Introduction
Periodontal disease is one of the most common oral
diseases in adults in China (1). It
causes periodontal ligament, cementum and alveolar bone
descruction, gingiva, tooth loosening and may leads to the loss of
teeth. Previous studies have demonstrated that chronic
periodontitis can aggravate or directly affect cardiovascular
disease, diabetes, rheumatism, digestive system disease, eye
disease and immune function in the body (2-4).
The existing periodontal treatment methods include periodontal
basic treatment, flap curettage, root planing, application of
growth factors (5,6), natural material grafts and guided
tissue regeneration (GTR) (7),
which can only partially regenerate periodontal tissue.
Additionally, the curative effect is not stable. Therefore, it is
necessary to elucidate novel and more effective periodontal
treatment methods in the clinic (8). Human periodontal mesenchymal stem
cells (hPDLSCs) derived from periodontal ligament are a suitable
source of stem cells for periodontal tissue regeneration due to the
ease of acquisition, simple means of culturing and isolation,
multilineage potential and low immunogenicity (9). The release of inflammatory mediators,
such as TNF-α, not only accelerates the progression of
periodontitis, but also affects the regeneration of hPDLSCs
(10). It was reported that TNF-α
inhibits the osteogenic differentiation of mesenchymal stem cells
(MSCs), such as bone marrow stem cells (11,12).
However, studies on the effect of TNF-α on the osteogenic ability
of hPDLSCs are relatively rare and have provided contradictory
results (13,14). Therefore, the aim of the present
study was to determine the effect of TNF-α on the proliferation and
osteogenic ability of hPDLSCs, providing an experimental basis for
exploring antagonistic targets of TNF-α and improving the
understanding of regeneration and repair of hPDLSCs in
periodontitis.
Materials and methods
Isolation, purification and culture of
hPDLSCs
The acquisition and cultivation of hPDLSCs was
approved by the Ethics Committee of the Ninth People's Hospital,
Shanghai Jiao Tong University School of Medicine (Shanghai, China)
and informed consent was provided by the patients in writing. The
patients agreed to the use of their teeth for scientific research.
A total of 10 teeth were extracted during routine dental care in
the Ninth People's Hospital, Shanghai Jiao Tong University School
of Medicine were collected from 6 female and 4 male patients. Teeth
with no periodontal abnormalities, no inflammation and no caries
were obtained from July 2018 to July 2019. The age of the patients
ranged from 18-25 years, with a mean age of 20.7±3 years. Premolars
were washed with PBS three times, and the periodontal ligament
tissue from the middle and lower part of the root was scraped,
shredded, centrifuged (400 x g for 5 min at 37˚C), digested with
trypsin and placed in 10% FBS α-MEM culture medium (HyClone;
Cytiva) containing penicillin-streptomycin, and cultured at 37˚C
with 5% CO2 in a humidified incubator. The culture
medium was changed every 3 days. After the cells reached ~80%
confluence, they were digested with trypsin and sub-cultured at a
ratio of 1:3.
Identification of cell surface
antigens
Cells were cultured in vitro for three
passages, after which they were digested using trypsin-EDTA, and
centrifuged at 400 x g for 5 min at 37˚C, and the supernatant was
removed. The cell suspension was prepared by trypsin digestion and
the cell density was adjusted to 1x106/ml. Subsequently,
the cell suspension was divided into five 1.5 ml Eppendorf tubes
containing the following antibodies: 5 µl mouse anti-human STOR-1
(1:1,000; cat. no. ab92395; Abcam), mouse anti-human FITC-CD 90
(1:500; cat. no. BD-561969, BD Pharmingen; BD Biosciences) mouse
anti-human FITC-CD34 (1:500; cat. no. BD-555821; BD Pharmingen; BD
Bioseciences), mouse anti-human FITC-CD45 (1:500; cat. no.
BD-555482; BD Pharmingen; BD Biosciences), and FITC labeled mouse
anti-human IgG1 (1:1,000; cat. no. ab99773; Abcam). After
incubation for 1 h at room temperature, the expression of cell
surface markers was detected by flow cytometry (Attune NxT; Thermo
Fisher Scientific, Inc.) using Invitrogen Attune NxT software
version 4.2 (Invitrogen; Thermo Fisher Scientific, Inc.).
Effect of TNF-α on the proliferation
of hPDLSCs
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assay was used to determine the effect of
different concentrations of TNF-α (PeproTech, Inc.) on the
proliferation of hPDLSCs. Cell cultures were divided into four
groups. Osteogenic induction solution (α-MEM containing 10 mmol/l
dexamethasone, 10 mmol/l insulin, 0.2 mmol/l indomethacin and 0.5
mmol/l 3-isobutyl-1-methylxanthine) was added to the control group
without TNF-α. The other three experimental groups were treated
with osteogenic induction medium containing 0.1 ng/ml TNF-α, 1
ng/ml TNF-α and 10 ng/ml TNF-α, respectively.
The original culture medium was discarded, cells
were washed with PBS three times, digested with trypsin and
centrifuged at 400 x g for 5 min at 37˚C. The cells were cultured
in 100 µl medium at a density of 2x104 cells/well in a
96-well plate and cultured at 37˚C for 24 h. The culture medium was
changed every 3 days and cultured continuously for 7 days. On day
7, 10 µl of CCK-8 solution was added to each well, and cells were
further incubated for 2 h and gently mixed on a shaker for 5 min to
ensure uniform reagent distribution. The optical density at 450 nm
was measured using a microplate reader. The growth curve was drawn
with observation time on the x-axis and the OD value on the y-axis,
to analyze and compare cell proliferation.
Effect of TNF-α on osteogenic
differentiation of hPDLSCs
Quantitative alkaline phosphatase (ALP) enzyme ELISA
kit (cat. no. YM-S2942) and alizarin red staining were used to
detect the effect of different concentrations of TNF-α on the
osteogenic induction of hPDLSCs. The osteogenic induction solution
without TNF-α was added to the control group and the osteogenic
induction solution containing 10 ng/ml TNF-α was added to the
experimental group. After 7 days of induction culture at 37˚C,
there was intact cell morphology of spindle or triangular shape in
the 96-well plate when observed under a light microscope. The cells
were washed using 10%PBS. A total of 50 µl from each well was
collected and transferred to another 96-well plate at a density of
1x104 cells/ml. Buffer, substrate and chromogenic
solution (provided by the ELISA kit) were added in turn, and the
ALP activity of each well was measured at 520 nm.
After osteogenic induction and culture for 21 days
as aforementioned, the culture medium was discarded, the cells were
fixed with 4% paraformaldehyde for 10 min at 4˚C, and stained with
alizarin red S solution for 15 min at 37˚C. The formation of
mineralized nodules was observed under an inverted phase contrast
microscope (Olympus Corporation) and imaged.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Following spectrophotometric
quantification using a NanoDrop spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.), 1 µg total RNA in a
final volume of 20 µl was used for RT with a PrimeScript RT Reagent
kit (Takara Bio, Inc.) according to the manufacturer's protocol.
Aliquots of cDNA were used for mRNA quantification by qPCR using a
LightCycler 96 Real-time Quantitative PCR Detection system (Roche
Applied Science). The thermocycling conditions were as follows: 35
cycles of denaturation at 94˚C for 30 sec, annealing at 37˚C for 30
sec, and extension at 72˚C for 1 min. The reaction system (25 µl)
contained the cDNA, forward and reverse primers, and SYBR-Green PCR
MasterMix (Roche Applied Science). Data were analyzed using β-actin
gene expression as the internal standard, and the 2-ΔΔCq
method was used for quantification (15). The sequences of the primers were
based on a previous study (16):
TNF-α forward, 5'-CCCCTCAGCAAACCACCAAG-3' and reverse,
5'-CTTGGCAGATTGACCTCAGC-3'; β-actin forward,
5'-CCACACCCGCCACCAGTTCG-3' and reverse, 5'-CCCATTCCCACCATCACACC-3';
Runx2 forward, 5'-TGAAATAGGCATCAGACAAA-3' and reverse,
5'-CAGTAGCAAACCGAAACACT-3'; Col-I forward,
5'-AGTGGTTTGGATGGTGCCAA-3' and reverse, 5'-GCACCATCATTTCCACGAGC-3';
OCN forward, 5'-ATGAGAGCCCTCACACTCCT-3' and reverse,
5'-CTTGGACACAAAGGCTGCAC-3'.
Western blotting
The effect of TNF-α on the expression of
osteogenesis-related marker proteins was detected by western
blotting. Total proteins were extracted, and the concentration of
each protein sample was determined. Cells were harvested and lysed
on ice in RIPA Lysis Buffer (Beyotime Institutte of Biotechnology).
A bicinchoninic acid assay (Thermo Fisher Scientific, Inc.) was
performed to measure the concentration of protein. Equal quantities
of protein (50 µg) were loaded on an SDS gel, resolved using
SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked
in 5% skimmed milk for 2 h. Subsequently the membranes were
incubated with primary antibodies against runt-related
transcription factor 2 (Runx2), osteocalcin (OCN) and collagen type
I α 1 (COL-I) and β-actin (all 1:1,000) overnight at 4˚C.
Subsequently, the membranes were washed three times with PBS
containing 0.05% Tween-20 and incubated with a horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no.
sc-2005; Santa Cruz Biotechnology, Inc.) at room temperature for 2
h. The membranes were washed again, and target bands and detected
using an enhanced chemiluminescence reagent (Thermo Fisher
Scientific, Inc.) and analyzed using ImageJ software v1.8.0
(National Institutes of Health). β-actin was used as the internal
control. The experiments were repeated three times.
Statistical analysis
Data were analyzed using SPSS version 13 (SPPSS,
Inc.). Data are presented as the mean ± standard deviation of three
repeats. Differences between two groups were compared using a
paired Student's t-test. The significance of differences among more
than two groups was assessed using the one-way ANOVA followed by
Tukey-Kramer multiple comparisons test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Isolation of hPDLSCs
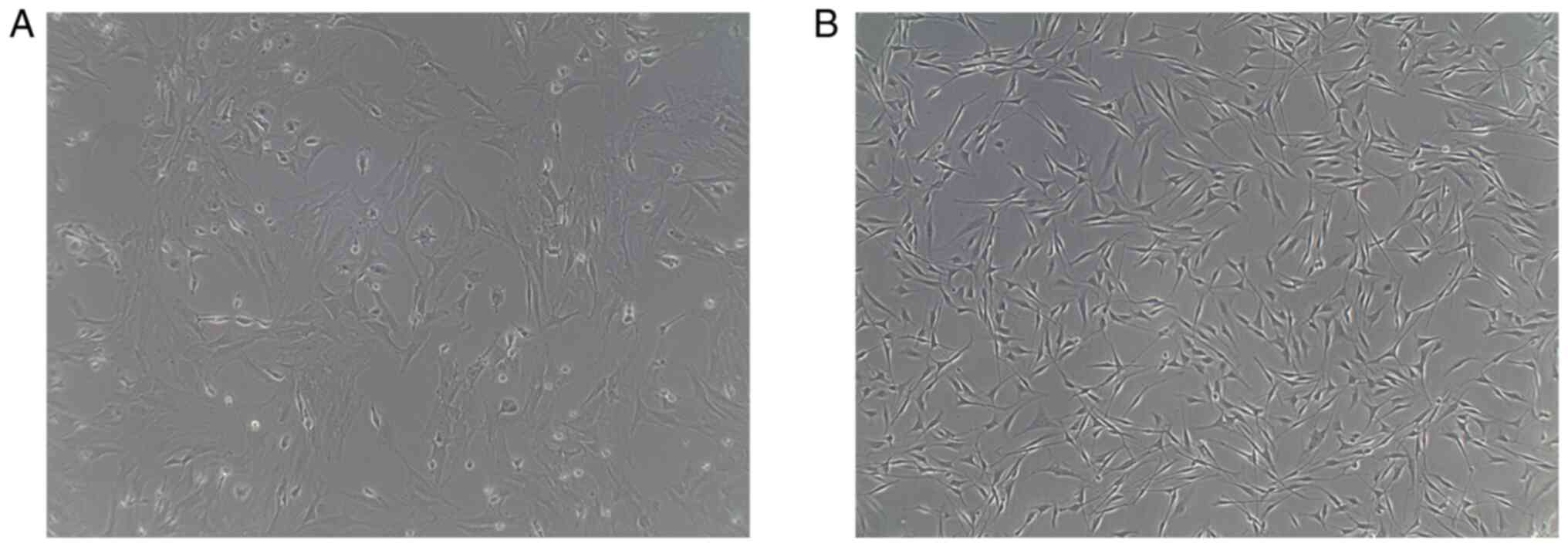
When the primary hPDLSCs cells were cultured for 3
days, a few cells could be obtained from the tissue block, with an
oval and polygonal shape, and of different sizes. The number of the
cells began to increase after 5 days, with cells exhibiting a long
spindle or polygonal morphology. After 6-8 days of cultivation,
occurring prior to osteogenic induction, the number of the cells
markedly increased, with the majority of cells exhibiting a long
spindle, short spindle or polygonal morphology (Fig. 1A). When the cell confluence rate
reached ~80%, they were sub-cultured by selective digestion. The
purity and number of the cells after three passages was higher
compared with the primary cells, and the growth was considered to
be healthy, with no visible abnormalities. The size of the cells
was even, they were arranged closely, and predominantly exhibited a
long spindle and fiber-like morphology (Fig. 1B).
Evaluation of surface antigens on the
hPDLSCs
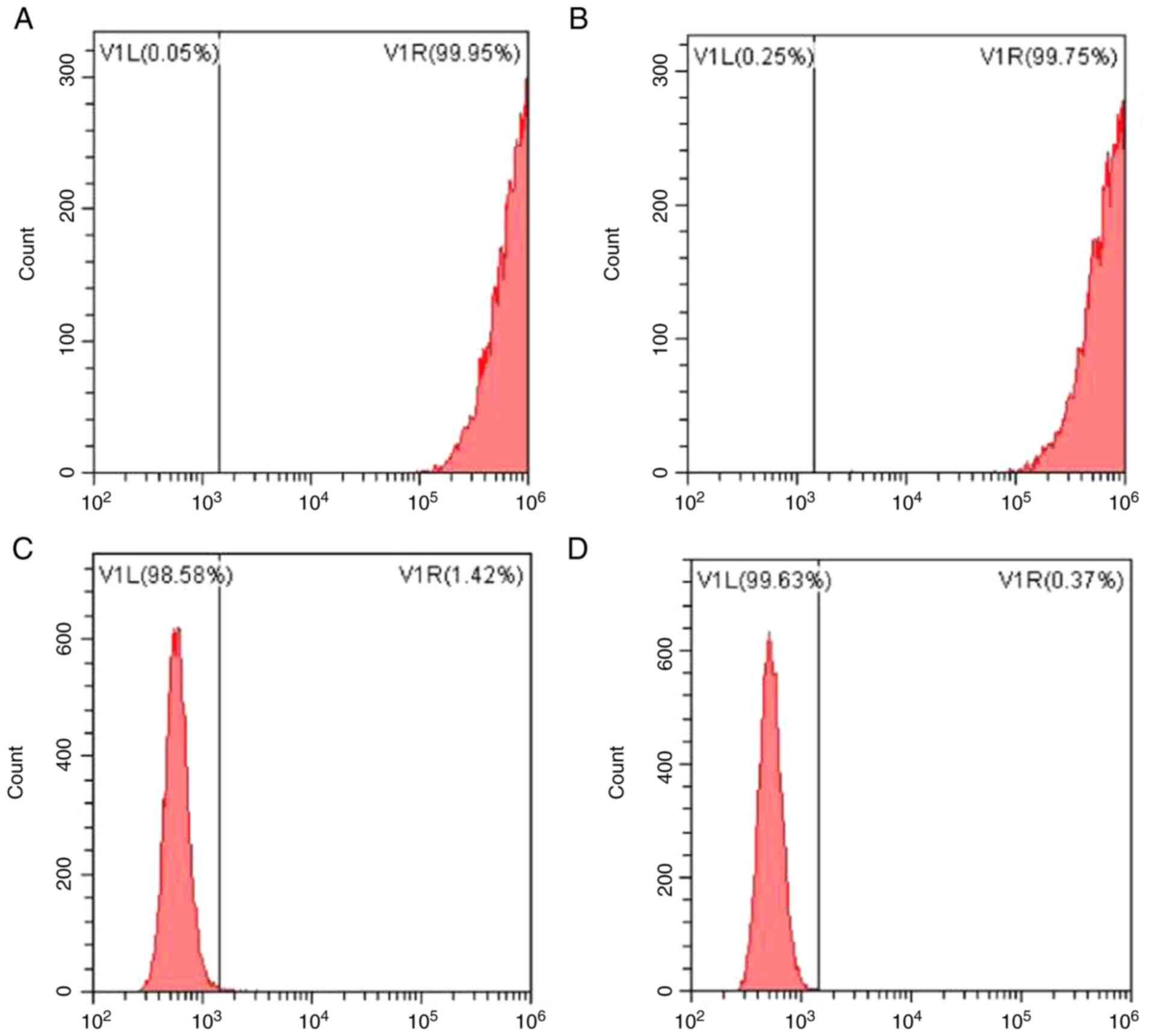
The expression of surface antigen molecules on
hPDLSCs was assessed using flow cytometry (Fig. 2). The results showed that amongst
the established markers of MSCs assessed, the proportion of cells
expressing STOR-1 was 99.95%, and those expressing CD90 accounted
for 99.75% of the cells. Amongst the markers of blood cell growth,
the proportion of cells expressing CD34 was 1.42 and 0.37% cells
were positive for CD45. The results of flow cytometry indicated
that hPDLSCs cells were successfully isolated and extracted.
Effect of TNF-α on hPDLSCs
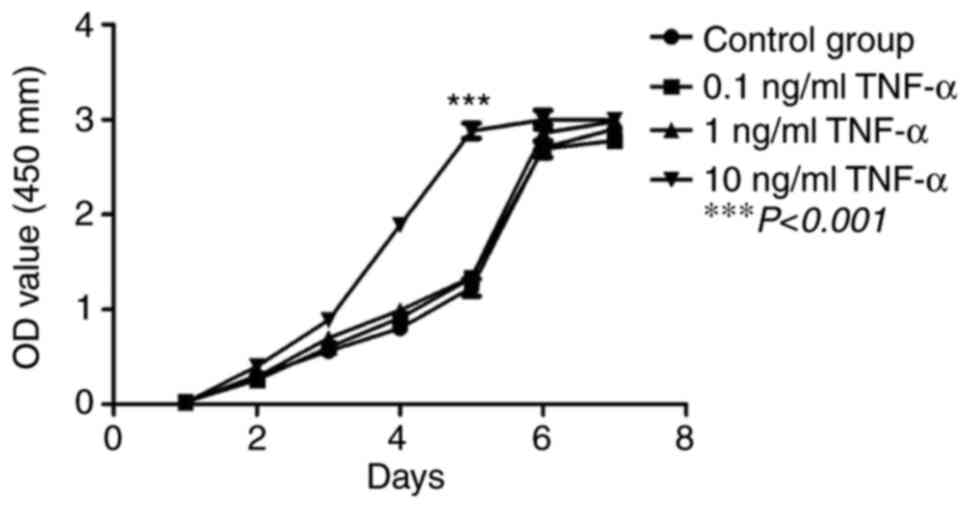
The proliferative capacity of third generation
hPDLSCs treated with different concentrations of TNF-α was assessed
using a CCK-8 assay. The results showed that hPDLSCs exhibited
relatively strong proliferative capacity in vitro when
compared with the control. On days 1-3, the hPDLSC cells were in
linear growth and were proliferating slowly. On days 4-5, cell
proliferation was in the logarithmic growth phase, whereas on day
6, cells entered a stable phase with no apparent proliferation
being observed. Compared with the control group, 10 ng/ml TNF-α
significantly increased the proliferation of hPDLSCs on days 4-5
(P<0.001), whereas 0.1 and 1 ng/ml TNF-α did not have a
significant effect on proliferation compared with the control group
at any time point (Fig. 3).
Effect of TNF-α on the osteogenic
ability of hPDLSCs
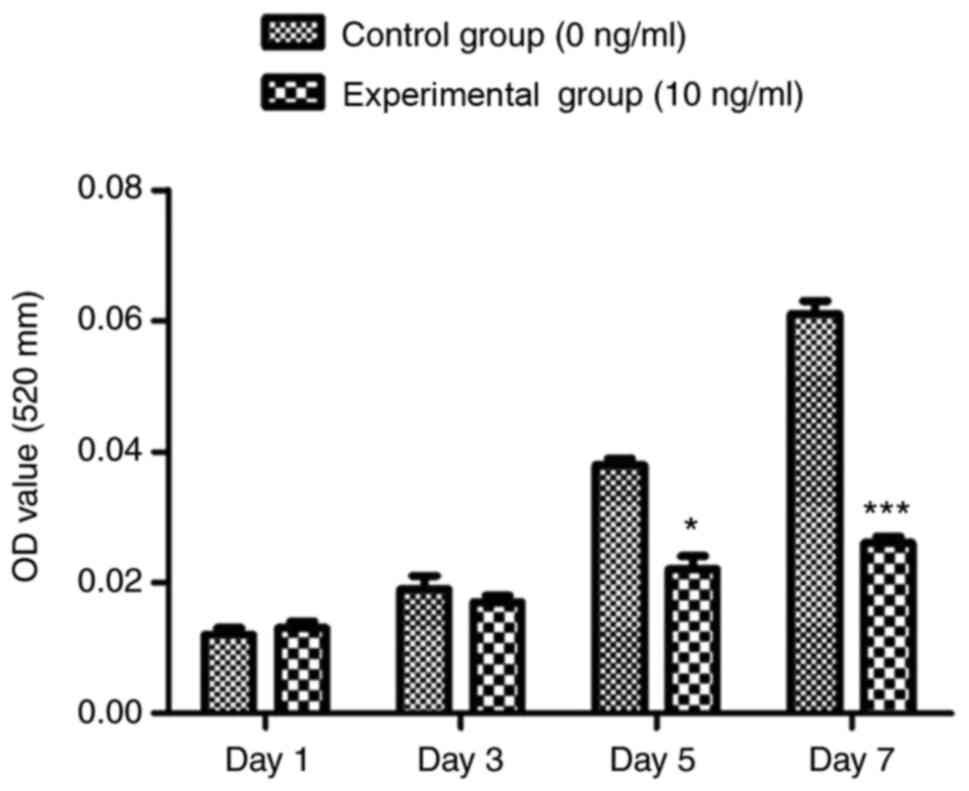
Induction of osteogenesis begun on day 7, and the
detection of ALP activity showed that in both groups (treated and
untreated with TNF-α), ALP activity gradually increased with
cultivation time when compared with the control group, and 10 ng/ml
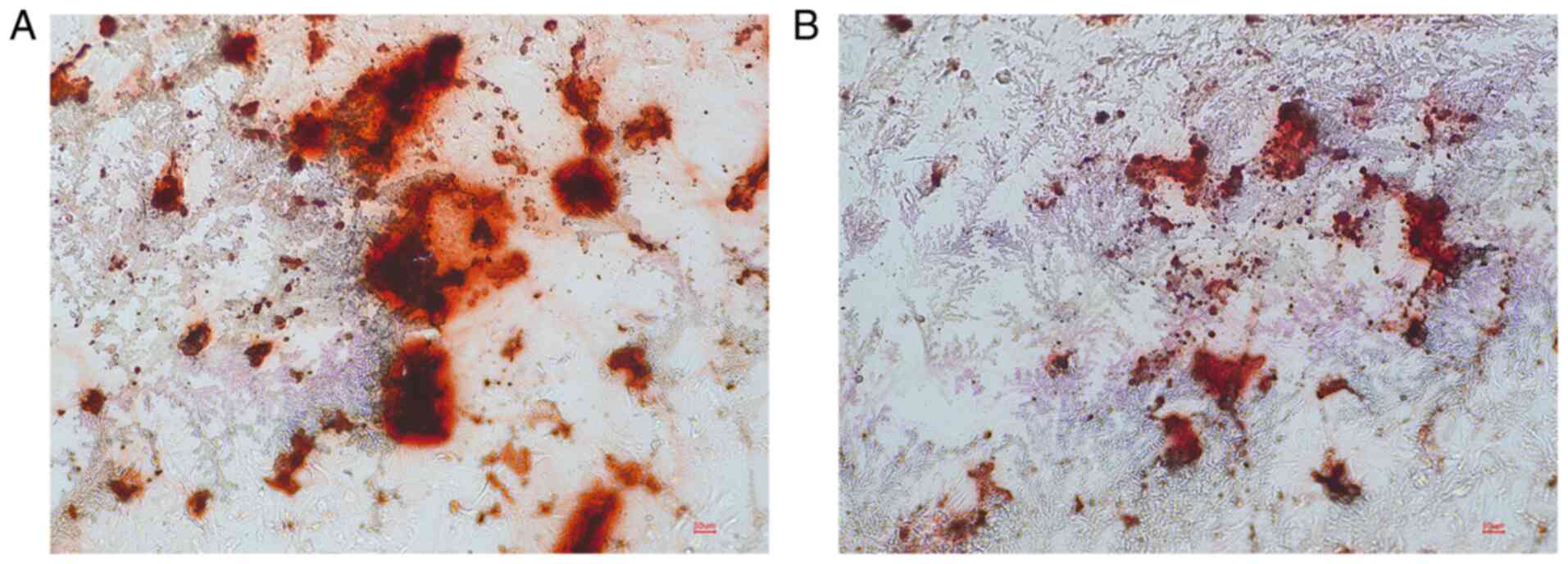
TNF-α significantly reduced ALP activity (Fig. 4). After 21 days of osteogenesis
induction, alizarin red staining showed that compared with the
control group, the number and size of red mineralized nodules
decreased in the test group (10 ng/ml TNF-α), and the staining was
lighter (Fig. 5).
Effect of TNF-α on mRNA and protein
expression of osteogenesis-associated genes
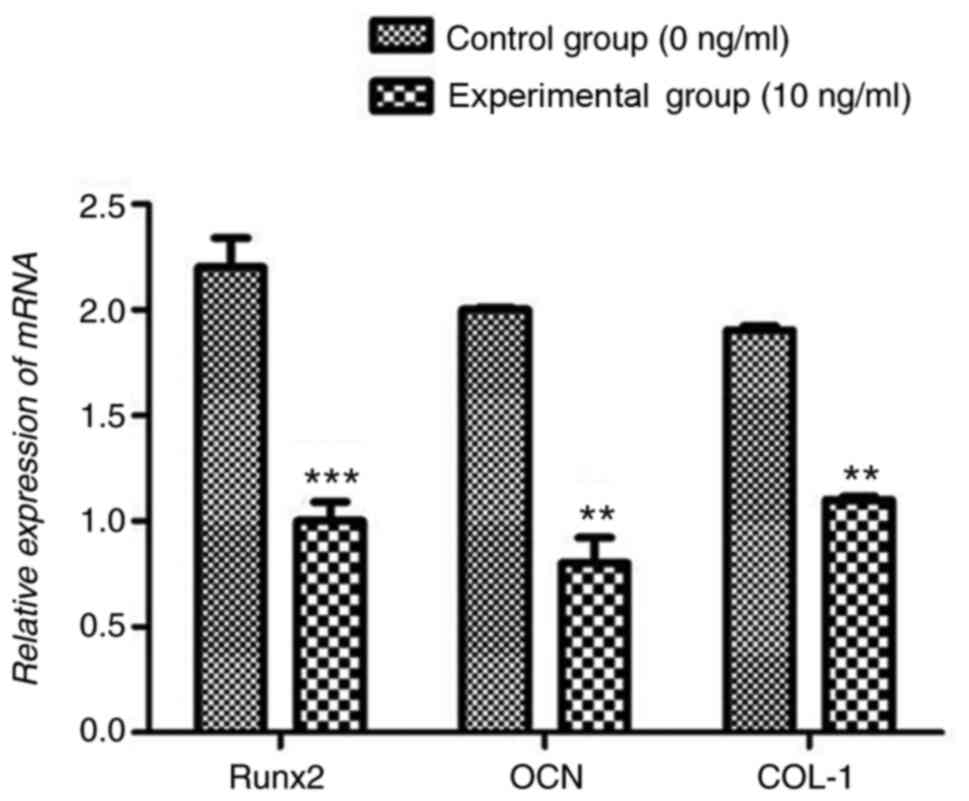
Compared with the control group, 10 ng/ml TNF-α
significantly reduced Runx2, OCN and COL-I mRNA expression levels
(P<0.001 Fig. 6). Similarly, 10
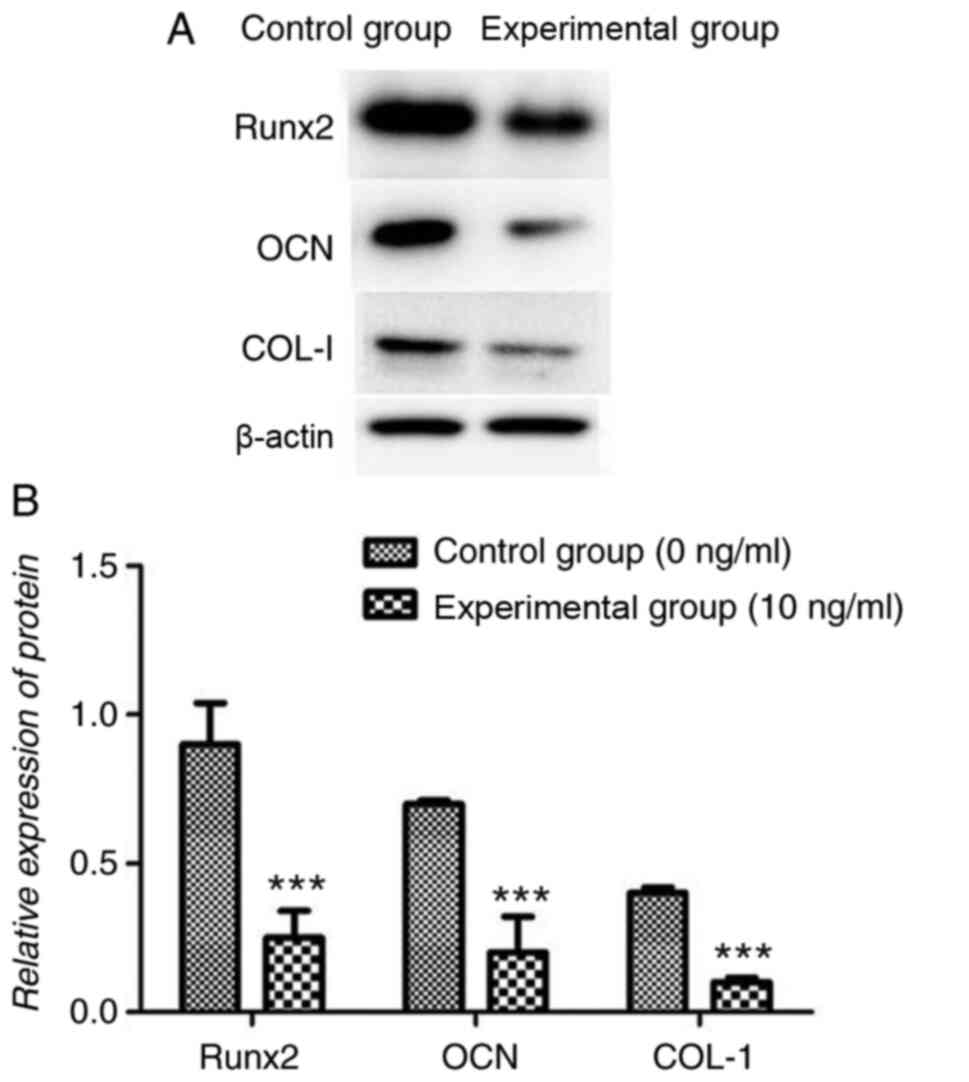
ng/ml TNF-α significantly reduced Runx2, OCN and COL-I protein
expression levels compared with the control group (all P<0.001;
Fig. 7).
Discussion
Periodontitis can result in swelling of the gums,
pain, alveolar bone destruction, occlusal function weakness, tooth
loss and/or complete loss of occlusal function, and may thus
severely affect a patient's quality of life and health (17). MSCs exhibit self-replication and
multi-linage differentiation potential, and are considered a type
of adult stem cells that are widely present in human tissues. Under
specific conditions, MSCs can differentiate into bone, cartilage,
fat, muscle and nerve tissue (18).
hPDLSCs are a type of MSCs widely used and studied in periodontal
regeneration engineering, and can survive, proliferate and
differentiate into cementum, alveolar bone and periodontal
ligament-like tissue, and may thus serve as a source of stem cells
for periodontal tissue regeneration (19,20).
Previous clinical studies have shown that periodontal ligament stem
cells were safe and effective in the treatment of periodontal
diseases (21).
Enzyme digestion, tissue culture, or a combination
of both are commonly used in studies involving acquisition,
purification and culture of PDLSCs (22). In the present study, the combined
method of enzyme digestion and tissue culture was used to isolate
and culture hPDLSCs. This approach was superior to the use of
enzyme digestion alone, as it enabled extraction of a greater
number of cells and avoided the difficulties in having to adjust
the extent of digestion (22). The
method used in the current study was also superior to the use of
tissue culture alone, which often results in incomplete digestion
and low cell survival rates (23).
As a result, primary hPDLSCs were obtained and sub-cultured
successfully in the present study. At present, to the best of our
knowledge, there are no specific identification methods for
hPDLSCs. Characteristics of MSCs include high expression of stem
cell growth-related proteins, such as antigen molecules including
STOR-1, CD105, CD90 and CD73; and almost no expression of blood
cell growth-related proteins, such as CD34, CD45 and HLA-DR, and
these were used to validate whether the cells obtained in the
present study were indeed hPDLSCs. After successful sub-culturing
of hPDLSCs, the cells were shown to be of a high level of purity,
with a high proliferation capacity, with no visible abnormalities,
uniform cell size and compact arrangement, exhibiting a long
fusiform and fibrous morphology. Flow cytometry was used to confirm
the expression of surface antigen molecules, and it was found that
the stem cell-related proteins STOR-1 and CD105 were abundantly
expressed, whereas expression of the blood cell growth-related
proteins CD34 and CD45 were detected at low levels. Together these
results provided confidence that the cells extracted and isolated
were hPDLSCs.
Periodontitis is a ubiquitous periodontal tissue
immune inflammatory disease caused by bacteria (24). The regenerative ability of hPDLSCs
is readily affected by the presence of inflammatory mediators such
as TNF-α, IL-6, IL-11 and IL-17 in the periodontal environment
(25,26). Among these, TNF-α is an important
member of the TNF family, showing its effect primarily through the
NF-κB or MAPK signaling pathways. It is also the primary
inflammatory factor regulating tissue destruction in periodontitis,
and is involved in several different inflammatory reactions
(27).
In the present study, the effects of different
concentrations of TNF-α on the proliferation of hPDLSCs in
vitro were compared, and it was found that only 10 ng/ml TNF-α
could promote the proliferation of hPDLSCs in vitro. It has
been previously shown that 10 ng/ml TNF-α significantly promoted
the proliferation of dental pulp stem cells and hPDLSCs (28). In the present study, the effect of
TNF-α on cell proliferation was detected using a CCK-8 assay, and
the results were consistent with the previous study, where 10 ng/ml
TNF-α promoted the proliferation of hPDLSCs (28). However, it has also been reported
that 10 ng/ml TNF-α can significantly induce apoptosis in dental
pulp stem cells and hPDLSCs (29).
The effect of TNF-α on the proliferation of hPDLSCs in vitro
is complex and diverse, and this may be related to the source and
method of acquisition of hPDLSCs, culture conditions and detection
methods.
ALP, OCN, COL-I and Runx2 are important factors
associated with osteogenesis, and serve important roles in the
formation and development of osteocytes, and are thus used as an
index to assess the osteogenic induction and differentiation
ability of MSCs. Previous studies have shown that TNF-α can inhibit
osteoblast bone formation, promote bone resorption, inhibit ALP
activity and the mRNA levels of ALP, Runx2 and osterix (30,31).
In the present study, 10 ng/ml TNF-α was used to inhibit the
osteogenic differentiation of normal periodontal ligament stem
cells. The decrease in ALP activity and alizarin red staining, and
the decreased mRNA and protein expression levels of Runx2, COL-I
and OCN showed that 10 ng/ml TNF-α could inhibit the osteogenic
differentiation of hPDLSCs.
In conclusion, the present study indicated that 10
ng/ml TNF-α significantly promoted the proliferation of hPDLSCs and
inhibited osteogenic differentiation. These results highlight novel
potential means of regulating regeneration of hPDLSCs through the
use of specific concentrations of inflammatory factors such as
TNF-α for the prevention and treatment of periodontitis.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 31800816), and Fundamental Research
Program Funding of the Ninth People's Hospital affiliated to
Shanghai Jiao Tong University School of Medicine (grant nos.
JYZZ109 and JYZZ038).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QJ and LZ conceived and designed the study. QJ and
YC performed the experiments. QJ, YC, YW and CL analyzed the data.
YC and CL wrote the manuscript. All authors read and approved the
final manuscript. QJ and YC confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The protocols used in the present study were
approved by the Ethics Committee of the Ninth People's Hospital,
Shanghai Jiao Tong University School of Medicine (Shanghai, China)
and informed consent was provided by the patients. The ethics
approval reference number is [2018] 376. The patients agreed to the
use of their teeth for scientific research.
Patient consent for publication
All patients provided written informed consent for
the publication of their anonymized clinical data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheng ML, Xu MR, Xie YY, Gao XL, Wu HJ,
Wang X, Feng XP, Tai BJ, Hu DY, Lin HC, et al: Utilisation of oral
health services and economic burden of oral diseases in China. Chin
J Dent Res. 21:275–284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Carrizales-Sepúlveda EF, Ordaz-Farías A,
Vera-Pineda R and Flores-Ramírez R: Periodontal disease, systemic
inflammation and the risk of cardiovascular disease. Heart Lung
Circ. 27:1327–1334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liccardo D, Cannavo A, Spagnuolo G,
Ferrara N, Cittadini A, Rengo C and Rengo G: Periodontal disease: A
risk factor for diabetes and cardiovascular disease. Int J Mol Sci.
20(1414)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gheorghe ND, Foia L, Toma V, Surdu A,
Herascu E, Popescu DM, Surlin P, Vere CC and Rogoveanu I: Hepatitis
C infection and periodontal disease: Is there a common
immunological link? J Immunol Res. 2018(8720101)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Panezai J, Ghaffar A, Altamash M,
Sundqvist KG, Engström PE and Larsson A: Correlation of serum
cytokines, chemokines, growth factors and enzymes with periodontal
disease parameters. PLoS One. 12(e0188945)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ravi S, Malaiappan S, Varghese S,
Jayakumar ND and Prakasam G: Additive effect of plasma rich in
growth factors with guided tissue regeneration in treatment of
intrabony defects in patients with chronic periodontitis: A
split-mouth randomized controlled clinical trial. J Periodontol.
88:839–845. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kao RT, Nares S and Reynolds MA:
Periodontal regeneration-intrabony defects: A systematic review
from the AAP Regeneration Workshop. J Periodontol. 86 (Suppl
2):S77–S104. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mlachkova AM and Popova CL: Efficiency of
nonsurgical periodontal therapy in moderate chronic periodontitis.
Folia Med (Plovdiv). 56:109–115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dave JR and Tomar GB: Dental
tissue-derived mesenchymal stem cells: Applications in tissue
engineering. Crit Rev Biomed Eng. 46:429–468. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zheng CX, Chen J, Liu SY and Jin Y: Stem
cell-based bone and dental regeneration: A view of
microenvironmental modulation. Int J Oral Sci. 11:23–28.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qin ZJ, Fang ZX, Zhao L, Chen J, Li YT and
Liu GY: High dose of TNF-α suppressed osteogenic differentiation of
human dental pulp stem cells by activating the Wnt/β-catenin
signaling. Mol Histol. 46:409–420. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng GJ, Shen QJ, Lian M, Gu ZF, Xing J,
Lu XH, Huang D, Li LR, Huang S, Wang Y, et al: RAC1 regulate tumor
necrosis factor-α-mediated impaired osteogenic differentiation of
dental pulp stem cells. Dev Growth Differ. 57:497–506.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang C, Wang Q, Song MM, Wang M, Zhao L
and Huang Y: Coronarin D affects TNF-α induced proliferation and
osteogenic differentiation of human periodontal ligament stem
cells. Arch Oral Biol. 108(104519)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhao B, Zhang WJ, Xiong YX, Zhang YP, Jia
LL and Xu X: Rutin protects human periodontal ligament stem cells
from TNF-α induced damage to osteogenic differentiation through
suppressing mTOR signaling pathway in inflammatory environment.
Arch Oral Biol. 109(104584)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hwang JH, Chen JC, Yang SY, Wang MF and
Chan YC: Expression of tumor necrosis factor-α and interleukin-1β
genes in the cochlea and inferior colliculus in salicylate-induced
tinnitus. J Neuroinflammation,. 8(30)2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hienz SA, Paliwal S and Ivanovski S:
Mechanisms of bone resorption in periodontitis. J Immunol Res.
2015(615486)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Almalki SG and Agrawal DK: Key
transcription factors in the differentiation of mesenchymal stem
cells. Differentiation. 92:41–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ge Y, Li J, Hao Y, Hu Y, Chen D, Wu B and
Fang F: MicroRNA-543 functions as an osteogenesis promoter in human
periodontal ligament-derived stem cells by inhibiting transducer of
ERBB2. Periodontal Res. 53:832–841. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Corrêa NCR, Kuligovski C, Paschoal ACC,
Abud APR, Rebelatto CLK, Leite LMB, Senegaglia AC, Dallagiovanna B
and Aguiar AM: Human adipose-derived stem cells (ADSC) and human
periodontal ligament stem cells (PDLSC) as cellular substrates of a
toxicity prediction assay. Regul Toxicol Pharmacol. 9:75–82.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Su F, Liu SS, Ma JL, Wang DS, E LL and Liu
HC: Enhancement of periodontal tissue regeneration by
transplantation of osteoprotegerin-engineered periodontal ligament
stem cells. Stem Cell Res Ther. 6(22)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iwasaki K, Komaki M, Yokoyama N, Tanaka Y,
Taki A, Kimura Y, Takeda M, Oda S, Izumi Y and Morita I:
Periodontal ligament stem cells possess the characteristics of
pericytes. J Periodontol. 84:1425–1433. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wen Y, Yang HX, Wu JJ, Wang A, Chen XD, Hu
SJ, Zhang YX, Bai D and Jin ZL: COL4A2 in the tissue-specific
extracellular matrix plays important role on osteogenic
differentiation of periodontal ligament stem cells. Theranostics.
9:4265–4286. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bartold PM and Van Dyke TE: An appraisal
of the role of specific bacteria in the initial pathogenesis of
periodontitis. J Clin Periodontol. 46:6–11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Misawa MYO, Silvério Ruiz KG, Nociti FH
Jr, Albiero ML, Saito MT, Nóbrega Stipp R, Condino-Neto A,
Holzhausen M, Palombo H and Villar CC: Periodontal ligament-derived
mesenchymal stem cells modulate neutrophil responses via paracrine
mechanisms. J Periodontol. 90:747–755. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao S, Cheng Y and Kim JG: MicroRNA-146a
downregulates IL-17 and IL-35 and inhibits proliferation of human
periodontal ligament stem cells. J Cell Biochem. 120:13861–13866.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao B, Zhang YP and Xu X: Rutin promotes
osteogenic differentiation of periodontal ligament stem cells under
inflammatory microenvironment. Shanghai Kou Qiang Yi Xue.
28:356–361. 2019.PubMed/NCBI(In Chinese).
|
|
28
|
Yuan P, Li SH, Zhao L, Yu L, Zhou CM and
Wu PL: Biological properties of human periodontal ligament stem
cells under inflammatory microenvironment. Chin J Tissue Eng Res.
20:898–905. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ma Y, Li SH, Ding XX and Wu PL: Effects of
tumor necrosis factor-α on osteogenic differentiation and Notch
signaling pathway in human periodontal ligament stem cells. Hua Xi
Kou Qiang Yi Xue Za Zhi. 36:184–189. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
30
|
Jeong BC: ATF3 mediates the inhibitory
action of TNF-α on osteoblast differentiation through the JNK
signaling pathway. Biochem Biophys Res Commun. 499:696–701.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sui BD, Hu CH, Liu AQ, Zheng CX, Xuan K
and Jin Y: Stem cell-based bone regeneration in diseased
microenvironments: Challenges and solutions. Biomaterials.
19:18–30. 2019.PubMed/NCBI View Article : Google Scholar
|