Introduction
Cerebral infarction is a relatively common critical
disease in clinic, which may induce hypoxic-ischemic injury of
brain tissues and neurons to cause serious damage to the nervous
system. For patients with cerebral infarction, the central nervous
system injury induced by the disease brings about severe
consequences, and the patients often experience limb motor
dysfunction, speech disorder and even the risk of death (1,2).
Therefore, cerebral infarction seriously threatens the life, health
and quality of life of human beings and brings heavy economic
burdens to the patients' families and society. Therefore, it is
very important to effectively treat cerebral infarction, promote
repair of nervous system injury following cerebral infarction and
ameliorate limb motor dysfunction, speech disorder and other
complications of cerebral infarction (3,4).
At present, it is hypothesized that nerve cell
apoptosis, a crucial pathological response, is associated with the
repair of the nervous system following cerebral infarction injury
and functional restoration of the patients. In particular, massive
nerve cell apoptosis may severely affect the patients' limb motor
function, sensory function and speech function (5,6).
Decreasing nerve cell apoptosis following cerebral infarction is
conducive to protecting the nerve cells and facilitating the repair
of nervous system injury to a certain extent. Furthermore, it may
aid in restoring relevant functions, including limb motor function
and speech function following cerebral infarction. Therefore,
efficacious anti-apoptosis of nerve cells is regarded as one of the
keys to the treatment of cerebral infarction.
As a vital signal transduction pathway, the c-Jun
N-terminal kinase (JNK)/p38 mitogen-activated protein kinase (MAPK)
signaling pathway has correlations with multiple physiological and
pathological responses, including cell apoptosis, proliferation and
necrosis (7,8). It is argued at present that the
JNK/p38 MAPK signaling pathway serves as an important signaling
pathway regulating cell apoptosis. A large number of cytokines
following cerebral infarction injury may significantly activate the
JNK/p38 MAPK signaling pathway to promote the massive nerve cell
apoptosis in the brain, thereby triggering nervous system damage.
Butylphthalide, a brain protecting agent commonly used in clinic,
has certain protective effects on the nerve cells following
cerebral infarction, but its mechanism of action remains unclear.
Therefore, the present study aims to investigate the influence of
butylphthalide on nerve cell apoptosis in rats with cerebral
infarction through the JNK/p38 MAPK signaling pathway.
Materials and methods
Laboratory animals
A total of 36 male one-month-old SPF Laboratory
Sprague-Dawley (SD) rats, weighing 240-260 g, were purchased from
Shanghai Laboratory Animal Co., Ltd. (license no. SCXK; 2014-0003).
Rats were housed in a temperature-controlled room (21±2˚C) with a
relative humidity range of 30-40% on a 12/12 h light/dark cycle
(lights on at 06:00 a.m.). All rats had free access to water and
food. The present study was approved by the Animal Ethics Committee
of Zhejiang University School of Medicine Animal Center.
Experimental reagents and
instruments
Butylphthalide injection was obtained from NBP
Pharmaceutical Co. Ltd. The following primary antibodies:
Anti-phosphorylated (p)-p38 MAPK (cat. no. ab170099), p38 MAPK
(cat. no. ab31828), anti-p-JNK (cat. no. ab124956), anti-JNK (cat.
no. ab76125), anti-Bcl-2 (cat. no. ab182858) and anti-Bax (cat. no.
ab32503), and secondary antibodies, were obtained from Abcam. An
immunohistochemistry kit and fluorescence quantitative polymerase
chain reaction (qPCR) instrument were obtained from Applied
Biosystems; Thermo Fisher Scientific, Inc. A light microscope was
obtained from Leica Microsystems GmbH and qPCR-related kits
(including TRIzol reagent (Thermo Fisher Scientific, Inc.), Primer
Script real-time (RT) reagent kit with gDNA Eraser, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
miRNA measurement kit were from Thermo Fisher Scientific, Inc.
Animal grouping, processing and
modeling
The aforementioned 36 SD rats were randomly divided
into a sham-operation group (n=12), a model group (n=12) and a
butylphthalide group (n=12). The rats were fed in the Zhejiang
University Animal Center. Rats were housed in a
temperature-controlled room (21±2˚C) with a relative humidity range
of 30-40% on a 12/12 h light/dark cycle (lights on at 06:00 a.m.).
All rats had free access to water and food.
Only the common carotid artery, internal carotid
artery and external carotid artery were exposed in the
sham-operation group, while the model of cerebral infarction was
established in the model group and the butylphthalide group.
Following surgery, the rats in the butylphthalide group were
intraperitoneally injected with butylphthalide (4.5 mg/kg) every
day, and those in the sham-operation group and the model group were
subjected to a daily intraperitoneal injection of an equal volume
of normal saline. Specimens were obtained following 7 consecutive
days of intervention.
Establishment of cerebral infarction
model (9)
Pentobarbital sodium at a dose of 40 mg/kg was
intraperitoneally injected for anesthesia. Subsequently, the rats
were fixed in the supine position, and the neck was depilated and
then covered with a sterile towel. Next, a longitudinal incision
(~2 cm) was made at the anteromedian line of the neck, and the
common carotid artery, external carotid artery and internal carotid
artery were separated and exposed carefully. Subsequently, the
common carotid artery and external carotid artery were ligated with
silk sutures, and the internal carotid artery was clipped using
vascular forceps. The suture was inserted into the ligated site of
the common carotid artery, the vascular forceps at the internal
carotid artery were released, and the suture was slowly pushed into
the branch of middle cerebral artery. Later, the internal carotid
artery was ligated again and fixed by the suture, and the incision
was sutured following flushing with normal saline. Next, timing was
commenced and the suture was withdrawn slowly at 90 min after
vascular occlusion.
Specimen acquisition
Following successful anesthesia, specimens were
directly obtained from 6 rats in each group. Specifically, the
brain tissues were removed directly, washed with normal saline, put
into EP tubes and stored at -80˚C for subsequent use. As for the
remaining 6 rats in each group, the specimens were acquired via
perfusion-fixation. Specifically, the thoracic cavity of the rat
was cut open to expose the heart, and 400 ml 4% paraformaldehyde
was perfused from the left atrial appendage. After 24 h, the brain
tissues were removed and soaked in 4% paraformaldehyde solution at
20˚C for fixation for 24 h.
Zea-Longa score
At the beginning of feeding, 7 days after feeding,
following modeling and at 2 weeks following intervention, the
neurological deficits of the rats were assessed using the Zea-Longa
score (10) on the basis of the
symptoms and manifestations of the rats. The Zea-Longa scores are
presented in Table I.
 | Table IZea-Longa score. |
Table I
Zea-Longa score.
| Score | Symptom |
|---|
| 0 point | No neurological
deficits |
| 1 point | Mild: Unable to
extend the right front paw completely when the tail is lifted |
| 2 points | Moderate: Circle to
the right when walking |
| 3 points | Severe: Tumble to the
right when walking |
| 4 points | Unable to walk
spontaneously, with loss of consciousness |
Hematoxylin and eosin (H&E)
staining
The tissues previously embedded in paraffin were
sliced into 5 µm-thick sections, spread in warm water at 42˚C,
collected and baked, so as to prepare the paraffin-embedded
sections. Next, the sections obtained were soaked in xylene
solution and a gradient of alcohol for routine deparaffinization
until rehydration. Next, the sections were stained with hematoxylin
dye for 5 min at 25˚C using the H&E staining kit and then
placed in pure water for 10 min, followed by color separation in
95% ethanol for 5 sec, transparentizing with xylene for 10 sec and
mounting in neutral balsam.
Immunohistochemistry
The process of obtaining paraffin-embedded tissue
sections was the same as adorementioned. Subsequently, the
aforementioned sections were immersed in citric acid buffer
solution and heated repeatedly in a microwave oven for 3 times
(heating for 3 min and braising for 5 min every time), so as to
realize sufficient antigen retrieval. Following rinsing, 0.3%
endogenous peroxidase blocker (hydrogen peroxide; Beyotime
Institute of Biotechnology) was added dropwise onto the specimens
and reacted for 10 min. Next, the specimens were rinsed and goat
serum was added for sealing for 20 min. Anti-Bax primary antibody
(dilution, 1:200) and anti-Bcl-2 primary antibody (dilution, 1:200)
were added after the goat serum blocking buffer was shaken off,
followed by culture in a refrigerator at 4˚C overnight. The next
day, the specimens were rinsed and secondary goat anti-rabbit (HRP)
IgG antibody (1:1,000; cat. no. ab6721; Abcam,) was added dropwise
for 10 min. Following rinsing adequately, streptavidin-peroxidase
solution was added for reaction for 10 min, followed by color
development with 3,3'-diaminobenzidine added in drops,
counterstaining of the nucleus at 25˚C for 5 min with hematoxylin,
mounting and observation (magnification, x200). Images were
visualized and captured using a light microscopy (Nikon
Corporation).
Western blotting (WB) assay
The RIPA lysis buffer (Beyotime Institute of
Biotechnology) was added to the cryopreserved brain tissues in an
ice bath for 1 h. Next, the tissues were centrifuged at 4˚C, 14,000
x g for 10 min, and the protein was quantified using the
bicinchoninic acid method (Pierce; Thermo Fisher Scientific, Inc.).
Next, the absorbance and standard curve of the protein were
obtained through a microplate reader and were applied to calculate
the protein concentration in tissues. Subsequently, the proteins
(20 mg/ml) in the tissue specimens were subjected to denaturation
and separation via 10% SDS-PAGE. The position of the Marker
proteins was observed, and the electrophoresis was stopped when the
Marker proteins reached the bottom of glass plate in a straight
line. Next, the proteins were transferred onto a polyvinylidene
fluoride membrane (EMD Millipore) and reacted with blocking buffer
(skimmed milk) at 25˚C for 1.5 h. Subsequently, the membranes were
incubated overnight at 4˚C with anti-p-p38 MAPK (dilution,
1:1,000), anti-p38 MAPK (dilution, 1:1,000), anti-p-JNK (dilution,
1:1,000) and anti-JNK primary antibodies (dilution, 1:1,000), prior
to incubation at 25˚C for 2 h with secondary goat anti-rabbit (HRP)
IgG antibody (dilution: 1:1,000; cat. no. ab6721, Abcam). Finally,
the image was fully developed using a chemiluminescent reagent
(Beyotime Institute of Biotechnology) in the dark for 1 min after
rinsing.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The brain tissues stored for use were added into the
RNA extraction reagent (TRIzol, Invitrogen; Thermo Fisher
Scientific, Inc.) to extract the total RNA from the specimens.
Next, total RNA was reverse transcribed into cDNA at 50˚C for 45
min by using PrimeScript RT reagent kit (Takara Biotechnology Co.,
Ltd.), according to the manufacturer's protocol. RT-qPCR was then
performed based on the instructions of SYBR Premix Ex Taq™ (Takara
Bio, Inc.), with 3 replicates in each group. Thermocycling
conditions were as follows: Reaction at 53˚C for 5 min,
pre-denaturation at 95˚C for 10 min, denaturation at 95˚C for 10
sec and annealing at 62˚C for 30 sec, for 35 cycles in total. ∆Cq
was calculated first, and the difference in the expression of
target genes was calculated (11).
The detailed primer sequences are presented in Table II.
 | Table IIPrimer sequences. |
Table II
Primer sequences.
| Name | Primer sequence |
|---|
| Bax | F:
5'-GTGGCGGGACATAGTCAGCA-3' |
| | R:
5'-CCCATTGGGACAGCTTGGA-3' |
| Bcl-2 | F:
5'-AACCTTCCTTCCTGCTTGAG-3' |
| | R:
5'-TGCTGTTCCTCTTCCAACG-3' |
| GAPDH | F:
5'-ACGGCAAGTTCAACGGCACAG-3' |
| | R:
5'-GAAGACGCCAGTAGACTCCACGAC-3' |
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
Sections were fixed using 4% paraformaldehyde at
20˚C for 30 min. 50 µl TUNEL reagent (Beyotime Institute of
Biotechnology; cat. no. C1088) was added to each section for
incubation for 60 min at 37˚C. Nuclear staining reagent and
mounting medium used were also from One Step TUNEL Apoptosis Assay
Kit (Beyotime Institute of Biotechnology). The kit was used
according to the manufacturer's protocols. Nuclear stain was added
to each section for incubation for 5 min at 37˚C. After staining, a
fluorescent microscope (magnification, x200) (IX70, Olympus
Corporation) was used to observe the images (5 fields of view). The
tissues previously embedded in paraffin were prepared into 5
µm-thick paraffin-embedded sections following spreading in warm
water at 42˚C, collection and baking at 37˚C for 1 h, followed by
soaking in xylene solution and a gradient of ethanol for routine
deparaffinization until rehydration. Subsequently, the cell
apoptosis in the brain tissues was detected according to the
instructions of the TUNEL apoptosis kit.
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used for
statistical analysis, and the enumeration data are expressed as the
mean ± standard deviation. Comparison between multiple groups was
performed using one-way analysis of variance followed by the
Bonferroni post hoc test. P<0.05 was used to indicate a
statistically significant difference.
Results
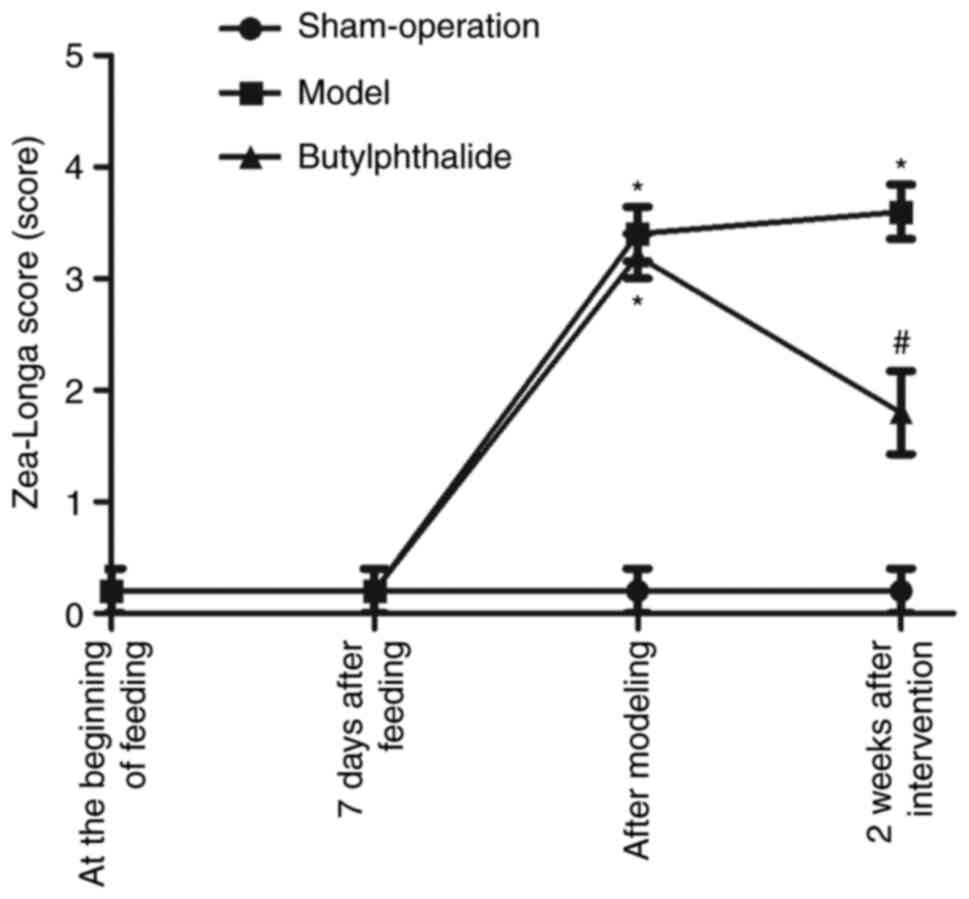
Zea-Longa score
At the beginning of feeding and 7 days after
feeding, there was no statistically significant difference in
Zea-Longa score among the three groups. Following modeling,
compared with the sham-operation group, the model group and the
butylphthalide group had notably increased Zea-Longa scores
(P<0.05), while there was no statistical difference between the
model group and the butylphthalide group. At 2 weeks after
intervention, compared with the sham-operation group, the model
group and the butylphthalide group had significantly increased
Zea-Longa scores (P<0.05), while the butylphthalide group
exhibited a significantly decreased Zea-Longa score in comparison
with the model group (P<0.05; Fig.
1).
Immunohistochemistry
The neuronal structure was clear and intact in the
sham-operation group, with abundant Nissl bodies. In the model
group, there was disturbance in the neuronal structure, fewer Nissl
bodies and partial cleavage. The neuronal morphology was improved
distinctly in the butylphthalide group compared with that in model
group, with a larger number of Nissl bodies and improved
morphology. The cells with positive expression were sepia. There
was low positive expression of Bax and high positive expression of
Bcl-2 in the sham-operation group. By contrast, the model group had
more positive expression of Bax and less positive expression of
Bcl-2. Compared with the model group, the positive expression of
Bax was weakened and the positive expression of Bcl-2 was enhanced
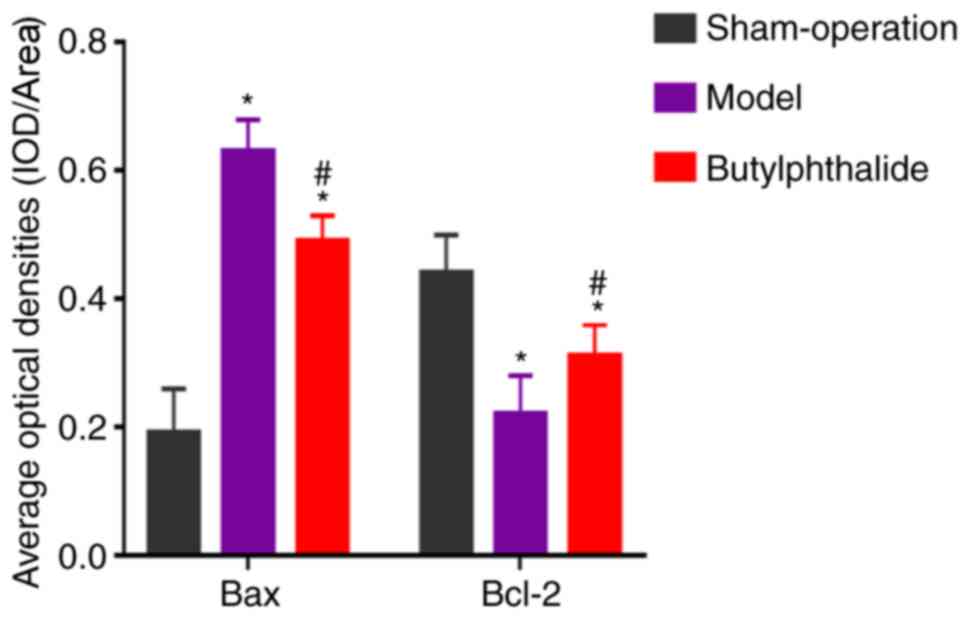
in the butylphthalide group. The statistical results (Fig. 2) indicated that the average optical
density of the positive expression of Bax was significantly
increased, while that of Bcl-2 was significantly decreased in the
model group and the butylphthalide group, compared with those in
sham-operation group (P<0.05). In comparison with the model
group, the butylphthalide group exhibited significantly decreased
average optical density of the positive expression of Bax and
significantly increased average optical density of the positive
expression of Bcl-2 (P<0.05).
Relative expression of associated
proteins detected via WB
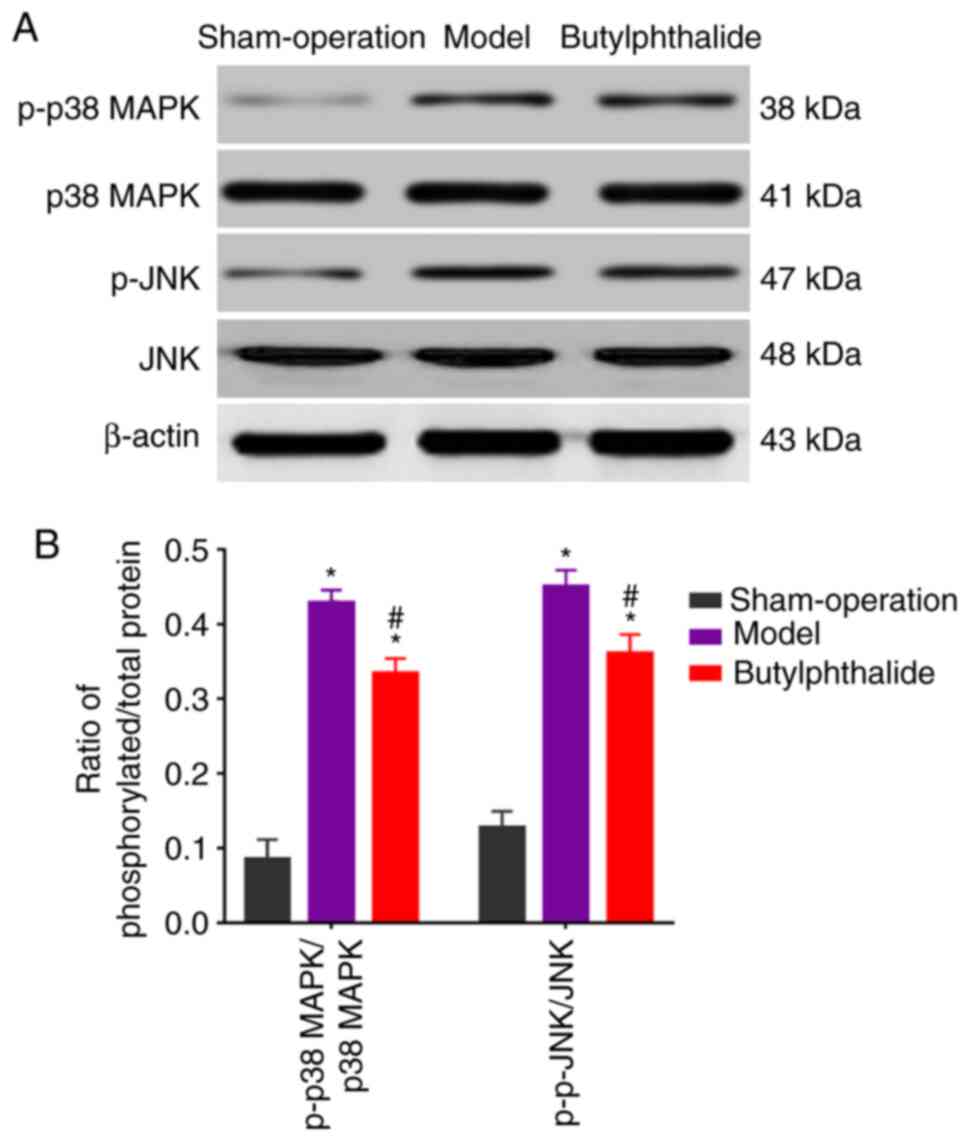
The protein expression of p-p38 MAPK and p-JNK were
low in the sham-operation group, but high in the remaining groups.
However, they were decreased in the butylphthalide group, compared
with those in the model group (Fig.
3A). The statistical results (Fig.
3B) demonstrated that the model group and the butylphthalide
group had significantly higher relative protein expression levels
of p-p38 MAPK and p-JNK than those in the sham-operation group
(P<0.05). Furthermore, the butylphthalide group displayed
notably lower relative protein expression levels of p-JNK and p-p38
MAPK than the model group (P<0.05).
mRNA expressions detected via
qPCR
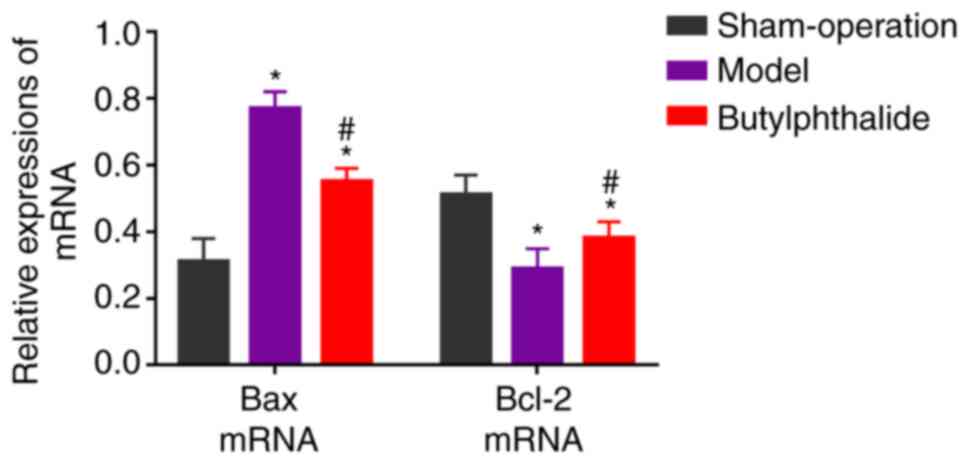
The relative mRNA expression level of Bax was
signficiantly increased, while that of Bcl-2 was significantly
decreased in the model group and the butylphthalide group, compared
with those in the sham-operation group (P<0.05; Fig. 4). Additionally, compared with those
in the model group, the relative mRNA expression level of Bax
decreased significantly, and that of Bcl-2 increased significantly
in the butylphthalide group (P<0.05).
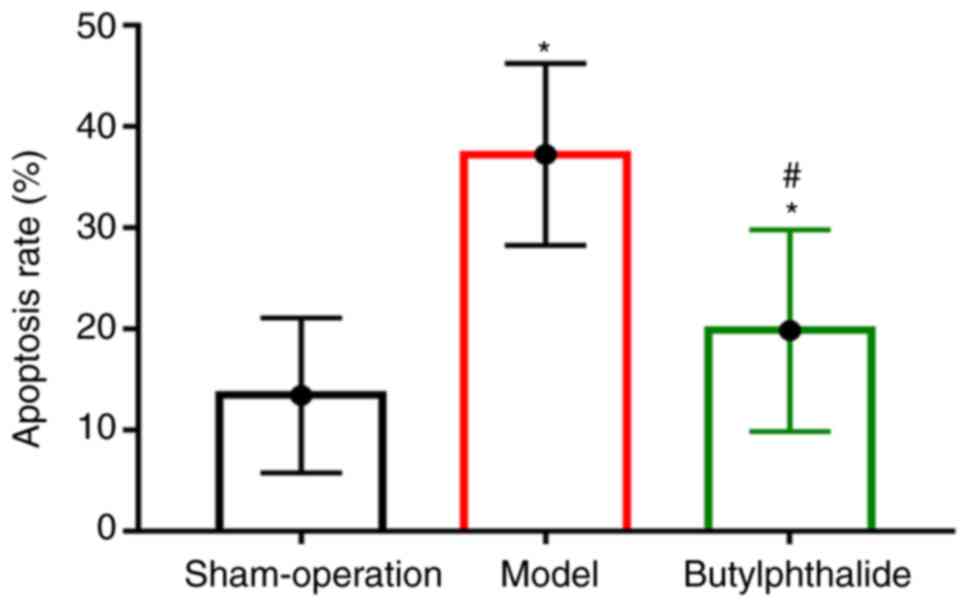
Cell apoptosis detected via TUNNEL
assay
There were fewer TUNEL-positive cells in the
sham-operation group and more TUNEL-positive cells in the remaining
groups. The apoptotic rate was significantly higher in the model
group and the butylphthalide group than that in the sham-operation
group (P<0.05), but it was significantly lower in the
butylphthalide group than that in the model group (P<0.05;
Fig. 5).
Discussion
Cerebral infarction, a clinically common
cerebrovascular disease, usually occurs in elderly patients and
leads to nervous system damage, including motor and sensory
dysfunctions of limbs, thereby triggering neurological deficits and
resulting in the death of patients in severe cases. Therefore, it
is a ubiquitous critical disease in clinic (12,13).
It is essential to effectively treat cerebral infarction,
accelerate the rehabilitation of nervous system injury and
neurological deficits following cerebral infarction and relieve the
limb motor dysfunction, sensory disturbance and speech disorder,
thereby significantly improving the quality of life of patients
with cerebral infarction and reducing the economic burdens on their
family and society. The nerve cell apoptosis, one of the critical
pathological responses and processes following cerebral infarction,
is considered an important action pathway that regulates the repair
of nervous system following the disease (14,15).
Studies have demonstrated that injury-induced numerous inflammatory
factors and cytokines may stimulate the abnormal expression of
pro-apoptotic Bax and anti-apoptotic Bcl-2 in the brain tissues
under hypoxic and ischemic conditions, and the proportion imbalance
between the two factors will ultimately lead to the nerve cell
apoptosis (16,17). A great amount of nerve cell
apoptosis may further destroy the nervous system, thereby causing
neurological deficits and even death. Therefore, efficiently
alleviating the nerve cell apoptosis following cerebral infarction
may protect the nerve cells and aid in the repair of the nervous
system following cerebral infarction (18,19).
Previous studies have proven that the JNK/p38 MAPK
signaling pathway serves as a crucial cell signal transduction
pathway to serve important regulatory roles in such physiological
and pathological processes as cell proliferation, differentiation,
apoptosis and necrosis (20-23).
Furthermore, it was also manifested through research that with the
presence of injury factors and hypoxic and ischemic conditions,
massive inflammatory factors and cytokines are able to
phosphorylate JNK in the JNK/p38 MAPK signaling pathway to activate
p-JNK. Additionally, p-JNK may further interact with p38 MAPK to
generate p-p38 MAPK via phosphorylation, activating the JNK/p38
MAPK signaling pathway and modulating the expression of downstream
apoptosis-related molecules Bax and Bcl-2. The results of this
study also confirmed that the apparently aberrant expression of Bax
and Bcl-2, namely high expression of Bax and low expression of
Bcl-2, were observed at the injury site following cerebral
infarction, suggesting that the aggravation of nerve cell apoptosis
is associated with the abnormal expression of Bax and Bcl-2.
Meanwhile, the expression of p-JNK and p-p38 MAPK were increased
following the occurrence of cerebral infarction, illustrating that
the JNK/p38 MAPK signaling pathway in the body was activated and
enhanced nerve cell apoptosis following cerebral infarction.
The results of the present study indicated that the
Zea-Longa score was significantly decreased following intervention
with butylphthalide for the rats with cerebral infarction in the
butylphthalide group, illustrating that butylphthalide may
favorably ameliorate neurological deficits and cognitive impairment
following cerebral infarction. It was also demonstrated that the
neuronal morphology was significantly improved in the
butylphthalide group via HE staining, indicating that
butylphthalide has a protective effect on neurons in cerebral
infarction. Additionally, butylphthalide group had decreased
expression of Bax, p-JNK and p-p38 MAPK, a decreased apoptotic rate
and increased Bcl-2 expression following intervention with
butylphthalide after the onset of cerebral infarction. These
results suggested that butylphthalide has an anti-apoptotic effect
in cerebral infarction, and this may be related to fact that
butylphthalide represses the activation of the JNK/p38 MAPK
signaling pathway. There are certain limitations to the present
study. For example, there was only one time point to determine the
mechanism; therefore, whether different time points have influence
on the experimental results requires further research to confirm.
In addition, no blockers or other methods were used to further
confirm the effect of the JNK/p38 MAPK signaling pathway on
cerebral infarction. Therefore, the specific mechanism of
butylphthalide on cerebral infarction and its association with the
JNK/p38 MAPK signaling pathway require further study to clarify in
the future.
Therefore, it may be concluded that butylphthalide
may inhibit the nerve cell apoptosis in rats with cerebral
infarction to exert a protective effect, which may be associated
with the JNK/p38 MAPK signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XB and YG designed the study and performed the
experiments, XB and WX collected the data, XW and SL analyzed the
data, XB and YG prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Zhejiang University School of Medicine Animal
Center.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang WT, Li YY, Lin WC, Chen JY, Lan KM,
Sun CK and Hung KC: Bilateral visual loss and cerebral infarction
after spleen embolization in a trauma patient with idiopathic
thrombocytopenic purpura: A case report. Medicine (Baltimore).
97(e332)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tao L, ShiChuan W, DeTai Z and Lihua H:
Evaluation of lipoprotein-associated phospholipase A2, serum
amyloid A, and fibrinogen as diagnostic biomarkers for patients
with acute cerebral infarction. J Clin Lab Anal.
34(e23084)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lu SS, Ge S, Su CQ, Xie J, Mao J, Shi HB
and Hong XN: MRI of plaque characteristics and relationship with
downstream perfusion and cerebral infarction in patients with
symptomatic middle cerebral artery stenosis. J Magn Reson Imaging.
48:66–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tajima H, Kasai H, Sugiura T and Tatsumi
K: Pulmonary arteriovenous fistula complicated by venous
thromboembolism and paradoxical cerebral infarction during early
pregnancy. BMJ Case Rep. 2018(bcr2017222519)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhao J, Mao Y and Qi J: Expression of
cytoskeleton and apoptosis related genes after cerebral infarction.
Neurol Res. 28:71–75. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li C, Che LH, Ji TF, Shi L and Yu JL:
Effects of the TLR4 signaling pathway on apoptosis of neuronal
cells in diabetes mellitus complicated with cerebral infarction in
a rat model. Sci Rep. 7(43834)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim SH, Yoo ES, Woo JS, Han SH, Lee JH,
Jung SH, Kim HJ and Jung JY: Antitumor and apoptotic effects of
quercetin on human melanoma cells involving JNK/P38 MAPK signaling
activation. Eur J Pharmacol. 860(172568)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kwon HC, Kim TY, Lee CM, Lee KS and Lee
KK: Active compound chrysophanol of Cassia tora seeds suppresses
heat-induced lipogenesis via inactivation of JNK/p38 MAPK signaling
in human sebocytes. Lipids Health Dis. 18(135)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kodata T, Kamata K, Fujiwara K, Kano M,
Yamakawa T, Yuki I and Murayama Y: A new infarction detection
method based on heart rate variability in rat middle cerebral
artery occlusion model. Conf Proc IEEE Eng Med Biol Soc.
2017:3061–3064. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu D, Tang ZY, Hu ZJ, Li WW and Yuan WN:
miR-940 regulates angiogenesis after cerebral infarction through
VEGF. Eur Rev Med Pharmacol Sci. 22:7899–7907. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang D, Wang L, Bai L, Du Y, Liu L and
Chen X: Effects of inhibition of miR-155-5p in neural stem cell
subarachnoid transplant on rats with cerebral infarction. Hum Gene
Ther Methods. 30:184–193. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu XH, Zhang SM, Yan WM, Li XR, Zhang HY
and Zheng XX: Development of cerebral infarction, apoptotic cell
death and expression of X-chromosome-linked inhibitor of apoptosis
protein following focal cerebral ischemia in rats. Life Sci.
78:704–712. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ji JF and Ma XH: Effect of baculovirus P35
protein on apoptosis in brain tissue of rats with acute cerebral
infarction. Genet Mol Res. 14:9353–9360. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun Y, Xu Y and Geng L: Caspase-3
inhibitor prevents the apoptosis of brain tissue in rats with acute
cerebral infarction. Exp Ther Med. 10:133–138. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xie YL, Zhang B and Jing L: MiR-125b
blocks Bax/Cytochrome C/Caspase-3 apoptotic signaling pathway in
rat models of cerebral ischemia-reperfusion injury by targeting
p53. Neurol Res. 40:828–837. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou L, Zhang J, Wang C and Sun Q:
Tanshinone inhibits neuronal cell apoptosis and inflammatory
response in cerebral infarction rat model. Int J Immunopathol
Pharmacol. 30:123–129. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee JB, Woo HG, Chang Y, Jin YM, Jo I, Kim
J and Song TJ: Plasma Klotho concentrations predict functional
outcome at three months after acute ischemic stroke patients. Ann
Med. 51:262–269. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu N, Gu C, Gu H, Hu H, Han Y and Li Q:
Metformin induces apoptosis of lung cancer cells through activating
JNK/p38 MAPK pathway and GADD153. Neoplasma. 58:482–490.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Su JC, Lin KL, Chien CM, Lu CM, Chen YL,
Chang LS and Lin SR: Novel indoloquinoline derivative, IQDMA,
induces G(2)/M phase arrest and apoptosis in A549 cells through
JNK/p38 MAPK signaling activation. Life Sci. 85:505–516.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Osone S, Hosoi H, Kuwahara Y, Matsumoto Y,
Iehara T and Sugimoto T: Fenretinide induces sustained-activation
of JNK/p38 MAPK and apoptosis in a reactive oxygen
species-dependent manner in neuroblastoma cells. Int J Cancer.
112:219–224. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mansouri A, Ridgway LD, Korapati AL, Zhang
Q, Tian L, Wang Y, Siddik ZH, Mills GB and Claret FX: Sustained
activation of JNK/p38 MAPK pathways in response to cisplatin leads
to Fas ligand induction and cell death in ovarian carcinoma cells.
J Biol Chem. 278:19245–19256. 2003.PubMed/NCBI View Article : Google Scholar
|