Introduction
Osteoarthritis (OA) is an age-related degenerative
joint disease that is characterized by the loss of articular
cartilage, synovitis, subchondral bone sclerosis and osteophyte
formation (1). With the
intensification of population ageing, the incidence of OA has
increased from 29.2 to 40.5/1,000 person-years between 1992 and
2013(2), and OA seriously
endangers the health of elderly people (3). At present, the aetiology and
pathogenesis of OA are still not completely clear; OA is generally
considered the result of the joint contribution of biological and
mechanical factors (4). The death
of cartilage cells, the only type of cell in articular cartilage,
is considered the main biological factor (5). A large amount of extracellular
matrix, known as type II collagen (Col II), surrounds chondrocytes
(6). Matrix metalloproteinase
(MMP)-3 and MMP-13 are two main enzymes responsible for the
degradation of cartilage matrix (7). Decreased anabolism of cartilage
extracellular matrix components, such as Col II and proteoglycans,
and increased cartilage-related catabolism components, such as
MMP-13 and MMP-3, are characteristic manifestations of cartilage
cell degradation (8). Therefore,
delaying the degradation and death of chondrocytes is of major
significance for delaying the pathological process of OA (5).
Pyroptosis is a newly discovered mechanism of cell
death; it is characterized by cell swelling and rupture, cell
membrane dissolution, release of cytoplasmic contents to the
outside of the cell and chromosomal DNA breakage (9). The typical activation pathway of
pyroptosis is mediated by the NOD-like receptor protein 3 (NLRP3)
inflammasome, which mainly includes NLRP3, apoptosis-associated
speck-like protein containing CARD (ASC) and pro-caspase-1(10). In the typical activation pathway,
NLRP3 is activated in response to stimuli inside and outside the
cell, recruits ASC and assembles into the NLRP3 inflammasome by
combining ASC with pro-caspase-1(11). The activated NLRP3 inflammasome
cleaves pro-caspase-1 into activated caspase-1, which can both
cleave the precursors of IL-1β and IL-18, and promote their
maturation and secretion. The inflammasome can also cleave
gasdermin D (GSDMD) and create GSDMD-N pores in the membrane,
thereby inducing cell pyroptosis (12).
There is evidence that the NLRP3 inflammasome is
involved in the pathogenesis of OA and can lead to cartilage
degeneration and synovial inflammation through the activation of
Toll-like receptors and NF-κB signal transduction (13). The latest research shows that
exogenous stromal cell-derived factor-1 inhibits the NLRP3
inflammasome by activating the adenosine 5'-monophosphate-activated
protein kinase (AMPK) signalling pathway, and that it inhibits the
pyroptosis of synovial cells in OA (14). The NLRP3 inflammasome has gradually
become a new therapeutic target for OA (15). The pathways that activate the NLRP3
inflammasome, such as potassium efflux and the production of
reactive oxygen species, have been extensively studied (16). Currently, the literature on the
involvement of the NLRP3 inflammasome in the pathogenesis of OA and
its potential use as a biomarker is limited.
Metformin is the first-line medication for diabetes
treatment (17). Studies have
shown that metformin, as an AMPK activator, can affect bone
metabolism (18) and enhance the
anti-inflammatory properties of experimental OA mesenchymal stem
cells (19). Moreover, it has been
indicated that metformin can reverse the interferon-inducible
protein AIM2-related pyroptosis of macrophages caused by diabetes
(20), and can protect against
myocardial ischaemia-reperfusion injury and decrease myocardial
cell pyroptosis through the AMPK/NLRP3 inflammasome pathway
(21). Metformin can also exert an
anti-periodontitis effect by targeting the NLRP3 inflammasome
(22). However, the mechanism
through which metformin affects the activation of NLRP3
inflammasome in OA chondrocytes and delays the progression of OA
remains unclear.
To the best of the authors' knowledge, the effect of
metformin on the pyroptosis of OA chondrocytes remains to be
elucidated. In the present study, an OA mouse model was constructed
through destabilization of the medial meniscus (DMM) surgery to
investigate the therapeutic effects of metformin on knee OA in mice
from a novel perspective.
Materials and methods
Ethics statement
All experiments were approved by the Animal
Experiment Ethics Committee of Ningxia Medical University
(Yinchuan, China; protocol no. 2020-115). All experiments were
conducted under the standard principles of animal experiment
ethics.
Animals and the DMM-induced OA
model
All animals in the present study were healthy,
wild-type, adult male C57BL/6 mice [specific pathogen-free (SPF)
grade], aged 8 weeks and weighing 20-25 g. The mice were obtained
from the Laboratory Animal Centre of Ningxia Medical University.
All experimental subjects were maintained in an SPF environment at
a temperature of 22±1˚C with a humidity of 55%, a 12-h light/dark
cycle and free access to food and water. In the experiment,
surgically induced OA was achieved through DMM. First, the mice
were weighed and anesthetized via intraperitoneal injection of 0.2%
pentobarbital sodium (40 mg/kg) in phosphate-buffered saline (PBS;
OriGene Technologies, Inc.). After cutting off the nodular sac, the
meniscus tibial ligament was cut open by microsurgery, and the
anterior horn of the medial meniscus was freed to ensure that the
tibial ligament of the inner meniscus was cut. The procedures for
the sham operation were the same as those for the DMM operation
except that the joint capsule was sutured directly after opening
and confirming the absence of abnormalities; that is, no treatment
was performed on the meniscus tibial ligament.
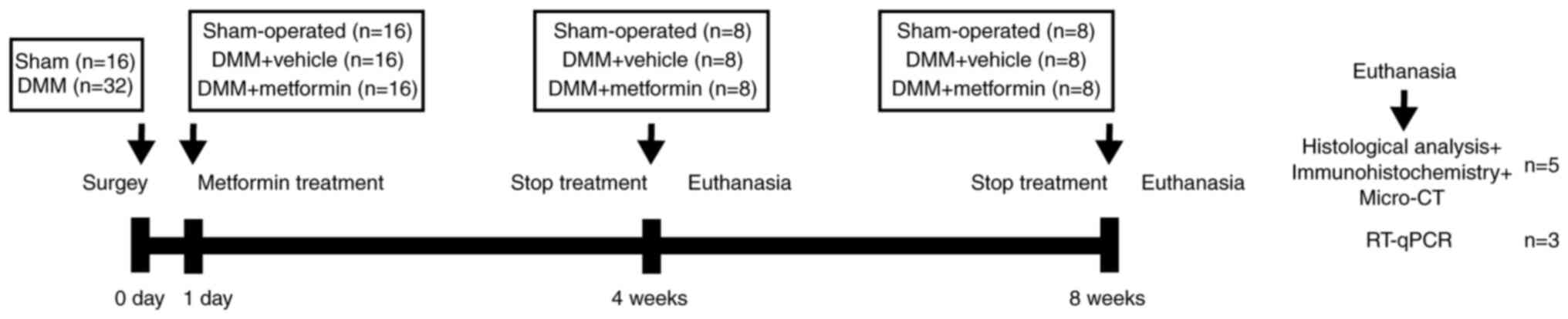
Experimental design and
processing
A total of 48 mice were randomly divided into three
groups (16 in each group) as follows: A control group (sham
operation group), an OA group (DMM group) and a metformin
(Sino-American Shanghai Squibb Pharmaceuticals Ltd.) treatment
group. The metformin treatment group was administered daily
metformin (200 mg/kg) via oral gavage based on the results of a
previous study (23), with
administration initiated on the second day after surgery (8 mice
for 4 weeks and another 8 mice for 8 weeks). The sham operation and
DMM groups were administered an equivalent dose of normal saline as
a drug-treatment control. At 4 and 8 weeks following surgery, 8
mice from each group were sacrificed by cervical dislocation, and
knee joint tissues were collected to evaluate the severity of OA.
The schematic diagram of the animal experiments is presented in
Fig. 1.
Histological evaluation of articular
cartilage degeneration
Follow-up experiments were performed on the right
knee joints of 5 mice in each group. The right knee joint of each
mouse was fixed in 4% paraformaldehyde for 24 h at 20˚C, rinsed
with PBS every hour for 6 h, and then decalcified with 10% EDTA
(cat. no. AR1071; Wuhan Boster Biological Technology Co., Ltd.) for
2 weeks at 20˚C. Next, the tissue was dehydrated with gradient
ethanol and embedded in paraffin. The thickness of the sagittal
section of the knee joint was 4 µm, and the sections were stained
with safranin O-fast green and H&E. According to H&E
staining kit (cat. no. G1005; Wuhan Servicebio Technology Co.,
Ltd.), the sections were placed in xylene twice for 15 min,
anhydrous ethanol twice (soaked for 7 min) and finally, in 75%
alcohol for 7 min. The sections were stained with hematoxylin at
20˚C for 5 min, rinsed in running water for 5 min, dehydrated in 85
and 95% alcohol for 10 min each, and finally stained with eosin at
20˚C for 5 min. For staining with safranin O-fast green (cat. no.
G1053; Wuhan Servicebio Technology Co., Ltd.), the
deparaffinization of slides were consistent with the description
above, and subsequently stained with Fast Green for 6 min, washed
at 20˚C, dehydrated and stained with Safranin O at 20˚C for 3 min.
Using the Osteoarthritis Research Society International (OARSI)
scoring system to evaluate the degeneration of articular cartilage,
as described previously (24). The
distance from the tidemark to the surface of the articular
cartilage was measured and recorded as the thickness of the hyaline
cartilage (HC), and the distance from the tidemark to the
subchondral bone plate was recorded as the thickness of the
calcified cartilage (CC). Then, five random views from three
sections per mouse were visualized using a DP71 light microscope
with DP controller 3.3.1.292 software (Olympus Corporation) and
quantified using ImageJ 1.48v software (National Institutes of
Health).
Immunohistochemistry
Paraffin-embedded mouse knee joint sections at a
thickness of 4 µm were deparaffinized with xylene and rehydrated
with a graduated ethanol series. For antigen retrieval, 0.1%
trypsin was applied to each section at 37˚C for 15 min, and the
sections were incubated with 3% hydrogen peroxide at 37˚C for 10
min to deactivate endogenous peroxidase. Next, the sections were
blocked with 5% normal goat serum (OriGene Technologies, Inc.) at
37˚C for 30 min and incubated overnight at 4˚C with the following
primary antibodies: Anti-MMP-13 (cat. no. ab39012; 1:300 dilution;
Abcam), anti-Col II (cat. no. ab34712; 1:300 dilution; Abcam),
anti-NLRP3 (cat. no. ab214185; 1:200 dilution; Abcam),
anti-caspase-1 (cat. no. 22915-1-AP; 1:200 dilution; ProteinTech
Group, Inc.), anti-GSDMD (cat. no. ab219800; 1:200 dilution; Abcam)
and anti-IL-1β (cat. no. ab205924; 1:300 dilution; Abcam). After
that, sections were processed using a two-step IHC detection
reagent (ZSGB-Bio; OriGene Technologies, Inc.). Briefly, sections
were incubated with reaction enhancement solution (reagent 1) at
37˚C for 30 min and then with enhanced enzyme-labeled goat
anti-rabbit IgG polymer (reagent 2) at 37˚C for 30 min. The
sections were then developed using 3,3'-diaminobenzidine (DAB)
(ZSGB-Bio; OriGene Technologies, Inc.), followed by counterstaining
with hematoxylin (ZSGB-Bio; OriGene Technologies, Inc.). Positively
stained cells in five random views from three sections per mouse
were visualized using a DP71 light microscope with DP controller
3.3.1.292 software (Olympus Corporation) and quantified using
ImageJ 1.48v software (National Institutes of Health).
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
RNA extraction was performed according to the
Minimum Information Published in Quantitative Real-time PCR
Experiments (MIQE) guidelines (25). A tissue extraction kit (cat. no.
AP-MN-MS-RNA-250; Axygen; Corning, Inc.) was used to extract total
RNA from knee joints of the mice in the 4 and 8 weeks groups.
Complementary DNA was synthesized from 1 µg total RNA using a
TransScript All-in-One First-Strand cDNA Synthesis kit (cat. no.
AT341-01; TransGen Biotech Co., Ltd.), according to the
manufacturer's protocol and a S1000 Thermal Cycler (Bio-Rad
Laboratories, Inc.). qPCR was performed with 10 µl 2X ChamQ SYBR
qPCR Master Mix (cat. no. Q311-02; Vazyme Biotech Co., Ltd.), 0.4
µl primers and 1 µl template cDNA (total reaction volume, 20 µl).
The qPCR parameters were as follows: 95˚C for 30 sec, 40 cycles of
95˚C for 10 sec and 60˚C for 1 min. qPCR was performed in
triplicate using an iQ5 system (Bio-Rad Laboratories, Inc.). The
primer sequences used in the present study are listed in Table I.
 | Table IPrimer sequences used in reverse
transcription-quantitative PCR. |
Table I
Primer sequences used in reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| NLRP3 |
TGCCGTGGTCTCTTCTCAAG |
GTCGAAGCAGCATTGATGGG |
| Caspase-1 |
TGATGGCATTAAGAAGGCCCA |
TCCAAGTCACAAGACCAGGC |
| GSDMD |
ATGGGAACATTCAGGGCAGAG |
ACCTCAGTGATCTGCACTTCC |
| IL-1β |
TGACGGACCCCAAAAGATGAAG |
AGCTCTTGTTGATGTGCTGC |
| β-actin |
GTGCTATGTTGCTCTAGACTTCG |
ATGCCACAGGATTCCATACC |
β-actin was used as a reference gene. Relative gene
expression was calculated using the 2-ΔΔCq method
(26).
Micro-CT
The mouse knee joint was scanned with a micro-CT
device (SkyScan 1176; Bruker Belgium S.A./N.V.) and NRecon v1.6
software (Bruker), and reconstructed micro-CT images were obtained.
Data analysis software (CTAn v1.9; Bruker) and three-dimensional
model visualization software (CTVol v2.0; Bruker) were used to
analyse the data. Visual evaluation of structure in the images was
performed, and the quantitative morphometric index was determined
based on microtomographic data obtained from the three-dimensional
morphometric measurements (27).
The area of interest was between the proximal tibial growth plate
and the tibial plateau. Evaluation indicators included the bone
volume fraction (BV/TV; %), bone mineral density (BMD;
g/cm3) and trabecular separation (Tb.Sp; mm).
Statistical analysis
All data are expressed as the mean ± SD. GraphPad
Prism 8 software (Graphpad Software, Inc.) was used for the
statistical analysis. One-way ANOVA followed by Tukey's multiple
comparison test was used to compare data among groups after testing
the data for homogeneity of variance. Non-parametric data (OARSI
scores) were analysed using the Kruskal-Wallis H test followed by
Dunn's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
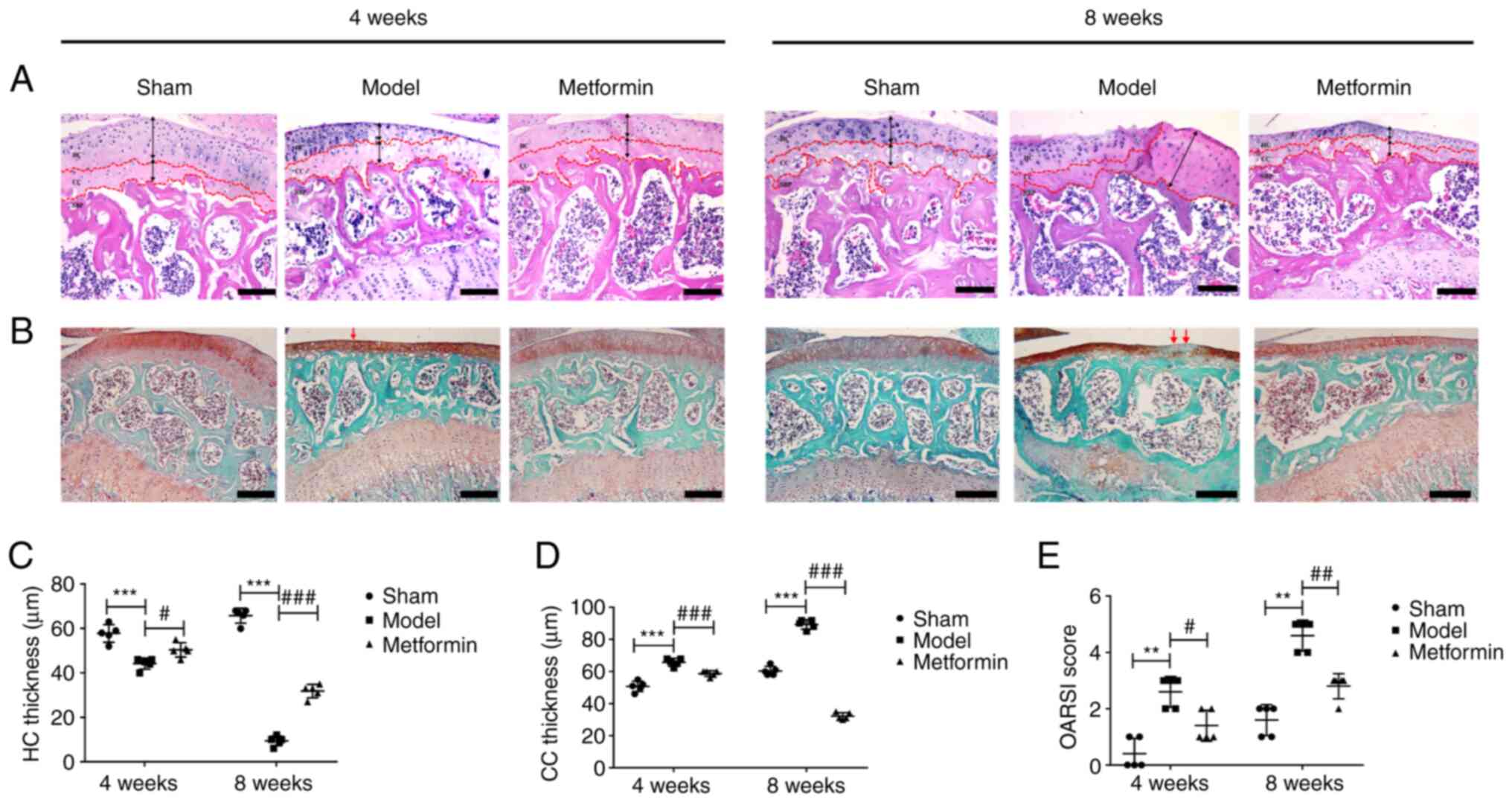
Metformin can decrease cartilage
degradation in a DMM model
To study the effect of metformin treatment on
cartilage degradation in the DMM-induced OA model, histological
analysis of safranin O-fast green staining was performed 4 and 8
weeks after DMM surgery. In the sham operation group, the articular
cartilage surface was complete and smoothly connected, the tide
line was obvious and clear, the cartilage thickness was moderate,
the number of cartilage cells was normal, and the structure was
regular. Compared with that of the sham operation group, the
cartilage of the DMM group exhibited surface destruction,
discontinuity, a decreased number of cells and a large amount of
proteoglycan loss. At 8 weeks after DMM surgery, HC was lost and CC
was evident. Metformin gavage treatment significantly increased the
thickness of the articular cartilage and decreased cartilage damage
compared with that in the DMM group (Fig. 2A and B). The OARSI histology scoring system was
used to evaluate the safranin O-fast green-stained sections. The
OARSI score of the DMM group was significantly higher than that of
the sham group (Fig. 2E). At 4
weeks after DMM surgery, the metformin treatment group showed a
lower OARSI score compared with the DMM group. At 8 weeks after DMM
surgery, a significant decrease in the OARSI score was observed in
the metformin-treated mice compared with that in the DMM-only mice.
It was also demonstrated that DMM surgery resulted in a significant
decrease in HC thickness and a significant increase in CC
thickness, and that treatment with metformin was able to reverse
these changes in mice at 4 and 8 weeks after DMM surgery (Fig. 2C and D).
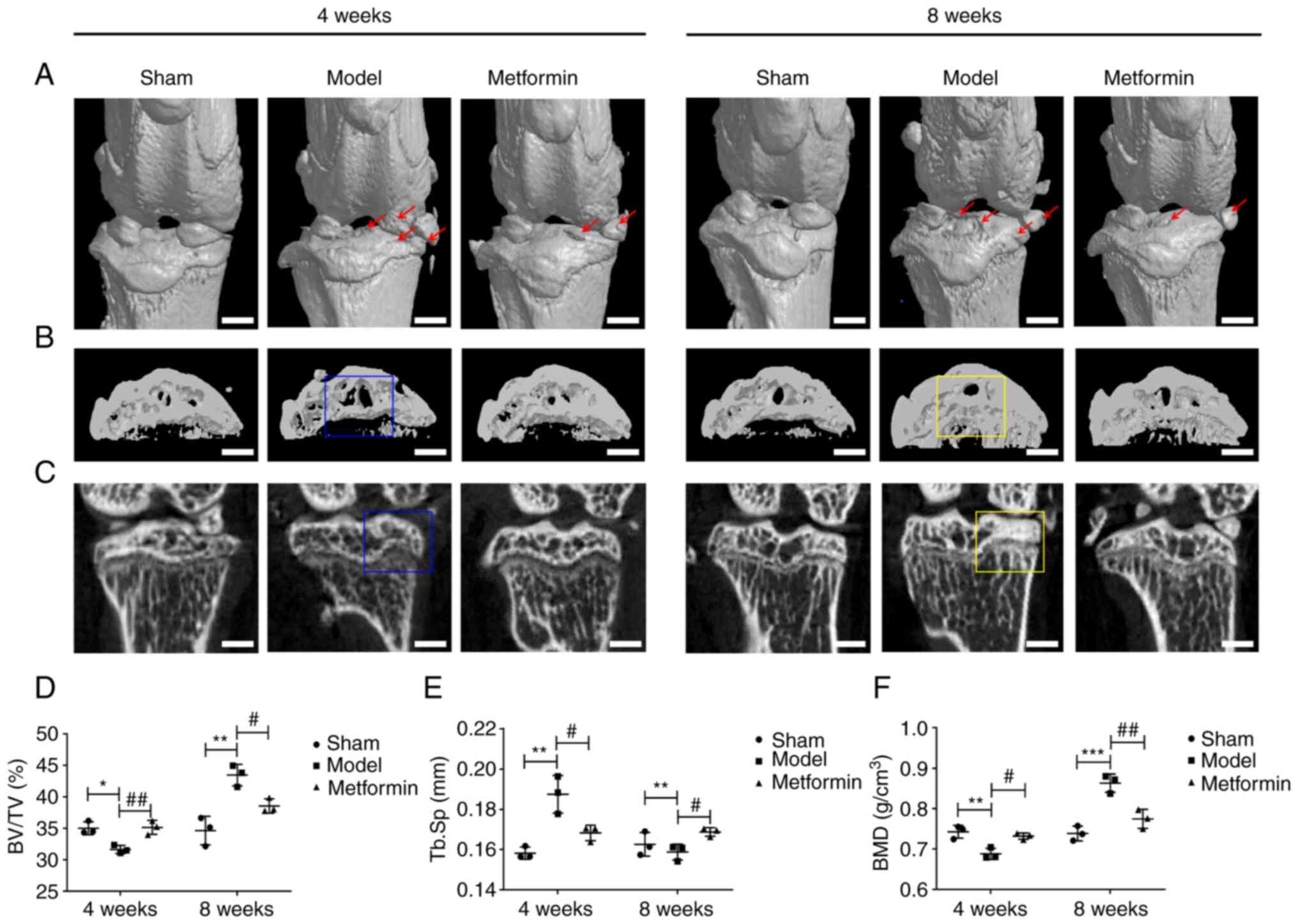
Metformin can improve subchondral bone
remodelling in a DMM model
To evaluate the structural changes in subchondral
bone in the metformin-treated OA model mice, three-dimensional
imaging was performed using micro-CT, and quantitative morphometric
indicators were analysed. Metformin prominently decreased
DMM-induced osteophyte formation and destruction of articular
cartilage (Fig. 3A). At 4 weeks,
compared with the sham group, the DMM group demonstrated a
pathological change, exhibiting subchondral bone loss in the tibia.
At 8 weeks, a notable increase in bone mass was observed in the DMM
group compared with that in the sham group, and bone sclerosis was
present in the DMM mice. In the metformin treatment group,
expansion of the bone marrow cavity and loss of bone mass were not
observed at 4 weeks. At 8 weeks, there was also no increase in
either bone mass or bone sclerosis in the metformin treatment group
compared with that in the DMM group (Fig. 3B and C). Furthermore, at 4 weeks, BV/TV and BMD
in the DMM group were decreased compared with those values in the
sham group, while Tb.Sp was increased in the DMM group compared
with that in the sham group. These changes were partially reversed
in the metformin treatment group. At 8 weeks, BV/TV and BMD were
significantly increased and Tb.Sp was decreased in the DMM group
compared with those values in the sham group, while in the
metformin treatment group, these changes were significantly
mitigated (Fig. 3D-F). The changes
in the aforementioned parameters indicated that metformin could
improve subchondral bone remodelling in DMM model mice.
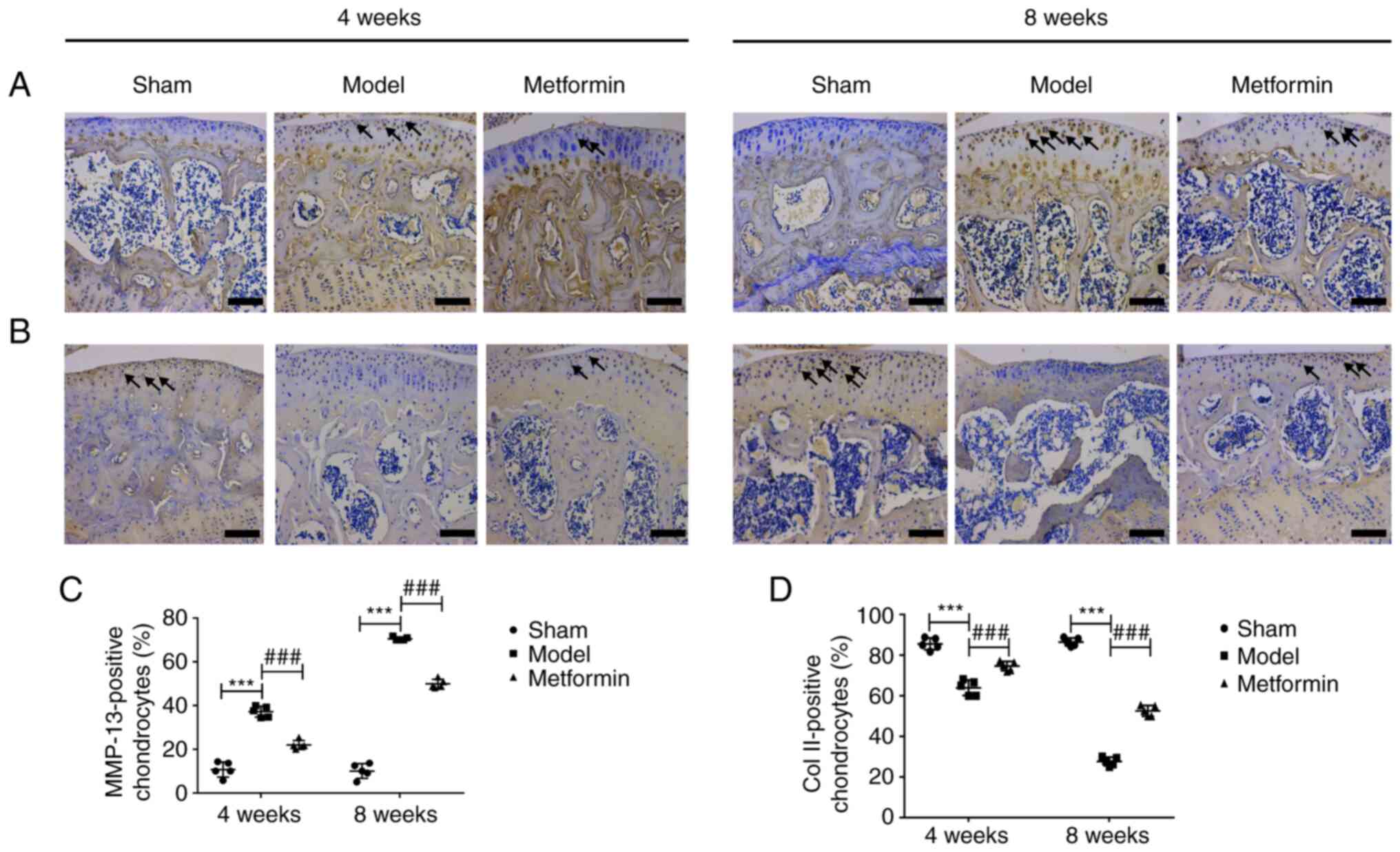
Metformin can enhance cartilage matrix
anabolism and inhibit its catabolism
To explore the mechanism by which metformin delays
cartilage damage in the mouse OA model, immunohistochemical
staining of Col II and MMP-13 was performed, as presented in
Fig. 4. The results of
immunohistochemical staining demonstrated that the expression of
MMP-13 was significantly increased after 4 and 8 weeks. Aberrantly
expressed MMP-13 was recovered in the metformin treatment group
compared with the DMM group at both 4 and 8 weeks following surgery
(Fig. 4A and C). In addition, the expression of Col II
was significantly decreased after 4 and 8 weeks. Aberrantly
expressed Col II was recovered in the metformin treatment group
compared with the DMM group at both 4 and 8 weeks following surgery
(Fig. 4B and D). In summary, metformin had a protective
effect in the DMM-induced OA model, which was mainly mediated
through the upregulation of Col II expression and the
downregulation of MMP-13 expression.
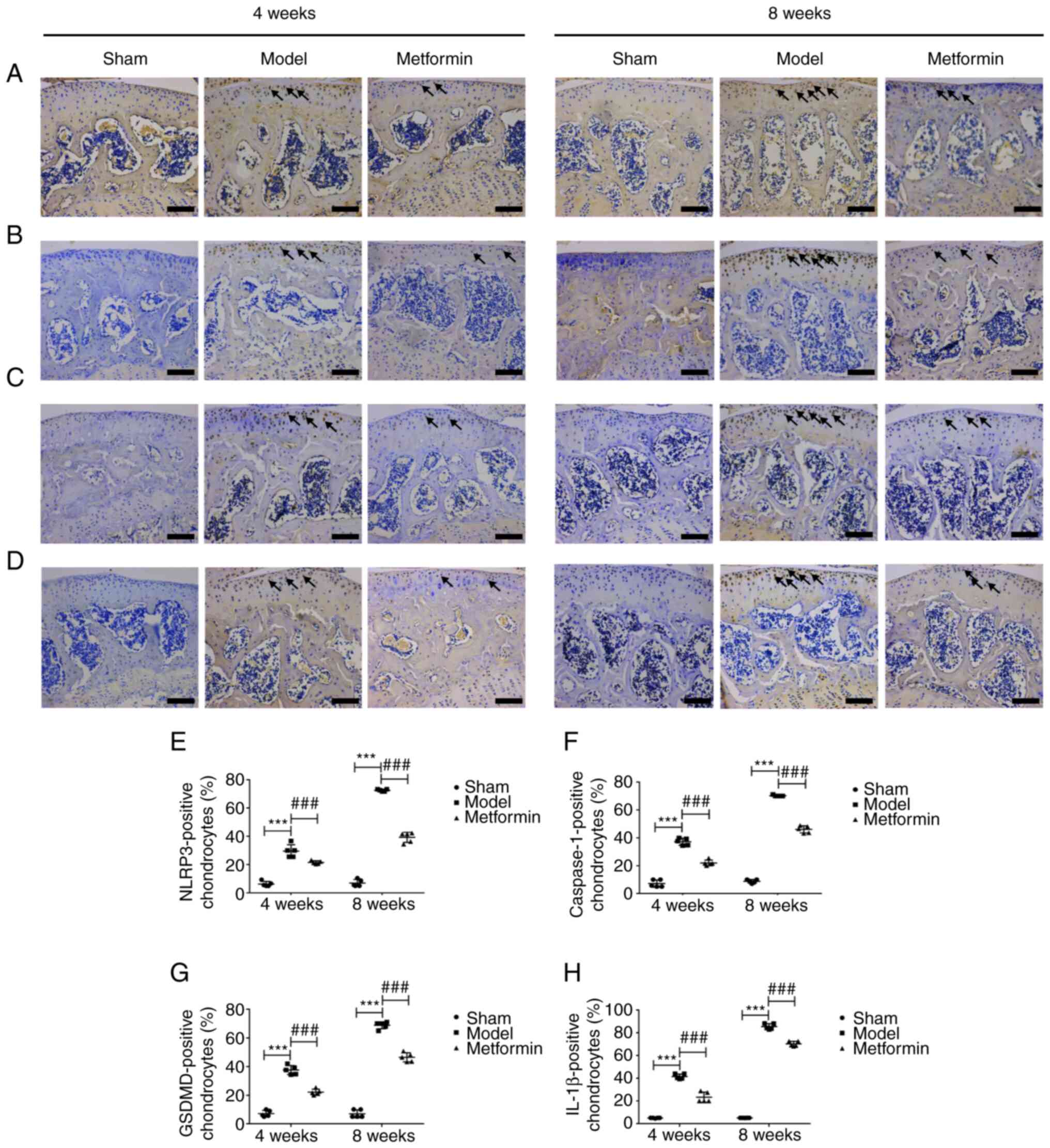
Metformin can decrease chondrocyte
pyroptosis in a DMM model
To investigate whether cartilage degradation is
related to chondrocyte pyrolysis, NLRP3, caspase-1, GSDMD and IL-1β
immunohistochemical staining and RT-qPCR detection was performed on
mouse knee joints collected at 4 and 8 weeks after DMM. The results
of immunohistochemical staining demonstrated that the expression of
NLRP3, caspase-1, GSDMD and IL-1β were significantly increased
after 4 and 8 weeks. Aberrantly expressed NLRP3, caspase-1, GSDMD
and IL-1β were recovered in the metformin treatment group compared
with the DMM group at both 4 and 8 weeks following surgery
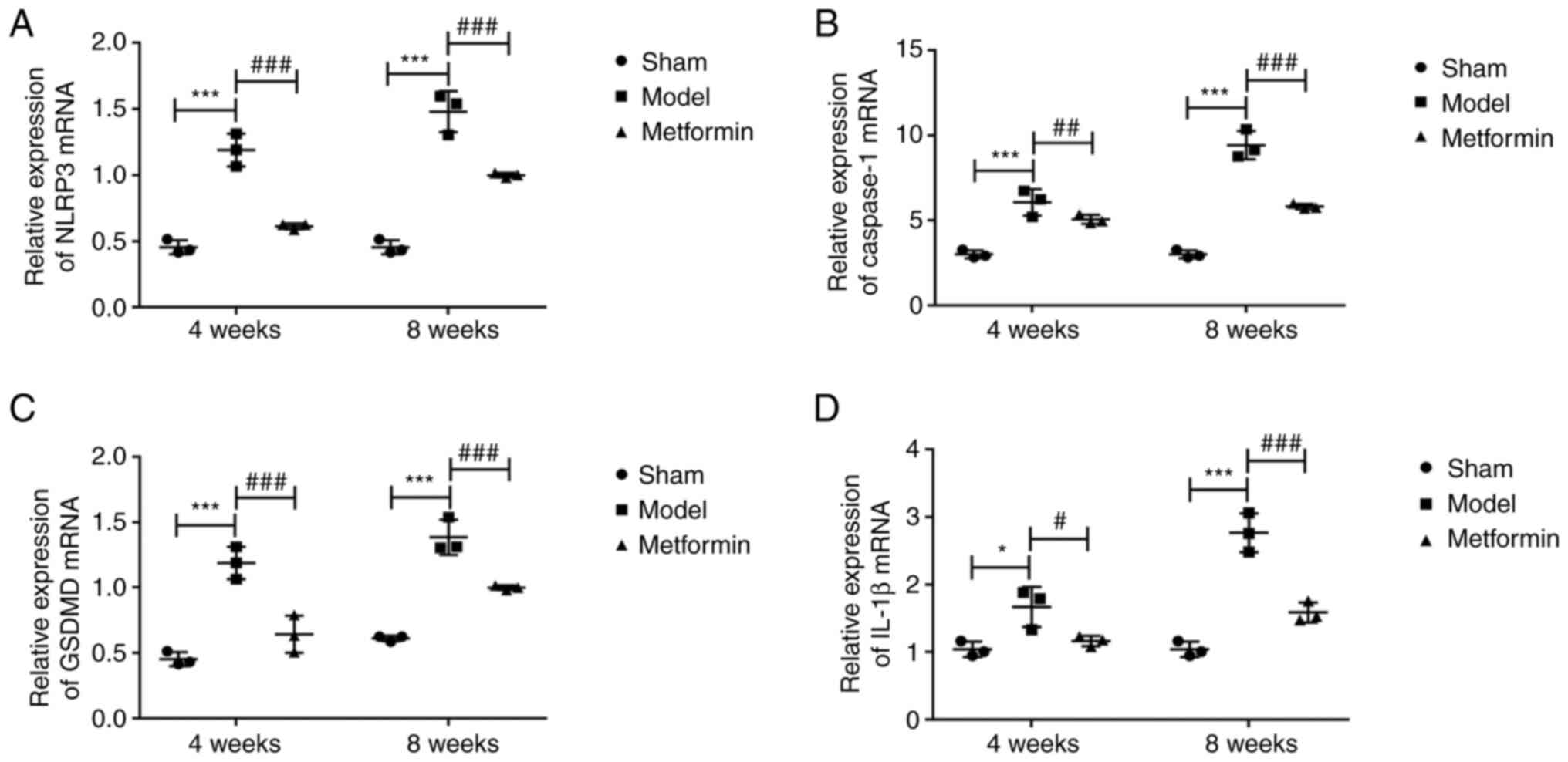
(Fig. 5). The RT-qPCR results were
consistent with those from immunohistochemical staining. The mRNA
levels of NLRP3, caspase-1, GSDMD and IL-1β were significantly
increased after 4 and 8 weeks. The abnormal mRNA levels of NLRP3,
caspase-1, GSDMD and IL-1β were recovered in the metformin
treatment group compared with the DMM group at both 4 and 8 weeks
following surgery (Fig. 6). These
results revealed that treatment with metformin can reduce the level
of pyroptosis in OA chondrocytes.
Discussion
To the best of the authors' knowledge, the present
study provides the first demonstration that metformin delays the
degeneration of articular cartilage after DMM by inhibiting the
activation of the NLRP3 inflammasome. The current study found that
metformin can decrease the occurrence of chondrocyte pyroptosis by
affecting the activation of NLRP3 inflammasome, and that it can
ultimately delay the progression of OA. Knee OA was induced in
C57BL/6 mice through DMM. Articular cartilage exhibited early
degenerative changes 4 weeks after DMM and late degenerative
changes after 8 weeks. The current study demonstrated that
metformin not only exerted a chondroprotective effect by increasing
HC thickness and decreasing CC thickness, cartilage degeneration
(as measured using the OARSI score) and osteophyte formation, but
also that it enhanced cartilage matrix anabolism, inhibited
cartilage catabolism and decreased the occurrence of chondrocyte
pyroptosis in articular cartilage.
Pyroptosis is increasingly being identified as
closely related to the pathophysiological changes in certain
chronic diseases including rheumatoid arthritis and osteoarthritis
(28). Activation of the NLRP3
inflammasome is the core driver of pyroptosis (29). To date, the occurrence of pyrolysis
mediated by NLRP3 inflammasome activation in knee OA and the
specific mechanisms involved have remained unclear. Denoble et
al (30) demonstrated that
uric acid in the synovium of patients with OA could activate
pre-IL-18 and pre-IL-1β by activating the NLRP3 inflammasome, and
that IL-18 and IL-1β levels in the synovium were positively
associated with the severity of OA. Therefore, we hypothesize that
pyroptosis mediated by NLRP3 inflammasome activation may be
involved in the process of OA. Our study revealed that in the early
stage of OA (4 weeks), cartilage degradation and cartilage matrix
catabolism had started to significantly increase compared with the
sham group, and anabolic metabolism had started to significantly
decrease compared with the sham group. During the same period,
chondrocyte pyrolysis was significantly increased. When OA
developed to the late stage (8 weeks), cartilage degradation and
cartilage matrix catabolism had increased further, and anabolic
metabolism had decreased further. During the same period,
chondrocyte pyroptosis was also significantly increased. Treatment
with metformin reversed the aforementioned changes. The present
results indicated that cell pyroptosis is involved in the
development of OA and that the degradation of OA chondrocytes may
be achieved through chondrocyte pyrolysis. The activation of the
NLRP3 inflammasome plays a key role in this process. According to a
previous report, the expression of NLRP3, IL-18 and IL-1β in the
synovial fluid of mice is markedly increased in mice with
collagen-induced arthritis, furthermore, the expression of NLRP3 is
associated with the severity of knee OA (31). These observations are consistent
with the present study results. Xyloside can decrease the synovitis
and fibrosis in knee OA by inhibiting HIF-1α and the NLRP3
inflammasome (32). This
observation confirms our study finding that inhibiting chondrocytes
pyrolysis may be a feasible strategy for alleviating cartilage
degeneration in knee OA. Anti-inflammatory therapy targeting the
NLRP3 inflammasome, as an important participant in pyroptosis, may
be a novel method for the treatment of OA (33).
Metformin was first used as a hypoglycaemic drug,
and it has an anti-inflammatory effect and a regulatory effect on
bone (28). Metformin may serve a
role in the treatment of OA. Numerous animal studies have shown
that metformin affects the development of OA by activating the AMPK
signalling pathway (23,34). At the cellular level, metformin
protects chondrocytes from IL-1β-induced damage by regulating the
AMPK/NF-κB signalling pathway (35). These observations are consistent
with our study findings. Metformin treatment decreased cartilage
degradation, inhibited cartilage matrix catabolism and enhanced
cartilage matrix anabolism in both the early and late stages of OA
in our study. Immunohistochemistry and RT-qPCR analyses were
employed, and indicated that metformin effectively inhibited the
occurrence of chondrocyte pyrolysis in OA mice. Previous studies
have confirmed that the inhibition of mitochondrial ATP and DNA
synthesis by metformin inhibits the activation of NLRP3
inflammasome and lung inflammation (36), and can improve depression-like
symptoms in mice by inhibiting peripheral and central NF-κB-NLRP3
inflammation activation (37).
Based on the aforementioned findings, we hypothesize that metformin
can decrease the occurrence of OA chondrocyte pyrolysis by
inhibiting the NLRP3 inflammasome, inhibiting cartilage
degradation, promoting cartilage anabolism and inhibiting cartilage
catabolism. This route may be another important pathway by which
metformin delays the progression of OA, in addition to the AMPK
pathway. The most important limitation of our study is that the
research was limited to the level of small animals. The role of
metformin in the development of knee OA through the inhibition of
chondrocyte pyroptosis requires further investigation at the
cellular level. Regarding the therapeutic effect of metformin in
the development of OA, further research is needed, including
clinical studies with large cohorts.
Subchondral bone is one of the basic units that
constitutes the structure and function of joints by maintaining the
normal structure and function of cartilage (38). A number of animal experiments have
demonstrated that early damage to the subchondral bone occurs
before cartilage degeneration and osteophyte formation, mainly via
enhanced bone resorption, which manifests as a decrease in bone
volume and a thickening of the CC layer. Later damage is mainly
caused by bone formation, manifested as subchondral bone sclerosis
(39,40). The present study revealed that
metformin could improve this abnormal bone remodelling of the
subchondral bone in a DMM model. Bone and cartilage are the
functional and structural units of the knee joint, and the present
results confirmed that metformin could directly act on chondrocytes
and decrease the pyroptosis of OA chondrocytes. It is hypothesized
that metformin may also act first on the OA subchondral bone to
improve abnormal bone remodelling and indirectly act on
chondrocytes through the subchondral bone. Research on the
mechanism underlying the effects of metformin on OA cartilage and
subchondral bone is required at the animal and cellular levels to
confirm this.
In conclusion, the present study demonstrated that
metformin improved the progression of OA in a mouse model of DMM
surgery-induced OA. Metformin inhibited the activation of NLRP3
inflammasome, decreased cartilage degradation, improved subchondral
bone remodelling and inhibited chondrocyte pyroptosis. These
findings enhance our understanding of the role of metformin as a
promising drug for the treatment of OA.
Acknowledgements
The authors would like to thank Dr Xueyu Hu (The
General Hospital of Ningxia Medical University, Yinchuan, China)
for providing experimental technical support.
Funding
Funding: The present study was supported by the Scientific
Research Project from Ningxia Province (grant no. 2021AAC03396) and
the Scientific Research Project of the Key Research and Development
Project from Ningxia Province (grant no. 2018BEG02005).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QJ and ZL designed the experiment. JY, DD and GF
conducted the experiment. YZ, YY, LM and HG analysed the data, and
drafted and revised the manuscript. QJ, ZL and JY confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Animal
Experiment Ethics Committee of Ningxia Medical University
(Yinchuan, China; protocol no. 2020-115). All experiments were
performed under the standard ethical principles of animal
experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Z, Huang Z and Bai L: The P2X7 receptor
in osteoarthritis. Front Cell Dev Biol. 9(628330)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yu D, Jordan KP, Bedson J, Englund M,
Blyth F, Turkiewicz A, Prieto-Alhambra D and Peat G: Population
trends in the incidence and initial management of osteoarthritis:
Age-period-cohort analysis of the clinical practice research
datalink, 1992-2013. Rheumatology (Oxford). 56:1902–1917.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fu C, Zheng C, Lin J, Ye J, Mei Y, Pan C,
Wu G, Li X, Ye H and Liu X: Cibotium barometz polysaccharides
stimulate chondrocyte proliferation in vitro by promoting G1/S cell
cycle transition. Mol Med Rep. 15:3027–3034. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fischer H, Koenig U, Eckhart L and
Tschachler E: Human caspase 12 has acquired deleterious mutations.
Biochem Biophys Res Commun. 293:722–726. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Charlier E, Relic B, Deroyer C, Malaise O,
Neuville S, Collée J, Malaise MG and De Seny D: Insights on
molecular mechanisms of chondrocytes death in osteoarthritis. Int J
Mol Sci. 17(2146)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liao L, Zhang S, Gu J, Takarada T, Yoneda
Y, Huang J, Zhao L, Oh CD, Li J, Wang B, et al: Deletion of Runx2
in articular chondrocytes decelerates the progression of
DMM-induced osteoarthritis in adult mice. Sci Rep.
7(2371)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen D, Shen J, Zhao W, Wang T, Han L,
Hamilton JL and Im HJ: Osteoarthritis: Toward a comprehensive
understanding of pathological mechanism. Bone Res.
5(16044)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang K, Li Y, Han R, Cai G, He C, Wang G
and Jia D: T140 blocks the SDF-1/CXCR4 signaling pathway and
prevents cartilage degeneration in an osteoarthritis disease model.
PLoS One. 12(e176048)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Samways DS, Li Z and Egan TM: Principles
and properties of ion flow in P2X receptors. Front Cell Neurosci.
8(6)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shao BZ, Xu ZQ, Han BZ, Su DF and Liu C:
NLRP3 inflammasome and its inhibitors: A review. Front Pharmacol.
6(262)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Swanson KV, Deng M and Ting JPY: The NLRP3
inflammasome: Molecular activation and regulation to therapeutics.
Nat Rev Immunol. 19:477–489. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xing Y, Yao X, Li H, Xue G, Guo Q, Yang G,
An L, Zhang Y and Meng G: Cutting edge: TRAF6 mediates TLR/IL-1R
signaling-induced nontranscriptional priming of the NLRP3
inflammasome. J Immunol. 199:1561–1566. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang S, Mobasheri A, Zhang Y, Wang Y, Dai
T and Zhang Z: Exogenous stromal cell-derived factor-1 (SDF-1)
suppresses the NLRP3 inflammasome and inhibits pyroptosis in
synoviocytes from osteoarthritic joints via activation of the AMPK
signaling pathway. Inflammopharmacology. 29:695–704.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McAllister MJ, Chemaly M, Eakin AJ, Gibson
DS and McGilligan VE: NLRP3 as a potentially novel biomarker for
the management of osteoarthritis. Osteoarthr Cartilage. 26:612–619.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Di Virgilio F: The therapeutic potential
of modifying inflammasomes and NOD-like receptors. Pharmacol Rev.
65:872–905. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pernicova I and Korbonits M:
Metformin-mode of action and clinical implications for diabetes and
cancer. Nat Rev Endocrinol. 10:143–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao Y, Li Y, Xue J, Jia Y and Hu J: Effect
of the anti-diabetic drug metformin on bone mass in ovariectomized
rats. Eur J Pharmacol. 635:231–236. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Park MJ, Moon SJ, Baek JA, Lee EJ, Jung
KA, Kim EK, Kim DS, Lee JH, Kwok SK, Min JK, et al: Metformin
augments anti-inflammatory and chondroprotective properties of
mesenchymal stem cells in experimental osteoarthritis. J Immunol.
203:127–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nie L, Zhao P, Yue Z, Zhang P, Ji N, Chen
Q and Wang Q: Diabetes induces macrophage dysfunction through
cytoplasmic dsDNA/AIM2 associated pyroptosis. J Leukoc Biol.
110:497–510. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang J, Huang L, Shi X, Yang L, Hua F, Ma
J, Zhu W, Liu X, Xuan R, Shen Y, et al: Metformin protects against
myocardial ischemia-reperfusion injury and cell pyroptosis via
AMPK/NLRP3 inflammasome pathway. Aging (Albany NY). 12:24270–24287.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tan Y, Chen J, Jiang Y, Chen X, Li J, Chen
B and Gao J: The anti-periodontitis action of metformin via
targeting NLRP3 inflammasome. Arch Oral Biol.
114(104692)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Feng X, Pan J, Li J, Zeng C, Qi W, Shao Y,
Liu X, Liu L, Xiao G, Zhang H, et al: Metformin attenuates
cartilage degeneration in an experimental osteoarthritis model by
regulating AMPK/mTOR. Aging (Albany NY). 12:1087–1103.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Glasson SS, Chambers MG, Van Den Berg WB
and Little CB: The OARSI histopathology initiative-recommendations
for histological assessments of osteoarthritis in the mouse.
Osteoarthr Cartilage. 18 (Suppl 3):S17–S23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kirschneck C, Batschkus S, Proff P,
Köstler J, Spanier G and Schröder A: Valid gene expression
normalization by RT-qPCR in studies on hPDL fibroblasts with focus
on orthodontic tooth movement and periodontitis. Sci Rep.
7(14751)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hildebrand T, Laib A, Müller R, Dequeker J
and Rüegsegger P: Direct three-dimensional morphometric analysis of
human cancellous bone: Microstructural data from spine, femur,
iliac crest, and calcaneus. J Bone Miner Res. 14:1167–1174.
1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Spel L and Martinon F: Inflammasomes
contributing to inflammation in arthritis. Immunol Rev. 294:48–62.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Toldo S, Mezzaroma E, Buckley LF, Potere
N, Di Nisio M, Biondi-Zoccai G, Van Tassell BW and Abbate A:
Targeting the NLRP3 inflammasome in cardiovascular diseases.
Pharmacol Ther. 236(108053)2021.PubMed/NCBI View Article : Google Scholar : (Online ahead of
print).
|
|
30
|
Denoble AE, Huffman KM, Stabler TV, Kelly
SJ, Hershfield MS, McDaniel GE, Coleman RE and Kraus VB: Uric acid
is a danger signal of increasing risk for osteoarthritis through
inflammasome activation. Proc Natl Acad Sci USA. 108:2088–2093.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Zheng Y and Li H: NLRP3
inflammasome plays an important role in the pathogenesis of
collagen-induced arthritis. Mediat Inflamm.
2016(9656270)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang L, Li X, Zhang H, Huang Z, Zhang N,
Zhang L, Xing R and Wang P: Agnuside alleviates synovitis and
fibrosis in knee osteoarthritis through the inhibition of HIF-1α
and NLRP3 inflammasome. Mediat Inflamm.
2021(5534614)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediat Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li J, Zhang B, Liu WX, Lu K, Pan H, Wang
T, Oh CD, Yi D, Huang J, Zhao L, et al: Metformin limits
osteoarthritis development and progression through activation of
AMPK signalling. Ann Rheum Dis. 79:635–645. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang M, Liu Y, Huan Z, Wang Y and Xu J:
Metformin protects chondrocytes against IL-1β induced injury by
regulation of the AMPK/NF-κB signaling pathway. Pharmazie.
75:632–636. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xian H, Liu Y, Rundberg Nilsson A,
Gatchalian R, Crother TR, Tourtellotte WG, Zhang Y, Aleman-Muench
GR, Lewis G, Chen W, et al: Metformin inhibition of mitochondrial
ATP and DNA synthesis abrogates NLRP3 inflammasome activation and
pulmonary inflammation. Immunity. 54:1463–1477.e11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Du RW and Bu WG: Metformin improves
depressive-like symptoms in mice via inhibition of peripheral and
central NF-κB-NLRP3 inflammation activation. Exp Brain Res.
238:2549–2556. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Muraoka T, Hagino H, Okano T, Enokida M
and Teshima R: Role of subchondral bone in osteoarthritis
development: A comparative study of two strains of guinea pigs with
and without spontaneously occurring osteoarthritis. Arthritis
Rheum. 56:3366–3374. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hayami T, Pickarski M, Zhuo Y, Wesolowski
GA, Rodan GA and Duong LT: Characterization of articular cartilage
and subchondral bone changes in the rat anterior cruciate ligament
transection and meniscectomized models of osteoarthritis. Bone.
38:234–243. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fang H, Huang L, Welch I, Norley C,
Holdsworth DW, Beier F and Cai D: Early changes of articular
cartilage and subchondral bone in the DMM mouse model of
osteoarthritis. Sci Rep. 8(2855)2018.PubMed/NCBI View Article : Google Scholar
|