Introduction
Cardiovascular and cerebrovascular diseases are the
predominant causes of human death (1). According to the current
epidemiological data, it has been revealed that the number of CVD
mortalities steadily increased from 12.1 million in 1990 to 18.6
million in 2019 globally (2).
Ischemic cardiomyopathy (ICM) is one such type of cardiovascular
disease (3). The main cause of
myocardial ischemia is coronary artery stenosis, resulting in
insufficient blood supply to the myocardium, which hinders aerobic
metabolism in myocardial cells and reduces contractile capacity
(4,5). Therefore, energy supply during heart
activity is rendered insufficient, which can cause arrhythmia and
angina pectoris (6). In severe
cases, it can result in myocardial infarction (7). For myocardial ischemia, the most
effective treatment strategy is currently to quickly restore blood
supply to the ischemic section of the heart. However, resuming
blood flow after myocardial ischemia for a period of time may lead
to myocardial contractile dysfunction and structural damage at the
ischemic site, which will aggravate myocardial injury (8). This phenomenon is known as myocardial
ischemia-reperfusion injury (MIRI) (8). MIRI is a condition that requires
close medical attention and implementation of management following
treatment, which mainly consists of coronary artery bypass or
cardiopulmonary bypass surgery (9,10).
In addition, alleviating MIRI has been hypothesized to be the main
cause for successful treatment and prolonged survival in patients
(11). Coupled with the continued
advancements in surgical techniques, antiplatelet and
antithrombotic agents, reperfusion therapy in a timely manner can
effectively preserve the patency of coronary arteries (10,12,13).
However, the treatment for MIRI has not been effectively improved,
evidenced by ~8.8 million mortalities in 2015 worldwide, accounting
for 15.5% of all deaths that year (14). Therefore, studying the growth state
and metabolic activity of cardiomyocytes is expected to yield
significant results for the prevention and treatment strategies for
complications caused by MIRI.
Previous studies have shown that the pathogenesis
during myocardial ischemia is associated with oxidative stress,
chronic inflammation and cardiomyocyte apoptosis (15-17).
Reperfusion in the ischemic myocardium results in the production of
oxygen free radicals (OFR), which can mediate myocardial damage
(18). In addition, production of
vast quantities of reactive oxygen species (ROS) during the early
stages of reperfusion can lead to dysfunction of the antioxidant
system in myocardial cells, causing widespread cell damage and
eventually necrosis and apoptosis (19). Therefore, improving the antioxidant
capacity of cells may reduce myocardial cell damage caused by MIRI.
Cbl proto-oncogene (CBL) is widely expressed in various tissues and
contains an N-terminal tyrosine-kinase-binding domain, which can
interact with a variety of proteins (20). Elsewhere, Cbl proteins are
implicated in the regulation of multiple signal transduction
(21). For instance, Cdc42
sequesters Cbl from the epidermal growth-factor receptor (EGFR)
through βPIX, thus inhibiting EGFR downregulation (22). In addition, c-Cbl, an adaptor
protein, has been reported to participate in the activation of MAP
kinases (23). Previous studies
have revealed that CBL inhibition can alleviate cardiomyopathy
injury. Using c-Cbl as an example, the silencing of c-Cbl helps to
improve cardiac function in response to myocardial ischemia
(24). Furthermore, CBL-knockout
mice exhibit improved abilities to recover heart function following
ischemia and reperfusion (24),
suggesting that CBL may exert negative regulatory effects on the
survival of myocardial cells in addition to angiogenesis following
MIRI.
At present, the pathogenesis of MIRI has not been
fully elucidated (25). Therefore,
it remains in demand to actively explore novel targets and their
underlying mechanism of action in MIRI. In the present study, an
in vitro MIRI model was constructed through
hypoxia-reoxygenation (H/R), where the target of CBL was predicted
using bioinformatics analysis. The aim of the present study was to
explore the effect of CBL on H/R-induced cardiomyocyte injury and
to determine the underlying mechanism.
Materials and methods
Clinical samples
All procedures were performed in accordance with the
Declaration of the Institutional Research Committee's Ethical
standards and the 1964 Declaration of Helsinki with its later
amendments. The Ethics Committee of the Second People's Hospital of
Chengdu approved the involvement of human participants and the
study ran from August 2017 to August 2019 (approval no.
2017081302). All patients or their parents/guardians provided
written informed consent for participation after they were fully
informed of the study details.
The plasma samples of 30 patients with ICM (age,
70.2±9.24 years old; 21:9 males and females) and 30 healthy
subjects who went to the hospital for health checkups (age, 69±8.12
years old; 21:9 males to females) admitted to The Second People's
Hospital of Chengdu (Chengdu, China) between August 2017 and August
2019 were obtained. A total of 5 ml fasting venous blood of the
study subjects was drawn into tubes containing vitamin K
antagonists and centrifuged at 1,200 x g for 10 min at 4˚C, before
the supernatant was collected and stored at -20˚C. According to
World Health Organization, ICM presents as a dilated cardiomyopathy
with impaired contractile performance (26). The diagnosis of ICM group and all
patients in the present study were based on this. MIRI inclusion
criteria: Left ventricular ejection fraction still <40% after
percutaneous coronary intervention surgery was judged as occurrence
of myocardial ischemia-reperfusion injury. MIRI exclusion criteria:
Patients have chest pain caused by menopausal syndrome, neurosis,
gastroesophageal reflux disease, combined autoimmune diseases,
malignant tumors and previous history of cardiovascular and
cerebrovascular diseases (27).
Cell culture and treatment
The H9c2 rat cardiomyoblasts were purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences and cultured in DMEM supplemented with 15% FBS (both
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin at 37˚C in a humidified incubator with 5%
CO2.
To established a cellular MIRI model,
serum/glucose-free DMEM was employed. H9c2 cells
(1.5x105 cells/well) were placed in an incubator
containing 94% N2, 5% CO2 and 1%
O2 at 37˚C to simulate hypoxia for 24 h. Subsequently,
the cells were placed in an incubator with 5% CO2 at
37˚C and cultured for another 24 and 48 h for reoxygenation at
37˚C. H9c2 cells cultured with 5% CO2 for the full 48 h
period were used as the control group. In addition, the group that
underwent H/R treatment after 24 h transfection was designated the
as H/R + small interfering RNA (si)/or Overexpression
(Ov)-group.
Cell transfection
H9c2 cells were seeded into six-well plates at a
density of 2x105 cells/well and transfected with 1 µg
pcDNA3.1-growth factor receptor-bound protein 2 (GRB2; accession
no. NM_030846.2) overexpression vector or pcDNA3.1 empty vector
(Hunan Fenghui Biotechnology Co., Ltd.) and/or 50 nM siRNAs against
CBL (si-CBL) or negative control (si-NC; Shanghai Genepharma Co.,
Ltd.) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Following transfection for 24 h, the cells were used for subsequent
experiments. si-CBL-1 target, 5'-GGAAGCCATCGCCAAATATGA-3'; si-CBL-2
target, 5'-GTGCTGGAAGCTCATGGACAA-3'; si-NC target,
5'-GGCAGACAATGCGAAACACTT-3'.
Cell Counting Kit 8 (CCK-8) assay
H9c2 cells (1.5x104 cells/well) were
cultured in 96-well plates before 10 µl CCK-8 reagent (Beyotime
Institute of Biotechnology) was added into each well 24 or 48 h
after plating the cells. Cells were then incubated at 37˚C for a
further 2 h. The absorbance in each well was measured at a
wavelength of 450 nm using a microplate reader (Molecular Devices,
LLC).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and then reverse transcribed into cDNA using Universal RT-PCR
kit (cat. no. QN0943; Beijing Baiao Laibo Technology Co., Ltd.)
according to the manufacturer's protocols. The mRNA expression
levels were measured using SYBR Green Master Mix (cat. no. MT0017;
Beijing Baiao Laibo Technology Co., Ltd.) on a
StepOnePlus™ Real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions were
as follows: Initial denaturation at 95˚C for 3 min; followed by 40
cycles of denaturation at 95˚C for 30 sec, annealing at 60˚C for 30
sec and extension at 72˚C for 30 sec. The primer sequences used are
listed in Table I. GAPDH was used
as internal reference and the relative mRNA expression levels were
calculated using the 2-ΔΔCq method (28).
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5'-3') | Product sizes
(bp) |
|---|
| CBL (Rat) | F:
GCTTCTCTTTGGCTTGTGCG | 71 |
| | R:
CTGGTGAAGCGAGGAATGGA | |
| Growth factor
receptor bound protein 2 (Rat) | F:
TGCCTAGAGCCACTAAGGGT | 373 |
| | R:
GGAGAGAAACGGTGGACAGG | |
| Proliferating cell
nuclear antigen (Rat) | F:
AGTTTTCTGCGAGTGGGGAG | 354 |
| | R:
AAGACCTCAGAACACGCTGG | |
| Ki67 (Rat) | F:
TTCCAGACACCAGACCATGC | 96 |
| | R:
GGTTCTAACTGGTCTTCCTGGTT | |
| GAPDH (Rat) | F:
TCTCTGCTCCTCCCTGTTCT | 104 |
| | R:
TACGGCCAAATCCGTTCACA | |
| CBL (Homo
sapiens) | F:
CAACGTGAAGAAGAGCTCTGGG | 472 |
| | R:
GTGGCTGAAGATGAGGGACA | |
| GAPDH (Homo
sapiens) | F:
GACTCATGACCACAGTCCATGC | 113 |
| | R:
AGAGGCAGGGATGATGTTCTG | |
Western blot analysis
Total proteins were extracted from each group of
H9c2 cells using RIPA lysis buffer (Beyotime Institute of
Biotechnology) and then quantified with bicinchoninic acid protein
assay kit (cat. no. P0012S; Beyotime Institute of Biotechnology).
Equal amount of proteins (20 µg per lane) were separated by 10%
SDS-PAGE and then transferred onto PVDF membranes. Following
blocking with 5% non-fat milk for 2 h at room temperature, the
membranes were incubated with primary antibodies against CBL (cat.
no. ab228785; 1:1,000; Abcam), GRB2 (cat. no. ab32111; 1:1,000;
Abcam), Ki67 (cat. no. ab16667; 1:1,000; Abcam), proliferating cell
nuclear antigen (PCNA; cat. no. ab18197; 1:1,000; Abcam), Bcl-2
(cat. no. ab32124; 1:1,000; Abcam), Bax (cat. no. ab32503; 1:1,000;
Abcam), caspase-3 (cat. no. ab32351; 1:5,000; Abcam) cleaved
caspase-3 (cat. no. ab2302; 1:500; Abcam) or GAPDH (cat. no.
ab8245; 1:1,000; Abcam) at 4˚C overnight. The membranes were then
incubated with HRP-conjugated goat anti-rabbit secondary antibodies
(cat. no. ab97080; 1:5,000; Abcam) for 2 h at room temperature.
Enhanced chemiluminescence reagent (Thermo Fisher Scientific, Inc.)
was used to visualize the protein bands and the gray values were
assessed using ImageJ (version 1.8; National Institutes of
Health).
Detection of oxidation stress
H9c2 cells were collected and lysed using a cell
lysis buffer (cat. no. P0013; Beyotime Institute of Biotechnology),
followed by centrifugation at 10,000 x g for 10 min at 4˚C. In
total, 200 µl supernatant was collected. Lactate dehydrogenase
(LDH; cat. no. A020-2-2), superoxide dismutase (SOD; cat. no.
A001-3-2) and malondialdehyde (MDA; cat. no. A003-4-1) detection
kits were used according to the manufacturers' protocols (Nanjing
Jiancheng Bioengineering Institute). The activity values were
calculated based on absorbance using a microplate reader (Thermo
Fisher Scientific, Inc.).
TUNEL assay
H9c2 cells (2x104 cells/well) were seeded
into a 24-well plate and assay was stained using a TUNEL kit (cat.
no. C1086; Beyotime Institute of Biotechnology) according to the
manufacturer's protocols. Briefly, cells were first stained by 4%
paraformaldehyde for 30 min at room temperature followed by
incubation with PBS containing 0.3% Triton X-100 for 5 min at room
temperature. TUNEL working fluid was added and cells were incubated
for another 1 h at 37˚C away from the light. Subsequently, Antifade
Mounting Medium with DAPI (cat. no. P0131; Beyotime Institute of
Biotechnology) was employed to treat the cells. A total of 10
randomly selected visual fields were observed using a fluorescence
microscope (magnification, x200; Olympus Corporation). Apoptosis
rate was calculated as follows: Apoptosis rate %=number of positive
apoptotic cells/total number of cells x100%.
Co-immunoprecipitation (co-IP)
assay
H9c2 cells were collected and lysed in pre-cooled
cell lysis buffer (cat. no. P0013; Beyotime Institute of
Biotechnology) containing protease inhibitors at 0˚C. The
supernatant was collected after centrifugation at 13,000 x g for 10
min at 4˚C. Subsequently, 0.2 mg protein A agarose beads (cat. no.
20366; Thermo Fisher Scientific, Inc.) that were washed with 100 µl
PBS buffer were added to 500 µg lysis buffer and incubated with 2
µg IgG antibody (1:1,000; cat. no. ab172730; Abcam) or CBL antibody
(cat. no. 2747; 1:50; Cell Signaling Technology, Inc.) overnight at
4˚C with slow shaking. Following the IP reaction and centrifugation
at 1,000 x g at 4˚C for 2 min, the agarose beads were washed with
lysis buffer and boiled for 5 min at 100˚C by adding 15 µl 2X SDS
sample buffer. This sample were then subjected to western
blotting.
Bioinformatics analysis
Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING, version 11.0; https://string-db.org/) (29) is an online database used for
searching known protein interactions. CBL and GRB2 were first
entered together at the multiple-proteins interface and the species
Homo sapiens was selected before the result was
automatically displayed.
Statistical analysis
Data are presented as the mean ± standard deviation
and statistical analysis was performed using GraphPad Prism version
8.0 (GraphPad Software, Inc.). Comparisons among multiple groups
were performed using a one-way ANOVA followed by a Tukey's post hoc
test and unpaired Student's t test between two groups. All cellular
experiments were performed ≥ three times from three different
cultures. P<0.05 was considered to indicate a statistically
significant difference.
Results
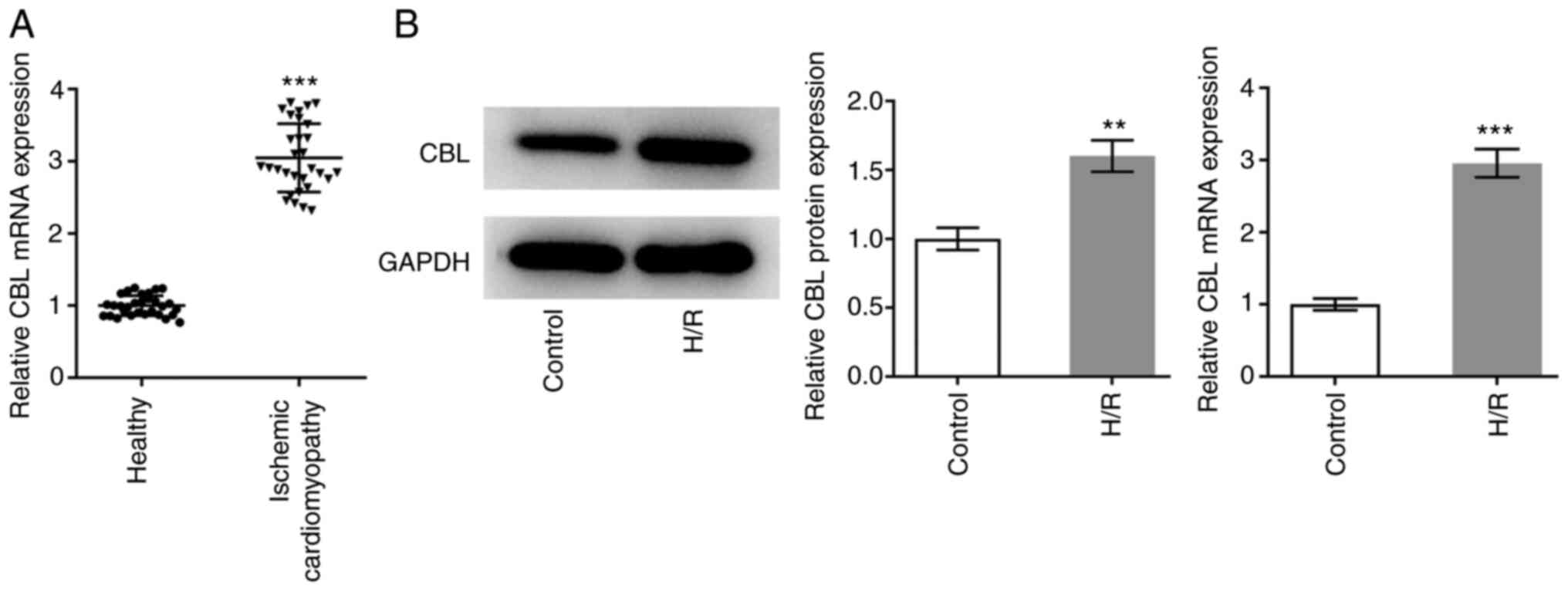
Analysis of CBL expression
The expression levels of CBL in patients with ICM
and healthy individuals were measured using RT-qPCR. There were 30
individuals in each group and the expression levels were
statistically analyzed. The expression levels of CBL in the plasma
of patients with ICM were significantly higher compared with those
in the healthy individuals (Fig.
1A). Subsequently, expression levels of CBL in H/R-induced H9c2
cells and normally cultured H9c2 cells were measured using RT-qPCR
and western blot analysis. The expression levels in H/R-induced
cells were significantly higher compared with those in cells
cultured normally (Fig. 1B).
CBL knockdown promotes the
proliferation and oxidative stress resistance of H/R-induced H9c2
rat cardiomyoblasts
To explore the specific role of CBL in H/R-induced
cells, CBL expression was knocked down by transfection with
specific siRNAs before the efficacy of knockdown was assessed using
RT-qPCR and western blot analysis. The expression levels of CBL
were significantly lower in cells transfected with si-CBL-1
compared with those in cells transfected with si-NC (Fig. 2A). si-CBL-1 was chosen for
subsequent experiments due to the higher potency compared with
si-CBL-2 (Fig. 2A). The H9c2 cells
were subsequently divided into the following four groups: Control
group, H/R group, H/R + si-NC group and the H/R + si-CBL group.
Viability in each group of cells was first detected using CCK-8
assay. After 24 and 48 h of treatment the results showed that cell
viability in the H/R group was significantly lower at both time
points compared with that in the control group. In addition,
viability in the H/R + si-CBL group was significantly higher
compared with that in the H/R + si-NC group, indicating that CBL
knockdown alleviated the damage caused by H/R (Fig. 2B). Similarly, PCNA and Ki67 protein
expression levels in the cells of each group were detected using
western blot analysis and RT-qPCR. Compared with the control group,
the expression levels of PCNA and Ki67 were significantly
downregulated in H9c2 cells after H/R induction. In addition, their
expression levels in the H/R + si-CBL group were significantly
higher compared with that in the H/R + si-NC group, suggesting that
CBL knockdown also promoted H/R-induced cell proliferation
(Fig. 2C). Subsequently, the
antioxidant capacity of each group of cells was assessed by
specifically measuring LDH, SOD and MDA. From the calculated
results, SOD levels were found to be significantly decreased in the
H/R group compared with those in the control, but were
significantly upregulated in the H/R + si-CBL group compared with
those in the H/R + si-NC group (Fig.
2D). By contrast, LDH and MDA levels exhibited an opposite
trend (Fig. 2D). This suggests
that CBL knockdown improved the antioxidant capacity of H/R-induced
cells.
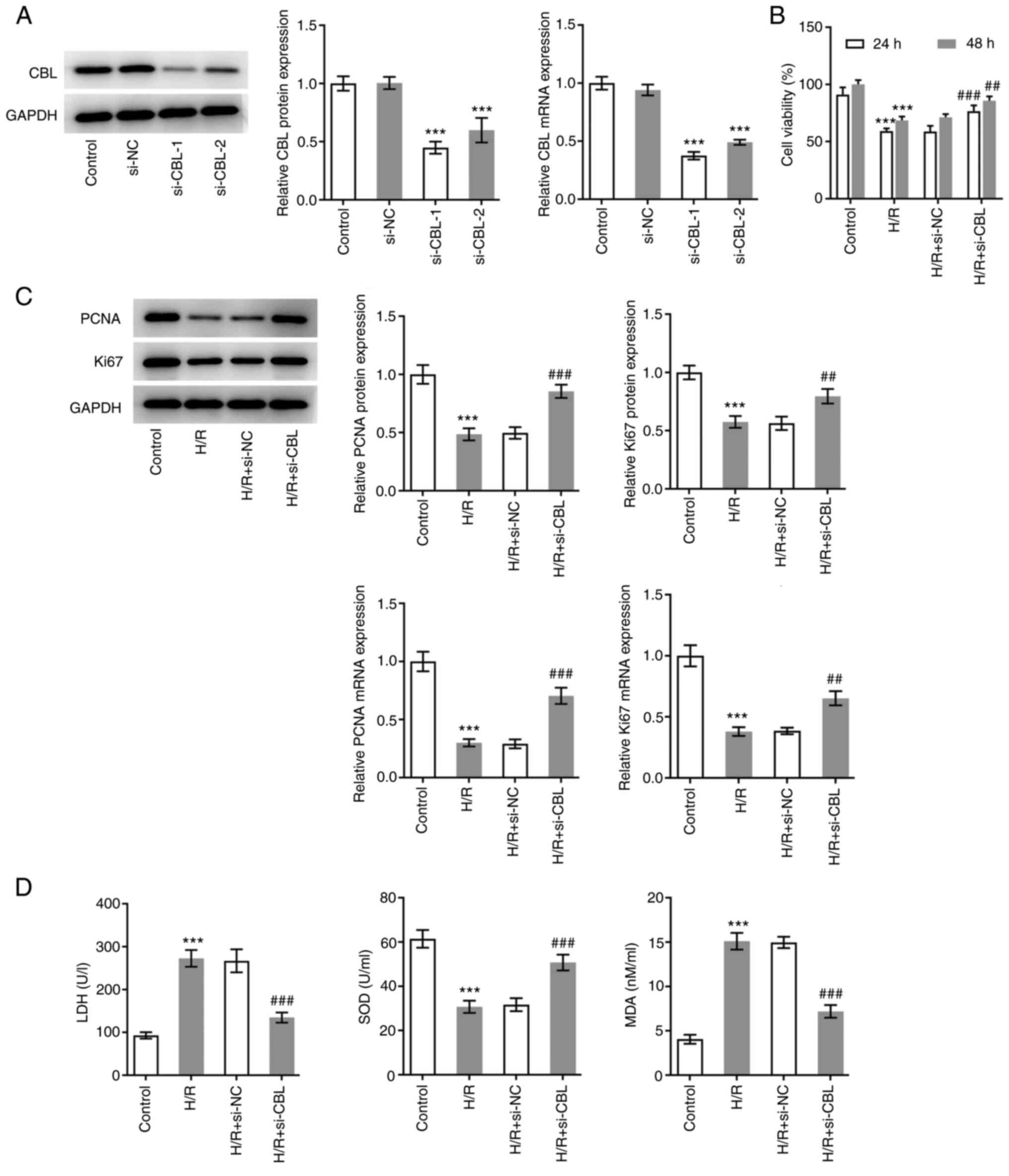 | Figure 2CBL knockdown promotes the
proliferation and oxidative stress resistance of H/R-induced
myocardiocytes. (A) Efficacy of transfection was detected using
RT-qPCR and western blot analysis. ***P<0.001 vs.
si-NC. (B) Cell viability of cells in each group was detected using
Cell Counting Kit-8 assay. (C) PCNA and Ki67 protein expression in
cells in each group were detected using RT-qPCR and western blot
analysis. (D) Antioxidant capacity of each group of cells was
assessed by measuring their LDH, SOD and MDA levels.
***P<0.001 vs. Control; ##P<0.01 and
###P<0.001 vs. H/R + si-NC. Si-, small interfering;
CBL, Cbl proto-oncogene; RT-qPCR, reverse
transcription-quantitative PCR; H/R, hypoxia-reoxygenation; si-NC,
small interfering negative control; PCNA, proliferating cell
nuclear antigen; LDH, lactate dehydrogenase; SOD, superoxide
dismutase; MDA, malondialdehyde. |
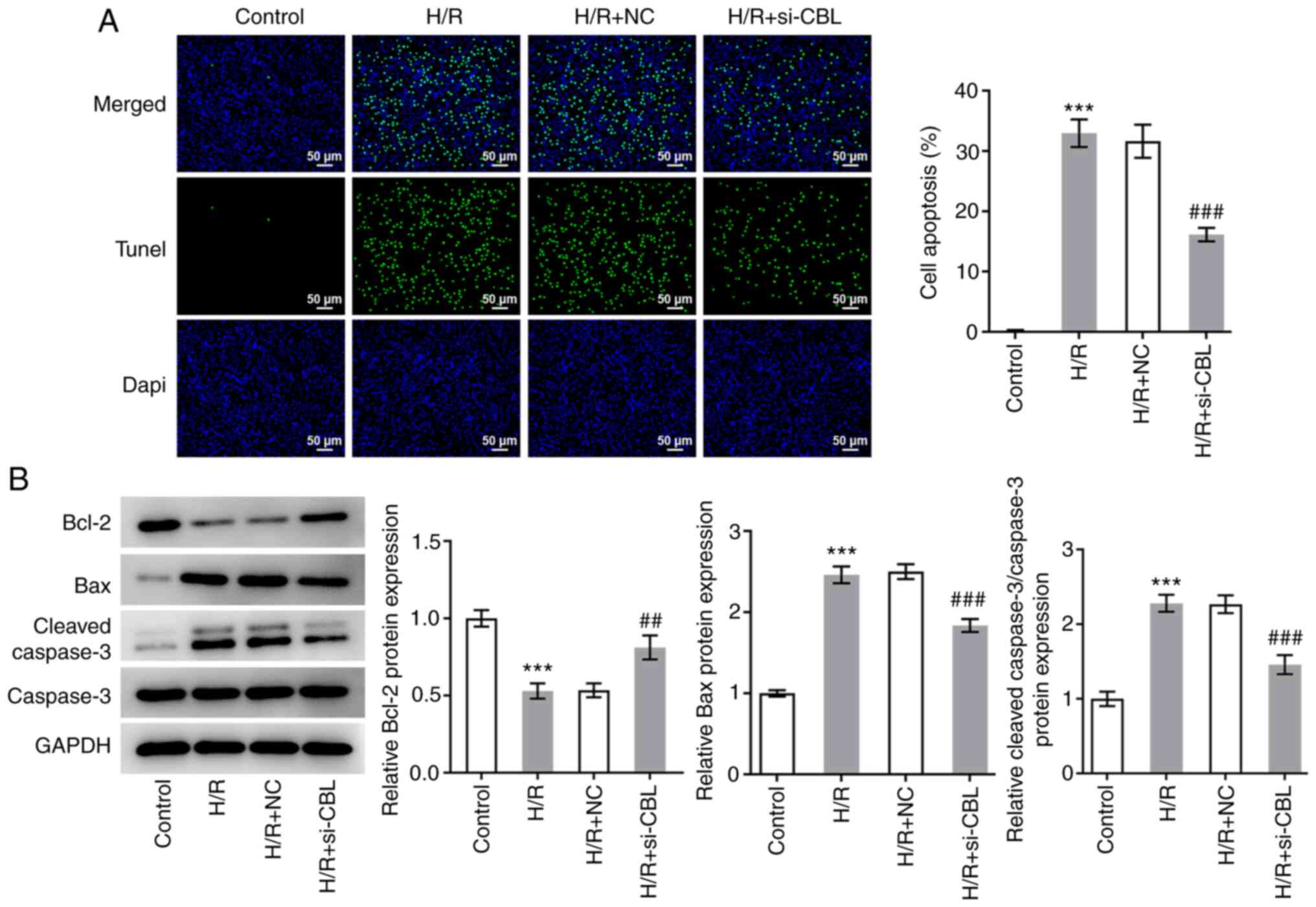
CBL knockdown inhibits H/R-induced
myocardial apoptosis
The aforementioned treatment groups were also
designated to explore the effects of CBL knockdown on cell
apoptosis. The results of the TUNEL assays revealed that the number
of apoptotic cells in the H/R group was significantly increased
compared with that in the control group (Fig. 3A). In the H/R + si-CBL group, the
number of apoptotic cells was significantly lower compared with
that in the H/R + si-NC group (Fig.
3A). According to the results of western blot analysis, the
expression levels of Bax and cleaved caspase-3 were found to be
significantly upregulated in the H/R group compared with those in
the control group, whilst knocking down CBL expression
significantly reversed this increase in Bax and cleaved caspase-3
expression (Fig. 3B).
Additionally, the trend in Bcl-2 expression was found to be
opposite to that shown by Bax and cleaved caspase-3 (Fig. 3B). These results suggest that CBL
knockdown can inhibit H/R-induced cell apoptosis.
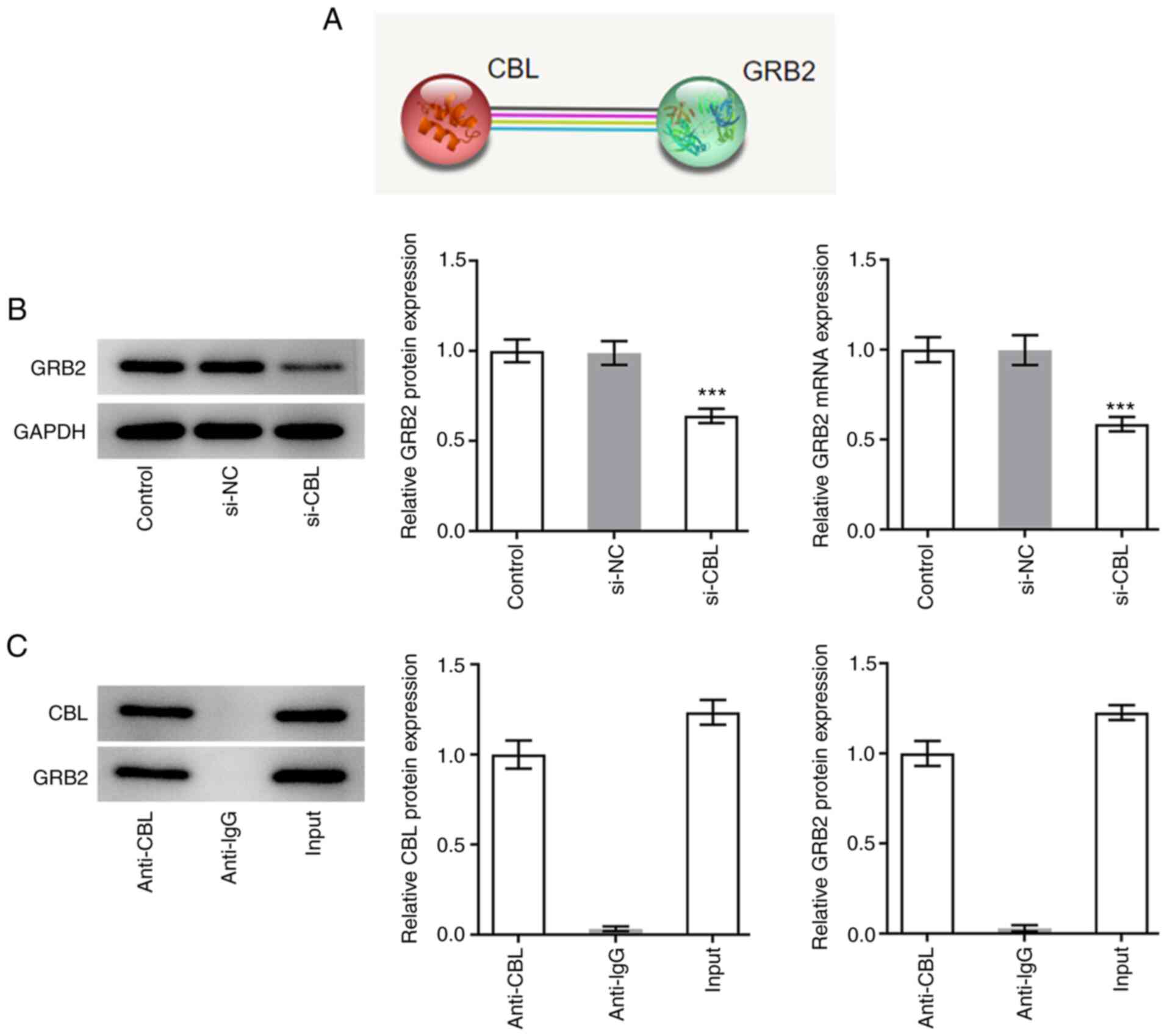
CBL knockdown reduces the expression
of GRB2 in H9c2 rat cardiomyoblasts
Notably, GRB2 was revealed to promote the
advancement of cardiac fibrosis. Moreover, according to the data on
STRING database, GRB2 was predicated to be associated with CBL
(Fig. 4A). The expression levels
of GRB2 were therefore measured using RT-qPCR and western blot
analysis. The expression levels of GRB2 in cells transfected with
si-CBL were markedly lower compared with those in the si-NC group
(Fig. 4B). The results of co-IP
and western blot analysis revealed that both CBL and GRB2 were
expressed in the input group and GRB2 protein was revealed to exist
in the anti-CBL group. However, IgG could not pull down CBL and was
used to rule out false positive results. This suggests that there
was an association between these two proteins (Fig. 4C).
CBL knockdown promotes the
proliferation and oxidative stress resistance of H/R-induced H9c2
rat cardiomyoblasts by downregulating GRB2 expression
To verify the mechanism by which GRB2 regulate CBL,
cells were transfected with pcDNA3.1-GRB2 before transfection
efficiency was verified using RT-qPCR and western blotting
(Fig. 5A). The expression of GRB2
was markedly enhanced after the cells transfection with GRB2
overexpression plasmids compared with that in the Ov-NC group. The
H9c2 cells were then divided into the following five groups:
Control group, H/R + si-NC group, H/R + si-CBL group, H/R + si-CBL
+ Ov-NC group and the H/R + si-CBL + Ov-GRB2 group. The viability
of cells in each group was first examined using a CCK-8 assay. As
depicted in Fig. 5B, the cell
viability in H/R-induced H9c2 cells were significantly increased by
CBL-silencing in comparison with that in H/R + si-NC group.
Notably, GRB2 overexpression slightly decreased the cell viability
in comparison with that in H/R + si-CBL + Ov-NC, revealing that
GRB2 overexpression didn't have significant influence on viability
of H/R-induced H9c2 cells transfection with siRNA specific CBL. In
addition, the expression levels of PCNA and Ki67 were both
significantly downregulated in the H/R + si-CBL + Ov-GRB2 group
compared with those in the H/R + si-CBL + Ov-NC group (Fig. 5C). LDH, SOD and MDA levels were
also assessed. SOD levels were found to be significantly
downregulated, whilst LDH and MDA levels were significantly
upregulated following GRB2 overexpression, compared with those in
the H/R + si-CBL + Ov-NC group (Fig.
5D).
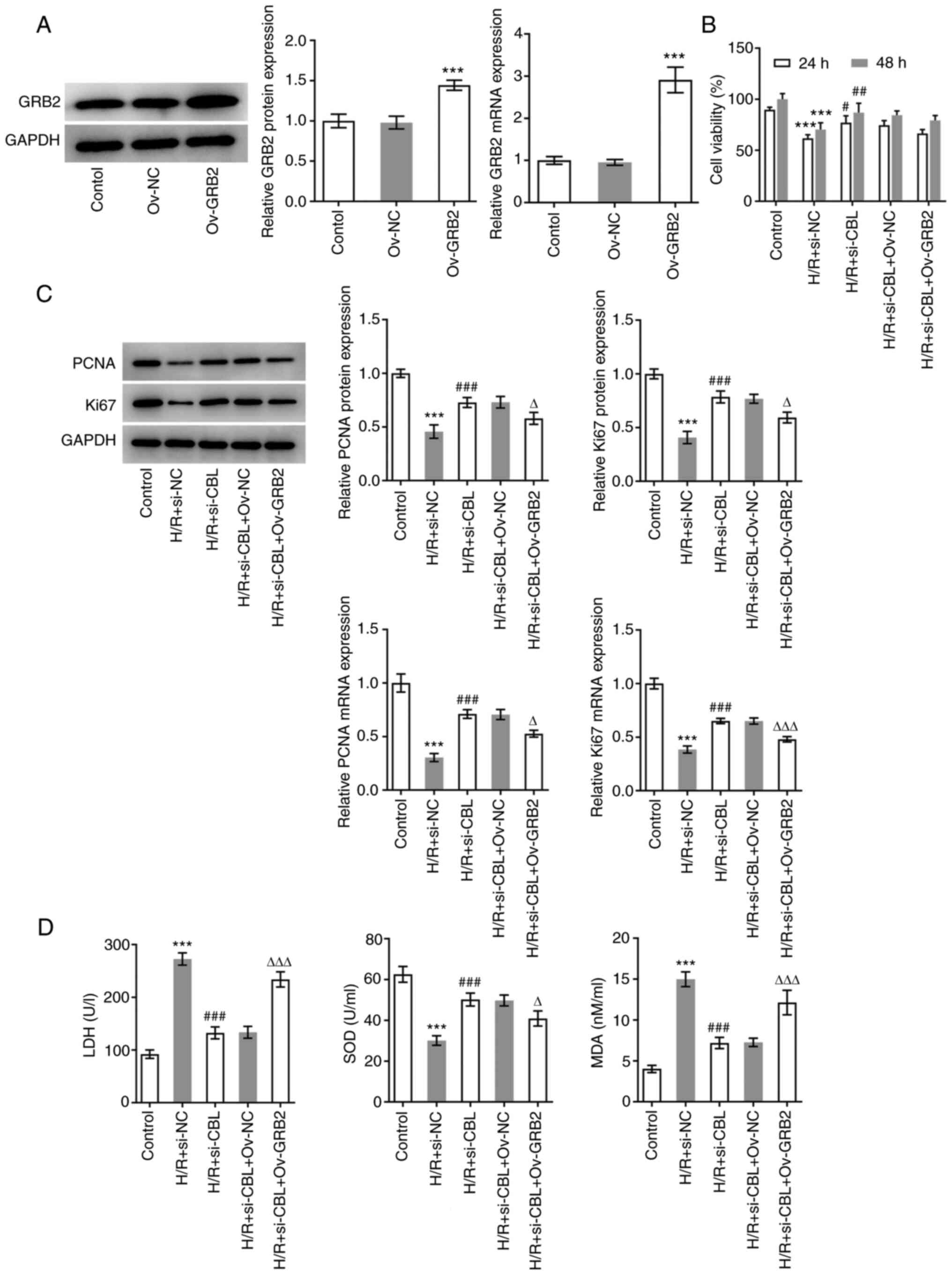 | Figure 5CBL knockdown promotes the
proliferation and oxidation resistance of H/R-induced H9c2 rat
cardiomyoblasts by downregulating of GRB2 expression. (A)
Transfection efficiency of Ov-GRB2 was verified using RT-qPCR and
western blot analysis. ***P<0.001 vs. Ov-NC. (B) Cell
viability in each group was examined using Cell Counting Kit-8
assay. (C) Expression levels of PCNA and Ki67 were measured using
western blot analysis. (D) Antioxidant capacity of each group of
cells was assessed by measuring their LDH, SOD and MDA levels.
***P<0.001 vs. Control; #P<0.05,
##P<0.01 and ###P<0.001 vs. H/R +
si-NC; ∆P<0.05 and ∆∆∆P<0.001 vs. H/R +
si-CBL + Ov-NC. Si-, small interfering; CBL, Cbl proto-oncogene;
NC, negative control; Ov, overexpression; H/R,
hypoxia-reoxygenation; GRB2, Growth factor receptor-bound protein
2; RT-qPCR, reverse transcription-quantitative PCR; PCNA,
proliferating cell nuclear antigen; LDH, lactate dehydrogenase;
SOD, superoxide dismutase; MDA, malondialdehyde. |
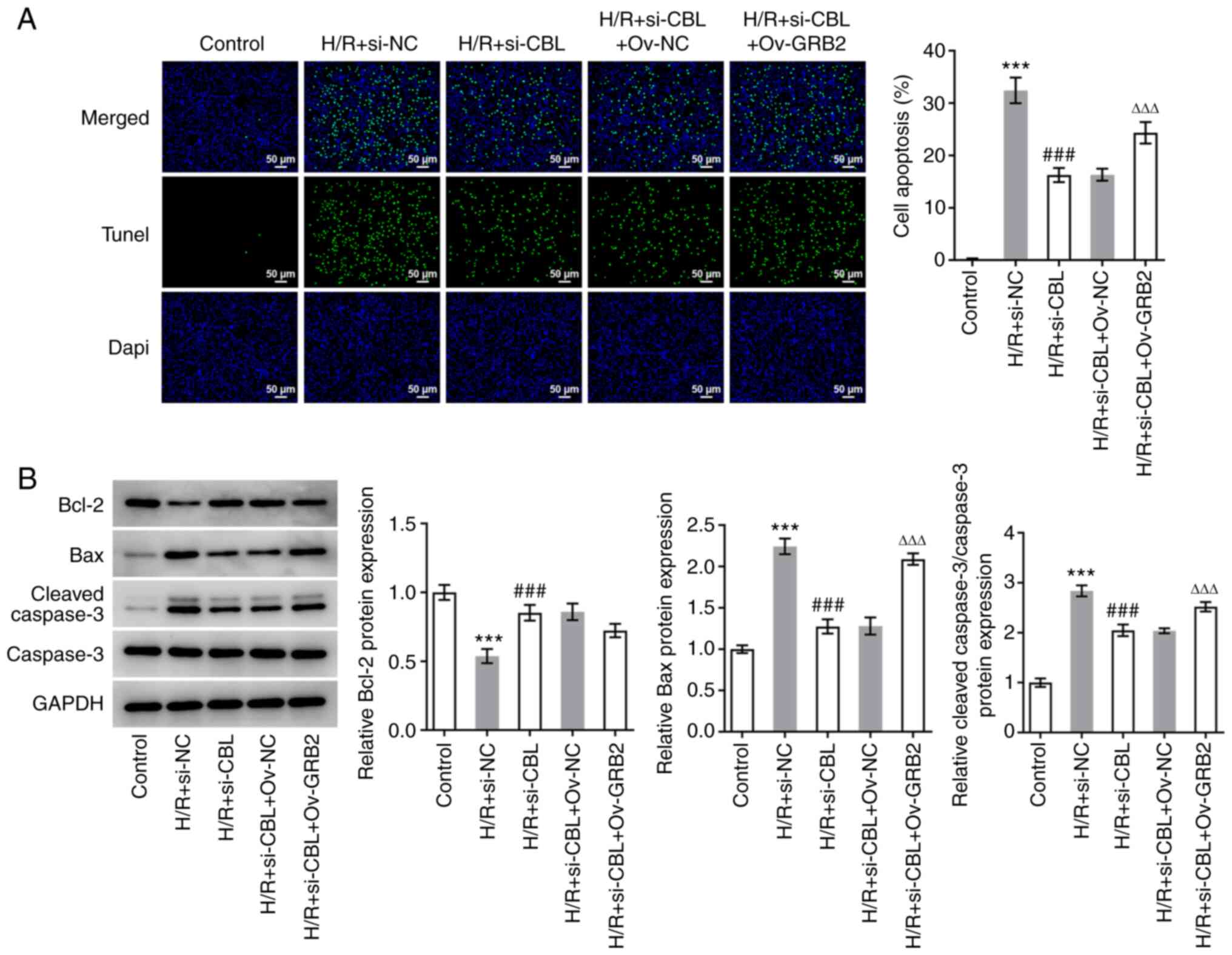
CBL knockdown inhibits H/R-induced
myocardial apoptosis by downregulating GRB2 expression
The same five groups of H9c2 cells aforementioned
were used to explore the effects of GRB2 and CBL on apoptosis. The
levels of apoptosis and the expression levels of apoptosis-related
proteins in each group were determined using a TUNEL assay and
western blot analysis, respectively. The number of apoptotic cells
in the H/R + si-CBL + Ov-GRB2 group was significantly greater
compared with that in the H/R + si-CBL + Ov-NC group (Fig. 6A). In addition, compared with H/R +
si-NC, CBL-silencing significantly upregulated Bcl-2 expression but
significantly downregulated the expression levels of Bax and
cleaved caspase-3; while GRB2 overexpression reversed the
inhibitory effects of CBL-silencing on apoptosis, evidenced by the
downregulated Bcl-2 expression but upregulated expression levels of
Bax and cleaved caspase-3 in contrast to that in the H/R + si-CBL +
Ov-NC group (Fig. 6B).
Discussion
In previous experimental and clinical studies, a
number of treatment strategies for preventing MIRI have been
proposed, including ischemic pre-conditioning (IPC), ischemic
post-conditioning (IPost), remote ischemic conditioning (RIC) and
drug intervention (30). IPC
entails multiple transient ischemia-reperfusion treatments in
coronary arteries to activate the endogenous protective mechanisms
and enhance ischemic tolerance (31). IPost consists of allowing the
coronary blood to flow back for a period of time before obstructing
it again (32). This
reperfusion/obstruction process is repeated several times to
complete the reperfusion treatment (32). RIC entails the prevention of MIRI
by simultaneously applying ≥ one brief intermittent reperfusion
cycles to another organ or tissue (33). In addition, various novel drug
targets have been discovered, where examples such as Exenatide and
Cyclosporin have demonstrated potential (30). However, to date no effective
therapeutic agent exist for the prevention and treatment of
reperfusion injury (34). A
previous study found that pre-administration of fish oil and
flaxseed oil to rats alleviated MIRI through regulation of the
mitochondrial membrane permeability transport pore (35). In addition, a number of genes have
been considered to be promising targets, such as sirtuin 3(36), melatonin receptor 2(37) and protein inhibitor of activated
STAT1(16). Due to the lack of
known therapeutic targets approved for clinical use, the present
study assessed the effects of CBL on the proliferation, antioxidant
capacity and apoptosis of H9c2 rat cardiomyoblasts induced by H/R.
The results suggested that CBL may serve to be an effective
therapeutic target in the future.
Under normal physiological conditions, OFR increases
lipid peroxidation, resulting in the production of MDA and LDH
(38,39). OFR can be scavenged by SOD and
glutathione, which maintains the balance between the generation and
elimination of OFR (40). The
oxidative capacity of cardiomyocytes is significantly enhanced
during MIRI, where unsaturated fatty acids on the membrane can be
attacked to trigger alterations in the cell membrane functional
unit structure and function (19).
The increased production of OFRs may cause cardiomyocyte necrosis
and dysfunction (41). Therefore,
the present study explored the effects of CBL on the antioxidant
capacity of cardiomyocytes following H/R, which found that CBL
knockdown improved the antioxidant capacity of this H9c2 cell
model. In addition, associated proteins were predicted using the
STRING database, where it was found that GRB2 and CBL may be
associated. Additionally, GRB2-silencing was reported to suppress
TGF-β1-induced collagen production and cell viability in cardiac
fibrosis, revealing that GRB2 participated in the promotion of
cardiac fibrosis (42). Therefore,
it is proposed that GRB2 may be involved in the role of CBL in
cardiomyocytes. Results in the present study revealed that GRB2 was
involved in the mechanism of action of CBL.
Apoptosis is the most common type of programmed cell
death and has been shown to be the major pathological mechanism
underlying MIRI (15). Aberrant
levels of apoptosis will aggravate the destruction of the
myocardium, resulting in a decrease in the number of viable
myocardial cells and thus cardiac function, thereby aggravating
myocardial damage (43).
Mitochondria are vital for the production of ATP and the induction
of cell apoptosis (44).
Cytochrome c, a marker of activation of the mitochondrial
apoptosis pathway, can activate pro-caspase 9, thereby promoting
the activation of caspase-3(45).
In addition, Bax is a pro-apoptotic protein (46). Following activation of Bax, it
enters the mitochondria from the cytoplasm, increases the
permeability of the mitochondrial membrane and promotes the release
of cytochrome c, thereby mediating cell apoptosis (47). Bcl-2 is an anti-apoptotic protein,
which can inhibit the release of cytochrome c from the
mitochondria and inhibit cell apoptosis (48). In the present study, CBL knockdown
downregulated the expression of Bax and caspase-3 whilst
upregulating the expression of Bcl-2, suggesting that it can
alleviate the apoptosis of cardiomyocytes induced by H/R.
In conclusion, the results of the present study
revealed that CBL expression was upregulated in the plasma of
patients with ICM. CBL knockdown promoted the proliferation and
increased the antioxidant capacity of damaged cardiomyocytes
induced by H/R whilst inhibiting cell apoptosis, by regulating the
expression of GRB2. CBL may thus serve as a target for the
management of MIRI. Whilst the underlying mechanism was elucidated
to a certain depth, the present article is limited in that only
in vitro experiments were performed. Future studies should
verify these findings using in vivo models.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and XL designed the study. ZL, XL, BH and QY
performed the experiments. JL revised the manuscript for important
intellectual content. JL and YH collected the clinical data and
analyzed the data. All authors have read and approved the final
manuscript. JL and YH can confirm the authenticity of the raw
data.
Ethics approval and consent to
participate
All procedures were performed in accordance with the
Declaration of the Institutional Research Committee's Ethical
standards, as well as the 1964 Declaration of Helsinki and its
later amendments. The Ethics Committee of the Second People's
Hospital of Chengdu approved the involvement of human participants,
and the study ran from August 2017 to August 2019 (approval no.
2017081302). All the patients or their parents/guardians provided
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brueck M, Koerholz D, Nuernberger W,
Juergens H, Goebel U and Wahn V: Elimination of l-asparaginase in
children treated for acute lymphoblastic leukemia. Dev Pharmacol
Ther. 12:200–204. 1989.PubMed/NCBI
|
|
2
|
Roth GA, Mensah GA, Johnson CO, Addolorato
G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ,
Benziger CP, et al: Global burden of cardiovascular diseases and
risk factors, 1990-2019: Update from the GBD 2019 study. J Am Coll
Cardiol. 76:2982–3021. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dang H, Ye Y, Zhao X and Zeng Y:
Identification of candidate genes in ischemic cardiomyopathy by
gene expression omnibus database. BMC Cardiovasc Disord.
20(320)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dixon SR, Henriques JP, Mauri L, Sjauw K,
Civitello A, Kar B, Loyalka P, Resnic FS, Teirstein P, Makkar R, et
al: A prospective feasibility trial investigating the use of the
Impella 2.5 system in patients undergoing high-risk percutaneous
coronary intervention (The PROTECT I Trial): Initial U.S.
experience. JACC Cardiovasc Interv. 2:91–96. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Henriques JP, Remmelink M, Baan J Jr, van
der Schaaf RJ, Vis MM, Koch KT, Scholten EW, de Mol BA, Tijssen JG,
Piek JJ and de Winter RJ: Safety and feasibility of elective
high-risk percutaneous coronary intervention procedures with left
ventricular support of the Impella recover LP 2.5. Am J Cardiol.
97:990–992. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kiyooka T and Satoh Y: Mid-ventricular
obstructive hypertrophic cardiomyopathy with an apical aneurysm
caused by vasospastic angina. Tokai J Exp Clin Med. 39:29–33.
2014.PubMed/NCBI
|
|
7
|
Fanari Z, Abraham N, Hammami S and Qureshi
WA: High-risk acute coronary syndrome in a patient with coronary
subclavian steal syndrome secondary to critical subclavian artery
stenosis. Case Rep Cardiol. 2014(175235)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang X, Chen J and Huang X: Rosuvastatin
attenuates myocardial ischemia-reperfusion injury via upregulating
miR-17-3p-mediated autophagy. Cell Reprogram. 21:323–330.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bulluck H and Hausenloy DJ: Ischaemic
conditioning: Are we there yet? Heart. 101:1067–1077.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ziegler M, Wang X and Peter K: Platelets
in cardiac ischaemia/reperfusion injury: A promising therapeutic
target. Cardiovasc Res. 115:1178–1188. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fanari Z, Weiss S and Weintraub WS: Cost
effectiveness of antiplatelet and antithrombotic therapy in the
setting of acute coronary syndrome: Current perspective and
literature review. Am J Cardiovasc Drugs. 15:415–427.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
World Health Organization. The top 10
causes of death. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/.
WHO website, 2018.
|
|
15
|
Huang ZQ, Xu W, Wu JL, Lu X and Chen XM:
MicroRNA-374a protects against myocardial ischemia-reperfusion
injury in mice by targeting the MAPK6 pathway. Life Sci.
232(116619)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xie B, Liu X, Yang J, Cheng J, Gu J and
Xue S: PIAS1 protects against myocardial ischemia-reperfusion
injury by stimulating PPARγ SUMOylation. BMC Cell Biol.
19(24)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liang S, Ping Z and Ge J: Coenzyme Q10
regulates antioxidative stress and autophagy in acute myocardial
ischemia-reperfusion injury. Oxid Med Cell Longev.
2017(9863181)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen S, Zhu Q, Ju H, Hao J, Lai Z, Zou C,
Zhang W, Zhao S, Chen X, Zhang H, et al: The role of oxygen free
radicals in myocardial ischemia/reperfusion injury. Chin Med Sci J.
6:127–131. 1991.PubMed/NCBI
|
|
19
|
Tian L, Cao W, Yue R, Yuan Y, Guo X, Qin
D, Xing J and Wang X: Pretreatment with Tilianin improves
mitochondrial energy metabolism and oxidative stress in rats with
myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha
signaling pathway. J Pharmacol Sci. 139:352–360. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Grøvdal LM, Stang E, Sorkin A and Madshus
IH: Direct interaction of Cbl with pTyr 1045 of the EGF receptor
(EGFR) is required to sort the EGFR to lysosomes for degradation.
Exp Cell Res. 300:388–395. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schmidt MHH and Dikic I: The Cbl
interactome and its functions. Nat Rev Mol Cell Biol. 6:907–918.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Wu WJ, Tu S and Cerione RA: Activated
Cdc42 sequesters c-Cbl and prevents EGF receptor degradation. Cell.
114:715–725. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Swaminathan G and Tsygankov AY: The Cbl
family proteins: Ring leaders in regulation of cell signaling. J
Cell Physiol. 209:21–43. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rafiq K, Kolpakov MA, Seqqat R, Guo J, Guo
X, Qi Z, Yu D, Mohapatra B, Zutshi N, An W, et al: c-Cbl inhibition
improves cardiac function and survival in response to myocardial
ischemia. Circulation. 129:2031–2043. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yu SY, Dong B, Fang ZF, Hu XQ, Tang L and
Zhou SH: Knockdown of lncRNA AK139328 alleviates myocardial
ischaemia/reperfusion injury in diabetic mice via modulating
miR-204-3p and inhibiting autophagy. J Cell Mol Med. 22:4886–4898.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Richardson P, McKenna W, Bristow M, Maisch
B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I,
et al: Report of the 1995 World Health Organization/international
society and federation of cardiology task force on the definition
and classification of cardiomyopathies. Circulation. 93:841–842.
1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu D: Effects of Nicorandil combined with
Danhong injection on SOD and MDA content in patients with
myocardial ischemia-reperfusion injury. Mod J Integr Tradit Chin
West Med. 1:78–80. 2016.
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Szklarczyk D, Gable AL, Nastou KC, Lyon D,
Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, et al:
The STRING database in 2021: Customizable protein-protein networks,
and functional characterization of user-uploaded gene/measurement
sets. Nucleic Acids Res. 49:D605–D612. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hausenloy DJ: Conditioning the heart to
prevent myocardial reperfusion injury during PPCI. Eur Heart J
Acute Cardiovasc Care. 1:13–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hausenloy DJ, Candilio L, Evans R, Ariti
C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J,
et al: Remote ischemic preconditioning and outcomes of cardiac
surgery. N Engl J Med. 373:1408–1417. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Miao W, Yan Y, Bao TH, Jia WJ, Yang F,
Wang Y, Zhu YH, Yin M and Han JH: Ischemic postconditioning exerts
neuroprotective effect through negatively regulating PI3K/Akt2
signaling pathway by microRNA-124. Biomed Pharmacother.
126(109786)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Heusch G, Bøtker HE, Przyklenk K,
Redington A and Yellon D: Remote ischemic conditioning. J Am Coll
Cardiol. 65:177–195. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Soares ROS, Losada DM, Jordani MC, Évora P
and Castro-E-Silva O: Ischemia/reperfusion injury revisited: An
overview of the latest pharmacological strategies. Int J Mol Sci.
20(5034)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ivary SHA, Jajarmy N, Shahri MK, Shokoohi
M, Shoorei H, Ebadi A, Moghimian M and Sigaroodi F: Effect of fish
and flaxseed oil supplementation on isoprenaline-induced myocardial
infarction in rats: Inhibition of mitochondrial permeability
transition pore opening. Crescent J Med Biol Sci. 6:158–163.
2019.
|
|
36
|
Zheng Y, Shi B, Ma M, Wu X and Lin X: The
novel relationship between Sirt3 and autophagy in myocardial
ischemia-reperfusion. J Cell Physiol. 234:5488–5495.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Han D, Wang Y, Chen J, Zhang J, Yu P,
Zhang R, Li S, Tao B, Wang Y, Qiu Y, et al: Activation of melatonin
receptor 2 but not melatonin receptor 1 mediates
melatonin-conferred cardioprotection against myocardial
ischemia/reperfusion injury. J Pineal Res.
67(e12571)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li J, Zheng X, Ma X, Xu X, Du Y, Lv Q, Li
X, Wu Y, Sun H, Yu L and Zhang Z: Melatonin protects against
chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2
pathway. J Inorg Biochem. 197(110698)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang D, Yang Q, Fu N, Li S, Han B, Liu Y,
Tang Y, Guo X, Lv Z and Zhang Z: Hexavalent chromium induced heart
dysfunction via Sesn2-mediated impairment of mitochondrial function
and energy supply. Chemosphere. 264(128547)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bouayed J and Bohn T: Exogenous
antioxidants-double-edged swords in cellular redox state: Health
beneficial effects at physiologic doses versus deleterious effects
at high doses. Oxid Med Cell Longev. 3:228–237. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Puente BN, Kimura W, Muralidhar SA, Moon
J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R,
Garcia JA, et al: The oxygen-rich postnatal environment induces
cardiomyocyte cell-cycle arrest through DNA damage response. Cell.
157:565–579. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sun F, Zhuang Y, Zhu H, Wu H, Li D, Zhan
L, Yang W, Yuan Y, Xie Y, Yang S, et al: LncRNA PCFL promotes
cardiac fibrosis via miR-378/GRB2 pathway following myocardial
infarction. J Mol Cell Cardiol. 133:188–198. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Peng X, Lin L, Zhou X, Yang D, Cao Y, Yin
T and Liu Y: miR-133b inhibits myocardial
ischemia-reperfusion-induced cardiomyocyte apoptosis and
accumulation of reactive oxygen species in rats by targeting YES1.
Nan Fang Yi Ke Da Xue Xue Bao. 40:1390–1398. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
44
|
Su C, Fan X, Xu F, Wang J and Chen Y:
Prostaglandin E1 attenuates post-cardiac arrest myocardial
dysfunction through inhibition of mitochondria-mediated
cardiomyocyte apoptosis. Mol Med Rep. 23(110)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chuang GC, Xia H, Mahne SE and Varner KJ:
Environmentally persistent free radicals cause apoptosis in HL-1
cardiomyocytes. Cardiovasc Toxicol. 17:140–149. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pirocanac EC, Nassirpour R, Yang M, Wang
J, Nardin SR, Gu J, Fang B, Moossa AR, Hoffman RM and Bouvet M:
Bax-induction gene therapy of pancreatic cancer. J Surg Res.
106:346–351. 2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dejean LM, Martinez-Caballero S, Guo L,
Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B,
Jonas EA and Kinnally KW: Oligomeric Bax is a component of the
putative cytochrome c release channel MAC, mitochondrial
apoptosis-induced channel. Mol Biol Cell. 16:2424–2432.
2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997.PubMed/NCBI View Article : Google Scholar
|