Introduction
Osteosarcoma is the most common primary bone
malignancy in children and adolescents worldwide (1). The majority of patients with
osteosarcoma display invasion and metastasis, leading to a
reduction in the efficiency of anticancer agents and the failure of
therapy (2). Therefore,
identification of potential therapeutic targets to suppress
osteosarcoma cell proliferation and invasion is required.
Various mechanisms underlying osteosarcoma cell
proliferation and invasion have been previously described (1,2). The
Wnt signaling pathway, which regulates cell proliferation,
differentiation and migration, serves important functions in
osteosarcoma cell proliferation and invasion (3,4).
Previous studies have reported that Wnt1 expression is increased in
osteosarcoma tissues (3,4). Activation of Wnt signaling activates
genes associated with cell proliferation, including c-Myc, matrix
metallopeptidases and cyclin D1 (3-5),
leading to osteosarcoma cell proliferation and invasion. Moreover,
it has been reported that SOX9 regulates hyperexpression of Wnt1 in
osteosarcoma tissues and cells (3).
Therefore, it was hypothesized that inhibition of
SOX9/Wnt1-mediated signaling might suppress osteosarcoma
metastasis.
Oleanolic acid (OA), a naturally occurring
triterpenoid, displays potential antitumor activity in several
tumor cells, and has also has been reported to inhibit osteosarcoma
cell proliferation and induce cell apoptosis (6-8).
However, the mechanisms underlying OA during osteosarcoma are not
completely understood. Previous studies have demonstrated that OA
inhibits the Wnt signaling pathway in hepatoma cells (9) and derivatives of OA decrease the
expression levels of SOX2(10). The
present study investigated the hypothesis that OA functions as an
anticancer agent to protect against osteosarcoma.
Materials and methods
Osteosarcoma cell lines culture
Osteosarcoma cell lines (KHOS and U2OS) and an
osteoblastic cell line (hFOB1.19) were purchased from American Type
Culture Collection. Osteosarcoma cells were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), and osteoblastic cells
were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS at 37˚C with 5% CO2. Cells
were incubated with 50 µM OA (cat. no. O5504; Sigma-Aldrich; Merck
KGaA) for 24 h at 37˚C.
Cell viability analysis
The Cell Counting Kit-8 (CCK-8) assay (cat. no.
C0038; Beyotime Institute of Biotechnology) was performed to
evaluate cell viability according to the manufacturer's protocol.
Briefly, cells (1x104 cells/well) were seeded into a
96-well plate and incubated with 10 µl CCK-8 reagent for 2 h at
37˚C. The absorbance of each well was measured at a wavelength of
450 nm using a microplate spectrophotometer.
Colony formation assay
Cell proliferation was assessed by conducting a
colony formation assay. Cells were seeded (1x103
cells/well) into a 6-well plate and cultured at 37˚C with 5%
CO2. Following culture for 21 days, cell colonies, which
were defined as 0.5-1 mm in diameter cell assemblies, were fixed
with 4% paraformaldehyde for 30 min at room temperature, stained
using crystal violet for 10 min at room temperature and observed
using a light microscope (magnification, x100).
Ki67 staining
Cell proliferation was also assessed by performing
Ki67 staining. Briefly, cells were grown on coverslips and fixed
with 4% paraformaldehyde for 30 min at room temperature.
Subsequently, cells were incubated with an Alexa Fluor
647-conjugated anti-Ki67 antibody (1:100; cat. no. ab196907; Abcam)
for 30 min at 37˚C and the nuclei were stained with DAPI for 1 min
at room temperature. Ki67-positive cells were counted in ten
randomly selected fields of view using a fluorescence microscope
(magnification, x100) and NIS-Elements Viewer software (version
4.2.0; Nikon Corporation).
Flow cytometry
The cell cycle distribution was analyzed by
performing by flow cytometry. Cells were harvested and washed with
PBS. Subsequently, cells were fixed with ice-cold 70% ethanol for 4
h at 4˚C. After permeabilization by 0.1% Triton X-100 for 30 min at
room temperature, the cells were stained with PI containing RNase A
(cat. no. C1052; Beyotime Institute of Biotechnology) at 37˚C for 1
h. The cell cycle distribution was analyzed via flow cytometry
using a BD flow cytometry system (FACSVerse; BD Biosciences) and
FlowJo software (version 10.0; BD Biosciences).
Wound healing assay
The wound healing assay was performed to assess cell
migration. Cells were cultured in a 6-well plate. At 90%
confluence, the cell layer was scratched using a 200-µl sterile
pipette tip. Subsequently, cells were cultured in DMEM or RPMI-1640
without FBS at 37˚C with 5% CO2. At 0 and 48 h, the
wounds were observed using a phase contrast light microscope
(magnification, x200). Taking the distance between the wound edges
at 0 h as a baseline, the image of the same location after 48 h was
captured. The percentage of the wound healing area was analyzed
using ImageJ software (version 1.46r; National Institutes of
Health).
Transwell assay
To assess cell invasion, cells (5x104)
were seeded into the upper chambers of Transwell plates with 50
mg/l Matrigel (pore size, 8 µm; cat. no. CLS3374; Corning Inc.) and
serum-free medium after Matrigel precoating at 37˚C for 1 h. DMEM
or RPMI-1640 medium supplemented with 10% FBS was filled into the
lower chambers. Following incubation for 8 h, cells on the upper
surface of the membrane were removed and invading cells were fixed
with 4% paraformaldehyde for 15 min at room temperature and stained
with crystal violet for 30 min at room temperature. Invading cells
were visualized using an inverted light microscope (magnification,
x100).
TUNEL assay
To detect osteosarcoma cell apoptosis, a TUNEL assay
(cat. no. C1088; Beyotime Institute of Biotechnology) was
performed. Briefly, 104 cells/well were cultured on
coverslips in DMEM or RPMI-1640 supplemented with 10% FBS at 37˚C
with 5% CO2. Subsequently, cells were fixed with 4%
paraformaldehyde for 30 min at room temperature. After rinsing with
PBS, cells were stained using the TUNEL assay kit at 37˚C for 60
min and the nuclei were stained with DAPI (0.3 mM, Vector
Laboratories, Inc.) at room temperature for 1 min. Following
mounting with an anti-fluorescence quenching mounting medium (cat.
no. P0126; Beyotime Institute of Biotechnology), the number of
apoptotic nuclei per section was calculated using the following
formula: The number of TUNEL-positive cell nuclei/the total number
of DAPI-positive nuclei. The number of apoptotic nuclei were
counted in ten randomly selected fields of view using a
fluorescence microscope (magnification, x100).
Western blotting
After washing twice with ice-cold PBS, total protein
was extracted from osteosarcoma cells using ice-cold lysis buffer
(cat. no. P0013; Beyotime Institute of Biotechnology) and
quantified using the bicinchoninic acid assay (cat. no. P0012;
Beyotime Institute of Biotechnology). Protein homogenates (30 µg)
were separated via 8-10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes. The membranes were washed with
TBS and blocked with 5% milk powder in TBS for 1 h at room
temperature. Subsequently, the membranes were incubated at 4˚C
overnight with the following primary antibodies: Rabbit
anti-caspase-3 (1:1,000; cat. no. 9662; Cell Signaling Technology,
Inc.), rabbit anti-cleaved- caspase-3 (1:1,000; cat. no. 9664; Cell
Signaling Technology, Inc.), rabbit anti-Bcl2 (1:1,000; cat. no.
3498; Cell Signaling Technology, Inc.), rabbit anti-SOX9 (1:1,000;
cat. no. 82630; Cell Signaling Technology, Inc.), rabbit
anti-β-catenin (1:1,000; cat. no. 9582; Cell Signaling Technology,
Inc.), rabbit anti-Wnt1 (1:1,000; cat. no. 2915; Cell Signaling
Technology, Inc.) and anti-GAPDH (1:1,000; cat. no. sc-47724; Santa
Cruz Biotechnology, Inc.). Following primary incubation, the
membranes were washed with TBS and incubated with an HRP-conjugated
goat anti-rabbit-IgG secondary antibody (1:4,000; cat. no. BA1039;
Boster Biological Technology) for 1 h. Protein bands were
visualized using enhanced chemiluminescence (EMD Millipore).
Densitometry levels were analyzed using Quantity One software
(version 4.6.6; Bio-Rad Laboratories, Inc.) with GAPDH as the
loading control.
Small interfering (si)RNA)
transfection
Cells in 6-well plates (2x105 cells/well)
were transfected with 10 nM SOX9-specific siRNA
(5'-GCGACGUCAUCUCCAACAU-3') or scramble siRNA
(5'-UAGAGCUAGAGCAAGGGUA-3') (both GE Healthcare Dharmacon, Inc.)
using 6 µl Oligofectamine in Opti-MEM medium (Invitrogen; Thermo
Fisher Scientific, Inc.). At 24 h post-transfection, cells were
cultured in DMEM supplemented with 10% FBS for 24 h at 37˚C with 5%
CO2. Subsequently, transfection efficiency was
determined via RT-qPCR and western blotting (Fig. S1).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 22.0; IBM Corp.). Comparisons among multiple
groups were analyzed using one-way ANOVA followed by Holm-Sidak's
post hoc test. Comparisons between two groups were analyzed using
unpaired Student's t-test. Data are presented as the mean ± SEM.
P<0.05 was considered to indicate a statistically significant
difference.
Results
OA inhibits osteosarcoma cell
proliferation and invasion
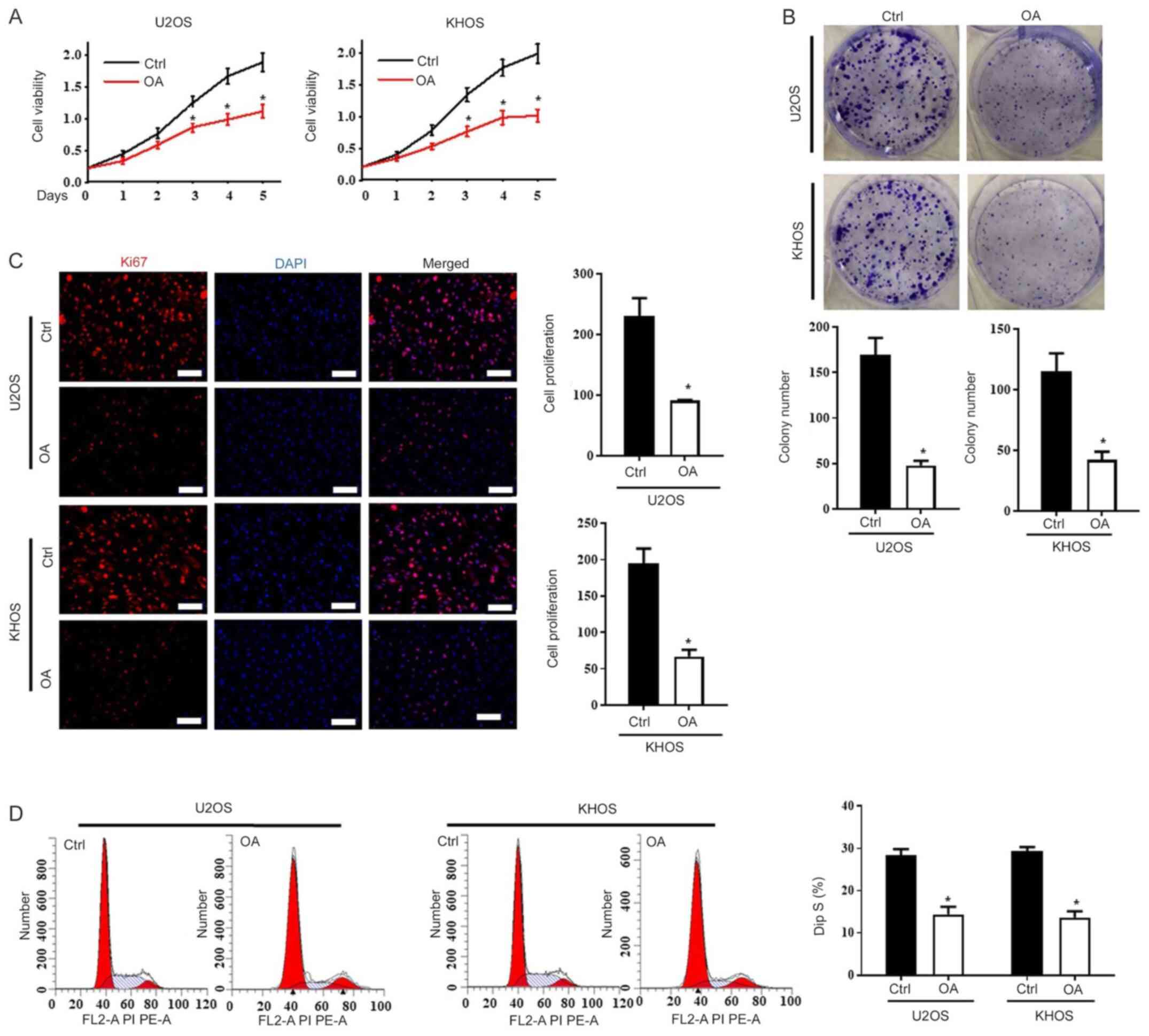
To investigate the protective effect of OA against
osteosarcoma, the primary low-metastatic U2OS (11) and high-metastatic KHOS (12) cell lines were treated with OA. The
CCK-8 assay was performed to assess cell viability, and the results
indicated that OA significantly inhibited cell viability compared
with the control (Ctrl) group on days 3, 4 and 5 in both U2OS and
KHOS cells (Fig. 1A). Similar
results were obtained in the colony formation assay (Fig. 1B). The Ki67 staining results
indicated that cell proliferation in the OA-treated group was
significantly lower compared with the Ctrl group in both
osteosarcoma cell lines (Fig. 1C).
Cell cycle analysis indicated that OA significantly reduced the
percentage of U2OS and KHOS cells in the S phase compared with the
Ctrl group (Fig. 1D).
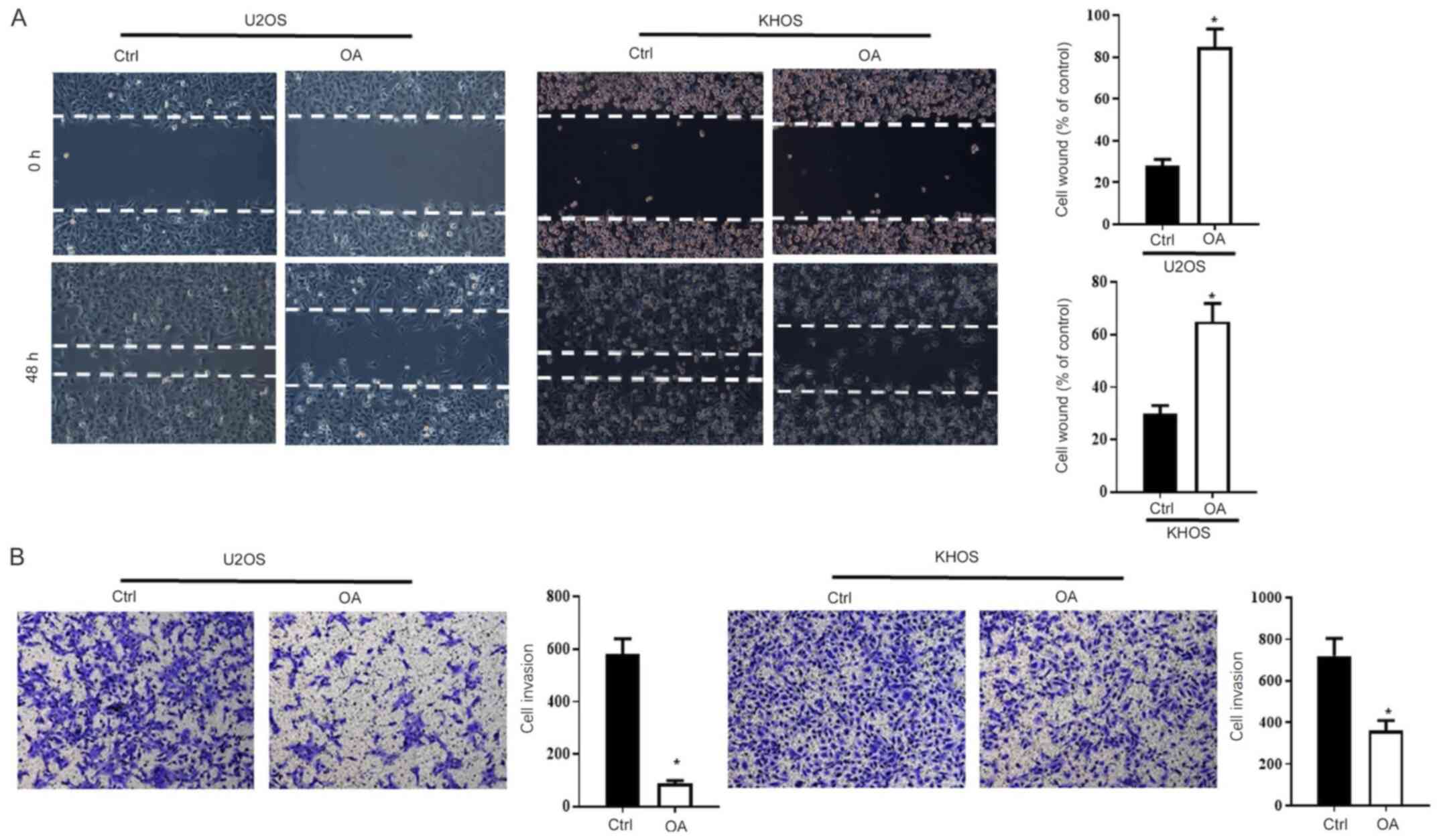
Further studies investigated the effects of OA on
osteosarcoma cell migration and invasion. The wound healing assay
results indicated that the OA group displayed significantly
decreased cell re-colonization of the wound after 48 h compared
with the Ctrl group (Fig. 2A).
Moreover, the Transwell assay results indicated that OA
significantly inhibited cell invasion compared with the Ctrl group
(Fig. 2B). An additional
osteoblastic cell line, hFOB1.19, was used to assess the effect of
OA on non-tumor cells. Compared with the Ctrl group, OA did not
significantly alter hFOB1.19 cell viability, colony formation,
proliferation, migration or invasion, as determined by performing
CCK-8, colony formation, Ki67 staining, wound healing and Transwell
assays, respectively (Fig.
S2).
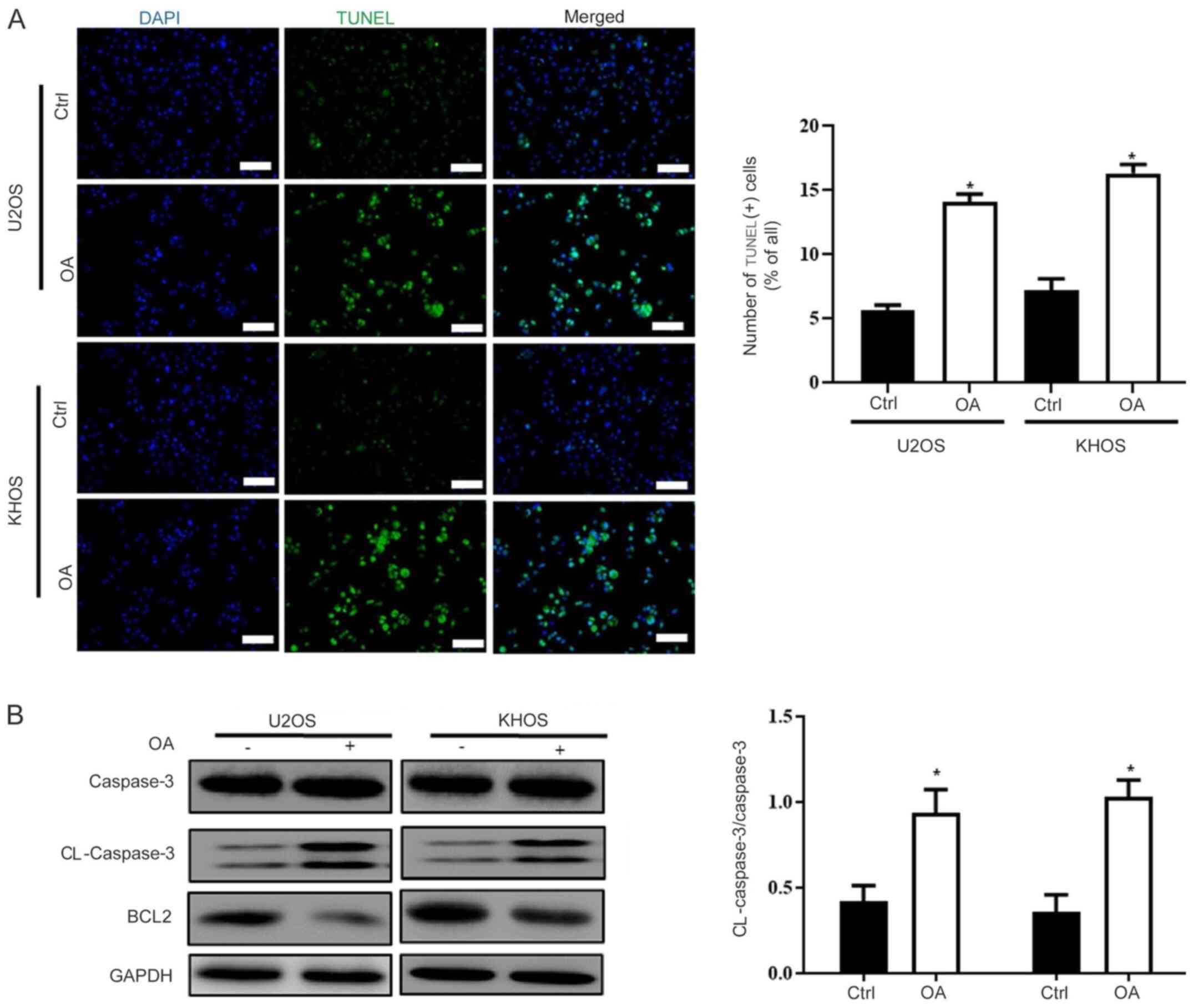
OA enhances osteosarcoma cell
apoptosis
Previous studies have reported that OA increased
cell apoptosis in other tumors (6,13);
therefore, the effects of OA on osteosarcoma cell apoptosis were
assessed. The TUNEL staining results suggested that OA
significantly increased osteosarcoma cell apoptosis compared with
the Ctrl group (Fig. 3A). The
western blotting results also suggested OA-mediated induction of
cell apoptosis. Caspase-3, a key enzyme involved in apoptosis
(14), was significantly activated
in OA-treated osteosarcoma cells compared with control osteosarcoma
cells. In addition, OA treatment markedly reduced Bcl-2 expression
compared with the Ctrl group (Fig.
3B and C).
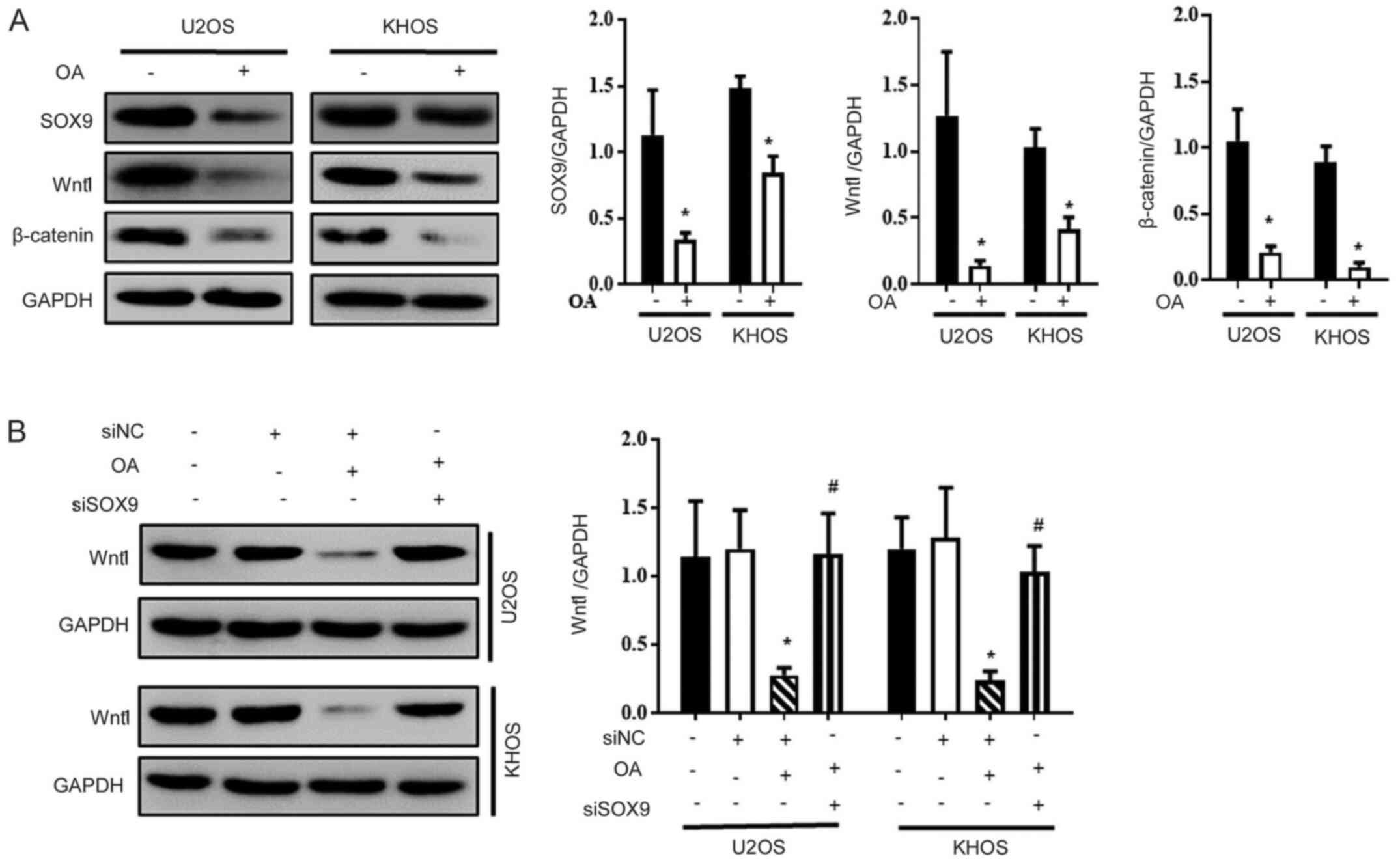
OA inactivates the SOX9/Wnt1 signaling
pathway in osteosarcoma cells
Subsequently, whether OA downregulated the SOX9/Wnt1
signaling pathway was investigated. The protein expression levels
of SOX9, β-catenin and Wnt1 were significantly decreased in
OA-treated U2OS and KHOS cells compared with Ctrl cells (Fig. 4A and B). Moreover, OA-mediated downregulation of
Wnt1 expression was significantly reversed by transfection with
SOX9 siRNA (Fig. 4C).
Discussion
The results of the present study were consistent
with the hypothesis that OA, the 3β-hydroxy-olean-12-en-28-oic acid
that is widely distributed in the plant kingdom as free acid or as
aglycone of triterpenoid saponins (13), reduced osteosarcoma cell
proliferation and invasion, and promoted osteosarcoma cell
apoptosis compared with the Ctrl group. Although OA displays
protective effects against inflammation and oxidative
stress-induced cell apoptosis (15), OA also displays a wide range of
anticancer pharmacological activities (16,17).
Similar to the results of the present study, previous studies also
demonstrated that OA inhibits cell proliferation and invasion, and
induces cell apoptosis in other tumor cells, such as thyroid,
prostate and breast cancer cells, as well as hepatocellular
carcinoma and glioblastoma cells (13,18-20).
OA serves an antioncogenic role during malignant
tumor development by inhibiting cancer cell proliferation,
migration and invasion, and triggering cell death via apoptosis,
autophagy or mitophagy (6,17,21).
Multiple signaling pathways are associated with OA-mediated
anticancer activities. Previous studies have indicated that OA
induces cancer cell apoptosis by inhibiting the Akt-mTOR signaling
cascade (22-24),
as well as ERK, STAT3 and NF-κB signaling pathways (21).
Previous studies have also reported that OA induces
osteosarcoma cell apoptosis and inhibits osteosarcoma cell
proliferation; however, the underlying mechanism is not completely
understood. Xu et al (6)
reported that OA inhibits osteosarcoma cell proliferation and
viability and interrupts the balance between proapoptotic and
antiapoptotic factors by inhibiting the Notch signaling pathway.
Moreover, the derivative of OA, N-formyl morpholine substituent of
CDDO (CDDO-NFM), leads to degradation of c-Myc and decreases
glucose uptake, lactate generation and adenosine triphosphate
production to block glycolysis (7).
In the present study, compared with the Ctrl group, OA inhibited
osteosarcoma cell proliferation and invasion, and enhanced cell
apoptosis by inactivating the SOX9/Wnt1 signaling pathway.
SOX9 is a member of the SOX family of transcription
factors, which is closely associated with the development of a
variety of malignant tumors (25).
SOX9 enhances tumorigenesis by reactivating Wnt signaling (26,27).
Moreover, the SOX9/Wnt1 signaling pathway also serves a key role in
osteosarcoma (3). Suh et al
(28) indicated that SOX9 regulates
hyperexpression of Wnt1 in human osteosarcoma tissues and cells,
and siRNA-mediated SOX9 knockdown inhibits human osteosarcoma cell
proliferation by downregulating Wnt1 expression. Furthermore, the
synthetic oleanane triterpenoids, CDDO-Imidazolide (CDDO-Im) and
CDDO-Ethyl amide (CDDO-EA), upregulate SOX9 expression in normal
cartilage and induce chondrogenic differentiation. OA displays a
contrary effect in normal and tumor cells (3,28-30);
however, the results of the present study indicated that the
expression levels of SOX9 and Wnt1 were significantly decreased in
OA-treated osteosarcoma cells compared with Ctrl cells, which
suggested that OA inhibited SOX9/Wnt1-associated osteosarcoma cell
proliferation, migration and invasion.
The mechanisms underlying OA-mediated regulation of
SOX9 expression have not been previously reported. Previous studies
have indicated that SOX9 activity is regulated via multiple layers,
including posttranslational modifications, such as Small
Ubiquitin-like Modifier (SUMO)ylation. SUMOylation represses the
gene transcriptional activity of SOX9, leading to decreased SOX9
expression (31-33).
Meanwhile, Momordin Ιc, an analog of OA, inhibits the activity of
SUMO-specific protease 1, a member of the de-SUMOylation protease
family (34), which might elevate
the level of SOX9 SUMOylation and decease SOX9 expression. Future
studies are required to identify the mechanisms underlying
OA-mediated regulation of SOX9 expression. The current study has
several limitations. Firstly, in vivo studies should be
performed to identify the role of OA in tumor growth. Secondly,
further research is required on the mechanisms that underlie the
role of OA in the inhibition of SOX9 expression and the
inactivation of the SOX9/Wnt1 signaling pathway.
In conclusion, the present study indicated that
OA-mediated inactivation of the SOX9/Wnt1 signaling pathway may
serve as a potential therapeutic strategy for osteosarcoma. The
results of the present study indicated that OA significantly
inhibited osteosarcoma cell proliferation, migration and invasion
compared with the Ctrl group; therefore, OA may display potent
antitumorigenic activities in osteosarcoma. For centuries, several
medicinal plant extracts containing OA have been used in Asian
countries for medical purposes (29) and have become registered drugs for
the treatment of liver diseases in China (30). Chemically modified OA has been
evaluated in several clinical trials for the treatment of various
types of cancer such as breast, colorectal and lung cancer among
others (8); however, further
clinical trials are required to confirm the potential therapeutic
application of OA.
Supplementary Material
Inhibitory effect of siSOX9. The
transfection efficiency of siRNA was determined by performing (A)
western blotting and (B) reverse transcription-quantitative PCR
(n=4). *P<0.05 vs. NC. si, small interfering RNA; NC,
negative control.
OA treatment does not inhibit
non-tumor osteoblastic cell viability, proliferation, migration and
invasion. (A) OA did not alter osteosarcoma cell viability, as
determined by performing the Cell Counting Kit-8 assay (n=8). (B)
OA did not alter osteosar-coma cell colony formation. Cell colonies
were stained using crystal violet and observed under a microscope
(magnification, x40; n=5). (C) Ki67 staining of osteosarcoma cells
cultured with or without OA treatment (scale bar, 40 μm;
n=5). Cell migration and invasion were assessed by performing (D)
wound healing and (E) Transwell assays, respectively
(magnification, x100; n=5). OA, oleanolic acid; Ctrl, control.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and ZW contributed to the conception or design of
the work and drafting the article or revising it for important
intellectual content. YZ, SZ, AW and QD performed the experiments
and contributed to the acquisition of data, analysis and
interpretation of data and drafting and revising the article. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sadykova LR, Ntekim AI, Muyangwa-Semenova
M, Rutland CS, Jeyapalan JN, Blatt N and Rizvanov AA: Epidemiology
and risk factors of osteosarcoma. Cancer Invest. 38:259–269.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Morrow JJ, Bayles I, Funnell APW, Miller
TE, Saiakhova A, Lizardo MM, Bartels CF, Kapteijn MY, Hung S,
Mendoza A, et al: Positively selected enhancer elements endow
osteosarcoma cells with metastatic competence. Nat Med. 24:176–185.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Liu H, Chen Y, Zhou F, Jie L, Pu L, Ju J,
Li F, Dai Z, Wang X and Zhou S: Sox9 regulates hyperexpression of
Wnt1 and Fzd1 in human osteosarcoma tissues and cells. Int J Clin
Exp Pathol. 7:4795–4805. 2014.PubMed/NCBI
|
|
4
|
Wang S, Zhang D, Han S, Gao P, Liu C, Li J
and Pan X: Fibulin-3 promotes osteosarcoma invasion and metastasis
by inducing epithelial to mesenchymal transition and activating the
Wnt/β-catenin signaling pathway. Sci Rep. 7(6215)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zou J, Zhang W and Li XL: Effects of SOST
gene silencing on proliferation, apoptosis, invasion, and migration
of human osteosarcoma cells through the wnt/β-catenin signaling
pathway. Calcif Tissue Int. 100:551–564. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu Y, Shu B, Tian Y, Wang G, Wang Y, Wang
J and Dong Y: Oleanolic acid induces osteosarcoma cell apoptosis by
inhibition of Notch signaling. Mol Carcinog. 57:896–902.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Gao F, Zuo Q, Jiang T, Song H and Zhou J:
A newly synthesized oleanolic acid derivative inhibits the growth
of osteosarcoma cells in vitro and in vivo by decreasing
c-MYC-dependent glycolysis. J Cell Biochem. 120:9264–9276.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Oleanolic acid and its synthetic
derivatives for the prevention and therapy of cancer: Preclinical
and clinical evidence. Cancer Lett. 346:206–216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fan X, Wang P, Sun Y, Jiang J, Du H, Wang
Z, Duan Z, Lei H and Li H: Oleanolic acid derivatives inhibit the
Wnt/β-catenin signaling pathway by promoting the phosphorylation of
beta-catenin in human SMMC-7721 cells. Pharmazie. 71:398–401.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang YY, Yang YX, Zhao R, Pan ST, Zhe H,
He ZX, Duan W, Zhang X, Yang T, Qiu JX and Zhou SF: Bardoxolone
methyl induces apoptosis and autophagy and inhibits
epithelial-to-mesenchymal transition and stemness in esophageal
squamous cancer cells. Drug Des Devel Ther. 9:993–1026.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Landers JE, Cassel SL and George DL:
Translational enhancement of mdm2 oncogene expression in human
tumor cells containing a stabilized wild-type p53 protein. Cancer
Res. 57:3562–3568. 1997.PubMed/NCBI
|
|
12
|
Rhim JS, Cho HY, Vernon ML, Arnstein P,
Huebner RJ and Gilden RV: Characterization of non-producer human
cells induced by Kirsten sarcoma virus. Int J Cancer. 16:840–849.
1975.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Duan L, Yang Z, Jiang X, Zhang J and Guo
X: Oleanolic acid inhibits cell proliferation migration and
invasion and induces SW579 thyroid cancer cell line apoptosis by
targeting forkhead transcription factor A. Anticancer Drugs.
30:812–820. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hotchkiss RS, Strasser A, McDunn JE and
Swanson PE: Cell death. N Engl J Med. 361:1570–1583.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang SL, Yang ZN, He C, Liao HB, Wang HS,
Chen ZF and Liang D: Oleanane-type triterpenoid saponins from
lysimachia fortunei maxim. Phytochemistry. 147:140–146.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Peng XP, Li XH, Li Y, Huang XT and Luo ZQ:
The protective effect of oleanolic acid on NMDA-induced MLE-12
cells apoptosis and lung injury in mice by activating SIRT1 and
reducing NF-kB acetylation. Int Immunopharmacol. 70:520–529.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Žiberna L, Šamec D, Mocan A, Nabavi SF,
Bishayee A, Farooqi AA, Sureda A and Nabavi SM: Oleanolic acid
alters multiple cell signaling pathways: Implication in cancer
prevention and therapy. Int J Mol Sci. 18(643)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu YY, Huang HY and Wu YL: Anticancer and
apoptotic activities of oleanolic acid are mediated through cell
cycle arrest and disruption of mitochondrial membrane potential in
HepG2 human hepatocellular carcinoma cells. Mol Med Rep.
12:5012–5018. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liese J, Hinrichs TM, Lange M and Fulda S:
Cotreatment with sorafenib and oleanolic acid induces reactive
oxygen species-dependent and mitochondrial-mediated apoptotic cell
death in hepatocellular carcinoma cells. Anticancer Drugs.
30:209–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim GJ, Jo HJ, Lee KJ, Choi JW and An JH:
Oleanolic acid induces p53-dependent apoptosis via the ERK/JNK/AKT
pathway in cancer cell lines in prostatic cancer xenografts in
mice. Oncotarget. 9:26370–26386. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhu B, Ren C, Du K, Zhu H, Ai Y, Kang F,
Luo Y, Liu W, Wang L, Xu Y, et al: Olean-28,13b-olide 2 plays a
role in cisplatin-mediated apoptosis and reverses cisplatin
resistance in human lung cancer through multiple signaling
pathways. Biochem Pharmacol. 170(113642)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Khan MW, Zhao P, Khan A, Raza F, Raza SM,
Sarfraz M, Chen Y, Li M, Yang T, Ma X and Xiang G: Synergism of
cisplatin-oleanolic acid co-loaded calcium carbonate nanoparticles
on hepatocellular carcinoma cells for enhanced apoptosis and
reduced hepatotoxicity. Int J Nanomedicine. 14:3753–3771.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shi Y, Song Q, Hu D, Zhuang X, Yu S and
Teng D: Oleanolic acid induced autophagic cell death in
hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent
pathway. Korean J Physiol Pharmacol. 20:237–243. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nie H, Wang Y, Qin Y and Gong XG:
Oleanolic acid induces autophagic death in human gastric cancer
cells in vitro and in vivo. Cell Biol Int. 40:770–778.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khurana N and Sikka SC: Interplay between
SOX9, Wnt/β-catenin and androgen receptor signaling in
castration-resistant prostate cancer. Int J Mol Sci.
20(2066)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu Y, Yin W, Yu ZH, Zhou YJ, Chi JR, Ge J
and Cao XC: miR-190 enhances endocrine therapy sensitivity by
regulating SOX9 expression in breast cancer. J Exp Clin Cancer Res.
38(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Domenici G, Aurrekoetxea-Rodriguez I,
Simoes BM, Rábano M, Lee SY, Millán JS, Comaills V, Oliemuller E,
López-Ruiz JA, Zabalza I, et al: A Sox2-Sox9 signalling axis
maintains human breast luminal progenitor and breast cancer stem
cells. Oncogene. 38:3151–3169. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Suh N, Paul S, Lee HJ, Yoon T, Shah N, Son
AI, Reddi AH, Medici D and Sporn MB: Synthetic triterpenoids,
CDDO-Imidazolide and CDDO-Ethyl amide, induce chondrogenesis.
Osteoarthritis Cartilage. 20:446–450. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Borella R, Forti L, Gibellini L, De
Gaetano A, De Biasi S, Nasi M, Cossarizza A and Pinti M: Synthesis
and anticancer activity of CDDO and CDDO-Me, two derivatives of
natural triterpenoids. Molecules. 24(4097)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin C, Wen X and Sun H: Oleanolic acid
derivatives for pharmaceutical use: A patent review. Expert Opin
Ther Pat. 26:643–655. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oh HJ, Kido T and Lau YF: PIAS1 interacts
with and represses SOX9 transactivation activity. Mol Reprod Dev.
74:1446–1455. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hattori T, Eberspaecher H, Lu J, Zhang R,
Nishida T, Kahyo T, Yasuda H and de Crombrugghe B: Interactions
between PIAS proteins and SOX9 result in an increase in the
cellular concentrations of SOX9. J Biol Chem. 281:14417–14428.
2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saotome H, Ito A, Kubo A and Inui M:
Generation of a quantitative luciferase reporter for Sox9
SUMOylation. Int J Mol Sci. 21(1274)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu J, Lei H, Zhang J, Chen X, Tang C, Wang
W, Xu H, Xiao W, Gu W and Wu Y: Momordin Ic, a new natural SENP1
inhibitor, inhibits prostate cancer cell proliferation. Oncotarget.
7:58995–59005. 2016.PubMed/NCBI View Article : Google Scholar
|