Introduction
Acute myocardial infarction (AMI) is caused by the
sudden occlusion of coronary blood flow and is a major cause of
morbidity and mortality in developed countries (1,2). AMI
leads to >4 million deaths in North Asia and Europe, and >1/3
of all deaths in developed countries every year (3,4). It
poses a major threat to human health (5). To date, significant advances have been
made in developing efficient treatment strategies, including
thrombolysis and direct coronary intervention (6,7).
However, myocardial injury as a result of ischemia followed by
restoration of blood flow remains to be a major cause of
cardiovascular disease and contributes to mortality associated with
cardiovascular events (8).
Therefore, identification and development of novel and effective
therapeutic methods for the treatment of myocardial injury remain
to be in demand.
Myocardial ischemia-reperfusion (I/R) injury is an
inherent response to the recovery of blood flow following ischemia
during myocardial infarction (MI) (9). This is a complex process that involves
numerous mechanisms, the most well studied of which is that
mediated by reactive oxygen species (ROS) (10). Excessive production of ROS leads to
oxidative stress, which in turn mediates the pathological process
of almost all cardiovascular diseases, including myocardial injury
post-MI (3,4). Hydrogen peroxide
(H2O2) is an exogenous form of ROS that is
produced as a by-product of I/R and a direct free radical donor
during oxidative stress injury (11). H2O2-induced
myocardial cell injury has been applied widely for the
investigation of AMI in vitro (12,13). A
growing body of evidence has demonstrated that the overload of ROS,
such as H2O2, during oxidative stress may
impair cell viability and trigger myocardial cell apoptosis by
inducing DNA, protein and lipid damage (14,15).
In addition, tt has been previously documented that cardiomyocyte
cell death is a prominent pathological change post-MI, which
results in irreversible cardiac dysfunction (16).
Forkhead box O3a (FOXO3a) is a member of the O
subclass of the Forkhead family of transcription factors that has
previously been investigated as a key protein involved in the
regulation of oxidative stress, inflammation and apoptosis
(17,18). Accumulating evidence has
demonstrated that FOXO3a is closely associated with the
pathogenesis of MI (19,20). It was previously documented that the
activity of FOXO3a may be regulated by the Sirtuin (SIRT) family of
proteins. In particular, trans-sodium crocetinate, a
derivative compound of the carotenoid crocetin, has been reported
to attenuate myocardial I/R injury through SIRT3/FOXO3a signaling
(21). SIRT4 is a deacetylase that
is normally localized to the mitochondrial matrix and is highly
expressed in cardiomyocytes (22).
SIRT4 has been previously found to alleviate oxidative stress and
apoptotic damage during myocardial I/R (23).
Traditional Chinese Medicine (TCM) has long been
used for the treatment of a number of diseases. Shenfu Qiangxin
Drink (SFQXD) was created by adding and subtracting ingredients
based on the Shenfu Qiangxin Decoction as described in Good
Remedies For Women, which has been reported to effectively relieve
the clinical symptoms of patients with heart failure (24,25).
SFQXD is mainly composed of a mixture of seven herbal Chinese
medicine, including Codonopsis codonopsis (30 g),
Aconitum carmichaeli Debx (4 g), Ophiopogon japonicus
(10 g), Schisandra fructus (10 g), Polygonatum
odoratum (20 g), Semen lepidii (20 g), Semen
plantaginis (20 g) and Radix paeoniae rubra (15 g) in
specific proportions (25). To
date, SFQXD has been used to treat a variety of cardiac diseases,
including AMI and chronic heart failure (26). In addition, Shenfu injection has
been developed based on this recipe and has become an important
procedure for AMI complicated by cardiac shock (27). However, the effect of SFQXD on
SIRT4/FOXO3a signaling for the treatment of MI remains to be fully
elucidated.
In the present study, the levels of ROS and
inflammatory factors were investigated in patients with MI before
and after the administration of SFQXD. In addition, an in
vitro model of H2O2-induced myocardial
injury was established in neonatal rat cardiomyocytes to explore
the effects of SFQXD on myocardial damage and possible underlying
regulatory mechanism. The ultimate aim was to elucidate the
mechanisms underlying the effect of SFQXD on myocardial injury
during MI.
Materials and methods
Clinical sample collection
The present study involved 30 patients with acute
non-ST segment elevation MI who fulfilled the diagnostic criteria
for patients with AMI. The inclusion criteria were as follows: i)
Electrocardiogram with characteristic alterations including the
emergence of Q, the spread of ST segment elevation and the dynamic
evolution of ST-T; ii) elevated serum biomarkers for myocardial
necrosis; myocardial necrosis detected by serum biomarkers; and
iii) an intracoronary thrombus identified by angiography (28). The following exclusion criteria
applied: i) Previous history of myocardial infarction; ii) having
received percutaneous coronary intervention treatment; iii) having
acute heart failure upon admission; iv) having myocardial disease,
infectious pericarditis or pericardial disease; v) having an
infectious disease, severe diabetes mellitus, malignant tumor,
liver or kidney disease, pulmonary fibrosis, bone metabolic
disorder, systemic immune disease or complications caused by
malignant tumors; and vi) having cardiac shock. The patients (male,
17; female, 13; mean age, 45.8±10.2 years) were recruited from The
Jiangsu Provincial Hospital of Integrated Chinese and Western
Medicine (Nanjing) between February 2019 and October 2019.
Compositions of SFQXD (Beijing Tongrentang Pharma Co., Ltd.) were
mixed and boiled in ddH2O twice, with the first boiling
processing (100˚C) lasting for 1.5 h and the second lasting for 30
min, before being finally concentrated using ddH2O into
a decoction at a concentration of 0.43 g/ml.
Blood samples (5 ml of each patient) were obtained
from the patients prior to any treatment. Subsequently, the
patients received SFQXD administration (300 ml) every day for 2
weeks. SFQXD was consistently prepared in the preparation room of
Jiangsu Provincial Hospital of Integrated Chinese and Western
Medicine. Blood samples (5 ml of each patient) were obtained from
the patients after 2 weeks of treatment. Samples were immediately
frozen in liquid nitrogen at -80˚C for storage. The protocol of the
present study was approved by the Ethics Committee of Jiangsu
Provincial Hospital of Integrated Traditional Chinese and Western
Medicine (approval no. 2018LW012; Nanjing, China). All study
participants were informed on the purpose of the study and provided
written informed consent.
Primary cultures of
cardiomyocytes
A total of 10 Sprague-Dawley (SD) rats aged 1-3 days
(weight, 8-10 g; 5 males and 5 females) were provided by the Model
Animal Research Center of Nanjing University (Nanjing, China;
license number: 20181221-63). All animal procedures were performed
according to the Guide for Care and Use of Laboratory Animals
published by the United States National Institutes of Health
(29) and experimental protocols
were approved by the Jiangsu Provincial Hospital of Integrated
Chinese and Western Medicine (Nanjing, China). Neonatal rat
cardiomyocytes were prepared and cultured as described previously
(30,31). Briefly, neonatal SD rats were
euthanized using carbon dioxide (CO2) with the flow rate
displacing 20% of the chamber volume/min. Rats were exposed to 50%
CO2 until they were euthanized, which was subsequently
confirmed by decapitation. Subsequently, hearts of the neonatal
rats were removed and placed in pre-cooled (4˚C) D-Hanks' Balanced
Salt Solution (Sigma-Aldrich; Merck KGaA) under sterile conditions.
The ventricles were first excised and cut into small pieces, which
were then digested three times with 0.08% trypsin solution for 8
min at 37˚C each. After centrifugation at 1,200 x g at 4˚C for 6
min, the supernatants were discarded. The pellet was then
re-suspended and cultured in DMEM/F12 (1:1; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) with 5-Bromo-2'-deoxyuridine (0.1 mM;
Sigma-Aldrich, Merck KGaA) to prevent fibroblast proliferation in
60-mm culture dishes. The cells were cultured at 37˚C in a 95%
O2 and 5% CO2 incubator and the medium was
replaced daily. After 72-96 h, rhythmic contractions could be
observed and cells were deemed ready for subsequent
experiments.
Experimental groups and
treatments
Cardiomyocytes were treated with a series of
H2O2 concentrations (25, 50, 100, 200, 400
and 600 µM; Sigma-Aldrich, Merck KGaA) to determine the optimal
dose of H2O2 for mimicking oxidative
stress-induced injury in cardiomyocytes. Compositions in SFQXD were
concentrated into the decoction to a concentration of 0.43 g/ml as
the aforementioned. For SFQXD treatment, SFQXD was diluted using
DMEM/F12 (1:1). Cardiomyocytes were pretreated with 25, 50, 100,
200, 400 and 800 µl/ml SFQXD for 12 h at 37˚C and then incubated in
medium containing 100 µM H2O2 for another 2 h
at 37˚C. Cells in the control group (con) were cultured in complete
DMEM/F12 (1:1) for 14 h at 37˚C. Doses of 25, 50 and 100 µl/ml
SFQXD were selected for subsequent experimentation, which were
designated as model + low dose (L), model + medium dose (M) and
model + high dose (H) groups, respectively.
Cell viability assay
Cell Counting Kit-8 (CCK-8) kit (Sigma-Aldrich;
Merck KGaA) was performed to measure cell viability. The cells were
plated into 96-well plates (3,000 cells/100 µl). After treatment
with H2O2 and/or SFQXD (mentioned in the
previous section) for 14 h at 37˚C, 10 µl CCK-8 solution was added
to each well and the cells were incubated for an additional 4 h at
37˚C. Absorbance was measured at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc.).
Test for inflammatory factors and
phosphorylated (p-)-FOXO3a
Levels of inflammatory cytokines, specifically
interleukin (IL)-6 (cat. no. F01310), IL-1β (cat. no. F01220) and
tumor necrosis factor-α (TNF-α; cat. no. F02810) in serum from
patients and culture media of the cultured myocytes
(3x105 cells/well) were measured using ELISA in
accordance with the manufacturer's protocols (Shanghai Xitang
Biotechnology Co., Ltd.; http://westang.bioon.com.cn/). The levels of p-FOXO3a
(cat. no. JL49691-96T) in the serum samples of patients before and
after SFQXD treatment was determined using an ELISA kit obtained
from Shanghai Jianglai Industrial Co., Ltd. according to the
manufacturer's protocol.
Detection of intracellular ROS
Generation of intracellular ROS was examined using
2,7-dichlorofluorescein diacetate (DCFH-DA) assay. Primary
cardiomyocytes (1x106/well) were first collected and
washed with PBS three times, followed by incubation in DMEM
containing 10 µM DCFH-DA (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37˚C for 20 min in a dark chamber. After centrifugation at
800 x g at 4˚C for 5 min, fluorescence was measured using a flow
cytometer (BD Biosciences; Becton, Dickinson and Company) at 488 nm
excitation and 525 nm emission wavelength. The data analysis was
performed using BD CellQuest™ Pro Software (version 5.1; BD
Biosciences; Becton, Dickinson and Company). The quadrant(s) from
Q3 reflected the ROS levels.
Measurement of oxidative stress
markers
Primary cardiomyocytes were seeded in 6-well plates
at a density of 5x105 cells/well l and treated as
mentioned above. The culture media or cell lysate supernatants were
first collected. Levels of oxidative stress-related markers, namely
malondialdehyde (MDA; cat. no. A003-4-1), catalase (CAT; cat. no.
A007-1-1) and total-superoxide dismutase (T-SOD; cat. no.
A001-1-2), were assessed using their respective commercial kits
(Nanjing Jiancheng Bioengineering Institute), according to
colorimetric methods.
Cell transfection
Small hairpin RNA (shRNA) specific against SIRT4 (50
nM; shRNA-SIRT4-1, shRNA-SIRT4-2 and shRNA-SIRT4-3) and a
non-targeting sequence serving as a negative control (shRNA-NC)
were synthesized by Guangzhou RiboBio Co., Ltd. For transfection,
primary neonatal rat cardiomyocytes were placed in the wells of
six-well plates at 2x105 cells/well and cultured at 37˚C
until reaching 70% confluence. Lipofectamine® 200
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was utilized
to perform the transfection experiments. At 48 h post-transfection,
transfection efficacy was assessed using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Subsequently, transfected cells were treated with
H2O2 and/or SFQXD mentioned in the previous
section.
Flow cytometry analysis
Cardiomyocytes (1x106/well) were
harvested and double-stained with Annexin V-FITC (5 µl) and
propidium iodide (PI; 10 µl). The mixture was placed in the dark
for 15 min at room temperature. Cardiomyocytes were then subjected
to apoptosis assay (Nanjing KeyGen Biotech Co., Ltd.) using flow
cytometry (BD Biosciences; Becton, Dickinson and Company) and
analyzed using the CellQuest™ Pro Software (version 5.1; BD
Biosciences; Becton, Dickinson and Company).
RT-qPCR analysis
After treatment, total RNA was extracted from
primary neonatal rat cardiomyocytes using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol. Complementary DNA (cDNA) was synthesized
using PrimeScript™ RT reagent (Takara Bio, Inc.; 16˚C for 30 min,
42˚C for 30 min and 85˚C for 5 min). iTaq™ Universal
SYBR® Green Supermix (Bio-Rad Laboratories, Inc.) was
employed to conduct qPCR according to the manufacturer's protocol
in the ABI 7500 PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were
used: Initial denaturation at 95˚C for 7 min; followed by 40 cycles
of 95˚C for 15 sec and 60˚C for 30 sec; and a final extension at
72˚C for 30 sec. Sequences of the gene-specific primers used in
this study were as follows: SIRT4 forward,
5'-ACCCTGAGAAGGTCAAAGAGTTAC-3' and reverse,
5'-TTCCCCACAATCCAAGCAC-3' and GAPDH forward, 5'-ACCACAGTCCATGAA
ATCAC-3' and reverse, 5'-AGGTTTCTCCAGGCGGCATG-3'. All primers used
in the present study were synthesized by Sangon Biotech Co., Ltd.
GAPDH served as the endogenous control. Relative expression was
calculated using the 2-ΔΔCq method (32).
Western blot analysis
Total proteins were extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) in the presence
of a protease inhibitor cocktail (Beyotime Institute of
Biotechnology). Bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) was used to measure protein
concentration. Subsequently, 40 µg protein per lane was separated
via 10% SDS-PAGE and transferred onto PVDF membranes. The membranes
were then blocked with 5% non-fat milk for 1.5 h at room
temperature and incubated with primary antibodies against the
target proteins at 4˚C overnight. Following incubation with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibodies
(cat. no. 7074S; 1:5,000; Cell Signaling Technology, Inc.) for 1.5
h at room temperature, the bands were visualized using an enhanced
chemiluminescence assay (EMD Millipore) and analyzed using ImageJ
software (version 1.52r; National Institutes of Health). Anti-Bcl-2
(1:1,000; cat. no. sc-7382), anti-Bax (1:1,000; cat. no. sc-7480),
anti-Bcl-2-like protein 11 (BIM; 1:1,000; cat. no. sc-374358) and
anti-SIRT4 (1:1,000; cat. no. sc-135797) were purchased from Santa
Cruz Biotechnology, Inc. Anti-cleaved caspase-3 (1:1,000; cat. no.
9664T), anti-FOXO3a (1:1,000; cat. no. 12829S), anti-p-FOXO3a
(1:1,000; cat. no. 5538S), anti-acetyl lysine (1:1,000; cat. no.
9441S) and anti-β-actin (1:1,000; cat. no. 4970S) antibodies were
obtained from Cell Signaling Technology, Inc. β-actin was
considered as the internal control.
Statistical analysis
All experiments were repeated independently in
triplicate. All data were represented as mean values ± SD and
statistical analysis was performed using GraphPad Prism 6 (GraphPad
Software, Inc.). Comparisons between two groups in the clinical
sample analysis was evaluated using paired student's t-test.
Comparisons involving two groups and multiple samples in the cell
experiments were analyzed by unpaired t-test or one-way analysis of
variance (ANOVA) followed by Tukey's post hoc test, respectively.
P<0.05 was considered to indicate a significantly different
difference.
Results
SFQXD treatment reduces the levels of
ROS, IL-1β and p-FOXO3a in the blood of patients with AMI
To investigate the effects of SFQXD in AMI, the
levels of ROS, IL-6 and TNF-α and IL-1β in the blood samples from
patients were measured before and after SFQXD administration. SFQXD
notably reduced ROS, IL-1β, IL-6 and TNF-α levels (Fig. 1A-D). Furthermore, a significant
increase in the level of phosphorylated FOXO3a was observed in the
SFQXD after treatment group (Fig.
1E).
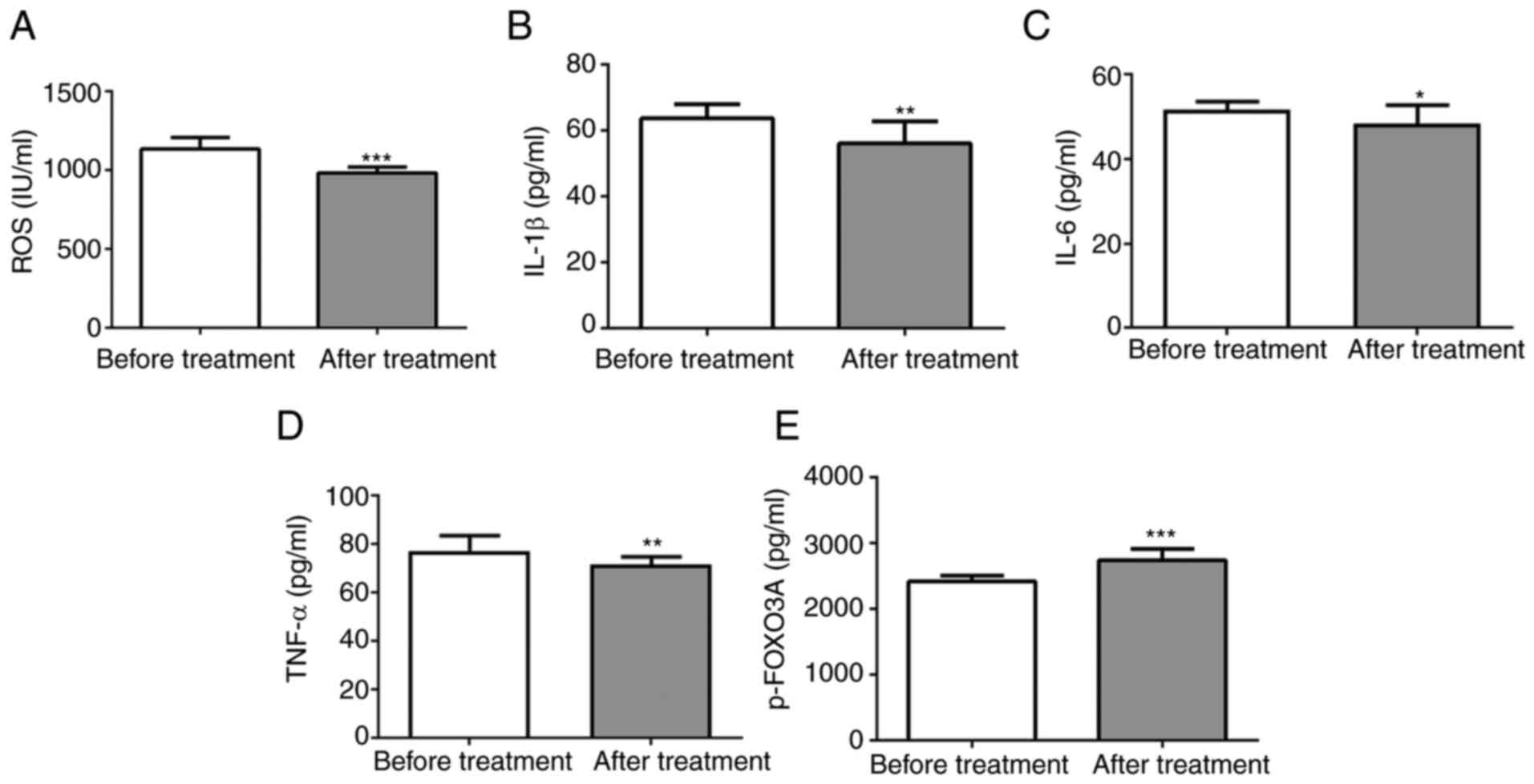 | Figure 1SFQXD treatment significantly reduces
the levels of ROS, inflammatory cytokines and FOXO3a
phosphorylation in blood samples of patients with acute myocardial
infarction. The levels of (A) ROS, (B) IL-1β (C) IL-6, (D) TNF-α
and (E) phosphorylated FOXO3a were measured using the corresponding
commercially available kits. Experimental results were generated
from three independent experimental repeats. *P<0.05,
**P<0.01 and ***P<0.001 vs. Before
treatment. SFQXD, Shenfu Qiangxin Drink; ROS, reactive oxygen
species; TNF-α, tumor necrosis factor-α; IL, interleukin; FOXO3a,
forkhead box O3a; p-, phosphorylated. |
SFQXD ameliorates oxidative stress and
inflammation in H2O2-treated neonatal rat
cardiomyocytes
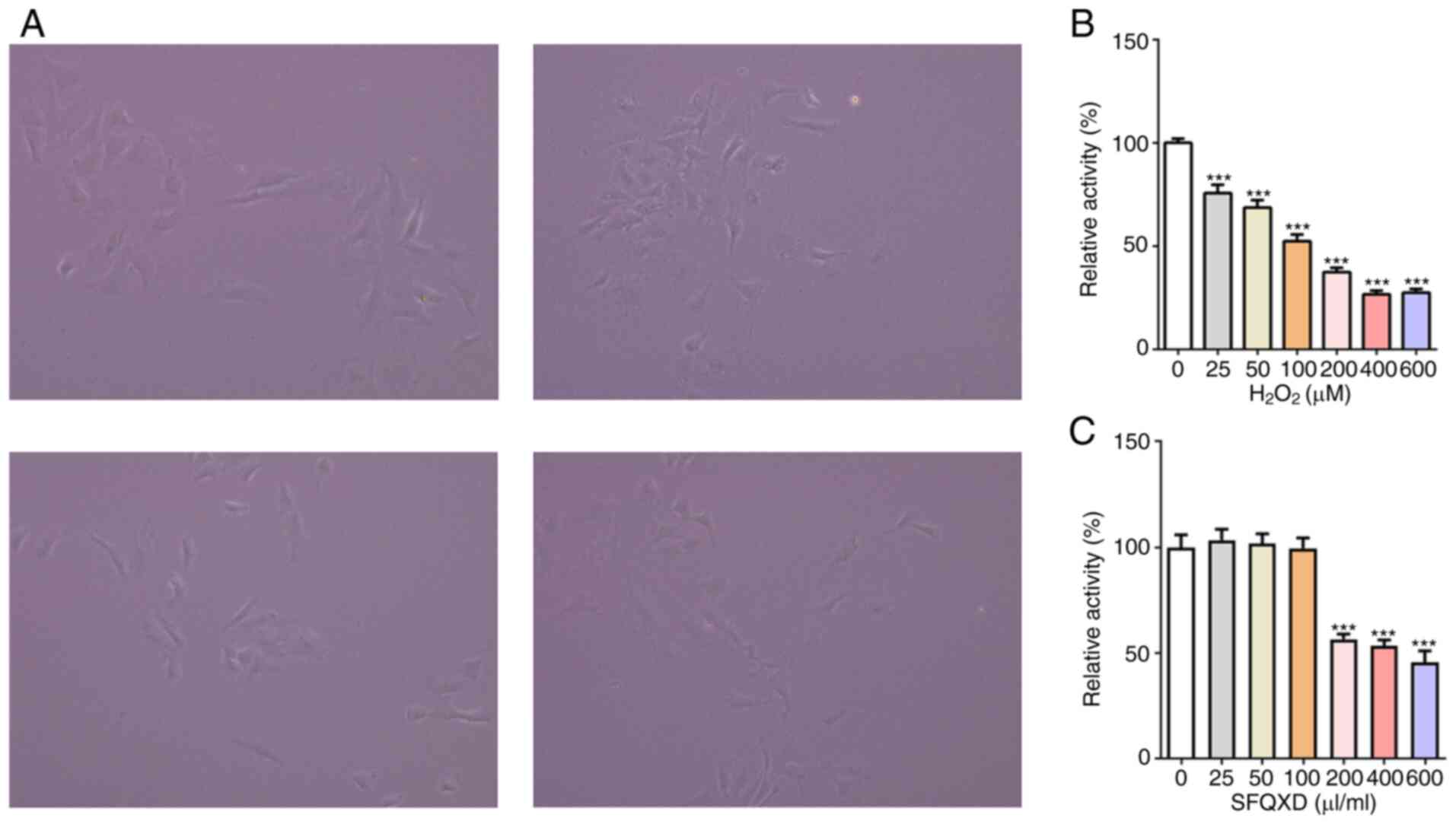
To study the mechanism of action of SFQXD in the
treatment of AMI, neonatal rat cardiomyocytes were isolated
(Fig. 2A) and a
H2O2-induced primary cardiomyocyte damage
model was established in vitro. Viability of cardiomyocytes
was reduced after stimulation with H2O2 in a
dose-dependent manner (Fig. 2B). At
100 µM H2O2, cell viability was reduced by
~50% (Fig. 2B). Therefore, this
concentration was selected for subsequent experiments. By contrast,
SFQXD at a concentration of ≥200 µl/ml exerted significant
inhibitory effects on the viability of primary cardiomyocytes
(Fig. 2C). Therefore, 25, 50 and
100 µl/ml SFQXD were selected for the following experiments.
The potential effects of SFQXD on
H2O2-induced oxidative stress was then
tested. Generation of ROS was markedly increased after
H2O2 induction, which was reversed with SFQXD
treatment in a dose-dependent manner (Fig. 3A). Consistently, SFQXD pre-treatment
reduced the concentration of MDA, whilst significantly increasing
the activities of T-SOD and CAT in a dose-dependent manner in
H2O2-induced primary cardiomyocytes compared
with those in the model group (Fig.
3B-D). Concurrently, medium and high dose SFQXD pre-treatment
group exhibited significantly lower levels of TNF-α, IL-1β and IL-6
compared with those in the model group (Fig. 3E-G). In summary, these data suggest
that SFQXD was able to ameliorate oxidative stress and inflammation
induced by H2O2 in neonatal rat
cardiomyocytes.
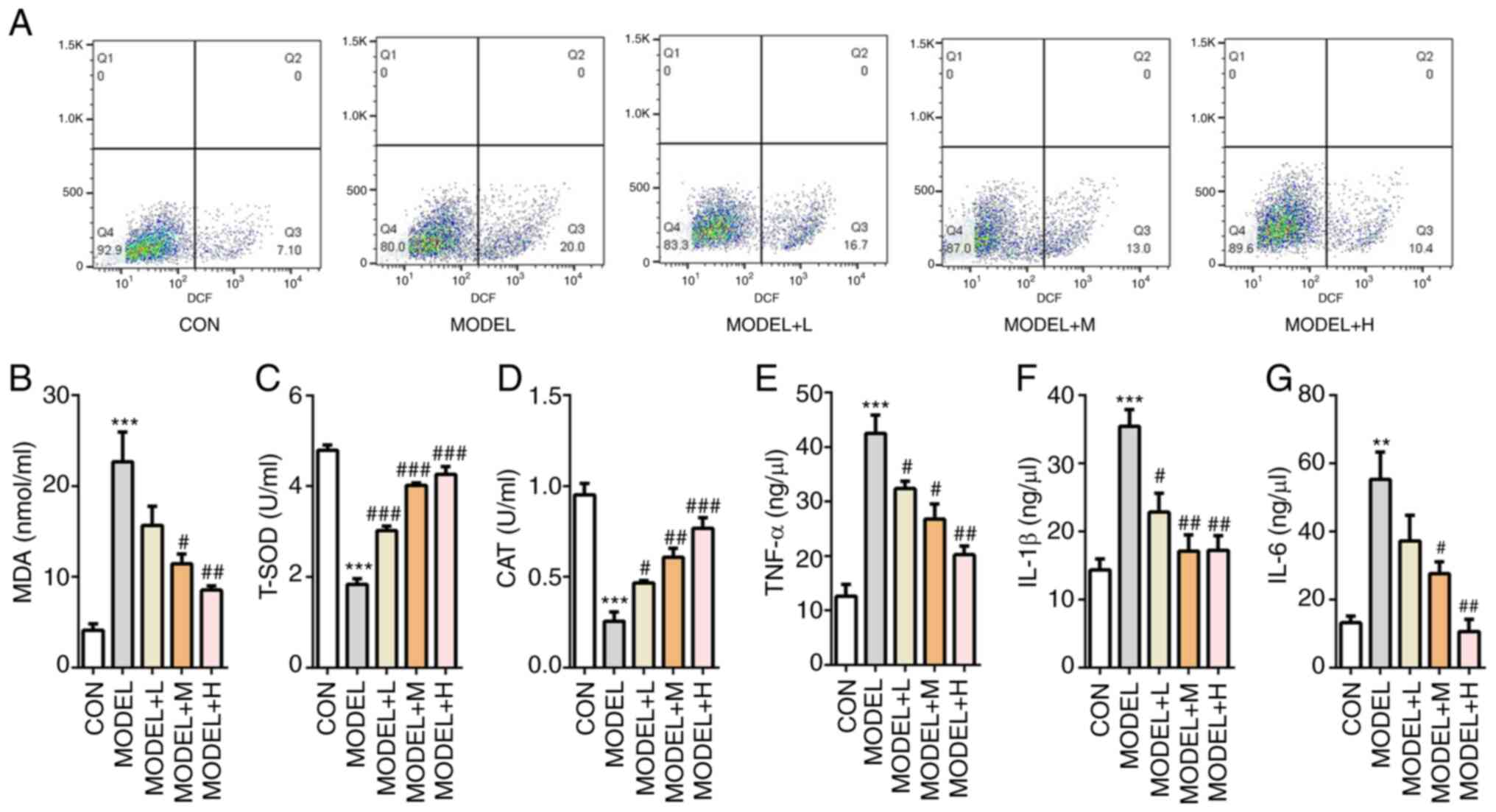 | Figure 3SFQXD ameliorates
H2O2-induced oxidative stress and
inflammation in primary cardiomyocytes. (A) Generation of
intracellular ROS was tested using 2,7-dichlorofluorescein
diacetate staining followed by flow cytometry. The levels of (B)
MDA and activities of (C) T-SOD and (D) CAT were measured using
commercially available kits by visible spectrophotometry.
Concentrations of (E) TNF-α, (F) IL-1β and (G) IL-6 were determined
using ELISA. The experimental results were generated from three
experimental independent repeats. **P<0.01 and
***P<0.001 vs. CON; #P<0.05,
##P<0.01 and ###P<0.001 vs. MODEL.
SFQXD, Shenfu Qiangxin Drink; MDA, malondialdehyde; T-SOD, total
superoxide dismutase; CAT, catalase; ROS, reactive oxygen species;
TNF, tumor necrosis factor; IL, interleukin; TNF, tumor necrosis
factor; CON, control; L, low dose; M, medium dose; H, high
dose. |
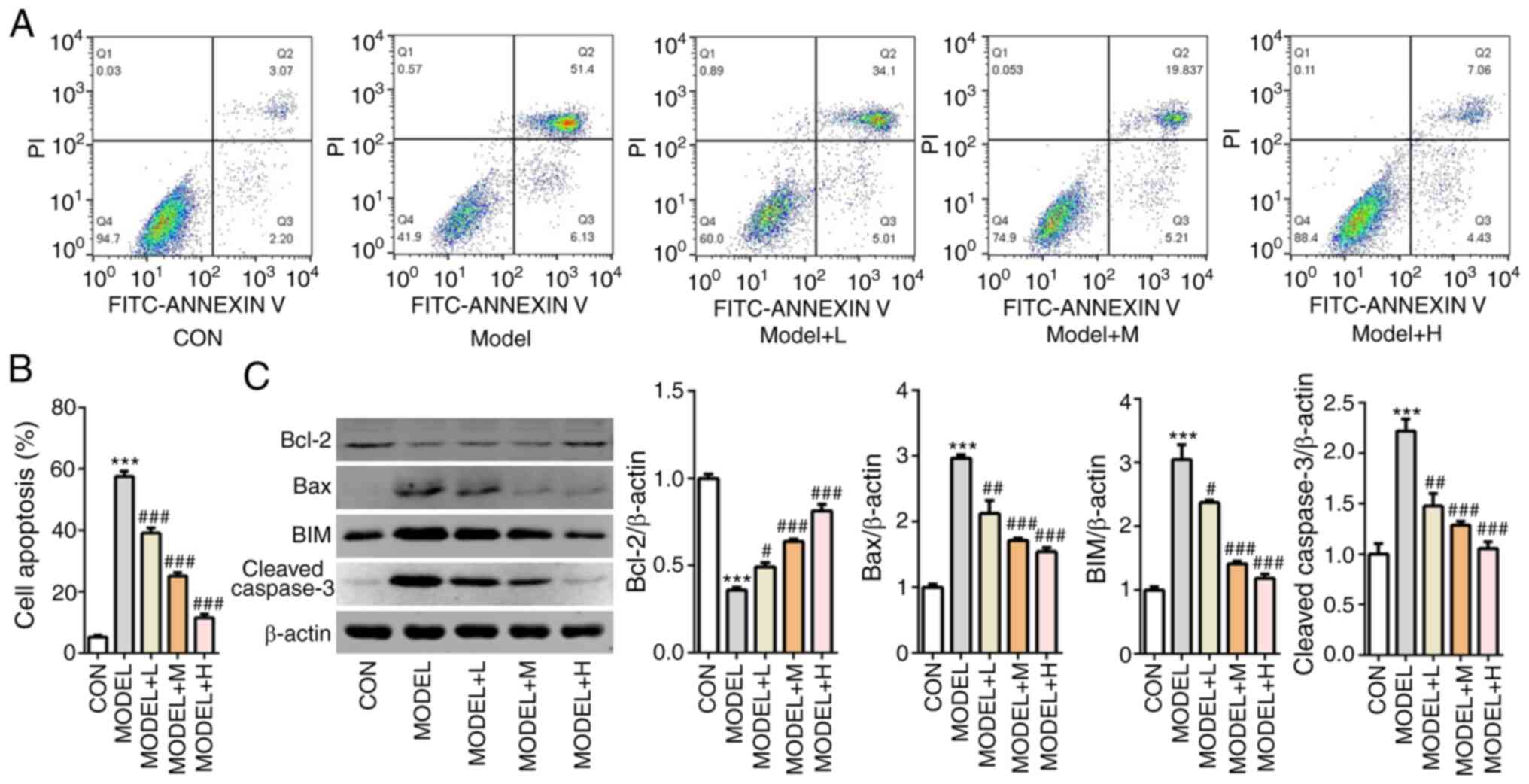
SFQXD alleviates apoptosis in
H2O2-treated neonatal rat cardiomyocytes
Subsequently, cell apoptosis was measured using flow
cytometry analysis. The number of apoptotic cells was markedly
increased in the model group compared with that the control group,
whilst SFQXD pre-treatment markedly prevented this in a
dose-dependent manner (Fig. 4A and
B). Compared with those in the
control group, the expression levels of Bcl-2 was significantly
downregulated, whilst those of Bax, BIM and cleaved caspase-3 were
significantly upregulated after the primary cardiomyocytes were
treated with H2O2 (Fig. 4C). There effects aforementioned were
significantly prevented by all three doses of SFQXD pre-treatment
(Fig. 4C). These observations
suggest that SFQXD suppressed H2O2-induced
apoptosis in primary cardiomyocytes.
SFQXD attenuates oxidative stress and
inflammation in H2O2-treated neonatal rat
cardiomyocytes via regulation of SIRT4/FOXO3a signaling
To investigate the potential regulatory mechanisms
by which SFQXD exerts its effects on
H2O2-treated neonatal rat cardiomyocytes, the
levels of SIRT4 expression, FOXO3a phosphorylation and acetylation
were measured using western blot analysis.
H2O2 treatment significantly downregulated
the levels of SIRT4 and p-FOXO3a, but significantly upregulated in
ace-FOXO3a levels compared with those in the control group
(Fig. 5). After the primary
cardiomyocytes were pretreated with SFQXD, reductions in the levels
of SIRT4 expression and FOXO3a phosphorylation and increments in
FOXO3a acetylation induced by H2O2 were
significantly prevented (Fig.
5).
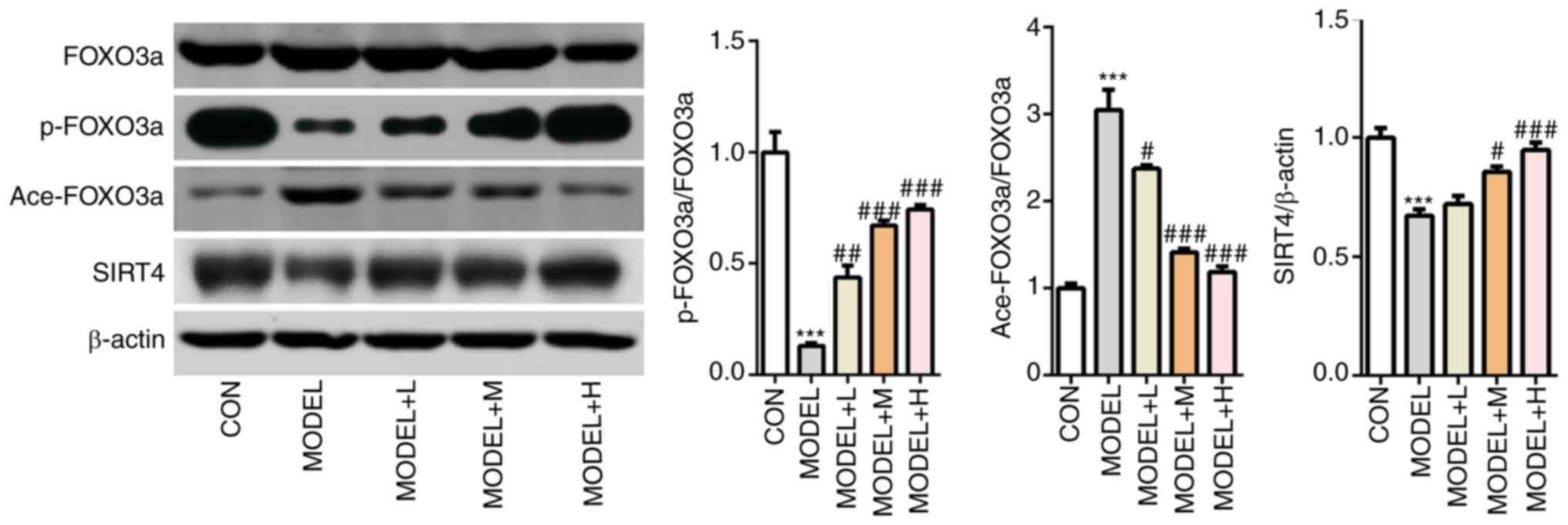 | Figure 5Shenfu Qiangxin Drink can regulate
SIRT4/FOXO3a signaling. Expression levels of SIRT4, FOXO3a
phosphorylation and FOXO3a acetylation were measured using western
blot analysis. Experimental results were obtained from three
independent experimental repeats. ***P<0.001 vs. CON;
#P<0.05, ##P<0.01,
###P<0.001 vs. MODEL. SIRT4, sirtuin-4; FOXO3a,
Forkhead box O3; p-, phosphorylated; ace-, acetylated; CON,
control; L, low dose; M, medium dose; H, high dose. |
Subsequently, SIRT4 expression was silenced by
transfection with shRNA-SIRT4. Cardiomyocytes that were transfected
with shRNA-SIRT4-1 were selected for the following experiments,
since they exhibited the lowest expression levels of SIRT4 compared
with those transfected with shRNA-NC (Fig. 6A). Among all
H2O2- and SFQXD-treated cells, it was
subsequently observed that SIRT4 silencing markedly enhanced the
levels of intracellular ROS and MDA (Fig. 6B and C), whilst significantly reducing the
activities of T-SOD and CAT (Fig.
6D and E), compared with those
in the model + H + shRNA-NC group. Similarly, the levels of the
inflammatory factors TNF-α, IL-1β and IL-6 exhibited similar trends
with ROS, all of which were significantly increased after SIRT4
knockdown compared with those after shRNA-NC transfection (Fig. 6F-H). The aforementioned findings
suggest that SFQXD was able to alleviate oxidative stress and
inflammation in H2O2-treated neonatal rat
cardiomyocytes through regulation of SIRT4/FOXO3a signaling.
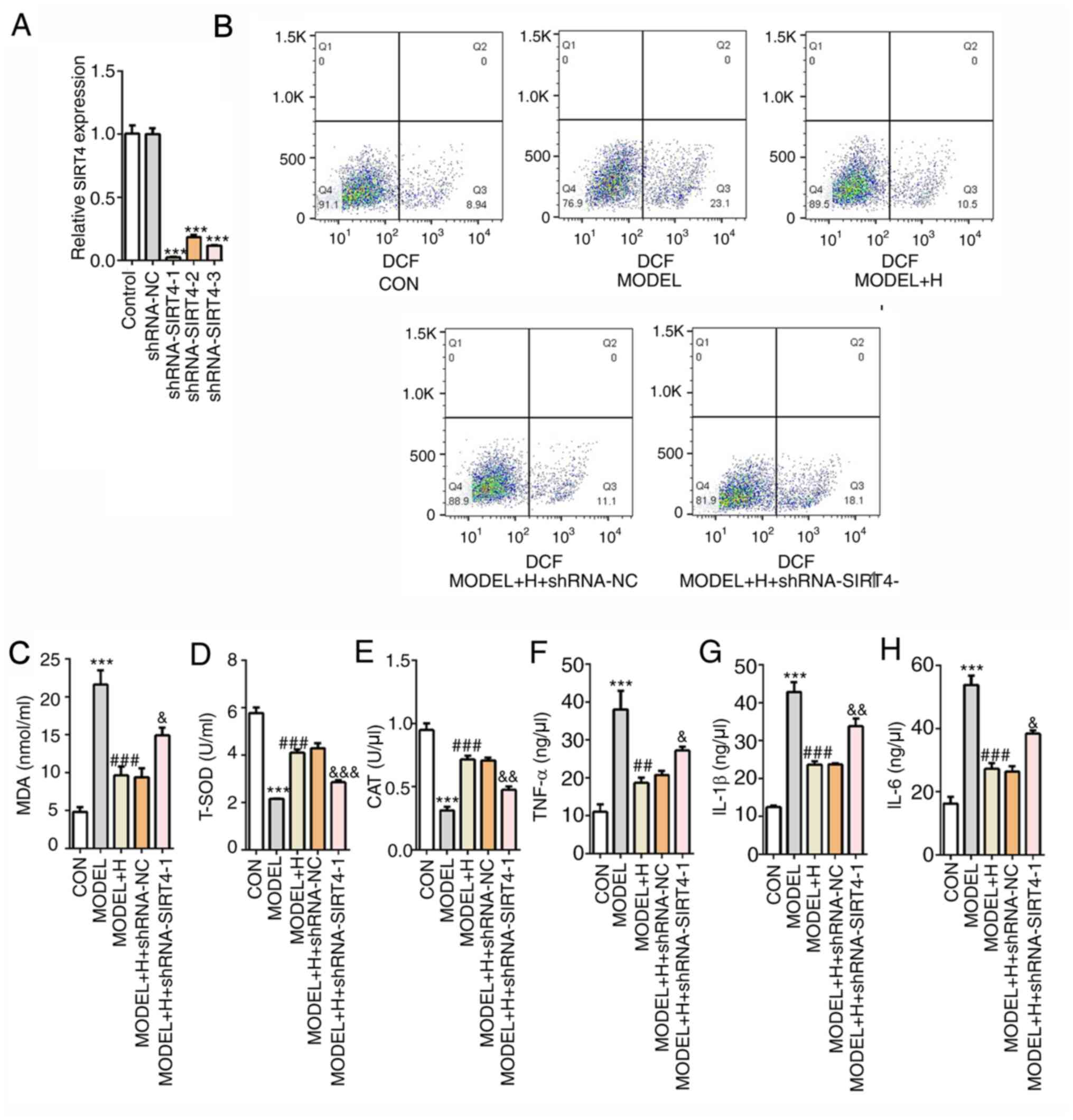 | Figure 6Shenfu Qiangxin Drink reduces
H2O2-induced oxidative stress and
inflammation in neonatal rat cardiomyocytes by regulating
SIRT4/FOXO3a signaling. (A) Expression of SIRT4 was measured using
reverse transcription-quantitative PCR after transfection with
shRNA-SIRT4. ***P<0.001 vs. shRNA-NC. (B) Generation
of intracellular reactive oxygen species was tested by staining
with 2,7-dichlorofluorescein diacetate follow by flow cytometry.
The levels of (C) MDA and activities of (D) T-SOD and (E) CAT were
determined using commercially available kits by visible
spectrophotometry, respectively. Concentrations of (F) TNF-α, (G)
IL-1β and (H) IL-6 were determined using ELISA. The experimental
results were generated from three independent experimental repeats.
***P<0.001 vs. CON; ##P<0.01 and
###P<0.001 vs. MODEL; &P<0.05,
&&P<0.01 and
&&&P<0.001 vs. MODEL + H + shRNA-NC.
SIRT4, sirtuin-4; FOXO3a, Forkhead box O3; shRNA, short hairpin
RNA; IL, interleukin; TNF, tumor necrosis factor; MDA,
malondialdehyde; T-SOD, total superoxide dismutase; CAT, catalase;
CON, control; H, high dose; NC, negative control. |
SFQXD inhibits apoptosis in
H2O2-treated neonatal rat cardiomyocytes by
regulating SIRT4/FOXO3a signaling
Next, cell apoptosis was examined by flow cytometry
analysis after SIRT4 knockdown in
H2O2-treated primary cardiomyocytes. After
H2O2 and SFQXD treatment, the apoptotic ratio
in the SIRT4-knockdown group was significantly increased compared
with that in the model + H + shRNA-NC group (Fig. 7A and B). Additionally, silencing of SIRT4
combined with SFQXD treatment in
H2O2-stimulated primary cardiomyocytes
significantly inhibited the expression of Bcl-2 whilst
significantly promoting the expression of Bax, BIM and cleaved
caspase-3 compared with those in the shRNA-NC group (Fig. 7C). The levels of SIRT4 expression
and FOXO3a phosphorylation were subsequently found to be
significantly decreased whilst FOXO3a acetylation was significantly
increased in the SIRT4-knockdown group compared with those
transfected with shRNA-NC (Fig. 8).
Collectively, these results suggest that SFQXD attenuates apoptosis
of H2O2-treated primary cardiomyocytes by
regulating SIRT4/FOXO3a signaling.
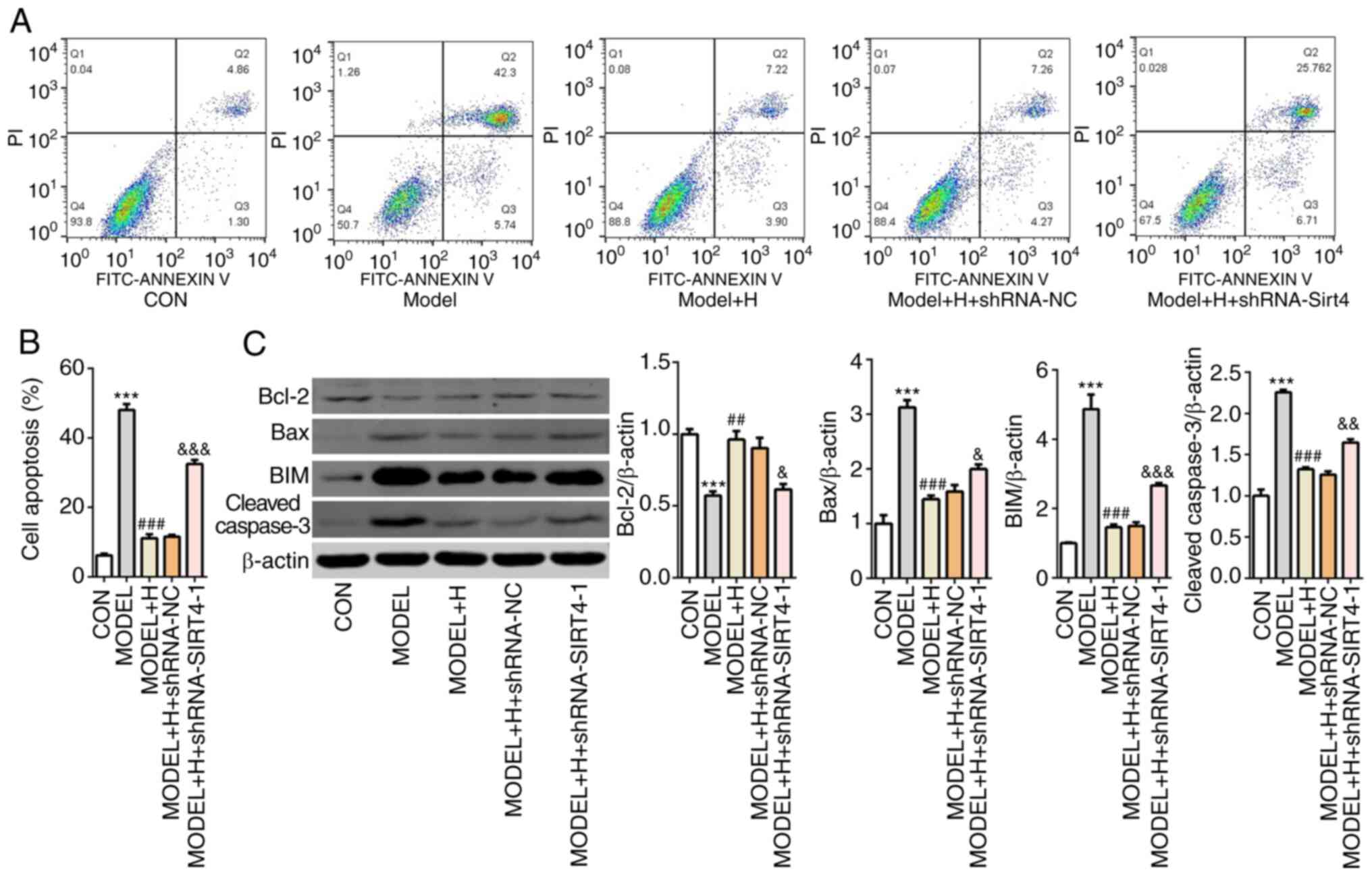 | Figure 7Shenfu Qiangxin Drink inhibits
apoptosis in H2O2-treated neonatal rat
cardiomyocytes by regulating SIRT4/FOXO3a signaling. (A) Cell
apoptosis was assessed using flow cytometry. (B) Quantification of
apoptotic rate. (C) Western blot analysis was used to measure the
expression of Bcl-2, Bax, BIM and cleaved caspase-3. Experimental
results were obtained from three independent experimental repeats.
***P<0.001 vs. CON; ##P<0.01 and
###P<0.001 vs. MODEL; &P<0.05,
&&P<0.01 and
&&&P<0.001 vs. MODEL + H + shRNA-NC.
SIRT4, sirtuin-4; FOXO3a, Forkhead box O3; shRNA, short hairpin
RNA; BIM, Bcl-2-like protein 11; CON, control; H, high dose; NC,
negative control. |
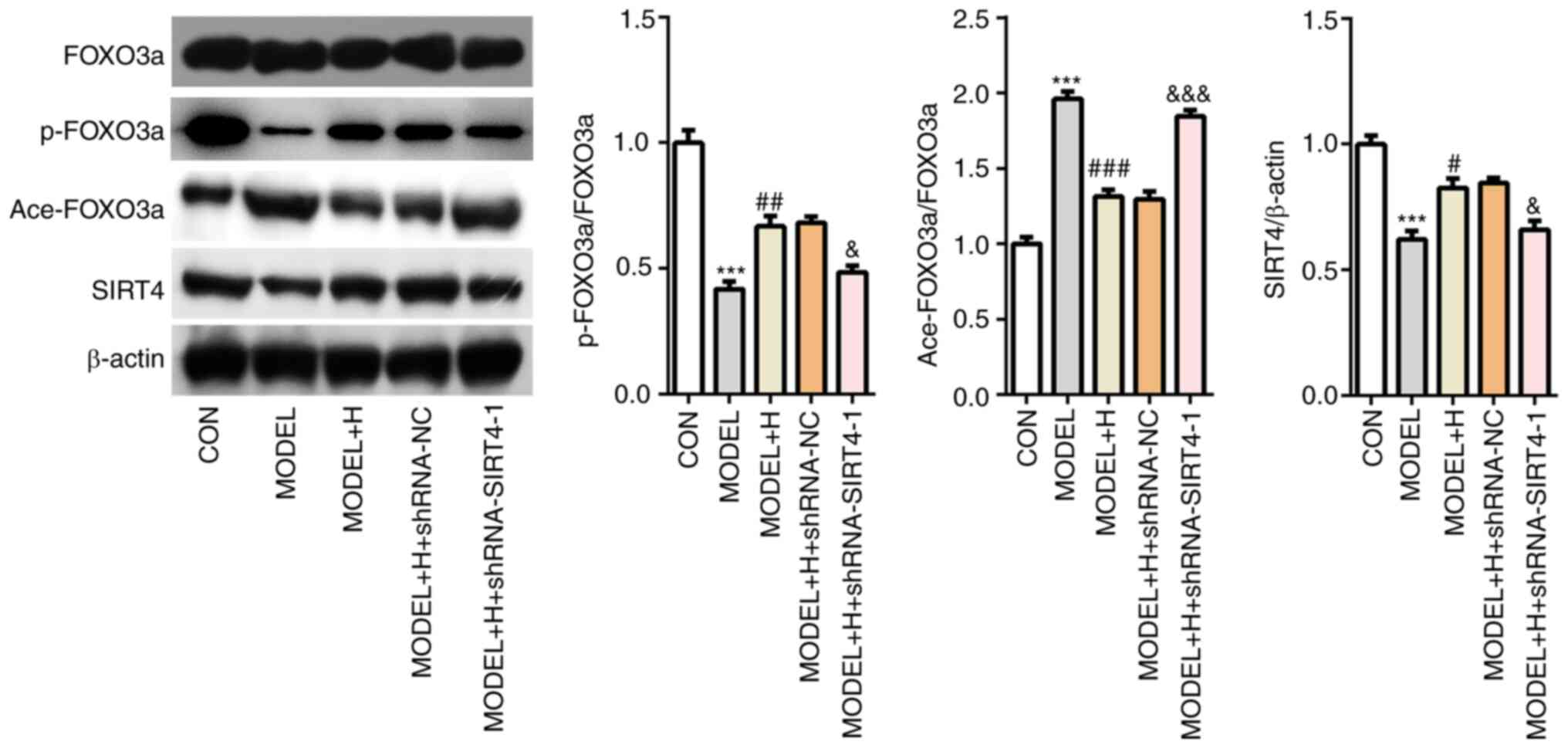 | Figure 8SIRT4 silencing reverses the effects
of Shenfu Qiangxin Drink on SIRT4/FOXO3a signaling in
H2O2-treated neonatal rat cardiomyocytes. The
expression of SIRT4, FOXO3a phosphorylation and FOXO3a acetylation
was detected using western blot analysis. The experimental results
were generated from three independent experimental repeats.
***P<0.001 vs. CON; #P<0.05,
##P<0.01 and ###P<0.001 vs. MODEL;
&P<0.05, &&&P<0.001 vs.
MODEL + H + shRNA-NC. SIRT4, sirtuin-4; FOXO3a, Forkhead box O3;
shRNA, short hairpin RNA; BIM, Bcl-2-like protein 11; CON, control;
H, high dose; NC, negative control. |
Discussion
Cardiovascular diseases, particularly AMI, have
become the leading cause of death worldwide (33). At present, TCM has been attracting
increasing attention due to its clinical application potential for
the treatment of various diseases, such as AMI. A previous study
demonstrated that the Baoyuan decoction can improve oxidative
stress-induced myocardial cell apoptosis in heart failure post-AMI
(34). In addition, Qili qiangxin
has been demonstrated to suppress the apoptosis of rat
cardiomyocytes following MI (35).
In the present study, it was demonstrated that SFQXD can alleviate
oxidative stress-induced myocardial damage, potentially through
regulation of the SIRT4/FOXO3a signaling pathway. This provides a
theoretical basis and partly elucidates the underlying mechanism
supporting the clinical application of SFQXD for the treatment of
AMI.
Oxidative stress results from the excessive
production of ROS, including O2, ·OH and
H2O2, which is implicated in the pathogenesis
of myocardial damage post-AMI (36). In particular, MDA is the end-product
of lipid peroxidation and serves a key role in the process of
oxidative stress. By contrast, T-SOD and CAT are vital antioxidant
enzymes that act against oxidative stress by removing free ROS
(37). In addition, excessive
oxidative stress can induce the overproduction of inflammatory
cytokines, including TNF-α, IL-6 and IL-1β (38). TNF-α has been reported to serve a
key role in inducing the production of free radicals, which in turn
further damages the myocardium (39). IL-6 and IL-1β are pivotal regulatory
markers that participate in the post-MI inflammatory response
(40). The present study revealed
that SFQXD alleviated the generation of ROS, MDA, TNF-α, IL-6 and
IL-1β, whilst enhancing the activities of T-SOD and CAT in a
dose-dependent manner, which was in agreement with a previous
finding which reported that SFQXD could effectively improve the
heart function of rats with heart failure (25). These results indicated the potential
protective effects of SFQXD against MI.
Accumulated evidence has demonstrated that the
imbalance between ROS production and clearance leads to myocardial
cell apoptosis by activating caspase-3 and subsequently the
mitochondria-dependent pathway (41,42).
Caspases are a family of cysteine-aspartic proteases serve a key
pro-apoptotic role (43). The Bcl-2
protein family serves as a crucial regulator of this apoptotic
pathway (44). Among these
proteins, Bax is a typical pro-apoptotic factor that can trigger
apoptosis by inducing the release of mitochondrial
apoptosis-inducing factors, such as cytochrome c, into the
cytoplasm under stress conditions, which further results in the
activation of the caspase cascade and cell apoptosis (45). Of note, the Shenfu Qiangxin
decoction has been previously shown to improve the cardiac function
of rats with heart failure (25).
The present study revealed that SFQXD dose-dependently inhibited
the apoptosis of H2O2-treated primary
cardiomyocytes.
The serine 253 residue of FOXO3a can be
phosphorylated, which subsequently results in its inactivation and
translocation into the cytoplasm, leading to the inhibition of its
transcriptional activity (46).
Acetylation of FOXO3a interrupts its nuclear translocation to
suppress its transactivation further (46). The activity of FOXO3a can be
regulated by the proteins of the Sirtuin family (47,48).
It has been reported that SIRT6 protects cardiomyocytes from I/R
injury by augmenting FOXO3a-dependent antioxidant defense
mechanisms (49). SIRT7
overexpression can reduce oxidative stress, prevent inflammatory
injury and apoptosis of cardiomyocytes in a mouse model of
cardiomyopathy (50). In addition,
results from a previous study also supported the notion that SIRT4
can alleviate oxidative stress and apoptosis damage following
myocardial I/R (23). In another
study, SIRT4 was reported to ameliorate myocardial I/R injury by
regulating mitochondria function and apoptosis (22). The present study revealed that
FOXO3a phosphorylation was significantly lower whilst FOXO3a
acetylation was notably enhanced in primary cardiomyocytes after
H2O2 treatment, suggesting that the
transcriptional activity of FOXO3a was inhibited. Additionally, the
expression of SIRT4 was markedly reduced following
H2O2 stimulation. Changes in SIRT4 expression
and post-translational modification of FOXO3 were blocked by SFQXD
treatment, suggested that the transcriptional activity of FOXO3a
was protected. SIRT4 expression was subsequently silenced to
investigate the regulatory mechanism between SIRT4 and FOXO3a. The
results indicated that the inhibitory effects of SFQXD on
H2O2-induced neonatal rat cardiomyocytes were
reversed following SIRT4 silencing. Taken together, these findings
provide evidence that SFQXD alleviates
H2O2-induced oxidative stress, inflammation
and apoptosis in neonatal rat cardiomyocytes by regulating
SIRT4/FOXO3a signaling.
In summary, to the best of our knowledge, the
present study was the first to investigate the mechanistic role of
SFQXD in H2O2-induced myocardial damage. It
was concluded that SFQXD was able to ameliorate
H2O2-induced oxidative stress, inflammation
and apoptosis in neonatal rat cardiomyocytes by regulating
SIRT4/FOXO3a signaling. However, whether this herbal mixture can
modify other signal pathways during myocardial damage remain a
topic that require further study. In addition, the lack of in
vivo animal experiments should be investigated in any future
investigations, which serve as limitations of the present study.
The findings of the present study may provide experimental data to
support the clinical application of SFQXD in restoring myocardial
function post-MI in the future.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Clinical Study
of Qiangxin Jieyu Prescription on Chronic Heart Failure Complicated
With Depressive Symptoms and its effect on Blood Serotonin fund
(grant no. JD201713).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ, YZ and XW searched the literature, designed the
experiments and conducted the experiments. LW, JS, MG and ZF
analyzed and interpreted the data. MG and ZF wrote the manuscript.
ZF revised the manuscript. SZ and ZF confirmed the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Jiangsu Province Hospital of Integrated Traditional
Chinese and Western Medicine (Nanjing, China). Written informed
consent was obtained from each patient or their legal
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mostofsky E, Maclure M, Sherwood JB,
Tofler GH, Muller JE and Mittleman MA: Risk of acute myocardial
infarction after the death of a significant person in one's life:
The determinants of myocardial infarction onset study. Circulation.
125:491–496. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Barnes M, Heywood AE, Mahimbo A, Rahman B,
Newall AT and Macintyre CR: Acute myocardial infarction and
influenza: A meta-analysis of case-control studies. Heart.
101:1738–1747. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nichols M, Townsend N, Scarborough P and
Rayner M: Cardiovascular disease in Europe 2014: Epidemiological
update. Eur Heart J. 35(2929)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yeh RW, Sidney S, Chandra M, Sorel M,
Selby JV and Go AS: Population trends in the incidence and outcomes
of acute myocardial infarction. N Engl J Med. 362:2155–2165.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Qiu H, Liu JY, Wei D, Li N, Yamoah EN,
Hammock BD and Chiamvimonvat N: Cardiac-generated prostanoids
mediate cardiac myocyte apoptosis after myocardial ischaemia.
Cardiovasc Res. 95:336–345. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dall C, Khan M, Chen CA and Angelos MG:
Oxygen cycling to improve survival of stem cells for myocardial
repair: A review. Life Sci. 153:124–131. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Asaria P, Elliott P, Douglass M, Obermeyer
Z, Soljak M, Majeed A and Ezzati M: Acute myocardial infarction
hospital admissions and deaths in England: A national follow-back
and follow-forward record-linkage study. Lancet Public Health.
2:e191–e201. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Y, Liu X, Zhang L, Li X, Zhou Z,
Jiao L, Shao Y, Li M, Leng B, Zhou Y, et al: Metformin protects
against H2O2-induced cardiomyocyte injury by
inhibiting the miR-1a-3p/GRP94 pathway. Mol Ther Nucleic Acids.
13:189–197. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mo Y, Tang L, Ma Y and Wu S: Pramipexole
pretreatment attenuates myocardial ischemia/reperfusion injury
through upregulation of autophagy. Biochem Biophys Res Commun.
473:1119–1124. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bugger H and Pfeil K: Mitochondrial ROS in
myocardial ischemia reperfusion and remodeling. Biochim Biophys
Acta Mol Basis Dis. 1866(165768)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bhatt DL and Pashkow FJ: Introduction.
Oxidative stress ad heart disease. Am J Cardiol. 101:1D–2D.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma R, Gao L, Liu Y, Du P, Chen X and Li G:
LncRNA TTTY15 knockdown alleviates
H2O2-stimulated myocardial cell injury by
regulating the miR-98-5p/CRP pathway. Mol Cell Biochem. 476:81–92.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang W, Zhang Q, Qi H, Shi P, Song C, Liu
Y and Sun H: Deletion of neuropeptide Y attenuates cardiac
dysfunction and apoptosis during acute myocardial infarction. Front
Pharmacol. 10(1268)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ning YZ, Li ZL and Qiu ZH: FOXO1 silence
aggravates oxidative stress-promoted apoptosis in cardiomyocytes by
reducing autophagy. J Toxicol Sci. 40:637–645. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tang Q, Li MY, Su YF, Fu J, Zou ZY, Wang Y
and Li SN: Absence of miR-223-3p ameliorates hypoxia-induced injury
through repressing cardiomyocyte apoptosis and oxidative stress by
targeting KLF15. Eur J Pharmacol. 841:67–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang J, Xu R, Wu J and Li Z: MicroRNA-137
negatively regulates H(2)O(2)-induced cardiomyocyte apoptosis
through CDC42. Med Sci Monit. 21:3498–3504. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen BY, Huang CC, Lv XF, Zheng HQ, Zhang
YJ, Sun L, Wang GL, Ma MM and Guan YY: SGK1 mediates the hypotonic
protective effect against H2O2-induced
apoptosis of rat basilar artery smooth muscle cells by inhibiting
the FOXO3a/Bim signaling pathway. Acta Pharmacol Sin. 41:1073–1084.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Holzhauser L, Stehr J, Jenke A, Savvatis
K, Scheibenbogen C, Schultheiss HP and Skurk C: Foxo3a dependent
adiponectin expression-implications for myocardial inflammation. J
Am Coll Cardiol. 59:E1003. 2012.
|
|
19
|
Chang GD, Chen YW, Zhang HW and Zhou W:
Trans sodium crocetinate alleviates ischemia/reperfusion-induced
myocardial oxidative stress and apoptosis via the SIRT3/FOXO3a/SOD2
signaling pathway. Int Immunopharmacol. 71:361–371. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang R, Li Y, Liu X, Qin S, Guo B, Chang
L, Huang L and Liu S: FOXO3a-mediated long non-coding RNA LINC00261
resists cardiomyocyte hypoxia/reoxygenation injury via targeting
miR23b-3p/NRF2 axis. J Cell Mol Med. 24:8368–8378. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang G, Chen Y, Zhang H and Zhou W: Trans
sodium crocetinate alleviates ischemia/reperfusion-induced
myocardial oxidative stress and apoptosis via the SIRT3/FOXO3a/SOD2
signaling pathway. Int Immunopharmacol. 71:361–371. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zeng GW, Liu H and Wang HY: Amelioration
of myocardial ischemia-reperfusion injury by SIRT4 involves
mitochondrial protection and reduced apoptosis. Biochem Biophys Res
Commun. 502:15–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zeng G, Liu H and Wang H: Amelioration of
myocardial ischemia-reperfusion injury by SIRT4 involves
mitochondrial protection and reduced apoptosis. Biochem Biophys Res
Commun. 502:15–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shaqura M, Mohamed DM, Aboryag NB, Bedewi
L, Dehe L, Treskatsch S, Shakibaei M, Schäfer M and Mousa SA:
Pathological alterations in liver injury following congestive heart
failure induced by volume overload in rats. PLoS One.
12(e0184161)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xiong L, Xie GB, Luo BH and Mei ZL: Effect
of Shenfu Qiangxin on the expression of TGF-beta/Smads signaling
pathway-related molecules in myocardium of rats with heart failure.
Eur J Inflamm. 17(7)2019.
|
|
26
|
Yan X, Wu H, Ren J, Liu Y, Wang S, Yang J,
Qin S and Wu D: Shenfu formula reduces cardiomyocyte apoptosis in
heart failure rats by regulating microRNAs. J Ethnopharmacol.
227:105–112. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jin YY, Gao H, Zhang XY, Ai H, Zhu XL and
Wang J: Shenfu injection () inhibits inflammation in patients with
acute myocardial infarction complicated by cardiac shock. Chin J
Integr Med. 23:170–175. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ren Y, Bao R, Guo Z, Kai J, Cai CG and Li
Z: miR-126-5p regulates H9c2 cell proliferation and apoptosis under
hypoxic conditions by targeting IL-17A. Exp Ther Med.
21(67)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guide for the Care and Use of Laboratory
Animals. NIH Publication: No. 85-23, 1996.
|
|
30
|
Chang JC, Lien CF, Lee WS, Chang HR, Hsu
YC, Luo YP, Jeng JR, Hsieh JC and Yang KT: Intermittent hypoxia
prevents myocardial mitochondrial Ca2+ overload and cell
death during ischemia/reperfusion: The role of reactive oxygen
species. Cells. 8(564)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qin X, Gao S, Yang Y, Wu L and Wang L:
MicroRNA-25 promotes cardiomyocytes proliferation and migration via
targeting Bim. J Cell Physiol. 234:22103–22115. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Han XJ, Li H, Liu CB, Luo ZR, Wang QL, Mou
FF and Guo HD: Guanxin danshen formulation improved the effect of
mesenchymal stem cells transplantation for the treatment of
myocardial infarction probably via enhancing the engraftment. Life
Sci. 233(116740)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang Y, Li C, Meng H, Guo D, Zhang Q, Lu
W, Wang Q, Wang Y and Tu P: BYD ameliorates oxidative
stress-induced myocardial apoptosis in heart failure post-acute
myocardial infarction via the P38 MAPK-CRYAB signaling pathway.
Front Physiol. 9(505)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xiao J, Deng SB, She Q, Li J, Kao GY, Wang
JS and Ma YU: Traditional Chinese medicine Qili qiangxin inhibits
cardiomyocyte apoptosis in rats following myocardial infarction.
Exp Ther Med. 10:1817–1823. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Riba A, Deres L, Eros K, Szabo A, Magyar
K, Sumegi B, Toth K, Halmosi R and Szabados E: Doxycycline protects
against ROS-induced mitochondrial fragmentation and ISO-induced
heart failure. PLoS One. 12(e0175195)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Raish M, Ahmad A, Ansari MA, Alkharfy KM,
Ahad A, Khan A, Ali N, Ganaie MA and Hamidaddin MAA: Beetroot juice
alleviates isoproterenol-induced myocardial damage by reducing
oxidative stress, inflammation, and apoptosis in rats. 3 Biotech.
9(147)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yu ZP, Yu HQ, Li J, Li C, Hua X and Sheng
XS: Troxerutin attenuates oxygen-glucose deprivation and
reoxygenation-induced oxidative stress and inflammation by
enhancing the PI3K/AKT/HIF-1α signaling pathway in H9C2
cardiomyocytes. Mol Med Rep. 22:1351–1361. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hu J, Cheng P, Huang GY, Cai GW, Lian FZ,
Wang XY and Gao S: Effects of Xin-Ji-Er-Kang on heart failure
induced by myocardial infarction: Role of inflammation, oxidative
stress and endothelial dysfunction. Phytomedicine. 42:245–257.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhou R, Gao J, Xiang C, Liu Z, Zhang Y,
Zhang J and Yang H: Salvianolic acid A attenuated myocardial
infarction-induced apoptosis and inflammation by activating Trx.
Naunyn Schmiedebergs Arch Pharmacol. 393:991–1002. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bashar T and Akhter N: Study on oxidative
stress and antioxidant level in patients of acute myocardial
infarction before and after regular treatment. Bangladesh Med Res
Counc Bull. 40:79–84. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li C, Chen L, Song M, Fang Z, Zhang L,
Coffie JW, Zhang L, Ma L, Wang Q, Yang W, et al: Ferulic acid
protects cardiomyocytes from TNF-α/cycloheximide-induced apoptosis
by regulating autophagy. Arch Pharm Res. 43:863–874.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mitupatum T, Aree K, Kittisenachai S,
Roytrakul S, Puthong S, Kangsadalampai S and Rojpibulstit P: mRNA
expression of Bax, Bcl-2, p53, Cathepsin B, Caspase-3 and Caspase-9
in the HepG2 cell line following induction by a novel monoclonal Ab
Hep88 mAb: Cross-talk for paraptosis and apoptosis. Asian Pac J
Cancer Prev. 17:703–712. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhao GC, Zhang XL, Wang H and Chen Z: Beta
carotene protects H9c2 cardiomyocytes from advanced glycation end
product-induced endoplasmic reticulum stress, apoptosis, and
autophagy via the PI3K/Akt/mTOR signaling pathway. Ann Transl Med.
8(647)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xin S and Ye X: Oxalomalate regulates the
apoptosis and insulin secretory capacity in streptozotocin-induced
pancreatic beta-cells. Drug Dev Res. 81:437–443. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Fu B, Zhao J, Peng W, Wu H and Zhang Y:
Resveratrol rescues cadmium-induced mitochondrial injury by
enhancing transcriptional regulation of PGC-1α and SOD2 via the
Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem Biophys Res Commun.
486:198–204. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sun W, Qiao WX, Zhou B, Hu Z, Yan Q, Wu J,
Wang R, Zhang Q and Miao D: Overexpression of Sirt1 in mesenchymal
stem cells protects against bone loss in mice by FOXO3a
deacetylation and oxidative stress inhibition. Metabolism.
88:61–71. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang H, Zhao Z, Pang X, Yang J, Yu H,
Zhang Y, Zhou H and Zhao J: MiR-34a/sirtuin-1/foxo3a is involved in
genistein protecting against ox-LDL-induced oxidative damage in
HUVECs. Toxicol Lett. 277:115–122. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang XX, Wang XL, Tong MM, Gan L, Chen H,
Wu SS, Chen JX, Li RL, Wu Y, Zhang HY, et al: SIRT6 protects
cardiomyocytes against ischemia/reperfusion injury by augmenting
FoxO3α-dependent antioxidant defense mechanisms. Basic Res Cardiol.
111(13)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Vakhrusheva O, Smolka C, Gajawada P,
Kostin S, Boettger T, Kubin T, Braun T and Bober E: Sirt7 increases
stress resistance of cardiomyocytes and prevents apoptosis and
inflammatory cardiomyopathy in mice. Circ Res. 102:703–710.
2008.PubMed/NCBI View Article : Google Scholar
|