Introduction
Myopia is one of the most common eye diseases,
affecting ~27% of the population worldwide in 2010(1). The sclera, which contributes to the
regulation of refractive status, is a critical structure involved
in the development of myopia (2).
Clinical and experimental studies have demonstrated that changes in
the biochemical and mechanical properties of the sclera during the
development of myopia can induce scleral remodeling and lengthening
of the ocular axis (3); with
scleral remodeling serving an important role in emmetropia and eye
development (4). Fibroblasts are
the major cell type within the sclera and the main cells affected
in myopia (5). Structural and
functional abnormalities in scleral fibroblasts have been
implicated in various pathologies (6).
The lumican gene encodes leucine glycan, a
regulator of scleral development that is present at high levels in
the extracellular matrix. The protein core interacts with collagen
molecules to regulate the diameter of collagen fibers (7) and the inter-fiber space (8). Lumican has been shown to inhibit the
lateral aggregation of collagen molecules and limit the diameter of
collagen fibers so as to maintain the biomechanical properties of
the sclera, such as elasticity and tension (9,10).
Mutations to the lumican gene are thought to be a cause of
myopia, as demonstrated by loss-of-function experiments in animal
models (11). However, whether
lumican expression is altered in myopia and the mechanisms of
action involved are unclear. The present study aimed to address
these areas using cultured scleral fibroblasts and a rat model of
myopia.
Materials and methods
Animals
Male Sprague-Dawley rats (21 days old; n=20; 120±5
g) were purchased from Shanghai Laboratory Animal Research Center
[license no. scxk (Lu) 2018-0006] and housed under specific
pathogen-free conditions at 23±2˚C, at a relative humidity of
45-65% and under a 12 h light/dark cycle. The animals were fed
rodent pellets and water ad libitum. For the cell culture,
an additional 6 male newborn rats (5 g) were also purchased from
Shanghai Laboratory Animal Research Center [license no. scxk (Lu)
2018-0006] All experimental protocol was approved by the Ethics
Committee of the Second Affiliated Hospital of Nanchang
University.
Establishment of the myopia model
The myopia model was established as previously
described (12). Rats were
anesthetized with isoflurane (5% for induction and 2% for
maintenance) and immobilized on a surgical table in the prone
position. The hair around the right eye was shaved with a razor and
the skin sterilized with 1% iodophor. A 2-mm long incision was made
with ophthalmic scissors, 3 mm inside the right eye, outside the
outer canthus and sutured with a 6-0 needle. The tip of the needle
was inserted through the incision of the outer canthus, lower
eyelid and inner canthus of the eye. The start and end points of
the suture were tightened until the eyelid was closed. The
uninjured left eye served as the control. Experiments using the
rats were performed 8 weeks after surgery.
Diopter detection
Tropicamide (0.5%, 5 µl, each eye) was applied to
the eyes as drops. After 3 min, the pupils were sufficiently
dilated, with a suitable working distance. The 3 hole/1 line was
maintained with a strip light detector and diopter was determined
while moving the light band back and forth. The final diopter was
calculated by adding and subtracting the positive and negative
diopters of different degrees for neutralization according to the
following formula: Final diopter = (degrees for examined eye) -
[working distance mirror (d)].
Axial measurement
Rats were anesthetized with isoflurane (5%) and then
sacrificed by decapitation. The eyeball was dissected, placed on a
sterile gauze moistened with normal saline on ice and the axis was
measured with Vernier calipers. The average value of 3 measurements
was reported.
Hematoxylin and eosin (H&E)
staining
Sclera tissue samples were fixed in 4%
paraformaldehyde for 30 min at room temperature, then dehydrated in
70, 80 and 90% ethanol solutions. The samples were then immersed in
pure ethanol and xylene for 15 min, xylene I for 15 min and xylene
II for 15 min at room temperature until the tissue became
transparent. This was followed by immersion in a mixture of xylene
and paraffin for 15 min and paraffin I and paraffin II for 50-60
min. Finally, the samples were embedded in paraffin at room
temperature for 5 min and sectioned at a thickness of 10 µm. The
sections were collected on a slide and baked at 60˚C for 2 h,
deparaffinized and rehydrated, then stained with hematoxylin for 3
min and eosin for 3 min at room temperature. The slides were sealed
and at least four fields of each slide were imaged using a light
microscope (magnification, x200; BX51; Olympus Corporation).
Immunohistochemistry
Eyeball tissue sections were prepared as
aforementioned followed by blocking in 5% bovin serum albumin
(Hyclone; GE Healthcare Life Science) at room temperature for 2 h
and incubated overnight at 4˚C with monoclonal antibodies against
MMP2 (1:250; cat. no. AF0577), tissue inhibitor of
metalloproteinases (TIMP)-2 (1:200; cat. no. AF7008; each from
Affinity Biosciences), MMP-14 (1:100; cat. no. ab51074) and lumican
(1:100, cat. no. ab168348; each purchased from Abcam), followed by
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
(1:10,000; cat. no. A16104; Thermo Fisher Scientific, Inc.) or
Alexa Fluor 593 goat anti-mouse IgG (1:200; cat. no. A-11001;
Thermo Fisher Scientific, Inc.) for 30 min at room temperature.
Immunoreactivity was visualized by incubation with
3,3'-diaminobenzidine for 3 min at room temperature. At least four
fields were taken from each image using a light microscope
(magnification, x200; BX51, Olympus Corporation). The grey value of
the staining was analyzed by Image-Pro Plus software v6.0 (National
Institutes of Health).
Transmission electron microscopy
Scleral tissue samples were fixed with 2.5%
glutaraldehyde at room temperature for 30 min and then dehydrated,
embedded in epoxy resin at 60˚C for 24 h and sectioned at a
thickness of 100 nm. The sections were stained with 3% uranyl
acetate and lead citrate at room temperature for 5 min, and then
imaged by transmission electron microscopy (magnification, x1,000;
80 kV; JEM-1230; JEOL, Ltd.).
Cell culture
The Scleral tissue was collected from six newborn
rats and cut into 1x1 mm sections that were digested in 0.1% type
II collagenase at 37˚C for 30 min. After filtering through a 70-µm
mesh, the cells were collected by centrifugation (1,000 x g for 5
min at 4˚C), resuspended in RPMI-1640 medium containing 20% FBS and
cultured at 37˚C and 5% CO2, to make primary scleral
fibroblast cultures.
Cell transfection
Lumican overexpression or silencing constructs (2
µg/µl) and a negative control were produced by Shanghai GenePharma
Co., Ltd using pcDNA3.1 plasmid (Invitrogen; Thermo Fisher
Scientific, Inc.). Scleral fibroblasts were cultured until they
reached 70% confluence, then transfected at room temperature with
1.25 µg of pcDNA3.1 plasmid (Invitrogen; Thermo Fisher Scientific,
Inc.) using 5 µl Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.). After 2 h, the culture medium was
refreshed and the cells were divided into the following five
groups: Untransfected (negative control), lumican overexpression
construct (2 µg/µl, Shanghai GenePharma Co., Ltd), empty vector
(negative control), small interfering (si)RNA construct, and
scrambled siRNA construct (negative control). After 48 h, the cells
were used to evaluate cell proliferation, apoptosis and protein
expression. The siRNA sequences for lumican are listed in Table I.
 | Table IsiRNA sequences. |
Table I
siRNA sequences.
| siRNA | siRNA sequence
(5'-3') |
|---|
| Lumican-siRNA1 |
ACAAUAAGCUCAAGAGUAUTTAUACUCUUGAGCUUAUUGUTT |
| Lumican-siRNA2 |
UGAAGAAGCUGCAUAUAAATTUUUAUAUGCAGCUUCUUCATT |
| Lumican-siRNA3 |
ACUCCAAGAUCAAAGGAAATTUUUCCUUUGAUCUUGGAGUTT |
| Scrambled siRNA
(siRNA-NC) |
UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT |
Reverse transcription-quantitative
(q)PCR
RNA was extracted from cells using
TRIzol® (Thermo Fisher Scientific, Inc.) and cDNA was
synthesized at 30˚C for 10 min according to the instructions of the
reverse transcriptase kit (CoWin Biosciences). cDNA was used as the
template for qPCR, which was performed on a 7500 Fast Real-Time PCR
System (Applied Biosystems). The level of β-actin was used as the
internal reference to calculate the mRNA expression levels of,
MMP-2, MMP-14 and TIMP-2. The qPCR reaction consisted of 9.5 µl
RNase-free dH2O, 1 µl cDNA/DNA, 2 µl primers and 12.5 µl
2X UltraSYBR mix (cat. no. A25778; Thermo Fisher Scientific, Inc.).
The cycling protocol was as follows: Pre-denaturation at 95˚C for
10 min, 40 cycles of 95˚C for 10 sec, 54.3˚C for 30 sec and 72˚C
for 30 sec. The target gene expression was normalized to β-actin as
previously described (13). Primer
sequences are listed in Table
II.
 | Table IIPrimer sequences. |
Table II
Primer sequences.
| Primer | Sequence (5'-3') | Primer length
(bp) | Product length
(bp) | Annealing temperature
(˚C) |
|---|
| Lumican F |
GCCTTTGAGAATGTAACGGAT | 21 | 169 | 57.1 |
| Lumican R |
CTTGTAGGGACTTTGGGAGC | 20 | | |
| MMP-2 F |
AGGACACCCTCAAGAAGATGC | 21 | 134 | 58.1 |
| MMP-2 R |
GCGGGGAAAGAAGTTGTAGTT | 21 | | |
| MMP-14 F |
GCAGTATGGCTACCTACCTCC | 21 | 118 | 58.4 |
| MMP-14 R |
CTTGCCTGTCACTTGTAAACC | 21 | | |
| TIMP-2 F |
GCAACCCCATCAAGAGGA | 18 | 212 | 56.6 |
| TIMP-2 R |
CCAGGGCACAATAAAGTCAC | 20 | | |
| β-actin F |
ACGGTCAGGTCATCACTATC | 20 | 90 | 56.5 |
| β-actin R |
TGCCACAGGATTCCATACC | 19 | | |
Western blotting
Total protein was extracted from scleral tissues or
cultured scleral fibroblasts. After determining the concentration
using the BCA method, the proteins (20 µg) were separated by 12%
SDS-PAGE and transferred to a PVDF membrane. Non-specific antibody
binding was blocked by incubating the membrane in 5% non-fat milk
at room temperature for 2 h. The membrane was then incubated
overnight at 4˚C with antibodies against MMP-2 (1:1,000), MMP-14
(1:1,000), TIMP-2 (1:1,000), lumican (1:1,000) and GAPDH (1:3,000;
cat. no. ab8245; Abcam), followed by incubation with HRP-conjugated
anti-rabbit IgG (1:10,000; cat. no. A16104SAMPLE; Thermo Fisher
Scientific, Inc.) at room temperature for 2 h. An enhanced
chemiluminescence reagent kit (cat. no. WP20005; Thermo Fisher
Scientific, Inc.) was used to visualize immunoreactivity. The blots
were scanned with a ChemiDoc XRS imaging system (Bio-Rad
Laboratories, Inc.) and signal intensity was analyzed with Quantity
One v1.4.6 software (Bio-Rad Laboratories, Inc.) as previously
described (14).
Cell Counting Kit (CCK)-8 assay
Transfected cells were digested, resuspended at a
density of 5x103 cells/well and counted under a light
microscope (magnification, x200; BX51; Olympus Corporation). After
24 h, 10 µl CCK-8 reagent (cat. no. C0037, Beyotime Institute of
Biotechnology) was added to each well, followed by incubation for 2
h at 37˚C according to the instruction of the kit. The absorbance
of each well was measured at a wavelength of 450 nm using a
microplate reader.
Flow cytometry
A total of 3x106 cells were collected
from each group and centrifuged with 1 ml PBS at 1,500 x g for 3
min at room temperature. After two washes with 0.1 M PBS, cells (in
1 ml) were incubated in the dark for 10 min with 3 µl FITC-annexin
V and 5 µl propidium iodide at room temperature (cat. no. C1062M,
Beyotime Institute of Biotechnology). Apoptotic cells were detected
by NovoCyte™ flow cytometer (NovoCyte 2060R; ACEA Bioscience, Inc.)
and analyzed using FlowJo v10 (FlowJo, LLC).
Statistical analysis
Data are expressed as the mean ± SD and were
analyzed using SPSS v19.0 software (IBM Corp.). The control and
modeled eyes were from the same animals; therefore, paired
Student's t-tests were used to analyze the differences. When there
were ≥3 groups, the differences were evaluated using one-way ANOVA
followed by a post-hoc Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Evaluation of the form-deprivation
myopia model
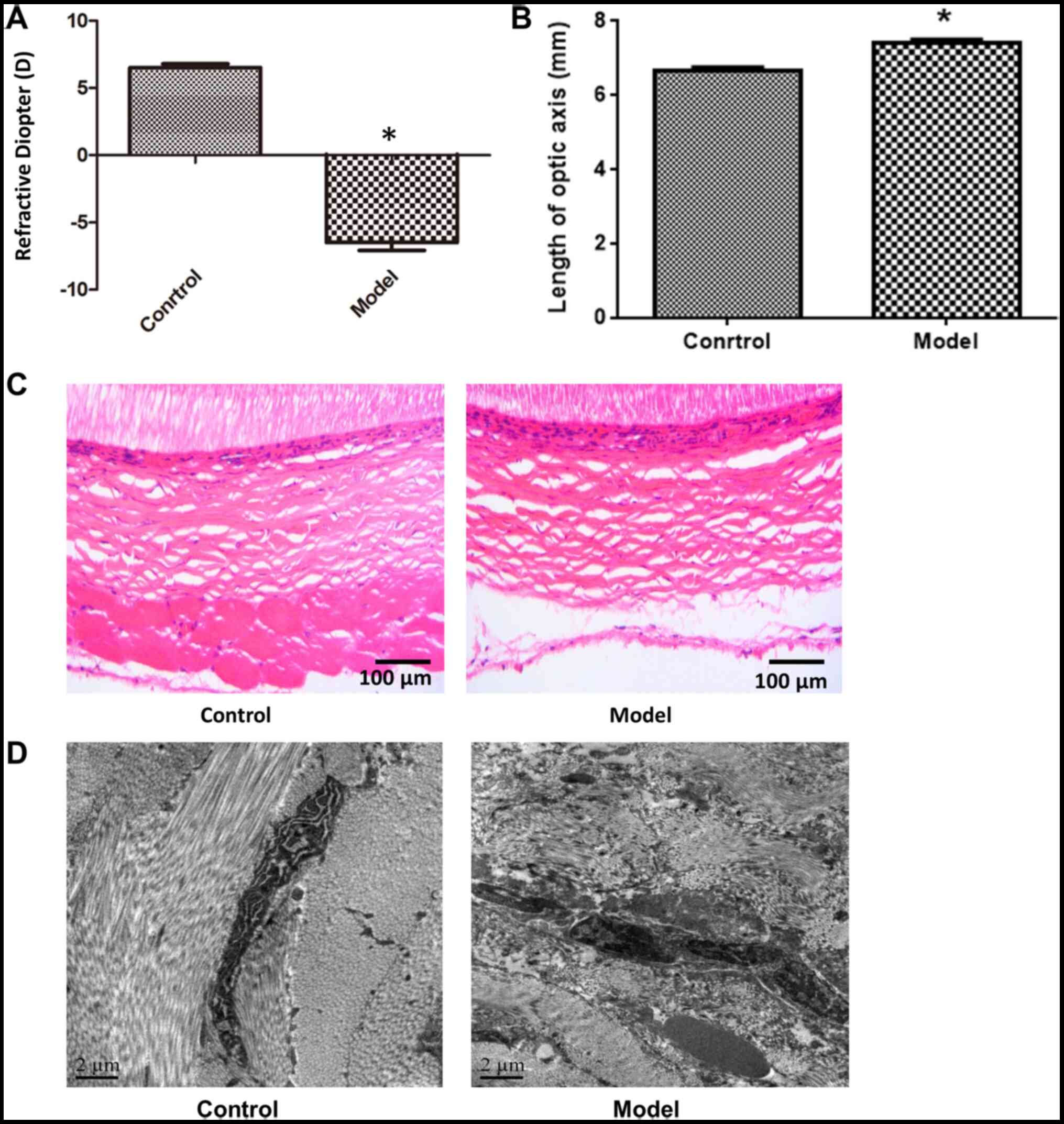
In myopia model eyes, the diopter was decreased
(Fig. 1A) and axial length was
increased (P<0.05; Fig. 1B)
compared with control eyes, indicating that the model was
successfully established. Furthermore, histologic analysis via
H&E staining revealed that in the control eyes, the collagen
fibers of scleral tissue were evenly distributed and had an orderly
arrangement, with little extracellular matrix. In contrast, in
myopia model eyes, the sclera was thinner and collagen fibers were
more sparse and disorganized; fractured fibers were also observed
(Fig. 1C). Electron microscopy
analysis confirmed the presence of partly disintegrated and
vacuolated collagen fibers with variable thickness and disordered
arrangement in the posterior sclera, as well as the enlargement of
inter-fiber space (Fig. 1D).
Expression of lumican, MMP-2, MMP-14
and TIMP-2 in rat scleral tissue
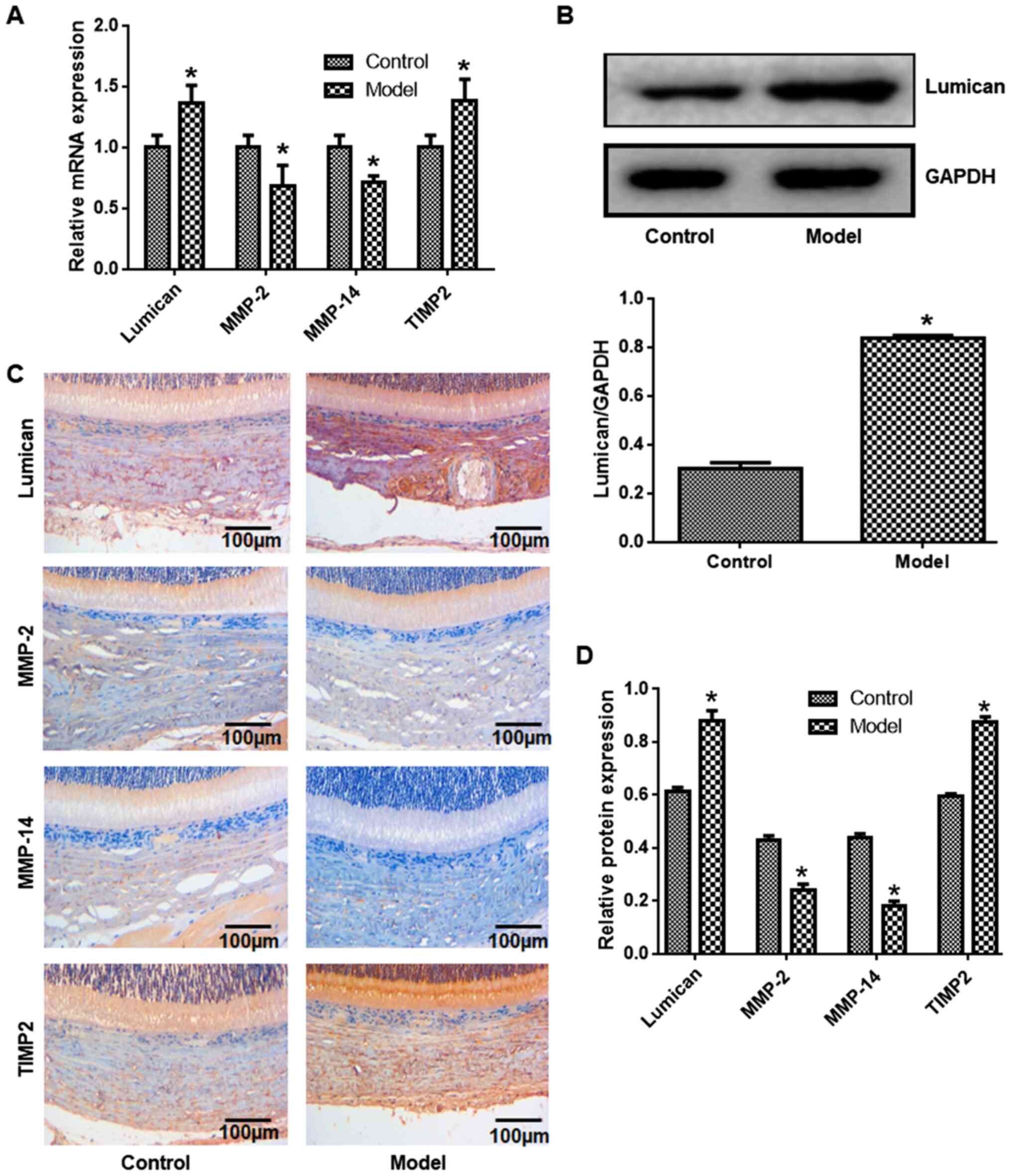
The expression levels of lumican, MMP-2, MMP-14 and
TIMP-2 in scleral tissue was evaluated at the mRNA and protein
level. Compared with control eyes, lumican and TIMP-2 transcript
levels were increased, whereas MMP-2 and MMP-14 levels were lower
in the myopia model (Fig. 2A).
Western blot analysis revealed that lumican expression was
increased in myopia model eyes compared with control eyes (Fig. 2B). Similar trends were observed by
immunohistochemistry, with higher expression of lumican and TIMP-2
and lower expression of MMP-2 and MMP-14 observed in myopia model
eyes relative to control eyes (Fig.
2C and D).
Lumican overexpression and knockdown
in cultured scleral fibroblasts
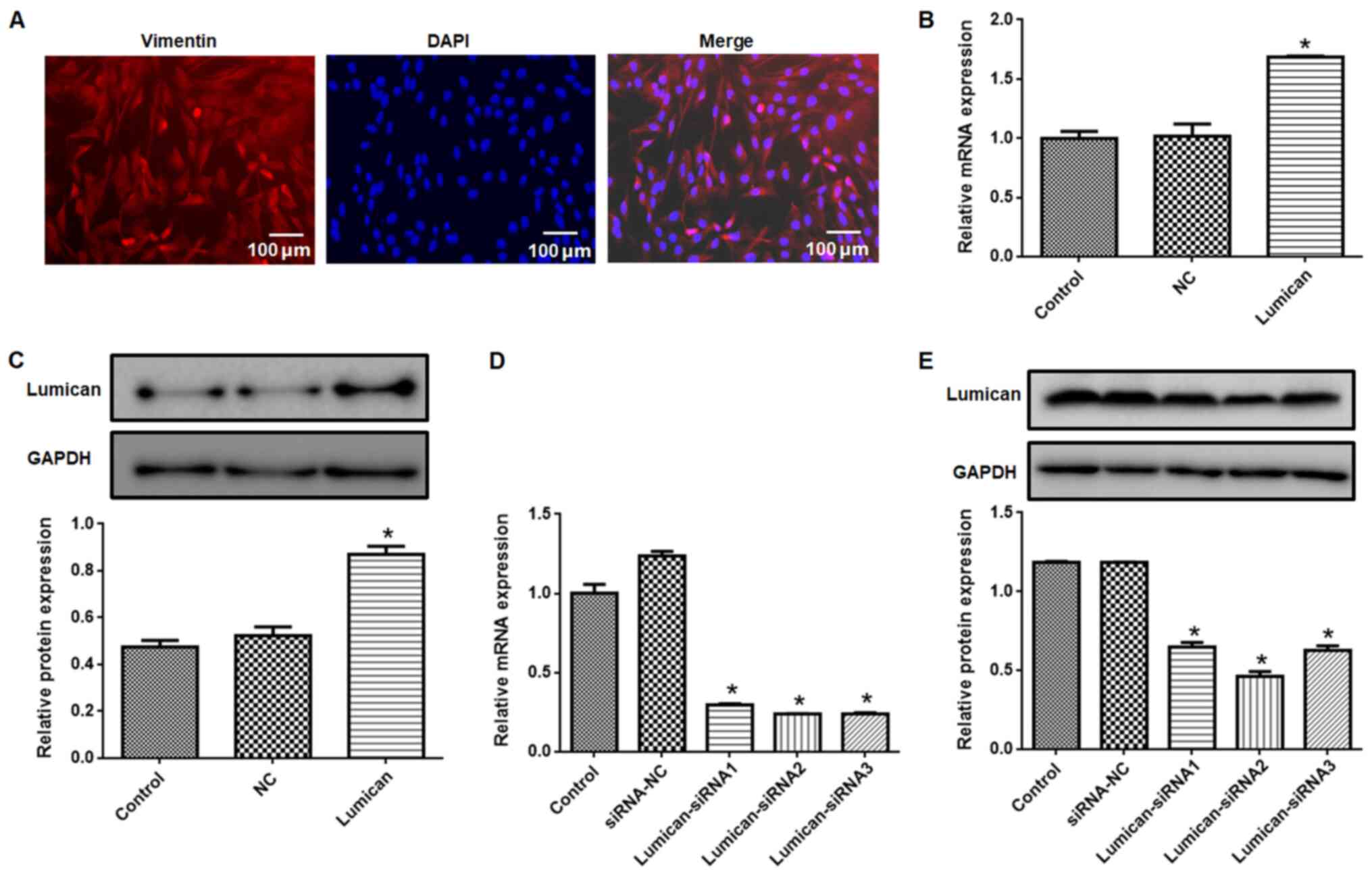
Vimentin expression was detected in primary cultured
scleral fibroblasts (Fig. 3A).
Compared with the control group, lumican expression at the mRNA
(Fig. 3B) and protein (Fig. 3C) levels were higher in the lumican
overexpression group, whereas lumican knockdown resulted in a loss
of expression (Fig. 3D and E). The lumican-siRNA2 construct had the
highest knockdown efficiency and was used for subsequent
experiments.
Lumican overexpression reduces cell
viability and promotes apoptosis in scleral fibroblasts
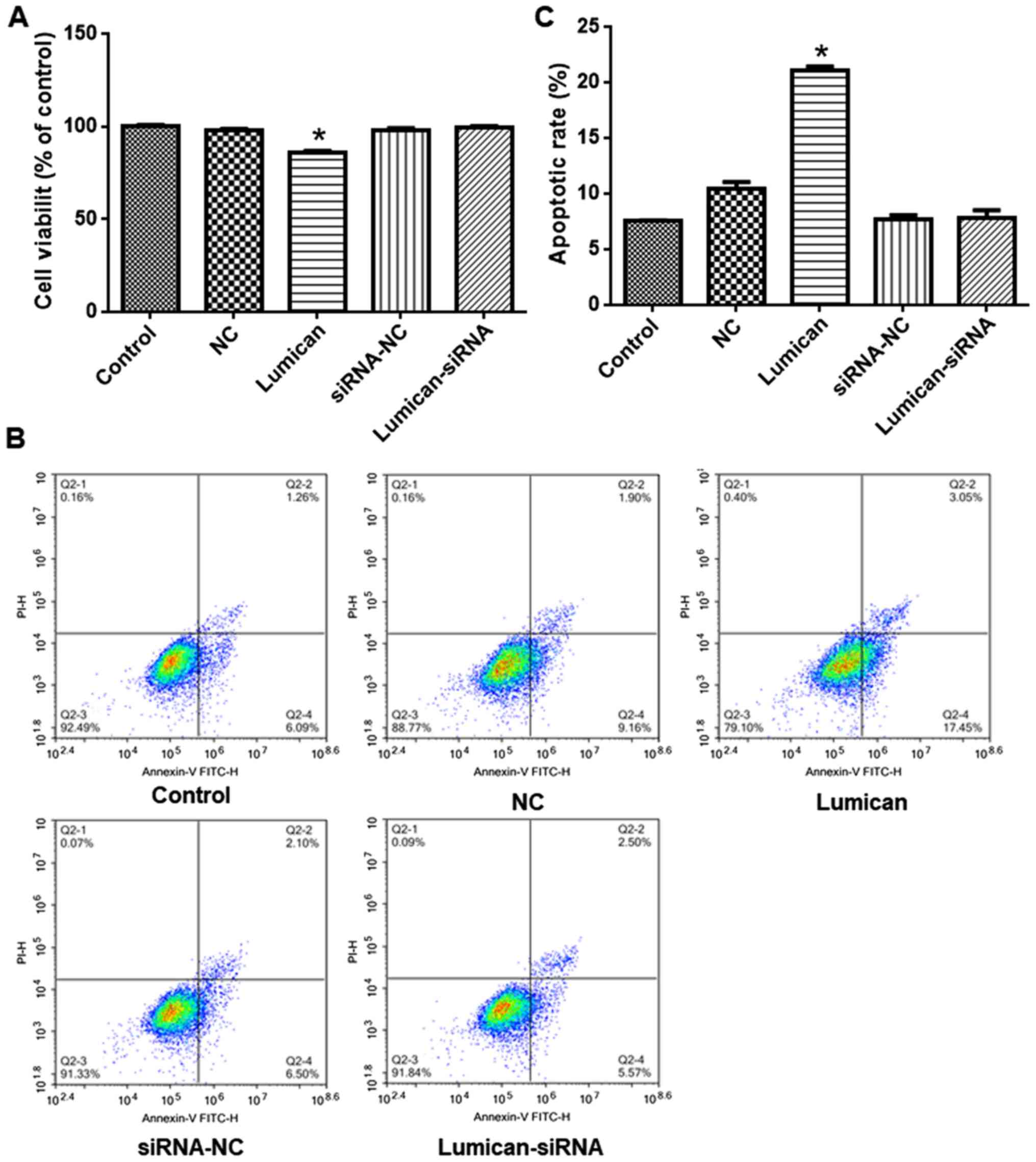
Compared with scleral fibroblasts transfected with
the empty vector, viability was reduced in cells overexpressing
lumican (Fig. 4A). However,
siRNA-mediated lumican knockdown had no effect on cell viability.
Similarly, apoptosis was enhanced relative to the control group by
lumican overexpression but was unaffected by lumican knockdown
(Fig. 4B and C).
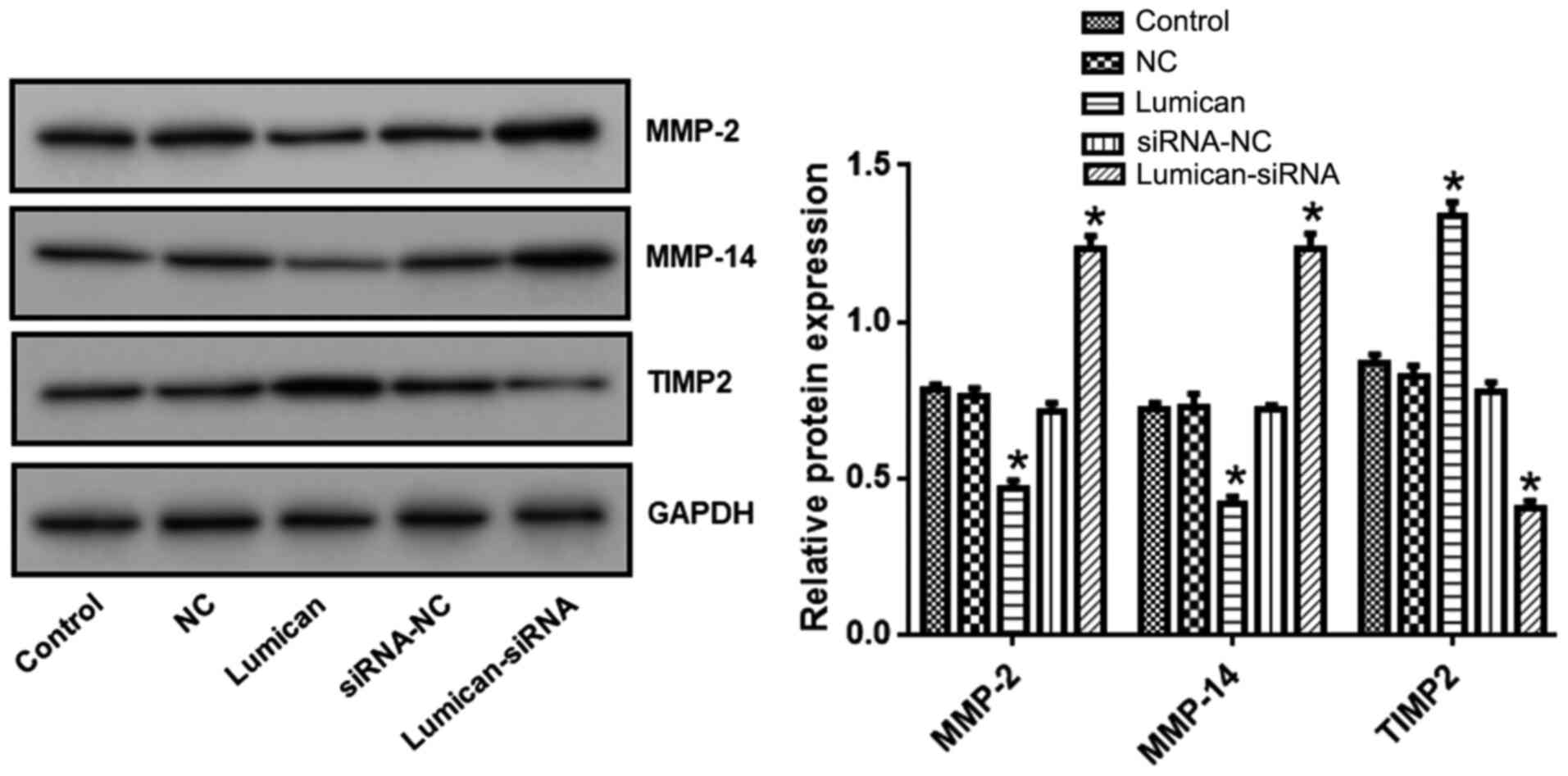
Lumican overexpression or knockdown
alters MMP-2, MMP-14 and TIMP-2 expression levels in rat scleral
fibroblasts
Compared with the control group, MMP-2 and MMP-14
levels were reduced while TIMP-2 levels were increased in cells
overexpressing lumican (Fig. 5). In
contrast, MMP-2 and MMP-14 were upregulated whereas TIMP-2 was
downregulated in cells transfected with lumican siRNA compared with
those transfected with the scrambled control siRNA.
Discussion
The results of the present study demonstrated that
lumican and TIMP-2 were upregulated whereas MMP-2 and MMP-14 were
downregulated in the scleral tissue of rats in a model of
form-deprivation myopia. It was also revealed that overexpressing
lumican in normal scleral fibroblasts induced apoptosis and reduced
cell viability, which was associated with decreased MMP-2 and
MMP-14 levels, as well as increased TIMP-2 expression. The results
implied that elevated levels of lumican in scleral tissue
contributed to the development of myopia.
The pathogenesis of myopia is mainly attributable to
genetic factors (15) but is also
related to the increase in diopter with age (16). Degeneration of the vitreous body may
be caused by lengthening of the ocular axis and enlargement of the
vitreous cavity (17). In the
present study, a form-deprivation myopia model was successfully
established, which was confirmed by an increased axial length and
diopter, and histopathologic changes such as decreased scleral
thickness. Ultrastructural analysis demonstrated that collagen
fibers in the posterior sclera of model eyes were disorganized,
with variable thickness and an enlarged space between fibers. These
results indicated that myopia is associated with pathologic
remodeling of collagen fibers in the sclera. Corneal thickness,
anterior chamber depth or lens thickness was not examined in the
present study due to technical limitations.
Lumican has been previously demonstrated to be
overexpressed in a rat model of form-deprivation myopia (18). Lumican contributes to the formation
of scleral collagen fibers; changes in fiber structure can lead to
abnormal eye shape and size (19).
Scleral remodeling involves extracellular matrix proteins and
enzymes including collagen, proteoglycan and proteases (20). MMP-2 and TIMP-2 are typical
proteases that serve an important role in scleral remodeling by
regulating the balance between the synthesis and degradation of
scleral fibroblast extracellular matrix (21). MMP-2 was found to be upregulated in
scleral tissue in a porcine model of form-deprivation myopia
(22). Additionally, MMP-2 mRNA
expression levels were increased, whereas TIMP-2 levels were
decreased in the posterior scleral fiber layer in form-deprivation
myopia (23). MMP-2 expression
levels in sclera have also been demonstrated to be altered in a
tree shrew myopia model (24). In
chickens, an imbalance between MMP-2 and TIMP-2 levels is linked to
abnormalities in the shape of the sclera (25). MMP-14 is expressed on the surface of
scleral fibroblasts and directly degrades the extracellular matrix
(26). MMP-2 is activated by
MMP-14, which in turn activates MMP-2 by forming a complex with
pro-MMP-2 and TIMP-2(27).
Fibroblasts synthesize collagen fibers and other
matrix components to form the outer wall of the eyeball. These
cells maintain the integrity of eyeball tissue and determine the
length of the ocular axis (28).
The present study investigated the function of lumican in myopia
using primary cultured scleral fibroblasts isolated from normal rat
eyes. Most of the cells were vimentin+, indicating the
successful isolation of scleral fibroblasts. Lumican expression has
been demonstrated to alter collagen synthesis, leading to scleral
remodeling, lengthening of the ocular axis and the development of
myopia (29). Lumican binds to
integrin on the cell surface to regulate cell proliferation and
apoptosis (30). The present
results revealed that proliferation was suppressed and apoptosis
was enhanced in scleral fibroblasts by overexpressing lumican.
Lumican induces apoptosis in endothelial cells through the
activation of Fas/Fas ligand signaling, which suppresses integrin
α2β1 activity and p38 MAPK signaling; reduces MMP-2 and MMP-14
expression, and increases that of TIMP-2, ultimately preventing the
formation of the vascular lumen (22). Lumican is essential for promoting
the growth and maintenance of corneal stromal transparency
(31). Its overexpression has been
demonstrated to reduce the expression of MMP-2 and MMP-14 in the
corneal matrix and increase that of TIMP-2, thereby altering
collagen fiber structure (32).
Similarly, it was revealed that lumican overexpression decreased
MMP-2 and MMP-14, and increased TIMP-2 expression in rat scleral
fibroblasts, while the opposite effects were observed following
lumican knockdown. These results are consistent with the role of
TIMP-2 as a negative regulator of MMP-2 and indicated that this
function may be perturbed in myopia, as suggested by previous
studies (27,32).
Inhibiting lumican expression may be an important
mechanism for preventing apoptosis in scleral fibroblasts and
stimulating MMP expression. In the present study, silencing lumican
expression did not affect cell viability or apoptosis in scleral
fibroblasts from normal rats although it altered TIMP-2 and MMP
expression. These results suggested that the regulation of MMP
expression is independent of fibroblast viability, which may be
maintained by compensatory mechanisms in the absence of
lumican.
In conclusion, the present results demonstrated that
lumican overexpression promotes myopia by inducing apoptosis of
scleral fibroblasts and TIMP-2 expression and reducing MMP-2 and
MMP-14 levels. As such, therapeutic strategies targeting lumican
may be effective for the treatment of myopia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, YZ, YF and SL performed the experiments and
analyzed the data. JW and XZ designed the study and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocol was approved by the Ethics
Committee of the Second Affiliated Hospital of Nanchang
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Foster PJ and Jiang Y: Epidemiology of
myopia. Eye (Lond). 28:202–208. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hayashi M, Ito Y, Takahashi A, Kawano K
and Terasaki H: Scleral thickness in highly myopic eyes measured by
enhanced depth imaging optical coherence tomography. Eye (Lond).
27:410–417. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Metlapally R and Wildsoet CF: Scleral
mechanisms underlying ocular growth and myopia. Prog Mol Biol
Transl Sci. 134:241–248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Harper AR and Summers JA: The dynamic
sclera: Extracellular matrix remodeling in normal ocular growth and
myopia development. Exp Eye Res. 133:100–111. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rada JA, Shelton S and Norton TT: The
sclera and myopia. Exp Eye Res. 82:185–200. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhan X, Zhu ZC, Sun SQ and Wen YC: Dynamic
changes of activator protein 1 and collagen I expression in the
sclera of myopia guinea pigs. Int J Ophthalmol. 12:1272–1276.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen L, Zhang Y, Zuo Y, Ma F and Song H:
Lumican expression in gastric cancer and its association with
biological behavior and prognosis. Oncol Lett. 14:5235–5240.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Austin BA, Coulon C, Liu CY, Kao WW and
Rada JA: Altered collagen fibril formation in the sclera of
lumican-deficient mice. Invest Ophthalmol Vis Sci. 43:1695–1701.
2002.PubMed/NCBI
|
|
9
|
Mouw JK, Ou G and Weaver VM: Extracellular
matrix assembly: A multiscale deconstruction. Nat Rev Mol Cell
Biol. 15:771–785. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Stuart K, Paderi J, Snyder PW, Freeman L
and Panitch A: Collagen-binding peptidoglycans inhibit MMP mediated
collagen degradation and reduce dermal scarring. PLoS One.
6(e22139)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang P, Karani R, Turner RL, Dufresne C,
Ferri S, Van Eyk JE and Semba RD: The proteome of normal human
retrobulbar optic nerve and sclera. Proteomics. 16:2592–2596.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xiao H, Fan ZY, Tian XD and Xu YC:
Comparison of form-deprived myopia and lens-induced myopia in
guinea pigs. Int J Ophthalmol. 7:245–250. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Song ZJ, Yang SJ, Han L, Wang B and Zhu G:
Postnatal calpeptin treatment causes hippocampal neurodevelopmental
defects in neonatal rats. Neural Regen Res. 14:834–840.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guggenheim JA, Ghorbani Mojarrad N,
Williams C and Flitcroft DI: Genetic prediction of myopia:
Prospects and challenges. Ophthalmic Physiol Opt. 37:549–556.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Steidl SM: How does visual acuity change
over time in adults with high myopia? Br J Ophthalmol.
90(524)2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stone RA, Lin T, Laties AM and Iuvone PM:
Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci
USA. 86:704–706. 1989.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun MS, Song YZ, Zhang FJ, Tao J and Liu
YB: Changes of ocular biological parameters and Lumican expression
in the monocularly deprivation myopic model of mutant Lumican
transgenic mice. Zhonghua Yan Ke Za Zhi. 52:850–855.
2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
19
|
Song Y, Zhang F, Zhao Y, Sun M, Tao J,
Liang Y, Ma L, Yu Y, Wang J and Hao J: Enlargement of the axial
length and altered ultrastructural features of the sclera in a
mutant lumican transgenic mouse model. PLoS One.
11(e0163165)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lu P, Takai K, Weaver VM and Werb Z:
Extracellular matrix degradation and remodeling in development and
disease. Cold Spring Harb Perspect Biol. 3(a005058)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vij N, Roberts L, Joyce S and Chakravarti
S: Lumican suppresses cell proliferation and aids Fas-Fas ligand
mediated apoptosis: Implications in the cornea. Exp Eye Res.
78:957–971. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Leung KH, Yiu WC, Yap MK, Ng PW, Fung WY,
Sham PC and Yip SP: Systematic investigation of the relationship
between high myopia and polymorphisms of the MMP2, TIMP2, and TIMP3
genes by a DNA pooling approach. Invest Ophthalmol Vis Sci.
52:3893–3900. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Siegwart JT Jr and Norton TT: Steady state
mRNA levels in tree shrew sclera with form-deprivation myopia and
during recovery. Invest Ophthalmol Vis Sci. 42:1153–1159.
2001.PubMed/NCBI
|
|
25
|
Dai SZ, Zeng JW and Wang LY: Effect of
pirenzepine on form deprivation myopia in chicks and its possible
mechanism. Zhonghua Yan Ke Za Zhi. 42:42–47. 2006.PubMed/NCBI(In Chinese).
|
|
26
|
Pietraszek K, Chatron-Colliet A, Brézillon
S, Perreau C, Jakubiak-Augustyn A, Krotkiewski H, Maquart F and
Wegrowski Y: Lumican: A new inhibitor of matrix
metalloproteinase-14 activity. FEBS Lett. 588:4319–4324.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lam C, Jamerson M, Cabral G, Carlesso AM
and Marciano-Cabral F: Expression of matrix metalloproteinases in
Naegleria fowleri and their role in invasion of the central nervous
system. Microbiology (Reading). 163:1436–1444. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Murata K, Hirata A, Ohta K, Enaida H and
Nakamura KI: Morphometric analysis in mouse scleral fibroblasts
using focused ion beam/scanning electron microscopy. Sci Rep.
9(6329)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen ZT, Wang IJ, Shih YF and Lin LL: The
association of haplotype at the lumican gene with high myopia
susceptibility in Taiwanese patients. Ophthalmology. 116:1920–1927.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lu YP, Ishiwata T, Kawahara K, Watanabe M,
Naito Z, Moriyama Y, Sugisaki Y and Asano G: Expression of lumican
in human colorectal cancer cells. Pathol Int. 52:519–526.
2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Niewiarowska J, Brézillon S,
Sacewicz-Hofman I, Bednarek R, Maquart F, Malinowski M, Wiktorska
M, Wegrowski Y and Cierniewski CS: Lumican inhibits angiogenesis by
interfering with alpha2beta1 receptor activity and downregulating
MMP-14 expression. Thromb Res. 128:452–457. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pietraszek K, Brézillon S, Perreau C,
Malicka-Błaszkiewicz M, Maquart F and Wegrowski Y: Lumican-derived
peptides inhibit melanoma cell growth and migration. PLoS One.
8(e76232)2013.PubMed/NCBI View Article : Google Scholar
|