Introduction
Myelodysplastic syndromes (MDSs) are incurable
malignant hematological diseases caused by malfunctioning
hematopoietic stem cells (HSC) or CD34+ progenitor cells
(1). A substantial proportion of
MDSs arise in the setting of exposure to environmental or
occupational toxins, including cytotoxic therapy for a prior
malignancy or other disorder (2).
Patients with MDS are classified as lower-risk or higher-risk
according to different prognostic scoring systems (3,4). The
incidence of MDS progression to acute leukemia is
3.3/100,000(5). According to the
revised International Prognostic Scoring System (2012), high-risk
and extremely high-risk patients often display multiple morbid
hematopoiesis and malignant clonal hyperplasia, which can easily
transform into leukemia, leading to a median survival of 1.6 and
0.8 years, respectively (6). Due to
its poor prognosis, the treatment of higher-risk MDS has received
increasing attention. Stem cell transplantation remains the most
effective treatment strategy for patients with higher-risk MDS, but
only a few patients are eligible for this treatment strategy
(3,7). The primary chemotherapy regimen for
patients with higher-risk MDS is hypomethylating agents (HMAs)
(8); however, as only ~50% of
patients with higher-risk MDS respond to HMA treatment and the
response is often transient, there is a need to improve the
treatment strategy for higher-risk MDS.
On the genomic level, MDS is classified by losses
and translocations involving certain key gene segments, with
disruption of the normal structure and function of genes that
control the balance of proliferation and differentiation of
hematopoietic precursors (9). The
evidence suggests that unidentified tumor suppressor genes may
serve important roles in the molecular mechanisms underlying MDS
(10,11). Further molecular approaches to
genetic lesions will aid with the identification of relevant tumor
suppressor genes. Over the past few years, major signal
transduction molecules, their genetic alteration molecules, cell
cycle regulators, and transcription factors have been identified
(9). In particular, transcription
factors such as EVI-1, that regulate both hematopoietic stem cell
proliferation and differentiation were identified (12). Disruption of signal transduction
pathways involving these molecules results in ineffective
multilineage hematopoiesis and bone marrow failure (13).
Activation of telomerase can lead to uncontrollable
cell proliferation or even tumorigenesis, and ~90% of malignant and
immortalized cells display abnormal telomerase activity (14). In contrast to normal cells, heat
shock protein 90 (HSP90) is continuously activated in cancer cells
(15) and highly expressed in
hematological malignancies, especially in acute leukemia (16). HSP90 and the co-chaperone p23 bind
to human telomerase reverse transcriptase (hTERT) to activate
telomerase during tumorigenesis (17,18).
HSP90 expression is higher in patients with higher-risk MDS
compared with patients with lower-risk MDS, and is therefore
positively correlated with the risk of MDS conversion to acute
leukemia and poor prognosis (19,20).
It was hypothesized that high HSP90 expression contributes to
higher-risk MDS-induced leukemia via reactivation of hTERT; hence,
inhibiting HSP90 activity and promoting substrate degradation with
specific inhibitors should inhibit MDS cell proliferation and
conversion. BIIB021, a derivative of the first sputum compound PU3,
is a new generation terpenoid that is currently in clinical trials
for the treatment of solid tumors and hematological malignancies
(21,22). Therapeutically, higher-risk MDS is
insensitive to common chemotherapy, and bone marrow transplantation
is often hindered by human leukocyte antigen (HLA) mismatch, high
costs, immune rejection and postoperative infections (23). Moreover, the use of demethylation
drugs, such as 5-aza cytidine and 5-aza-2 deoxycytidine, is
typically limited due to non-specific toxicity, potential
carcinogenicity and drug resistance (24). Therefore, novel treatment strategies
for higher-risk MDS with higher efficacy, reduced aggression, fewer
side effects and lower cost are urgently needed.
Based on the difficulties and risks of higher-risk
MDS in clinical treatment, the present study aimed to investigate
the pathogenesis of the disease and combine the advantages of
traditional Chinese medicine (for example, low toxicity and side
effects) to evaluate its advantages for the treatment of
higher-risk MDS. Oldenlandia diffusa Willd belongs to the
hedyotis genus of rubiaceous family, which grows in the south of
the Yangtze River in China (23).
Oldenlandia diffusa Willd is a widely used Chinese herbal
medicine that has been reported to display the following effects:
lowering heat, detoxification, promoting blood circulation,
clearing blood stasis and benefiting diuresis (23). Higher-risk and extreme higher-risk
MDS are diagnosed as visceral dysfunction and Shengqi deficiency by
the traditional Chinese medicine system (25). Oldenlandia diffusa Willd is a
herbal prescription for the treatment of visceral dysfunction, and
previous studies have indicated that that Oldenlandia
diffusa Willd displays anti-inflammatory, antibacterial,
immune-enhancing and antitumour effects (26-28).
It has been previously reported that the total
coumarins of Hedyotis diffusa (TCHD) could inhibit SKM-1
cell (MDS cell line) proliferation (23). The present study aimed to
preliminarily explore the potential use of HDE extracted from
Oldenlandia diffusa Willd combined with HSP90 inhibitor
BIIB021 for the treatment of MDS in vitro.
Materials and methods
Plant material and reagents
Authentic plant material of HDE was purchased from
Zhejiang University of Traditional Chinese Medicine Chinese
Medicine Decoction Pieces Co., Ltd. and identified by Dr Jianping
Jiang at Zhejiang Chinese Medical University (voucher specimen:
Jiang J.P., 141001, ZM). The dried and sliced HDE material was
ground into fine powder before extraction. FBS and RPMI-1640 medium
were purchased from Gibco (Thermo Fisher Scientific, Inc.). BIIB021
was purchased from Selleck Chemicals (cat. no. S1175). All other
reagents were obtained from Sigma-Aldrich (Merck KGaA).
Cell culture and preparation
The SKM-1 cell line was purchased from The Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences.
Cells were cultured with RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.) supplied with 10% (v/v) FBS (Gibco; Thermo Fisher
Scientific, Inc.) and penicillin-streptomycin under a humidified
atmosphere of 5% CO2 at 37˚C and used at the logarithmic
growth phase. Cells were digested and a cell suspension
(8x104 cells/ml) was prepared. Subsequently, 100 µl cell
suspension was added to each well of a 96-well culture plate.
Following culture for 24 h at 37˚C with 5% CO2, the
drugs were diluted to 0, 25, 50, 100, 200 µg/ml concentrations and
added to the SKM-1 cells with 100 µl per well. Following 48 h
incubation for test, bone marrow-derived stem cells (BMSCs; cat.
no. HUXMA-90011; Cyagen Biosciences, Inc.) were cultured in α-MEM
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 100 U/ml penicillin-streptomycin (Hyclone; GE Healthcare Life
Sciences) at 37˚C with 5% CO2 in a humidified
atmosphere.
Cytotoxicity assay
The drugs were diluted to the required working
concentrations in culture medium and 100 µl medium was added to
each well. A negative control (NC) group (standard curve reference)
and experiment control group were simultaneously set up. Cell
cytotoxicity was assessed using the Cell Counting Kit-8 (CCK-8)
assay (cat. no. 35003; Biosharp Life Sciences) according to the
manufacturer's protocol. Following incubation for 48 h, 10 µl CCK-8
solution was added to each well and incubated for 2-3 h at 37˚C.
The optical density (OD) was measured at a wavelength of 450 nm
using a microplate reader (BioTek Instruments, Inc.; EL-x800). Cell
proliferation was calculated according to the following formula:
proliferation (%) =
(Adrug-Ablank)/(A0-Ablank)
x 100, where Adrug represents the OD value of wells
containing treated cells, Ablank represents the OD value
of blank wells (no cells) and A0 represents the OD value
of wells containing untreated cells.
Small interfering (si)RNA
transfection
siRNA sequences targeting hTERT were designed as
follows: hTERT-siRNA-1728 sense, 5'-GGAAGAGUGUCUGGAGCAAGU-3' and
antisense, 5'-UUGCUCCAGACACUCUUCCGG-3'; hTERT-siRNA-966 sense,
5'-CGGUGUACGCCGAGACCAAGC-3' and antisense,
5'-UUGGUCUCGGCGUACACCGGG-3'; and hTERT-siRNA-1464 sense,
5'-GGAACACCAAGAAGUUCAUCU-3' and antisense,
5'-AUGAACUUCUUGGUGUUCCUG-3'); control siRNA (NC) sense,
5'-UUCUCCGAACGUGUCACGUTT-3' and antisense
5'-ACGUGACACGUUCGGAGAATT-3'. SKM-1 cells (8x104
cells/well) were cultured in 24-well plates for at least 12 h. A
total of 100 nM of each siRNA was transfected into SKM-1cells using
Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific, Inc.)
for up to 48 h.
Real-time quantitative PCR
analysis
Total RNA was extracted from cells using the
EasyPure® Blood RNA kit (TransGen BioTech Co., Ltd.)
according to the manufacturer's instructions. Total RNA was reverse
transcribed to cDNA using the One Step Goscript Reverse
Transcription System kit (Promega Corporation). The reaction
mixture contained: 15 µl RNA, 1.2 µl random primer and 1.2 µl Oligo
(dT). Following mixing and centrifugation at 1200 x g for 15 sec at
room temperature, cDNA was preheated at 70˚C for 5 min, placed on
ice for at least 5 min and centrifuged at 1200 x g for at least 10
sec at room temperature. The second strand cDNA synthesis mixture
contained: 6 µl 5X reaction buffer, 3.8 µl MgCl2 (25
mM), 1.5 µl PCR nucleotide mix, 0.3 µl RNA enzyme inhibitor and 1
µl reverse transcriptase. The following thermocycling conditions
were used: 25˚C for 5 min, 42˚C for 1 h and 70˚C for 15 min. The
cDNA products (5 µl) were subjected to electrophoresis using 1%
agarose gel (containing SYBR Green nucleic acid dye). cDNA bands
were visualized using a gel imaging system (Tanon Science and
Technology Co., Ltd.). The bands were quantified using Quantity One
image analysis software (Bio-Rad Laboratories, Inc.) with ACTB as
the internal reference gene.
Subsequently, cDNA was diluted using RNase-free
water up to 100 µl on ice. qPCR was performed using the following
reaction mixture (20 µl): 5 µl cDNA, 1 µl primer (4 pmol/µl), 1 µl
TaqMan Probe (6 pmol/µl; Genscript), 10 µl 2X Mix (Vazyme Biotech
Co., Ltd.) and RNase-free water. The following thermocycling
conditions were used for qPCR: 37˚C for 2 min, 95˚C for 5 min, and
denaturation at 95˚C for 10 sec and 60˚C for 30 sec, followed by 40
amplification cycles. The relative expression levels were analyzed
according to the 2-ΔΔCT method (29). The sequences of primers and probes
(NCBI Bank) were as follows: hTERT forward, 5'-GTGGTT
TCTGTGTGGTGTCA-3' and reverse, 5'-GGAGTAGAGGAA GTGCTTGG-3'; actin-β
(ACTB) forward, 5'-GATGAGATTG GCATGGCTTT-3' and reverse,
5'-GTCACCTTCACCGTT CCAGT-3'; HSP90 forward, 5'-CAGTTGGAATTCAGAGC
CCTTCT-3' and reverse, 5'-TCACGGGATATGTTTAGAGG GAG-3'; TaqMan
probe, 5'-6-FAM-TTTGTCCCACGACG TGCTCCTTTTG-BHQ1-3'; GAPDH forward,
5'-CTGACT TCAACAGCGACACC-3' and reverse, 5'-GTGGTCCAGGG
GTCTTACTC-3'; and TaqMan probe, 5'-6-FAM-CATTGCC
CTCAACGACCACTTTGTCA-BHQ1-3'.
Telomerase activity detection by
TRAP-ELISA
SKM-1 cell telomerase activity was assessed using
the TEELISA kit (cat. no. CSB-E08021h; Cusabio Technology LLC, USA)
according to the manufacturer's instructions. Following treatment
for 24 h, cells (1x106) were centrifuged at 4200 x g for
20 min at 4˚C. The cell pellet was incubated with cold lysis buffer
(200 µl) form the aforementioned kit, for 30 min on ice. The
supernatant (2 µl) was used as the TRAP reaction template.
Subsequently, 25 µl reaction mixture was added to the PCR reaction
tube and the total volume was adjusted to 50 µl with DEPC-treated
sterilized double distilled water to perform the TRAP reaction
according to the following protocol: primer extension for 10 min at
25˚C; telomerase inactivation for 5 min at 94˚C; 30 cycles of
denaturation for 30 sec at 94˚C, annealing for 30 sec at 50˚C and
extension for 90 sec at 72˚C; and extension for 10 min at 72˚C. The
amplified product (5 µl) and the denaturant (20 µl) were mixed and
maintained at room temperature for 10 min. Subsequently, 225 µl
hybridization solution (containing digoxin-labeled probe) was
added, and after mixing, 100 µl mixture was added to an anti-biotin
coated plate and incubated for 2 h at 37˚C (300 rpm). Then
peroxidase was added and developed with TMB substrate for 30 min at
room temperature. Finally, 100 µl stop solution was added. The A
value of each well (detection wavelength, 450 nm; reference
wavelength, 690 nm) and the magnitude of the A value represented
the level of telomerase activity.
HDE extraction
A total of 200 g Oldenlandia diffusa Willd
was weighed. Subsequently, the plant was incubated with 5.4 l 80%
ethanol for 30 min at room temperature, followed by
reflux-extraction for 110 min at 80˚C. Following filtering, the
filtrate was dried at 45˚C to obtain Hedyotis diffusa. The
extracts were suspended in 200 ml distilled water and separately
re-extracted four times with an equal volume of petroleum ether,
ethyl acetate or n-butanol. Subsequently, the extracts were
combined. The extracts of Hedyotis diffusa were named HDP
(petroleum ether extract), HDE (ethyl acetate extract), HDB
(n-butanol extract) and HDW (water fraction). HDE (5 g) was
suspended in 100 ml distilled water and HPD-300 macroporous resin
was used for dynamic adsorption at an adsorption flow rate of 0.5
ml/min (diameter:height = 1:10), and then eluted with water until
the residue was colorless. Dynamic elution was performed with 60%
ethanol at a flow rate of 1 ml/min. Finally, the eluate was
collected and dried under reduced pressure.
Chemical analysis of HDE
The ultra-performance liquid chromatography-tandem
mass spectrometry (UPLC-MS/MS) system (Waters Corporation) was used
to determine the chemical constituents of HDE. The filtered sample
(per 20 µl) was separated using an Inertsil C18 column (250x4.6 mm;
5 µm; Agilent Technologies Inc.) at 30˚C, with a controlled flow
rate of 1 ml/min at a set wavelength of 310 nm. The mobile phase
was composed of (A) acetonitrile and (B) 0.05% methanoic acid and
used for gradient elution of HDE at 0 min (85:15 v/v), 15-20 min
(80:20 v/v) and 30-60 min (75:15 v/v). For mass spectrometry,
ultra-high-purity argon (Ar) and high-purity nitrogen (N2) were
used as the collision gas and the nebulizing gas, respectively.
Ultra-high-purity argon (Ar) and high-purity nitrogen (N2) were
used as the collision gas and the nebulizing gas, respectively. The
ESI(-) source was operated under the following conditions: 2.0 kV
source voltage, 38psi nebulizer pressure, 350˚C nitrogen gas
temperature, with a controlled flow rate of 1 ml/min, 100˚C source
temperature, 300˚C capillary temperature, 500 l/h gas flow rate and
full scanning mode. The CA ion (163 m/z) produced three primary
fragments in MS/MS (119, 93 and 65 m/z) and the E-CSME ion (549
m/z) also produced three primary fragments in MS/MS (163, 119 and
89 m/z). Each chromatographic peak was identified by comparing the
mass spectrum with the NIST mass spectral database (http://www.nist.gov/nist). For further verification,
the retention time and mass data of each peak were compared with
the reference standard compound. All chromatograms and mass spectra
were analyzed using the Micromass MassLynx data system (MassLynx
4.1; Waters Corporation).
The total coumarin content of HDE was determined by
spectrophotometry using a UV-vis spectrophotometer (Shimadzu
Corporation) based on the method described by Wang et al
(30). Briefly, 1.0 g dried HDE was
dissolved in 100 ml 70% ethanol and soaked in the dark for 24 h at
room temperature. After filtration, the absorbance of HDE solution
at a wavelength of 310 nm was determined. The total coumarins were
calculated using a standard curve prepared with reference standard
p-coumaric acid (CA; Selleck Chemicals) and expressed in terms of
mg of CA equivalents per g of dried HDE.
Flow cytometry analysis
SKM-1 cells (8x104 cells/well) in the
logarithmic growth phase were seeded into 6-well plates. Cell
apoptosis was assessed using the Apoptosis detection kit
(Biouniquer Technology Co., Ltd.). Following drug
treatment/transfection for 24 h, BMSCs and SKM-1 cells were washed
twice with pre-cooled PBS. Subsequently, 300 µl binding buffer was
added to each well. Annexin V-FITC (5 µl) was added to each well,
gently mixed and incubated in the dark for 15 min at room
temperature. Subsequently, propidium iodide (10 µl) was added to
each well, gently mixed and incubated for 10 min at room
temperature in the dark. Stained cells were analyzed using a
FACSCalibur (BD Biosciences) and CellQuest software (version 1.2;
BD Biosciences).
Western blotting
Following treatment for 24 h, SKM-1 cells and
untreated BMSCs were harvested and total protein was extracted
using 2X SDS lysis buffer (Cell Signaling Technology, Inc.). Total
protein was quantified using the bicinchoninic acid assay (Sangon
Biotech Co., Ltd.). A total of 20 µl protein samples was separated
via 12% SDS-PAGE and transferred onto PVDF membranes. The membranes
were blocked with 5% skimmed milk in TBST for 2 h at room
temperature. Subsequently, the membranes were incubated with
primary antibodies (1:1,000) overnight at 4˚C. The primary
antibodies included hTERT (cat. no. ab32020; Abcam), caspase-3
(cat. no. ab184787; Abcam), caspase-8 (cat. no. ab108333; Abcam),
PARP (cat. no. ab139417; Abcam) and β-actin (cat. no. ab124964;
Abcam). Following primary incubation, the membranes were incubated
with a horseradish peroxidase-conjugated secondary antibody
(1:5,000, Bio-Rad Laboratories, Inc.) at room temperature for 2 h.
All antibodies were purchased from Cell Signaling Technology, Inc.
β-actin was used as a loading control. Protein bands were
visualized using Western blotting Luminol reagent (Biological
Industries).
Statistical analysis
Statistical analyses were conducted using SPSS
software (version 17.0; SPSS, Inc.). Comparisons between two groups
were analyzed using unpaired Student's t-tests. Comparisons among
multiple groups were analyzed using one-way ANOVAs followed by the
Dunnett's and Tukey's post hoc test with GraphPad Prism software
(version 5.0; GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference. Experiments were
performed in triplicates.
Results
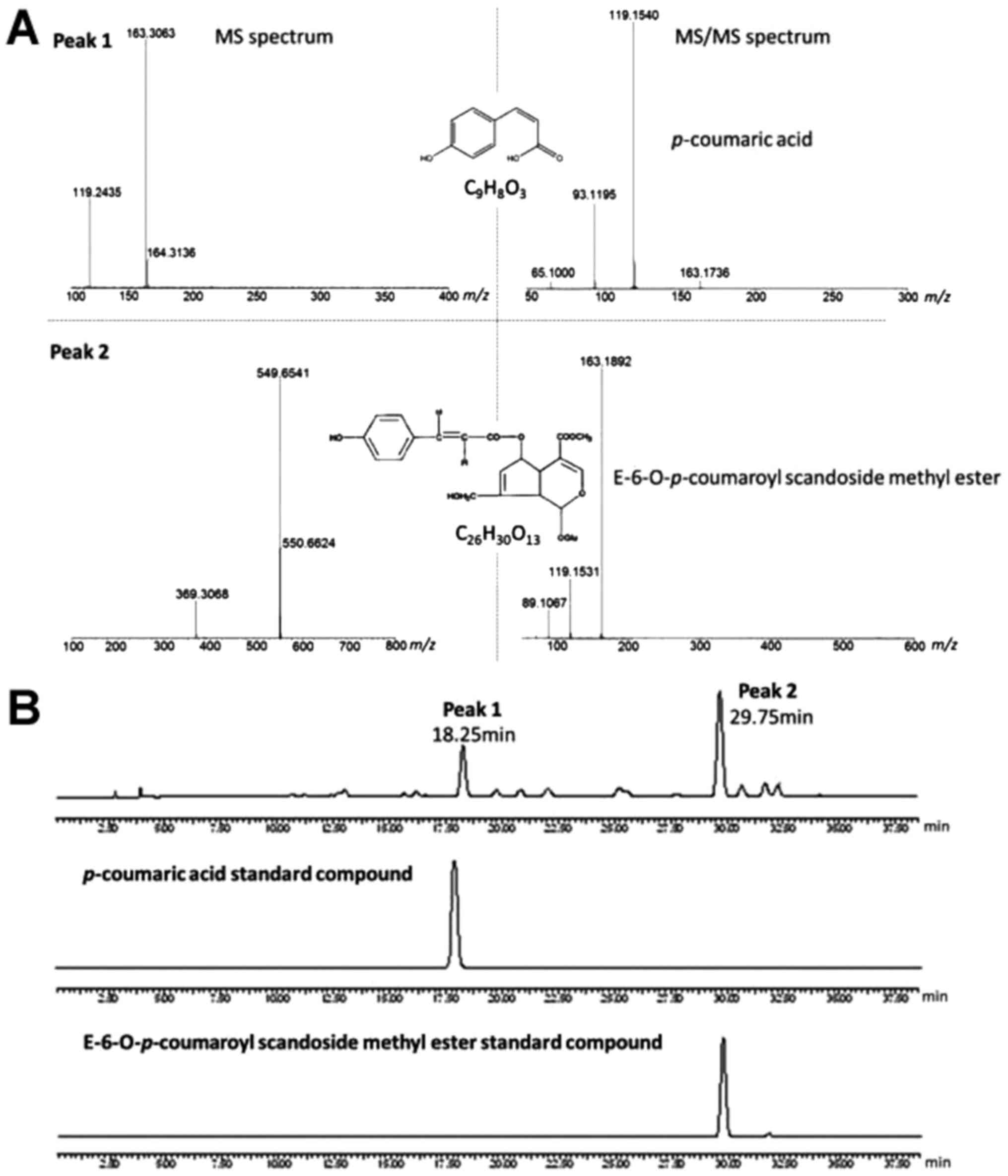
Chemical analysis of HDE
The UPLC-MS/MS method displayed high precision and
sensitivity, with mass accuracy of <10 ppm (data not shown). The
chromatogram (Fig. 1) displayed two
distinct chromatographic peaks with good resolution and response in
negative ion mode. Peak 1 and peak 2 were identified as CA and
E-6-O-p-coumaroyl scandoside methyl ester (E-CSME), respectively,
based on their MS and MS/MS spectra, as well as UV retention time,
compared with the reference standard compounds. The CA ion (163
m/z) produced three primary fragments in MS/MS (119, 93 and 65
m/z), and the E-CSME ion (549 m/z) also produced three primary
fragments in MS/MS (163, 119 and 89 m/z). The results indicated
that the total coumarin content of HDE was 87.4%.
HDE combined with BIIB021
significantly inhibits SKM-1 cell proliferation and telomerase
activity
To determine the anti-MDS efficacy of extracted HDE
and BIIB021, the inhibitory effects of the two drugs on SKM-1 cell
proliferation were assessed by performing the CCK-8 assay. The
concentration ranged from 0 to 200 µg/ml for HDE and 0 to 400
nmol/l for BIIB021. SKM-1 cell proliferation gradually decreased
with increasing HDE and BIIB021 concentrations (Fig. 2A and B). The IC50 of HDE was 98.03
µg/ml and the IC50 of BIIB021 was 171.7 nmol/l in SKM-1
cells, which were used for subsequent experiments.
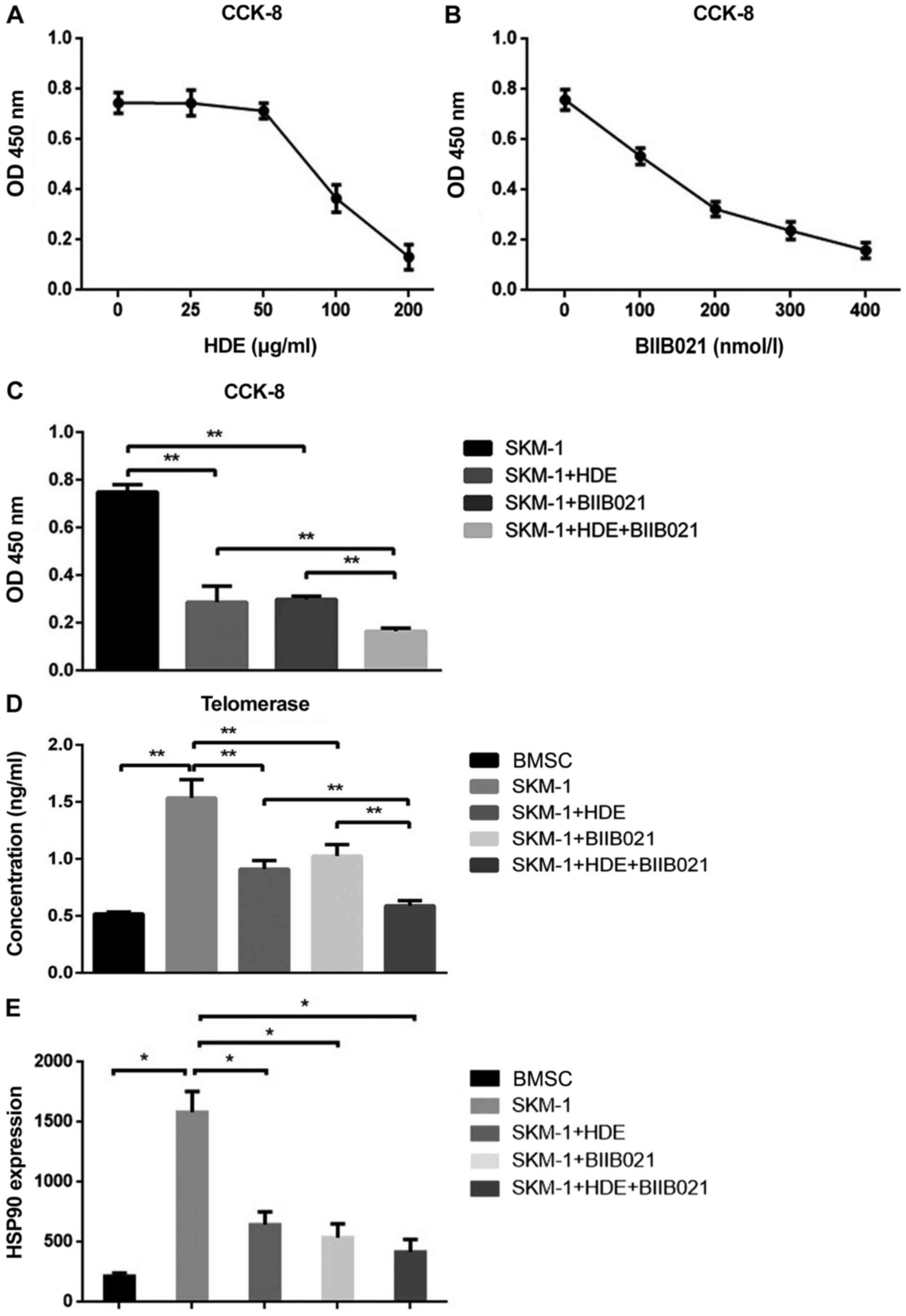 | Figure 2Effect of drug treatment on cell
proliferation and telomerase activity in SKM-1 cells. (A) Effects
of different concentrations of HDE on cell proliferation. The
IC50 of HDE was 98.03 µg/ml. (B) Effects of different
concentrations of BIIB021 on cell proliferation. The
IC50 of BIIB021 was 171.7 nmol/l. (C) Effects of HDE and
BIIB021 on cell proliferation (SKM-1+HDE vs. SKM-1, P=0.004;
SKM-1+BIIB021 vs. SKM-1, P=0.001; SKM-1+HDE+BIIB021 vs. SKM-1,
P=0.001). (D) Effects of HDE and BIIB021 on telomerase activity in
BMSCs and SKM-1 cells (BMSC vs. SKM-1, P=0.0004; SKM-1+HDE vs.
SKM-1, P=0.0036; SKM-1+BIIB021 vs. SKM-1, P=0.0094;
SKM-1+HDE+BIIB021 vs. SKM-1, P=0.0006; SKM-1+HDE vs.
SKM-1+HDE+BIIB021, P=0.0031; SKM-1+BIIB021 vs. SKM-1+HDE+BIIB021,
P=0.0022). (E) HSP90 expression levels in BMSCs and SKM-1 cells
(BMSC vs. SKM-1, P=0.00016; SKM-1 vs. SKM-1+HDE, P=0.0012; SKM-1
vs. SKM-1+BIIB021, P=0.0009; SKM-1 vs. SKM-1+HDE+BIIB021,
P=0.0005). hUC-MSC, human umbilical cord-mesenchymal stem cells;
BMSC, bone marrow-derived stem cell; OD, optical density.
*P<0.05, **P<0.01. |
Compared with the control group, HDE or BIIB021
treatment inhibited cell proliferation (P<0.01), whereas the
inhibitory effect of the two drugs on cell proliferation was
significantly increased in the HDE+BIIB021 combination group
(Fig. 2C; P<0.01). To explore
the mechanism underlying the inhibitory effects of HDE and BIIB021
on SKM-1 cell proliferation, the telomerase activity in BMSCs and
SKM-1 cells was assessed. The results indicated that telomerase was
highly activated in SKM-1 cells compared with BMSC (Fig. 2D; P<0.01), but significantly
inhibited by HDE, BIIB021 or combination treatment (Fig. 2D; P<0.01).
HSP90 expression
The expression of HSP90 in each group was assessed
via RT-qPCR. HSP90 was highly expressed in SKM-1 cells compared
with BMSC (P=0.00016). HDE (P=0.0012), BIIB021 (P=0.0009) or
combination (P=0.0005) treatment significantly reduced HSP90
expression levels in SKM-1 cells compared with untreated SKM-1
cells (Fig. 2E).
Decreased expression of hTERT and
treatment with HDE+BIIB021 enhances the inhibitory effect and
induces SKM-1 cell apoptosis
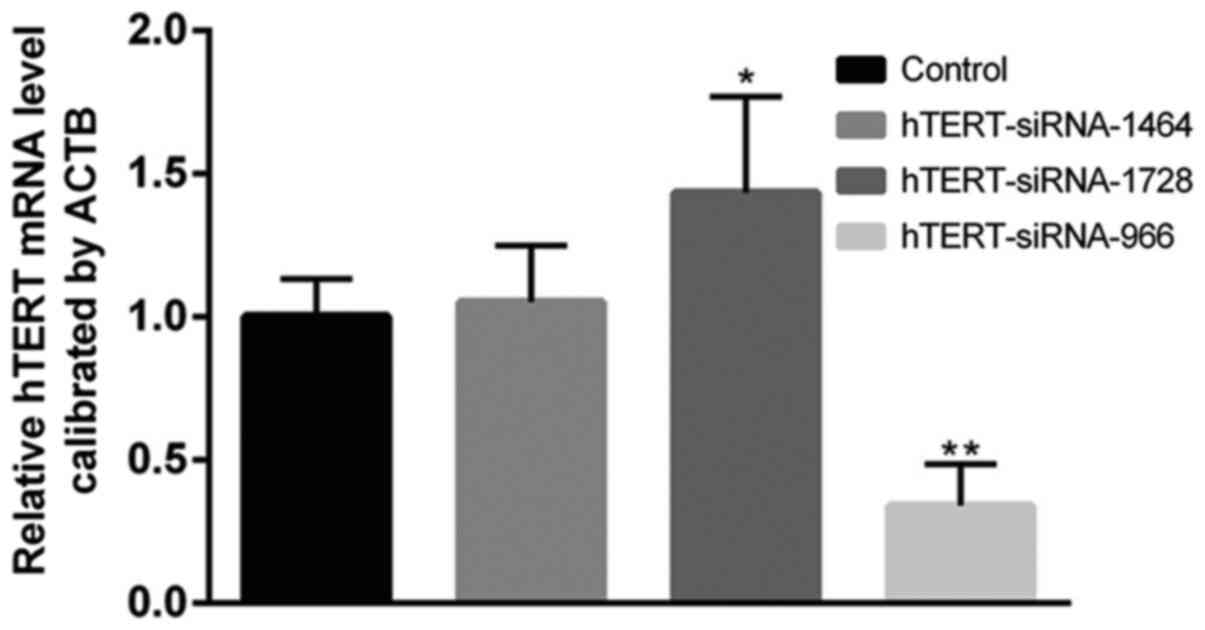
siRNAs were synthesized to inhibit hTERT expression.
The transfection efficiency of hTERT-siRNAs was assessed, which
indicated that hTERT-siRNA-966 significantly decreased hTERT
expression compared with the control group (Fig. 3); therefore, hTERT-siRNA-966 was
used for subsequent experiments. Subsequently,
hTERT-siRNA-transfected SKM-1 cells were treated with HDE, BIIB021
or combination treatment, and cell apoptosis was assessed via flow
cytometry (Fig. 4A). The results
indicated that HDE or BIIB021 treatment significantly induced SKM-1
cell apoptosis compared with non-treated SKM-1 cells (Fig. 4B; P<0.01). hTERT knockdown
combined with HDE or BIIB021 treatment significantly increased
SKM-1 cell apoptosis compared with SKM-1 cells treated with HDE and
hTERT knockdown combined with BIIB021 treatment also remarkably
increased cell apoptosis when compared with SKM-1 cells treated
with BIIB021 (Fig. 4B; P<0.01).
Moreover, HDE+BIIB021 combination treatment-induced SKM-1 cell
apoptosis was enhanced by hTERT knockdown (Fig. 4B; P<0.01).
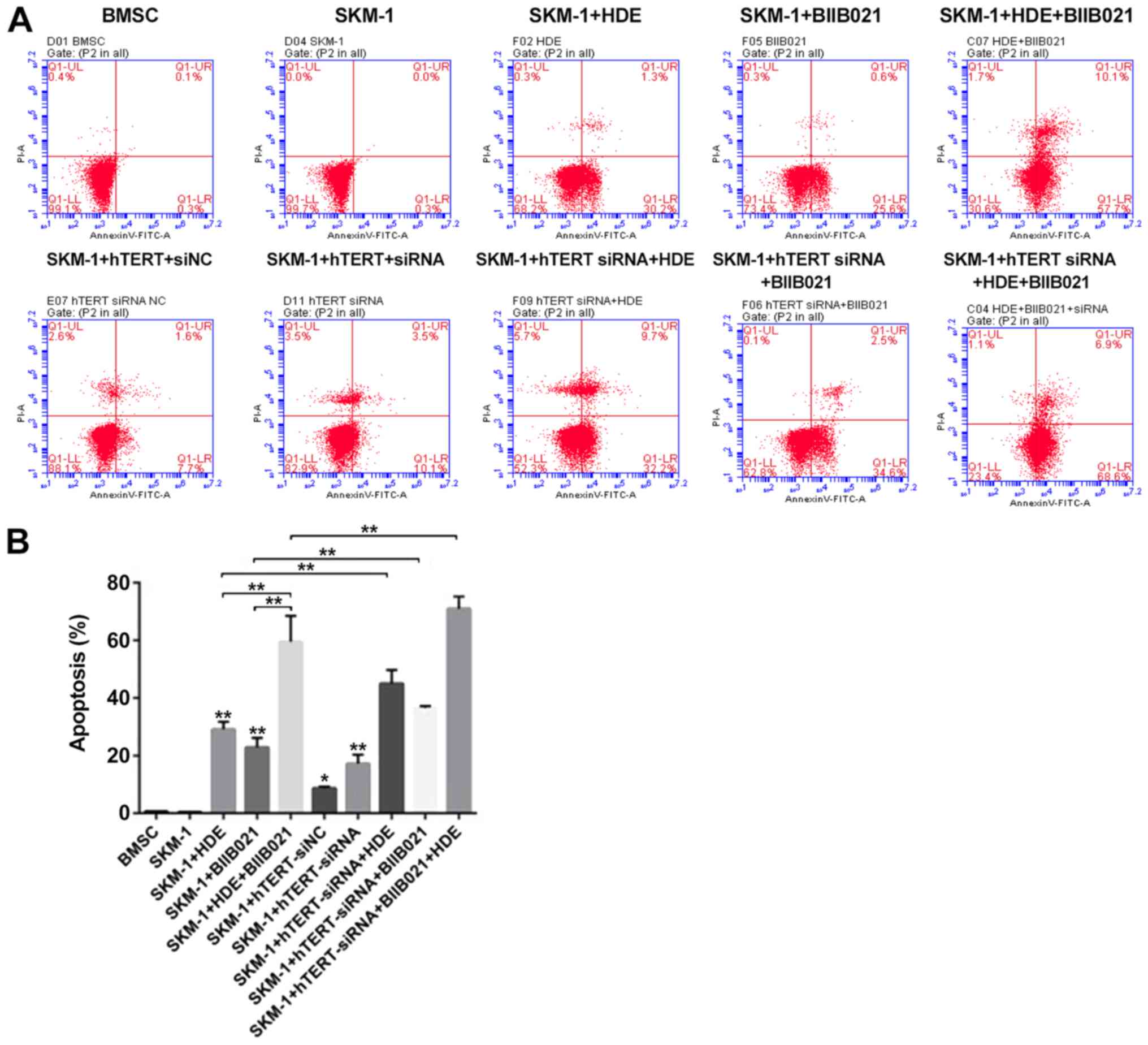 | Figure 4hTERT knockdown induces SKM-1 cell
apoptosis after treatment with HDE+BIIB021. SKM-1 cell apoptosis
was (A) determined via flow cytometry and (B) quantified (SKM-1+HDE
vs. SKM-1, P=0.0001; SKM-1+BIIB021 vs. SKM-1, P=0.0003;
SKM-1+HDE+BIIB021 vs. SKM-1, P=0.003; SKM-1+hTERT-siNC vs. SKM-1,
P=0.0001; SKM-1+hTERT-siRNA vs. SKM-1, P=0.0007;
SKM-1+hTERT-siRNA+HDE vs. SKM-1, P=0.0001;
SKM-1+hTERT-siRNA+BIIB021 vs. SKM-1, P=0.0001;
SKM-1+hTERT-siRNA+BIIB021+HDE vs. SKM-1, P=0.0007). hTERT, human
telomerase reverse transcriptase; si, small interfering RNA; NC,
negative control. *P<0.05,
**P<0.01. |
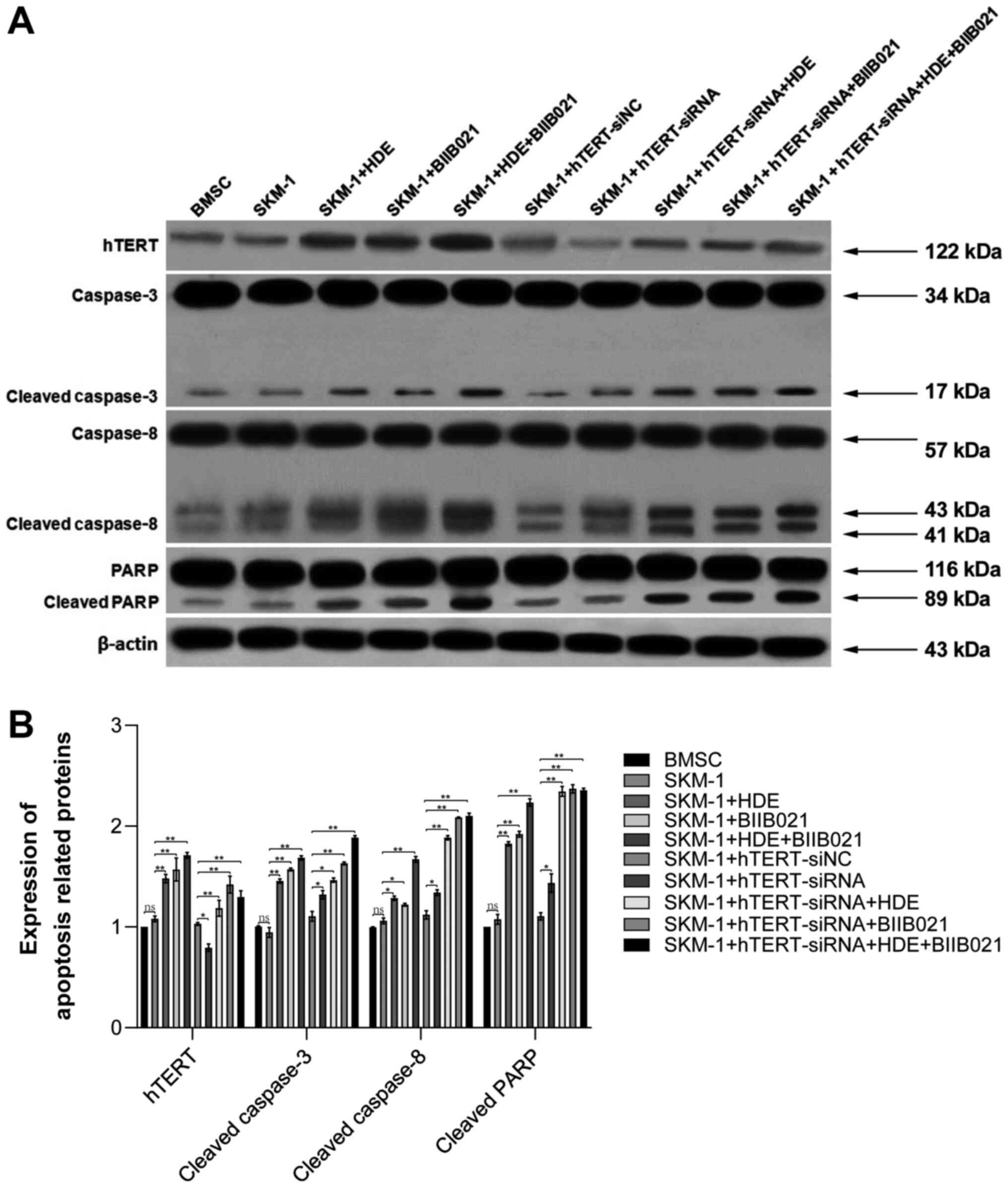
hTERT knockdown-induced SKM-1 cell
apoptosis is enhanced by treatment with single drug or combination
of two drugs
To investigate the mechanism underlying HDE in
promoting cancer cell apoptosis, treated BMSCs and SKM-1 cells were
homogenized to measure the protein expression levels of hTERT,
caspase 3, cleaved caspase 3, caspase 8, cleaved caspase 8, PARP
and cleaved PARP by western blotting (Fig. 5). Compared with BMSCs, protein
expression in SKM-1 cells was not notably altered. However, the
expression levels of hTERT, cleaved caspase 3, cleaved caspase 8
and cleaved PARP were increased following HDE, BIIB021 or
HDE+BIIB021 treatment compared with non-treated SKM-1 cells.
Additionally, following hTERT knockdown, the expression levels of
cleaved caspase 3, cleaved caspase 8 and cleaved PARP were slightly
increased compared with the hTERT-siNC group. By contrast, there
was no notable difference in protein expression levels among the
BMSC, SKM-1 and hTERT-siNC groups. Furthermore, following HDE,
BIIB021 or HDE+BIIB021 treatment, the expression levels of cleaved
caspase 3, cleaved caspase 8 and cleaved PARP were increased in
hTERT knockdown SKM-1 cells compared with hTERT-siNC-transfected
SKM-1 cells. The results further indicated that hTERT knockdown
facilitated the antitumor effects of HDE and BIIB021.
Discussion
Ineffective hematopoiesis with peripheral cytopenia
despite normo or hypercellular bone marrow is the hallmark of MDSs,
especially in the low-risk subgroups. It is caused by increased
secretion of inhibitory cytokines in the marrow; however, the
underlying mechanisms are not completely understood (31). Immunoinhibitory or modulatory
therapy aims to restore hematopoiesis by reversing inhibitory
processes, and includes anti-thymocyte globulin (ATG), cyclosporin
A (CSA), direct TNF-inhibitors and lenalidomide (32). A simple scoring system, using age,
duration of transfusion dependency and HLA-DR15 status, has been
developed, which facilitates identification of patients with a
high, intermediate or low probability of response to treatment with
ATG (33). Other immunosuppressive
agents that can also induce responses, such as CSA, have been
combined with ATG, but have not yet been fully evaluated for their
potential role in the treatment of MDS (32). Lenalidomide displays superior
efficacy in the 5q-syndrome, but low efficacy in other patients
with low-risk MDS (34). Additional
clinical trials are required to define the potential of
immunosuppressive and modulatory treatment in MDS (35,36).
Thus, identifying safer and more effective anti-MDS strategies
remains a major global health challenge. In our previous study,
TCHD displayed proapoptotic effects in SKM-1 cells, and the
underlying mechanism was associated with the inhibition of the
PI3K/Akt signaling pathway and activation of caspase proteins
(23). A further two new compounds,
CA and E-CSME, were identified in TCHD (23). Also, the newly extracted compound of
HDE efficiently inhibited leukemia cell proliferation and induced
cancer cell apoptosis with our patented extraction technology
(patent no. ZL201410324070.2).
Patients with higher-risk MDS with AML
transformation display higher hTERT mRNA expression levels compared
with healthy individuals and patients with low-risk MDS (37). hTERT-positive patients can easily
develop leukemia (37), indicating
that hTERT is positively related to the progression of MDS. Several
natural drugs can inhibit the function of HSP90, such as
benzoquinone ansamycin antibiotics, radicicol and neomycin, by
competing with ATP for binding to the N-terminal conserved domain
of HSP90 or interfering with the interaction between HSP90 and
other accessory chaperone proteins, thus reducing the stability of
chaperone protein (38).
Previously, the synergistic effect of traditional
Chinese medicine extract and chemotherapy drugs has been reported
(39). Similarly, a previous study
has also demonstrated that both HDE and BIIB021 downregulated
telomerase activity, and combined treatment further enhanced the
tumor apoptosis-inducing effects of the compounds (40). BIIB021 outperforms other drugs as it
displays independent anticancer effects with expression of
multidrug resistance (MDR) proteins, such as P-gp and/or
MDR-related protein 1, which typically confer resistance to a broad
range of chemotherapeutic agents and molecularly targeted drugs
(41-44).
The prolonged inhibitory effects of BIIB021 on proliferation could
increase its clinical applicability. In vitro experiments
indicated that BIIB021 can competitively bind to the N-terminal
ATP/ADP domain of HSP90, downregulate the expression of chaperones
HER2, Akt, and Raf-1, and upregulate HSP70 and HSP27, with a broad
spectrum of antitumor activities (20,45).
HDE may share a similar anticancer mechanism with BIIB021, but
further investigation is required. In addition, HDE is extracted
from the natural herb Hedyotis diffusa, which has been used
for cancer treatment in China for centuries (46). Previous studies have demonstrated
that TCHD could induce apoptosis in leukemia cell lines Kasumi-1,
KG-1, THP-1, U937 and K562 (47,48).
The present study in SKM-1 cells further demonstrated the
broad-spectrum antileukemia benefits of TCHD. In the future, a
preclinical study should be conducted to identify other purified
coumarin components with fewer side effects and improved
efficacy.
The present study indicated that downregulation of
hTERT by siRNA might serve as a more direct strategy to inhibit
cancer cell proliferation. Following hTERT knockdown, the flow
cytometry results suggested that the apoptotic rate was increased
in HDE- or BIIB021-treated SKM-1 cells. Antitumor effects were
observed with a combination of the two drugs following hTERT
knockdown. The western blotting results further indicated that
cleaved caspase 3, cleaved caspase 8 and downstream target cleaved
PARP, which are involved in apoptosis pathways, were upregulated
following hTERT knockdown compared with the hTERT-siNC group.
Collectively, the results indicated an additive effect between the
two drugs. However, the lack of cell proliferation analysis with
trypan blue was a key limitation of the present study. Therefore,
further mechanisms underlying HDE require investigation in future
studies.
In conclusion, the present study identified a new
active component HDE in Oldenlandia diffusa Willd, which
displayed apoptosis-inducing effects on the MDS-derived leukemia
cell line SKM-1, and the underlying mechanism, which involved
downregulation of hTERT expression. The present study indicated
that HDE, due to its potent anti-MDS activity, may be valuable for
the development of new therapies for patients with MDS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Great Item of Science and
Technology Planning Project of Zhejiang Province (grant no.
2019C03047), the Medical and Health Science and Technology Plan of
Zhejiang Province of China (grant no. 2017RC022) and the
Traditional Chinese Medicine Science and Technology Plan Projects
of Zhejiang Province (grant no. 2017ZQ012).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL, YunZ and BW supervised the project. BW, JJ and
YS designed the study. YunZ, JJ, ST, SL, YS and LW performed the
experiments. BW, ST and SL wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adès L, Itzykson R and Fenaux P:
Myelodysplastic syndromes. Lancet. 383:2239–2252. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mitani K: Molecular pathogenesis of MDS.
Rinsho Ketsueki. 50:1325–1331. 2009.PubMed/NCBI(In Japanese).
|
|
3
|
Malcovati L, Hellström-Lindberg E, Bowen
D, Adès L, Cermak J, Del Cañizo C, Della Porta MG, Fenaux P,
Gattermann N, Germing U, et al: European Leukemia Net: Diagnosis
and treatment of primary myelodysplastic syndromes in adults:
Recommendations from the European LeukemiaNet. Blood.
122:2943–2964. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cheson BD, Greenberg PL, Bennett JM, et
al: Clinical application and proposal for modification of the
International Working Group (IWG) response criteria in
myelodysplasia. Blood. 108:419–425. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cogle CR, Craig BM, Rollison DE and List
AF: CR C. Incidence of the myelodysplastic syndromes using a novel
claims-based algorithm: High number of uncaptured cases by cancer
registries. Blood. 117:7121–7125. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Greenberg PL, Tuechler H, Schanz J, Sanz
G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus
F, et al: Revised international prognostic scoring system for
myelodysplastic syndromes. Blood. 120:2454–2465. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Killick SB, Carter C, Culligan D, Dalley
C, Das-Gupta E, Drummond M, Enright H, Jones GL, Kell J, Mills J,
et al: British Committee for Standards in Haematology: Guidelines
for the diagnosis and management of adult myelodysplastic
syndromes. Br J Haematol. 164:503–525. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Greenberg PL, Stone RM, Al-Kali A, et al:
Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice
Guidelines in Oncology. J Natl Compr Canc Netw. 15:60–87.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Macedo LC, Silvestre AP, Rodrigues C, de
Alencar JB, Zacarias JM, Ambrosio-Albuquerque EP, Sell AM and
Visentainer JE: Genetics factors associated with myelodysplastic
syndromes. Blood Cells Mol Dis. 55:76–81. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grisendi S, Bernardi R, Rossi M, Cheng K,
Khandker L, Manova K and Pandolfi PP: Role of nucleophosmin in
embryonic development and tumorigenesis. Nature. 437:147–153.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schneider F, Hoster E, Unterhalt M,
Schneider S, Dufour A, Benthaus T, Mellert G, Zellmeier E,
Bohlander SK, Feuring-Buske M, et al: NPM1 but not FLT3-ITD
mutations predict early blast cell clearance and CR rate in
patients with normal karyotype AML (NK-AML) or high-risk
myelodysplastic syndrome (MDS). Blood. 113:5250–5253.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wimmer K, Vinatzer U, Zwirn P, Fonatsch C
and Wieser R: Comparative expression analysis of the antagonistic
transcription factors EVI1 and MDS1-EVI1 in murine tissues and
during in vitro hematopoietic differentiation. Biochem Biophys Res
Commun. 252:691–696. 1998.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Graubert T and Walter MJ: Genetics of
myelodysplastic syndromes: New insights. Hematology (Am Soc Hematol
Educ Program). 2011:543–549. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bagatell R and Whitesell L: Altered Hsp90
function in cancer: A unique therapeutic opportunity. Mol Cancer
Ther. 3:1021–1030. 2004.PubMed/NCBI
|
|
15
|
Kamal A, Thao L, Sensintaffar J, Zhang L,
Boehm MF, Fritz LC and Burrows FJ: A high-affinity conformation of
Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature.
425:407–410. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Flandrin P, Guyotat D, Duval A, Cornillon
J, Tavernier E, Nadal N and Campos L: Significance of heat-shock
protein (HSP) 90 expression in acute myeloid leukemia cells. Cell
Stress Chaperones. 13:357–364. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Forsythe HL, Jarvis JL, Turner JW, Elmore
LW and Holt SE: Stable association of hsp90 and p23, but Not hsp70,
with active human telomerase. J Biol Chem. 276:15571–15574.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pinkerton DM, Chow S, Eisa NH, Kainth K,
Vanden Berg TJ, Burns JM, Guddat LW, Savage GP, Chadli A and
Williams CM: Synthesis of the seco-Limonoid BCD Ring System
Identifies a Hsp90 Chaperon Machinery (p23) Inhibitor. Chemistry.
25:1451–1455. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Duval A, Olaru D, Campos L, Flandrin P,
Nadal N and Guyotat D: Expression and prognostic significance of
heat-shock proteins in myelodysplastic syndromes. Haematologica.
91:713–714. 2006.PubMed/NCBI
|
|
20
|
Flandrin-Gresta P, Solly F, Aanei CM,
Cornillon J, Tavernier E, Nadal N, Morteux F, Guyotat D, Wattel E
and Campos L: Heat Shock Protein 90 is overexpressed in high-risk
myelodysplastic syndromes and associated with higher expression and
activation of Focal Adhesion Kinase. Oncotarget. 3:1158–1168.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chiosis G, Timaul MN, Lucas B, Munster PN,
Zheng FF, Sepp-Lorenzino L and Rosen N: A small molecule designed
to bind to the adenine nucleotide pocket of Hsp90 causes Her2
degradation and the growth arrest and differentiation of breast
cancer cells. Chem Biol. 8:289–299. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lundgren K, Zhang H, Brekken J, Huser N,
Powell RE, Timple N, Busch DJ, Neely L, Sensintaffar JL, Yang YC,
et al: BIIB021, an orally available, fully synthetic small-molecule
inhibitor of the heat shock protein Hsp90. Mol Cancer Ther.
8:921–929. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jiang J, Wang B, Li J, Ye B, Lin S, Qian
W, Shan L and Efferth T: Total coumarins of Hedyotis diffusa
induces apoptosis of myelodysplastic syndrome SKM-1 cells by
activation of caspases and inhibition of PI3K/Akt pathway proteins.
J Ethnopharmacol. 196:253–260. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qin T, Castoro R, El Ahdab S, Jelinek J,
Wang X, Si J, Shu J, He R, Zhang N, Chung W, et al: Mechanisms of
resistance to decitabine in the myelodysplastic syndrome. PLoS One.
6(e23372)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Di H, Liu D, Gao X, Xu Y, Wang Y and Wang
Z: [TCM Diagnosis and Treatment Status of Myelodysplastic
Syndrome]. World Chinese Medicine. 9:1557–1560. 2014.
|
|
26
|
Lee HZ, Bau DT, Kuo CL, Tsai RY, Chen YC
and Chang YH: Clarification of the phenotypic characteristics and
anti-tumor activity of Hedyotis diffusa. Am J Chin Med.
39:201–213. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meng QX, Roubin RH and Hanrahan JR:
Ethnopharmacological and bioactivity guided investigation of five
TCM anticancer herbs. J Ethnopharmacol. 148:229–238.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Niu Y and Meng QX: Chemical and
preclinical studies on Hedyotis diffusa with anticancer
potential. J Asian Nat Prod Res. 15:550–565. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang M, Jia M, Ma Y, Jiang G, Tang S and
Xia L: Determination of coumarins content in Radix Angelicae
dahuricae by HPLC and UV. Zhong Yao Cai. 27:826–828.
2004.PubMed/NCBI(In Chinese).
|
|
31
|
Cazzola M: Myelodysplastic Syndromes. N
Engl J Med. 383:1358–1374. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Garg R, Faderl S, Garcia-Manero G, Cortes
J, Koller C, Huang X, York S, Pierce S, Brandt M, Beran M, et al:
Phase II study of rabbit anti-thymocyte globulin, cyclosporine and
granulocyte colony-stimulating factor in patients with aplastic
anemia and myelodysplastic syndrome. Leukemia. 23:1297–1302.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saunthararajah Y, Nakamura R, Wesley R,
Wang QJ and Barrett AJ: A simple method to predict response to
immunosuppressive therapy in patients with myelodysplastic
syndrome. Blood. 102:3025–3027. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Santini V: Treatment of low-risk
myelodysplastic syndromes. Hematology (Am Soc Hematol Educ
Program). 2016:462–469. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gibbs JB: Mechanism-based target
identification and drug discovery in cancer research. Science.
287:1969–1973. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ganser A, Passweg J, Stadler M,
Dobbelstein C and Weissinger EM: Immunosuppressive treatment
strategies in low-risk MDS. Cancer Treat Rev. 33:S11–S14. 2007.
|
|
37
|
Cogulu O, Kosova B, Gunduz C, Karaca E,
Aksoylar S, Erbay A, Karapinar D, Vergin C, Vural F, Tombuloglu M,
et al: The evaluation of hTERT mRNA expression in acute leukemia
children and 2 years follow-up of 40 cases. Int J Hematol.
87:276–283. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Taldone T, Sun W and Chiosis G: Discovery
and development of heat shock protein 90 inhibitors. Bioorg Med
Chem. 17:2225–2235. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang B, Lin SY, Shen YY, Wu LQ, Chen ZZ,
Li J, Chen Z, Qian WB and Jiang JP: Pure total flavonoids from
Citrus paradisi Macfadyen act synergistically with arsenic trioxide
in inducing apoptosis of Kasumi-1 leukemia cells in vitro. J
Zhejiang Univ Sci B. 16:580–585. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang B, Zhang Y, Shen Y, Wu L, Zhou Y and
Lin S: [Downregulation of hTERT with Drug Combination of HDE and
BIIB021 Efficiently Inhibit Cell Proliferation of and Induce
Apoptosis in Myelodysplastic Syndromes]. 2019 Annual Meeting of the
Hematology Specialty Committee of Zhejiang Integrative Medicine
Association: 233, 2019.
|
|
41
|
Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE
and Gottesman MM: P-glycoprotein: From genomics to mechanism.
Oncogene. 22:7468–7485. 2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Glavinas H, Krajcsi P, Cserepes J and
Sarkadi B: The role of ABC transporters in drug resistance,
metabolism and toxicity. Curr Drug Deliv. 1:27–42. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Larsen AK, Escargueil AE and Skladanowski
A: Resistance mechanisms associated with altered intracellular
distribution of anticancer agents. Pharmacol Ther. 85:217–229.
2000.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Varma MV, Ashokraj Y, Dey CS and
Panchagnula R: P-glycoprotein inhibitors and their screening: A
perspective from bioavailability enhancement. Pharmacol Res.
48:347–359. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang H, Neely L, Lundgren K, Yang YC,
Lough R, Timple N and Burrows F: BIIB021, a synthetic Hsp90
inhibitor, has broad application against tumors with acquired
multidrug resistance. Int J Cancer. 126:1226–1234. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Fang T, Yan YX, Yang Y, Lv YX, Chang QQ
and Zhang DD: Ethyl Acetate Fraction from Hedyotis diffusa
plus Scutellaria barbata Suppresses Migration of
Bone-Metastatic Breast Cancer Cells via OPN-FAK/ERK/NF-κB Axis.
Evid Based Complement Alternat Med. 2020(3573240)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen Z, Wang B, Qiu M, Lin SY and Xiong H:
[Proliferation Inhibition and Its Mechanism of Total Coumarins on
Leukemia Cells]. Chinese Pharmaceutical Journal. 52:1503–1509.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen Z, Lin S, Jiang J, et al: [Inhibitory
Effect of Total Coumarins of Hedyotis diffusa Willd on the Growth
of AML Cell Line Kasumi-1]. Chinese Journal of Integrated
Traditional and Western Medicine. 37:1089–1094. 2017.
|