Introduction
Anesthetic agents serve an important role in
relieving pain in patients with traumatic injuries and in surgical
procedures, but managing the utilization of anesthetic agents is a
challenge for the anesthesiologist (1). A variety of anesthetic agents have
been developed, including volatile anesthetics, nitrous oxide,
xenon, propofol, ketamine, etomidate, dexmedetomidine, opioids,
benzodiazepines, barbiturates, lidocaine and regional anesthesia
(1). Different anesthetic agents
exert distinct biological and physiological functions in the body,
whereas determining the molecular and cellular functions of
anesthetic agents is a challenge due to their pleiotropic effect
(2). Besides their anesthetic
effects, a number of anesthetic agents exert other functions, such
as antimicrobial effects (3),
synaptic inhibition (4), disruption
of brain circuit formation (5) and
left ventricular systolic function (ketamine and xylazine
increasing the left ventricular ejection fraction and decreasing
the left ventricular end diastolic diameter) (6). It has also been reported that certain
anesthetic agents display cytotoxicity, neurotoxicity and
genotoxicity (7). Inflammation is
an innate physiological process in the body, which may have both
favorable and unfavorable consequences for an individual's health.
Inflammation protects against harmful pathogens and is activated
during acute and chronic diseases (8). Inflammation is a complex biological
network that involves a number of transcription factors, including
NF-κB and STAT3, inflammatory enzymes and inflammatory cytokines
(8). The NF-κB signaling pathway
serves a central role in regulating inflammation, and functions in
various organs and tissues. Emerging evidence indicates that
inflammation is related to surgical processes, which should be
considered during the management of surgery. For example, if
trauma-induced inflammation is not appropriately regulated,
neuro-inflammation may interfere with synaptic plasticity to affect
learning and memory aspects of cognition (9). By contrast, surgical manipulation
causes stress responses, inhibits immune cells and suppresses
cell-mediated immunity (10).
Increasing evidence indicates that anesthetic agents regulate
immune reactions in the body (11).
Therefore, the optimal choice of anesthetic agents serves an
important role in health management during surgery. However, how
anesthetic agents affect the immune system is not completely
understood.
It has been reported that several anesthetic agents
exert immunosuppressive functions by suppressing the viability of
immune cells (11). Different
anesthetic agents exert different effects on a variety of immune
cells, for example, ketamine and thiopental, but not propofol,
inhibit natural killer cells, whereas ketamine, but not midazolam,
causes T-lymphocyte apoptosis (11). Investigating the effects of
different anesthetic agents on inflammation is important due to the
link between surgery and inflammation, and understanding this link
is important for regulating the effects of anesthetic agents on
inflammation (10).
The present study aimed to investigate the effects
of five anesthetic agents, including sodium barbiturate, midazolam,
etomidate, ketamine and propofol, on inflammation in three cell
lines (Caco-2, HK-2 and HepG2). The results of the present study
may aid with choosing the suitable anesthetic agent for surgical
procedures.
Materials and methods
Study design
To investigate the effects of various commonly used
anesthetic agents on different cell lines, five commonly used
anesthetic agents including sodium barbiturate, midazolam,
etomidate, ketamine and propofol at different concentrations were
used to treat Caco-2 (intestine), HK-2 (kidney) and HepG2 (liver)
cells. Caco-2 cell line is a continuous line of heterogeneous human
epithelial colorectal adenocarcinoma cells, which has been used for
intestine studies (12,13). HK-2 cell line is an immortalized
proximal tubular cell line derived from normal kidney, which has
been widely used for kidney studies (14,15).
HepG2 is a liver cancer cell line, which has been used for liver
studies (16,17). Reverse transcription-quantitative
PCR (RT-qPCR) and western blotting assays were used to determine
the expression levels of NF-κB and its downstream cytokines in
cells treated with different anesthetic agents.
Chemicals and drugs
The following anesthetic agents were used: Sodium
barbiturate
[C4H3N2NaO3; molecular
weight, 150.07 g/mol; Chemical Abstracts Service (CAS) no.
4390-16-3], midazolam
(C18H13ClFN3; molecular weight,
325.77 g/mol; CAS no. 59467-70-8), etomidate
(C14H16N2O2; molecular
weight, 244.29; CAS no. 33125-97-2), ketamine
(C13H17Cl2NO; molecular weight,
274.19; CAS no. 1867-66-9) and propofol
[(CH3)2CH2C6H3OH;
molecular weight, 178.27; CAS no. 2078-54-8]. All chemicals were
supplied by Sigma-Aldrich; Merck KGaA, diluted according to
manufacturer's instruction, aliquoted and frozen at -80˚C prior to
use. Control groups were non-treated cells.
Cell culture
Caco-2, HK-2 and HepG2 cells were purchased from
American Type Culture Collection and cryopreserved until use. After
thawing, cells were sub-cultured at least twice prior to
experimental use. All cells were cultured according to
manufacturer's instructions. Briefly, cells were cultured in DMEM
(cat. no. 10567014; Thermo Fisher Scientific, Inc.) supplemented
with 10% (v/v) FBS (Invitrogen; Thermo Fisher Scientific, Inc.) and
1% penicillin-streptomycin (cat. no. 15140163; Thermo Fisher
Scientific, Inc.). The culture medium was refreshed every 3 days.
Cells were maintained at 37˚C with 5% CO2 in a
humidified atmosphere and routinely sub-cultured twice a week at
70-80% confluence using 0.5% trypsin-EDTA (cat. no. 25200072;
Thermo Fisher Scientific, Inc.).
Drug treatment
At >90% confluence in T75 flasks, cells were
dissociated using TrpyLE (cat. no. 12604013; Thermo Fisher
Scientific, Inc.) and seeded into 48-well plates
(2.5x104 cell per well). At 30-40% confluence, cells
were treated with 0, 0.1, 1.0, 2.0, 5.0 or 10.0 µM sodium
barbiturate, midazolam, etomidate, ketamine or propofol for 48 h at
37˚C with 5% CO2 in a humidified atmosphere. The doses
of drugs were selected based on the clinical experience of the
authors. A previous study indicated that the plasma concentration
of barbiturate should be below 4.4 µg/ml (~24 µM; measured on the
day when burst-suppression pattern had disappeared) (18). In addition, concentration of
midazolam in patients was between 20 and 100 µM (blood was
collected at 30-45 min before the patient came to the operating
room) (19), the steady state
plasma concentration of etomidate was 158 µg/l (~0.65 µM; measured
for periods of up to 24 h after stopping the infusion) (20), the plasma concentration of ketamine
at steady-state was 1,018.7 ng/ml (~4.29 µM; the average of the
three plasma samples collected at 20, 42, and 54 half-lives during
continuous infusion) (21) and the
concentration of propofol was 4-6 µg/l (22.50-33.75 µM; the time
point of the measurement was not mentioned) (22). According to the aforementioned
clinical studies, the plasma concentration of anesthetic agents
should be between 0.65 to 33.75 µM. In combination with the
experience of the current authors, the maximum concentration of the
anesthetic agents used was 10.0 µM
For the co-treatment of TNFα (100 nM) and anesthetic
agents (10 µM) including sodium barbiturate, ketamine and propofol,
cells were treated with a cocktail of TNFα and sodium barbiturate,
TNFα and ketamine, and TNFα and propofol for 48 h at 37˚C with 5%
CO2 in a humidified atmosphere. Medium without any drugs
was set as the control.
RNA isolation and RT-qPCR
Total RNA from cells including Caco-2, HK-2 and
HepG2 cells was isolated using the RNAeasy™ kit (Beyotime Institute
of Biotechnology) according to the manufacturer's protocol. Total
RNA was eluted with nuclease-free water and treated with DNase I
(Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, total
RNA (1 µg) was reverse transcribed into cDNA using the ReverTra
Ace® RT system (Toyobo Life Science) according to the
manufacturer's protocol. qPCR was performed using the iCycler iQ
system (Bio-Rad Laboratories, Inc.) and IQ SYBR Green Supermix
(Bio-Rad Laboratories, Inc.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95˚C for
5 min; 40 cycles of 95˚C for 15 sec and 60˚C for 35 sec. The
sequences of the primers used for qPCR are presented in Table I. The primers were designed using
NCBI Pick Primers (https://www.ncbi.nlm.nih.gov/tools/primer-blast/)
and manufactured by Sangon Biotech Co., Ltd. The mRNA expression
levels were quantified using the 2-ΔΔCq method and
normalized to the internal reference gene GAPDH according to a
previous study (12). RT-qPCR was
performed in triplicate.
 | Table IPrimers for reverse
transcription-quantitative PCR. |
Table I
Primers for reverse
transcription-quantitative PCR.
| Primer | Sequence
(5'-3') | Product size,
nt |
|---|
| NF-κB | F:
ACAGCGGGGAAAGACACATC | 221 |
| | R:
TCTGCCATTCTGAAGCTCTCTC | |
| IL-1B | F:
AGCCATGGCAGAAGTACCTG | 116 |
| | R:
CCTGGAAGGAGCACTTCATCT | |
| IL-18 | F:
TGCAGTCTACACAGCTTCGG | 99 |
| | R:
GCAGCCATCTTTATTCCTGCG | |
| GAPDH | F:
AATGGGCAGCCGTTAGGAAA | 166 |
| | R:
GCCCAATACGACCAAATCAGAG | |
Western blot analysis
Caco-2, HK-2 and HepG2 cells treated with 10 µM
sodium barbiturate, ketamine and propofol were lysed using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) for 30 min on ice. The supernatant was aspirated for
the determination of protein concentration using a bicinchoninic
acid assay kit, after centrifugation at 1,500 x g and 4˚C for 10
min. The samples were heated for 10 min at 95˚C. Afterwards, the
samples (30 µg/lane) were subjected to SDS-PAGE (10% gel) at 110 V,
followed by protein transfer to polyvinylidene difluoride membranes
on ice at 100 V for 2 h. The membranes were blocked with 5% skimmed
milk diluted in ddH2O at room temperature for 1 h and
subsequently incubated with rabbit anti-IL-1β (D3U3E; cat. no.
12703), anti-IL-18 (D2F3B; cat. no. 54943) and anti-β-actin (13E5;
cat. no. 4970) antibodies at 4˚C overnight (all at 1:1,000
dilution; Cell Signaling Technology, Inc.). Then, the membranes
were washed three times with PBS supplemented with 0.1% Tween-20
for 15 min, followed by an incubation with HRP-conjugated goat
anti-rabbit IgG H&L (1:5,000; cat. no. ab6721; Abcam) for 1 h
at room temperature before washing with PBS with 0.1% Tween-20
three times for 15 min. β-actin was used as the endogenous control.
The bands were visualized using Odyssey CLx Imager (LI-COR
Biosciences) and quantified using ImageJ software (64-bit Java
1.8.0_172; National Institutes of Health).
Cell Counting Kit-8 (CCK-8)
Caco-2, HK-2 and HepG2 cells at a density of
1x104 cells/well were seeded into a 96-well-plate
(Corning Inc.) and cultured as aforementioned for 24 h. Then, the
cells were treated with the aforementioned concentrations of sodium
barbiturate, ketamine and propofol for 48 h at 37˚C. Subsequently,
the cells were treated with 10 µl CCK-8 reagent (cat. no. C0037;
Beyotime Institute of Biotechnology) for an additional 1 h at 37˚C
in the dark. The absorbance was measured using a microplate reader
(Bio-Rad Laboratories, Inc.) at a wavelength of 450 nm.
Statistical analysis
Statistical analyses were performed using one-way
ANOVA followed by Tukey's test using GraphPad Prism software
(version 5.0; GraphPad Software, Inc.). Data are presented as the
mean ± SEM of at least three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
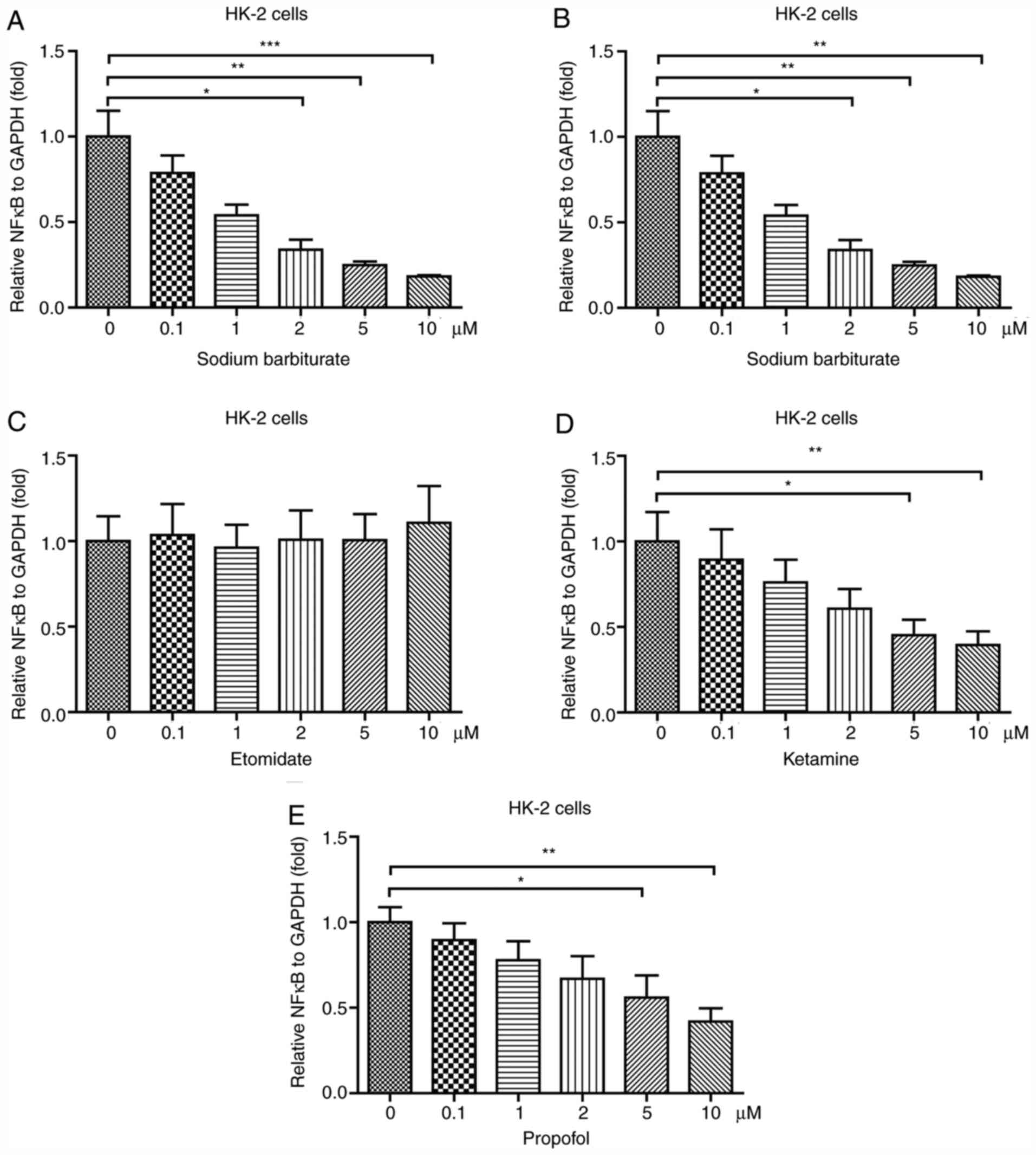
Sodium barbiturate, ketamine and
propofol decrease NF-κB expression in HK-2 cells
To investigate the effects of different anesthetic
agents on inflammation in the kidney, an immortalized proximal
tubule epithelial cell line HK-2 was used. HK-2 cells were treated
with different concentrations of five different anesthetic agents,
including sodium barbiturate, midazolam, etomidate, ketamine and
propofol. The results indicated that sodium barbiturate (2, 5 and
10 µM) significantly decreased the mRNA expression levels of NF-κB
in a dose-dependent manner (Fig.
1A). However, midazolam (Fig.
1B) and etomidate (Fig. 1C) did
not significantly alter NF-κB mRNA expression levels in HK-2 cells
compared with the control group. Ketamine and propofol (5 and 10
µM) significantly reduced mRNA expression levels of NF-κB in HK-2
cells (Fig. 1D and E). Collectively, the results suggested
that anesthetic agents, including sodium barbiturate, ketamine and
propofol, decreased NF-κB mRNA expression levels in HK-2 cells,
whereas midazolam and etomidate displayed no effect on NF-κB mRNA
expression levels compared with the control cells.
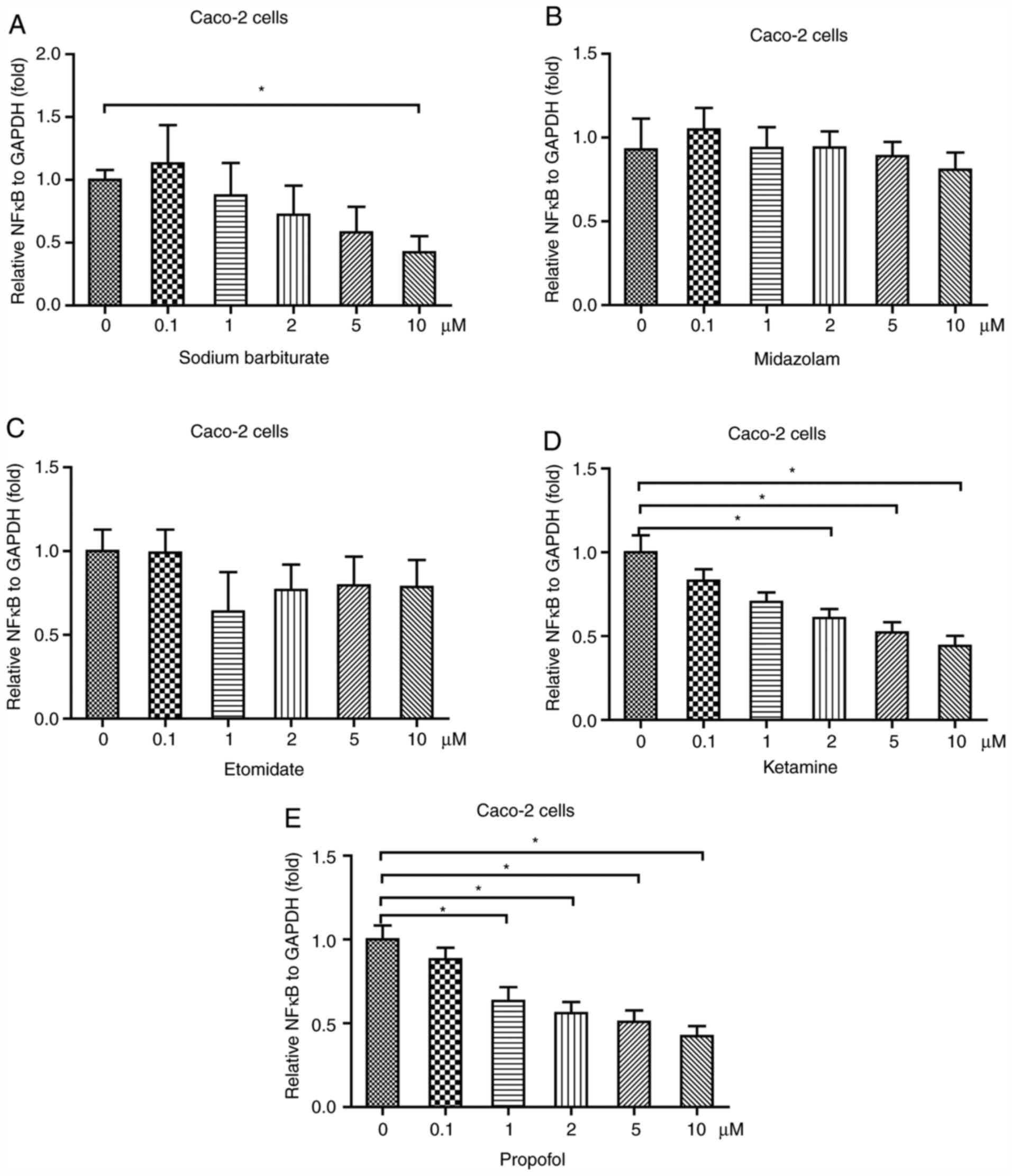
Sodium barbiturate, ketamine and
propofol decrease NF-κB expression in Caco-2 cells
The intestine is a location where inflammation often
occurs (23). To investigate the
effects of anesthetic agents on inflammation in the intestine, a
commonly used intestinal cell line (Caco-2) was used (12,24).
The results indicated that 10 µM sodium barbiturate significantly
decreased the mRNA expression levels of NF-κB in Caco-2 cells
(Fig. 2A). Similar to in HK-2
cells, in Caco-2 cells, the results suggested that midazolam
(Fig. 2B) and etomidate (Fig. 2C) did not significantly alter the
mRNA expression levels of NF-κB. At 2, 5 and 10 µM, ketamine
significantly decreased NF-κB mRNA expression levels in Caco-2
cells in a dose-dependent manner (Fig.
2D). Moreover, at 1, 2, 5 and 10 µM, propofol significantly
reduced the mRNA expression levels of NF-κB in HK-2 cells in a
dose-dependent manner (Fig. 2E).
Therefore, the results indicated that anesthetic agents, including
sodium barbiturate, ketamine and propofol, rather than midazolam
and etomidate, decreased the mRNA expression levels of NF-κB in
Caco-2 cells.
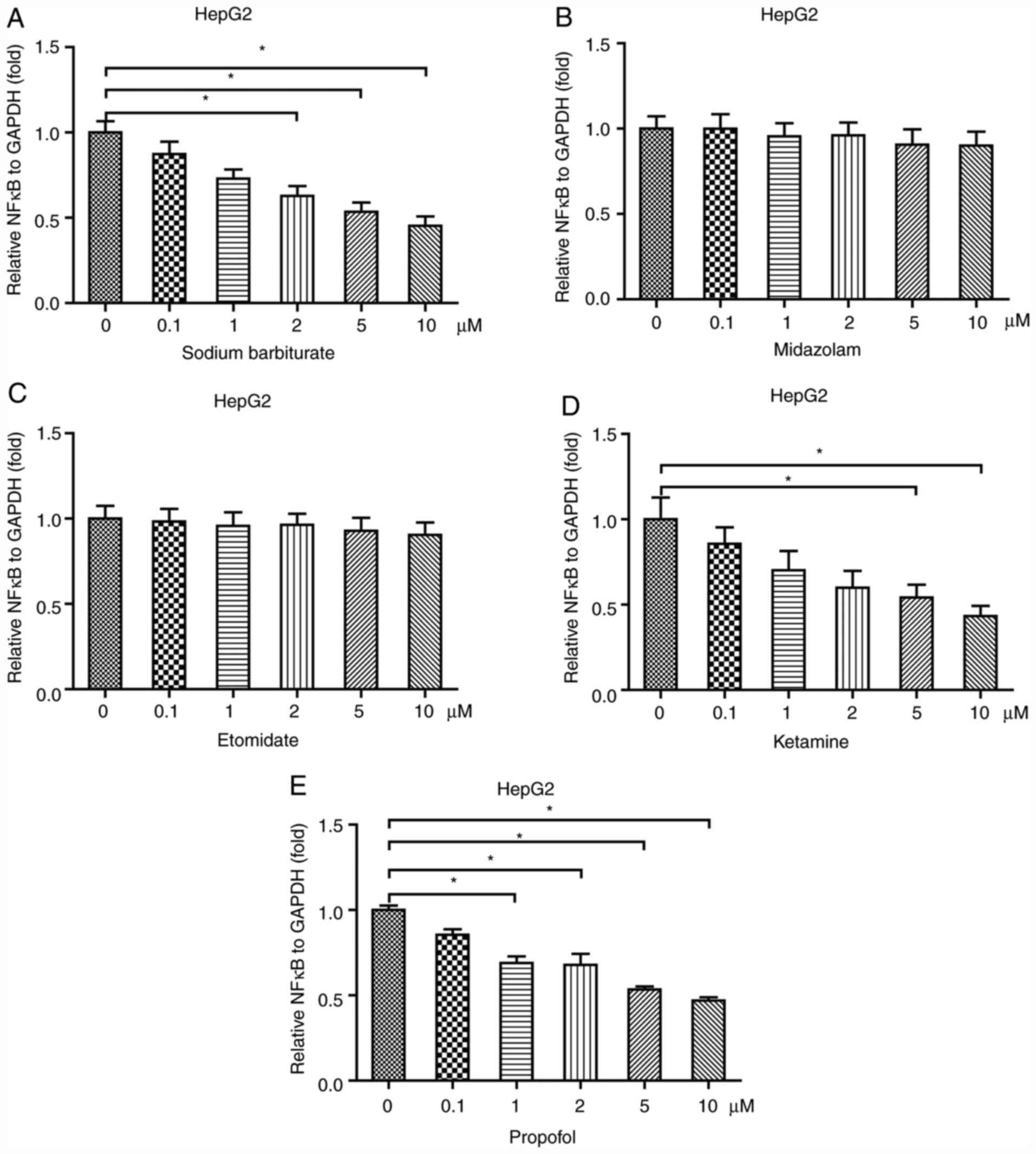
Sodium barbiturate, ketamine and
propofol reduce NF-κB expression in HepG2 cells
The liver is also an important organ where
inflammation may occur (25). To
examine the effects of anesthetic agents on inflammation in the
liver, a liver cell line HepG2 was treated with different
concentrations of sodium barbiturate, midazolam, etomidate,
ketamine and propofol. Sodium barbiturate (2, 5 and 10 µM)
significantly decreased the mRNA expression levels of NF-κB in
HepG2 cells (Fig. 3A). Similar to
in HK-2 and Caco-2 cells, the results indicated that midazolam
(Fig. 3B) and etomidate (Fig. 3C) had no significant effect on the
mRNA expression levels of NF-κB in HepG2 cells. Ketamine (5 and 10
µM) significantly decreased NF-κB mRNA expression levels in HepG2
cells in a dose-dependent manner (Fig.
3D). Propofol (1, 2, 5 and 10 µM) significantly reduced the
mRNA expression levels of NF-κB in HepG2 cells in a dose-dependent
manner (Fig. 3E). Collectively, the
results indicated that anesthetic agents, including sodium
barbiturate, ketamine and propofol, but not midazolam and
etomidate, decreased the mRNA expression levels of NF-κB in HepG2
cells.
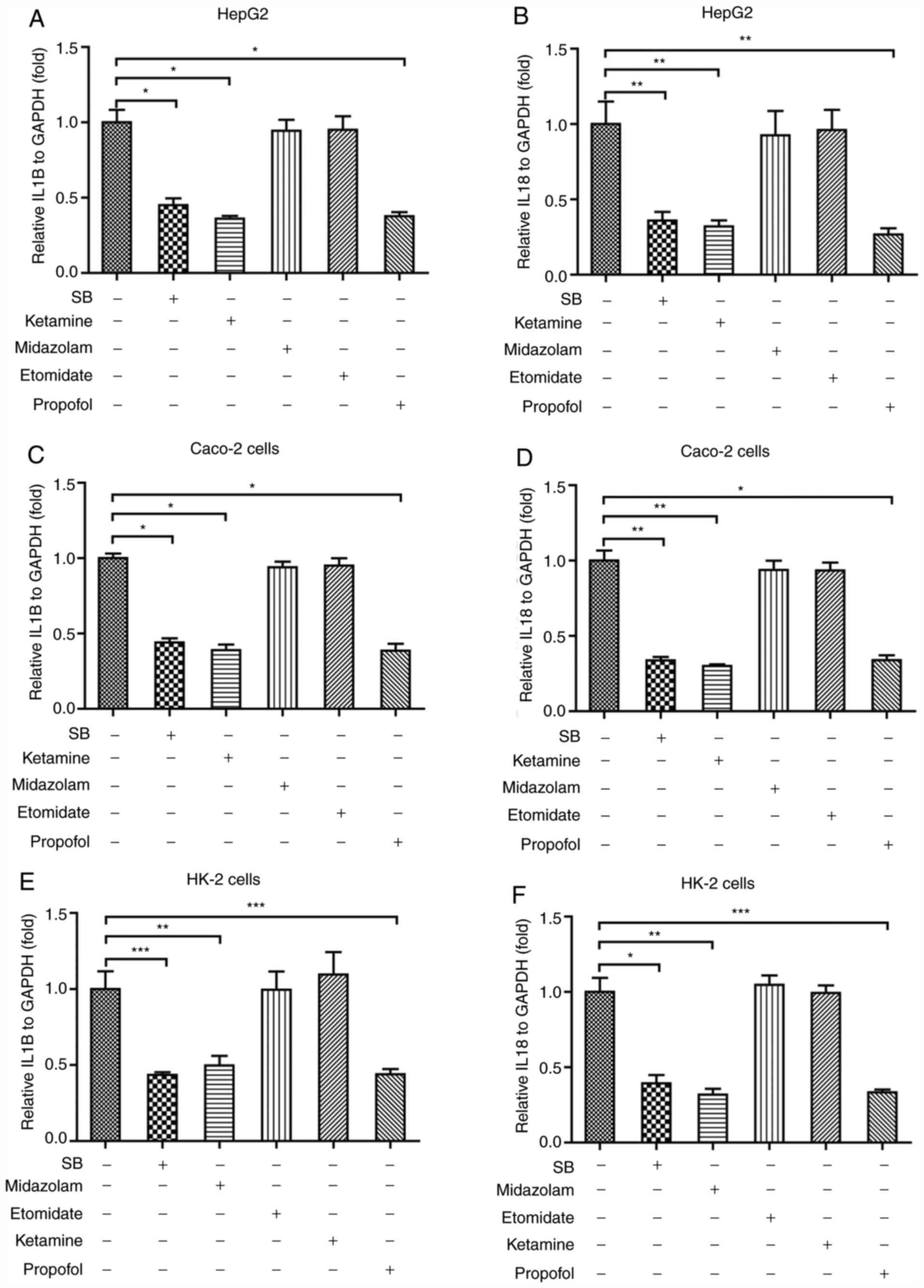
Sodium barbiturate, ketamine and
propofol downregulate NF-κB downstream cytokines in the three cell
lines
The NF-κB signaling pathway serves a central role in
regulating inflammatory activities in the body, and can activate a
variety of downstream cytokines, including IL1β and IL18, to result
in inflammation (26). To further
verify the effects of anesthetic agents on inflammation, the
effects of sodium barbiturate, midazolam, etomidate, ketamine and
propofol on IL-1β and IL-18 expression levels were assessed in the
three different cell lines. Sodium barbiturate, ketamine and
propofol significantly decreased the mRNA expression levels of
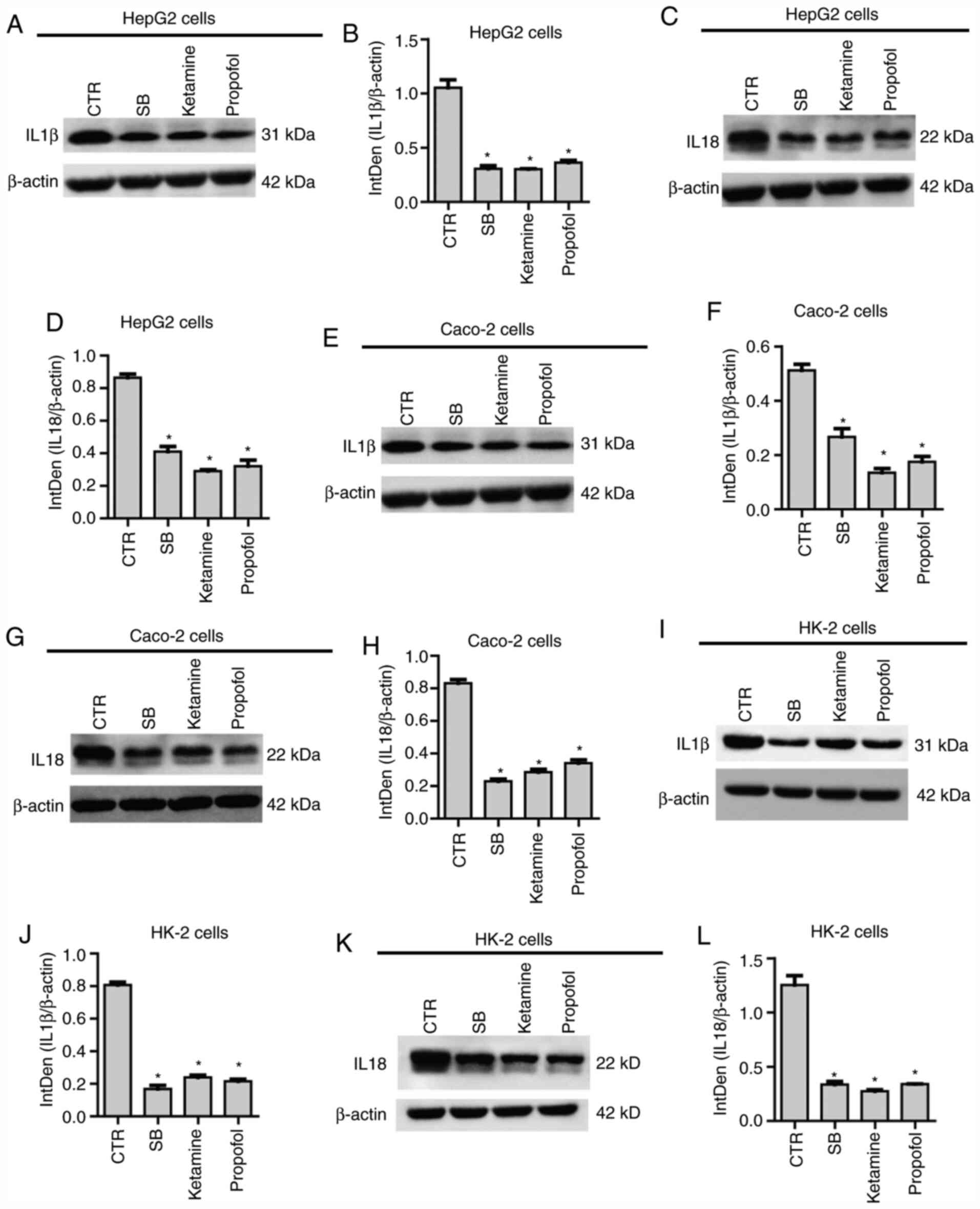
IL-1β (Fig. 4A) and IL-18 (Fig. 4B) in HepG2 cells, which was
consistent with the observations that the three anesthetic agents
downregulated NF-κB expression. As in HepG2 cells, sodium
barbiturate, ketamine and propofol, but not midazolam and
etomidate, significantly reduced the mRNA expression levels of
IL-1β (Fig. 4C) and IL-18 (Fig. 4D) in Caco-2 cells. Finally, the
results indicated that sodium barbiturate, ketamine and propofol,
but not midazolam and etomidate, significantly decreased the mRNA
expression levels of IL-1β (Fig.
4E) and IL-18 (Fig. 4F) in HK-2
cells. To further verify the effects of ketamine, midazolam and
propofol on IL-1β and IL-18 expression levels, western blotting was
performed. The results indicated that sodium barbiturate, midazolam
and propofol decreased the protein expression levels of IL-1β
(Fig. 5A and B) and IL-18 (Fig. 5C and D) in HepG2 cells. In parallel, sodium
barbiturate, midazolam and propofol decreased the protein
expression levels of IL-1β (Fig. 5E
and F) and IL18 (Fig. 5G and H) in Caco-2 cells. Similarly, sodium
barbiturate, midazolam and propofol decreased the protein
expression levels of IL-1β (Fig. 5I
and J) and IL-18 (Fig. 5K and L) in HK-2 cells. Therefore, the results
indicated that sodium barbiturate, ketamine and propofol suppressed
inflammation in the three different cell lines used in the present
study.
Sodium barbiturate, ketamine and
propofol inhibit TNFα-mediated activation of NF-κB signaling in the
three different cell lines
TNF-α is an activator of inflammation (27). To further investigate the effects on
anesthetic agents on inflammation, cells were co-treated with TNF-α
and sodium barbiturate, ketamine or propofol. The three
aforementioned anesthetic agents were used as the results indicated
that these agents significantly reduced the expression levels of
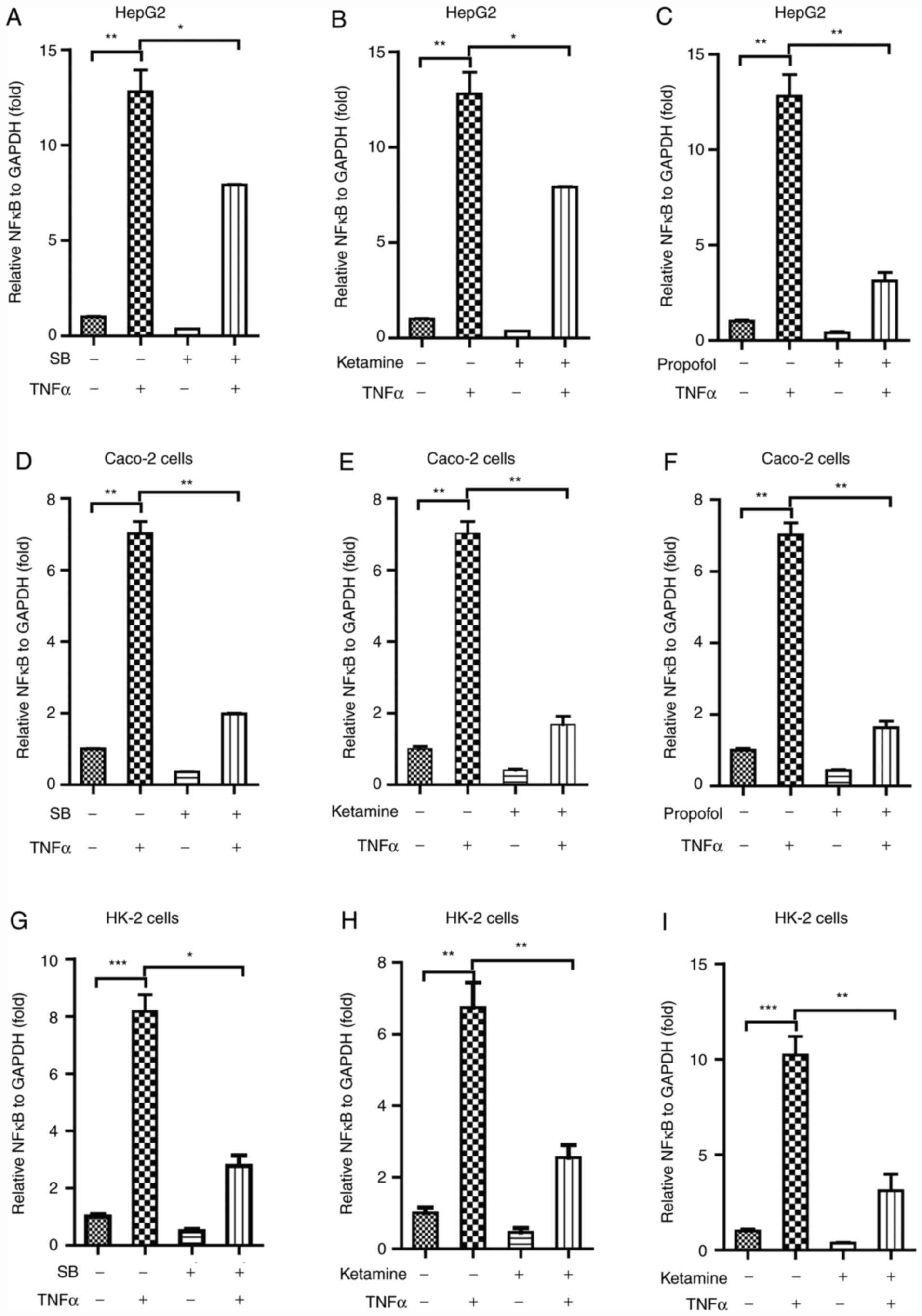
NF-κB and its downstream effectors. TNFα (100 nM) markedly
increased the mRNA expression level of NF-κB in HepG2 cells, as
determined using RT-qPCR (Fig.
6A-C). Of note, sodium barbiturate (Fig. 6A), ketamine (Fig. 6B) and propofol (Fig. 6C) significantly inhibited
TNFα-mediated NF-κB expression in HepG2 cells. Similarly, TNFα
markedly increased the mRNA expression levels of NF-κB in Caco-2
cells (Fig. 6D-F), and sodium
barbiturate (Fig. 6D), ketamine
(Fig. 6E) and propofol (Fig. 6F) significantly inhibited
TNFα-mediated NF-κB expresion in Caco-2 cells, as determined using
RT-qPCR. TNFα significantly increased the mRNA expression levels of
NF-κB in HK-2 cells (Fig. 6G-I),
and sodium barbiturate (Fig. 6G),
ketamine (Fig. 6H) and propofol
(Fig. 6I) significantly inhibited
TNFα-mediated NF-κB expression in HK-2 cells, as determined using
RT-qPCR. Collectively, the results indicated that sodium
barbiturate, ketamine and propofol inhibited TNFα-mediated
activation of NF-κB signaling in HepG2, Caco-2 and HK-2 cells.
Effects of sodium barbiturate,
ketamine and propofol on HepG2, Caco-2 and HK-2 cell viability
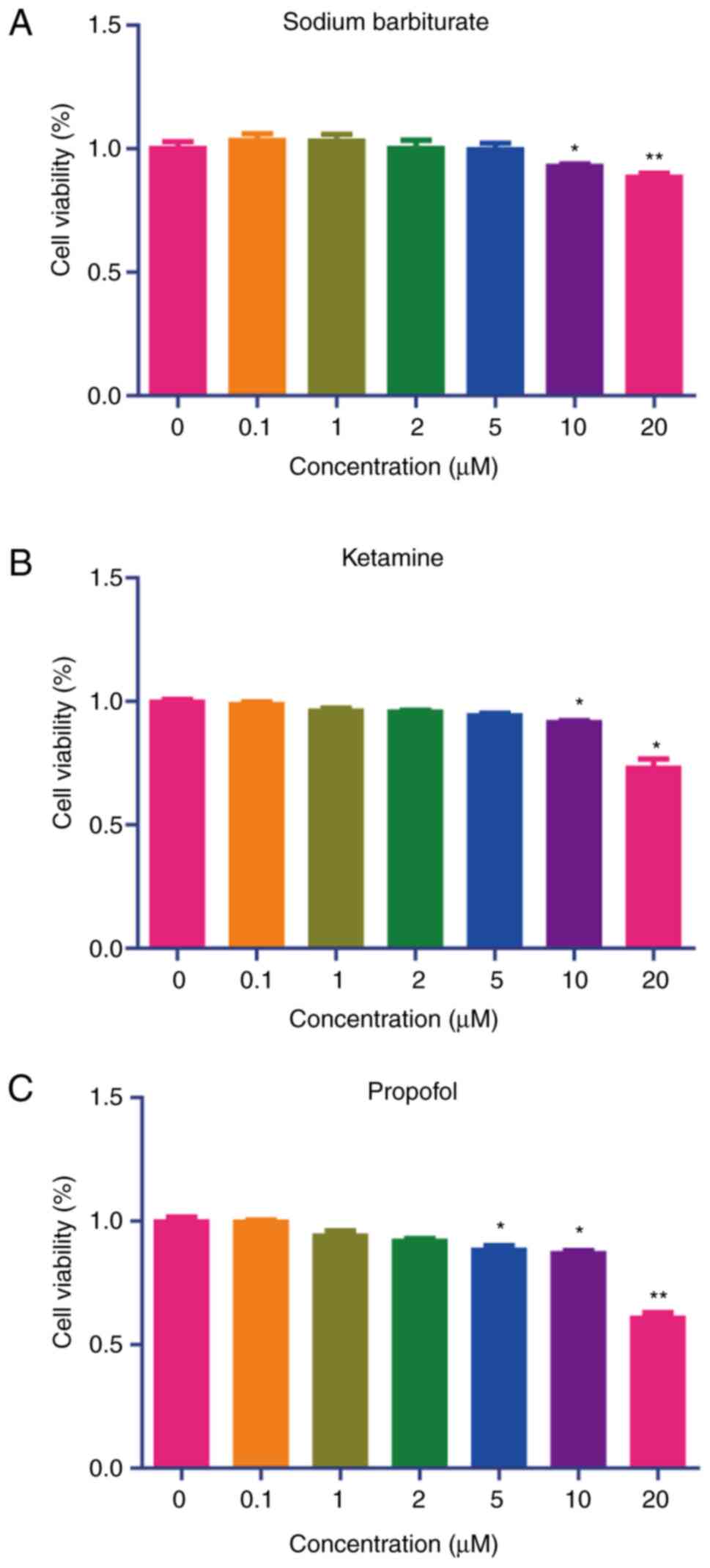
To determine the effects of sodium barbiturate,
ketamine and propofol on the viability of HepG2, Caco-2 and HK-2
cells, a CCK-8 assay was performed, and it was indicated that 10
and 20 µM sodium barbiturate had slight cytotoxicity on HepG2 and
Caco-2 cells, respectively (Fig. 7A
and B). Furthermore, 20 µM sodium
barbiturate exhibited cytotoxicity on HK-2 cells (Fig. 7C). High concentrations (10 and 20
µM) of ketamine showed significant cytotoxicity on HepG2, Caco-2
and HK-2 cells, respectively (Fig.
7D, E and F). Similarly, 5, 10 and 20 µM of propofol
showed significant cytotoxicity on HepG2 cells (Fig. 7G). Furthermore, 10 and 20 µM
propofol produced cytotoxicity on Caco-2 (Fig. 7H), and 20 µM propofol produced
cytotoxicity on HK-2 cells (Fig.
7I). Thus, low concentrations (<10 µM) of sodium
barbiturate, ketamine and propofol had minor cytotoxic effects
while high concentrations (≥10 µM) had significant effects on the
viability of HepG2, Caco-2 and HK-2 cells.
Discussion
The association between surgical procedures and
inflammation has received increasing attention (28). Since anesthetic agents are widely
used during surgical procedures to manage the pain and comfort of
patients, the effects of anesthetic agents on inflammation have
received increasing attention. Therefore, the present study
investigated the effects of five commonly used anesthetic agents,
including sodium barbiturate, midazolam, etomidate, ketamine and
propofol, on inflammation in Caco-2, HK-2 and HepG2 cells. The
results indicated that three out of the five anesthetic agents,
sodium barbiturate, ketamine and propofol, significantly decreased
the expression levels of NF-κB and its downstream cytokines,
including IL-1β and IL-18, in the three cell lines. Moreover, the
results indicated that sodium barbiturate, ketamine and propofol
inhibited TNFα-mediated activation of NF-κB signaling in HepG2,
Caco-2 and HK-2 cells. The present study provided novel insight
into the molecular mechanisms underlying anesthetic agent-mediated
regulation of inflammation and may aid in selecting an appropriate
anesthetic agent for surgical procedures.
Surgery can cause inflammation, for example, it was
reported that colorectal surgery often causes systemic inflammatory
response syndrome, which may cause post-operative morbidity and
mortality (28). The pathogenesis
underlying inflammation induced by surgery is not completely
understood. Numerous anesthetic agents are used in surgical
procedures. Several studies have demonstrated that certain
anesthetic agents are linked to inflammation. Getachew et al
(29) reported that ketamine
exerted antidepressant and anti-inflammatory effects via
interacting with specific gut bacteria in rats. Ketamine was
reported to control innate immunity in the body and regulate the
functions of a number of cellular effectors in the inflammatory
reaction (30). Chang et al
(31) reported that ketamine could
affect macrophages to suppress NF-κB-mediated responses to
lipopolysaccharide (LPS). Chang et al (32) also reported that ketamine inhibited
hypoxia-induced inflammatory responses in late-gestation ovine
fetal kidney cortex. In the present study, ketamine markedly
decreased NF-κB expression in kidney, intestine and liver cell
lines, and further downregulated the expression levels of NF-κB
downstream cytokines, which was consistent with previous reports.
The present study also indicated that ketamine significantly
suppressed TNFα-mediated activation of NF-κB signaling in kidney,
intestine and liver cell lines. The results of the present study
demonstrated the inhibitory effects of ketamine on inflammation.
The kidney, intestine and liver contain rich immune cells, which
often generate immune reactions in response physiological changes
(33). The results of the present
study may aid with the identification of a suitable anesthetic
agent for surgical procedures on the kidney, intestine and
liver.
Propofol has been used in surgery for a number of
years (34). Accumulating evidence
demonstrated that propofol exerted regulatory effects on
inflammation. Jia et al (35) confirmed that propofol could suppress
LPS-induced inflammation via the PI3K signaling pathway in
microglia. Jia et al (35)
reported that propofol suppressed the secretion of cytokines,
including IL8, IL6 and TNFα, in LPS-treated RAW 264.7 cells.
Consistently, the present study indicated that propofol
downregulated the expression levels of TNFα, IL1β and IL18 in
Caco-2, HK-2 and HepG2 cells. The results also indicated that
propofol inhibited TNFα-mediated activation of NF-κB signaling in
the three cell lines.
Furthermore, the present study suggested that sodium
barbiturate exerted anti-inflammatory effects in different types of
cells. A previous study reported that a barbituric acid derivative
exerted an inhibitory effect on the NF-κB signaling pathway in
hepatic stellate cells (36). In
addition, O'Sullivan et al (37) reported that dinitrate-barbiturate
served as an anti-inflammatory agent, which could be used for the
treatment of inflammatory bowel disease.
It has been reported that several anesthetic agents
exert immune regulatory effects in in vitro models. However,
the present study had a number of limitations: i) There are other
types of anesthetic agents in addition to sodium barbiturate,
midazolam, etomidate, ketamine and propofol, thus, screening the
effects of a large number of anesthetic agents on inflammation to
obtain a profile of the effects of anesthetic agents on immunity is
important; ii) cell line models are less reliable compared with
more advanced in vitro models, such as organoid models
(38), therefore, validating the
results of the present study in more advanced organoids is
required; and iii) although the preliminary mechanism underlying
the effects of anesthetic agents on inflammation was identified in
the present study, future studies should investigate the precise
mechanism of action.
In conclusion, the inflammatory reaction serves a
key role in maintaining body homeostasis and survival, especially
in the context of surgical procedures (39). Moreover, three out of the five
anesthetic agents, including sodium barbiturate, ketamine and
propofol, displayed anti-inflammatory effects in kidney, intestine
and liver cells. Midazolam and etomidate did not display
significant effects on inflammation in kidney, intestine and liver
cells. Sodium barbiturate, ketamine and propofol decreased
TNFα-mediated effects on inflammatory gene expression levels. The
results of the present study provide further understanding of the
effects of anesthetic agents on inflammation and may aid in
selecting a suitable anesthetic agent in surgical procedures.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL and XH contributed to study conception, design
and management. WL, YL, TT, HX and LJ performed the experiments and
collected and analyzed the data. WL contributed to manuscript
writing and draft preparation. XH contributed to manuscript
revision. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Slupe AM and Kirsch JR: Effects of
anesthesia on cerebral blood flow, metabolism, and neuroprotection.
J Cereb Blood Flow Metab. 38:2192–2208. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Armstrong R, Riaz S, Hasan S, Iqbal F,
Rice T and Syed N: Mechanisms of anesthetic action and
neurotoxicity: Lessons from molluscs. Front Physiol.
8(1138)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kaewjiaranai T, Srisatjaluk RL,
Sakdajeyont W, Pairuchvej V and Wongsirichat N: The efficiency of
topical anesthetics as antimicrobial agents: A review of use in
dentistry. J Dent Anesth Pain Med. 18:223–233. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
MacIver MB: Anesthetic agent-specific
effects on synaptic inhibition. Anesth Analg. 119:558–569.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wagner M, Ryu YK, Smith SC, Patel P and
Mintz CD: Review: Effects of anesthetics on brain circuit
formation. J Neurosurg Anesthesiol. 26:358–362. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tanaka DM, Romano MM, Carvalho EE,
Oliveira LF, Souza HC, Maciel BC, Salgado HC, Fazan-Junior R and
Simoes MV: Effect of different anesthetic agents on left
ventricular systolic function assessed by echocardiography in
hamsters. Braz J Med Biol Res. 49(e5294)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Braz MG and Karahalil B: Genotoxicity of
anesthetics evaluated in vivo (animals). Biomed Res Int.
2015(280802)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kunnumakkara AB, Sailo BL, Banik K, Harsha
C, Prasad S, Gupta SC, Bharti AC and Aggarwal BB: Chronic diseases,
inflammation, and spices: How are they linked? J Transl Med.
16(14)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saxena S and Maze M: Impact on the brain
of the inflammatory response to surgery. Presse Med. 47:e73–e81.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chiang N, Schwab JM, Fredman G, Kasuga K,
Gelman S and Serhan CN: Anesthetics impact the resolution of
inflammation. PLoS One. 3(e1879)2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim R: Effects of surgery and anesthetic
choice on immunosuppression and cancer recurrence. J Transl Med.
16(8)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kampfer AAM, Urban P, Gioria S, Kanase N,
Stone V and Kinsner-Ovaskainen A: Development of an in vitro
co-culture model to mimic the human intestine in healthy and
diseased state. Toxicol In Vitro. 45:31–43. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ryan MJ, Johnson G, Kirk J, Fuerstenberg
SM, Zager RA and Torok-Storb B: HK-2: An immortalized proximal
tubule epithelial cell line from normal adult human kidney. Kidney
Int. 45:48–57. 1994.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang L, Liu N, Xue X and Zhou S: The
effect of overexpression of the enhancer of zeste homolog 1 (EZH1)
gene on aristolochic acid-induced injury in HK-2 human kidney
proximal tubule cells in vitro. Med Sci Monit. 25:801–810.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Y, Wang C, Yu B, Jiang JD and Kong
WJ: Gastrodin protects against ethanol-induced liver injury and
apoptosis in HepG2 cells and animal models of alcoholic liver
disease. Biol Pharm Bull. 41:670–679. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shah UK, Mallia JO, Singh N, Chapman KE,
Doak SH and Jenkins GJS: Reprint of: A three-dimensional in vitro
HepG2 cells liver spheroid model for genotoxicity studies. Mutat
Res Genet Toxicol Environ Mutagen. 834:35–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Saito T, Kurashima A, Oda T, Aoki S, Endo
H, Nashimoto T and Yamada R: Quantitative analysis of plasma
concentration of barbiturate for diagnosis of brain death. No
Shinkei Geka. 30:593–599. 2002.PubMed/NCBI(In Japanese).
|
|
19
|
Steiner C, Steurer MP, Mueller D, Zueger M
and Dullenkopf A: Midazolam plasma concentration after anesthesia
premedication in clinical routine-an observational study: Midazolam
plasma concentration after anesthesia premedication. BMC
Anesthesiol. 16(105)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hebron BS: Plasma concentrations of
etomidate during an intravenous infusion over 48 hours.
Anaesthesia. 38 (Suppl):S39–S43. 1983.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lam E, Rochani A, Kaushal G, Thoma BN,
Tanjuakio J, West FM and Hirose H: Pharmacokinetics of ketamine at
dissociative doses in an adult patient with refractory status
asthmaticus receiving extracorporeal membrane oxygenation therapy.
Clin Ther. 41:994–999. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Casati A, Fanelli G, Casaletti E, Cedrati
V, Veglia F and Torri G: The target plasma concentration of
propofol required to place laryngeal mask versus cuffed
oropharyngeal airway. Anesth Analg. 88:917–920. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen S, Feng C, Fang Y, Zhou X, Xu L, Wang
W, Kong X, Peppelenbosch MP, Pan Q and Yin Y: The eukaryotic
translation initiation factor 4F complex restricts rotavirus
infection via regulating the expression of IRF1 and IRF7. Int J Mol
Sci. 20(1580)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yin Y, Bijvelds M, Dang W, Xu L, van der
Eijk AA, Knipping K, Tuysuz N, Dekkers JF, Wang Y, de Jonge J, et
al: Modeling rotavirus infection and antiviral therapy using
primary intestinal organoids. Antiviral Res. 123:120–131.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Koyama Y and Brenner DA: Liver
inflammation and fibrosis. J Clin Invest. 127:55–64.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen Z, Amro EM, Becker F, Holzer M, Rasa
SMM, Njeru SN, Han B, Di Sanzo S, Chen Y, Tang D, et al:
Cohesin-mediated NF-kB signaling limits hematopoietic stem cell
self-renewal in aging and inflammation. J Exp Med. 216:152–175.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hakim MS, Ding S, Chen S, Yin Y, Su J, van
der Woude CJ, Fuhler GM, Peppelenbosch MP, Pan Q and Wang W: TNF-α
exerts potent anti-rotavirus effects via the activation of
classical NF-kB pathway. Virus Res. 253:28–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wolfer AM, Scott AJ, Rueb C, Gaudin M,
Darzi A, Nicholson JK, Holmes E and Kinross JM: Longitudinal
analysis of serum oxylipin profile as a novel descriptor of the
inflammatory response to surgery. J Transl Med.
15(83)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Getachew B, Aubee JI, Schottenfeld RS,
Csoka AB, Thompson KM and Tizabi Y: Ketamine interactions with
gut-microbiota in rats: Relevance to its antidepressant and
anti-inflammatory properties. BMC Microbiol. 18(222)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yin Y, Metselaar HJ, Sprengers D,
Peppelenbosch MP and Pan Q: Rotavirus in organ transplantation:
Drug-virus-host interactions. Am J Transplant. 15:585–593.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chang EI, Zarate MA, Rabaglino MB,
Richards EM, Arndt TJ, Keller-Wood M and Wood CE: Ketamine
decreases inflammatory and immune pathways after transient hypoxia
in late gestation fetal cerebral cortex. Physiol Rep.
4(e12741)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chang EI, Zarate MA, Rabaglino MB,
Richards EM, Keller-Wood M and Wood CE: Ketamine suppresses
hypoxia-induced inflammatory responses in the late-gestation ovine
fetal kidney cortex. J Physiol. 594:1295–1310. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sahinovic MM, Struys M and Absalom AR:
Clinical pharmacokinetics and pharmacodynamics of propofol. Clin
Pharmacokinet. 57:1539–1558. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Luo J, Huang B, Zhang Z, Liu M and Luo T:
Delayed treatment of propofol inhibits lipopolysaccharide-induced
inflammation in microglia through the PI3K/PKB pathway.
Neuroreport. 29:839–845. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jia J, Sun Y, Hu Z, Li Y and Ruan X:
Propofol inhibits the release of interleukin-6, 8 and tumor
necrosis factor-α correlating with high-mobility group box 1
expression in lipopolysaccharides-stimulated RAW 264.7 cells. BMC
Anesthesiol. 17(148)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang YH, Suk FM, Liu CL, Chen TL, Twu YC,
Hsu MH and Liao YJ: Antifibrotic effects of a barbituric acid
derivative on liver fibrosis by blocking the NF-kB signaling
pathway in hepatic stellate cells. Front Pharmacol.
11(388)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
O'Sullivan S, Wang J, Pigott MT, Docherty
N, Boyle N, Lis SK, Gilmer JF and Medina C: Inhibition of matrix
metalloproteinase-9 by a barbiturate-nitrate hybrid ameliorates
dextran sulphate sodium-induced colitis: Effect on
inflammation-related genes. Br J Pharmacol. 174:512–524.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yin YB, de Jonge HR, Wu X and Yin YL:
Mini-gut: A promising model for drug development. Drug Discov
Today. 24:1784–1794. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Staff N, Engelstad J, Klein C, Amrami K,
Spinner R and Dyck P, Warner M, Warner M and Dyck P: Post-surgical
inflammatory neuropathy. Brain. 133:2866–2880. 2010.PubMed/NCBI View Article : Google Scholar
|