Introduction
Ginseng (Panax ginseng) is a traditional
Chinese herbal medicine that has been widely used for thousands of
years in East Asia, including China, Korea and Bhutan (1). The main components responsible for its
therapeutic effects are ginsenosides, which are divided into
protopanaxadiol and protopanaxatriol ginsenoside groups (2). Ginsenoside Rg2 is one of the compounds
in the protopanaxatriol group (3).
Ginsenoside Rg2 improved neurological performance and enhanced
memory by inhibiting neuronal apoptosis in a rat model of vascular
dementia (3). Ginsenoside Rg2
improved neuronal and metabolic activities by inducing autophagy in
a protein kinase AMP-activated catalytic subunit α2 (AMPK)/unc-51
like autophagy activating kinase 1-dependent and mTOR-independent
manner (4). Furthermore,
ginsenoside Rg2 increased autophagy in MCF-7 cells in vitro
(5). Additionally, it was reported
that ginsenoside Rg2 exhibited protective effects against hydrogen
peroxide-induced injury and apoptosis in human cardiomyocytes
(HCMs) (6).
Trastuzumab (TZM) is a humanized monoclonal antibody
that targets the extracellular domain of human epidermal growth
factor receptor 2 (HER2) and exerts significant therapeutic effects
in early-stage HER2-positive breast cancer (7). TZM significantly improves the overall
survival of the majority of patients with HER2-positive breast
cancer (8). However, despite its
beneficial effects, TZM is associated with several cardiac side
effects, including congestive heart failure, hypertension,
thromboembolic disease, ischemic heart disease, QT prolongation and
bradycardia (9,10).
The present study aimed to investigate whether
ginsenoside Rg2 could protect HCMs against TZM-induced toxicity
in vitro. The results may improve the care of patients with
breast cancer treated with TZM.
Materials and methods
Cell culture
Human primary HCMs (Applied Biological Materials,
Inc.) were cultured in DMEM (Sigma-Aldrich; Merck KGaA) containing
10% FBS (Thermo Fisher Scientific, Inc.) and 1%
streptomycin-penicillin, and maintained at 37˚C and 5%
CO2. Upon reaching 80% confluency, HCMs were pretreated
with 200 µM ginsenoside Rg2 [purity >98% (by high-performance
liquid chromatography analysis); MedChemExpress] for 12 h at 37˚C,
followed by exposure to 100 µg/ml TZM (Roche Diagnostics) for 24 h
at 37˚C.
Cell proliferation assay
HCMs (5x103 cells/well) were seeded in a
96-well plate in triplicate. The cells were subsequently treated
with TZM (0, 10, 50, 100 or 200 µg/ml), ginsenoside Rg2 (0, 50,
100, 200, or 300 µM), a combination of 200 µM ginsenoside Rg2 and
100 µg/ml TZM or a combination of ginsenoside 200 µM Rg2, 100 µg/ml
TZM and 5 mM 3-methyladenine (3-MA; Sigma-Aldrich; Merck KGaA) for
24 h at 37˚C. Following treatment, the cells were further incubated
with 10 µl Cell Counting Kit-8 (CCK-8) solution (Dojindo Molecular
Technologies, Inc.) at 37˚C for 3 h and the absorbance was measured
at a wavelength of 490 nm using a microplate reader. The cells in
the control group were not given any treatment. IC50
values were determined using GraphPad Prism software (version 7.0;
GraphPad Software, Inc.).
Apoptosis assay
Cell apoptosis was detected using a propidium iodide
(PI) and Annexin V staining kit (BD Biosciences) according to the
manufacturer's protocol. Briefly, 2x105 HCMs were seeded
in a 6-well plate and then treated with 50 or 100 µg/ml TZM for 24
h and were subsequently harvested and washed twice with cold PBS.
Subsequently, the cells were resuspended in binding buffer and
stained with 2 µl Annexin V and 2 µl PI for 15 min at 25˚C in the
dark. The cell apoptosis rate was determined using a FACSCanto II
flow cytometer (BD Biosciences) and BD CellQuest™ Pro
software (version 5.1; BD Biosciences).
Western blotting
HCMs were rinsed and lysed using RIPA lysis buffer
(EMD Millipore) containing a protease and phosphatase inhibitor.
Subsequently, the cell lysates were vortexed on ice five times
within 20 min and centrifuged for 10 min at 10,000 x g at 4˚C. The
protein concentration was determined using a bicinchoninic acid
protein quantification kit (Promega Corporation). Protein aliquots
(30 µg) were subjected to SDS-PAGE on a 10% gel and subsequently
transferred onto PVDF membranes (EMD Millipore). The membranes were
blocked using 5% non-fat milk for 1 h at room temperature. The
membranes were then incubated with the following primary
antibodies: Anti-phosphorylated (p)-AKT (cat. no. ab38449),
anti-p-mTOR (cat. no. ab84400), anti-autophagy protein 5 (ATG5,
cat. no. ab109490), anti-beclin 1 (cat. no. ab210498),
anti-microtubule associated protein 1 light chain 3α (LC3, cat. no.
ab62721) overnight at 4˚C. All primary antibodies were used at a
1:200 dilution and were purchased from Abcam. Following washing
with PBS, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (Abcam) for 1 h at room
temperature. An ECL reagent kit (Santa Cruz Biotechnology, Inc.)
was used to visualize the immunoreactive bands according to the
manufacturer's protocol. Protein band intensities were quantified
using ImageJ software (v1.8.0.112; National Institutes of Health).
β-actin (1:200 dilution; cat. no. ab8227; Abcam) acted as the
internal control.
Immunofluorescence staining
Following exposure to the aforementioned treatments,
HCMs were washed with PBS three times and fixed with 100% methanol
for 10 min at room temperature. Next, cells were washed three times
with PBS and permeabilized with 1% Triton X-100 (Sigma-Aldrich;
Merck KGaA) for 10 min at room temperature. The cells were
subsequently blocked with 4% BSA (Sigma-Aldrich; Merck KGaA) in PBS
for 1 h at room temperature and incubated with primary antibodies
against Ki67 (1 µg/ml; cat. no. ab15580; Abcam) or LC3 (1 µg/ml;
cat. no. ab192890; Abcam) for 2 h at 4˚C. Following primary
antibody incubation, cells were incubated with a FITC-conjugated
anti-rabbit IgG secondary antibody (1:5,000; cat. no. 150077;
Abcam) for 1 h at room temperature. Finally, the cells were washed
three times with PBS and stained with DAPI (Vector Laboratories,
Inc.) for 5 min at room temperature. The cells were imaged using a
fluorescence microscope (magnification, x200) and the number of
nuclei and Ki67-postive cells were counted in three
randomly-selected fields.
Monodansylcadaverine (MDC)
staining
A total of 2x105 HCMs were seeded in a
6-well plate and then treated with different concentrations of TZM
or a combination of TZM and ginsenoside Rg2. Cells were then
labeled with MDC (50 µM) in PBS for 10 min at 37˚C in the dark.
After washing with PBS three times, cells were fixed in 4% PFA for
30 min at room temperature. Subsequently, the cells were visualized
under a fluorescence microscope (magnification, x200).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 17.0; SPSS, Inc.). Data are expressed as the mean
± SD of three replicates. Comparisons among multiple groups were
made with the one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TZM inhibits the proliferation of HCMs
by inhibiting the Akt/mTOR signaling pathway
HCMs were treated with increasing concentrations of
TZM (0, 10, 50, 100 or 200 µg/ml) for 24 h, and the effects of TZM
on cell proliferation were measured using a CCK-8 assay. The
results revealed that 50, 100 or 200 µg/ml TZM significantly
decreased cell proliferation compared with the control group
(Fig. 1A). These data suggested
that TZM inhibited the proliferation of HCMs in a dose-dependent
manner, with an IC50 value of 88 µg/ml. Annexin V/PI
staining was subsequently performed to determine the percentage of
apoptotic cells following treatment with 50 or 100 µg/ml TZM. As
indicated in Fig. 1B and C, a significant increase in apoptotic
cells was observed in TZM-treated cells compared with the controls.
Furthermore, western blotting revealed that the levels of p-Akt and
p-mTOR in cells were significantly decreased following treatment
with TZM compared with controls (Fig.
1D-F). Therefore, 100 µg/ml TZM was used for subsequent
experiments. These results suggested that TZM could induce
apoptosis of HCMs by inhibiting the Akt/mTOR signaling pathway.
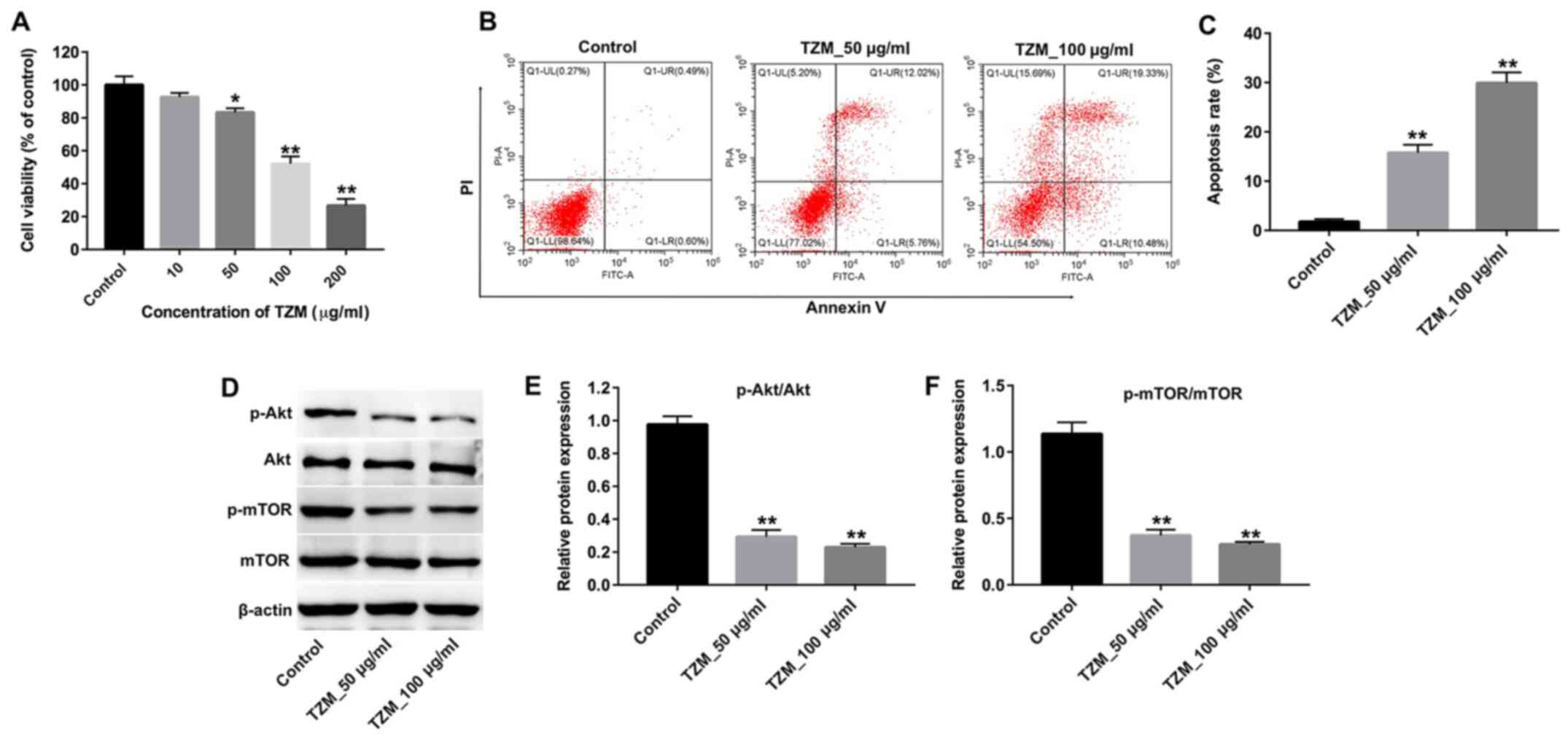 | Figure 1TZM inhibits the proliferation of HCMs
by inhibiting the Akt/mTOR signaling pathway. (A) HCMs were treated
with different concentrations of TZM (0, 10, 50, 100 or 200 µg/ml)
for 24 h, and cell proliferation was examined using a Cell Counting
Kit-8 assay. (B) HCMs were incubated with 0, 50 or 100 µg/ml TZM
for 24 h, and apoptotic cells were detected with Annexin V/PI
staining and flow cytometry. (C) Quantification of Annexin
V-positive cells. (D) Cells were treated with 0, 50 or 100 µg/ml
TZM for 24 h, and the expression of p-Akt and p-mTOR protein was
measured by western blotting. The relative levels of (E) p-Akt and
(F) p-mTOR were normalized to Akt and mTOR, respectively and
quantified using ImageJ software. Data were representative of three
separate experiments. *P<0.05 and
**P<0.01 vs. the control group. TZM, trastuzumab;
HCM, human cardiomyocyte; PI, propidium iodide; p,
phosphorylated. |
TZM-induced cytotoxicity in HCMs is
reversed by ginsenoside Rg2
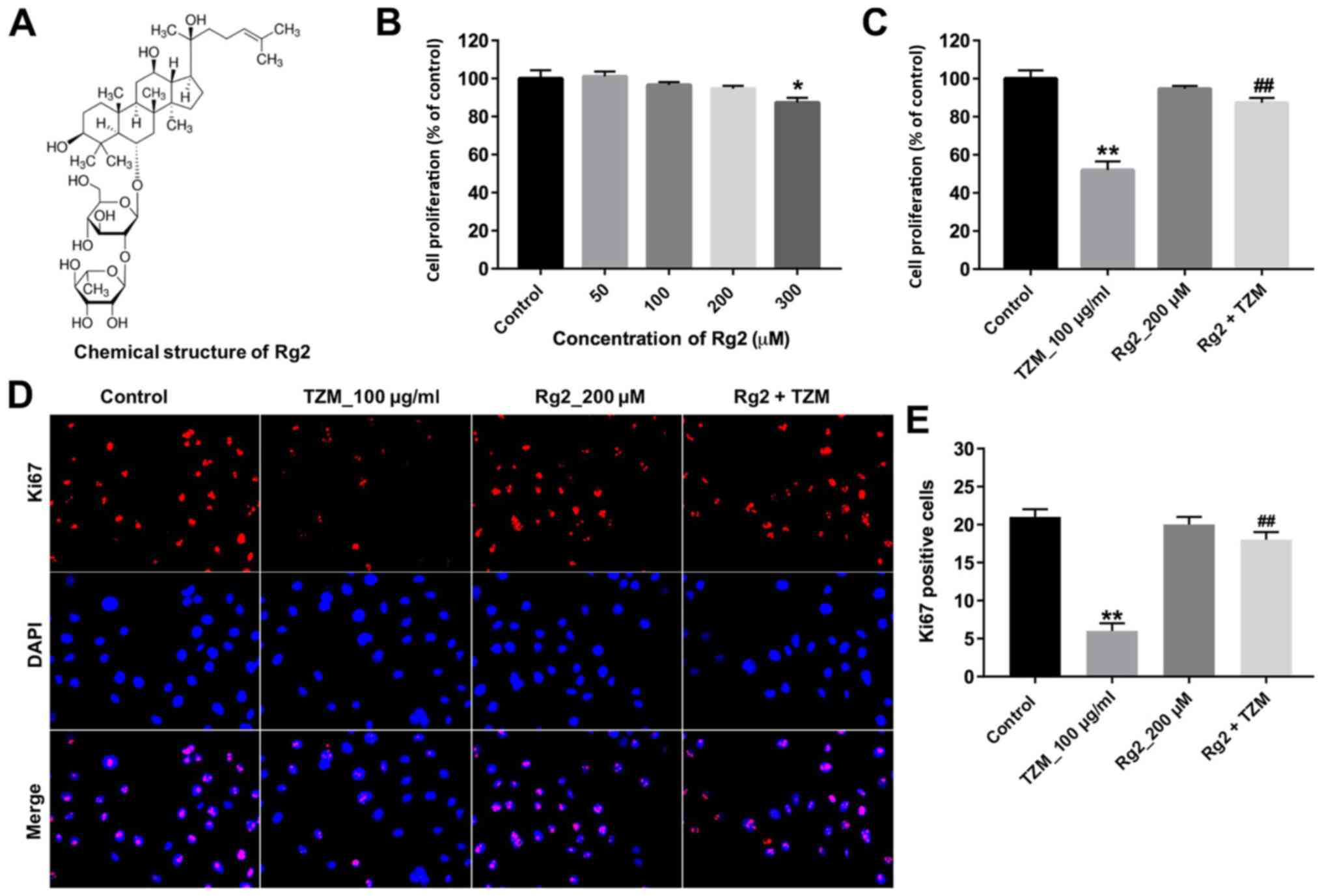
The chemical structure of ginsenoside Rg2 is
presented in Fig. 2A. A CCK-8 assay
was performed to evaluate the effects of ginsenoside Rg2 or the
combination of ginsenoside Rg2 and TZM on HCM proliferation. The
results indicated that ginsenoside Rg2 (at concentrations between
0-200 µM) exhibited no cytotoxicity (Fig. 2B). Therefore, 200 µM ginsenoside Rg2
was used for subsequent experiments. Pretreatment with ginsenoside
Rg2 significantly reversed TZM-induced cytotoxicity compared with
TZM alone (Fig. 2C).
HCMs were subsequently subjected to
immunofluorescence staining. Ki67 expression was significantly
increased in the ginsenoside Rg2-pretreated group compared with the
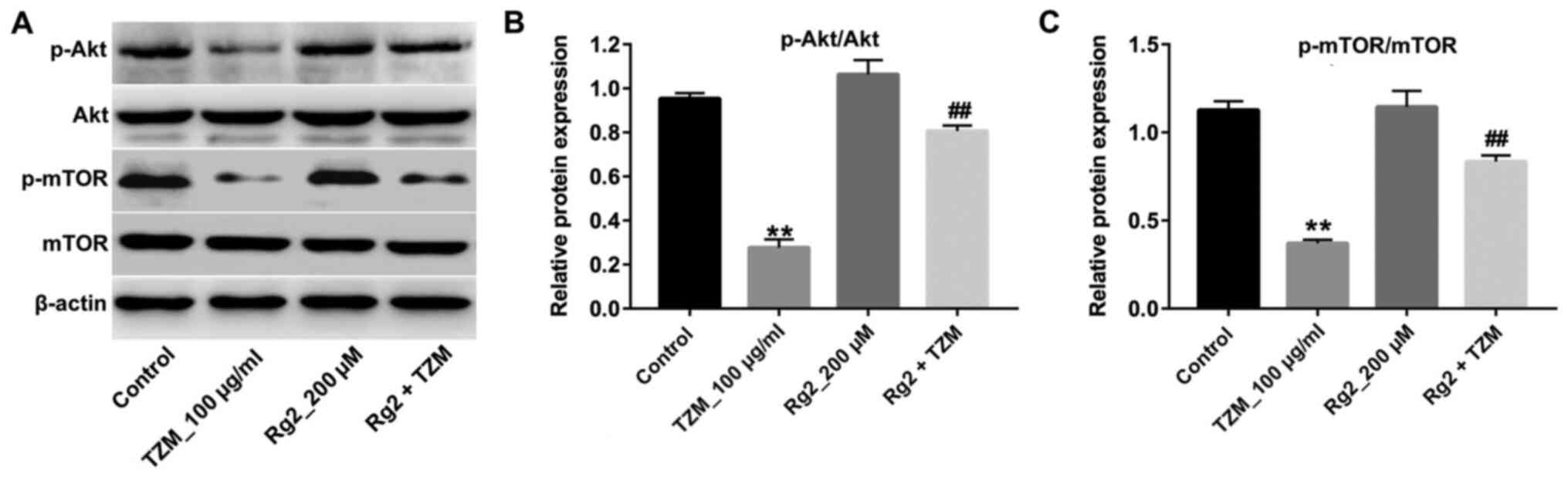
TZM group (Fig. 2D and E). Furthermore, TZM-induced p-Akt and
p-mTOR downregulation was significantly reversed by ginsenoside Rg2
compared with the TZM group (Fig.
3A-C). Collectively, these results suggested that ginsenoside
Rg2 may significantly alleviate TZM-induced cardiotoxicity by
upregulating the Akt/mTOR signaling pathway.
Ginsenoside Rg2 induces autophagy of
HCMs
MDC is a selective fluorescent marker that labels
autophagic vacuoles (11). To
further explore the mechanisms underlying the protective effect of
ginsenoside Rg2 against TZM cytotoxicity, MDC fluorescence staining
was performed. The results revealed that MDC fluorescence was
barely detected in HCMs incubated with TZM (Fig. 4A and B), whereas strong MDC fluorescence was
observed in ginsenoside Rg2-treated cells (Fig. 4C and D). Furthermore, the combination of
ginsenoside Rg2 and TZM significantly increased the levels of MDC
fluorescence compared with the control group, which were
subsequently significantly decreased in the presence of the
autophagy inhibitor 3-MA (Fig. 4E
and F). Therefore, the results
suggested that ginsenoside Rg2 may significantly induce autophagy
in HCMs.
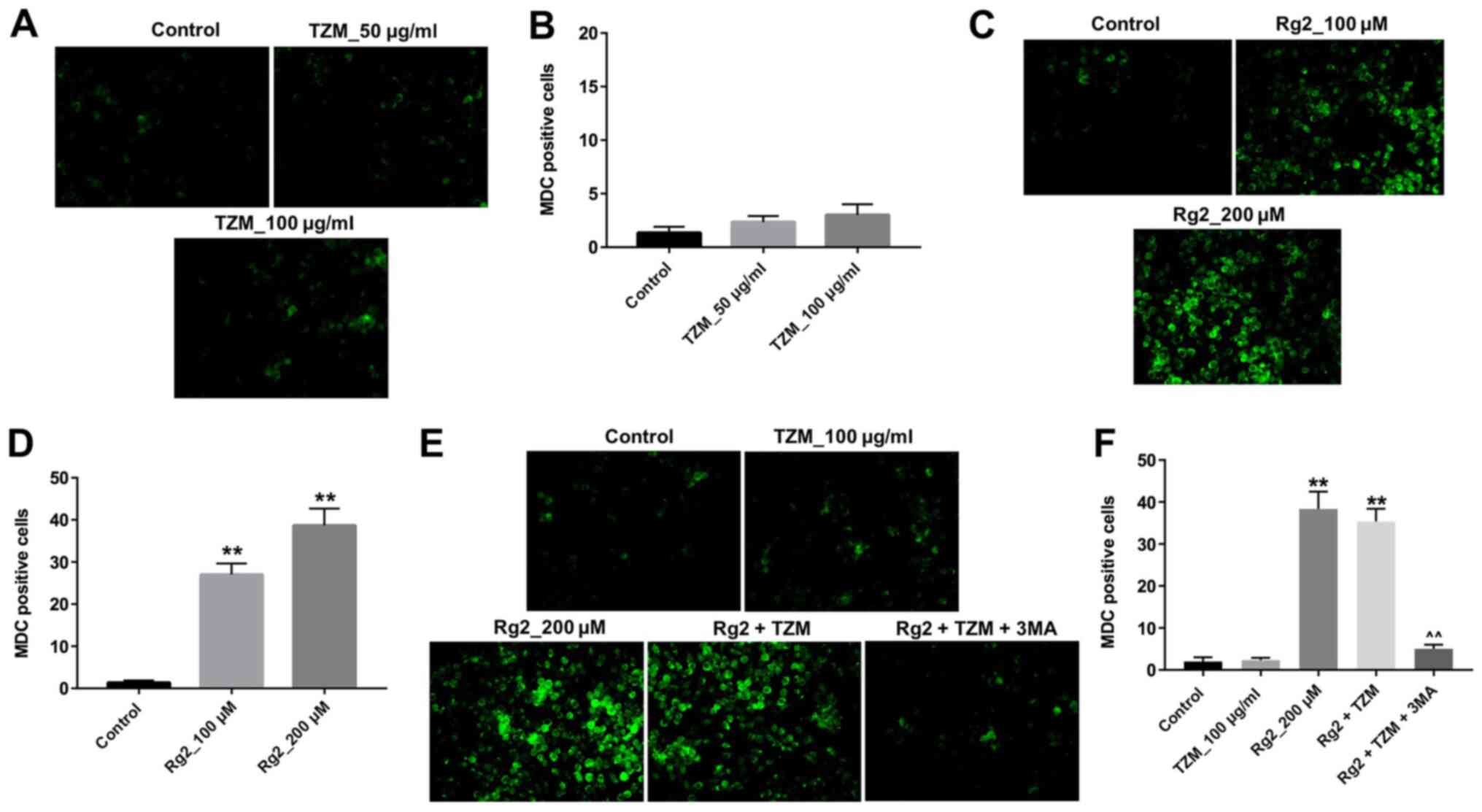 | Figure 4Ginsenoside Rg2 induces autophagy of
HCMs. (A) HCMs were labeled with MDC after incubation with 0, 50 or
100 µg/ml TZM for 24 h. Magnification, x200. (B) Quantitative
analysis of MDC-positive cells in (A). (C) HCMs were labeled with
MDC after incubation with 0, 100 or 200 µM ginsenoside Rg2 for 12
h. Magnification, x200. (D) Quantitative analysis of MDC-positive
cells in (C). (E) HCMs were exposed to 200 µM ginsenoside Rg2 with
or without 5 mM 3-MA for 12 h and exposed to 100 µg/ml TZM for
another 24 h. Cell autophagy was then detected with MDC staining.
Magnification, x200. (F) Quantitative analysis of MDC-positive
cells from. (E) Data are representative of three independent
experiments. **P<0.01 vs. the control group.
^^P<0.01 vs. the Rg2 + TZM group. TZM, trastuzumab; HCM, human
cardiomyocyte; MDC, monodansylcadaverine; 3-MA, 3-methyladenine;
Rg2, ginsenoside Rg2. |
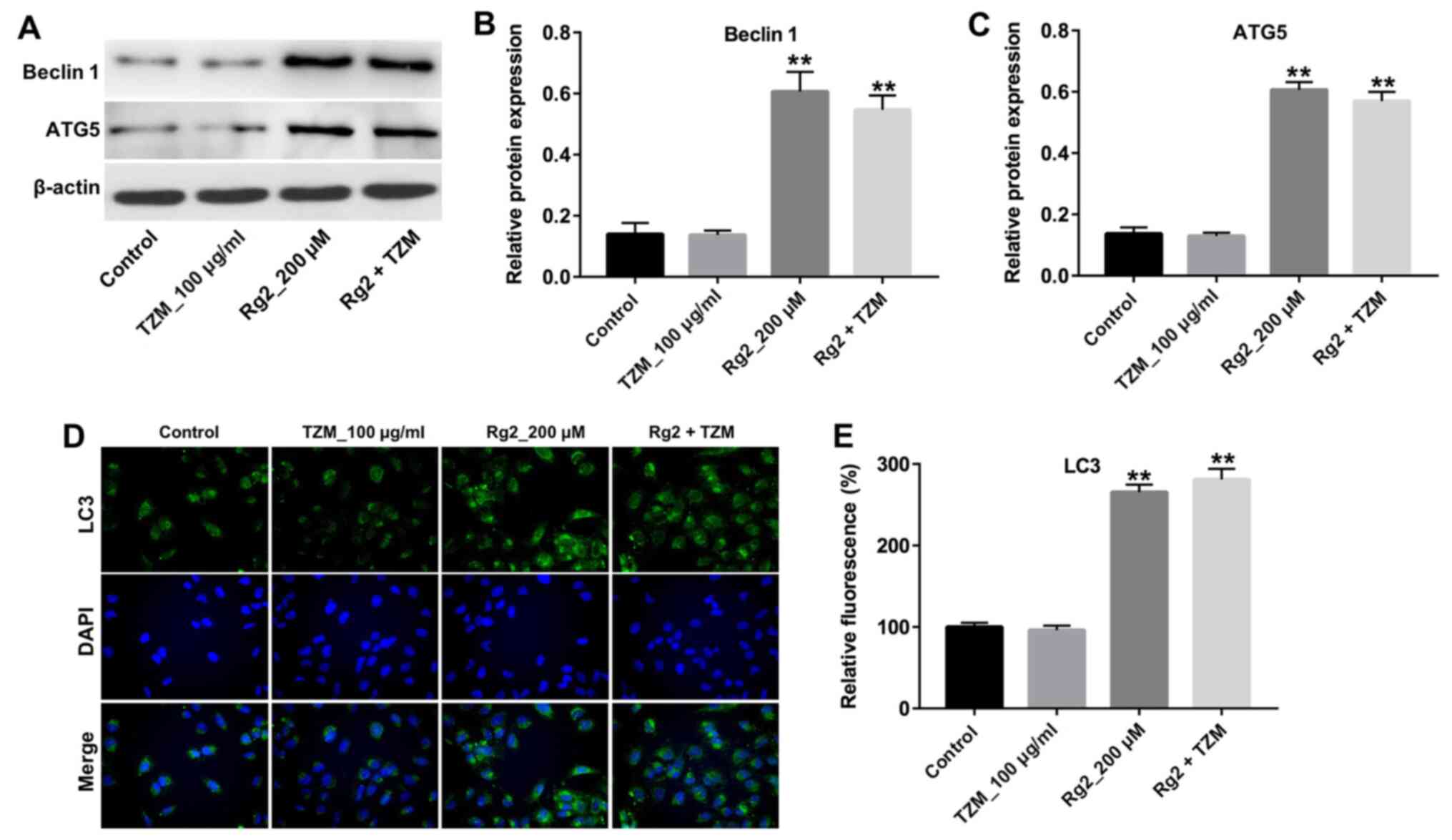
Ginsenoside Rg2 increases the
expression of the autophagy-related proteins beclin 1, LC3 and ATG5
in HCMs
To further explore the mechanisms underlying
ginsenoside Rg2-induced autophagy, the expression of the key
autophagy markers beclin 1, LC3 and ATG5 was detected by western
blotting. As indicated in Fig.
5A-C, treatment with TZM did not significantly affect the
expression of beclin 1 and ATG5 in cells compared with controls;
however, the levels of beclin1 and ATG5 were significantly
increased in ginsenoside Rg2-treated cells compared with controls.
In addition, the results of immunofluorescence staining indicated
the nuclear expression of LC3 was significantly increased following
Rg2 treatment (Fig. 5D and E). These data further demonstrated that
ginsenoside Rg2 increased autophagy in HCMs by increasing the
expression of the autophagy-related proteins beclin 1, LC3 and
ATG5.
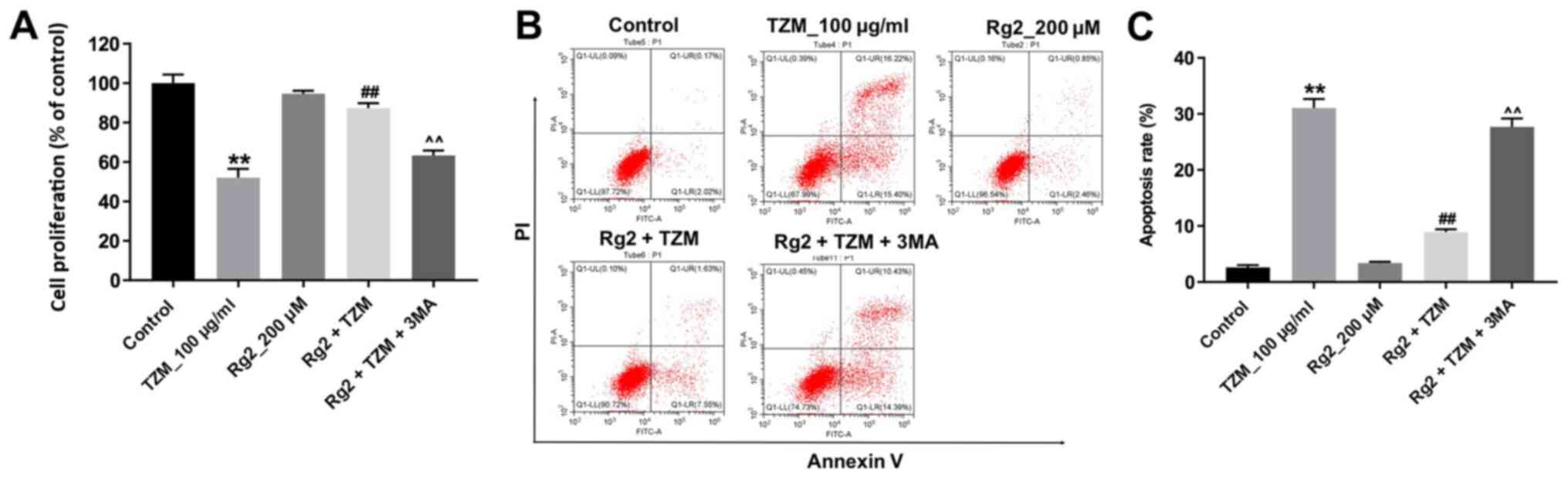
Inhibition of autophagy in HCMs
abolished the protective effect of ginsenoside Rg2 against TZM
To verify whether ginsenoside Rg2 exerted a
protective effect against TZM in HCMs by inducing autophagy, the
effects of the autophagy inhibitor 3-MA were investigated. As
indicated in Fig. 6A, the
protective effects of ginsenoside Rg2 against TZM-induced
cytotoxicity in HCMs were reversed by 3-MA. Meanwhile, the
antiapoptotic effects of ginsenoside Rg2 in TZM-stimulated HCMs
were also alleviated by 3-MA (Fig.
6B and C). These data indicated
that ginsenoside Rg2 could attenuate TZM-induced cytotoxicity in
HCMs by inducing autophagy.
Discussion
The present study established an in vitro
model of TZM- induced toxicity in HCMs. TZM decreased cell
proliferation, increased apoptosis and decreased the expression of
p-Akt and p-mTOR. However, TZM-induced cytotoxicity and p-Akt and
p-mTOR downregulation were reversed by ginsenoside Rg2. In
addition, ginsenoside Rg2 significantly induced autophagy in HCMs
by increasing the levels of beclin 1, LC3 and ATG5. The present
study revealed that ginsenoside Rg2 may protect HCMs from
TZM-induced toxicity by activating autophagy.
It was hypothesized that ginsenoside Rg2 has
antioxidant, antidiabetic, antiapoptotic and neuroprotective
activities (3,12,13). A
previous study reported that ginsenoside Rg2 exerted protective
effects against hydrogen peroxide-induced injury and apoptosis in
HCMs (6). Furthermore, ginsenoside
Rg2 increased autophagy and activated the p53/AMPK signaling
pathway in MCF-7 breast cancer cells (5). Moreover, ginsenoside Rg2 decreased
lipopolysaccharide-induced Bax and caspase-3 and -9 expression, and
exerted an antiapoptotic effect in neurons (14). Kang et al (15) found that ginsenoside Rg2 could
protect HaCaT cells from UV-B-induced cell damage. Meanwhile, Wang
et al (16) indicated that
ginsenoside Rg2 could inhibit the proliferation of breast cancer
cells in vitro. The aforementioned studies indicated that
ginsenoside Rg2 exhibited dual functions in protecting cells from
DNA damage and inducing apoptosis in cancer cells. The present
study investigated whether pretreatment with ginsenoside Rg2 could
reverse TZM-induced toxicity in HCMs. The results revealed that
ginsenoside Rg2 exhibited protective effects against TZM-induced
toxicity by inducing autophagy in HCMs. Moreover, to the best of
our knowledge, the present study was the first to demonstrate that
ginsenoside Rg2 exhibited protective effects against TZM-induced
toxicity in HCMs.
Beclin 1, LC3 and ATG5 are autophagy-related
proteins required for autophagosome elongation (17). In the present study, pretreatment
with ginsenoside Rg2 increased the levels of beclin 1, LC3 and
ATG5, which indicated that TZM-induced toxicity was reversed by
ginsenoside Rg2 by increasing autophagy. A previous study revealed
that p-53 and p-AMPK were involved in ginsenoside Rg2-induced
autophagy in MCF-7 cells (5).
However, in the present study, pretreatment with ginsenoside Rg2
reversed TZM-induced downregulation of p-Akt and p-mTOR in HCMs. It
was reported that canonical autophagy requires mTOR inhibition
(18). Moreover, the Akt/mTOR
signaling pathway is one of the main downstream effectors of
HER2(19). Previous studies
demonstrated that TZM exerted antitumor activities by inhibiting
the Akt/mTOR signaling pathway in HER2-overexpressing breast cancer
cells (20,21). In the present study, treatment with
ginsenoside Rg2 alone had no effect on the Akt/mTOR signaling
pathway in HCMs. The inhibition of the Akt/mTOR signaling pathway
induced by TZM was reversed by ginsenoside Rg2. Evidence has shown
that the AKT/mTOR pathway plays an important role in several
cellular processes, including proliferation, survival and autophagy
(22). Hu et al (23) found that inhibition of autophagy
promoted advanced glycation end product-induced apoptosis in
cardiomyocytes by inhibiting the AKT/mTOR signaling. In the present
study, TZM-induced p-Akt and p-mTOR protein decreases were
significantly reversed by ginsenoside Rg2 treatment, indicating
that ginsenoside Rg2 could induce autophagy of HCM cells by
activating the AKT/mTOR pathway. Meanwhile, the anti-apoptotic
effects of ginsenoside Rg2 in TZM-stimulated HCMs cells were
reversed by 3-MA treatment, indicating that inhibition of autophagy
abolished the protective effect of ginsenoside Rg2 against TZM in
HCMs. The results suggested that the mechanism by which ginsenoside
Rg2 protected against TZM-induced cardiotoxicity was reversed by
inhibition of the Akt/mTOR signaling pathway.
Collectively, the results obtained in the present
study revealed that ginsenoside Rg2 exhibited protective effects
against TZM-induced toxicity in HCMs by activating autophagy and
increasing the expression of Akt and mTOR. Ginsenoside Rg2 may
serve as a potential clinical agent to prevent TZM-related
cardiotoxicity and may provide significant beneficial effects for
patients with breast cancer. However, the data presented in this
study requires further in vivo validation prior to the
clinical application of ginsenoside Rg2.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL made major contributions to the conception,
design and manuscript drafting of this study. GL, XL and FS were
responsible for data acquisition, data analysis, data
interpretation and manuscript revision. XQ made substantial
contributions to conception and design of the study and revised the
manuscript. All authors agreed to be accountable for all aspects of
the work. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu QM, Jia D, Gao HW, Zhang MM, He WJ, Pan
S, Liu YL, Li XR, Cui JH and Yang SL: In vitro and in vivo
protective effects of gingenosides on acute renal injury induced by
cantharidin. J Functional Foods. 5:2012–2018. 2013.
|
|
2
|
Chen XJ, Zhang XJ, Shui YM, Wan JB and Gao
JL: Anticancer activities of protopanaxadiol- and
protopanaxatriol-type ginsenosides and their metabolites. Evid
Based Complement Alternat Med. 2016(5738694)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang G, Liu A, Zhou Y, San X, Jin T and
Jin Y: Panax ginseng ginsenoside-Rg2 protects memory
impairment via anti-apoptosis in a rat model with vascular
dementia. J Ethnopharmacol. 115:441–448. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fan Y, Wang N, Rocchi A, Zhang W, Vassar
R, Zhou Y and He C: Identification of natural products with
neuronal and metabolic benefits through autophagy induction.
Autophagy. 13:41–56. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chung Y, Jeong S, Choi HS, Ro S, Lee JS
and Park JK: Upregulation of autophagy by Ginsenoside Rg2 in MCF-7
cells. Anim Cells Syst (Seoul). 22:382–389. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fu W, Sui D, Yu X, Gou D, Zhou Y and Xu H:
Protective effects of ginsenoside Rg2 against
H2O2-induced injury and apoptosis in H9c2
cells. Int J Clin Exp Med. 8:19938–19947. 2015.PubMed/NCBI
|
|
7
|
Gershon N, Berchenko Y, Hall PS and
Goldstein DA: Cost effectiveness and affordability of trastuzumab
in sub-Saharan Africa for early stage. Cost Eff Resour Alloc.
17(5)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Slamon D, Eiermann W, Robert N, Pienkowski
T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et
al: Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J
Med. 365:1273–1283. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mazzotta M, Krasniqi E, Barchiesi G,
Pizzuti L, Tomao F, Barba M and Vici P: Long-term safety and
real-world effectiveness of trastuzumab in breast cancer. J Clin
Med. 8(254)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sato A, Yoshihisa A, Miyata-Tatsumi M,
Oikawa M, Kobayashi A, Ishida T, Ohtake T and Takeishi Y: Valvular
heart disease as a possible predictor of trastuzumab-induced
cardiotoxicity in patients with breast cancer. Mol Clin Oncol.
10:37–42. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Biederbick A, Kern HF and Elsässer HP:
Monodansylcadaverine (MDC) is a specific in vivo marker for
autophagic vacuoles. Eur J Cell Biol. 66:3–14. 1995.PubMed/NCBI
|
|
12
|
Jeong SJ, Han SH, Kim DY, Lee JC, Kim HS,
Kim BH, Lee JS, Hwang EH and Park JK: Effects of mRg2, a mixture of
ginsenosides containing 60% Rg2, on the ultraviolet B-induced DNA
repair synthesis and apoptosis in NIH3T3 cells. Int J Toxicol.
26:151–158. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ye J, Yao JP, Wang X, Zheng M, Li P, He C,
Wan JB, Yao X and Su H: Neuroprotective effects of ginsenosides on
neural progenitor cells against oxidative injury. Mol Med Rep.
13:3083–3091. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chung YH, Jeong SA, Choi HS, Ro S, Lee JS
and Park JK: Protective effects of ginsenoside Rg2 and astaxanthin
mixture against UVB-induced DNA damage. Anim Cells Syst (Seoul).
22:400–406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kang HJ, Huang YH, Lim HW, Shin D, Jang K,
Lee Y, Kim K and Lim CJ: Stereospecificity of ginsenoside Rg2
epimers in the protective response against UV-B radiation-induced
oxidative stress in human epidermal keratinocytes. J Photochem
Photobiol B. 165:232–239. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang CZ, Aung HH, Zhang B, Sun S, Li XL,
He H, Xie JT, He TC, Du W and Yuan CS: Chemopreventive effects of
heat-processed Panax quinquefolius root on human breast cancer
cells. Anticancer Res. 28:2545–2551. 2008.PubMed/NCBI
|
|
17
|
Mondaca-Ruff D, Riquelme JA, Quiroga C,
Norambuena-Soto I, Sanhueza-Olivares F, Villar-Fincheira P,
Hernández-Díaz T, Cancino-Arenas N, San Martin A, García L, et al:
Angiotensin II-regulated autophagy is required for vascular smooth
muscle cell hypertrophy. Front Pharmacol. 9(1553)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gatica D, Chiong M, Lavandero S and
Klionsky DJ: Molecular mechanisms of autophagy in the
cardiovascular system. Circ Res. 116:456–467. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sukawa Y, Yamamoto H, Nosho K, Ito M,
Igarashi H, Naito T, Mitsuhashi K, Matsunaga Y, Takahashi T, Mikami
M, et al: HER2 expression and PI3K-Akt pathway alterations in
gastric cancer. Digestion. 89:12–17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang Y, Ren F, Tian Z, Song W, Cheng B and
Feng Z: Osthole synergizes with HER2 inhibitor, trastuzumab in
HER2-overexpressed N87 Gastric cancer by inducing apoptosis and
inhibition of AKT-MAPK pathway. Front Pharmacol.
9(1392)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Han S, Meng Y, Tong Q, Li G, Zhang X, Chen
Y, Hu S, Zheng L, Tan W, Li H, et al: The ErbB2-targeting antibody
trastuzumab and the small-molecule SRC inhibitor saracatinib
synergistically inhibit ErbB2-overexpressing gastric cancer. MAbs.
6:403–408. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
143:3050–3060. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hu P, Zhou H, Lu M, Dou L, Bo G, Wu J and
Huang S: Autophagy plays a protective role in advanced glycation
end product-induced apoptosis in cardiomyocytes. Cell Physiol
Biochem. 37:697–706. 2015.PubMed/NCBI View Article : Google Scholar
|