1. Introduction
Gadolinium (Gd) has the atomic number 64. It is a
heavy metal and belongs to the family of lanthanides. The oxidation
state of gadolinium that is met often is +3. Gadolinium has an
ionic radius of 0.99 Å and is almost identical to the one of
Ca2+ (1).
Gd3+ and Ca2+ can become toxic to biological
systems if it compete. When administered to humans in chelated
forms the presence of free gadolinium is avoided, thereby reducing
its toxicity (2,3). Gd is capable of inducing a strong
magnetic field that influences the degree of relaxivity of the
protons of water molecules, resulting in a signal increase in MRI
(4-6).
2. Development of gadolinium complexes
Runge first introduced the term of gadolinium-based
contrast agent (GBCA) in 1982 at the Radiologic Society of North
America meeting in Chicago (7,8). GBCAs
were thereafter produced commercially because this increases
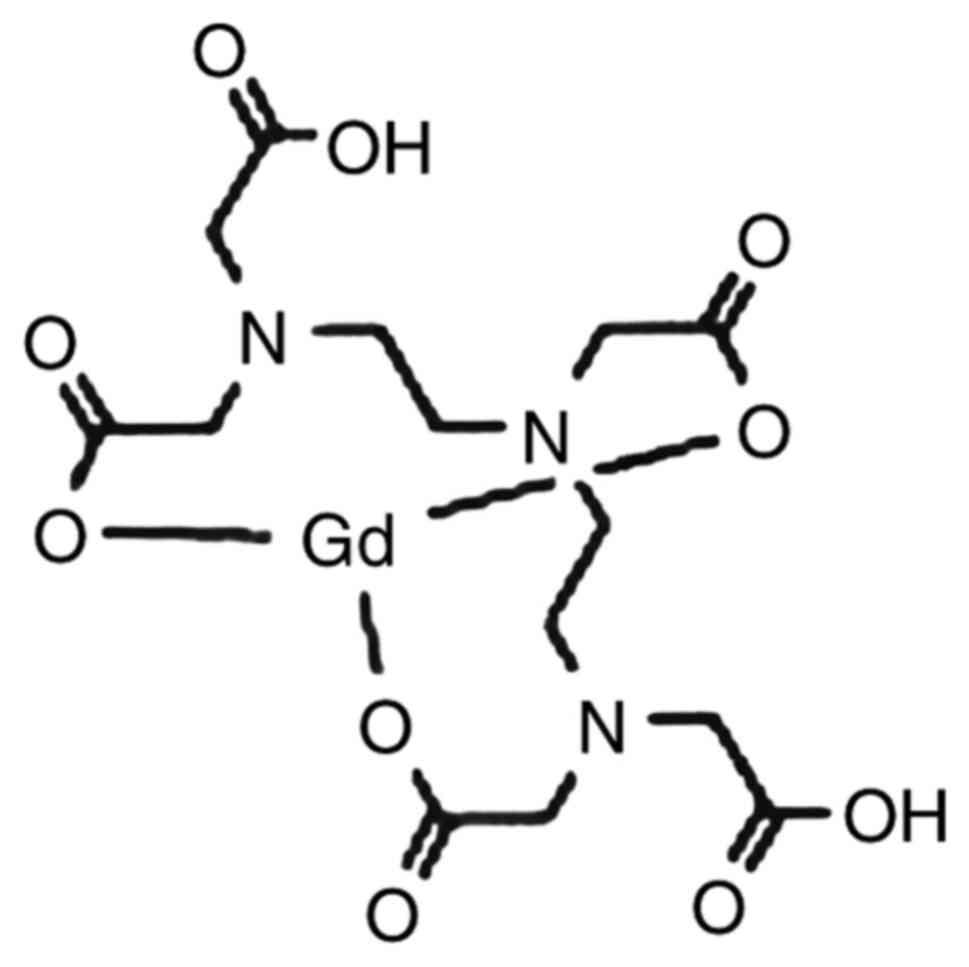
detection of the lesion. Fig. 1
shows the combined form of Gd-DTPA-Magnevist (also known as
gadopentetate dimeglumine). In 1988, this complex was first
authorized for use. Subsequently, gadolinium was used in the
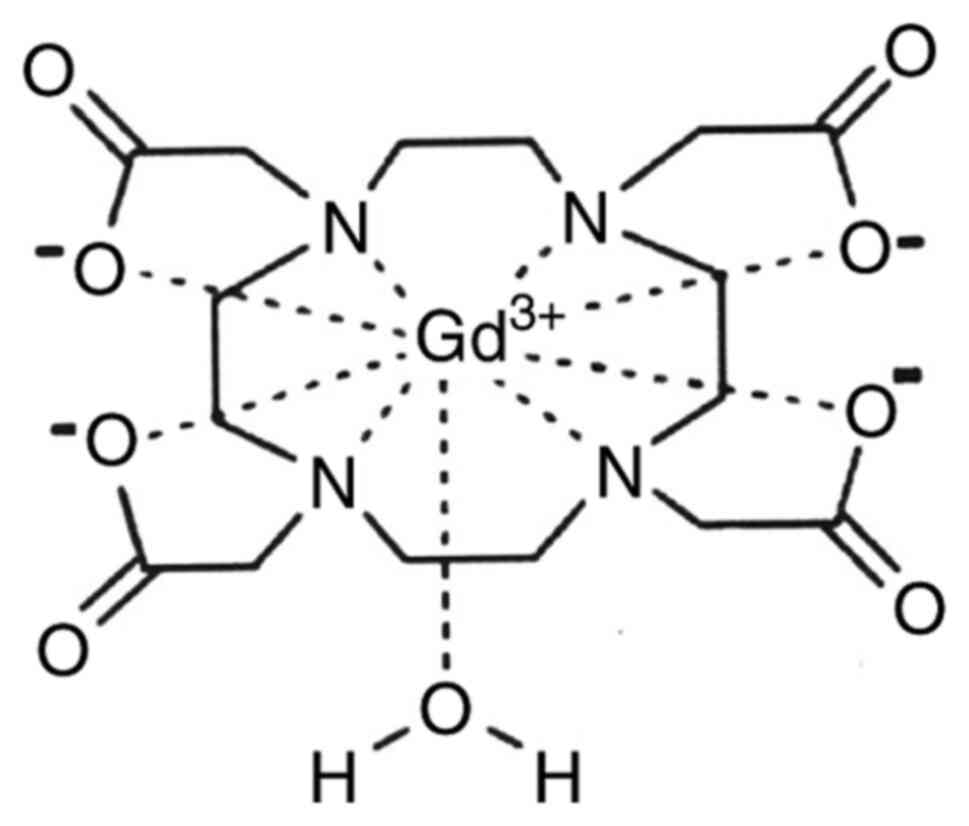
following complexes: Gd-DOTA-Dotarem (also known as gadoterate
meglumine) (Fig. 2),
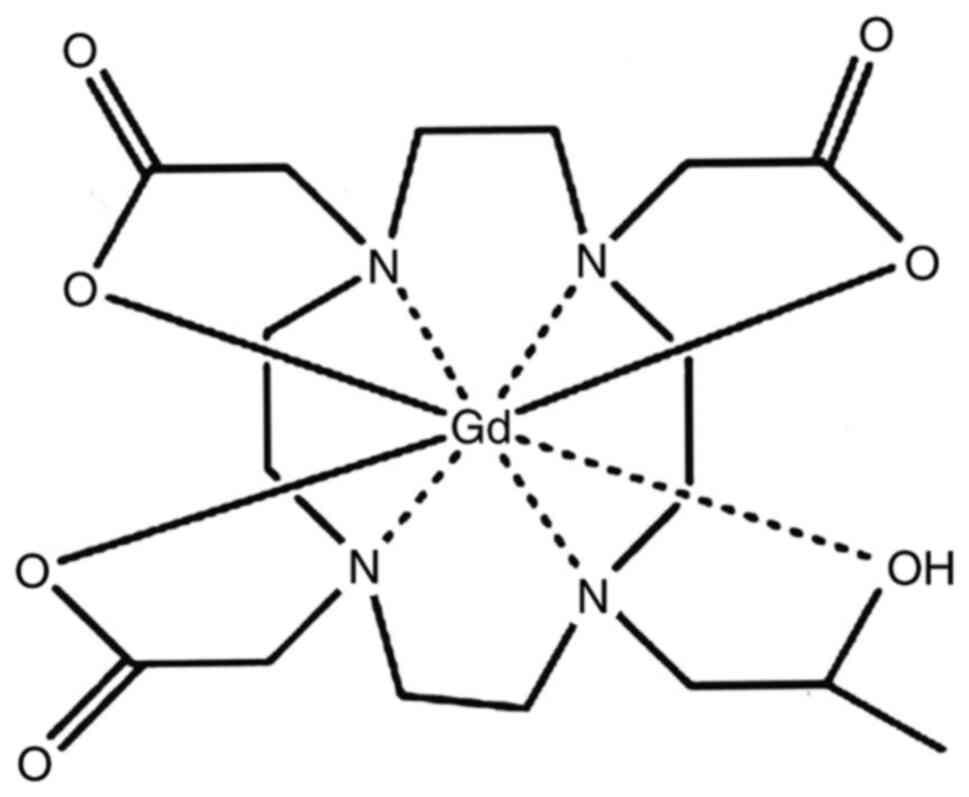
Gd-HP-DO3A-ProHance (gadoteridol) (Fig.
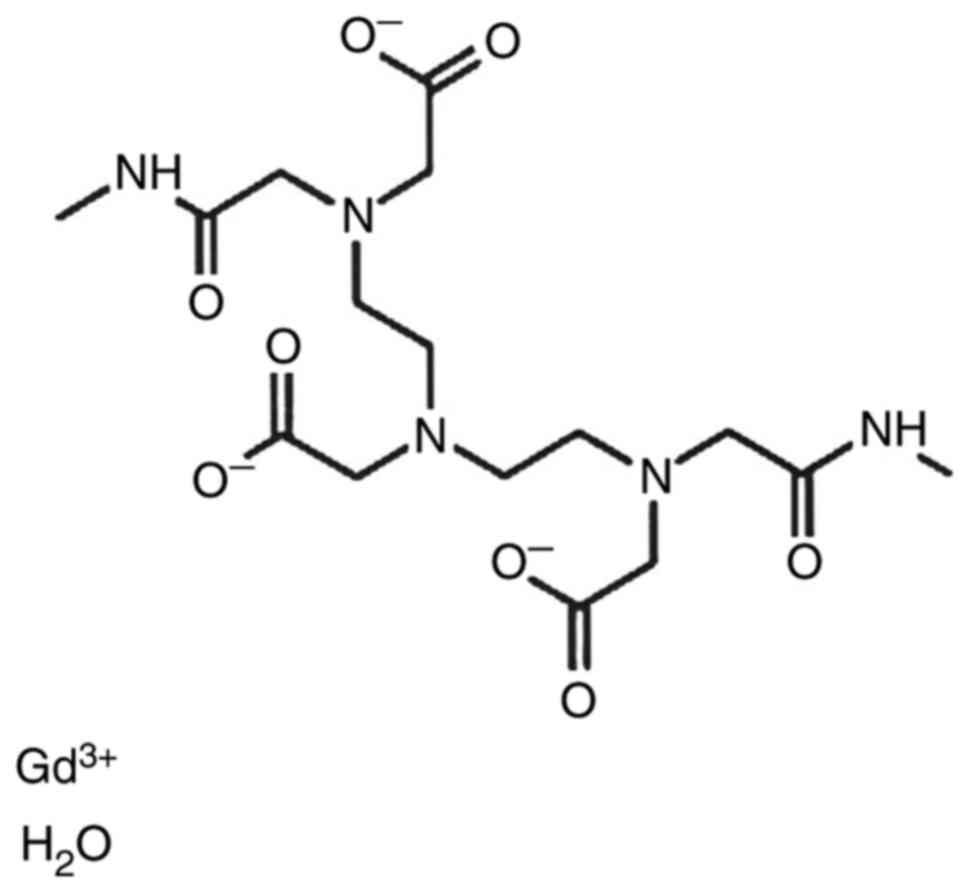
3), and Gd-DTPABMA-Ominiscan (gadodiamide) (Fig. 4) (9). Following identification of the
complexes, GBCAs were used for more than 30 years in more than
100,000,000 patients (1).
3. Findings
The administration of gadolinium complexes rarely
induces side effects and they can be divided into two groups: i)
non-allergic reactions (fatigue, headache, arthralgia, gustatory
perversion, flushed feeling, nausea, vomiting) and ii)
idiosyncrasic allergy-like reactions (periorbital edema, rash,
erythema, respiratory failure, chest pressure) (10,11).
The frequency of side effects is similar to medical systemic or
topical drugs used for various clinical conditions (12-21).
Classified by the severity, the acute reactions to GBCA are: Mild,
moderate and severe (10). The mild
ones are auto-limited events, show no progress, are the most common
and do not require medical treatment, except for skin reactions for
which an antihistamine drug can be administered. Skin reactions
occur in 0.07 and 2.4% of cases. Moderate reactions require medical
treatment such as antihistamines, or transport to emergency room
and occur in 0.004-0.7% of cases. The patient life is subjected to
immediate risk by severe reaction; however, such reactions do not
exceed 0.001-0.01% (10,11). If patients have a history of
allergy, hypersensitivity to a gadolinium-based contrast agent or
if they receive the contrast agent at a rapid rate, complications
may occur (6).
Neurofibromatosis type 1 (NF1) or von
Recklinghausen's disease is an autosomal dominant disorder
involving tissues of ectodermal origin and represents over 90% of
neurofibromatosis cases. Initially, the diagnosis is clinical
(22). Symptoms manifest
differently in each patient with a highly variable expression, even
those within the same family (23,24).
The diagnostic criteria take into account the cutaneous,
neurological, ocular and skeletal manifestations to which is added
the genetic component. These criteria were established in 1988
during the Neurofibromatosis - NIH Consensus Development Conference
(22). If two or more criteria out
of the seven are present, a clinical diagnosis can be made. The
criteria are: i) prepubertal: Six or more than six 'café-au-lait'
spots >5 mm and >15 mm, postpubertal; ii) two or more
neurofibromas or one or more plexiform neurofibroma; iii) axillary
or inguinal freckle (Crowe sign); iv) glioma of the optic nerve; v)
two or more than two iris hamartomas; vi) sphenoid wing dysplasia,
cortex of long bones thin(with/without pseudarthrosis); vii) a 1st
degree relative with neurofibromatosis type 1 diagnosed using the
above criteria (25).
The central nervous system is commonly involved in
NF1. The screening of the brain with magnetic resonance (MR)
imaging is utilised to evaluate the disease progression and as an
aid in the diagnosis of asymptomatic patients when clinical
criteria are not met (26-29).
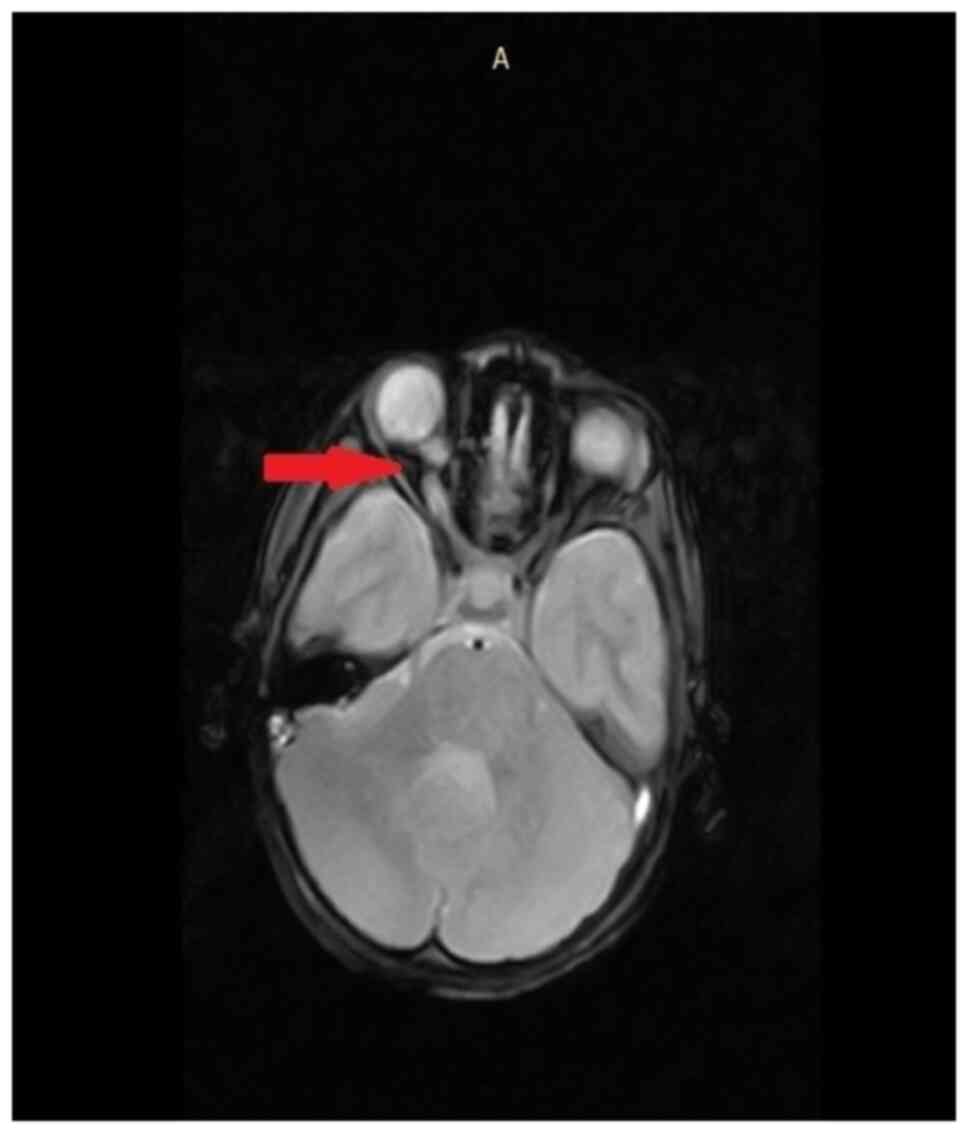
The most common intracranial abnormalities in NF1 patients are
astrocytomas of the optic pathways, as indicated in the MRI of an
8-year-old female patient (Fig. 5).
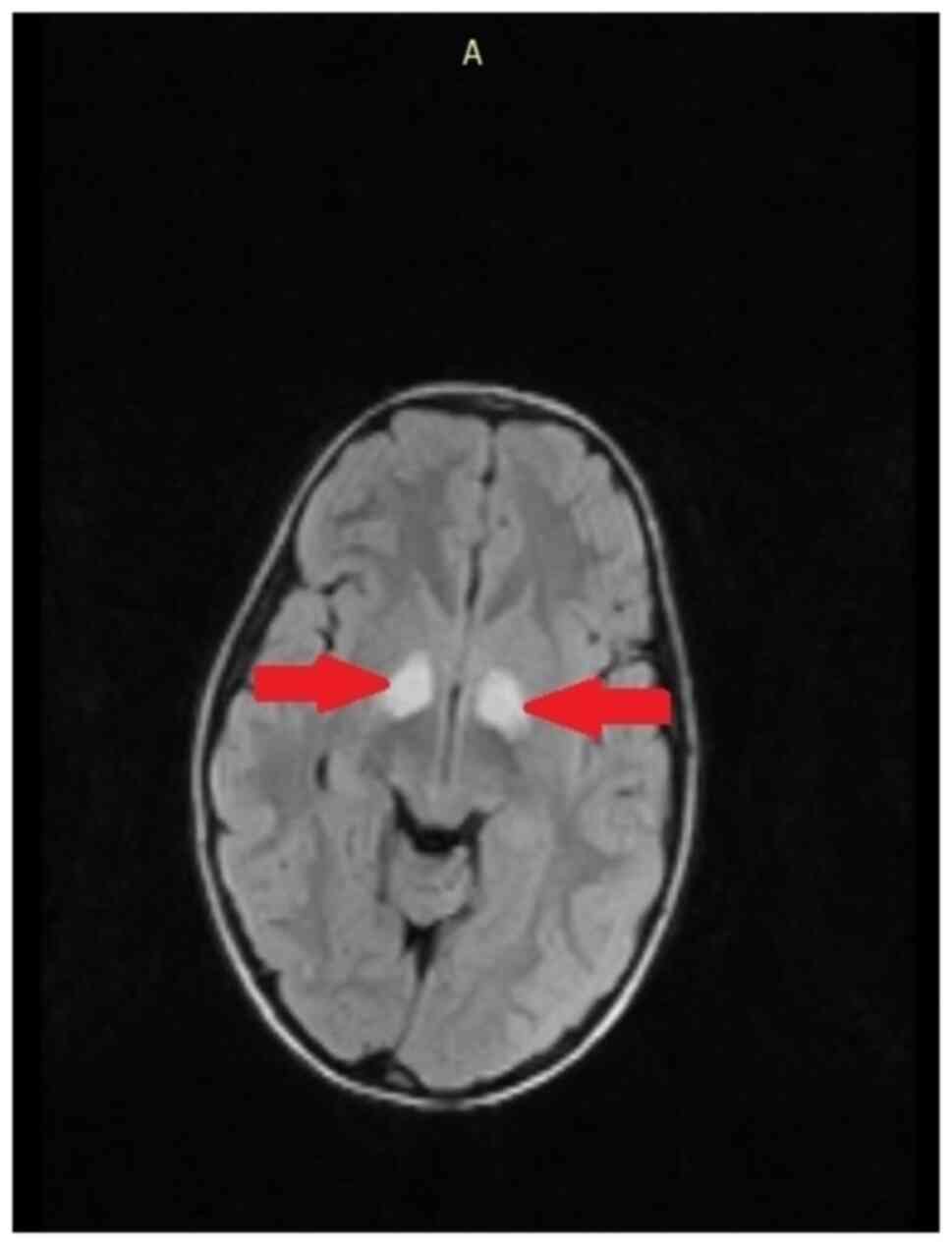
Surfaces with abnormal T-2 signal intensity are observed with high
frequency and represent hamartomas or heterotopias as identified in
an 8-year-old patient (Fig. 6).
4. Discussion
Progression of these lesions in the second decade of
life dictates the need for strict monitoring to exclude neoplasia.
In adults the safety of contrast material has been well
established, and according to preliminary data that it is also safe
for use in children. Contrast administration is recommended when
pre-contrast studies show abnormalities, when tumor is suspected,
when improved lesion delineation is necessary, and when
postoperative evaluation is required to ascertain tumor recurrence
(30). Approximately 15% of all
patients with NF1 have brain anomaly on MRI (27). The lesions are often multiple
(29,30), in characteristic locations: The
pons, cerebellar white matter, midbrain, splenium of the corpus
callosum and internal capsule. Cerebellar tumors can compress the
brain and the fourth ventricle (appearing hydrocephalus) and
treatment may require surgical resection. On suspicion of a tumor,
administrating contrast material can be helpful to fully delineate
and help characterize it, with other investigations being performed
for genetic or congenital disorders (30-34).
Rehabilitation of patients can be applied at any
stage of disease; consequently, the objectives change as the
disease advances. The use of preventive rehabilitation ensures the
maintenance of maximum functional independence. A decline in
functional skills due to tumor progression leads to rehabilitation
playing a supportive role via accommodating patients with anatomic
and physiologic limitations. Palliative rehabilitation is
recommended for the terminal stages of illness and isused to
improve and maintain comfort and quality of life.
5. Conclusions
Contrast administration may be used to maximize
tumor detection in basic MR and to determine the stability of
neoplasms in follow-up exams. However, if there no new symptoms
develop the contrast may not be necessary in patients with, for
example, myelin vacuolization. Nevertheless, GBCA is safe and the
patients tolerate it well.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The information generated and analyzed during the
current study is available from the corresponding author on
reasonable request.
Authors' contributions
All authors have had equal participation and equal
rights to this article. FN and ALT were major contributors in
writing the manuscript. FN, ALT, DM, AM, LB, DSR, BIS, AN and EN
contributed to the conception and design of the work, as well as
revising the manuscript. DM, BIS, AN and LB helped analyze the data
for the work. AM revised it for important intellectual content. ALT
and AN approved the final version to be published. All authors
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tomonori K, Hiroshi O, Toyoda K and
Kitajima K: Brain gadolinium deposition after administration of
gadolinium-based contrast agents. Jpn J Radiol. 34:3–9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sherry AD, Caravan P and Lenkinski RE: A
primer on gadolinium chemistry. J Magn Reson Imaging. 30:1240–1248.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thomsen HS, Morcos SK, Almen T, Almén T,
Bellin MF, Bertolotto M, Bongartz G, Clement O, Leander P,
Heinz-Peer G, Reimer P, et al: Nephrogenic systemic fibrosis and
gadolinium-based contrast media: Updated ESUR Contrast Medium
Safety Committee guidelines. Eur Radiol. 23:307–318.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bellin MF: MR contrast agents, the old and
the new. Eur J Radiol. 60:314–323. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Niendorf HP, Alhassan A and Balzer TH:
Safety and risk of gadolinium-DTPA: Extended clinical experience
after more than 20 million applications. Magn Monograph. 1–38.
1998.
|
|
6
|
Granata V, Cascella M, Fusco R,
dell'Aprovitola N, Catalano O, Filice S, Schiavone V, Izzo F, Cuomo
A, Petrillo A, et al: Immediate adverse reactions to
gadolinium-based MR contrast media: A retrospective analysis on
10.608 examinations. Biomed Res Int. 2016(3918292)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Runge VM, Ai T, Hao D and Hu X: The
developmental history of the gadolinium chelates as intravenous
contrast media for magnetic resonance. Invest Radiol. 46:807–816.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Runge VM, Steward RG, Clanton JA, Jones
MM, Lukehart CM, Partain CL and James AE Jr: Work in progress:
Potential oral and intravenous paramagnetic NMR contrast agents.
Radiology. 147:789–791. 1983.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hao D, Ai T, Goerner F, Hu X, Runge VM and
Tweedle M: MRI contrast agents: Basic chemistry and safety. J Magn
Reson Imaging. 36:1060–1071. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
The American College of Radiology. Manual
on Contrast Media Version. Version 10.3.2018. Accessed from:
http://www.acr.org/.
|
|
11
|
Ersoy H and Rybicki FJ: Biochemical safety
profiles of gadolinium-based extracellular contrast agents and
nephrogenic systemic fibrosis. J Magn Reson Imaging. 26:1190–1197.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Brănișteanu DE, Pintilie A, Dimitriu A,
Cerbu A, Ciobanu D, Oanta A and Tatu AL: Clinical, laboratory and
therapeutic profile of lichen planus. Medical Surgical J.
121:25–32. 2017.
|
|
13
|
Tatu AL, Ciobotaru OR, Miulescu M, Buzia
OD, Elisei AM, Mardare N, Diaconu C, Robu S and Nwabudike LC:
Hydrochlorothiazide: Chemical structure, therapeutic, phototoxic
and carcinogenetic effects in dermatology. Rev Chim. 69:2110–2114.
2018.
|
|
14
|
Fekete GL and Fekete L: Cutaneous
leukocytoclastic vasculitis associated with erlotinib treatment: A
case report and review of the literature. Exp Ther Med.
17:1128–1131. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ciobotaru OR, Lupu MN, Rebegea L,
Ciobotaru OC, Earar K and Miulescu M: Dexamethasone-chemical
structure and mechanisms of action in prophylaxis of postoperative
side effects. Rev Chim. 70:843–847. 2019.
|
|
16
|
Tatu AL and Cristea VC: Unilateral
blepharitis with fine follicular scaling. J Cutan Med Surg.
21(442)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lupu M, Miulescu M, Sandu MN, Filip I,
Rebegea L, Ciobotaru O, Stoleriu G, Earar K, Voinecsu CD, Oana R
and Ciobotaru OR: Cannabinoids: Chemical structure, mechanisms of
action, toxicity and implications in everyday life. Rev Chim.
70(627)2019.
|
|
18
|
Nwabudike LC, Elisei AM, Buzia OD,
Miulescu M and Tatu AL: Statins. A review on structural
perspectives, adverse reactions and relations with non-melanoma
skin cancer. Rev Chim. 69:2557–2562. 2018.
|
|
19
|
Ardeleanu V, Dobre M and Georgescu AM:
Deep facial wrinkle treatment outcome after first injection of
reticulated hyaluronic acid. Rev Chim. 66:2129–2131. 2015.
|
|
20
|
Nwabudike L and Tatu AL: Response to -
chronic exposure to tetracyclines and subsequent diagnosis for
non-melanoma skin cancer in a large Mid-Western US population. J
Eur Acad Dermatol Venereol. 32(e159)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tatu AL, Ionescu MA and Nwabudike LC:
Contact allergy to topical mometasone furoate confirmed by
rechallenge and patch test. Am J Ther. 25:e497–e498.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Neurofibromatosis. Conference statement.
National Institutes of Health Consensus Development Conference.
Arch Neurol. 45:575–578. 1988.
|
|
23
|
Antônio JR, Goloni-Bertollo EM and Trídico
L: Neurofibromatosis: Chronological history and current issues. An
Bras Dermatol. 88:329–343. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tatu AL and Nwabudike LC: The treatment
options of male genital lichen sclerosus et atrophicus short title
for a running head: Treatments of Genital Lichen Sclerosus
Conference: 14th National Congress of Urogynecology
(Urogyn)Location: Eforie, Romania Date: SEP 07-09, 2017.
Proceedings of the 14th national congress of Urogynecology and the
national conference of the Romanian association for the study of
pain, pp262-264, 2017.
|
|
25
|
Nica SC, Mihailescu G, Nica SM, Baetu C,
Clatici VG and Buruga I: Neurofibromatosis-one disease for a
multidisciplinary team. RoJCED. 3:38–49. 2016.
|
|
26
|
Lund AM and Skobby F: Optic gliomas in
children with neurofibromatosis type 1. Eur J Pediatr. 150:835–838.
1990.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Elster AD: Radiologic screening in
neurocutaneous syndromes: Strategies and controversies. AJNR Am J
Neuroradiol. 13:1078–1082. 1992.PubMed/NCBI
|
|
28
|
Truhan AP and Filipek PA: Magnetic
resonance imaging: Its role in the neuroradiologic evaluation of
neurofibromatosis, tuberous sclerosis and Sturge-Weber syndrome.
Arch Dermatol. 129:219–226. 1993.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bognanno JR, Edwards MK, Lee TA, Dunn DW,
Roos KL and Klatte EC: Cranial MR imaging in neurofibromatosis. Am
J Roentgenol. 151:381–388. 1988.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bonawitz C, Castillo M, Chin CT, Mukherji
SK and Barkovich AJ: Usefulness of contrast material in MR of
patients with Neurofibromatosis type 1. AJNR Am J Neuoradiol.
19:541–546. 1998.PubMed/NCBI
|
|
31
|
Tatu AL: Umbilicated blue black lesion on
the lateral thorax. J Cutan Med Surg. 21(252)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ardeleanu V, Frincu LL and Georgescu C:
Neoangiogenesis-assessment in esophageal adenocarcinomas. Indian J
Surg. 77 (Suppl 3):S971–S976. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ardeleanu V, Chebac GR, Georgescu C, Vesa
D, Frâncu L, Frîncu LD and Păduraru D: The modifications suffered
by the peri-esophageal anatomical structures in the hiatal hernia
disease: A qualitative and quantitative microanatomic study. Rom J
Morphol Embryol. 51:765–770. 2010.PubMed/NCBI
|
|
34
|
Fekete GL and Fekete JE: Steatocystoma
multiplex generalizata partial suppurativa-case report. Acta
Dermatovenerol Croat. 18:114–119. 2010.PubMed/NCBI
|