Introduction
Non-lactational mastitis refers to a group of
nonspecific inflammatory diseases with unknown pathogeneses, which
mainly occur in females during non-breastfeeding periods. The
pathological types include mammary duct ectasia (MDE)/periduct
mastitis (PDM) and granulomatous lobular mastitis (GLM) (1). Typical clinical symptoms are lacking
and at times, the only symptom may be a breast lump without any
obvious inflammatory signs, making it difficult to distinguish the
disease from breast cancer (2).
Abscess, sinus or ulcer formation are frequent and the condition is
unlikely to be cured by conventional antibiotics. As the surgical
methods are not uniform in consensus, it is difficult for surgeons
to make clinical decisions when patients require surgical
intervention (3-5).
Certain patients lose their breasts due to having non-lactational
mastitis, which seriously affects their physical and mental health.
At present, surgery is the most common and effective method for
non-lactational mastitis (3,6).
However, the current conventional surgical treatment, particularly
for abscess incision drainage, has several disadvantages, including
a long time interval over which the patient requires dressing
changes, nonhealing wounds, obvious breast deformation, high
recurrence rate and poor efficacy. Simple lumpectomy is suitable
for patients with localized lesions, but the disease easily recurs
(7). However, total mastectomy
causes patients to lose their breasts and there are still cases of
residual skin ulceration after the operation. Therefore, surgical
treatment is still the focus and challenge of current research.
Vacuum sealing drainage (VSD) technology refers to
the debridement of lesions, the placement of medical sponge
parceling drainage tubes into the lacuna, the sealing of wounds
with a semipermeable membrane and the connection of the drainage
tube to a device for negative pressure drainage. This technology
may intermittently or continuously generate a pressure lower than
atmospheric pressure at the wound surface and promote wound healing
through a series of physiological mechanisms (8). VSD may be applied to various types of
wounds, including infected wounds, skin graft wounds and wounds
without skin or soft tissue (9,10).
This technique has outstanding advantages in clinical application,
e.g. it is easy to operate, is able to avoid cross-infection and
accurately observe the amount and character of drainage, and it may
relieve wound pain and significantly shorten the treatment time
(11-13).
VSD is rarely reported for the treatment of non-lactational
mastitis and no prospective or controlled trials have been
performed to confirm the short-term and long-term efficacies of VSD
in the treatment of non-lactational mastitis.
Precise ultrasound-guided debridement refers to the
intraoperative use of ultrasound to locate the scope and depth of
lesions, and instruments such as probes and spatulas are employed
to remove pus and necrosis, as well as to clear the sinus tract.
Compared with traditional debridement, this technology has numerous
advantages, such as its accuracy, small incision and thorough
debridement. To the best of our knowledge, there are currently no
research studies on the use of this technology in the treatment of
non-lactational mastitis or comparisons with VSD in terms of
clinical efficacy. At our department, VSD and precise
ultrasound-guided debridement are both used to treat
non-lactational mastitis, with satisfactory clinical efficacies.
However, due to a lack of sufficient clinical data and objective
research to discuss the indications for the two treatment methods,
it is still in the exploratory stage. In the present study, the
efficacies of VSD and precise ultrasound-guided debridement in the
treatment of non-lactational mastitis were analyzed and the
advantages and disadvantages were compared to identify the optimal
surgical treatment for non-lactational mastitis.
Materials and methods
Subjects
A total of 60 patients diagnosed with
non-lactational mastitis who received surgical treatment at the
Department of Thoracic and Breast Surgery of Xiamen Hospital of
Traditional Chinese Medicine (Xiamen, China) between July 2017 and
June 2019 were included.
The adjustable negative pressure drainage device
(SAC-A3-D2; Sacco Medical Technology Co., Ltd.) consisted of four
parts: i) An adsorption sponge; ii) a semipermeable membrane; iii)
a sucking disc and pipeline; and iv) a negative pressure device. A
negative pressure of 140-150 mmHg was maintained.
The pathologic types included MDE/PDM and GLM.
According to the diagnostic criteria reported in literatures
(14-17),
the following diagnostic criteria were applied (all cases were in
accordance with the following two points): i) Clinical symptoms of
breast mass, with or without an inverted nipple, nipple discharge
and breast pain; an abscess may occur when the acute infection is
secondary to the disease; end-stage abscesses may be divided into
fistulas, sinus tracts or ulcers of the breast skin and they
require a long time period to heal because of recurrent chronic
inflammation; ii) Histopathological features: Hollow-needle
puncture biopsy was used to obtain specimens; microscopically, for
MDE/PDM, the breast duct is highly dilated and the lumen is filled
with pink granular thick material; infiltrating lymphocytes, plasma
cells and neutrophils are present around the dilated duct. The main
features of GLMs are non-caseous granulomas centered on lobular
units of the mammary gland and they are multifocal and unequal in
size, with or without micro-abscesses (1). Recurrence was defined as follows:
Recurrence at the primary site after the primary disease has been
cured for a period of time. The definition of new incidence was as
follows: After the disappearance or stabilization of the original
lesion, new lesions appear in other parts of the ipsilateral breast
or in the contralateral breast under certain conditions, not caused
by any direct spread of the original lesion. Clinical cure was
defined as disappearance of systemic symptoms, clinically
untouchable primary inflammatory lesions and healing of ulcers or
wounds. Exclusion criteria were as follows: i) Pregnant and breast
feeding patients, ii) patients with acute infectious mastitis and
iii) patients with malignant breast tumors.
Preoperative preparation
All patients were examined by color doppler
ultrasound before the operation to determine the location, breadth
and depth of lesions, and to mark the skin to avoid any omission
during the operation. In patients with skin tension caused by
abscess, a cut should be performed to discharge pus. The pus and
secretions of skin ulceration were subjected to bacterial culture
and drug sensitivity tests. Operation style was selected on an
individual basis after the surgeon clarified the advantages and
disadvantages of each surgery. A total of 30 patients received VSD
and the other 30 patients received precise ultrasound-guided
debridement. Full anesthesia was applied in the two groups before
surgery. Patients were put in the supine position with abduction of
upper limbs.
VSD
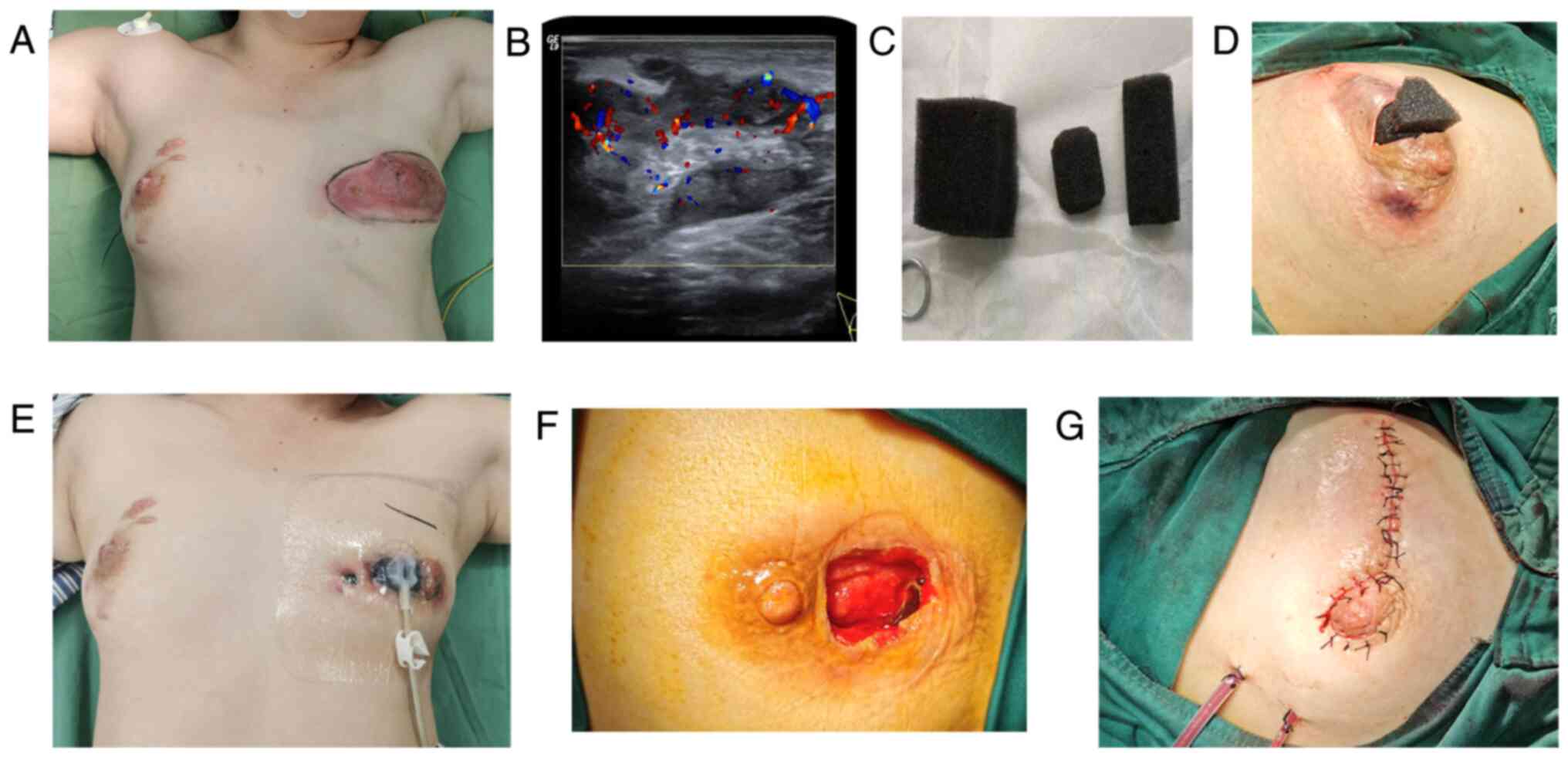
Representative images of the procedure are presented
in Fig. 1 (8,18).
Stage I operation: The location and size of the incision were
selected according to the location, scope and skin condition of the
lesion. On the basis of ensuring that the lesions were cleared to
the greatest extent, the surgical incision was inconspicuous as
much as possible for example incision around the areola and was
approximately 3-4 cm in length, as showed in Fig. 1D. For those cases with skin
ulcerations, the skin incision was extended directly and was at
least 2 cm away from the nipple to avoid nipple ischemia and
necrosis caused by subsequent compression of the sucking disc on
the nipple. For patients with multiple abscesses, intraoperative
ultrasound was used to open each abscess cavity to facilitate
drainage, and to remove pus and necrotic tissue. In cases of
combined inverted nipples or abscesses involving the large lacteal
duct, the large lacteal duct was disconnected intraoperatively and
the nipple was reconstructed with purse-string sutures behind the
nipple. For subcutaneous abscesses, the subcutaneous necrotic
tissue was scraped off with a spatula; chronic fistulas and sinus
tracts were resected intraoperatively as much as possible. The
wound was washed repeatedly with hydrogen peroxide (3%), dilute
iodine and normal saline. The pus was extracted and sent for
bacterial culture.
Placement of negative pressure drainage: After the
surgical wound was fully hemostatic, the size and shape of the
wound were initially estimated and a medical sponge was cut to fit
the size and shape of the wound to avoid the formation of a remnant
cavity (Fig. 1C). After the medical
sponge was placed, the whole wound was sealed with a medical
semipermeable membrane, with the coverage exceeding the scope of
the wound (Fig. 1E). If there was a
large extent of exudation at the site of the skin laceration, gauze
was used to cover the wound first and subsequently, the membrane
was applied. The connecting sucking disc was placed on the surface
of the wound and connected to the negative pressure device. A
continuous negative pressure of 140-150 mmHg was maintained. During
the treatment, obstruction of the negative pressure drainage tube
was avoided, the amount and characteristics of the drainage fluid
were observed and the negative pressure drainage material was
replaced once a week. Traditional Chinese Medicine, such as Tounong
Powder (19), was administered
according to the symptoms.
Stage Ⅱ operation for wound repair: The granulation
after vacuum drainage was evaluated over 3 weeks. If the
granulation tissue was fresh, the drainage fluid was clear and the
purulent fluid was small, a second-stage operation was performed.
The material was removed during the operation and the purulent
cavity was cleaned and explored again (Fig. 1F). If there was still a fistula or
sinus tract, it was removed. If the wound was too large to suture
directly, the mammary gland flap was transferred to fill the defect
area and a drainage tube was then placed.
Precise ultrasound-guided
debridement
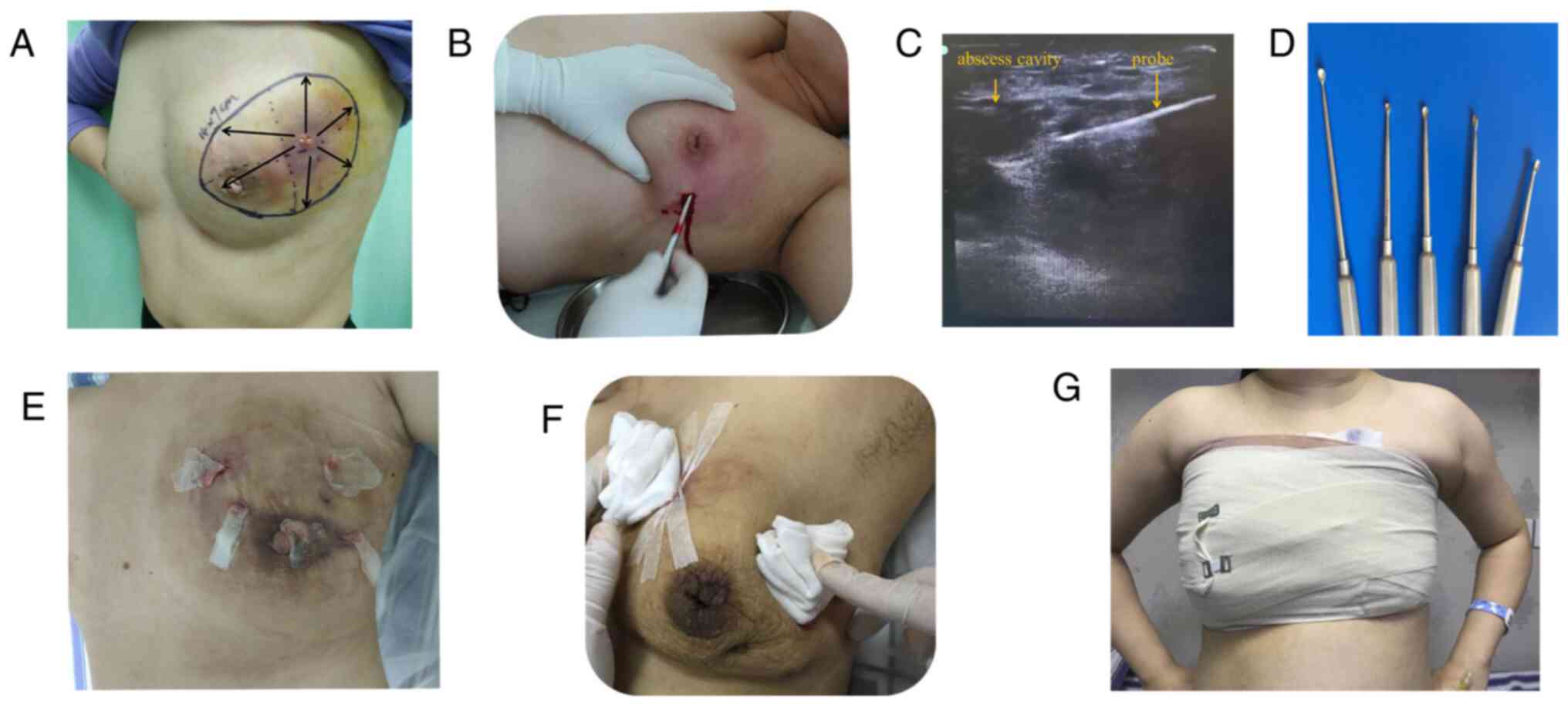
Representative images of the procedure are presented
in Fig. 2. The procedure of the
surgery was similar to that of minimally invasive rotary cutting
surgery as previously reported (20). During the operation, the breadth and
depth of the abscess were located by color Doppler ultrasound. The
incision was made as small as possible, approximately 1-2 cm long,
as showed in Fig. 2B. Under the
guidance of color Doppler ultrasound, a probe was used to explore
the purulent cavity and the necrotic tissue was scraped off with a
spatula (Fig. 2B and C). Multiple incisions were made for those
cases with multiple sinuses or a long distance from the purulent
cavity. During the operation, purulent fluid was extracted for
bacterial culture. After debridement, the wound was washed
repeatedly with hydrogen peroxide, dilute iodophor and normal
saline. Vaseline gauze was placed in the sinus or pus cavity
(Fig. 2E), which was covered with
imbricated triangular gauze after surgery (Fig. 2F). Subsequently, elastic bandages
were applied under pressure to cover the wound (Fig. 2G). For patients with excessive
bleeding during the operation, the bandage was maintained for 2
days and the dressing was changed on the second day after the
operation. The dressing was changed every day or every 2 days
according to the exudation situation. Gauze was placed for drainage
each time. After there was no purulent exudation, the gauze strip
was removed and the wound was closed with elastic bandages and
gauze under pressure. Prior to and after the operation, all
patients were treated with Traditional Chinese Medicine (19).
Post-operation and follow-up
After operation, dressing were changed every day in
the first week, and after that period, a compression bandage was
applied to reduce the abscess cavity of breast. Pain numerical
score (NRS) (21) was used to
evaluate the level of pain of the wound on the first, third, and
fifth days after operation. Patient follow-ups were performed via
outpatient appointment or telephone until the diseases were cured.
During follow-up, outcomes such as recurrent, new incidence, and
clinical cure were evaluated and recorded, as defined at the
‘subjects’ section.
Statistical analysis
All data were statistically processed with the SPSS
18.0 statistical software package (SPSS, Inc.). Continuous
variables were expressed as the mean ± SD after the testing of the
normal distribution and compared using unpaired t-tests. Count data
were expressed as n (%) and compared by Chi-square tests.
P<0.05 was considered to indicate a statistically
significant difference. Mann-Whitney U tests were used to compare
the postoperative pain scores.
Results
General information
All patients were female. One patent was lost to the
follow-up in the precise ultrasound-guided debridement group. The
average age at onset in the entire cohort was 31.1 years (23-44
years), there was no significant age different between groups and
the average preoperative disease course was 2.7 months and the
average time from delivery to onset was 2.8 years. Except for one
case of positive pus culture (Corynebacterium minimus), the
remainder of cases was negative. A total of 18.6% (11/59) of the
patients had inverted nipples. The follow-up rate was 98.3%
(59/60), the average follow-up time was 16.9 months, the total
recurrence rate was 6.8% (4/59), the total new incidence rate was
8.5% (5/59) and the average disease course was 5.3 months. All
patients were cured after the operation, as shown in Table I.
 | Table IComparison of clinicopathological data
between the two groups. |
Table I
Comparison of clinicopathological data
between the two groups.
| Item | VSD group, n=30 | Precise
ultrasound-guided debridement, group n=29 | P-value |
|---|
| Age (years) | 31.90±1.85 | 30.24±4.50 | 0.177 |
| Time from morbidity
to surgery (months) | 3.02±3.26 | 2.36±2.25 | 0.369 |
| Time from last birth
to morbidity (years) | 2.85±1.48 | 2.74±1.16 | 0.748 |
| Location | | | 0.992 |
|
Left | 16 | 15 | |
|
Right | 13 | 13 | |
|
Bilateral | 1 | 1 | |
| Inverted nipple | | | 0.287 |
|
Yes | 4 | 7 | |
|
No | 26 | 23 | |
| Birth history | | | 0.513 |
|
Yes | 28 | 28 | |
|
No | 2 | 1 | |
| Maximum diameter of
lesion (cm) | 9.83±3.78 | 9.00±4.09 | 0.419 |
| Skin ulceration | | | 0.937 |
|
Yes | 22 | 21 | |
|
No | 8 | 8 | |
| Pathology | | | 0.233 |
|
MDE/PDE | 8 | 12 | |
|
GLM | 22 | 17 | |
| Blood loss (ml) | 64±33.38 | 76.9±41.93 | 0.196 |
| Hospitalization time
(days) | 24.80±3.32 | 8.62±3.80 | <0.001 |
| Recurrence | | | 0.681 |
|
Yes | 2 (6.7) | 2 (6.9) | |
|
No | 28 (93.3) | 27 (93.1) | |
| New incidence | | | 0.516 |
|
Yes | 3(10) | 2 (6.9) | |
|
No | 27(90) | 27 (93.1) | |
| Postoperative pain
score (0-10) | | | |
|
Day 1 | 2.33±0.71 | 3.10±1.08 | 0.008 |
|
Day 3 | 1.57±0.68 | 2.14±0.52 | 0.001 |
|
Day 5 | 0.93±0.58 | 0.79±0.49 | 0.348 |
| Cure rate (%) | 100 (30/30) | 100 (29/29) | |
| Postoperative
disease course (months) | 2.97±1.49 | 2.40±0.93 | 0.084 |
| Total disease
course (months) | 5.98±3.69 | 4.75±2.50 | 0.141 |
Comparison of efficacy
The clinicopathological data of the two groups were
compared (Table I). Except for the
hospitalization time and postoperative pain score, the
clinicopathological data of the VSD group and precise
ultrasound-guided debridement group were similar, with no
significant differences. The hospitalization time in the VSD group
was significantly longer than that in the other group (24.80±3.32
vs. 8.62±3.80 days, P<0.001). The pain scores on the first and
third days after the operation in the precise ultrasound-guided
debridement group were significantly higher than those in the VSD
group (P=0.008 and 0.001, respectively), while the pain scores on
the fifth day after the operation were similar between the two
groups. There was no significant difference in the recurrence rate,
new incidence rate, disease course after the operation or total
disease course between the two groups.
Representative case
The patient was 33 years old, had 2 pregnancies and
2 births and was 3 years postpartum. She had left breast
non-lactational mastitis 3 months previously and the mass
disappeared after taking Traditional Chinese Medicine and applying
Traditional Chinese Medicine ointment externally for 1 month. The
right breast mass accompanied by pain and skin ulceration occurred
for 2 weeks. No significant relief was achieved after treatment
with Traditional Chinese Medicine or incision and drainage. The
right nipple was indicated to be inverted. The right breast was
obviously larger than the left breast. The size of the mass was
~10x8 cm in the upper and lower quadrants of the right breast. The
skin surface was ulcerated and purulent, with unclear boundaries
and poor mobility. The puncture pathology indicated ‘GLM’ and no
bacterial growth was detected in the pus culture. After admission,
‘right mammary abscess debridement + VSD drainage + inverted nipple
correction’ was performed. After negative pressure drainage for 21
days, granulation was fresh without obvious pus exudation, so VSD
equipment was removed, debridement and suture were performed and a
rubber drainage tube was placed. Prior to and after the operation,
the patient was treated with Traditional Chinese Medicine. The
drainage tube was removed 2 weeks after the operation, the wound
healed and the mass subsided. At four months after this, a mass was
observed again at the periphery of the primary lesion with skin
redness and ulceration. The biopsy again indicated ‘GLM’. After 2
months of incision, drainage and treatment with Traditional Chinese
Medicine, the abscess still repeatedly recurred. Subsequently,
precise ultrasound-guided debridement surgery was performed. After
2 months of conventional dressing change and treatment with
Traditional Chinese Medicine (19),
the lesion had healed and the mass had subsided.
Discussion
In recent years, the incidence rate of non-lactation
mastitis has been obviously increasing, particularly in coastal
cities (5). To date, the
pathogenesis of the disease has remained elusive (2,22,23).
It is characterized by various types, a long disease course and a
high recurrence rate, particularly for refractory non-lactational
mastitis, which is a focus and challenge in the clinic (24). At present, there are various
treatment methods for this disease. Traditional Chinese Medicine
emphasizes the concept of combining overall and local syndrome
differentiation, combining internal and external treatment and
treating both the symptoms and root causes. Internal and external
treatment methods are mostly used and external treatment methods
are also diversified. To date, Western medicine has not formulated
any systematic treatment standard. The common treatment methods
include surgical treatment, hormone therapy, immunosuppressive
therapy, antibiotic therapy (including antimycobacterial drug
therapy) and expectant therapy (3).
Among them, surgery is the preferred treatment. At present, there
are various surgical methods used at home and abroad, including
lumpectomy, incision and drainage, segmental resection, simple
mastectomy and subcutaneous mastectomy (4,5).
However, there is no unified surgical method. For simple lump-type
non-lactational mastitis, Traditional Chinese Medicine plus
external therapy has been adopted in our department. For refractory
non-lactation mastitis, particularly for cases of multiple
abscesses or sinuses, VSD or precise ultrasound-guided debridement
combined with Traditional Chinese Medicine has been adopted for
treatment. The purpose of the present study was to compare the
short-term and long-term effects of the two surgical methods, to
explore their clinical application value and to provide an optimal
treatment for refractory non-lactational mastitis.
Classic inflammatory lesions require long-term
dressing changes after incision and pus discharge (6). When dressings are changed, patients
suffer from intense pain. Open wounds are prone to secondary
bacterial infections and require a long time to heal. Compared with
traditional dressing changes, VSD is able to shorten the time
required for wound healing (25)
and markedly reduces the level of pain (26). The surgeon is able to trim the
medical sponge according to the size and shape of the purulent
cavity to make it fit into the cavity. After surgery, the sponge
may be adjusted according to the specific situation of the wound
and it may also be washed. It may not only ensure the timely
removal of necrotic tissue and exudate, but also promote the growth
of granulation and accelerate wound repair (9,25,27).
During precise ultrasound-guided debridement, it is easy to
determine the location, scope and depth of the lesions, which makes
it convenient to detect and deal with purulent cavities or sinuses
of various sizes by using color ultrasound, thereby avoiding
missing any lesions. This technology has the advantages of accurate
location, limited trauma and sufficient debridement. Comparing the
clinical data of the two groups of the present study indicated that
the hospitalization time in the VSD group was longer and the
hospitalization costs were higher due to the high cost of
consumables, but the postoperative pain score in this group was
significantly lower. In the VSD group, the incision was larger and
it was possible to excise the sinus simultaneously, trim the skin
lesion and correct the inverted nipple. The amount of
intraoperative bleeding in the precise ultrasound-guided
debridement group was slightly higher than that in the VSD group
but the difference was not statistically significant. The reason
was that the surgical wound was small, it was not possible to
achieve accurate hemostasis during the operation and the dressing
required to be changed more frequently after the operation. In
addition, the pus and necrotic tissue required to be cleared
manually during dressing changes, leading to a significantly higher
pain score on the first and third days after the operation than in
the VSD group. Therefore, VSD technology is suitable for patients
with multiple abscesses, sinus tracts, inverted nipples or obvious
skin ulcerations, while precise ultrasound-guided debridement is
more suitable for patients with abscesses or patients who require
small incisions, short hospitalization times and low
hospitalization costs. As reported in the literature, the course of
GLM may be as long as 10-22 months (28,29),
but the average disease course in the present study was only 5.3
months (1.25-19 months). This suggests that surgery with VSD and
precise ultrasound-guided debridement may be able to markedly
shorten the disease course of non-lactational mastitis. The total
recurrence rate was 6.8% (4/59), which was lower than that reported
in the literature (30).
Non-lactational mastitis is complex. As for refractory
non-lactational mastitis, VSD or precise ultrasound-guided
debridement combined with Traditional Chinese Medicine may achieve
satisfactory clinical effects, avoiding total mastectomy. Of note,
the VSD is suitable for patients with inverted nipples and obvious
skin ulcerations, while the precise ultrasound-guided debridement
is mainly suitable for patients with abscesses, small surgical
incisions and those who require short hospital stays.
However, a limitation of the present study is the
low number of patients enrolled. Including more participants may
help to confirm the findings of the present study. However, the
follow-up rate was 98% in total, the quality and reliability of the
data was good despite small sample size. As another limitation of
the present study, other diagnostic information (such as blood
tests and more detailed clinical stages of the disease) was not
available; however, future studies may be able to include such
additional parameters. Furthermore, the present study was a
clinical study lacking any mechanistic analysis of the two
techniques from a pathophysiological perspective. In addition,
traditional incision and drainage is a therapeutic option for the
disease (6), the present study
lacked a control group subjected to the traditional incision and
drainage and the length of the follow-up time was not sufficient.
These points will be addressed in future studies.
Acknowledgements
Not applicable.
Funding
This project was supported by the research project of Fujian
University of Traditional Chinese Medicine (grant no. XB2017069),
the National Natural Science Foundation of China (grant no.
81704094) and the National Talent Training Program of Innovation in
Traditional Chinese Medicine (grant no. 81704094).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RC and JC designed the study. RC, JC, AP, LY and RZ
performed the trial and collected the clinical data. RC analyzed
and interpreted the data. All authors wrote the manuscript and
revised it for important intellectual content. RC and JC checked
and approved the authenticity of the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee on Scientific Research of Xiamen Hospital of Traditional
Chinese Medicine (Xiamen, China). Written informed consent was
acquired from all patients.
Patient consent for publication
The patients provided written informed consent for
the publication of their data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang L, Li X, Sun B, Ma T, Kong X and
Yang Q: Clinicopathological features of granulomatous lobular
mastitis and mammary duct ectasia. Onco Lett. 19:840–848.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bashir MU, Ramcharan A, Alothman S,
Beaugris S, Khan SA, Sbeih MA and Engdahl R: The enigma of
granulomatous mastitis: A series. Breast Dis. 37:17–20.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ma X, Min X and Yao C: Different
treatments for granulomatous lobular mastitis: A systematic review
and meta-analysis. Breast Care (Basel). 15:60–66. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shin YD, Park SS, Song YJ, Son SM and Choi
YJ: Is surgical excision necessary for the treatment of
Granulomatous lobular mastitis? BMC Womens Health.
17(49)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Y, Song J, Tu Y, Chen C and Sun S:
Minimally invasive comprehensive treatment for granulomatous
lobular mastitis. BMC Surg. 20(34)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li J: Diagnosis and treatment of 75
patients with idiopathic lobular granulomatous mastitis. J Invest
Surg. 32:414–420. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Azlina AF, Ariza Z, Arni T and Hisham AN:
Chronic granulomatous mastitis: Diagnostic and therapeutic
considerations. World J Surg. 27:515–518. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ellis G: How to apply vacuum-assisted
closure therapy. Nurs Stand. 30:36–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Argenta LC and Morykwas MJ:
Vacuum-assisted closure: A new method for wound control and
treatment: Clinical experience. Ann Plast Surg. 38:563–577.
1997.PubMed/NCBI
|
|
10
|
Lozano-Balderas G, Ruiz-Velasco-Santacruz
A, Díaz-Elizondo JA, Gómez-Navarro JA and Flores-Villalba E:
Surgical site infection rate drops to 0% using a vacuum-assisted
closure in contaminated/dirty infected laparotomy wounds. Am Surg.
83:512–514. 2017.PubMed/NCBI
|
|
11
|
Weed T, Ratliff C and Drake DB:
Quantifying bacterial bioburden during negative pressure wound
therapy: Does the wound VAC enhance bacterial clearance? Ann Plast
Surg. 52:276–280. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Orgill DP and Bayer LR: Update on
negative-pressure wound therapy. Plast Reconstr Surg. 127 (Suppl
1):S105–S115. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu C, Zhang T, Deng Z, Ren B, Cai L, Zhang
Y and Yan L: Study on the effect of vacuum sealing drainage on the
repair process of rabbit sciatic nerve injury. Int J Neurosci.
125:855–860. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dixon JM: Mammary duct ectasia-periductal
mastitis complex. Br J Surg. 83:1017–1019. 1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Akcan A, Akyildiz H, Deneme MA, Akgun H
and Aritas Y: Granulomatous lobular mastitis: A complex diagnostic
and therapeutic problem. World J Surg. 30:1403–1409.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baslaim MM, Khayat HA and Al-Amoudi SA:
Idiopathic granulomatous mastitis: A heterogeneous disease with
variable clinical presentation. World J Surg. 31:1677–1781.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gautier N, Lalonde L, Tran-Thanh D, El
Khoury M, David J, Labelle M, Patocskai E and Trop I: Chronic
granulomatous mastitis: Imaging, pathology and management. Eur J
Radiol. 82:e165–e175. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou ZY, Liu YK, Chen HL and Liu F: Wound
management with vacuum assisted closure in surgical site infection
after ankle surgery. Int J Surg. 17:15–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fang LH, Liu SL, Wang RP, Hu SY, Ju WZ and
Li CY: Tounong Powder () extracts induce G1 cell cycle arrest and
apoptosis in LoVo cells. Chin J Integr Med: Jun 29, 2016 (Epub
ahead of print). doi: 10.1007/s11655-016-2597-8.
|
|
20
|
Liao H, Guo J, Chen X, Hua Z, Lin J and
Weng Y: Ultrasound classification-guided minimally invasive rotary
cutting in granulomatous lobular mastitis. BMC Womens Health.
20(252)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hawker GA, Mian S, Kendzerska T and French
M: Measures of adult pain: Visual Analog Scale for Pain (VAS Pain),
Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire
(MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain
Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS),
and Measure of Intermittent and Constant Osteoarthritis Pain
(ICOAP). Arthritis Care Res (Hoboken). 63 (Suppl 11):S240–S252.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Barreto DS, Sedgwick EL, Nagi CS and
Benveniste AP: Granulomatous mastitis: Etiology, imaging,
pathology, treatment, and clinical findings. Breast Cancer Res
Treat. 171:527–534. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nikolaev A, Blake CN and Carlson DL:
Association between hyperprolactinemia and granulomatous mastitis.
Breast J. 22:224–231. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gurleyik G, Aktekin A, Aker F, Karagulle H
and Saglamc A: Medical and surgical treatment of idiopathic
granulomatous lobular mastitis: A benign inflammatory disease
mimicking invasive carcinoma. J Breast Cancer. 15:119–123.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tsuji T, Satoh K, Okuno E, Sobue A,
Nishide Y, Tanaka S and Kogo M: The utility of vacuum-assisted
closure therapy for skin necrosis secondary to cervical abscess in
the elderly. Auris Nasus Larynx. 44:749–753. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Krasner DL: Managing wound pain in
patients with vacuum-assisted closure devices. Ostomy Wound Manage.
48:38–43. 2002.PubMed/NCBI
|
|
27
|
Silvis RS, Potter LE, Robinson DW and
Hughes WF: The use of continuous suction negative pressure instead
of pressure dressing. Ann Surg. 142:252–256. 1955.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mahlab-Guri K, Asher I, Allweis T, Diment
J, Sthoeger ZM and Mavor E: Granulomatous lobular mastitis. Isr Med
Assoc J. 17:476–480. 2015.PubMed/NCBI
|
|
29
|
Bouton ME, Jayaram L, O'Neill PJ, Hsu CH
and Komenaka IK: Management of idiopathic granulomatous mastitis
with observation. Am J Surg. 210:258–262. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yılmaz TU, Gürel B, Güler SA, Baran MA,
Erşan B, Duman S and Utkan Z: Scoring idiopathic granulomatous
mastitis: An effective system for predicting recurrence? Eur J
Breast Health. 14:112–116. 2018.PubMed/NCBI View Article : Google Scholar
|