Introduction
To date, effective treatments, such as negative
pressure wound therapy (NPWT), are commonly used to treat various
intractable wounds (1-4).
NPWT produces a conducive microenvironment for the stimulation of
granulation tissue and subsequent wound healing via open-cell foam
dressing and negative pressure (5).
Several mechanisms of action underlying NPWT have been proposed,
including the reduction of wound tissue edema and bacterial
colonization, promotion of cell proliferation and increased local
blood perfusion (5-8).
In addition, NPWT is used as an adjuvant therapy in orthopedic
surgery for the treatment of traumatic wounds and surgical
incisions (9-12).
However, the role of NPWT in bone tissue healing remains to be
elucidated.
Bone repair process represents a highly orchestrated
series of physiological events, including cellular recruitment,
proliferation and differentiation and the involvement of many
growth factors (13). Mesenchymal
stem cells (MSCs) serve a critical role in fracture healing.
Endogenous MSCs are primarily derived from the periosteum,
endosteum and marrow cavity (13).
Furthermore, MSCs are differentiated into osteoblasts and
chondrocytes, which in turn release soluble factors to regulate
bone regeneration, ultimately inducing local and distant
osteoprogenitor cell activation (13). In addition, mechanical stimulation
serves a key role in bone regeneration and remodeling (14,15). A
previous study revealed that porous polyurethane foam and suction
of NPWT induce microdeformations of the wound surface and cellular
mechanotransduction. These micromechanical forces may alter the
cell shape and increase fibroblast proliferation and
differentiation (16). Another
study reported that mechanical strain, caused by NPWT, might affect
mature dura matter, resulting in activation of bone tissue
formation in a rabbit cranial critical-sized defect model. The
results revealed that negative pressure-induced mechanical signals
(tissue stretching) may promote the differentiation of progenitor
cells from the dura to osteoblasts, and then synthesize bone matrix
with subsequent mineralization (17). In our previous study, where an in
vitro NPWT bioreactor was used, it was demonstrated that
short-term NPWT application at -125 mmHg promoted
periosteal-derived mesenchymal stem cell (P-MSCs) and osteogenic
differentiation in rats. Additionally, when NPWT is adopted,
expression levels of the mechanotransduction molecule integrin β5
are increased, suggesting that NPWT promotes bone formation while
concurrently reducing bone resorption and inducing fracture healing
(18). These findings support the
hypothesis that NPWT may result in mechanical strain transduction
to the underlying periosteum, mechanical stretching of
osteoprogenitor cells and stimulated bone regeneration in traumatic
wounds with fractures or segmental bone defects.
In the present study, a rabbit radial gap-healing
model was used to investigate the efficacy of NPWT on the bone
regeneration process. Following treatment with negative pressure,
changes in the expression levels of several critical factors
involved in bone formation, such as bone morphogenetic protein
(BMP)-2, osteopontin (OPN) and vascular endothelial growth factor
(VEGF) were measured. The findings of the present study may provide
additional insights regarding the potential mechanism of NPWT
action in bone healing.
Materials and methods
Establishment of an animal model
The present study was approved by the Institutional
Animal Care and Use Committee of Huazhong University of Science and
Technology. A total of 2 New Zealand rabbits (age, 21-23 weeks;
body weight, 3.0-3.5 kg; sex, 18 males and 10 females) were
purchased from the experimental animal center of Huazhong
University of Science and Technology (Wuhan, China). The rabbits
were housed in a light (12 h light/dark cycle), temperature (16˚C)
and relative humidity (40%) controlled room with free access to
food and water for at least 1 week prior to any procedures. All
rabbits exhibited mature bone tissue and were intramuscularly
anesthetized using 5 mg/kg xylazine and 35 mg/kg ketamine (19). The skin preparation and disinfection
of the left forelimb were performed according to standard protocols
(20). In brief, a 2-3 cm vertical
skin incision was performed on the lateral side of the limb at
approximately the same distance from the elbow and carpal joint.
Subsequently, the surrounding muscles were detached with artery
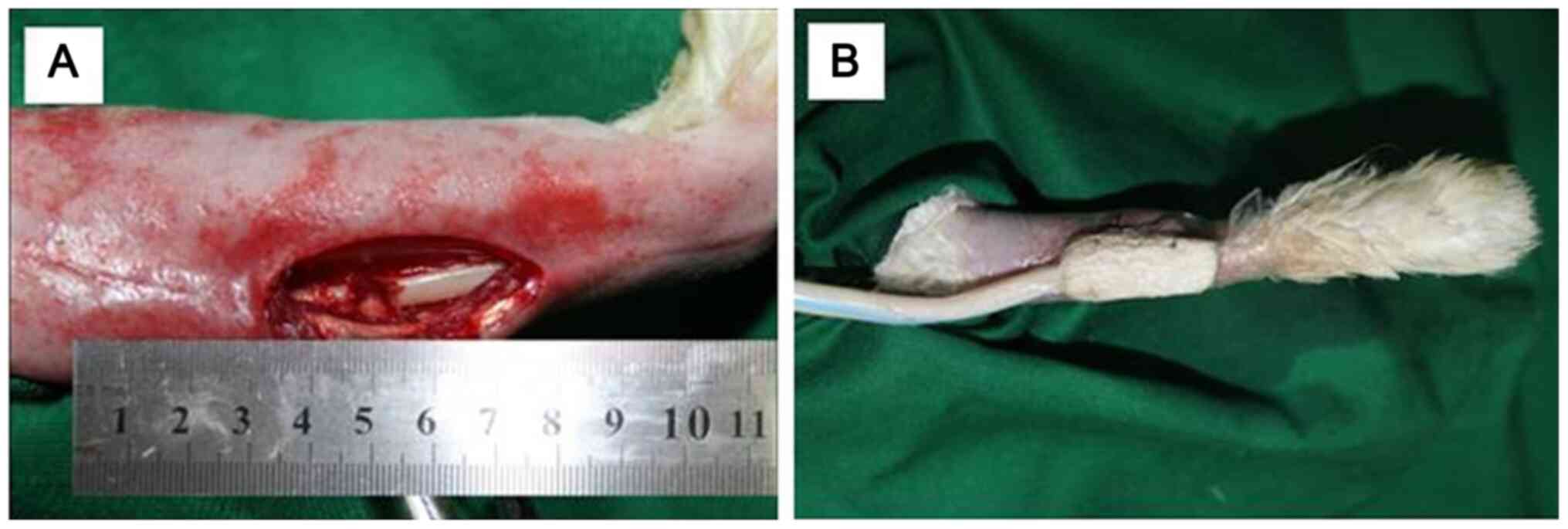
forceps and the radial bone was exposed. A 6-mm long bone section
of the central diaphysis of the radius was cut using an electric
saw and removed to create a segmental defect (Fig. 1). In addition, the osteotomy site
was thoroughly rinsed with saline to remove residual tissue,
including fascia, bone and muscle, in the gap. The soft tissue was
then reapproximated and the skin was not closed. All wounds were
randomly divided into two groups. In the first and second group,
the wound was covered with vacuum sealing drainage (VSD) foam
(Wuhan VSD Medical Science & Technology Co., Ltd.) and sterile
gauze (control group), respectively. A continuous negative pressure
of -125 mmHg was applied using a tubular suction pump and drainage
collection system. For VSD-covered wounds, dressings were replaced
on days 3, 7 and 14 following surgery and every 3 days for the
gauze-covered wounds. Finally, all rabbits were treated with
intramuscular injections of 400,000 units of penicillin daily for 3
days to prevent infection and for analgesia.
X-ray imaging and scoring
Mediolateral radiography of the affected sites was
performed immediately after surgery and subsequently on days 7, 14,
21 and 28. Each radiography was performed under the same conditions
(1.2 sec, 50 kV, 5.4 mA, 50 cm film-focus distance) using a
standard protocol (20). The
Lane-Sandhu X-ray scoring system (19) was used for quantitative analysis of
new bone formation, extent and size of the callus, bridging of the
gap and remodeling signs (n=5).
Histological examination
The bones were collected on the 2nd and 4th week
following surgery. The rabbits were sacrificed via air embolism,
during which all rabbits were anesthetized by xylazine and ketamine
as described earlier. At 10 min after anesthesia, 10 ml/kg air was
injected into the ear vein. Animal death was confirmed by checking
respiration and palpebral, pedal and postural reflexes. A 2.5-cm
long section of the radius, including the sites with bone defect
and normal bone tissue on both sides, was removed. Subsequently,
saline and 10% neutral formalin solutions were used to rinse and
fix samples at 4˚C for 24 h, respectively. The samples were
incubated at 4˚C for one week and decalcified using 20% EDTA. The
samples were then dehydrated using a gradient of ethanol solutions.
Following paraffin embedding, the samples were longitudinally cut
into 5 µm-thick sections and were subsequently stained with
hematoxylin and eosin (H&E) and Masson's trichrome staining at
room temperature for 5 min. Finally, the stained sections were
observed using an optical microscope (magnification, x100).
Immunohistochemical examination
The periosteum adjacent to the radial gap was
collected on day 3 post-surgery. The specimens were fixed, embedded
and sectioned using conventional methods (18). Subsequently, specimens were
permeabilized with 0.5% Triton X-100 for 10 min at room temperature
and non-specific epitopes were blocked using 10% goat serum for 1 h
at 4˚C. Finally, the samples were first incubated with a specific
rabbit anti-vimentin antibody (1:100; Wuhan Boster Biological
Technology, Ltd. cat. no. M00235-1) overnight at 4˚C and
subsequently with a goat anti-rabbit secondary antibody labeled
with streptavidin-biotin complex (1:200; Wuhan Boster Biological
Technology, Ltd.; cat. no. BA1034) for 30 min at 37˚C.
Western blot analysis
Western blot analysis was performed as previously
described (18). The bone tissues
were collected from the radial gaps on the 2nd and 4th week
post-surgery. Following freezing of the tissue samples in liquid
nitrogen, samples were then ground into powder using a pestle and
mortar. The cells were lysed using RIPA buffer (Beyotime Institute
of Biotechnology) supplemented with phosphatase-inhibitor cocktail
(Sigma-Aldrich; Merck KGaA; cat. no. 11873580001) and 1 mM
phenylmethylsulfonyl fluoride (PMSF; both Sigma-Aldrich; Merck
KGaA). The protein extracts were separated via 6% SDS-PAGE and
subsequently electrotransferred onto PVDF membranes (Hybond-P; GE
Healthcare). The membranes were then incubated overnight at 4˚C
with TBS-Tween-20 supplemented with one of the following primary
antibodies: Mouse anti-β-actin (1:1,000; Boster Biological
Technology; cat. no. P60709), mouse anti-VEGF (1:200; Santa Cruz
Biotechnology, Inc. cat. no. sc-7269), mouse anti-BMP-2 (1:400;
Santa Cruz Biotechnology, Inc. cat. no. sc-137087) or mouse
anti-OPN (1:400; Abcam; cat. no. ab228748). Following incubation
with secondary horseradish peroxidase-conjugated goat anti-mouse
IgG antibody (1:1,000; Santa Cruz Biotechnology, Inc. cat. no.
sc-2005), protein bands were detected using the enhanced
chemiluminescence method. The results were quantified using ImageJ
software (version no. 1.48 National Institutes of Health).
Statistical analysis
The results are presented as the mean ± SD.
Statistically significant differences were determined using two-way
ANOVA or mixed ANOVA followed by Bonferroni's post hoc test. All
statistical analyses were performed using GraphPad Prism 6
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
X-ray examination
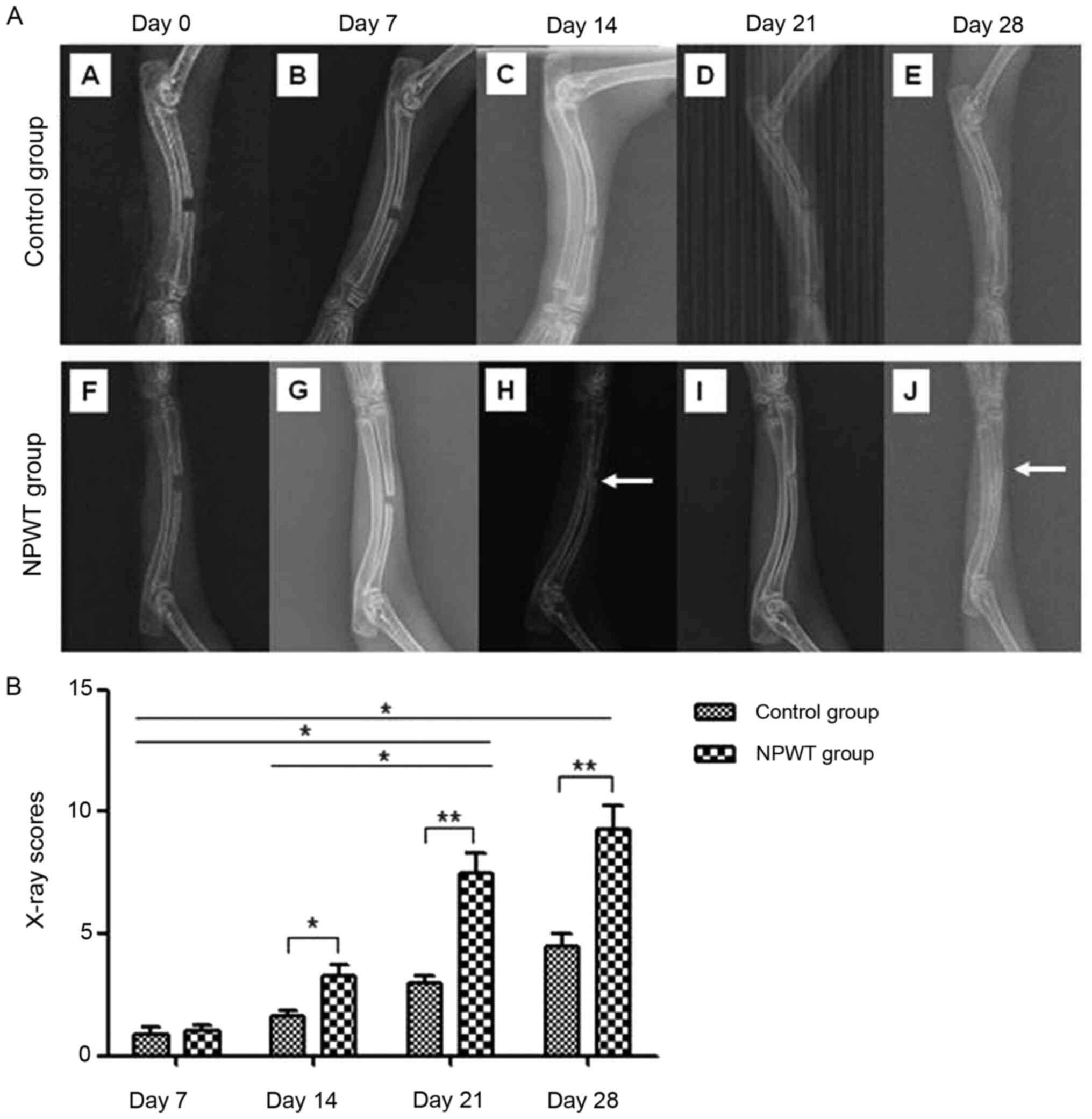
The fracture gap-healing processes was monitored in
all rabbits using radiographic examination on days 0, 7, 14, 21 and
28 (Fig. 2A). The results revealed
that calluses were formed around the defects in the NPWT group οn
day 14. By day 28, callus formation was increased on the proximal
and distal radial defects. The bone defects were radiographically
proven to be united. In addition, roentgenographic examination of
the control group revealed a small number of peripheral calluses on
day 14 and day 21 following surgery. However, on day 28, the bone
callus formation was poor and none of the bone defects completely
healed. Subsequent analysis on days 14, 21 and 28 after surgery
using the Lane-Sandhu X-ray scoring system demonstrated a
significantly higher score in the NPWT group compared with the
control group (Fig. 2B). The
results indicated that compared with the control group, the
osseointegration rate of the NPWT group was significantly
increased.
Histological examination
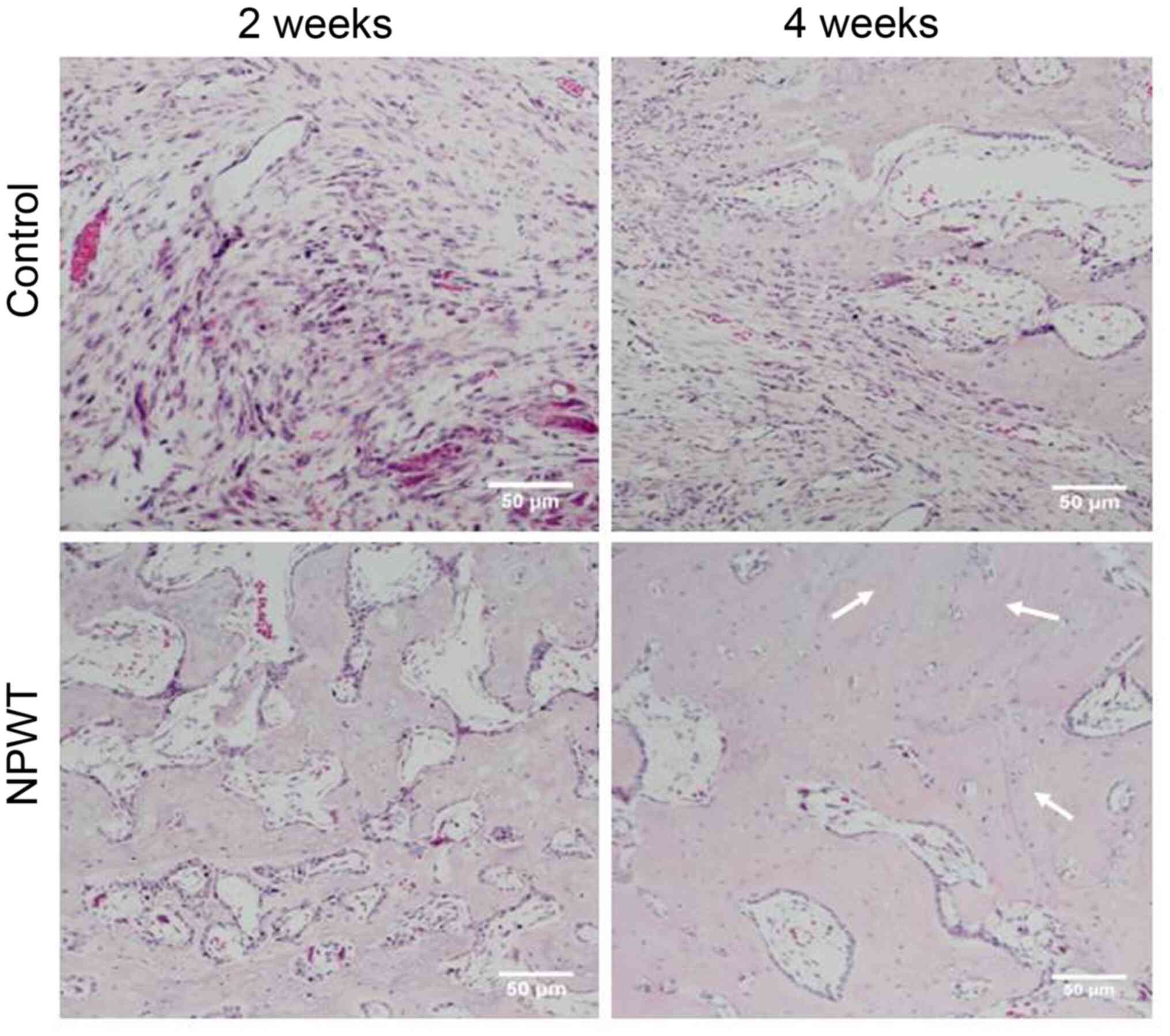
H&E histological staining was performed at 2 and
4 weeks following surgery. Histopathological examination revealed
that in the control group, the majority of bone defects were filled
with fibrous tissue, while in the NPWT group, new bone and
cartilage island formation was observed (Fig. 3). At the 4th week, small and sparse
trabecular bones, mixed cartilage islands and new bone tissue
formation were detected in the control group. However, in the NPWT
group, the trabecular bones were thicker and denser, with some
trabecular bone transforming into mature lamellar bone tissue
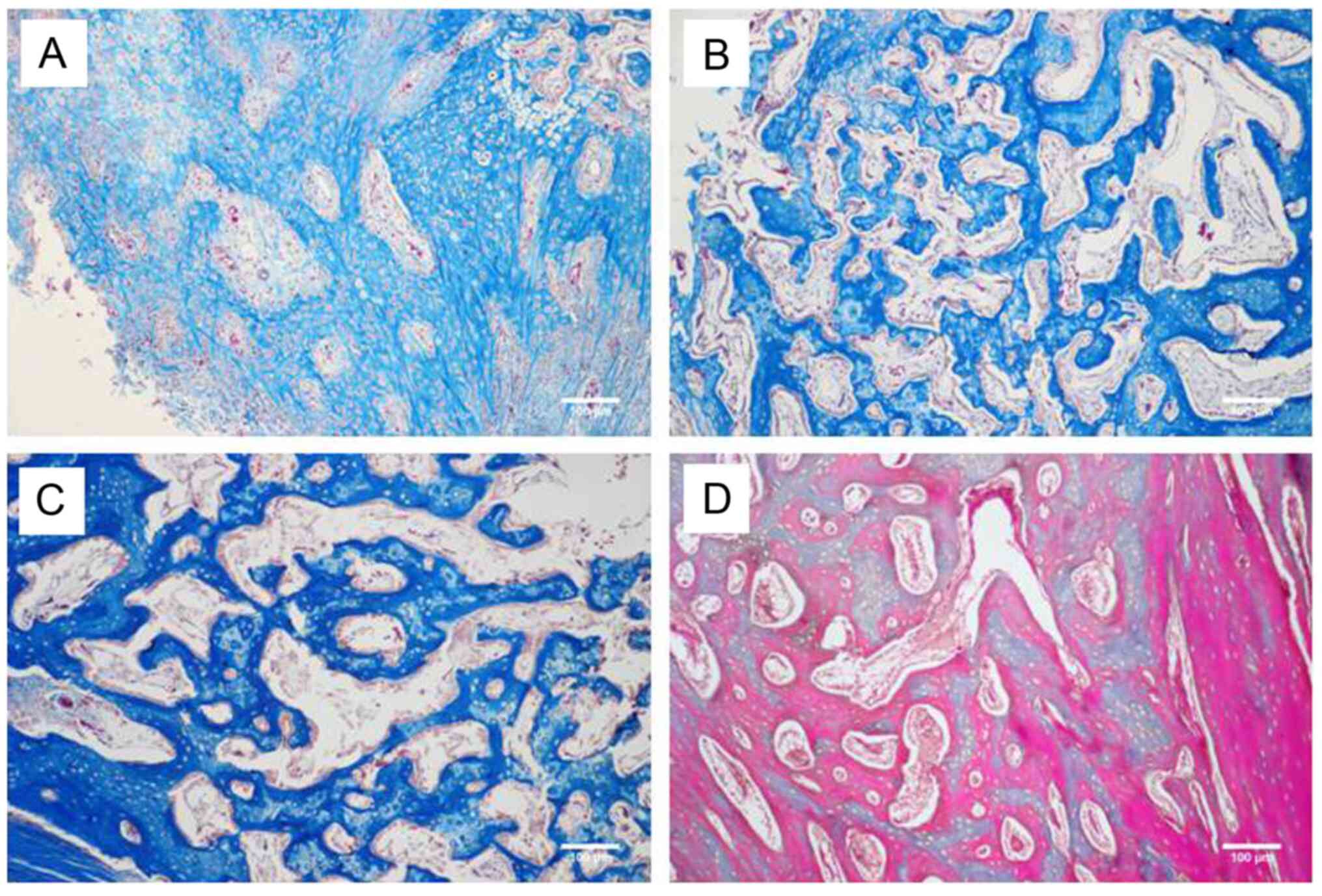
(Fig. 3). Furthermore, Masson's
trichrome staining revealed that connective tissue proliferation
occurred at the 2nd week post-operation in the control group
(Fig. 4A), while lots of cartilage
islands emerged in the NPWT group (Fig.
4B). By the 4th week, increased mature bone formation (stained
red) was observed in the NPWT group (Fig. 4D) compared with the control group
(Fig. 4C). All these outcomes were
consistent with H&E results.
Immunohistochemical examination
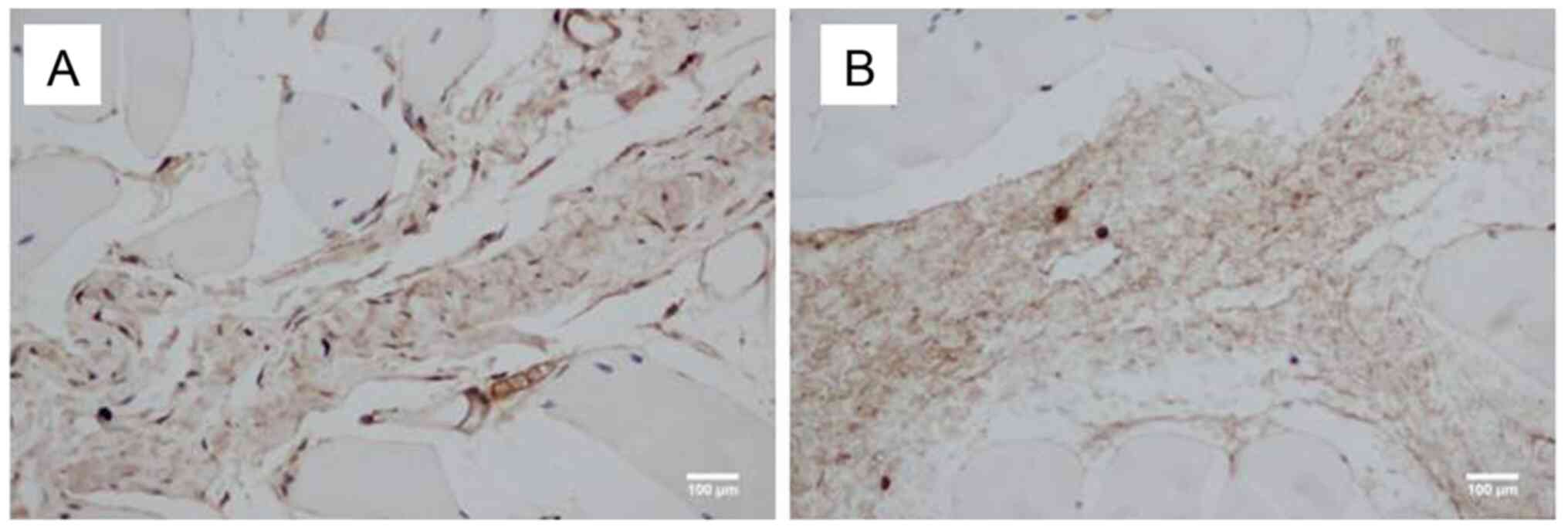
Histological sections of the periosteum adjacent to
the defected sites were collected following surgery and
subsequently incubated with antibodies specific against vimentin.
On day 3 after surgery, the number of vimentin-positive cells
(stained brown) was higher in the NPWT group (Fig. 5A) compared with the control group
(Fig. 5B).
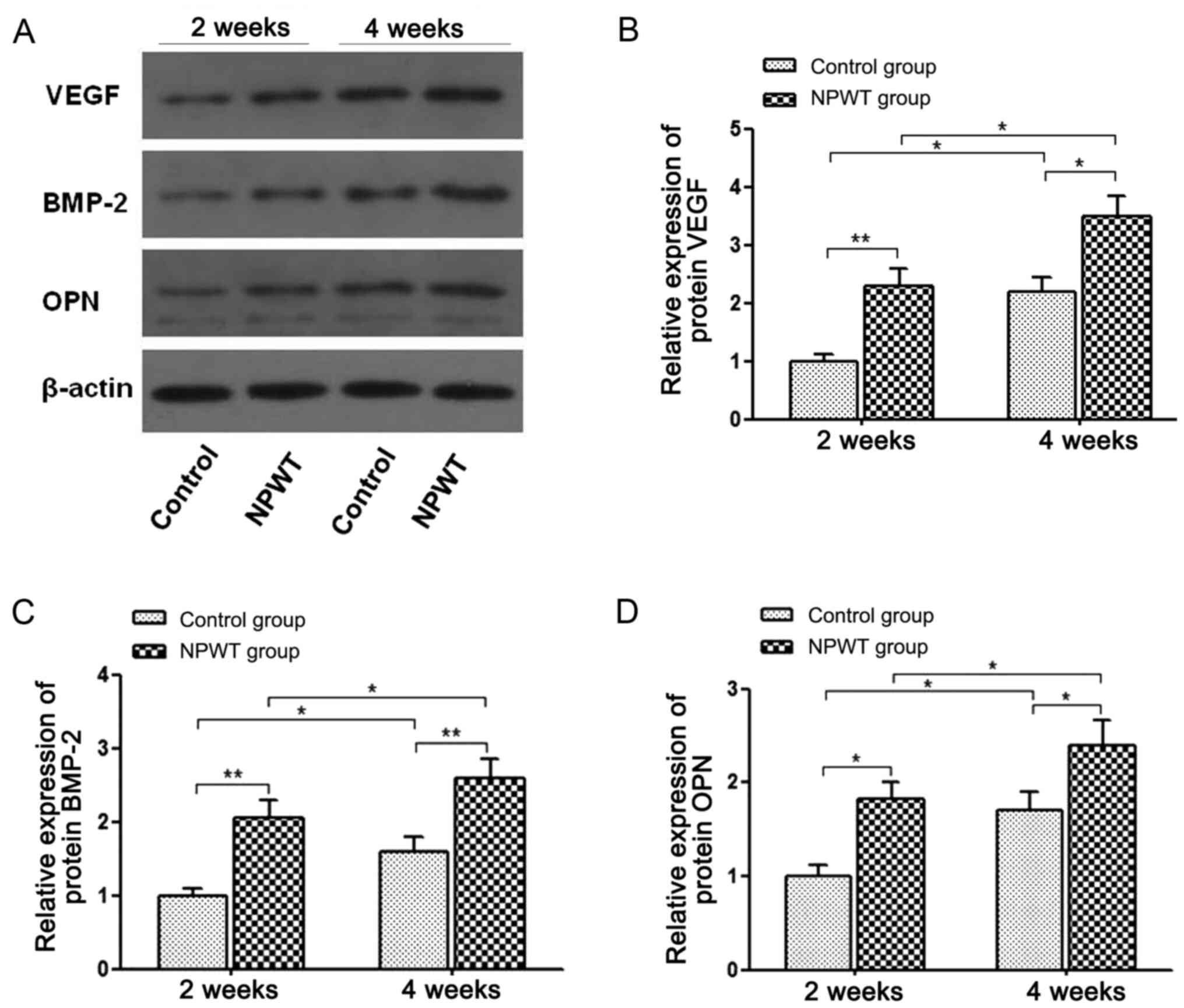
VEGF, BMP-2 and OPN expression
Western blot analysis revealed that the protein
expression levels of OPN, VEGF and BMP-2 significantly increased in
the NPWT group compared with the control group at weeks 2 and 4
post operation (Fig. 6). At week 2
following surgery, the expression levels of the aforementioned
proteins in the NPWT group were 2-fold higher compared with the
control group. Furthermore, at week 4 post-surgery, the relative
expression levels of OPN, VEGF and BMP-2 in the NPWT group were
also significantly higher than the control group.
Discussion
The NPWT approach was first applied in orthopedic
surgery in 1993 by Fleischmann et al (21) and was originally used to improve
gradual treatment of open fractures associated with soft-tissue
defects. More specifically, NPWT has been used to treat Gustilo
type III open fractures to achieve primary closure of wounds, which
is generally unattainable (22,23).
Currently, NPWT has been considered an important adjunct for the
treatment of traumatic wounds and surgical incisions associated
with orthopedic trauma (9-12).
It was reported that NPWT provided wound coverage and maintained
incision edges, thus eliminating edema, removing cytotoxic factors
and promoting granulation tissue formation (2,24,25).
However, its precise mechanism of action needs to be further
investigated. Although the advantages of NPWT have been reported,
controversy still exists. A number of systematic reviews have
reported that NPWT significantly decreases the risk of infection
and accelerates the wound healing process (26,27).
However, other studies suggested that negative pressure dressings
do not confer any advantage over conventional wound dressings for
open fracture treatment (28-30).
In addition, several factors may influence the complication rate of
NPWT, such as the degree of soft and bone tissue injury, adequate
debridement, nutritional support, patients' baseline health status
and antibiotic therapy (27).
To date, few studies have investigated the effects
of NPWT on bone tissue. Zhang et al (31) demonstrated that intermittent
negative pressure promoted bone regeneration in a rabbit skull
defect model. Our previous study demonstrated that NPWT induced
P-MSC proliferation and osteogenic differentiation (18). In the present study, a rabbit radial
gap-healing model was employed to further investigate the role of
NPWT in bone tissue healing. Bone regeneration was detected
following surgery using X-ray and histological staining. The
results showed that NPWT, with a continuous suction at -125 mmHg,
significantly accelerated bone regeneration compared with the
conventional gauze approach. In addition, on the 3rd day following
surgery, immunohistochemical staining revealed that the number of
vimentin-positive cells was higher in the NPWT group compared with
the control group. Vimentin is an intermediate filament protein
that is typically expressed in MSCs and is considered a main marker
of rabbit MSCs (32). These
findings indicated that NPWT may enhance MSC proliferation in the
periosteum, which is consistent with our previous in vitro
study (18).
Western blot analysis results revealed that VEGF
protein expression was significantly increased during the bone
healing process following negative pressure application.
VEGF-induced angiogenesis serves a critical role in bone
regeneration and fracture repair. The new capillaries provide the
cells in the fracture site with the necessary nutrients to mediate
the healing process (33). In
addition, it was previously demonstrated that VEGF serves a key
role in the recruitment and differentiation of osteoclasts and
osteoblasts, respectively (33).
The present study also revealed that NPWT upregulated BMP-2
expression during the bone healing process (on the 3rd-4th week
post-surgery). BMPs belong to the transforming growth factor-β
superfamily. Among the members of the superfamily, BMP-7, BMP-4 and
BMP-2 are the best-characterized BMPs with osteoinductive
properties (34). The
aforementioned proteins influence the induction of osteoblasts and
chondrocytes differentiation, resulting in membrane enhancement and
cartilage ossification, which in turn accelerate bone formation
(34). OPN, a key non-collagenous
protein, is involved in bone remodeling and mediates the binding of
osteoclasts to the bone surface (35). In addition, OPN serves several
important roles in the biomineralization process, including
modulation of osteoclastic function, osseous cell adherence and
matrix mineralization (35). The
results of the present study indicate that protein expression
levels were increased in the NPWT group compared with the control
group at three weeks after surgery. This finding indicated that
NPWT-mediated OPN upregulation may influence bone remodeling.
Furthermore, OPN and BMP-2 are considered as markers of different
stages of MSC differentiation into osteoblasts (36,37).
Therefore, NPWT may induce bone formation and osteoblastic
differentiation of MSCs via upregulating VEGF, BMP-2 and OPN
expression in a rabbit bone healing model.
In the present study, the rabbit segmental bone
defect model was directly established using a sharp saw. In
patients with open fracture, a high-energy trauma is often
associated with severe soft tissue injury, extensive contamination
and decreased viability (38).
Therefore, further studies on NPWT-mediated bone formation in an
open fracture model under high-energy trauma should be performed to
verify its osteoinductive effects. Although NPWT exhibits
beneficial effects on bone tissue regeneration in rabbits, further
research on its mechanisms of action is required. It is also
essential to test other pressure values and effect time of NPWT to
find optimal conditions for promoting osteogenesis.
In conclusion, the present study revealed that NPWT,
with a continuous -125 mmHg suction, accelerated bone regeneration
by upregulating VEGF, BMP-2 and OPN expression levels, and
promoting osteoblast differentiation and MSC proliferation. NPWT is
considered a promising technique that may be advantageous for wound
healing treatment. Finally, further basic and clinical studies
focusing on optimizing NPWT are required. It is also important to
try other negative pressure waveforms and find the most beneficial
conditions for osteogenesis. The current study may help to confirm
the effectiveness and elucidate the clinical indications for NPWT
in open fracture treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science Fund of
Hubei Provincial Health Commission (grant no. 2020CFB464) and Wuhan
Municipal Health Commission (grant no. WX20Q15).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceived and designed the experiments: JZ, LY.
Performed the experiments: JZ, FW, MW and YA. Analyzed the data:
JZ, FW, JW and RH. Contributed to the writing of the manuscript: JZ
and FW. JZ and FW confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Institutional
Animal Care and Use Committee of Huazhong University of Science and
Technology, and the animal procedures were performed in strict
accordance with institutional and national guidelines. All efforts
were made to minimize suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bee TK, Croce MA, Magnotti LJ, Zarzaur BL,
Maish GO III, Minard G, Schroeppel TJ and Fabian TC: Temporary
abdominal closure techniques: A prospective randomized trial
comparing polyglactin 910 mesh and vacuum-assisted closure. J
Trauma. 65:337–344. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Morykwas MJ, Argenta LC, Shelton-Brown EI
and McGuirt W: Vacuum-assisted closure: A new method for wound
control and treatment: Animal studies and basic foundation. Ann
Plastic Surg. 38:553–562. 1997.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Armstrong DG and Lavery LA: Diabetic Foot
Study Consortium. Negative pressure wound therapy after partial
diabetic foot amputation: A multicentre, randomised controlled
trial. Lancet. 366:1704–1710. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fleck T, Gustafsson R, Harding K,
Ingemansson R, Lirtzman MD, Meites HL, Moidl R, Price P, Ritchie A,
Salazar J, et al: The management of deep sternal wound infections
using vacuum assisted closure (V.A.C.) therapy. Int Wound J.
3:273–280. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Argenta LC and Morykwas MJ:
Vacuum-assisted closure: A new method for wound control and
treatment: Clinical experience. Ann Plastic Surg. 38:563–577.
1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Javed AA, Teinor J, Wright M, Ding D,
Burkhart RA, Hundt J, Cameron JL, Makary MA, He J, Eckhauser FE, et
al: Negative pressure wound therapy for surgical-site infections: A
randomized trial. Ann Surg. 269:1034–1040. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu Y, Zhou Q, Wang Y, Liu Z, Dong M, Wang
Y, Li X and Hu D: Negative pressure wound therapy decreases
mortality in a murine model of burn-wound sepsis involving
Pseudomonas aeruginosa infection. PLoS One.
9(e90494)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Orgill DP and Bayer LR: Update on
negative-pressure wound therapy. Plast Reconstr Surg. 127 (Suppl
1):S105–S115. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Iheozor-Ejiofor Z, Newton K, Dumville JC,
Costa ML, Norman G and Bruce J: Negative pressure wound therapy for
open traumatic wounds. Cochrane Database Syst Rev.
7(CD012522)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Agarwal A: Management of closed incisions
using negative-pressure wound therapy in orthopedic surgery. Plast
Reconstr Surg. 143:S21–S26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
A N, Khan WS and J P: The evidence-based
principles of negative pressure wound therapy in trauma &
orthopedics. Open Orthop J. 8:168–177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stannard JP, Volgas DA, McGwin G III,
Stewart RL, Obremskey W, Moore T and Anglen JO: Incisional negative
pressure wound therapy after high-risk lower extremity fractures. J
Orthop Trauma. 26:37–42. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang X, Wang Y, Gou W, Lu Q, Peng J and Lu
S: Role of mesenchymal stem cells in bone regeneration and fracture
repair: A review. Int Orthop. 37:2491–2498. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Maycas M, Esbrit P and Gortazar AR:
Molecular mechanisms in bone mechanotransduction. Histol
Histopathol. 32:751–760. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Duncan RL and Turner CH:
Mechanotransduction and the functional response of bone to
mechanical strain. Calcif Tissue Int. 57:344–358. 1995.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lu F, Ogawa R, Nguyen DT, Chen B, Guo D,
Helm DL, Zhan Q, Murphy GF and Orgill DP: Microdeformation of
three-dimensional cultured fibroblasts induces gene expression and
morphological changes. Ann Plast Surg. 66:296–300. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Swain LD, Cornet DA, Manwaring ME, Collins
B, Singh VK, Beniker D and Carnes DL: Negative pressure therapy
stimulates healing of critical-size calvarial defects in rabbits.
Bonekey Rep. 2(299)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu J, Yu A, Qi B, Li Z and Hu X: Effects
of negative pressure wound therapy on mesenchymal stem cells
proliferation and osteogenic differentiation in a fibrin matrix.
PLoS One. 9(e107339)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Udehiya RK, Amarpal Aithal HP, Kinjavdekar
P, Pawde AM, Singh R and Taru Sharma G: Comparison of autogenic and
allogenic bone marrow derived mesenchymal stem cells for repair of
segmental bone defects in rabbits. Res Vet Sci. 94:743–752.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu J, Zhou P, Long Y, Huang C and Chen D:
Repair of bone defects in rat radii with a composite of allogeneic
adipose-derived stem cells and heterogeneous deproteinized bone.
Stem Cell Res Ther. 9(79)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fleischmann W, Strecker W, Bombelli M and
Kinzl L: Vacuum sealing as treatment of soft tissue damage in open
fractures. Der Unfallchirurg. 96:488–492. 1993.PubMed/NCBI(In German).
|
|
22
|
Takeuchi N, Mae T, Hotokezaka S, Sasaki K,
Matsushita A, Miake G, Kuchiishi R and Noguchi Y: A Gustilo type
IIIB open forearm fracture treated by negative pressure wound
therapy and locking compression plates: A case report. Fukuoka
Igaku Zasshi. 102:293–297. 2011.PubMed/NCBI
|
|
23
|
Babiak I: Open tibial fractures grade IIIC
treated successfully with external fixation, negative-pressure
wound therapy and recombinant human bone morphogenetic protein 7.
Int Wound J. 11:476–482. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Suzuki T, Minehara A, Matsuura T, Kawamura
T and Soma K: Negative-pressure wound therapy over surgically
closed wounds in open fractures. J Orthop Surg (Hong Kong).
22:30–34. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stannard JP, Singanamala N and Volgas DA:
Fix and flap in the era of vacuum suction devices: What do we know
in terms of evidence based medicine? Injury. 41:780–786.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim JH and Lee DH: Negative pressure wound
therapy vs. conventional management in open tibia fractures:
Systematic review and meta-analysis. Injury. 50:1764–1772.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu X, Zhang H, Cen S and Huang F:
Negative pressure wound therapy versus conventional wound dressings
in treatment of open fractures: A systematic review and
meta-analysis. Int J Surg. 53:72–79. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Costa ML, Achten J, Bruce J, Davis S,
Hennings S, Willett K, Petrou S, Jeffery S, Griffin D, Parker B, et
al: Negative-pressure wound therapy versus standard dressings for
adults with an open lower limb fracture: The WOLLF RCT. Health
Technol Assess. 22:1–162. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Crist BD, Oladeji LO, Khazzam M, Della
Rocca GJ, Murtha YM and Stannard JP: Role of acute negative
pressure wound therapy over primarily closed surgical incisions in
acetabular fracture ORIF: A prospective randomized trial. Injury.
48:1518–1521. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cook R, Thomas V and Martin R: Negative
pressure dressings are no better than standard dressings for open
fractures. BMJ. 364(k4411)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang YG, Yang Z, Zhang H, Liu M, Qiu Y
and Guo X: Negative pressure technology enhances bone regeneration
in rabbit skull defects. BMC Musculoskeletal Disord.
14(76)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mazurkevych A, Malyuk M, Bezdieniezhnykh
N, Starodub L, Kharkevych Y, Jakubczak A and Gryzinska M:
Immunophenotypic characteristics and karyotype analysis of bone
marrow-derived mesenchymal stem cells of rabbits during in vitro
cultivation. Pol J Vet Sci. 20:687–695. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hu K and Olsen BR: Osteoblast-derived VEGF
regulates osteoblast differentiation and bone formation during bone
repair. J Clin Invest. 126:509–526. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Majidinia M, Sadeghpour A and Yousefi B:
The roles of signaling pathways in bone repair and regeneration. J
Cell Physiol. 233:2937–2948. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Icer MA and Gezmen-Karadag M: The multiple
functions and mechanisms of osteopontin. Clin Biochem. 59:17–24.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tang Z, Wang Z, Qing F, Ni Y, Fan Y, Tan Y
and Zhang X: Bone morphogenetic protein Smads signaling in
mesenchymal stem cells affected by osteoinductive calcium phosphate
ceramics. J Biomed Mater Res A. 103:1001–1010. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang CY, Zhao BH, Ai HJ and Wang YW:
Comparison of biological characteristics of mesenchymal stem cells
grown on two different titanium implant surfaces. Biomed Mater.
3(015004)2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Streubel PN, Stinner DJ and Obremskey WT:
Use of negative-pressure wound therapy in orthopaedic trauma. J Am
Acad Orthop Surg. 20:564–574. 2012.PubMed/NCBI View Article : Google Scholar
|