Introduction
Breast cancer (BC) is one of the most common and
deadly cancers in women worldwide (1-3),
and >20 distinct subtypes of breast cancer have been identified
(4). Next-generation sequencing
studies have drawn comprehensive molecular BC portraits, and
>1600 driver mutations have been identified in 93 BC genes
(5). Among all cases,
hormone-receptor-positive BCs account for half of the disease
subtypes (6). According to the
presence or absence of molecular markers for estrogen or
progesterone receptors and human epidermal growth factor 2 (ERBB2;
formerly HER2), BC is divided into three major subtypes (luminal,
basal-like and Her-2+) (7). The subtype would determine the type of
systemic therapy given to patient, including endocrine therapy,
chemotherapy, and ERBB2-targeted antibody or small-molecule
inhibitor therapy combined with chemotherapy (8). In addition, surgical resection is also
considered for patients with non-metastatic BC (9). At present, palliative care can improve
the quality of life and prolong the life in patients with
metastatic BC treated according to subtypes (10). It is therefore necessary to identify
new therapeutic targets and to determine the underlying mechanisms
of BC.
Arctigenin, a bioactive lignin, can be isolated from
the seeds of Asteraceae lappa and has exhibited some
anti-inflammatory and anti-viral effects (11). Furthermore, arctigenin has been
reported to increase the chemosensitivity of several cancer cells,
including HepG2, HeLa and K562(12). Arctigenin has also been applied to
the treatment of various types of cancer, and the anti-tumor
function has been illustrated in various cancers, including
gallbladder cancer (13), human
retinoblastoma cells (14), lung
cancer (15) and prostate tumor
(16). Wang et al (17) reported that arctigenin could trigger
autophagy, induce apoptosis and enhance the sensitivity of
colorectal cancer cell to chemotherapy. In addition, arctigenin can
inhibit the migratory and invasive abilities of breast cancer cells
by downregulating heparanase and matrix metalloproteinases (MMPs) 2
and 9 in MDA-MB-231 cells (18).
Huang et al (12) also
demonstrated that arctigenin could promote the anti-metastasis
effect and inhibit triple-negative breast cancer by downregulating
the protein cancerous inhibitor of protein phosphatase 2A. In
addition, it was demonstrated that arctigenin can target the
transcription factor signal transducer and activator of
transcription 3, which is involved in epithelial to mesenchymal
transition (EMT) (19). However,
limited studies have focused on the underlying mechanism of
arctigenin on metastasis, migration and EMT in BC.
Eukaryotic translation initiation factor 4E binding
protein 1 (4EBP1) is a type of translation-repressor protein and
represents one of the main downstream effector of mammalian target
of rapamycin (mTOR) (20). As a
tumor suppressor, 4EBP1 serves crucial roles in various types of
cancer. For example, 4EBP1 can be reactivated by mTOR inhibition
and act as a tumor suppressor in head and neck squamous cell
carcinomas (21). Furthermore,
overexpressed 4EBP1 is an independent predictor of outcome for
patients with ovarian cancer (22).
There is also some evidence that 4EBP1 is overexpressed in BC cells
where it might serve as an oncogene (23,24).
However, the underlying mechanism of 4EBP1 in BC remains
unknown.
Therefore, the present study aimed to investigate
the effect and underlying mechanisms of arctigenin on BC cells and
to explore the regulation relationship between arctigenin and
4EBP1.
Materials and methods
Chemicals and reagents
Arctigenin (purity, up to 98%) was obtained from
Shanghai Yuanye Bio-Technology Co., Ltd. Arctigenin was dissolved
in DMSO at a stock solution of 50 mM and stored at -20˚C. The
solution was then diluted in culture medium to the appropriate
final concentrations prior to use (5, 10, 20 and 40 µM).
Cell treatment and transfection
The human breast cancer cell lines MDA-MB-231 and
BT549 were purchased from the American Type Culture Collection.
Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.), containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
and 100 µg/ml streptomycin and 100 U/ml penicillin (Gibco; Thermo
Fisher Scientific, Inc.) and placed at 37˚C in a humidified
incubator containing 5% CO2. MDA-MB-231 and BT549 cells
were treated with arctigenin at various concentrations (5, 10, 20
and 40 µM) or vehicle as the control for 24, 48 and 72 h.
For 4EBP1 overexpression, 50 nM pcDNA3.1-4EBP1 or
pcDNA3.1-NC (Invitrogen; Thermo Fisher Scientific, Inc.) were
diluted by 250 µl of serum-free Opti-MEM and incubated at room
temperature for 5 min and mixed with 5-µl aliquot of
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for another 20 min at room temperature and added
to culture well of MDA-MB-231 or BT549 cell
(5x104/well). Subsequently, the cells were cultured for
6-8 h at 37˚C with 5% CO2, the complete medium was
refreshed and the cells were cultured for a further 48 h prior to
the following experiments.
Cell proliferation assay
MDA-MB-231 and BT549 cells were seeded at the
density of 5x103 cells per well in 96-well plates and
cultured in DMEM medium containing 10% FBS overnight. Subsequently,
cells were treated with different concentrations of arctigenin and
cultured for various times (24, 48 and 72 h). Cell Counting Kit-8
(CCK8; 10 µl; Dojindo Molecular Technologies, Inc.) reagent, which
was used to assess cell proliferation, was added to the wells and
cells were incubated for 3 h at 37˚C. Absorbance was measured at a
wavelength of 450 nm on a microplate reader.
Transwell migration and invasion
assay
To evaluate the migratory and invasive abilities of
cells, 24-well Transwell chambers (8-µm pore size; Corning Inc.)
were used. MDA-MB-231 and BT549 cells were treated with different
concentrations of arctigenin for 48 h. For the migration assay,
1x105 treated cells were resuspended in serum-free
medium containing 1% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) and seeded in the upper chamber of the Transwell, while DMEM
with 10% FBS was added to the lower chamber. After incubation at
37˚C for 24 h, cells in the lower chamber were fixed with 4%
paraformaldehyde and stained with 1% crystal violet for 15 min at
37˚C (JRDUN Biotechnology Co., Ltd.), whereas cells in the upper
wells were removed. For the invasion assay, the method was similar
to the cell migration assay, but the Transwell membrane was
pre-treated with Matrigel (BD Biosciences) at a concentration of 2
mg/ml. In addition, the results were assessed 36 h after
incubation. For qualification, five random fields per filter were
counted under a light microscope at a magnification of x100 (Leica
Microsystems GmbH).
RNA extraction and reverse
transcription quantitative (RT-q) PCR
MDA-MB-231 and BT549 cells were collected and total
RNA was extracted using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). PrimeScript RT reagent Kit (Takara
Biotechnology Co., Ltd.) was used for reverse transcription.
RT-qPCR was conducted with SYBR green master reagent (Toyobo Life
Science). The PCR reactions were conducted as follows: Initial
denaturation at 95˚C for 2 min followed by 28 cycles at 95˚C for 30
sec, 58˚C for 30 sec, and 72˚C for 30 sec. The sequences of the
primers used were as follows: 4EBP1, forward
5'-GATACCTCCTTGTGCCTCCA-3', reverse 5'-TCGTTCTTGTCCACTTCCTG-3'; and
GAPDH, forward 5'-ATCCCATCACCATCTTCCAG-3' and reverse
5'-TTCTAGACGGCAGGTCAGGT-3'. The relative expression levels of
4EBP1were normalized to endogenous control GAPDH and were expressed
as 2-ΔΔCq (25).
Western blotting
Western blotting was performed to detect the protein
expression of E-cadherin, N-cadherin, vimentin and 4EBP1. Cells
were harvested, washed with PBS and lysed in RIPA buffer
(Sigma-Aldrich; Merck KGaA) and 1% protease inhibitors cocktail
(Merck KGaA). Protein concentration was determined using BCA
protein reagent (Pierce; Thermo Fisher Scientific, Inc.). Proteins
(20 µg) were separated by 10% SDS-PAGE gel and transferred onto
PVDF membranes (Merck KGaA). Membranes were blocked with 5% skimmed
milk for 1 h at 37˚C and were incubated overnight at 4˚C with
primary antibodies against E-cadherin (cat. no. ab1416; 1:100),
N-cadherin (cat. no. ab76057; 1:1,000), vimentin (cat. no. ab92547;
1:1,000), 4EBP1 (cat. no. ab32024; 1:5,000) and GAPDH (cat. no.
ab181602; 1:10,000; all from Abcam). Membranes were then incubated
with a diluted horseradish peroxidase-labeled goat anti-rabbit
secondary antibody (cat. no. SE134; 1:2,000, Beijing Solarbio
Science and Technology Co., Ltd.) at room temperature for 1 h.
SuperSignal® West Pico Trial kit (Pierce; Thermo Fisher
Scientific, Inc.) was used to detect the signal on the membrane and
optical densities of the bands were measured using ImageJ software
(version 1.38; National Institutes of Health).
Statistical analysis
Statistical analyses were performed using SPSS 22.0
software (IBM Corp.). The data were presented as the means ±
standard deviation. Comparison among three or more groups was
conducted using one-way ANOVA followed by Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
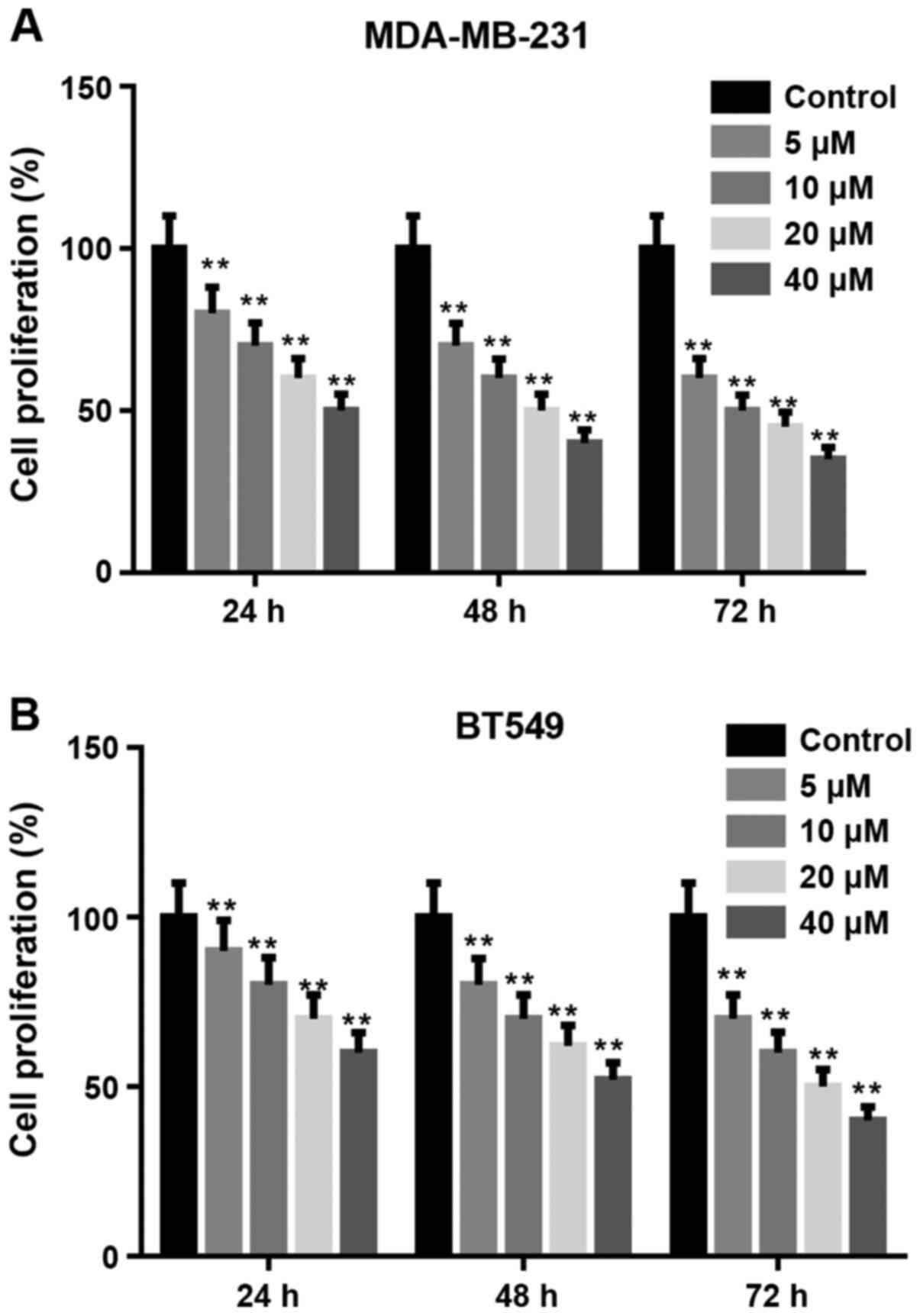
Arctigenin inhibits the proliferation
of MDA-MB-231 and BT549 cells
CCK-8 assay was used to measure the proliferation of
MDA-MB-231 and BT549 cells treated with arctigenin (0, 5, 10, 20,
and 40 µM) for 24, 48 and 72 h. As presented in Fig. 1A and B, arctigenin significantly decreased the
proliferation of MDA-MB-231 and BT549 cells in a
concentration-dependent manner compared with the control.
Subsequently, the concentrations of 20 and 40 µM were selected to
further evaluate the effects of arctigenin on the migratory and
invasive abilities and EMT of cells.
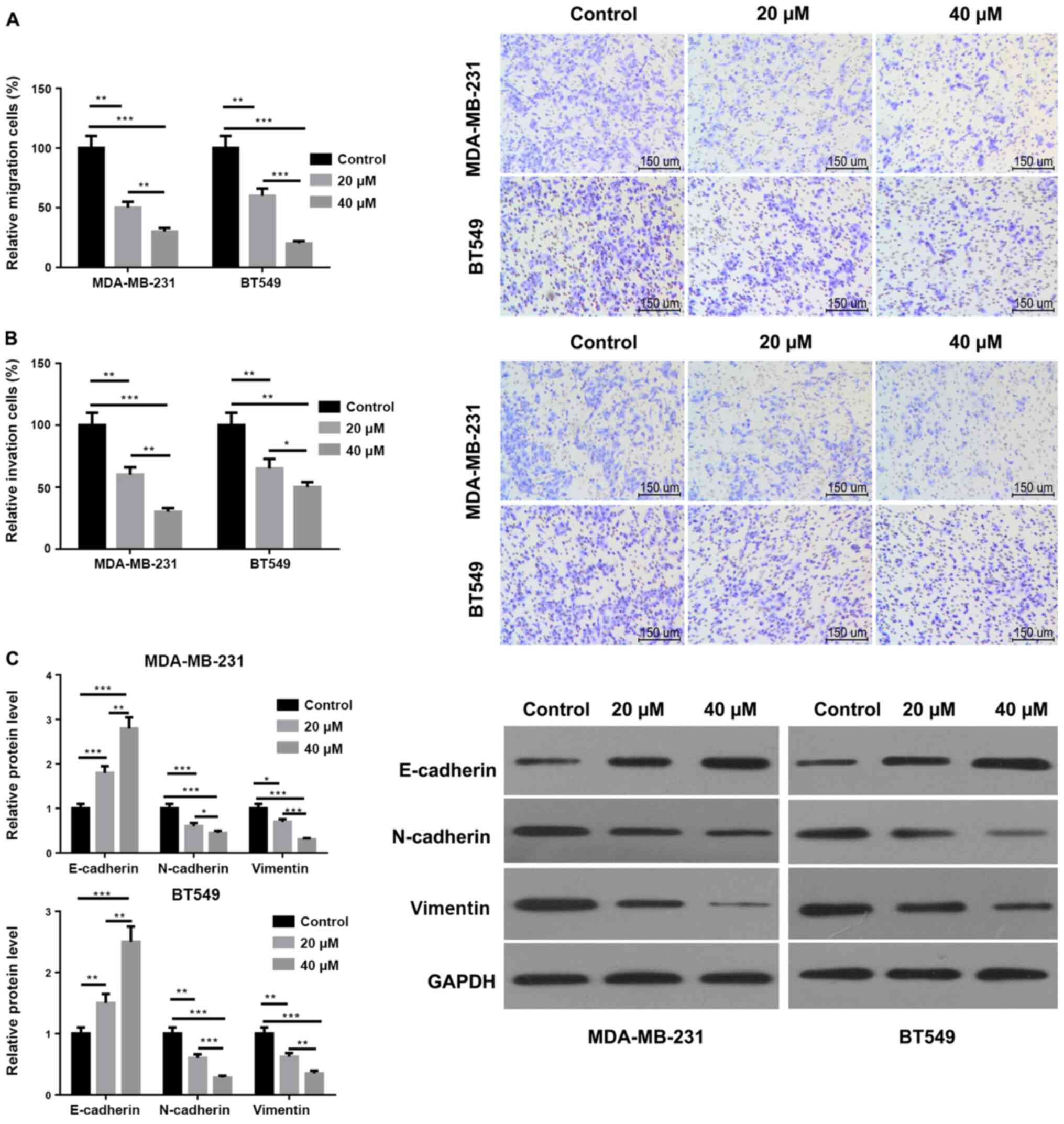
Arctigenin inhibits the migratory and
invasive abilities and EMT of MDA-MB-231 and BT549 cells
As presented in Fig.
2A, the migration ability of MDA-MB-231 and BT549 cells
following treatment with 20 and 40 µM arctigenin was significantly
decreased compared with the control. Furthermore, the migration
ability of MDA-MB-231 and BT549 cells treated with 40 µM arctigenin
was significantly decreased compared with cells treated with 20 µM
arctigenin. The results from Fig.
2B demonstrated that arctigenin could also inhibit the invasive
ability of MDA-MB-231 and BT549 cells in a concentration-dependent
manner. In addition, as presented in Fig. 2C, the protein expression of
N-cadherin and vimentin was significantly decreased in MDA-MB-231
and BT549 cells following treatment with 20 and 40 µM arctigenin
compared with the control. The expression of E-cadherin was
significantly increased in MDA-MB-231 and BT549 cells. These
results demonstrated also that arctigenin may inhibit EMT in a
dose-dependent manner. Taken together, these findings indicated
that arctigenin may serve a crucial role in the processes of
migration, invasion and EMT of MDA-MB-231 and BT549 cells.
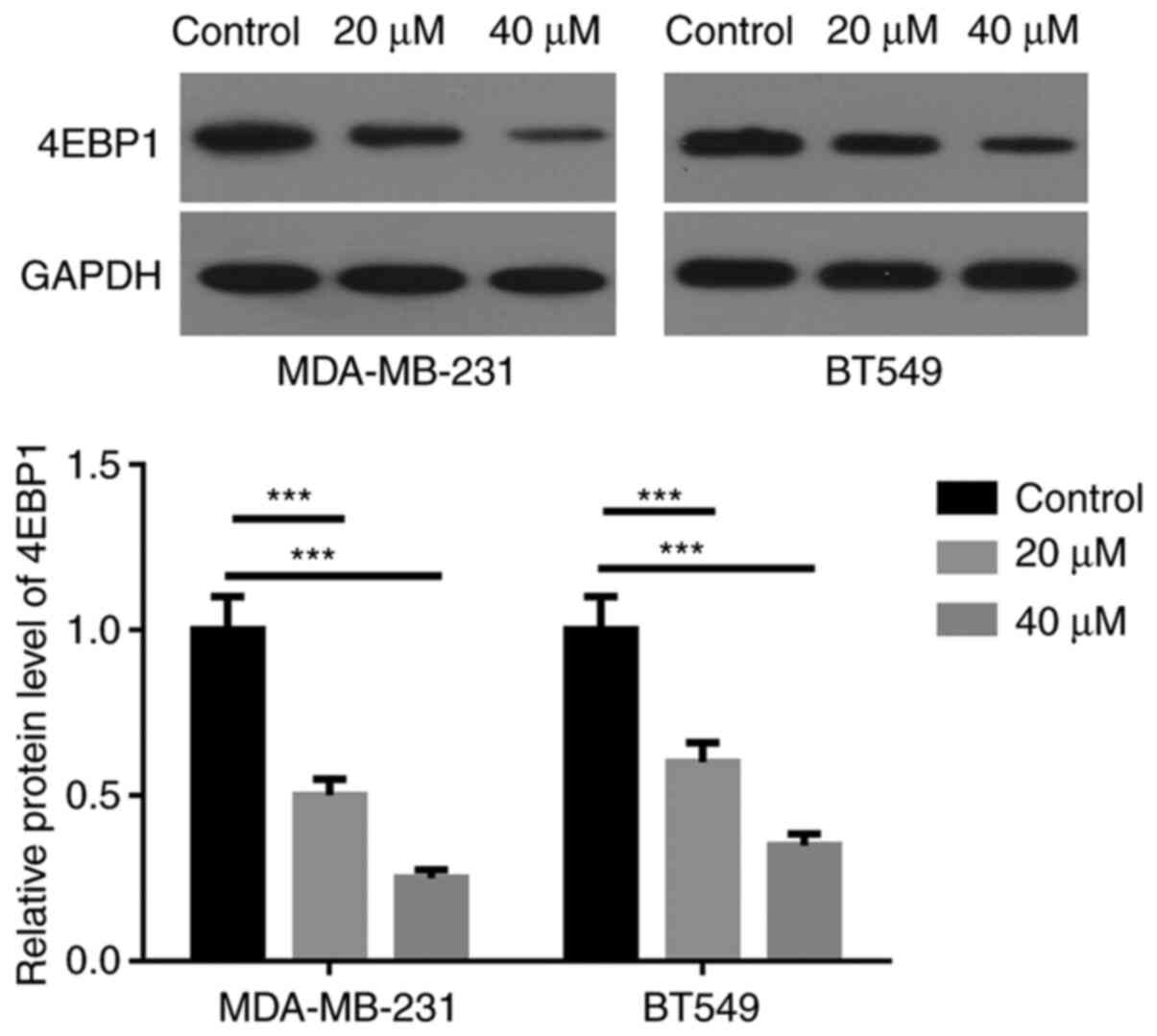
Arctigenin downregulates the
expression of 4EBP1 in MDA-MB-231 and BT549 cells
The effect of arctigenin on 4EBP1 expression in BC
cells was evaluated. As seen in Fig.
3, the expression of 4EBP1 was significantly decreased in
MDA-MB-231 and BT549 cells following treatment with 20 and 40 µM
arctigenin compared with the control. In addition, 4EBP1 expression
in cells treated with 40 µM arctigenin was significantly decreased
compared with cells treated with 20 µM arctigenin.
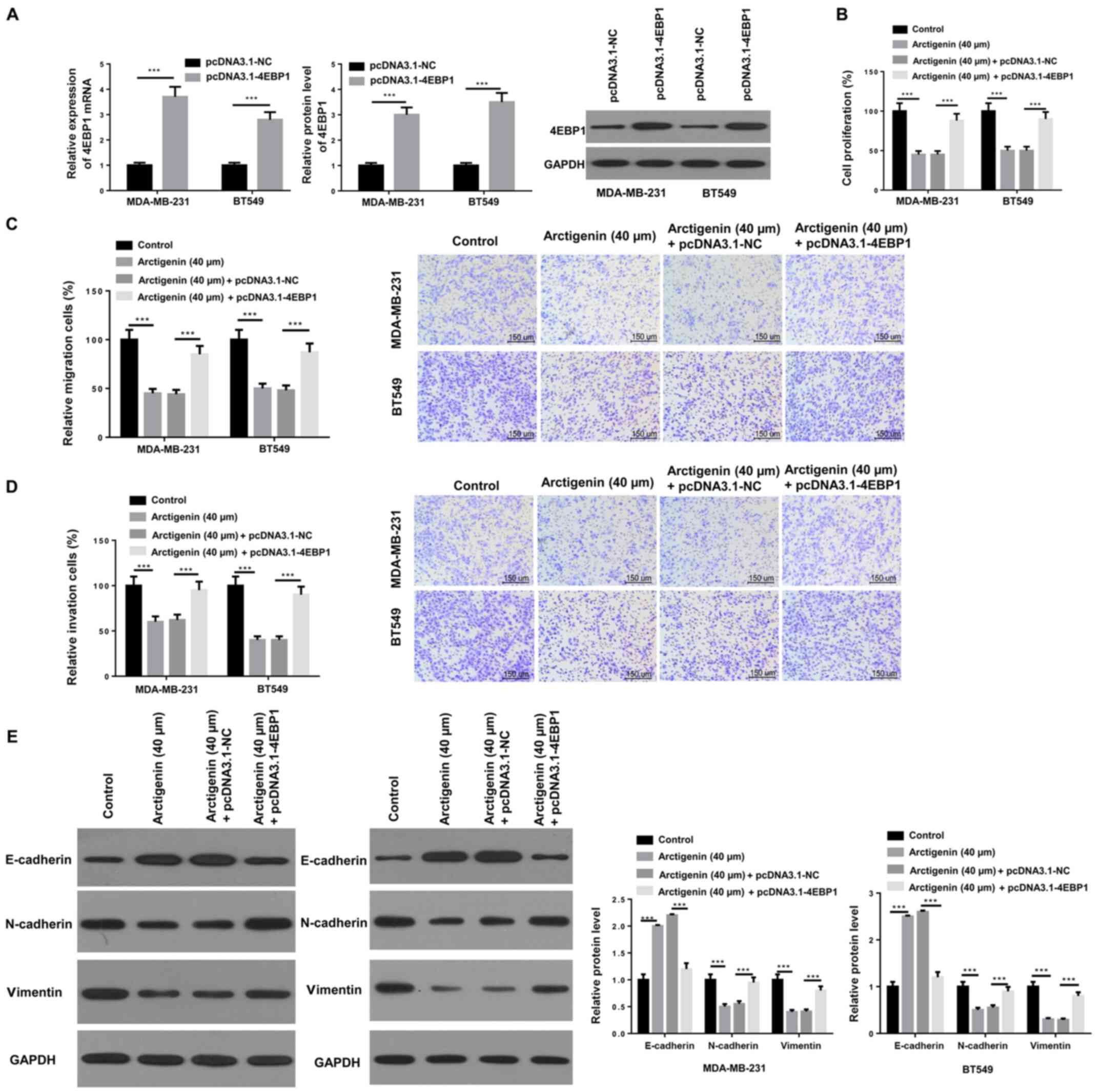
4EBP1 overexpression can reverse the
inhibitory effect of arctigenin on the proliferation, migratory and
invasive abilities and EMT in MDA-MB-231 and BT549 cells
The effect of 4EBP1 on cell migratory and invasive
abilities and EMT in MDA-MB-231 and BT549 cells was subsequently
further investigated. 4EBP1 was overexpressed by transfection with
pcDNA-4EBP1, and the transfection efficiency was examined by
RT-qPCR and western blotting. As presented in Fig. 4A, the mRNA and protein expression of
4EBP1 was significantly increased in transfected MDA-MB-231 and
BT549 cells compared with NC. Furthermore, MDA-MB-231 and BT549
cell proliferation was significantly decreased following treatment
with arctigenin, which was reversed following 4EBP1 overexpression
(Fig. 4B). The results from
Transwell assays demonstrated that 4EBP1 overexpression could
reverse the inhibitory effect of arctigenin on the cell migratory
and invasive abilities (Fig. 4C and
D). In addition, 4EBP1
overexpression significantly increased the expression of E-cadherin
but decreased the expression of N-cadherin and vimentin in
MDA-MB-231 and BT549 cells treated with arctigenin (Fig. 4E). These findings suggested that
overexpression of 4EBP1 may reverse the inhibitory effect of
arctigenin on the proliferation, migratory and invasive abilities
and EMT in MDA-MB-231 and BT549 cells.
Discussion
BC is one of the most prevalent carcinomas in women
worldwide, and the development of BC metastasis leads to a high
mortality rate. However, there is no Food and Drug
Administration-approved targeted therapy for BC (26). It is therefore urgent to identify
new targeting therapy and drugs against BC. In the last decades,
arctigenin and 4EBP1 have been reported in several studies. As
previously reported, arctigenin inhibits the degradation of
topoisomerase IIα and reduces the expression of GRP78 in solid
tumors, which can attenuate anticancer drug resistance (27). Maheshwari et al (28) also found that arctigenin shows
anti-tumor activity against a set of human solid tumor cell lines,
including pancreatic-PANC-1, colon-H116, lung-H125, liver-HepG2,
OVC-5 and brain-U251N. However, the role and underlying mechanism
of arctigenin and 4EBP1 in the proliferation, migratory and
invasive abilities and EMT of BC cells remain unclear. The present
study demonstrated that arctigenin could inhibit the proliferation,
migratory and invasive abilities and EMT of BC cells, which was
reversed following 4EBP1 overexpression.
Arctigenin is a member of the Asteraceae family that
could inhibit the growth of several cancer cells (29). Previous studies revealed that
arctigenin has anti-viral, anti-inflammatory and anti-tumor
activities (30,31). Furthermore, arctigenin was reported
to be a therapeutic agent against cancer and to inhibit some
oncogenic signaling pathways (32).
As demonstrated by Maxwell et al (33), arctigenin has some anti-metastatic
effects on human BC cells by inhibiting MMP-9 and urokinase
plasminogen activator via the Akt, NF-κB, and MAPK signaling
pathways. Lee et al (34)
also demonstrated that arctigenin can decrease the proliferation of
MCF-7 and MDA-MB-231 human BC cells and induce apoptosis in MCF-7
cells. In the present study, arctigenin inhibited the proliferation
and migratory and invasive abilities of MDA-MB-231 and BT549 cells.
These results were consistent with the study of Lou et al
(18), in which the effect of
arctigenin on the inhibition of BC cell migration and invasion is
confirmed. Another study reported that arctigenin can inhibit the
proliferation of MDA-MB-231 cells in a dose-dependent manner and
from a concentration as low as 0.4 µΜ (19). In the present study, arctigenin was
found to inhibit BC cell proliferation at the low concentration of
5 µΜ and to inhibit BC cell migratory and invasive abilities at the
low concentration at 20 µΜ. The difference may be due to detection
methods and cell culture conditions. As reported by Xu et al
(15), arctigenin can inhibit
TGF-β-induced EMT and suppress the progression and metastasis of
lung cancer cells. Lu et al (35) also demonstrated that arctigenin can
attenuate tumor metastasis by inhibiting EMT in hepatocellular
carcinoma. However, only a few studies have investigated the effect
of arctigenin on EMT in BC cells. To the best of our knowledge, the
present study was the first to demonstrate that arctigenin could
inhibit EMT in MDA-MB-231 and BT549 cells.
As a major substrate of mTORC1, 4EBP1 plays an
essential role in the regulation of cancer cell proliferation
(36). In addition, 4EBP1 can slow
tumor progression in phosphatase and tensin homolog (PTEN)-driven
prostate cancer (37). Significant
upregulation and dephosphorylation of 4EBP1 serve an important role
in the promotion of pancreatic cancer cell death (38). The results from these studies
suggest that 4EBP1 might serve as a tumor suppressor factor and
inhibit the migratory and invasive abilities of various cancer
cells. However, only limited studies have investigated the role and
underlying mechanism of 4EBP1 in BC cells in the last decades.
Conversely, it was reported that integrated analysis of PTEN and
p4EBP1 protein levels could be considered as predictors for
pathological complete response in patients with HER2-positive BC
receiving neoadjuvant therapy (39). Besides, 4EBP1 is considered as an
oncogene and was found to be upregulated in BC cells (23,24).
The present study demonstrated for the first time that
overexpressing 4EBP1 could reverse the inhibitory effect of
arctigenin on the proliferation, migratory and invasive abilities
and EMT of BC cells. These result suggested that 4EBP1 may promote
tumor progression and act as an oncogene in BC. All these results
indicated that 4EBP1 might serve different roles in cell
proliferation, migration, invasion and EMT in various types of
cancer cell. Further investigation is therefore essential.
In summary, the present study demonstrated that
arctigenin could inhibit human BC cell proliferation, migratory and
invasive abilities and EMT by targeting 4EBP1. These findings may
bring a new direction for the development of targeting therapy
against BC.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and material
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL conducted the majority of the experiments and
wrote the manuscript; FW and HLuo conducted experiments, analyzed
the data and confirm the authenticity of all the raw data. HLiu
designed the study and revised the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mavaddat N, Michailidou K, Dennis J, Lush
M, Fachal L, Lee A, Tyrer JP, Chen TH, Wang Q, Bolla MK, et al:
Polygenic risk scores for prediction of breast cancer and breast
cancer subtypes. Am J Hum Genet. 104:21–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shi J, Wu L, Zheng W, Wen W, Wang S, Shu
X, Long J, Shen CY, Wu PE, Saloustros E, et al: Genetic evidence
for the association between schizophrenia and breast cancer. J
Psychiatry Brain Sci. 3(7)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
den Bossche JV: Lipid-laden macrophages
cross the border to cancer. Immunometabolism. 2(e200006)2020.
|
|
4
|
Sudharshan PJ, Petitjean C, Spanhol F,
Oliveira LE, Heutte L and Honeine P: Multiple instance learning for
histopathological breast cancer image classification. Exp Syst
Appl. 117:103–111. 2019.
|
|
5
|
Sachs N, de Ligt J, Kopper O, Gogola E,
Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H,
et al: A living biobank of breast cancer organoids captures disease
heterogeneity. Cell. 172:373–386.e10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Turner NC, Slamon DJ, Ro J, Bondarenko I,
Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al:
Overall survival with palbociclib and fulvestrant in advanced
breast cancer. N Engl J Med. 379:1926–1936. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang X, Qi Y, Kong X, Zhai J, Li Y, Song
Y, Wang J, Feng X and Fang Y: Immunological therapy: A novel
thriving area for triple-negative breast cancer treatment. Cancer
Lett. 442:409–428. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang X, Song W, Zhou Y, Mao F, Lin Y,
Guan J and Sun Q: Expression and function of MutT homolog 1 in
distinct subtypes of breast cancer. Oncol Lett. 13:2161–2168.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Toi M, Tanaka S, Bando M, Hayashi K and
Tominaga T: Outcome of surgical resection for chest wall recurrence
in breast cancer patients. J Surg Oncol. 64:23–26. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maxwell T, Lee KS, Kim S and Nam KS:
Arctigenin inhibits the activation of the mTOR pathway, resulting
in autophagic cell death and decreased ER expression in ER-positive
human breast cancer cells. Int J Oncol. 52:1339–1349.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang Q, Qin S, Yuan X, Zhang L, Ji J, Liu
X, Ma W, Zhang Y, Liu P, Sun Z, et al: Arctigenin inhibits
triple-negative breast cancers by targeting CIP2A to reactivate
protein phosphatase 2A. Oncol Rep. 38:598–606. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang M, Cai S, Zuo B, Gong W, Tang Z,
Zhou D, Weng M, Qin Y, Wang S, Liu J, et al: Arctigenin induced
gallbladder cancer senescence through modulating epidermal growth
factor receptor pathway. Tumour Biol.
39(1010428317698359)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ke N, Liu Q, Pi L, Fang J, Chen L and Chen
X: The antitumor function of arctigenin in human retinoblastoma
cells is mediated by jagged-1. Mol Med Rep. 19:3642–3648.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu Y, Lou Z and Lee SH: Arctigenin
represses TGF-β-induced epithelial mesenchymal transition in human
lung cancer cells. Biochem Biophys Res Commun. 493:934–939.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang P, Diaz T, Henning S and Vadgama J:
Abstract 5253: Arctigenin inhibits prostate tumor growth in vitro
and in vivo in obese state. Cancer Res. 77 (Suppl
13)(S5253)2017.
|
|
17
|
Wang Y, Lina L, Xu L, Yang Z, Qian Z, Zhou
J and Suoni L: Arctigenin enhances the sensitivity of cisplatin
resistant colorectal cancer cell by activating autophagy. Biochem
Biophys Res Commun. 520:20–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lou C, Zhu Z, Zhao Y, Zhu R and Zhao H:
Arctigenin, a lignan from Arctium lappa L., inhibits
metastasis of human breast cancer cells through the downregulation
of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol Rep.
37:179–184. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Feng T, Cao W, Shen W, Zhang L, Gu X, Guo
Y, Tsai HI, Liu X, Li J, Zhang J, et al: Arctigenin inhibits STAT3
and exhibits anticancer potential in human triple-negative breast
cancer therapy. Oncotarget. 8:329–344. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang T, Guo J, Li H and Wang J:
Meta-analysis of the prognostic value of p-4EBP1 in human
malignancies. Oncotarget. 9:2761–2769. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Z, Feng X, Molinolo AA, Martin D,
Vitale-Cross L, Nohata N, Ando M, Wahba A, Amornphimoltham P, Wu X,
et al: 4E-BP1 Is a tumor suppressor protein reactivated by mTOR
inhibition in head and neck cancer. Cancer Res. 79:1438–1450.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alabdullah ML, Ahmad DA, Moseley P,
Madhusudan S, Chan S and Rakha E: The mTOR downstream regulator
(p-4EBP1) is a novel independent prognostic marker in ovarian
cancer. J Obstet Gynaecol. 39:522–528. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rutkovsky AC, Yeh ES, Guest ST, Findlay
VJ, Muise-Helmericks RC, Armeson K and Ethier SP: Eukaryotic
initiation factor 4E-binding protein as an oncogene in breast
cancer. BMC Cancer. 19(491)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cristina A, Bartolacci C, Wijnant K,
Crinelli R, Bianchi M, Magnani M, Hysi A, Iezzi M, Amici A and
Marchini C: Resveratrol fuels HER2 and ERα-positive breast cancer
behaving as proteasome inhibitor. Aging (Albany NY). 9:508–520.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yoon SB and Park HR: Arctigenin inhibits
etoposide resistance in HT-29 colon cancer cells during
microenvironmental stress. J Microbiol Biotechnol. 29:571–576.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Maheshwari M, Jia Q and Valeriote FA:
Arctin and arctigenin as a potential treatment for solid tumors.
Cancer Res. 79 (Suppl 13)(S366)2019.
|
|
29
|
Naoe A, Tsuchiya T, Kondo Y, Uga N,
Watanabe S, Yasui T, Hara F and Suzuki T: Arctigenin induces
apoptosis in human hepatoblastoma cells. Pediatr Surg Int.
35:723–728. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun Y, Tan YJ, Lu ZZ, Li BB, Sun CH, Li T,
Zhao LL, Liu Z, Zhang GM, Yao JC and Li J: Arctigenin inhibits
liver cancer tumorigenesis by inhibiting gankyrin expression via
C/EBPα and PPARα. Front Pharmacol. 9(268)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lin CY, Hsieh PL, Liao YW, Peng CY, Yu CC
and Lu MY: Arctigenin reduces myofibroblast activities in oral
submucous fibrosis by LINC00974 inhibition. Int J Mol Sci.
20(1328)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
He Y, Fan Q, Cai T, Huang W, Xie X, Wen Y
and Shi Z: Molecular mechanisms of the action of arctigenin in
cancer. Biomed Pharmacother. 108:403–407. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Maxwell T, Chun SY, Lee KS, Kim S and Nam
KS: The anti-metastatic effects of the phytoestrogen arctigenin on
human breast cancer cell lines regardless of the status of ER
expression. Int J Oncol. 50:727–735. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee J, Imm JY and Lee SH: β-catenin
mediates anti-adipogenic and anticancer effects of arctigenin in
preadipocytes and breast cancer cells. J Agric Food Chem.
65:2513–2520. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lu Z, Chang L, Zhou H, Liu X, Li Y, Mi T
and Tong D: Arctigenin attenuates tumor metastasis through
inhibiting epithelial-mesenchymal transition in hepatocellular
carcinoma via suppressing GSK3β-dependent Wnt/β-catenin signaling
pathway in vivo and in vitro. Front Pharmacol.
10(937)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang H, Huang F, Zhang Z, Wang P, Luo Y,
Li H, Li N, Wang J, Zhou J, Wang Y and Li S: Feedback activation of
SGK3 and AKT contributes to rapamycin resistance by reactivating
mTORC1/4EBP1 axis via TSC2 in breast cancer. Int J Biol Sci.
15:929–941. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ding M, Van der Kwast TH, Vellanki RN,
Foltz WD, McKee TD, Sonenberg N, Pandolfi PP, Koritzinsky M and
Wouters BG: The mTOR targets 4E-BP1/2 restrain tumor growth and
promote hypoxia tolerance in PTEN-driven prostate cancer. Mol
Cancer Res. 16:682–695. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Elia A, Henry-Grant R, Adiseshiah C,
Marboeuf C, Buckley RJ, Clemens MJ, Mudan S and Pyronnet S:
Implication of 4E-BP1 protein dephosphorylation and accumulation in
pancreatic cancer cell death induced by combined gemcitabine and
TRAIL. Cell Death Dis. 8(3204)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Loibl S, Darb-Esfahani S, Huober J,
Klimowicz A, Furlanetto J, Lederer B, Hartmann A, Eidtmann H,
Pfitzner B, Fasching PA, et al: Integrated analysis of PTEN and
p4EBP1 protein expression as predictors for pCR in HER2-positive
breast cancer. Clin Cancer Res. 22:2675–2683. 2016.PubMed/NCBI View Article : Google Scholar
|