Introduction
Stress is a pivotal factor for inflammation and
reactive oxygen species (ROS) production, which can in turn induce
damage to the epithelial barrier (1,2). Heat
stress induced by continuous high ambient temperatures or strenuous
exercise in humans and animals can also lead to epithelial damage
in the digestive tract due to the induction of cellular stress
responses (3-5).
Damage to the intestinal barrier is an important cause of bacterial
translocation, inflammation, sepsis and multiple organ dysfunction
(3). In particular, tight junction
proteins are important for the maintenance of intestinal barrier
integrity. For instance, zonula occludens-1 (ZO-1) and Occludin can
both enhance steady-state barrier function in primary cultured
Sertoli cells (4). Occludin
function appear to require the cytoplasmic C terminus, which is
highly phosphorylated in tight junction-associated Occludin, which
binds to ZO-1, ZO-2 and ZO-3(5).
Occludin or ZO-1 knockdown increases the leak pathway permeability
in cultured epithelial monolayers (6). In addition, the expression pattern and
intracellular localization of Occludin and ZO-1 can change under
stress, ischemic or inflammation conditions (1,7).
Protease-activated receptor 2 (PAR-2) is highly
expressed in the gastrointestinal tract, which has dual effects on
inflammation and serves a key role in visceral hypersensitivity
(8). Expression levels of
intestinal tight junction proteins are reduced following
water-avoidance stress (9).
Furthermore, PAR-2 expression and mast cells are elevated under
acute or chronic restraint stress, which contributes to the
impaired epithelial barrier function in the colon and esophagus
(10,11). It has also been reported previously
that increased mast cell numbers and mucosal PAR2 expression in the
colon is mediated by the release of corticotrophin-releasing factor
(10). Subsequently, the activation
of PAR2 disrupts tight junctions and increases barrier permeability
through the activation of p38 MAPK (12). PAR2 activation can also compromise
vascular endothelial barrier function by suppressing the expression
of vascular endothelial (Ve)-cadherin (13).
Transient receptor potential ankyrin 1 channel
(TRPA1) serves as a key sensor for temperature and is permeable to
Ca2+ (14). In addition,
TRPA1 can be activated by mustard oil, cinnamic acid, garlicin,
oxidative stress products and inflammatory mediators, such as
prostanoids (15,16). TRPA1 is mainly expressed on sensory
neurons, afferent nerve endings and some non-neuronal cells,
including immune cells, where it is involved in the process of
nociception and inflammatory responses (17). Although TRPA1 is sensitive to
temperature, this differs among species. For instance, mouse TRPA1
has been implicated in noxious cold detection but was also
identified as one of the prime noxious heat sensors (18). Moreover, human TRPA1, which was
originally considered to be temperature-insensitive, is also
capable of sensing both hot and cold, where it is suggested that an
allosteric mechanism could account for the variability in TRPA1
temperature responsiveness (18).
TRPA1 can also serve as a sensor of cellular stress and tissue
damage (19). This channel has been
found to be upregulated in various tumors and is associated with
tumor proliferation and metastasis, as well as promoting ROS and
chemotherapy tolerance through the Ca2+-dependent
anti-apoptotic pathway (20).
Hypoxia and ischemia are associated with oxidative stress, which
can activate TRPA1 in cerebral artery endothelial cells, leading to
vasodilation, thereby reducing ischemic damage (21). These previous findings suggest a
protective role of TRPA1. However, TRPA1 can also induce
stress-induced duodenal lesions in a water immersion restraint
stress rat model by promoting the release of substance P (22).
Norepinephrine (NE) is a stress hormone that is
elevated due to activation of the hypothalamic-pituitary-adrenal
axis, the locus coeruleus (LC) and involves noradrenergic neurons,
the sympathetic adrenal medulla and the
renin-angiotensin-aldosterone system during stress (23). NE constricts the blood vessels to
change blood distribution, which is important during heat stress
(24). In addition, previous
studies have reported the effect of NE in the regulation of barrier
function. Degeneration of noradrenergic fibers from the LC causes
tight-junction disorganization in the rat brain (25). Moreover, another previous study
revealed that in the presence of NE, some Campylobacter
strains show increased invasion into T84 epithelial cells and
induced a greater breakdown of tight junctions (26). However, whether NE can directly
contribute to the regulation of barrier function remains poorly
understood.
Therefore, the present study aimed to investigate
the expression changes in tight junction proteins Occludin and
ZO-1, in addition to PAR-2 and TRPA1, in the rat colon after heat
stress. Additionally, the present study aimed to evaluate the
effects of NE on the expression levels of these proteins in
cultured Caco-2 cells.
Materials and methods
Animals
A total of 14 male Sprague-Dawley rats (weight,
220±20 g, age, 8 weeks) were randomly divided into control and heat
exposure groups (n=7 per group). Rats were provided with standard
laboratory diet and tap water ad libitum. The experimental
procedures were approved by the Animal Ethics Committee of the
Ningxia Medical University and Use Committee (Yinchuan, China).
After an adaptation period of 3 days, rats were acclimatized to
heat exposure with increasing durations from 2 h (day 1), 6 h (day
2), 14 h (day 3) and 18 h (day 4) at a temperature of 32±0.1˚C in a
closed, temperature-controlled chamber with a relative humidity of
54±5%, 12-h light/dark cycle. After a rest on day 5, the rats
received continuous 24-h heat exposure (32˚C) for 9 days. The rats
in the control group were raised in normal conditions (24±0.1˚C,
relative humidity of 54±5%, 12-h light/dark cycle).
Tissue preparation
All animals were anesthetized with intraperitoneal
injections of 2% sodium pentobarbital (40 mg/kg) and sacrificed by
exsanguination immediately after the 9-day 32˚C heat exposure
procedure. After anesthesia, the rats exhibited no signs of
peritonitis, pain or discomfort. The abdominal cavity was rapidly
opened and the distal colon (1-cm from the rectum; length, 2-3 cm)
was carefully excised, which was then placed into modified cold
Krebs' solution (120.6 mM NaCl, 5.9 mM KCl, 2.5 mM
CaCl2, 1.2 mM KH2PO4, 1.2 mM
MgCl2, 15.4 mM NaHCO3 and 11.5 mM glucose)
for rinsing. Part of the distal colon was fixed with 4%
paraformaldehyde for 24 h at room temperature. Blood was collected
from the inferior vena cava into a heparinized tube and centrifuged
at 1,449 x g for 10 min at 4˚C to obtain plasma samples, which was
then frozen at -80˚C to measure NE levels.
Hematoxylin and eosin (H&E)
staining
Full-thickness (4 µm) paraffin-embedded sections of
the distal colon from control and heat exposed rats were stained
with H&E staining for the evaluation of histological structural
change. Xylene followed by a descending ethanol gradient was used
for deparaffinization. Hematoxylin and eosin staining were both
performed at room temperature for 60-70 min. 60-70 min refers to
the total time of HE staining, from deparaffinization to the end.
For just Hematoxylin and eosin staining need 10 minutes (5 min for
H,and 5 min for E). Light microscopy was used for observation
(Magnification, x400).
Immunohistochemistry staining
Expression levels of Occludin, ZO-1, TRPA1 and PAR-2
were examined in rat distal colon full-thickness paraffin-embedded
sections (4 µm). Briefly, the sections were washed three times in
PBS after deparaffinization using the same protocol as that used
for in H&E staining aforementioned and incubated with 3%
hydrogen peroxide for 10 min at room temperature to block the
activity of endogenous peroxidase. EDTA buffer antigen retrieval
was used for Occludin and ZO-1, whilst citrate buffer antigen
retrieval was used for TRPA-1 and PAR-2. Microwave-treated antigen
retrieval was used, microwave heating in EDTA or citrate buffer was
performed for 15 min under high fire, following by another 10-min
heating after boiling. The sections were washed again with PBS and
blocked with 10% normal goat non-immune serum (cat. no. C01-03001;
BIOSS) for 30 min at room temperature, which was followed by
incubation with primary antibodies against rabbit polyclonal
anti-PAR-2 (1:250; cat. no. bs-1178R; BIOSS), rabbit monoclonal
anti-Occludin (1:200; Abcam; cat. no. ab216327), rabbit polyclonal
anti-ZO-1 (1:500; cat. no. bs-1329-R; BIOSS) and rabbit polyclonal
anti-TRPA1 (1:1,000; cat. no. ab58844; Abcam) overnight at 4˚C. The
slices were then stained using a two-step IHC detection reagent kit
(cat. no. PV-9001; ZSGB-BIO; http://www.zsbio.com/product/PV-9001) according to
manufacturer's protocol. DAB chromogenic kit (cat. no. ZLI-9018;
ZSGB-BIO) was used for detection. Hematoxylin was used at room
temperature for 5 min for counterstaining. Light microscopy was
used to image the sections (magnification, x200).
ELISA
The plasma level of NE was determined using an ELISA
kit (cat. no. EL-0047C; Elabscience) according to the
manufacturer's protocol.
Cell culture
Caco-2 cells were used for detection of the cell
permeability (27). Caco-2 cells
were kindly gifted by Professor Jingxin Li (Cheeloo College of
Medicine, Shangdong University, Jinan, China). The cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (cat. no. FB35015; Clark Bio Office;
https://www.clarkbio.com/index.php?m=Content&c=Index&a=show&catid=9&id=6),
100 U/ml penicillin and 100 µg/ml streptomycin (Beijing Solarbio
Science & Technology Co., Ltd.) at 37˚C under a humidified
atmosphere of 5% CO2. Caco-2 cells were collected when
they reached 70-80% confluence and cells from passages 2-15 were
used for all subsequent experiments.
Cell viability detection using a Cell
Counting Kit (CCK)-8 assay
Relative viability of Caco-2 cells after NE
treatment was detected using a CCK-8 (cat. no. BB-4202-2; BestBio
Science) assay. The kit was used according to the manufacturer's
protocols. Briefly, Cells (5x103 cells/well) in 100 µl
medium were added into 96-well plates. After 12 h, cells were
cultured with NE (cat. no. S25926; Shanghai Yuanye Bio-Technology
Co., Ltd. http://www.shyuanye.com/goods-S25926.html) at
different final concentrations (0, 5, 10, 20, 40, 60 and 80 µM) for
6 h and (10, 20, 40, 60, 80, 100, 120 and 160 µM) 24 h at 37˚C. In
total, 10 µl CCK-8 solution was added to the 100 µl culture medium
per well and incubated at 37˚C for 2 h. The optical density (OD)
was measured at 450 nm using a microplate spectrophotometer (1420
Victor3; Thermo Fisher Scientific, Inc.). Relative cell viability
(%)=[(OD of NE treatment group-OD of blank)/(OD of control group-OD
of blank)] x100%. Viability was represented as the percentage of
culture without NE that was set to 100%.
Western blot analysis
The expression levels of proteins were determined by
western blotting. Cells (1x106) were plated into 6-cm
dishes and cultured until reaching 80% confluence. Cells were then
separated into the control group, NE-treated 6 h group and
NE-treated 24 h group. After 6 and 24 h culture at 37˚C, cells were
washed three times with cold PBS and collected. The cell samples
were lysed in lysis buffer supplemented with 0.1% protease
inhibitor, 1% phosphatase inhibitor and 1% PMSF for 30 min on the
ice. All reagents were from the BCA whole protein extraction kit
(cat. no. KGP250; Nanjing KeyGen Biotech Co., Ltd.). Samples were
centrifuged at 13,684 x g for 5 min at 4˚C. Protein concentration
in the lysate was measured using the bicinchoninic acid protein
assay kit (cat. no. KGPBCA; Nanjing KeyGen Biotech Co., Ltd.).
After boiling the samples with SDS sample buffer for 5 min, equal
amounts of protein (40 µg) were separated by 10% SDS-PAGE (Nanjing
KeyGen Biotech Co., Ltd) and were transferred onto PVDF membranes
(EMD Millipore). The membranes were blocked for 2 h at room
temperature with 5% non-fat dry milk diluted in PBS. The membranes
were then incubated with primary antibodies against Occludin
(1:1,000; cat. no. ab216327; Abcam), ZO-1 (1:500; cat. no.
bs-1329-R; BIOSS), TRPA1 (1:1,000; cat. no. ab58844; Abcam) and
PAR-2 (1:500; cat. no. bs-1178R; BIOSS) at 4˚C overnight. β-actin
was used as an internal loading control (1:1,000; cat. no. TA09;
ZSGB-BIO; OriGene Technologies, Inc.). Following three washes with
TBS-T (0.2% Tween-20), membranes were then incubated with the
horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary
antibody (1:5,000; cat. no. ZB2301; ZSGB-BIO; OriGene Technologies,
Inc.) and anti-mouse IgG secondary antibody (1:5,000; cat. no.
ZB2305; ZSGB-BIO; OriGene Technologies, Inc.) respectively for 1 h
at room temperature. Immunoreactive bands were visualized using
enhanced chemiluminescence detection reagents (Affinity
Biosciences). Protein expression levels were analyzed using an
ImageJ Imaging System (version 1.37; National Institutes of
Health).
Immunofluorescence
Caco-2 cells were seeded into 24-well plates
containing 15-mm slides. The complete medium was replaced by fresh
complete medium (fully-supplemented DMEM) before further treatment
with NE at a final concentration of 10 µM. After incubation for 6
and 24 h at 37˚C, the cells were washed three times with cold PBS
and fixed in ice-cold 4% paraformaldehyde at room temperature for
20 min. After washing with cold PBS, the cells were blocked with 1%
BSA (cat. no. B1010; Biotopped;; http://www.bjbiotopped.com/showinfo-20-98588-0.html)
for 30 min at room temperature. The cells were then incubated with
primary antibodies against PAR-2 (1:300; cat. no. bs-1178R; BIOSS),
Occludin (1:100; cat. no. ab216327; Abcam), ZO-1 (1:300; cat. no.
bs-1329-R; BIOSS) and TRPA1 (1:200; cat. no. ab58844; Abcam) at 4˚C
overnight, followed by incubation with FITC-conjugated
goat-anti-rabbit secondary antibodies (1:50; cat. no.
bs-0295G-FITC; BIOSS) for 1 h at room temperature in the dark after
washing in cold PBS. The slices were sealed with mounting medium
containing DAPI (cat. no. DZ0125; Beijing Leagene Biotech Co.,
Ltd.; https://www.leagene.com/Catalogue/DZ0125-DAPIrsfpj_ID482.html).
Images were captured using an Olympus fluorescence microscope
(Olympus Corporation) and analyzed using Adobe Photoshop CS3 10.0.1
version (Adobe Systems, Inc.) at x400 magnification.
Statistical analysis
Data are presented as the mean ± SD, where n refers
to the number of animals or the number of duplicates. Unpaired
Student's t-test was performed for comparisons between two groups
and one-way ANOVA followed by Tukey's multiple comparisons test.
Mixed two-way ANOVA followed by a Sidak corrections were used for
comparisons among multiple groups with SigmaStat 3.5 software
(Systat Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
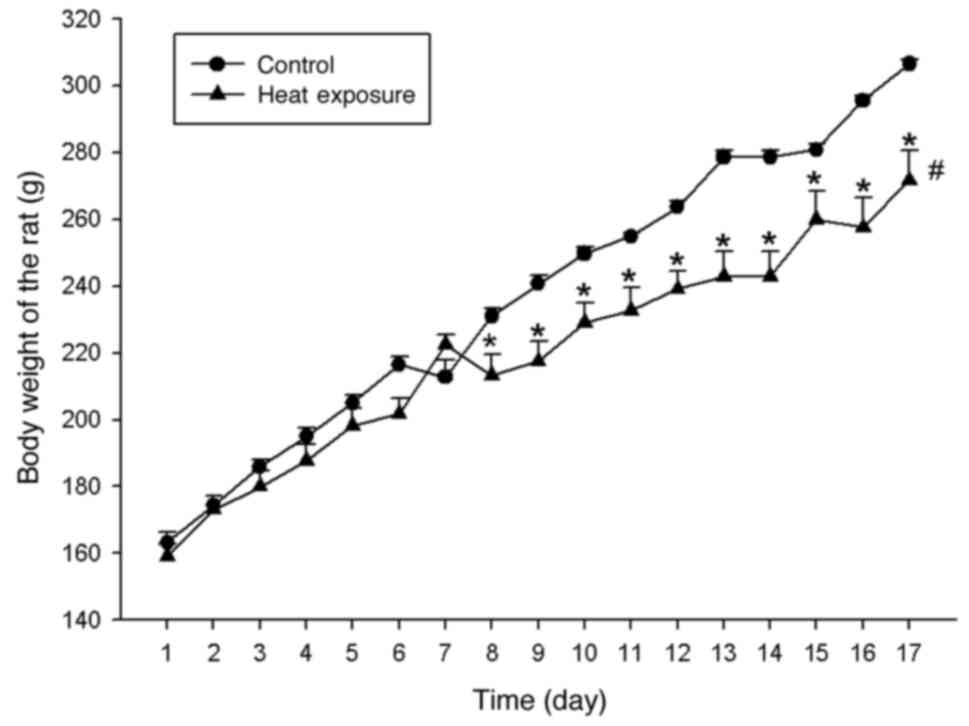
Heat exposure animal model
All rats survived prior to sacrifice. According to
the mixed two-way ANOVA (with animal treatment type as a
between-subjects factor and time as the within-subjects factor)
followed by post hoc testing with Sidak correction, the body weight
increase of the rats in the heat exposure groups was lower compared
with that in the control group (F=8.209, P<0.05), whilst time
also affected the weight of the rats (F=844.208; P<0.001), with
the difference more significant from day 8 onwards (F=28.15;
P<0.001) and weights in rats exposed to heat lower compared with
those in rats in the control group (Fig. 1). This suggests that long lasting
heat exposure adversely affected the rat weight.
According to the H&E staining images, the mucosa
in both control and heat-exposed rat colon tissues were found to be
intact, with no notable structural changes (Fig. 2).
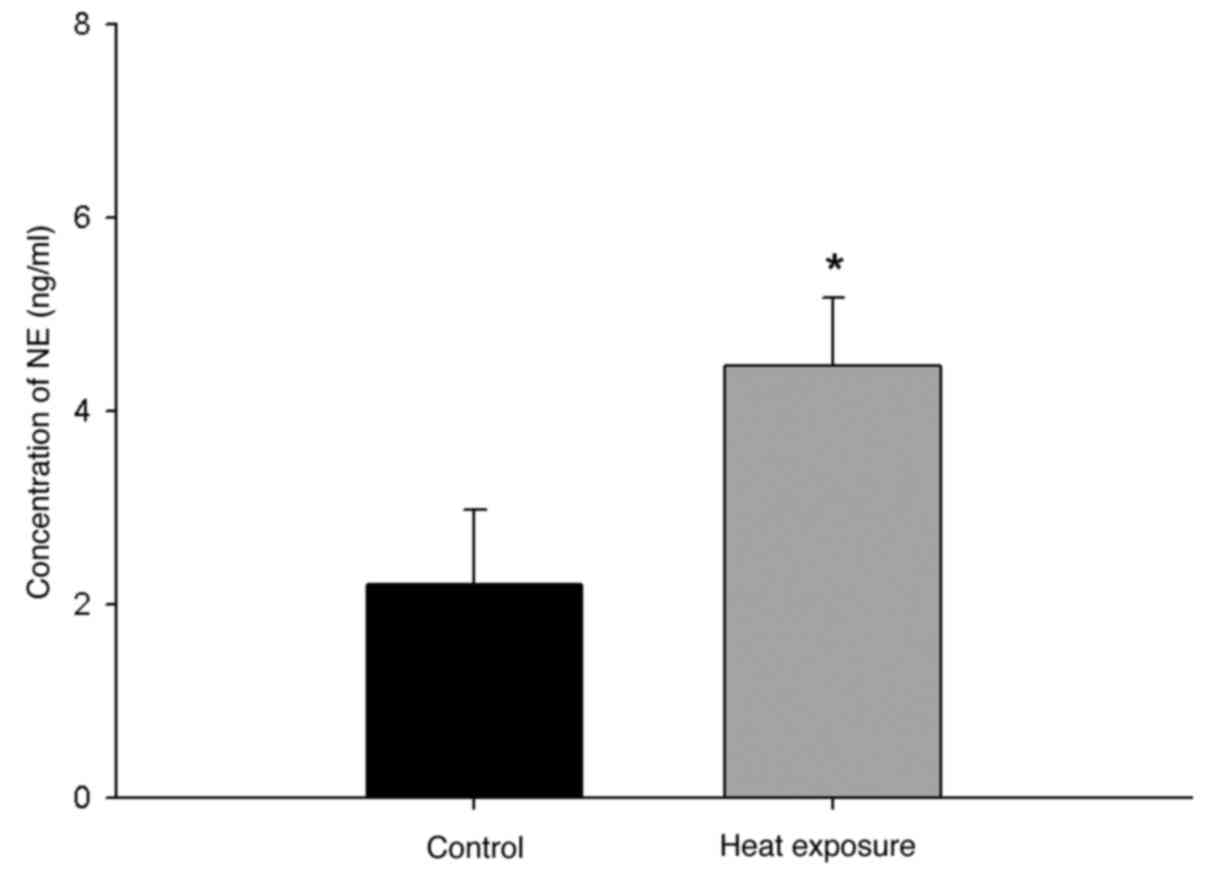
The rats were treated for 9 days continuous 24-h
heat exposure (32˚C) after 5 days of acclimatization. Plasma
samples were collected on day 17 and the plasma NE level was
detected. In the heat exposure group, the average plasma NE level
was significantly elevated compared with that in the control group
(P<0.05; n=6; Fig. 3).
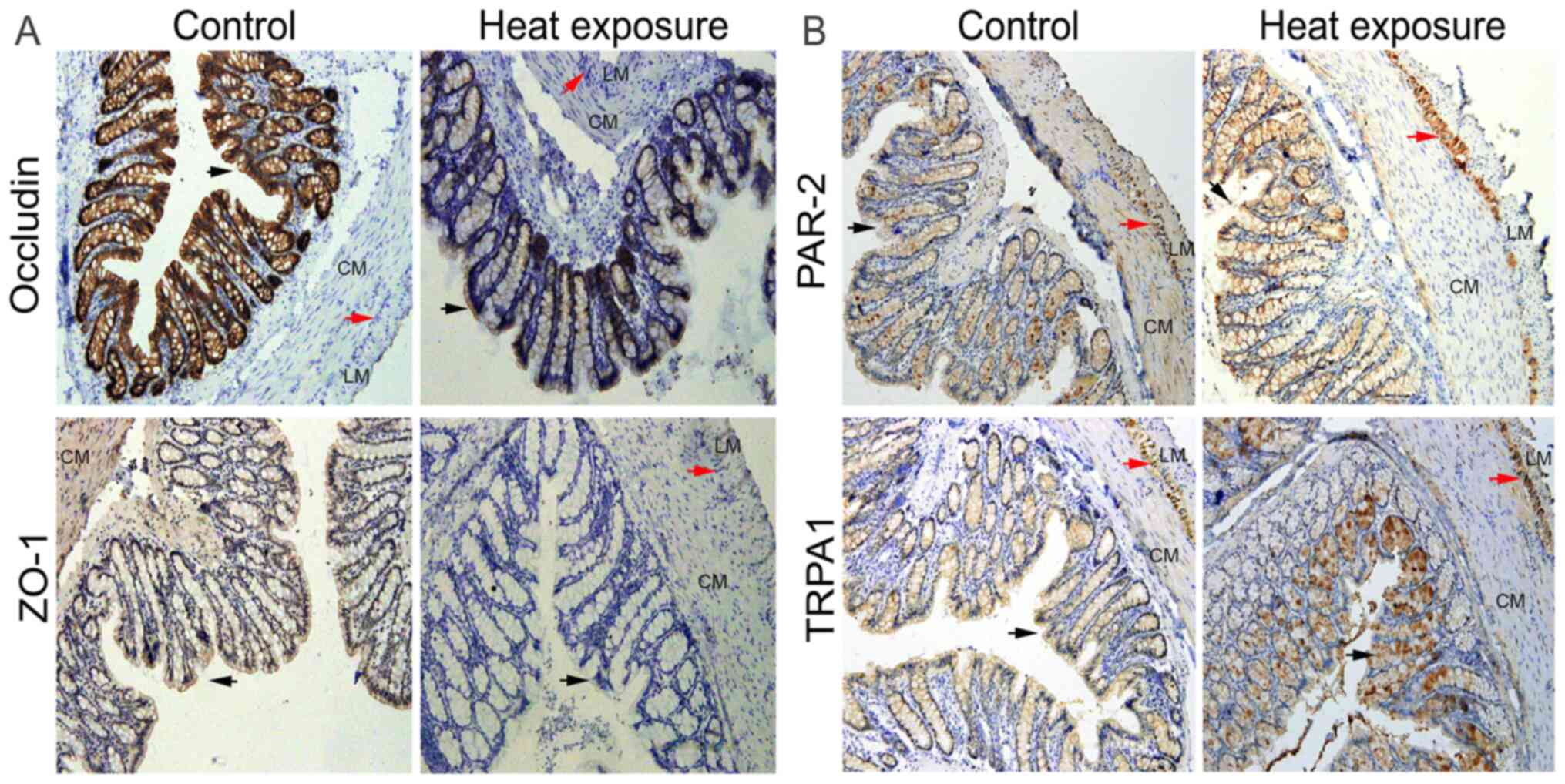
Expression levels of Occludin, ZO-1,
PAR-2 and TRPA1 in rat distal colon
In the control group, strong positive staining of
Occludin was observed in the mucosa, which was mainly located on
the cell membranes of epithelial cells and was decreased after heat
exposure (Fig. 4A). ZO-1 was
observed in the mucosa and smooth muscle cells, the expression of
which was found to be attenuated in the heat exposure group in
comparison with that in the control group (Fig. 4A). PAR-2 staining was detected in
the mucosa and the myenteric nerve plexus, in addition to within
smooth muscles cells (Fig. 4B). It
was found that heat exposure increased PAR-2 expression, where a
weaker expression was observed in the smooth muscle layer (Fig. 4B). Additionally, TRPA1 staining was
identified in the epithelial cells of the colon mucosa and
myenteric nerve plexus (Fig. 4B).
After heat exposure, the expression pattern of TRPA1 in mucosal
epithelial cells was different compared with that in control, with
enhanced expression observed in the myenteric nerve plexus.
Furthermore, increased positive staining was observed in the
luminal side of the mucosa, which appeared to be concentrated in
the cells of the luminal side (Fig.
4B).
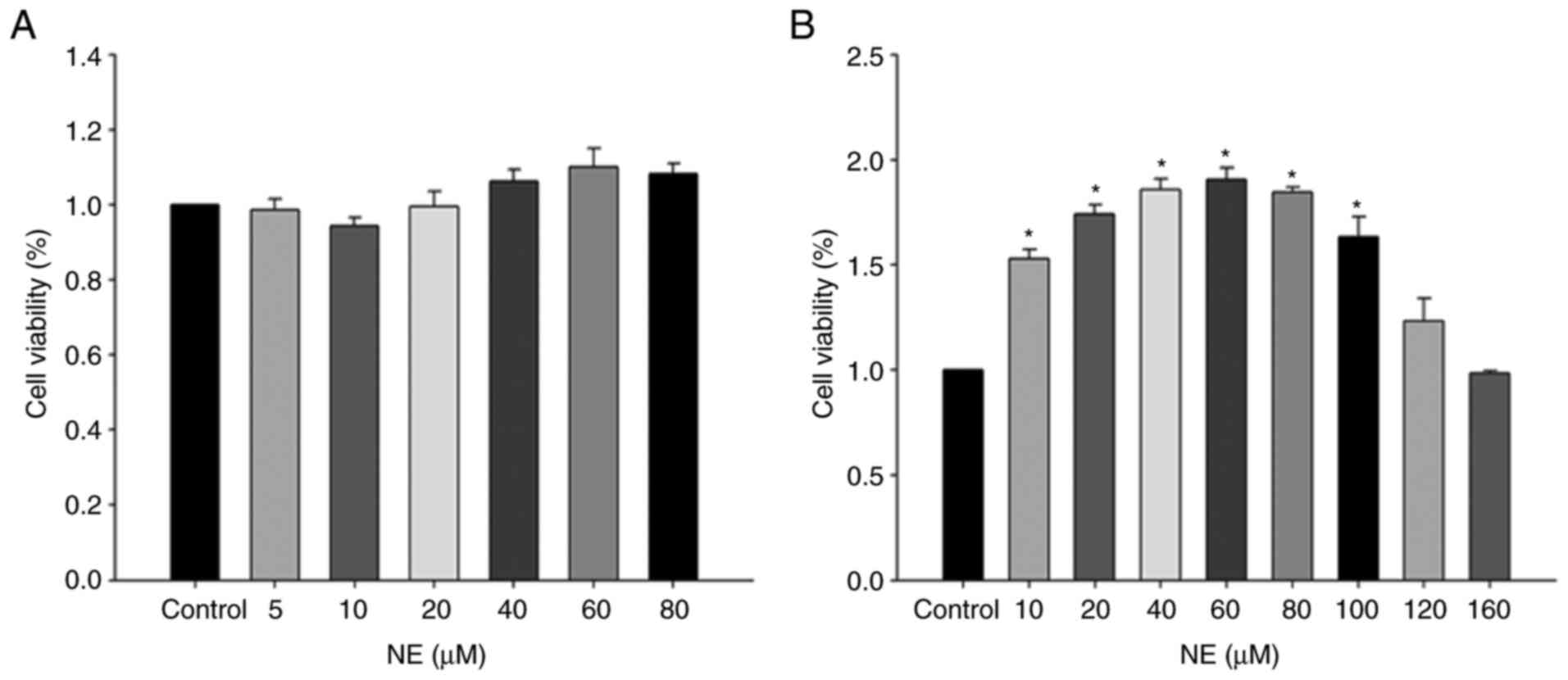
Cell viability detection using a CCK-8
assay
Caco-2 cells were treated with different
concentrations of NE for 6 and 24 h, respectively. Treatment with
increasing concentrations of NE for 6 h did not affect cell
viability (Fig. 5A). However, the
cell viability gradually increased as the NE concentration elevated
from 10 to 100 µM in the 24 h group, but there was no statistical
difference when cells were treated with 120 and 160 µM NE compared
with that in control (Fig. 5B).
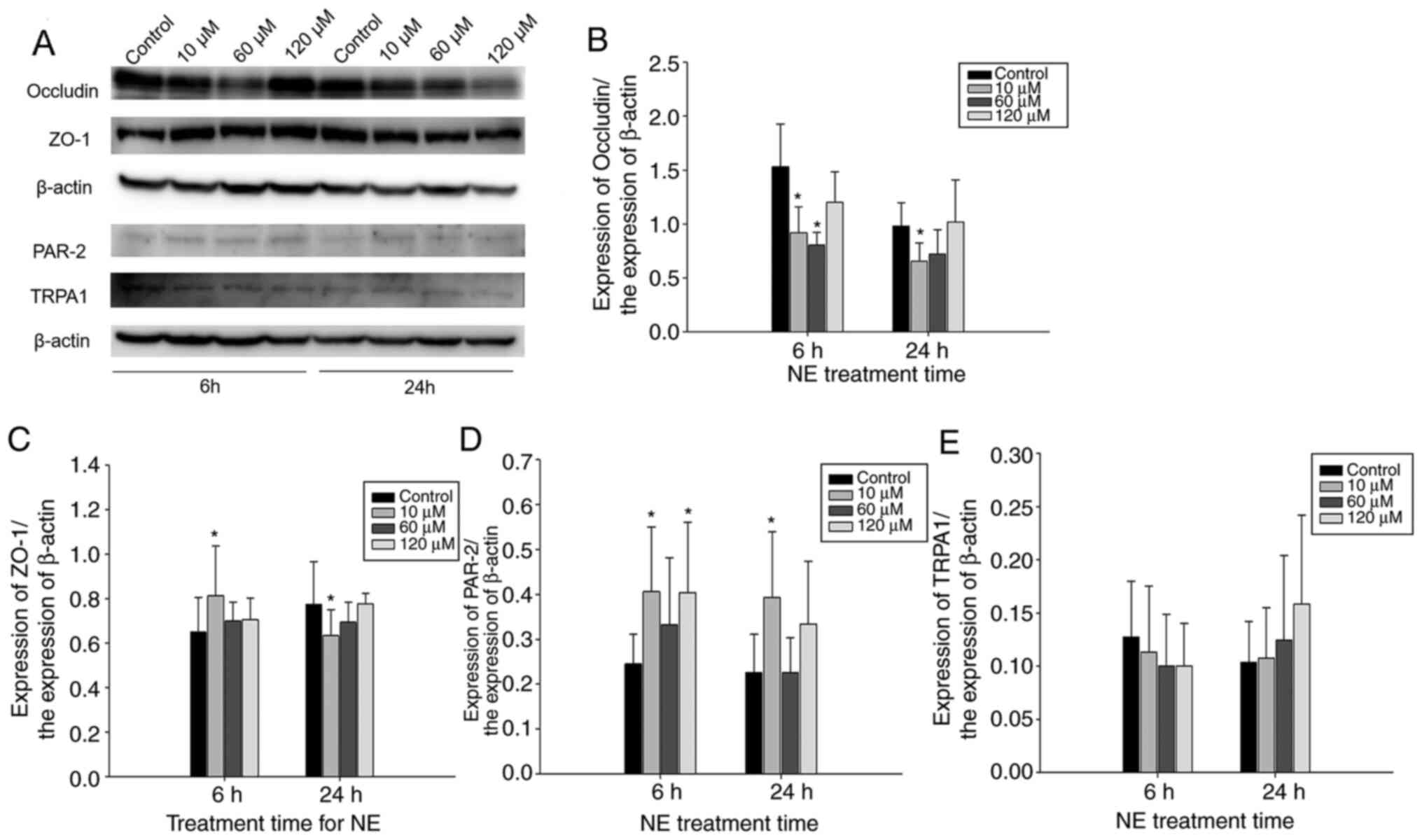
Protein expression levels of Occludin,
ZO-1, TRPA1 and PAR-2 after the administration of NE
To examine the regulatory effect of NE on the
expression levels of tight junction proteins, as well as PAR-2 and
TRPA1, 10, 60 and 120 µM were selected as the final NE
concentration to stimulate Caco-2 cells for 6 and 24 h. In the 6 h
treatment group, Occludin expression was significantly decreased
when 10 and 60 µM NE were applied compared with that in control
(n=4; P<0.05; Fig. 6A and
B). After 24 h treatment, 10 µM NE
also significantly downregulated Occludin expression compared with
that in control (n=4; P<0.05; Fig.
6A and B). Although
administration of 60 µM NE also decreased Occludin expression in
the 24 h group, the difference was not statistically significant
(Fig. 6A and B). Occludin expression was not
significantly affected by treatment with 120 µM NE for 6 h, 120 µM
NE appeared to have reduced Occludin expression after 24 h,
however, there was no significant difference (Fig. 6A and B). Significant upregulation of ZO-1
expression was observed after administration of 10 µM NE for 6 h
compared with that in the control group (n=4; P<0.05), but its
expression was downregulated by 10 µM NE after 24 h treatment
compared with that in the control group (P<0.05; n=4; Fig. 6A and C). PAR-2 protein expression was
significantly increased after 10 µM NE treatment for 6 h
(P<0.05; n=4) and 24 h (P<0.05; n=4) compared with that in
control, but not after 60 µM NE treatment (Fig. 6A and D). However, 120 µM NE treatment for 6 h
increased PAR-2 expression (P<0.05; n=4). There were no
statistical significant changes in TRPA1 protein expression when
different concentrations of NE were applied to the Caco-2 cells for
the different time periods (Fig. 6A
and E).
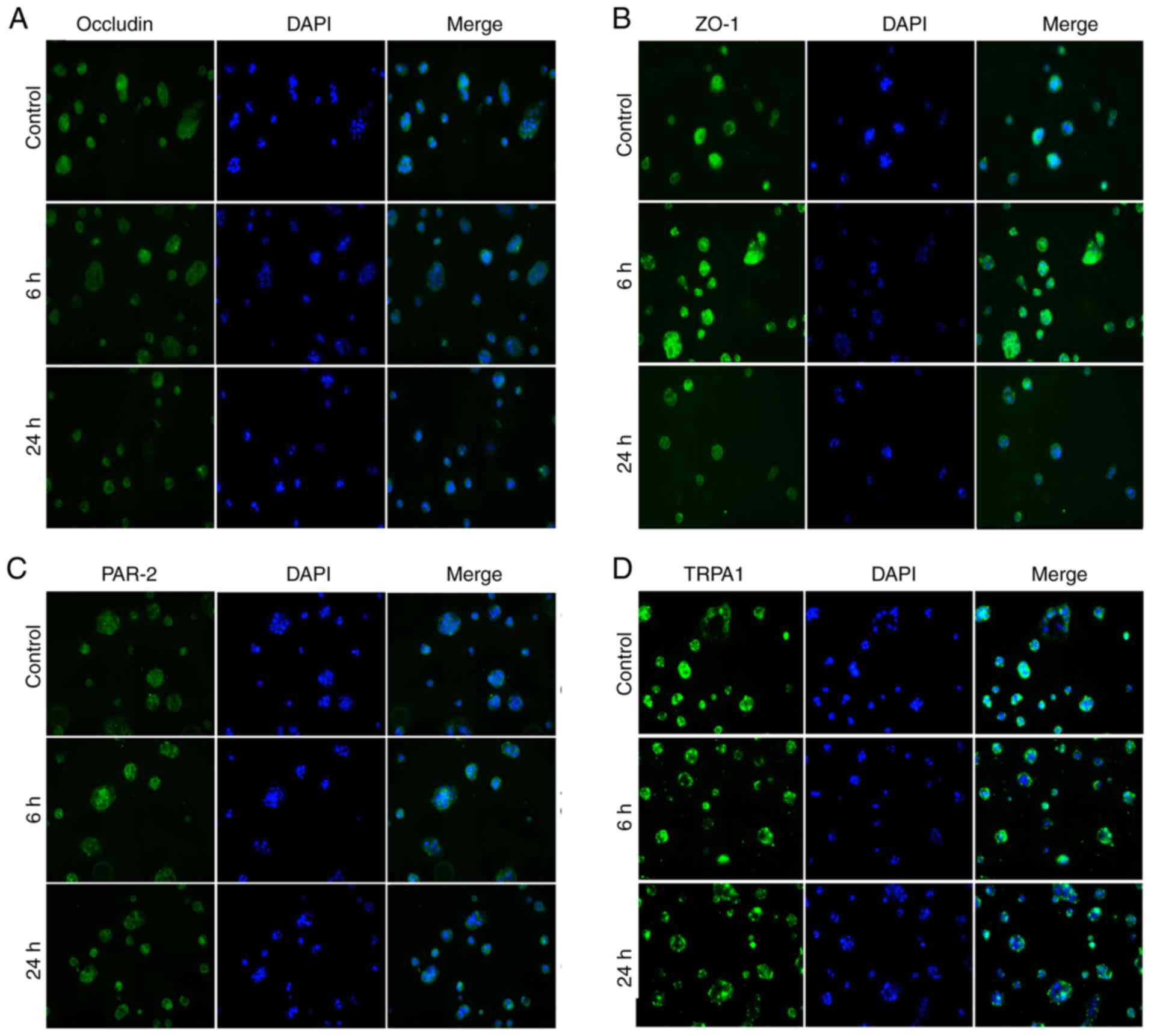
Immunofluorescence
The present study also evaluated the expression
levels of tight junction proteins, in addition to PAR-2 and TRPA1
by immunofluorescence after the application of 10 µM NE for 6 and
24 h. Occludin-positive staining was located on the cell membrane
of Caco-2 cells, which was reduced by NE treatment in both 6 and 24
h groups (Fig. 7A). ZO-1 was also
found to be expressed on the surface of Caco-2 cells, where it was
found that 6 h treatment with NE increased the expression of ZO-1,
but 24 h treatment reduced its expression (Fig. 7B). PAR-2 was observed on the cell
membrane, where its staining was stronger in NE-treated 6 h and 24
h cells (Fig. 7C). The staining
pattern of TRPA1 was different compared with that of the
aforementioned proteins (Fig. 7D).
The positive staining was discontinuous, which became more obvious
after NE treatment for 6 and 24 h. It appeared that TRPA1 gathered
on the cell membrane of Caco-2 cells, which was similar with the
results observed from immunohistochemistry in the rat colon mucosal
epithelium.
Discussion
In the present study, heat stress induced by heat
exposure attenuated body weight gain. Histological examination of
the rat colon tissues after heat exposure demonstrated that there
was no obvious damage to the rat colonic mucosa, but the protein
expression levels of tight junction proteins Occludin and ZO-1 were
decreased. These findings were consistent with a previous study,
which reported that psychological stress reduced brain and
intestinal expression levels of tight junction proteins, including
Claudin 5, Occludin, α-actin and ZO-1(1),However, whether elevated NE levels
regulated the expression levels of tight junction proteins was not
previously investigated (1). The
present study observed elevated plasma NE levels and found that the
administration of 10 µM NE for 6 and 24 h downregulated the
expression of Occludin in Caco-2 cells, whilst NE treatment
upregulated ZO-1 expression after 6 h treatment but reduced ZO-1
expression after 24 h. These data indicated that NE directly
regulated the expression levels of tight junction proteins, which
can contribute to altered gut permeability under stress.
In patients with septic shock, NE use is associated
with increased enterocyte damage (28), where the reason for this could be
the direct regulation by NE on tight junction proteins. In a
previous study on vascular endothelial cells, the inhibitory effect
of angiotensin II on Occludin and ZO-1 expression was identified
(29). In addition, our unpublished
data also revealed the significant inhibitory action of 6 h NE
treatment on the expression levels of Occludin and ZO-1 in thoracic
aortic endothelial cells. Taken together, these findings indicate a
direct regulatory effect of this stress hormone on tight junction
proteins in epithelial cells. Although there have been a few
reports that evaluated the direct regulatory effect of NE on the
tight junction proteins (25,30),
it has been suggested that in bovine aortic endothelial cells,
treatment with NE to concentrations ranging from normal to
pathophysiological circulating plasma levels significantly impedes
trypan blue dye-bovine serum albumin conjugate diffusion, compared
with that in untreated controls (31). The difference between the present
study and this previous report in the barrier-modulating effects of
NE may be due to the dose and cell type. As a chronic stress
hormone, NE promotes tumor progression by stimulating
β2-adrenoreceptors in oral cancer (32). In the present study, treatment of
Caco-2 cells with different concentrations of NE for 6 h did not
affect cell viability, but NE increased cell viability 24 h after
treatment. This finding was in accordance with a previous
observation that 24 h NE treatment enhanced cell viability and
invasion of pancreatic cancer cells (33).
The activation of PAR-2 and mast cell is involved in
increased epithelial permeability due to changes in tight junction
proteins under heat stress (11,34,35),
PAR-2 modulates Ve-Cadherin expression to affect human vascular
endothelial barrier function (13),
such that activation of PAR2 changes the localization of the tight
junction proteins and increases barrier permeability (12). However, it has also been reported
that treatment with a PAR-2 agonist prevents the downregulation of
tight junction proteins after P. aeruginosa elastase
treatment in human nasal epithelial cells (36). According to the present study,
expression of PAR-2 in colonic epithelial cells was increased after
heat exposure, which may be a reason for the altered expression of
tight junction proteins in the heat-exposed rats colon.
Furthermore, PAR-2 expression was upregulated after application of
10 µM NE for both 6 and 24 h, whereas NE level was increased in
heat-exposed rats. These findings suggested that NE can regulate
the expression of PAR-2 under stress.
TRP channels are non-selective cation channels that
act as biosensors for environmental and noxious stimuli, including
capsaicin and allicin, in addition to changes in temperature and
conditions inside the cell (14,15).
The TRPA1 receptor is highly expressed in the intestinal mucosa and
can be activated by oxidative stress products, where the cell
damage signals can induce oxidative stress (19), which implicates its possible
association with intestinal disfunction. Cold stress increases ROS
production by TRPA1 activation in A549 cells (37). In addition, upregulation of TRPA1
expression and function on vagal afferents is associated with
stress-exaggerated visceral mechanonociception after antral cold
(4˚C) stress (38). TRPA1 also
mediates cigarette smoke extract-induced damage of bronchial and
alveolar epithelial cells via the modulation of oxidative stress,
inflammation and mitochondrial damage (39). It has been reported that substance P
may initiate the earliest changes observed in blood-brain barrier
permeability (40). In addition, it
has also been documented that TRPA1 mediates the development of
gastric mucosal and duodenal lesions in a water immersion restraint
stress rat model by promoting the release of substance P (22,41).
Since TRPA1 is involved in Ca2+ influx and increases in
tight junction permeability (42),
TRPA1 may contribute to damage in epithelial barrier function by
regulating oxidative stress induced by stress. The present study
demonstrated that the expression of TRPA1 was changed, with
increased positive staining observed in the luminal side of the
mucosa in heat exposed rats, which was consistent with previous
results reported following water avoidance stress (43). Furthermore, the expression pattern
was altered after heat exposure and the expression of TRPA1 was
concentrated on the luminal surface. The present study investigated
the regulatory mechanism mediated by NE on expression of TRPA1.
However, there was no change in its protein expression level after
treatment with NE for either 6 or for 24 h (Fig. 6D). It was found that higher levels
of TRPA1 were gathered or recruited onto the cell membrane to form
dot staining after NE treatment. This may be the reason for the
failure in detecting changes in protein expression using western
blotting, therefore further investigation is required to confirm
the role of NE on the expression of TRPA1. Furthermore, in the
present study, the detailed regulatory mechanisms of NE on tight
junction proteins and PAR-2 function were not investigated. Further
experiments are needed to address these questions in the
future.
In conclusion, the present study demonstrated the
changed expression levels of tight junction proteins, PAR-2 and
TRPA after heat exposure, which was implicated in intestinal
barrier function under stress. The present results suggested that
NE directly regulated the expression of tight junction proteins and
PAR-2 in vitro. It was also indicated that NE may be
directly responsible for the altered levels of tight junction
proteins and PAR-2 under stress.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant nos. 81760265 and 81760055) and Ningxia
High School first-class Disciplines (grant no. NXYLXK2017B07; West
China first-class Disciplines Basic Medical Sciences at Ningxia
Medical University).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL contributed to the conception and design of the
study and completed the western blotting and immunohistochemistry
experiments. HM contributed to cell culture and acquisition of data
and performed IF analysis. SN established the animal model. XL
performed the Cell Counting Kit-8 assay. LN contributed to the
interpretation of data and revised the article for important
intellectual content. GL contributed to the conception and design
of the study. YL and GL can authenticate the raw data of this
study. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The experimental procedures were approved by the
Animal Ethics Committee of the Ningxia Medical University and Use
Committee (Yinchuan, China) and were performed in accordance with
the Guidelines of the Council of the Physiological Society of
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Geng S, Yang L, Cheng F, Zhang Z, Li J,
Liu W, Li Y, Chen Y, Bao Y, Chen L, et al: Gut microbiota are
associated with psychological stress-induced defections in
intestinal and blood-brain barriers. Front Microbiol.
10(3067)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lu S, Wu D, Sun G, Geng F, Shen Y, Tan J,
Sun X and Luo Y: Gastroprotective effects of Kangfuxin against
water-immersion and restraint stress-induced gastric ulcer in rats:
Roles of antioxidation, anti-inflammation, and pro-survival. Pharm
Biol. 57:770–777. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dokladny K, Zuhl MN and Moseley PL:
Intestinal epithelial barrier function and tight junction proteins
with heat and exercise. J Appl Physiol (1985). 120:692–701.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu B, Shen LJ, Zhao TX, Sun M, Wang JK,
Long CL, He DW, Lin T, Wu SD and Wei GH: Automobile exhaust-derived
PM2.5 induces blood-testis barrier damage through ROS-MAPK-Nrf2
pathway in sertoli cells of rats. Ecotoxicol Environ Saf.
189(110053)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Elias BC, Suzuki T, Seth A, Giorgianni F,
Kale G, Shen L, Turner JR, Naren A, Desiderio DM and Rao R:
Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its
interaction with ZO-1 and destabilizes its assembly at the tight
junctions. J Biol Chem. 284:1559–1569. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Buckley A and Turner JR: Cell biology of
tight junction barrier regulation and mucosal disease. Cold Spring
Harb Perspect Biol. 10(a029314)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sunagawa M, Wolf-Johnston A, Nomiya M,
Sawada N, Andersson KE, Hisamitsu T and Birder LA: Urinary bladder
mucosal responses to ischemia. World J Urol. 33:275–280.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Du L, Long Y, Kim JJ, Chen B, Zhu Y and
Dai N: Protease activated receptor-2 induces immune activation and
visceral hypersensitivity in post-infectious irritable bowel
syndrome mice. Dig Dis Sci. 64:729–739. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lu L, Yan L, Yuan J, Ye Q and Lin J:
Shuganyin decoction improves the intestinal barrier function in a
rat model of irritable bowel syndrome induced by water-avoidance
stress. Chin Med. 13(6)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim DH, Cho YJ, Kim JH, Kim YB and Lee KJ:
Stress-induced alterations in mast cell numbers and
proteinase-activated receptor-2 expression of the colon: Role of
corticotrophin-releasing factor. J Korean Med Sci. 25:1330–1335.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhong CJ, Wang K, Zhang L, Yang CQ, Zhang
K, Zhou SP and Duan LP: Mast cell activation is involved in
stress-induced epithelial barrier dysfunction in the esophagus. J
Dig Dis. 16:186–196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Enjoji S, Ohama T and Sato K: Regulation
of epithelial cell tight junctions by protease-activated receptor
2. J Vet Med Sci. 76:1225–1229. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang R and Ge J: Proteinase-activated
receptor-2 modulates Ve-cadherin expression to affect human
vascular endothelial barrier function. J Cell Biochem.
118:4587–4593. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Talavera K, Startek JB, Alvarez-Collazo J,
Boonen B, Alpizar YA, Sanchez A, Naert R and Nilius B: Mammalian
transient receptor potential TRPA1 channels: From structure to
disease. Physiol Rev. 100:725–803. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bamps D, Vriens J, de Hoon J and Voets T:
TRP channel cooperation for nociception: Therapeutic opportunities.
Annu Rev Pharmacol Toxicol. 61:655–677. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Taylor-Clark TE, Undem BJ, Macglashan DW
Jr, Ghatta S, Carr MJ and McAlexander MA: Prostaglandin-induced
activation of nociceptive neurons via direct interaction with
transient receptor potential A1 (TRPA1). Mol Pharmacol. 73:274–281.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gouin O, L'Herondelle K, Lebonvallet N,
Gall-Ianotto CL, Sakka M, Buhé V, Plée-Gautier E, Carré JL,
Lefeuvre L, Misery L and Le Garrec R: TRPV1 and TRPA1 in cutaneous
neurogenic and chronic inflammation: Pro-inflammatory response
induced by their activation and their sensitization. Protein Cell.
8:644–661. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sinica V, Zimova L, Barvikova K, Macikova
L, Barvik I and Vlachova V: Human and mouse TRPA1 are heat and cold
sensors differentially tuned by voltage. Cells.
9(57)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Viana F: TRPA1 channels: Molecular
sentinels of cellular stress and tissue damage. J Physiol.
594:4151–4169. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Wang Y, Yin S, Mei L, Yang Y, Xu S, He X,
Wang M, Li M, Zhang Z and He Q: A dual receptors-targeting and
size-switchable ‘cluster bomb’ co-loading chemotherapeutic and
transient receptor potential ankyrin 1 (TRPA-1) inhibitor for
treatment of triple negative breast cancer. J Control Release.
321:71–83. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pires PW and Earley S: Neuroprotective
effects of TRPA1 channels in the cerebral endothelium following
ischemic stroke. Elife. 7(e35316)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu Y, Huang C, Deng HI, Jia J, Wu Y, Yang
J and Tu W: TRPA1 and substance P. mediate stress induced duodenal
lesions in water immersion restraint stress rat model. Turk J
Gastroenterol. 29:692–700. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Morris LS, McCall JG, Charney DS and
Murrough JW: The role of the locus coeruleus in the generation of
pathological anxiety. Brain Neurosci Adv.
4(2398212820930321)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sawka MN, Leon LR, Montain SJ and Sonna
LA: Integrated physiological mechanisms of exercise performance,
adaptation, and maladaptation to heat stress. Compr Physiol.
1:1883–1928. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kalinin S, Feinstein DL, Xu HL, Huesa G,
Pelligrino DA and Galea E: Degeneration of noradrenergic fibres
from the locus coeruleus causes tight-junction disorganisation in
the rat brain. Eur J Neurosci. 24:3393–3400. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Aroori SV, Cogan TA and Humphrey TJ:
Effect of noradrenaline on the virulence properties of
campylobacter species. Int J Microbiol. 2014(279075)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zong Y, Zhu S, Zhang S, Zheng G, Wiley JW
and Hong S: Chronic stress and intestinal permeability:
Lubiprostone regulates glucocorticoid receptor-mediated changes in
colon epithelial tight junction proteins, barrier function, and
visceral pain in the rodent and human. Neurogastroenterol Motil.
31(e13477)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Habes Q, van Ede L, Gerretsen J, Kox M and
Pickkers P: Norepinephrine contributes to enterocyte damage in
septic shock patients: A prospective cohort study. Shock.
49:137–143. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang W, Yang H, Zhu L, Luo Y, Nie L and
Li G: Role of EGFR/ErbB2 and PI3K/AKT/e-NOS in lycium barbarum
polysaccharides ameliorating endothelial dysfunction induced by
oxidative stress. Am J Chin Med. 47:1523–1539. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu J, Zheng M, Zhao X, Zha YJ, Li HN and
Huang GQ: Effects of vasoactive drugs on hepatic and intestinal
circulation and intestinal barrier in patients with septic shock. J
Investig Med: Jan 13, 2021 (Epub ahead of print). doi:
10.1136/jim-2020-001685.
|
|
31
|
Bottaro D, Shepro D, Peterson S and
Hechtman HB: Serotonin, norepinephrine, and histamine mediation of
endothelial cell barrier function in vitro. J Cell Physiol.
128:189–194. 1986.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang B, Wu C, Chen W, Qiu L, Li S, Wang
T, Xie H, Li Y, Li C and Li L: The stress hormone norepinephrine
promotes tumor progression through beta2-adrenoreceptors in oral
cancer. Arch Oral Biol. 113(104712)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Qian W, Lv S, Li J, Chen K, Jiang Z, Cheng
L, Zhou C, Yan B, Cao J, Ma Q and Duan W: Norepinephrine enhances
cell viability and invasion, and inhibits apoptosis of pancreatic
cancer cells in a Notch1dependent manner. Oncol Rep. 40:3015–3023.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jin H, Li Z, Guo X, Tong H, Liu Z, Chen Y,
Su L and Huang Q: Microcirculatory disorders and protective role of
antioxidant in severe heat stroke: A rat study. Shock. 46:688–695.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shivers RR, Pollock M, Bowman PD and
Atkinson BG: The effect of heat shock on primary cultures of brain
capillary endothelium: Inhibition of assembly of zonulae
occludentes and the synthesis of heat-shock proteins. Eur J Cell
Biol. 46:181–195. 1988.PubMed/NCBI
|
|
36
|
Nomura K, Obata K, Keira T, Miyata R,
Hirakawa S, Takano KI, Kohno T, Sawada N, Himi T and Kojima T:
Pseudomonas aeruginosa elastase causes transient disruption of
tight junctions and downregulation of PAR-2 in human nasal
epithelial cells. Respir Res. 15(21)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun W, Wang Z, Cao J, Cui H and Ma Z: Cold
stress increases reactive oxygen species formation via TRPA1
activation in A549 cells. Cell Stress Chaperones. 21:367–372.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen X, Luo Q, Yan X, Li W and Chen S:
Vagal transient receptor potential ankyrin 1 mediates
stress-exacerbated visceral mechanonociception after antral cold
exposure. J Neurogastroenterol Motil. 25:442–460. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang M, Zhang Y, Xu M, Zhang H, Chen Y,
Chung KF, Adcock IM and Li F: Roles of TRPA1 and TRPV1 in cigarette
smoke-induced airway epithelial cell injury model. Free Radic Biol
Med. 134:229–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Corrigan F, Mander KA, Leonard AV and Vink
R: Neurogenic inflammation after traumatic brain injury and its
potentiation of classical inflammation. J Neuroinflammation.
13(264)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xu Y, Jia J, Xie C, Wu Y and Tu W:
Transient receptor potential ankyrin 1 and substance P mediate the
development of gastric mucosal lesions in a water immersion
restraint stress rat model. Digestion. 97:228–239. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kanda Y, Yamasaki Y, Sasaki-Yamaguchi Y,
Ida-Koga N, Kamisuki S, Sugawara T, Nagumo Y and Usui T:
TRPA1-dependent reversible opening of tight junction by natural
compounds with an α,β-unsaturated moiety and capsaicin. Sci Rep.
8(2251)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pierce AN, Di Silvestro ER, Eller OC, Wang
R, Ryals JM and Christianson JA: Urinary bladder hypersensitivity
and dysfunction in female mice following early life and adult
stress. Brain Res. 1639:58–73. 2016.PubMed/NCBI View Article : Google Scholar
|