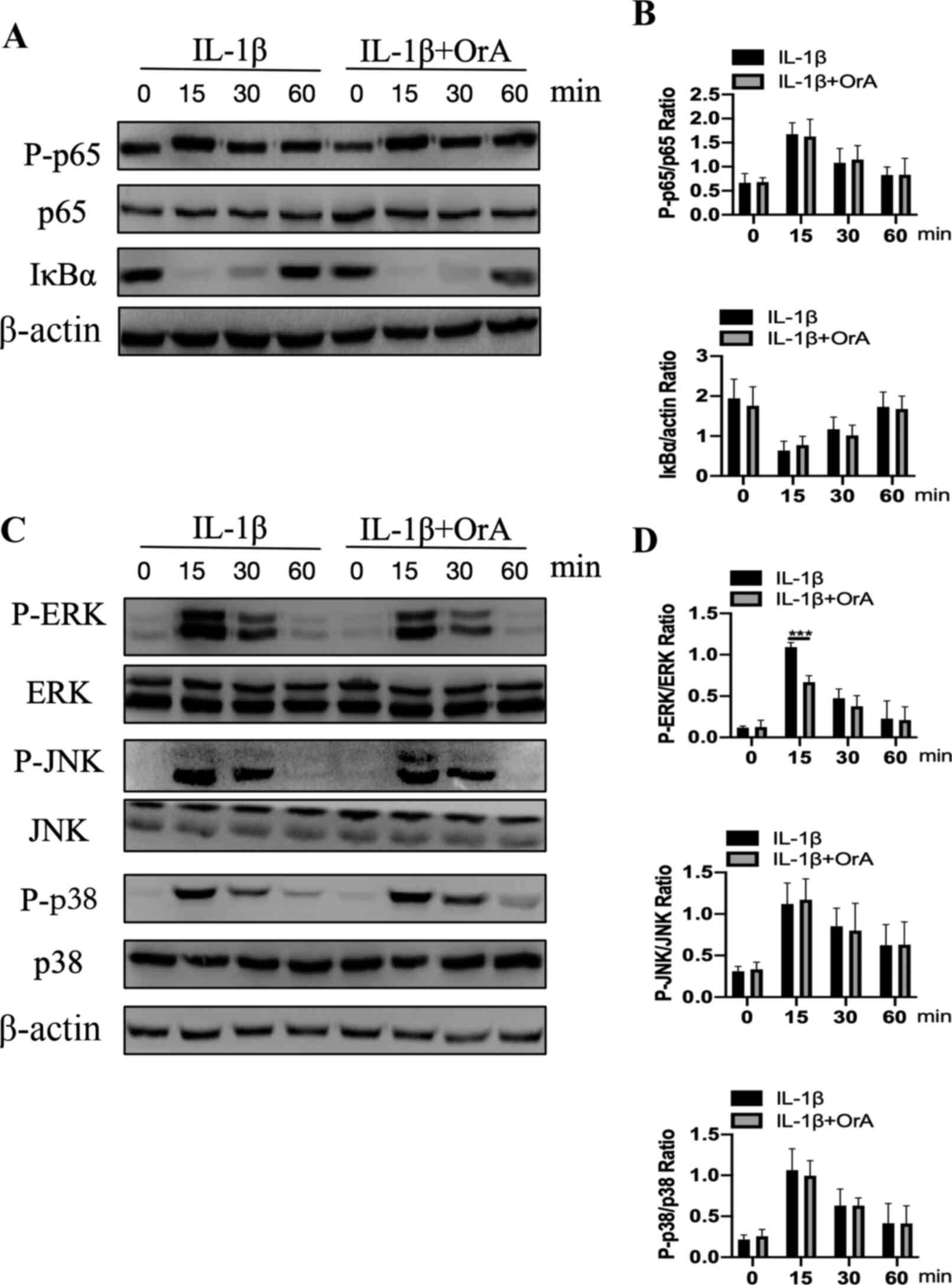 | Figure 5Effect of OrA on IL-1β-mediated NF-κB
and MAPK activation. Chondrocytes were pre-treated with or without
16 µM OrA for 2 h, and then stimulated with or without IL-1β (10
ng/ml) for different time periods (0, 15, 30 and 60 min). (A) The
protein expression levels of p65 and IκBα, in addition to p65
phosphorylation were determined by western blot analysis. (B) which
was then quantified. (C) The protein expression levels of ERK, JNK,
JNK and p38, in addition to their corresponding phosphorylation
levels, were determined by western blot analysis and (D)
quantified. Data are expressed as the mean ± standard deviation
from three experimental repeats.
***P<0.001 vs. the IL-1β only
group. OrA, oroxylin A; IL-1β, interleukin-1β; IκBα, NF-κB
inhibitor α; p-, phosphorylated. |
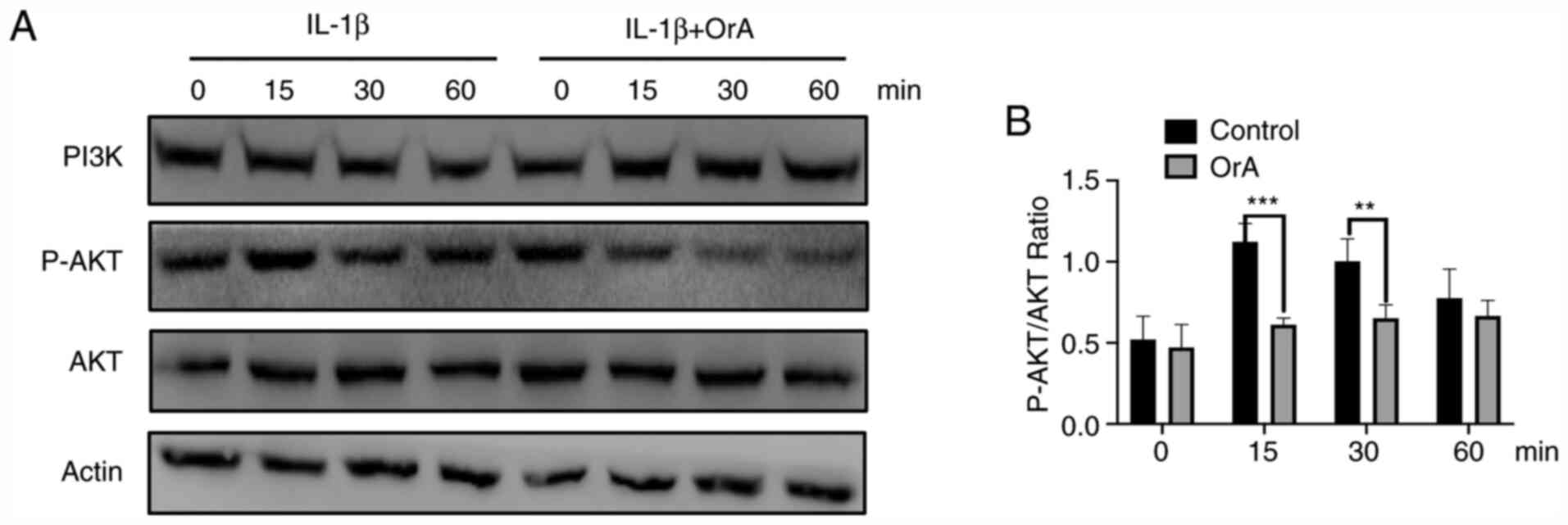
Effect of OrA on IL-1β-mediated
PI3K/AKT activation
To further explore the potential anti-inflammatory
effects of OrA in chondrocytes, western blot analysis was applied
to evaluate the phosphorylation levels of AKT. The results
demonstrated that at 15 and 30 min, IL-1β-mediated AKT
phosphorylation was significantly lower in chondrocytes pre-treated
with OrA compared with cells there were not pre-treated (Fig. 6A and B).
Discussion
OA is a chronic age-associated degenerative joint
disease with a complex pathology that imposes severe socio-economic
burdens on the patients (35).
According to statistics, ~10% men and 13% women aged ≥60 years are
afflicted with knee OA in the USA (36). At present, OA treatment strategies
for relieving the pain symptoms are limited, where surgery is
considered to be the final option in cases of advanced disease
progression (37). Although agents
are available to clinically relieve pain, severe side effects
frequently occur. For instance, NSAIDs, which are used widely in OA
to clinically relieve pain and swelling, do not ameliorate
cartilage degeneration and are associated with gastrointestinal
side effects, such as gastrorrhagia (38,39).
Therefore, novel, safe and effective alternative strategies are
urgently sorted for OA treatment. OrA is a natural mono-flavonoid
that can be extracted from Scutellariae radix (40). Previous studies have reported the
anti-inflammatory effects of OrA (21,41).
The present study investigated the potential effects of OrA in
IL-1β-induced inflammation in murine chondrocytes. The results
revealed that OrA pre-treatment resulted in the suppression of
inflammation by inhibiting the ERK and PI3K/AKT signaling
pathways.
Accumulating evidence has indicated that OA is
characterized by cartilage degeneration (42). Under normal conditions, the joint
cartilage is maintained through a delicate balance between the
synthesis and degradation of ECM (43). However, inflammatory cytokines,
especially IL-1β, can perturb this balance, which leads to
cartilage degradation (44). In the
present study, iNOS and COX-2 were found to be significantly
upregulated following stimulation with IL-1β. It has been
previously reported that the production of iNOS and COX-2 serves an
important role in the pathophysiology of OA (45). Several studies demonstrated that
iNOS and COX-2 downregulation ameliorated the progression of OA
(46,47). The present findings showed that OrA
significantly prevented the IL-1β-induced expression of iNOS and
COX-2. ECM is the main component of articular cartilage (48). Increased catabolism of ECM is
considered to be a crucial factor in the progression of OA
(49). Previous studies have
provided evidence that the activation of MMPs, especially MMP3 and
MMP13, promotes the degradation of ECM (12,13).
In the present study, stimulation with IL-1β markedly upregulated
the expression of MMP3 and MMP13, whilst pre-treatment with OrA
prevented this effect. Aggrecan and type II collagen are the main
components of ECM, such that downregulation of both of these
molecules leads to cartilage degradation (50). In the present study, IL-1β
significantly attenuated the expression of aggrecan and type II
collagen, whilst pre-treatment with OrA prevented this effect. In
addition, the ADAMTS enzymes, especially ADAMTS-4 and ADAMTS-5, are
considered to be the primary aggrecanases with the ability to
cleave aggrecans (51,52). In the present study, treatment with
OrA attenuated IL-1β-induced expression of ADAMTS-5 and protected
against IL-1β-induced cartilage degradation.
The MAPK and PI3K/AKT signaling pathways serve a
crucial role in the pathogenesis of OA (53,54).
Inhibition of IL-1β-induced activation of ERK has been previously
reported to attenuate the progress of OA (55). Accumulating evidence has suggested
that the activation of ERK mediates the production of MMPs, thereby
promoting cartilage degradation (56,57).
The present study demonstrated that treatment with OrA
significantly inhibited the activation of ERK. Another previous
study reported that inhibition of the PI3K/AKT signaling pathway
relieved IL-1β-induced inflammatory response in chondrocytes
(58). In addition, it has been
also reported that PI3K/AKT signaling regulates the expression of
aggrecan (59). Therefore, the
present study investigated the effect of OrA on IL-1β-mediated
activation of the PI3K/AKT signaling pathway and confirmed that OrA
could also inhibit the PI3K/AKT signaling pathway.
However, there are still several limitations in the
current study. First, as the present research was based on murine
chondrocytes, which were obtained from neonatal mice, the mice were
not weighed or sexed upon purchase. Additionally, the current study
lacks in vivo results, which should be assessed in future
work.
Taken together, the results of the present study
suggested that OrA exerted protective effects against IL-1β-induced
inflammatory response by inhibiting the activation of the ERK and
PI3K/AKT signaling pathways. The current study indicated the
therapeutic potential of OrA in osteoarthritis treatment and may
therefore provide a novel candidate for OA therapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Medical and
Health Technology Project of Zhejiang Province (grant no.
2019PY073); Science and Technology Research on Public Welfare
Project of Ningbo, Zhejiang Province (grant no. 2019C50050) and the
Scientific Technology Project of Agriculture and Social Development
of Yinzhou, Ningbo, Zhejiang Province (grant no. 20180137).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YZ and QW conceived the study; YZ and JC conducted
the experiments; JH wrote the manuscript and performed statistical
analysis; QW and ML analyzed the results and created the figures.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were conducted in accordance with
the international ethical guidelines and the National Institutes of
Health Guide for Care and Use of Laboratory Animals (NIH Pub No
85-23, revised 1996). The procedures were approved by the Ethics
Committee of Ningbo No. 6 Hospital (Ningbo, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ji Q, Xu X, Kang L, Xu Y, Xiao J, Goodman
SB, Zhu X, Li W, Liu J, Gao X, et al: Hematopoietic PBX-interacting
protein mediates cartilage degeneration during the pathogenesis of
osteoarthritis. Nat Commun. 10(313)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ashford S and Williard J: Osteoarthritis:
A review. Nurse Pract. 39:1–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Abramson SB: Inflammation in
osteoarthritis. J Rheumatol Suppl. 70:70–76. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen YF, Jobanputra P, Barton P, Bryan S,
Fry-Smith A, Harris G and Taylor RS: Cyclooxygenase-2 selective
non-steroidal anti-inflammatory drugs (etodolac, meloxicam,
celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for
osteoarthritis and rheumatoid arthritis: A systematic review and
economic evaluation. Health Technol Assess. 12:1–278.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Lane NE: Pain management in
osteoarthritis: The role of COX-2 inhibitors. J Rheumatol Suppl.
49:20–24. 1997.PubMed/NCBI
|
|
6
|
Hungin APS and Kean WF: Nonsteroidal
anti-inflammatory drugs: Overused or underused in osteoarthritis?
Am J Med. 110 (Suppl):S8–S11. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Felson DT: Risk factors for
osteoarthritis: Understanding joint vulnerability. Clin Orthop
Relat Res. 427:16–21. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mokuda S, Nakamichi R, Matsuzaki T, Ito Y,
Sato T, Miyata K, Inui M, Olmer M, Sugiyama E, Lotz M and Asahara
H: Wwp2 maintains cartilage homeostasis through regulation of
Adamts5. Nat Commun. 10(2429)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee JH, Shehzad O, Ko SK, Kim YS and Kim
HP: Matrix metalloproteinase-13 downregulation and potential
cartilage protective action of the Korean Red Ginseng preparation.
J Ginseng Res. 39:54–60. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gao Y, Liu S, Huang J, Guo W, Chen J,
Zhang L, Zhao B, Peng J, Wang A, Wang Y, et al: The ECM-cell
interaction of cartilage extracellular matrix on chondrocytes.
Biomed Res Int. 2014(648459)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang M, Sampson ER, Jin H, Li J, Ke QH, Im
HJ and Chen D: MMP13 is a critical target gene during the
progression of osteoarthritis. Arthritis Res Ther.
15(R5)2013.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Kubota E, Imamura H, Kubota T, Shibata T
and Murakami KI: Interleukin-1 beta and stromelysin (MMP3) activity
of synovial fluid as possible markers of osteoarthritis in the
temporomandibular joint. J Oral Maxillofac Surg. 55:20–28.
1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Studer R, Jaffurs D, Stefanovic-Racic M,
Robbins PD and Evans CH: Nitric oxide in osteoarthritis.
Osteoarthritis Cartilage. 7:377–379. 1999.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Smith RL: Degradative enzymes in
osteoarthritis. Front Biosci. 4(D704-D712)1999.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Tchetina EV, Squires G and Poole AR:
Increased type II collagen degradation and very early focal
cartilage degeneration is associated with upregulation of
chondrocyte differentiation related genes in early human articular
cartilage lesions. J Rheumatol. 32:876–886. 2005.PubMed/NCBI
|
|
17
|
Vertel BM: The ins and outs of aggrecan.
Trends Cell Biol. 5:458–464. 1995.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ding J, Ghali O, Lencel P, Broux O,
Chauveau C, Devedjian JC, Hardouin P and Magne D: TNF-alpha and
IL-1beta inhibit RUNX2 and collagen expression but increase
alkaline phosphatase activity and mineralization in human
mesenchymal stem cells. Life Sci. 84:499–504. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang J, Markova D, Anderson DG, Zheng Z,
Shapiro IM and Risbud MV: TNF-α and IL-1β promote a
disintegrin-like and metalloprotease with thrombospondin type I
motif-5-mediated aggrecan degradation through syndecan-4 in
intervertebral disc. J Biol Chem. 286:39738–39749. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zou M, Hu C, You Q, Zhang A, Wang X and
Guo Q: Oroxylin A induces autophagy in human malignant glioma cells
via the mTOR-STAT3-Notch signaling pathway. Mol Carcinog.
54:1363–1375. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Yao J, Hu R, Sun J, Lin B, Zhao L, Sha Y,
Zhu B, You QD, Yan T and Guo QL: Oroxylin a prevents
inflammation-related tumor through down-regulation of inflammatory
gene expression by inhibiting NF-κB signaling. Mol Carcinog.
53:145–158. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Ye M, Wang Q, Zhang W, Li Z, Wang Y and Hu
R: Oroxylin A exerts anti-inflammatory activity on
lipopolysaccharide-induced mouse macrophage via Nrf2/ARE
activation. Biochem Cell Biol. 92:337–348. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li J, Tong D, Liu J, Chen F and Shen Y:
Oroxylin A attenuates cigarette smoke-induced lung inflammation by
activating Nrf2. Int Immunopharmacol. 40:524–529. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Han Q, Wang H, Xiao C, Fu BD and Du CT:
Oroxylin A inhibits H2O2-induced oxidative
stress in PC12 cells. Nat Prod Res. 31:1339–1342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zou M, Lu N, Hu C, Liu W, Sun Y, Wang X,
You Q, Gu C, Xi T and Guo Q: Beclin 1-mediated autophagy in
hepatocellular carcinoma cells: Implication in anticancer
efficiency of oroxylin A via inhibition of mTOR signaling. Cell
Signal. 24:1722–1732. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun Y, Lu N, Ling Y, Gao Y, Chen Y, Wang
L, Hu R, Qi Q, Liu W, Yang Y, et al: Oroxylin A suppresses invasion
through down-regulating the expression of matrix
metalloproteinase-2/9 in MDA-MB-435 human breast cancer cells. Eur
J Pharmacol. 603:22–28. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Choi HW, Shin PG, Lee JH, Choi WS, Kang
MJ, Kong WS, Kang MJ, Kong WS, Oh MJ, Seo YB and Kim GD:
Anti-inflammatory effect of lovastatin is mediated via the
modulation of NF-κB and inhibition of HDAC1 and the PI3K/Akt/mTOR
pathway in RAW264. 7 macrophages. Int J Mol Med. 41:1103–119.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen YC, Yang LL and Lee TJF: Oroxylin A
inhibition of lipopolysaccharide-induced iNOS and COX-2 gene
expression via suppression of nuclear factor-κB activation. Biochem
Pharmacol. 59:1445–1457. 2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sherwin CM, Christiansen SB, Duncan IJ,
Erhard HW, Lay DC, Mench JA, O'Connorg CE and Petherick CJ:
Guidelines for the ethical use of animals in applied ethology
studies. Appl Anim Behav Sci. 81:291–305. 2003. View Article : Google Scholar
|
|
31
|
Derrell CJ, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: Special Report: The 1996 Guide for the care and use
of laboratory animals. ILAR J. 38:41–48. 1997.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Glasson SS, Askew R, Sheppard B, Carito B,
Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al:
Deletion of active ADAMTS5 prevents cartilage degradation in a
murine model of osteoarthritis. Nature. 434:644–648.
2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Poole AR, Kobayashi M, Yasuda T, Laverty
S, Mwale F, Kojima T, Sakai T, Wahl C, El-Maadawy S, Webb G, et al:
Type II collagen degradation and its regulation in articular
cartilage in osteoarthritis. Ann Rheum Dis. 61 (Suppl 2):ii78–ii81.
2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Song RH, Tortorella MD, Malfait AM, Alston
JT, Yang Z, Arner EC and Griggs DW: Aggrecan degradation in human
articular cartilage explants is mediated by both ADAMTS-4 and
ADAMTS-5. Arthritis Rheum. 56:575–585. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Glyn-Jones S, Palmer AJR, Agricola R,
Price AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis.
Lancet. 386:376–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang Y and Jordan JM: Epidemiology of
osteoarthritis. Clin Geriatr Med. 26:355–369. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sarzi-Puttini P, Cimmino MA, Scarpa R,
Caporali R, Parazzini F, Zaninelli A, Atzeni F and Canesi B:
Osteoarthritis: An overview of the disease and its treatment
strategies. Semin Arthritis Rheum. 35:1–10. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Giercksky KE, Huseby G and Rugstad HE:
Epidemiology of NSAID-related gastrointestinal side effects. Scand
J Gastroenterol Suppl. 163:3–8. 1989.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lichtenberger LM, Zhou Y, Dial EJ and
Raphael RM: NSAID injury to the gastrointestinal tract: Evidence
that NSAIDs interact with phospholipids to weaken the hydrophobic
surface barrier and induce the formation of unstable pores in
membranes. J Pharm Pharmacol. 58:1421–1428. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Song X, Chen Y, Sun Y, Lin B, Qin Y, Hui
H, Li Z, You Q, Lu N and Guo Q: Oroxylin A, a classical natural
product, shows a novel inhibitory effect on angiogenesis induced by
lipopolysaccharide. Pharmacol Rep. 64:1189–1199. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sun X, Chang X, Wang Y, Xu B and Cao X:
Oroxylin A suppresses the cell proliferation, migration, and EMT
via NF-κB signaling pathway in human breast cancer cells. Biomed
Res Int. 2019(9241769)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Roos EM and Arden NK: Strategies for the
prevention of knee osteoarthritis. Nat Rev Rheumatol.
12(92)2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Luo Y, Sinkeviciute D, He Y, Karsdal M,
Henrotin Y, Mobasheri A, Önnerfjord P and Bay-Jensen A: The minor
collagens in articular cartilage. Protein Cell. 8:560–572.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bonassar LJ, Sandy JD, Lark MW, Plaas AHK,
Frank EH and Grodzinsky AJ: Inhibition of cartilage degradation and
changes in physical properties induced by IL-1β and retinoic acid
using matrix metalloproteinase inhibitors. Arch Biochem Biophys.
344:404–412. 1997.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Needleman P and Manning PT: Interactions
between the inducible cyclooxygenase (COX-2) and nitric oxide
synthase (iNOS) pathways: Implications for therapeutic intervention
in osteoarthritis. Osteoarthritis Cartilage. 7:367–370.
1999.PubMed/NCBI View Article : Google Scholar
|
|
46
|
More AS, Kumari RR, Gupta G, Lingaraju MC,
Balaganur V, Pathak NN, Kumar D, Kumar D, Sharma AK and Tandan SK:
Effect of iNOS inhibitor S-methylisothiourea in monosodium
iodoacetate-induced osteoathritic pain: Implication for
osteoarthritis therapy. Pharmacol Biochem Behav. 103:764–772.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Watson DJ, Harper SE, Zhao PL, Quan H,
Bolognese JA and Simon TJ: Gastrointestinal tolerability of the
selective cyclooxygenase-2 (COX-2) inhibitor rofecoxib compared
with nonselective COX-1 and COX-2 inhibitors in osteoarthritis.
Arch Intern Med. 160:2998–3003. 2000.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wong M and Carter DR: Articular cartilage
functional histomorphology and mechanobiology: A research
perspective. Bone. 33:1–13. 2003.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rahmati M, Nalesso G, Mobasheri A and
Mozafari M: Aging and osteoarthritis: Central role of the
extracellular matrix. Ageing Res Rev. 40:20–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pattoli MA, MacMaster JF, Gregor KR and
Burke JR: Collagen and aggrecan degradation is blocked in
interleukin-1-treated cartilage explants by an inhibitor of IκB
kinase through suppression of metalloproteinase expression. J
Pharmacol Exp Ther. 315:382–388. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Stanton H, Rogerson FM, East CJ, Golub SB,
Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, et
al: ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and
in vitro. Nature. 434:648–652. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Song RH, Tortorella MD, Malfait AM, Alston
JT, Yang Z, Arner EC and Griggs DW: Aggrecan degradation in human
articular cartilage explants is mediated by both ADAMTS-4 and
ADAMTS-5. Arthritis Rheum. 56:575–585. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shi J, Zhang C, Yi Z and Lan C: Explore
the variation of MMP3, JNK, p38 MAPKs, and autophagy at the early
stage of osteoarthritis. IUBMB Life. 68:293–302. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chow YY and Chin KY: The role of
inflammation in the pathogenesis of osteoarthritis. Mediators
Inflamm. 2020(8293921)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Gao SC, Yin HB, Liu HX and Sui YH:
Research progress on MAPK signal pathway in the pathogenesis of
osteoarthritis. Zhongguo Gu Shang. 27:441–444. 2014.PubMed/NCBI(In Chinese).
|
|
56
|
Liu Z, Cai H, Zheng X, Zhang B and Xia C:
The involvement of mutual inhibition of ERK and mTOR in
PLCγ1-mediated MMP-13 expression in human osteoarthritis
chondrocytes. Int J Mol Sci. 16:17857–17869. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Hashizume M and Mihara M: High molecular
weight hyaluronic acid inhibits IL-6-induced MMP production from
human chondrocytes by up-regulating the ERK inhibitor, MKP-1.
Biochem Biophys Res Commun. 403:184–189. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Xue JF, Shi ZM, Zou J and Li XL:
Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of
articular chondrocytes and attenuates inflammatory response in rats
with osteoarthritis. Biomed Pharmacother. 89:1252–1261.
2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Cheng CC, Uchiyama Y, Hiyama A, Gajghate
S, Shapiro IM and Risbud MV: PI3K/AKT regulates aggrecan gene
expression by modulating Sox9 expression and activity in nucleus
pulposus cells of the intervertebral disc. J Cell Physiol.
221:668–676. 2009.PubMed/NCBI View Article : Google Scholar
|