Introduction
Premature rupture of membranes (PROM) refers to a
spontaneous rupture of fetal membranes that occurs at least 2 h
prior to the onset of labor. PROM that occurs in patients with
>37 weeks gestation is referred to as term premature rupture of
membranes (TPROM). Preterm premature rupture of membranes (PPROM)
occurs at <37 weeks gestation and has an incidence of ~3%,
accounting for approximately one third of preterm births in Nigeria
during 1993-2003(1). PROM is often
associated with puerperal and neonatal infection, fetal
malformation, premature infants and maternal complications
(2-6).
Obstetric disease often occurs due to multiple risk factors,
including amnionitis, multifetal pregnancy, polyhydramnios,
cervical relaxation or surgery, trauma, maternal smoking status,
poor maternal nutrition, abnormal pH values, congenital amniotic
dysplasia, hereditary amniotic membrane thinning, and vitamin C,
trace element zinc and copper deficiency (7-10).
Fetal membranes consist of chorion, amniotic membrane, basement
membrane and extracellular matrix (ECM) (11,12).
The nature of the membrane structure allows PROM to cause the
membranes to become thin and brittle, and eventually rupture
(13).
Matrix metalloproteinases (MMPs) are zinc and
calcium-dependent proteolytic enzymes that participate in the
degradation of ECM. MMPs are divided into six categories as
follows: Collagenase, stromal lysin, gelatinase, membrane
metalloproteinase, matrix enzyme and secreted MMP (14). The MMP family is involved in
physiological and pathological processes, and has attracted
attention in the field of gynecological and obstetric diseases
(15,16). MMPs are located between the
chorionic and amniotic membranes, and the enzymes MMP9, MMP2, MMP13
and MMP8 may serve a key role in the rupture of the fetal membranes
(17-20).
The histone octamer consists of two copies of each
histone, including H2A, H2B, H3 and H4, which are involved in DNA
organization and folding in the nucleus (21). Acetylation modification is an
important mechanism by which DNA may be unfolded and it is a
reversible dynamic process (22).
Histone acetylation is largely catalyzed by histone
acetyltransferases (HATs) and histone deacetylases (HDACs), which
activate and inhibit gene transcription, respectively (22). HDAC inhibitors are a class of agents
designed to block HDACs, therefore allowing HATs to induce histone
acetylation and to promote gene transcription (23). The structure of four core histones
(H2A, H2B, H3 and H4) and their associated modifications are
associated with gynecologic and obstetric diseases (24).
MMPs serve an important role in mediating
degradation of the ECM in pathologic conditions such as PROM
(25,26). In addition, Poljak et al
(27) suggested that HDACs regulate
MMP-9 expression in primary amnion cells. The current study
employed the HDAC inhibitors droxinostat and chidamide to block
HDACs and to investigate whether the levels of histone acetylation
are associated with MMPs in human amniotic epithelial cells.
Materials and methods
Patients and tissue samples
Patients with PROM were diagnosed according to the
American College of Obstetricians and Gynecologists (ACOG)
guidelines on the diagnosis and treatment of PROM (version 2013)
(28). The diagnostic criteria were
as follows: i) Leaking of amniotic fluid prior to labor, ii) an
alkaline pH test and iii) a positive amniotic fluid crystallization
test. A total of 7,630 puerperant, among them 4,230 who were
spontaneously delivered, were identified between May 2016 and March
2017 at the Maternal and Child Health Hospital of Tangshan
(Tangshan, China). A total of 180 puerperants were selected using
the following inclusion criteria: i) Patients agreed to give birth
naturally, ii) single fetus with head-first presentation, iii)
patients conformed with the aforementioned ACOG diagnostic
criteria, iv) patients delivered within 48 h following admission,
v) patients with normal body temperature on admission, vi) no
mechanical interventions used during pregnancy and vii) patients
without complications such as hypertension, diabetes, heart disease
and asthma. Patients were divided into three groups, PPROM, TPROM
and full-term labor (FTL), depending on whether the duration of
gestation exceeded 37 weeks prior to membrane rupture. The basic
information of the patients is presented in Table I. The current study was approved by
the Ethics Committee of the Maternal and Child Health Hospital of
Tangshan (Tangshan, China) and written informed consent was
obtained from each patient.
 | Table IBasic clinical information of the
patients enrolled in the current study. |
Table I
Basic clinical information of the
patients enrolled in the current study.
| Variable | PPROM (n=60) | TPROM (n=60) | FTL (n=60) | P-value |
|---|
| Age | 28.26±5.13 | 27.79±3.44 | 27.5±3.67 | 0.600 |
| BMI |
28.95±5.24a,b | 26.98±3.71 | 27.28±4.26 | 0.035 |
| Gestational age
(week) | 33.87±1.83 | 39.06±1.90 | 39.65±0.93 | <0.001 |
| TMRD (h) | 38.6±59.05 | 21.93±22.27 | 0.00 | <0.001 |
| One-child
ratio | 71.67% | 76.67% | 75.00% | 0.815 |
| IAH | 0.00% | 8.33% | 80.00% | <0.001 |
| Eosinophils
(%) | 0.92±0.28 | 1.03±1.74 | 1.36±0.38 | 0.057 |
| Neutrophils
(%) |
75.65±11.56a |
74.11±10.51a | 71.34±13.85 | 0.143 |
| Glucose
(mmol/l) | 6.06±1.72 | 5.84±1.73 | 6.13±1.87 | 0.647 |
| Albumin (g/l) |
31.84±5.56a |
33.22±5.31a | 35.52±6.65 | 0.003 |
Approximately 10 g of fetal membrane tissue was cut
close to the area of rupture from which amniotic fluid was released
within 15 min of natural delivery. The tissues collected were
rinsed three to four times in physiological saline solution
containing 0.9% sodium chloride and stored at -40˚C.
Cell culture
Human amniotic epithelial (HAEpi) cells were
obtained from Sciencell Research Laboratories, Inc. The cells were
cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented
with 10% fetal bovine serum (FBS), 1% L-glutamine, 1%
penicillin/streptomycin (all Thermo Fisher Scientific, Inc.) and 2
mM glutamine at 37˚C and 5% CO2. The cells were grown to
80% confluence and trypsinized using phosphate-buffered saline
(PBS) containing 0.25% trypsin (29).
Immunofluorescence staining
Immunofluorescence staining was used to verify the
presence of HAEpi cells, which were seeded in six-well plates
(2x105 cells/well) and fixed with 4% paraformaldehyde at
room temperature for 20 min. Cell were subsequently incubated with
PBS containing 0.3% Triton X-100 at 4˚C for 15 min. The cells were
blocked with 10% normal goat serum (cat. no. ab7481; 1:50; Abcam)
at 37˚C for 20 min and then incubated with an anti-cytokeratin
(CK)-19 antibody (cat. no. ab15463; 1:100; Abcam) at 4˚C overnight
(30). Cells were washed using PBS
and incubated with a horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (cat. no. 10285-1-AP; 1:1,000;
ProteinTech Group, Inc.) at room temperature for 1 h. Nuclei were
counterstained using 4',6-diamidino-2-phenylindole (DAPI) at room
temperature for 10 min. An anti-fluorescence quenching agent
(ProLong® Antifade kit; cat. no. P7481; Thermo Fisher
Scientific, Inc.) was then added to cells in the dark at room
temperature for 30 min. Images were subsequently taken using a
fluorescence microscope (magnification, x400) with NIS-Elements BR
software version 4.60.
Treating cells with HDAC
inhibitors
The HDAC inhibitors droxinostat (cat. no. S1422) and
chidamide (cat. no. S8567) were purchased from Selleck Chemicals.
In order to determine the effects of the HDAC inhibitors on cell
viability, HAEpi cells were exposed to droxinostat (0, 1, 5, 10 and
20 µM) and chidamide (0, 0.1, 0.2, 0.5 and 1 µM) and dissolved in
DMSO at room temperature for 24, 48 and 72 h. For other in
vitro experiments (detection of H4K5ac and H4K8ac protein
expression and the detection of MMP-2, MMP-9 and HDAC-1, 2 and 6),
HAEpi cells were treated with the DMSO (control) or with HDAC
inhibitors, droxinostat (5 µM) and chidamide (0.5 µM), for 48 h to
assess other in vitro experiments.
Cell Counting Kit-8 (CCK-8) assay
HAEpi cells were plated into 96-well plates at a
seeding density of 1x104 cells per well and incubated at
37˚C for 24 h. The cells were exposed to the HDAC inhibitors at the
aforementioned concentrations for 24, 48 and 72 h. A total of 10 µl
CCK-8 solution was subsequently added to each well and incubated
for an additional 3 h at 37˚C. Cell viability was determined by
measuring the optical density at a wavelength of 450 nm using a
microplate reader.
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of MMP2, MMP9, HDAC1, HDAC2 and
HDAC6 in fetal membrane tissues and HAEpi cells were measured using
ELISA kits. A total of 100 mg of fetal membrane tissues were
homogenized in 1 ml protein lysate buffer (comprising 2.5 ml Triton
X and one tablet of Roche Complete EDTA-free Protease Inhibitor
(Sigma-Aldrich; Merck KGaA) to a volume of 50 ml), centrifuged at
4,042 x g at room temperature for 10 min and supernatants were
collected. HAEpi cells (1x106 cells/well) were seeded on
a 24-well plate at 37˚C. Cell-free culture media harvested after 3
h. ELISA kits were used to detect the concentrations of MMP2 (cat.
no. MMP-200), MMP9 (cat. no. DMP-900; both R&D Systems, Inc.),
HDAC1 (cat. no. ml037173; Shanghai Enzyme-linked Biotechnology Co.,
Ltd), HDAC2 (cat. no. IT4052; G-Biosciences), HDAC6 (cat. no.
IT4738; G-Biosciences) in the culture media according to the
manufacturer's instructions and as described previously (31,32).
Transwell assay
Cell migration and invasion were performed using
uncoated and Matrigel-coated transwell respectively. Following
exposure to droxinostat (5 µM), chidamide (0.5 µM) or DMSO (control
group; 5 µM or 0.5 µM) for 48 h, HAEpi cells were resuspended in
serum-free DMEM, and 1x104 cells were added into the
upper chamber of the transwell insert (Corning, Inc.). DMEM
supplemented with 10% FBS was added to the lower chamber and the
cells were incubated at 37˚C for 24 h. The cells were subsequently
fixed with 1% formaldehyde for 10 min at room temperature and
stained with 0.5% crystal violet at room temperature for 5 min
after the Matrigel coated filter membrane was removed. The number
of migratory cells in five randomly selected fields were imaged
using a light microscope (magnification, x200) and counted using
ImageJ software (Version 1.49; National Institutes of Health).
Western blot analysis
Proteins were extracted from fetal membrane tissues
or HAEpi cells using radioimmunoprecipitation assay buffer (Thermo
Fisher Scientific, Inc.). Total protein was quantified using a
bicinchoninic acid kit (Beyotime Institute of Biotechnology).
Aliquots of protein (20 µg/lane) were separated via SDS-PAGE on a
12% gel. The separated proteins were transferred onto a
polyvinylidene fluoride membrane (EMD Millipore). The membrane was
blocked with 5% milk in PBS and 0.1% Triton X-100 at room
temperature for 2 h. The membrane was incubated with primary
antibodies against acetylated histone H4 lysine H4K5 (cat. no.
ab114146; 1:1,000), acetylated H4K8 (cat. no. ab45166; 1:5,000) and
H4 (cat. no. ab109463; 1:1,000; all Abcam) overnight at 4˚C.
Following primary antibody incubation, the membrane was incubated
with horseradish peroxidase-conjugated secondary antibodies (cat.
no. SA00001-2; 1:2,000; ProteinTech Group, Inc.). Blots were
visualized using an enhanced chemiluminescence (ECL) kit (cat. no.
WP20005; Thermo Fisher Scientific, Inc.) and an ECL system (GE
Healthcare). Protein expression was quantified using Quantity One
software (version 2.4; Bio-Rad Laboratories, Inc.). Each experiment
was performed in triplicate and H4 was used as the loading
control.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 6.0; GraphPad Software, Inc.). Data are
expressed as the mean ± standard deviation. Statistical differences
between groups were analyzed using the one-way analysis of variance
followed by the Tukey's post hoc test or student's t test. The
basic information of the patients presented in Table I was analyzed using the
χ2 and Student-Newman-Keuls tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Basic information of investigated
subjects
A total of 180 puerperant were recruited and divided
into three groups: FTL, TPROM and PPROM. Each group consisted on 60
patients. The mean age of the patients in the FTL, TPROM and PPROM
groups was 27.5±3.67, 27.79±3.44 and 28.26±5.13 years,
respectively. No difference in age distribution among the three
groups was observed (P>0.05). There were no significant
differences in one-child ratios (the number of babies born in the
current study as the first baby of the mother over the number of
all parturients included in the present study) x100%, and
eosinophil and glucose levels among the three groups (P>0.05;
Table I). However, significant
differences in the body mass index, gestational age, time of
membrane rupture to delivery, incidence of antepartum hemorrhage
and serum albumin levels were observed between PPROM and FTL or
between PPROM and TPROM (P<0.05; Table I). Moreover, neutrophil and albumin
levels significantly affected premature rupture of membranes in the
TPROM and PPROM groups, compared with the FTL group using SNK test
(P<0.05). These results indicated that obesity, inflammation and
infection may result in premature rupture of membranes.
Expression of MMPs, H4K5ac and H4K8ac
in fetal membrane tissues
The current study investigated the associations
between MMPs, H4K5ac and H4K8ac, and PROM. Compared with the FTL
group, MMP9 concentration was significantly decreased in the TPROM
group (P<0.05; Fig. 1A), and
MMP2 and MMP9 concentrations significantly increased in the PPROM
group (P<0.01; Fig. 1A). While
the concentration of HDAC2 and HDAC6 appeared to be decreased in
the PPROM group, no significant differences among the three groups
were observed (P>0.05; Fig. 1B).
HDAC1 concentration was decreased in the PPROM and TPROM groups
compared with the FTL group, although only significantly so in the
PPROM group (P<0.05; Fig. 1B).
As presented in Fig. 1C, the levels
of H4K5 and H4K8 acetylation in the PPROM and TPROM groups were
significantly increased compared with the FTL group (all P<0.01
except H4K8 in TPROM, P<0.05). The levels of H4K5ac and H4K8ac
were significantly increased in the PPROM group compared with the
TPROM group (P<0.05; Fig.
1C).
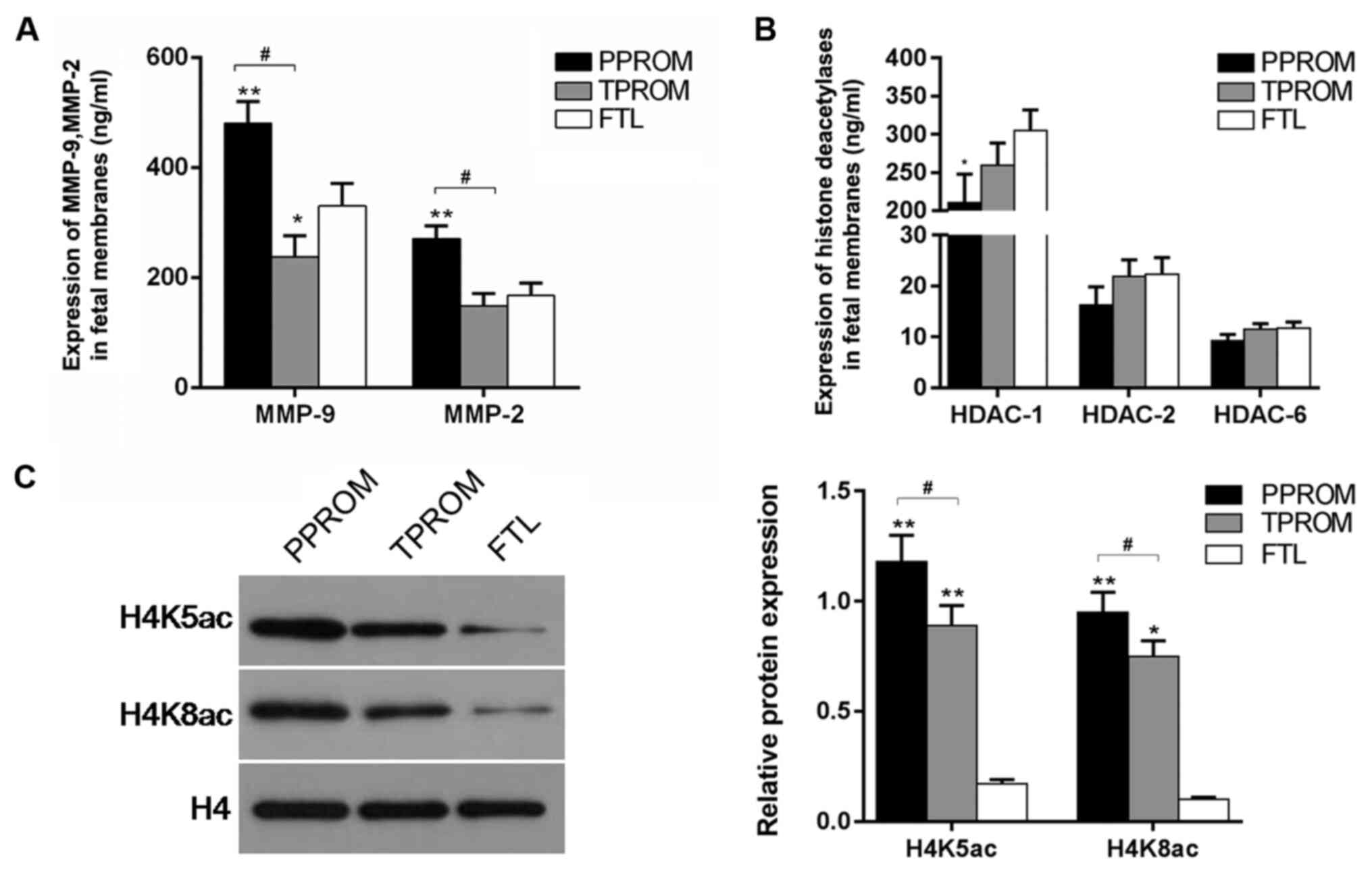 | Figure 1Expression levels of MMPs and histone
acetylation-associated factors (H4K5ac and H4K8ac) in fetal
membrane tissues. Fetal membranes were divided into three groups
(FTL, TPROM and PPROM), and levels of (A) MMP2 and MMP9, and (B)
HDAC1, HDAC2 and HDAC6 were detected using ELISA kits. (C) Western
blotting was performed to assess the protein levels of H4K5ac and
H4K8ac in FTL, TPROM and PPROM groups. H4 served as an internal
control. Data are expressed as mean ± standard deviation from three
independent experiments. *P<0.05 and
**P<0.01 vs. the FTL group. #P<0.05 vs.
the TPROM group. MMPs, matrix metalloproteinases; H4K, histone H4
lysine; FTL, full-term labor; PROM, premature rupture of membranes;
TPROM, term-PROM; PPROM, pre-term-PROM; HDAC, histone deacetylase;
ac, acetylated. |
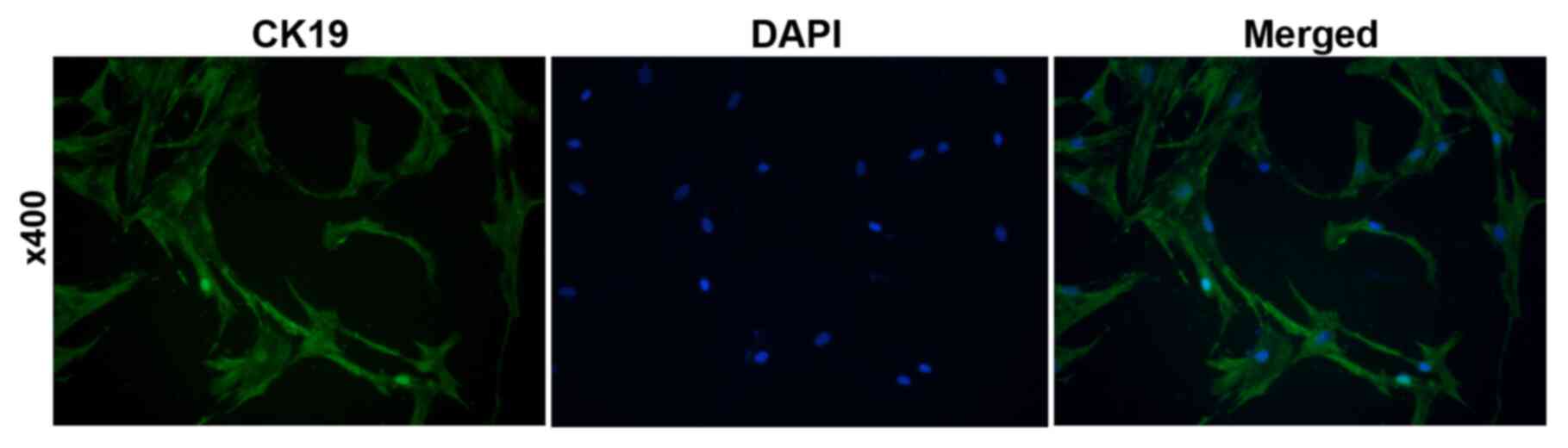
Verification of HAEpi cells
To verify HAEpi cells, the epithelial cell marker
CK19 was detected by immunofluorescence. As presented in Fig. 2, 98% of the cells were CK-19
positive, indicating that the cells were human amniotic epithelial
cells.
Effects of droxinostat on cell
viability and the levels of MMPs, H4K5ac and H4K8ac in HAEpi
cells
The effect of increasing concentrations of
droxinostat on the viability of HAEpi cells was investigated
following 24, 48 and 72 h of incubation. The results revealed that
as the droxinostat concentration increased the cell viability
decreased (Fig. 3A). Droxinostat
treated at 5 µM for 48 h was selected for subsequent
experimentation as this concentration and incubation time
significantly reduced the viability of HAEpi cells (P<0.05).
Western blotting revealed that 5 µM droxinostat significantly
enhanced the expression of H4K5ac and H4K8ac in HAEpi cells
compared with the control (P<0.01; Fig. 3B). Compared with the control, 5 µM
droxinostat significantly decreased the levels of MMP2 and MMP9
(P<0.01; Fig. 3C). Droxinostat
did not significantly affect the levels of HDAC1 and HDAC2 compared
with the control (P>0.05; Fig.
3D). However, 5 µM droxinostat significantly decreased the
level of HDAC6 compared with the control (P<0.01; Fig. 3D).
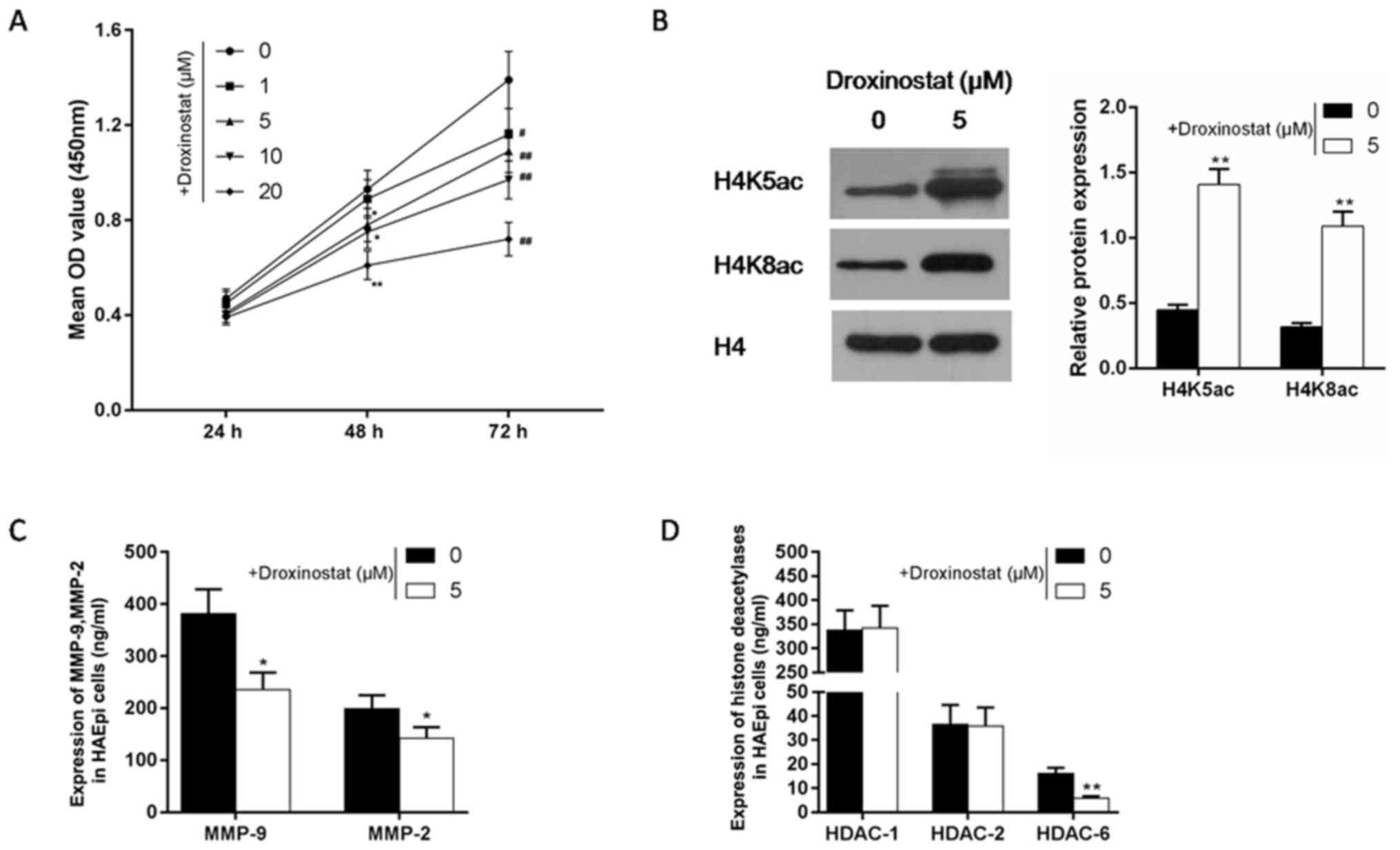 | Figure 3Effects of droxinostat on cell
viability and levels of MMPs and histone acetylation related
factors (H4K5ac and H4K8ac) in HAEpi cells. (A) A Cell Counting
Kit-8 assay was performed to detect the effect of different
concentrations (1, 5, 10 and 20 µM) of droxinostat on HAEpi cell
viability following 24, 48 and 72 h. *P<0.05 and
**P<0.01 vs. the control group at 48 h;
#P<0.05 and ##P<0.01 vs. the control
group at 72 h. (B) The effect of droxinostat on the levels of
H4K5ac and H4K8ac in HAEpi cells was investigated using western
blotting. H4 served as an internal control. The effects of
droxinostat on (C) MMP2 and MMP9, and (D) HDAC1, HDAC2 and HDAC6
were detected by ELISA kits. Data are expressed as the mean ±
standard deviation from three independent experiments
*P<0.05 and **P<0.01 vs. the control
group. MMPs, matrix metalloproteinases; H4K, histone H4 lysine;
HAEpi, human amniotic epithelial; HDAC, histone deacetylase; ac,
acetylated. |
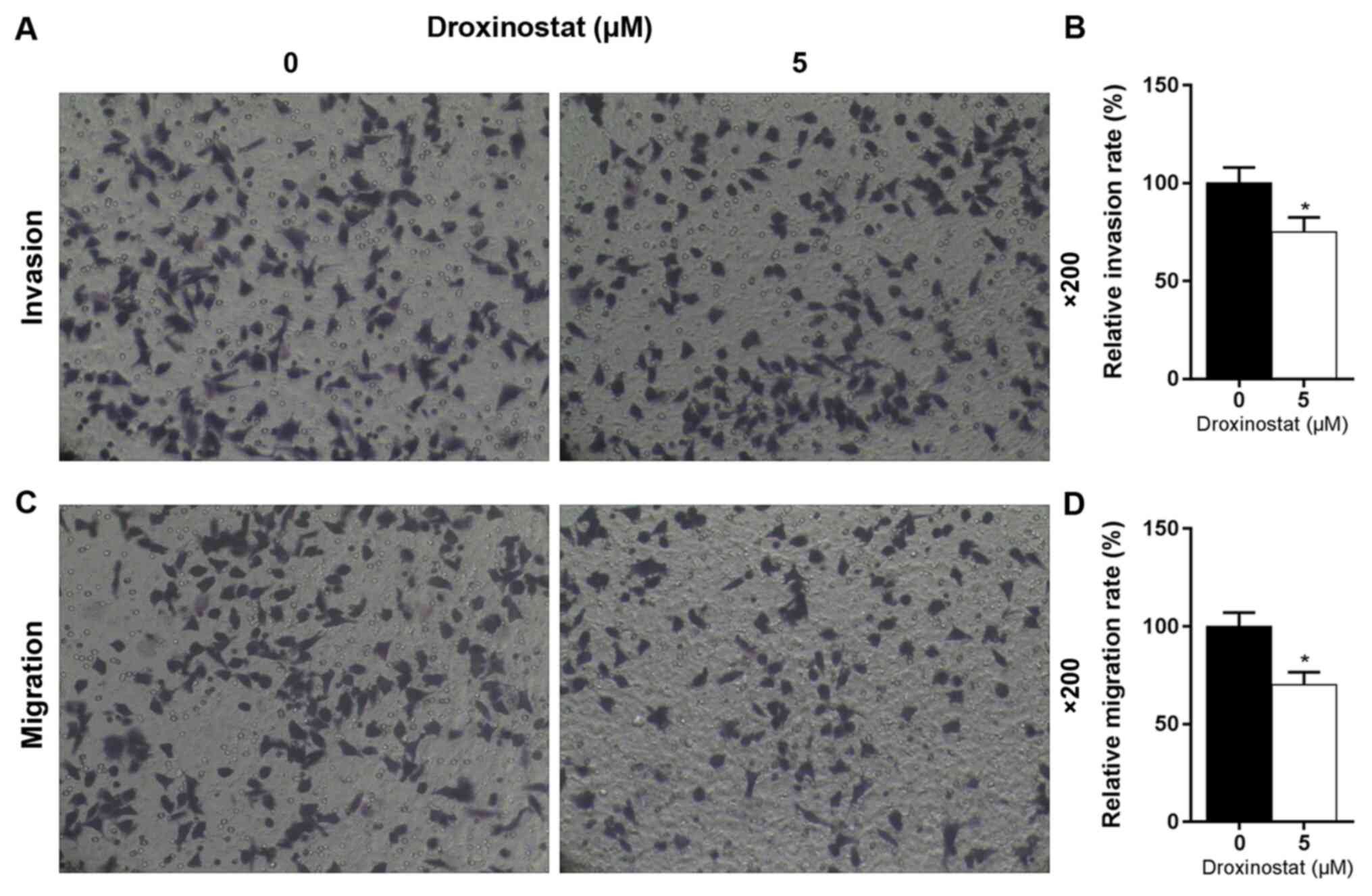
Effects of droxinostat on HAEpi cell
invasion and migration
The effect of droxinostat on HAEpi cell invasion and
migration was investigated using transwell assays. The results
revealed that droxinostat significantly inhibited HAEpi cell
invasion and migration compared with the control (P<0.05;
Fig. 4).
Effects of chidamide on cell viability
and the levels of MMPs, H4K5ac and H4K8ac in HAEpi cells
The effects of chidamide on cell viability, MMP
concentrations, and protein expression levels of H4K5ac and H4K8ac
in HAEpi cells were investigated in the current study. Increasing
concentrations (0.1, 0.2, 0.5 and 1 µM) of chidamide were used to
detect cell viability following 24, 48 and 72 h. The results
revealed that chidamide decreased HAEpi cell viability in a manner
similar to droxinostat (Fig. 5A). A
concentration of 0.5 µM chidamide and incubated of 48 h was
selected as this concentration/temperature significantly reduced
HAEpi cell viability (P<0.05). As presented in Fig. 5B, chidamide significantly increased
the levels of H4K5ac and H4K8ac compared with the control
(P<0.01). The concentrations of MMP2 and MMP9 were significantly
decreased in 0.5 µM chidamide-treated cells compared with controls
(P<0.01; Fig. 5C). Compared with
droxinostat, chidamide had different effects on the levels of
HDAC1, HDAC2 and HDAC6 in HAEpi cells. The levels of HDAC1 and
HDAC2 were significantly decreased following exposure to 0.5 µM
chidamide compared with control (P<0.01; Fig. 5D). Chidamide did not significantly
affect the level of HDAC6 compared with controls.
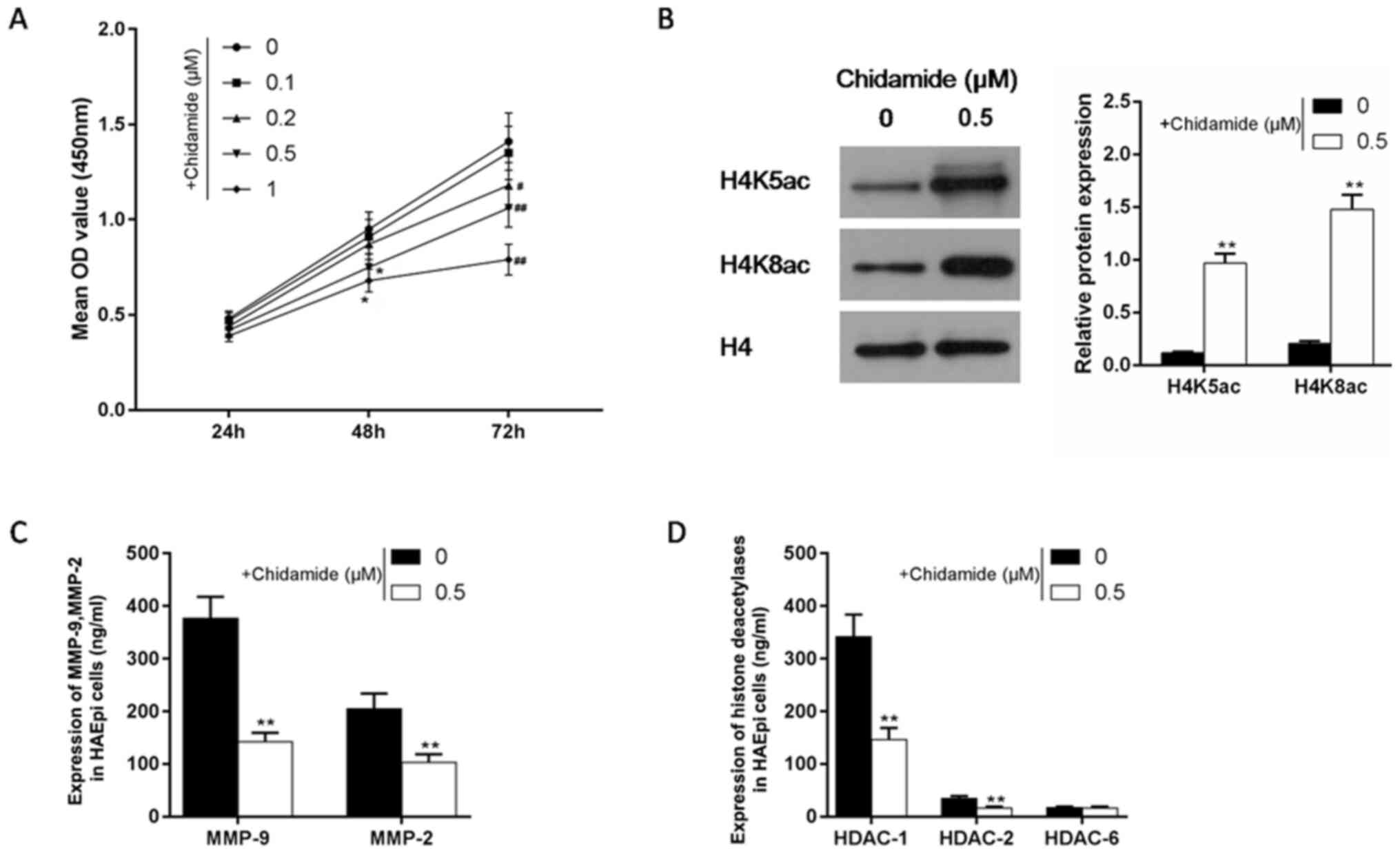 | Figure 5Effects of chidamide on cell
viability and levels of MMPs and histone acetylation related
factors (H4K5ac and H4K8ac) in HAEpi cells. (A) A Cell Counting
Kit-8 assay was performed to detect the effect of different
concentrations (0.1, 0.2, 0.5 and 1 µM) of chidamide on HAEpi cell
viability following 24, 48 and 72 h. *P<0.05 vs. the
control group at 48 h; #P<0.05 and
##P<0.01 vs. the control group at 72 h. (B) The
effect of chidamide on the levels of H4K5ac and H4K8ac in HAEpi
cells was investigated using western blotting. H4 served as an
internal control. The effects of chidamide on (C) MMP2 and MMP9,
and (D) HDAC1, HDAC2 and HDAC6 were detected by ELISA kits. Data
are expressed as mean ± standard deviation from three independent
experiments. *P<0.05 vs. the control group. MMPs,
matrix metalloproteinases; H4K, histone H4 lysine; HAEpi, human
amniotic epithelial; HDAC, histone deacetylase; ac, acetylated. |
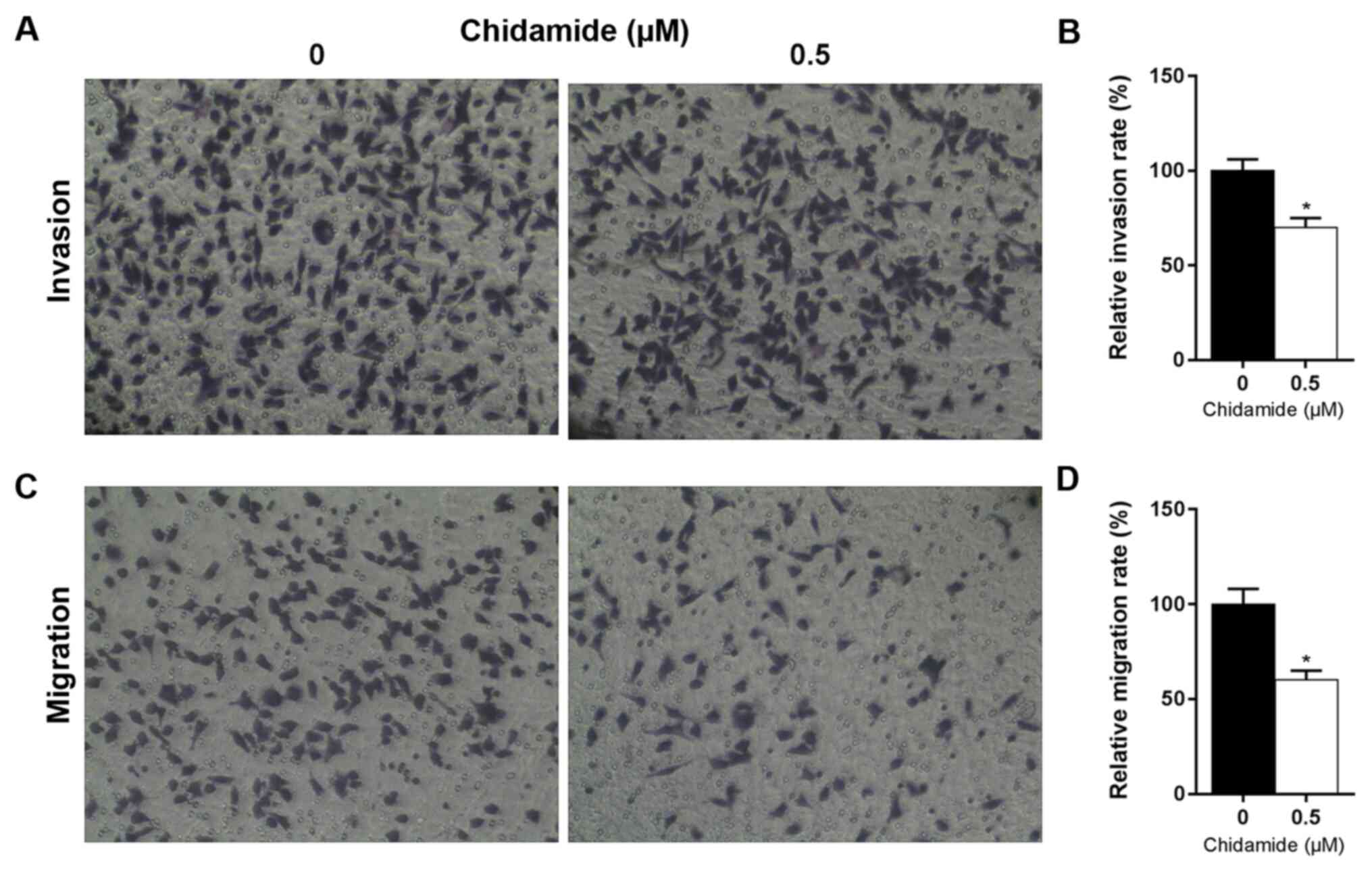
Effects of chidamide on HAEpi cell
invasion and migration
Similar to the results obtained for 5 µM
droxinostat, 0.5 µM chidamide significantly inhibited HAEpi cell
invasion and migration compared with the control (P<0.05;
Fig. 6).
Discussion
Premature rupture of fetal membranes may result in
various adverse effects in the puerperant and fetus, including
cerebral palsy, intellectual handicap and chronic lung disease
(33). An increased understanding
of the mechanisms underlying PROM and the development of novel
interventions may prolong the gestational age of the parturient and
improve the survival rate of newborns. Amnion and chorion exhibit
collagen-dissolving reconstitution processes, which allow the
membranes to overcome the effect of uterine growth (34). This process is closely associated
with the activation of MMPs (34),
which degrade the majority of the ECM components consisting of
collagen, proteoglycan and glycoprotein (16). Therefore, MMPs may serve important
roles in PROM (35).
The present study investigated 180 puerperant with
FTL, TPROM or PPROM. The concentrations of MMP2 and MMP9 in fetal
membranes were significantly different in PPROM and TPROM compared
with FTL. Specifically, MMP concentrations were increased and
decreased in the PPROM and TPROM groups, respectively, compared
with the FTL group. Previous studies reported an increased
expression of MMPs in PPROM (36-38).
Certain inflammatory factors (including interleukin-1b and tumor
necrosis factor-α) promote chorioamnionitis, which enhances the
secretion of MMPs, degrades ECM components of the fetal membrane,
decidua and cervix, and induces PROM and premature delivery
(36-40).
In the current study, lower concentrations of MMP2 and MMP9 in the
TPROM groups suggested that other MMPs, for example MMP7, may serve
a leading role in TPROM and that low levels of MMP2 are expressed
at term (41,42).
The present study demonstrated that while HDAC1
expression was decreased in the PPROM and TPROM groups compared
with the FTL group, the expression of acetylated histones
increased. Histone modification serves an important role in the
development of diseases such as malignant neoplasms, nephropathy,
respiratory diseases, blood system diseases and reproductive
diseases (21,22,43).
Additionally, in chronic obstructive pulmonary disease, high
histone H4 acetylation may cause excessive release of inflammatory
factors, inducing an inflammatory reaction and accelerating the
progression of the disease (44). A
total of 18 mammalian HDACs have been identified and may be divided
into four categories as follows: Class I (HDAC1-3 and HDAC8), class
II (IIa, HDAC4, HDAC5, HDAC7 and HDAC9; IIb, HDAC6 and HDAC10),
class III (sirtuin 1-7) and class IV (HDAC11) (45,46).
HDAC1 is expressed in human endometrium and is closely associated
with embryonic development (47).
The present study investigated the effects of
histone acetylation on regulating the expression levels of MMPs in
PROM by exposing HAEpi cells to histone deacetylase inhibitors
in vitro. The results revealed that different concentrations
of the histone deacetylase inhibitors droxinostat and chidamide
significantly inhibited cell viability compared with controls.
Droxinostat and chidamide are widely used antitumor agents
(48-51)
and reduced HAEpi cell viability, invasion and migration in the
current study, potentially by decreasing cell growth and increasing
apoptosis (52,53). Furthermore, the present study
revealed that droxinostat and chidamide significantly promoted the
levels of H4K5 and H4K8 acetylation, and inhibited the secretion of
MMP2 and MMP9, similar to what was observed in the TPROM group.
Moreover, 5 µM droxinostat significantly decreased the expression
level of HDAC6, and 0.5 µM chidamide significantly decreased the
expression levels of HDAC1 and HDAC2 in HAEpi cells. The
aforementioned results were in accordance with previous studies,
which suggested that droxinostat selectively decreased HDAC3, HDAC6
and HDAC8 expression levels and promoted histone acetylation, while
chidamide selectively decreased the expression levels of HDAC1,
HDAC2, HDAC3 and HDAC10 (54,55).
Therefore, future studies investigating different histone
deacetylase inhibitors are required to demonstrate that histone
deacetylase inhibitors decrease the expression of HDACs, and induce
acetylation of H4K5 and H4K8. In the current study, exposure to
droxinostat and chidamide increased histone H4 hyperacetylation in
HAEpi cells, and an accompanying decrease in cell viability, and
cell invasion and migration was observed. Furthermore, droxinostat
and chidamide decreased the expression levels of MMP2 and MMP9,
suggesting that histone H4 hyperacetylation may suppress MMP2 and
MMP9 in a manner similar to that observed in TPROM.
The current study had a number of limitations.
Western blotting was not performed to detect MMP and HDAC proteins.
Additionally, in vitro experiments were performed using
HAEpi and not PROM cells. Further investigations to validate the
results obtained in the current study are required.
In conclusion, the present study demonstrated that
high expression levels of MMP2 and MMP9 were observed in
PPROM. Additionally, in vitro experiments suggested
that the effect of histone H4 hyperacetylation on inhibiting MMP2
and MMP9 activities in HAEpi cell was similar to that observed in
TPROM. The results obtained in the current study may guide clinical
treatment for PROM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS, FF and WH conceived and designed the study. LY,
LZ and JY acquired the data and performed the data analysis and
interpretation. ZS drafted the article and critically revised it
for important intellectual content. All authors gave final approval
for the manuscript to be published.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the Maternal and Child Health Hospital of Tangshan
(Tangshan, China) and written informed consent was obtained from
each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eleje GU, Adinma JI, Ghasi S, Ikechebelu
JI, Igwegbe AO, Okonkwo JE, Okafor CI, Ezeama CO, Ezebialu IU and
Ogbuagu CN: Antibiotic susceptibility pattern of genital tract
bacteria in pregnant women with preterm premature rupture of
membranes in a resource-limited setting. Int J Gynaecol Obstet.
127:10–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mercer BM: Preterm premature rupture of
the membranes. Obstet Gynecol. 101:178–193. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Raines DA, Wagner A and Salinas A:
Intraamniotic infection and the term neonate. Neonatal Netw.
36:385–387. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu T, Shi J, Bao S, Qu Y and Mu DZ: Effect
of premature rupture of membranes on maternal infections and
outcome of preterm infants. Zhongguo Dang Dai Er Ke Za Zhi.
19:861–865. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
5
|
Patriota AF, Guerra GV, de Melo BC, Santos
AC, Torres Junior AC and Souza AS: Amniotic fluid volume and
maternal outcomes in women with preterm premature rupture of
membranes. Rev Bras Ginecol Obstet. 36:146–151. 2014.PubMed/NCBI View Article : Google Scholar : (In Portuguese).
|
|
6
|
Romero R, Espinoza J, Goncalves LF, Gomez
R, Medina L, Silva M, Chaiworapongsa T, Yoon BH, Ghezzi F, Lee W,
et al: Fetal cardiac dysfunction in preterm premature rupture of
membranes. J Matern Fetal Neonatal Med. 16:146–157. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Thomas W and Speer CP: Chorioamnionitis:
Important risk factor or innocent bystander for neonatal outcome?
Neonatology. 99:177–187. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Biggio JR Jr, Ramsey PS, Cliver SP, Lyon
MD, Goldenberg RL and Wenstrom KD: Midtrimester amniotic fluid
matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile
are a marker for subsequent preterm premature rupture of membranes.
Am J Obstet Gynecol. 192:109–113. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shaarawy M and El-Minawi AM: Prolactin and
calcitropic hormones in preterm premature rupture of membranes. Int
J Gynaecol Obstet. 84:200–207. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Osaikhuwuomwan JA, Okpere EE, Okonkwo CA,
Ande AB and Idogun ES: Plasma vitamin C levels and risk of preterm
prelabour rupture of membranes. Arch Gynecol Obstet. 284:593–597.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bou-Resli MN, Al-Zaid NS and Ibrahim ME:
Full-term and prematurely ruptured fetal membranes. An
ultrastructural study. Cell Tissue Res. 220:263–278.
1981.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Niknejad H, Yazdanpanah G and Kakavand M:
Extract of fetal membrane would inhibit thrombosis and hemolysis.
Med Hypotheses. 85:197–202. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mehats C, Schmitz T, Marcellin L and
Breuiller-Fouche M: Biochemistry of fetal membranes rupture.
Gynecol Obstet Fertil. 39:365–369. 2011.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
14
|
Rodriguez Faba O, Palou-Redorta J,
Fernandez-Gomez JM, Algaba F, Eiró N, Villavicencio H and Vizoso
FJ: Matrix metalloproteinases and bladder cancer: What is new? ISRN
Urol. 2012(581539)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Choo QY, Ho PC, Tanaka Y and Lin HS:
Histone deacetylase inhibitors MS-275 and SAHA induced growth
arrest and suppressed lipopolysaccharide-stimulated NF-kappaB p65
nuclear accumulation in human rheumatoid arthritis synovial
fibroblastic E11 cells. Rheumatology (Oxford). 49:1447–1460.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bajracharya D, Shrestha B, Kamath A, Menon
A and Radhakrishnan R: Immunohistochemical correlation of matrix
metalloproteinase-2 and tissue inhibitors of metalloproteinase-2 in
tobacco associated epithelial dysplasia. Dis Markers.
2014(197813)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fortunato SJ, Menon R and Lombardi SJ:
MMP/TIMP imbalance in amniotic fluid during PROM: An indirect
support for endogenous pathway to membrane rupture. J Perinat Med.
27:362–368. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fortunato SJ, Menon R, Ahmed NU, Bourgeois
M and Dildy GA: Amniotic fluid concentrations of collagenase-1 and
collagenase-3 are increased in polyhydramnios. J Perinat Med.
32:122–125. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Park CW, Yoon BH, Park JS and Jun JK: A
fetal and an intra-amniotic inflammatory response is more severe in
preterm labor than in preterm PROM in the context of funisitis:
Unexpected observation in human gestations. PLoS One.
8(e62521)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dubicke A, Akerud A, Sennstrom M, Hamad
RR, Bystrom B, Malmstrom A and Ekman-Ordeberg G: Different
secretion patterns of matrix metalloproteinases and IL-8 and effect
of corticotropin-releasing hormone in preterm and term cervical
fibroblasts. Mol Hum Reprod. 14:641–647. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sundar IK, Yao H and Rahman I: Oxidative
stress and chromatin remodeling in chronic obstructive pulmonary
disease and smoking-related diseases. Antioxid Redox Signal.
18:1956–1971. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Legube G and Trouche D: Regulating histone
acetyltransferases and deacetylases. EMBO Rep. 4:944–947.
2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiong C, Guan Y, Zhou X, Liu L, Zhuang MA,
Zhang W, Zhang Y, Masucci MV, Bayliss G, Zhao TC and Zhuang S:
Selective inhibition of class IIa histone deacetylases alleviates
renal fibrosis. FASEB J. 33:8249–8262. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vincent ZL, Mitchell MD and Ponnampalam
AP: Regulation of TIMP-1 in human placenta and fetal membranes by
lipopolysaccharide and demethylating agent 5-aza-2'-deoxycytidine.
Reprod Biol Endocrinol. 13(136)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vadillo-Ortega F, Gonzalez-Avila G, Furth
EE, Lei H, Muschel RJ, Stetler-Stevenson WG and Strauss JF III:
92-kd type IV collagenase (matrix metalloproteinase-9) activity in
human amniochorion increases with labor. Am J Pathol. 146:148–156.
1995.PubMed/NCBI
|
|
26
|
Vadillo-Ortega F, Hernandez A,
Gonzalez-Avila G, Bermejo L, Iwata K and Strauss JF III: Increased
matrix metalloproteinase activity and reduced tissue inhibitor of
metalloproteinases-1 levels in amniotic fluids from pregnancies
complicated by premature rupture of membranes. Am J Obstet Gynecol.
174:1371–1376. 1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Poljak M, Lim R, Barker G and Lappas M:
Class I to III histone deacetylases differentially regulate
inflammation-induced matrix metalloproteinase 9 expression in
primary amnion cells. Reprod Sci. 21:804–813. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Practice bulletins No. 139. Premature
rupture of membranes. Obstet Gynecol. 122:918–930. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hua Y, Ding S, Zhang W, Zhou Q, Ye W, Chen
M and Zhu X: Expression of AQP3 protein in hAECs is regulated by
Camp-PKA-CREB signalling pathway. Front Biosci (Landmark Ed).
20:1047–1055. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Miki T, Lehmann T, Cai H, Stolz DB and
Strom SC: Stem cell characteristics of amniotic epithelial cells.
Stem Cells. 23:1549–1559. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhu P, Liu Z, Zhou J and Chen Y: Tanshinol
inhibits the growth, migration and invasion of hepatocellular
carcinoma cells via regulating the PI3K-AKT signaling pathway. Onco
Targets Ther. 12:87–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Witkin SS, Nasioudis D, Leizer J, Minis E,
Boester A and Forney LJ: Epigenetics and the vaginal microbiome:
Influence of the microbiota on the histone deacetylase level in
vaginal epithelial cells from pregnant women. Minerva Ginecol.
71:171–175. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saigal S and Doyle LW: An overview of
mortality and sequelae of preterm birth from infancy to adulthood.
Lancet. 371:261–269. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Polimeni M and Prato M: Host matrix
metalloproteinases in cerebral malaria: New kids on the block
against blood-brain barrier integrity? Fluids Barriers CNS.
11(1)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Strauss JF III: Extracellular matrix
dynamics and fetal membrane rupture. Reprod Sci. 20:140–153.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Romero R, Chaiworapongsa T, Espinoza J,
Gomez R, Yoon BH, Edwin S, Mazor M, Maymon E and Berry S: Fetal
plasma MMP-9 concentrations are elevated in preterm premature
rupture of the membranes. Am J Obstet Gynecol. 187:1125–1130.
2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yan H, Zhu L, Zhang Z, Li H, Li P, Wang Y
and Leng M: HMGB1-RAGE signaling pathway in pPROM. Taiwan J Obstet
Gynecol. 57:211–216. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mano Y, Shibata K, Sumigama S, Hayakawa H,
Ino K, Yamamoto E, Kajiyama H, Nawa A and Kikkawa F: Tocilizumab
inhibits interleukin-6-mediated matrix metalloproteinase-2 and -9
secretions from human amnion cells in preterm premature rupture of
membranes. Gynecol Obstet Invest. 68:145–153. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Oner C, Schatz F, Kizilay G, Murk W,
Buchwalder LF, Kayisli UA, Arici A and Lockwood CJ:
Progestin-inflammatory cytokine interactions affect matrix
metalloproteinase-1 and -3 expression in term decidual cells:
Implications for treatment of chorioamnionitis-induced preterm
delivery. J Clin Endocrinol Metab. 93:252–259. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kumar D, Schatz F, Moore RM, Mercer BM,
Rangaswamy N, Mansour JM, Lockwood CJ and Moore JJ: The effects of
thrombin and cytokines upon the biomechanics and remodeling of
isolated amnion membrane, in vitro. Placenta. 32:206–213.
2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kwon JS, Kim YS, Cho AS, Cho HH, Kim JS,
Hong MH, Jeong HY, Kang WS, Hwang KK, Bae JW, et al: Regulation of
MMP/TIMP by HUVEC transplantation attenuates ventricular remodeling
in response to myocardial infarction. Life Sci. 101:15–26.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Nishihara S, Someya A, Yonemoto H, Ota A,
Itoh S, Nagaoka I and Takeda S: Evaluation of the expression and
enzyme activity of matrix metalloproteinase-7 in fetal membranes
during premature rupture of membranes at term in humans. Reprod
Sci. 15:156–165. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Franz MB, Poterauer M, Elhenicky M, Stary
S, Birner P, Vinatzer U, Husslein P, Streubel B and Husslein H:
Global and single gene DNA methylation in umbilical cord blood
cells after elective caesarean: A pilot study. Eur J Obstet Gynecol
Reprod Biol. 179:121–124. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang F, Zhang N, Li B, Liu L, Ding L, Wang
Y, Zhu Y, Mo X and Cao Q: Heparin defends against the toxicity of
circulating histones in sepsis. Front Biosci (Landmark Ed).
20:1259–1270. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
45
|
de Ruijter AJ, van Gennip AH, Caron HN,
Kemp S and van Kuilenburg AB: Histone deacetylases (HDACs):
Characterization of the classical HDAC family. Biochem J.
370:737–749. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gregoretti IV, Lee YM and Goodson HV:
Molecular evolution of the histone deacetylase family: Functional
implications of phylogenetic analysis. J Mol Biol. 338:17–31.
2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Krusche CA, Vloet AJ, Classen-Linke I, von
Rango U, Beier HM and Alfer J: Class I histone deacetylase
expression in the human cyclic endometrium and endometrial
adenocarcinomas. Hum Reprod. 22:2956–2966. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huang Y, Yang W, Zeng H, Hu C, Zhang Y,
Ding N, Fan G, Shao L and Kuang B: Droxinostat sensitizes human
colon cancer cells to apoptotic cell death via induction of
oxidative stress. Cell Mol Biol Lett. 23(34)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu J, Li G, Wang X, Wang L, Zhao R, Wang
J, Kong Y, Ding J, Li J and Zhang L: Droxinostat, a histone
deacetylase inhibitor, induces apoptosis in hepatocellular
carcinoma cell lines via activation of the mitochondrial pathway
and downregulation of FLIP. Transl Oncol. 9:70–78. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Xu Y, Zhang P and Liu Y: Chidamide
tablets: HDAC inhibition to treat lymphoma. Drugs Today (Barc ).
53:167–176. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gao S, Li X, Zang J, Xu W and Zhang Y:
Preclinical and clinical studies of chidamide (CS055/HBI-8000), an
orally available subtype-selective HDAC inhibitor for cancer
therapy. Anticancer Agents Med Chem. 17:802–812. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mann BS, Johnson JR, Cohen MH, Justice R
and Pazdur R: FDA approval summary: Vorinostat for treatment of
advanced primary cutaneous T-cell lymphoma. Oncologist.
12:1247–1252. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Coiffier B, Pro B, Prince HM, Foss F,
Sokol L, Greenwood M, Caballero D, Borchmann P, Morschhauser F,
Wilhelm M, et al: Results from a pivotal, open-label, phase II
study of romidepsin in relapsed or refractory peripheral T-cell
lymphoma after prior systemic therapy. J Clin Oncol. 30:631–636.
2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wood TE, Dalili S, Simpson CD, Sukhai MA,
Hurren R, Anyiwe K, Mao X, Suarez Saiz F, Gronda M, Eberhard Y, et
al: Selective inhibition of histone deacetylases sensitizes
malignant cells to death receptor ligands. Mol Cancer Ther.
9:246–256. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lu X, Ning Z, Li Z, Cao H and Wang X:
Development of chidamide for peripheral T-cell lymphoma, the first
orphan drug approved in China. Intractable Rare Dis Res. 5:185–191.
2016.PubMed/NCBI View Article : Google Scholar
|