Introduction
Cancer is a leading cause of death worldwide
(1). Despite the development of
various drugs for cancer therapy, numerous anticancer agents offer
little therapeutic benefit. This, together with their associated
adverse effects, limit their clinical outcomes (2). A reversed extracellular/intracellular
pH gradient is associated with tumor growth and metastasis
(3). These phenotypes have been
ascribed, mechanistically, to effects of extracellular acidosis on
several processes (4). Disrupting
extracellular/intracellular pH gradient by inhibiting membrane
transporters may be a therapeutic strategy (5). In addition, inhibiting these
transporters induces toxic intracellular acidosis (6); therefore, maintaining an alkaline
intracellular environment is necessary for cancer cell survival
(7).
Lactate is a bioenergetic metabolite formed in the
absence (fermentation) or presence of oxygen and is used by cells
as an oxidative substrate (8).
Lactate, in addition to being an energy substrate, is a
gluconeogenic and signaling factor in multiple cell types (9). Cancer cells produce high levels of
intracellular lactate, inducing an increase in lactate extrusion to
compensate for cytosolic acidity, which causes the cytosol to
become alkalinized (10). However,
inefficient lactate release caused by the functional disruption of
monocarboxylate transporters (MCTs) decreases intracellular pH
(pHi) and slows tumor growth (11). This suggests that targeting MCTs
may represent a new strategy for anticancer treatment.
Dipeptidyl-peptidase-4 (DPP-4) is a ubiquitously
expressed transmembrane exopeptidase found on the surface of
numerous hematopoietic cells (12). DPP-4 has sparked scientific
interest over the last 10 years, with numerous studies describing
its role in tumor immunology and the prognosis of patients with
cancer (13-16).
Various DPP-4 inhibitors are used to treat type II diabetes with an
absence of serious side effects (17). However, it remains unclear whether
DPP-4 inhibitors are beneficial or detrimental to existing tumors.
In the present study, animal and cell experiments were conducted to
verify whether anagliptin could inhibit cancer cells growth through
MCT-4 signaling pathway. In addition, the suppressive mechanism of
anagliptin was further explored.
Materials and methods
Cell culture
Murine colon carcinoma CT-26 cells (obtained from
American Type Culture Collection) were maintained in RPMI-1640
medium (HyClone; Cytiva) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 mg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
CT-26 cells were cultured at 37˚C in 5% CO2.
Experimental animals
A total of 45 healthy male BALB/c mice (weight,
25-28 g; age, 6-8 weeks) were obtained from the Animal Center of
The Second Affiliated Hospital of Harbin Medical University
(Harbin, China; license no. SCXK2019-001). Mice were maintained in
groups of five animals per cage under a 12-h light/dark cycle under
controlled conditions (23±1˚C and 55±5% humidity). Autoclaved water
and food were available ad libitum to mice. The experimental
protocol was designed in accordance with Institutional Laboratory
Animal Care and Use Committee standards. All animal-involving
experimental procedures performed in the present study were in
accordance with and approved by the Institutional Animal Care and
Use Committee of Harbin Medical University Cancer Hospital (Harbin,
China; approval no. KY2016-16).
Animal models
The 45 healthy male BALB/c mice were randomly
assigned to the following experimental groups: i) Model group
(n=15), ii) anagliptin group (n=15) and 5-fluorouracil (5-Fu) group
(n=15). A total of 5x105 CT-26 cells (re-suspended in
phosphate buffer saline) were injected subcutaneously in the right
flank. All mice were weighed daily. Tumor growth was monitored by
palpation, and the onset when tumors were detectable was noted. If
no visible nodules were observed at the site of injection within 2
weeks, it was considered that this tumor sample could not form a
tumor nodule. The tumor nodule was measured every day after
appearance. Individual tumor volumes were measured with calipers
and calculated using the following formula: Volume=[π/6 x
(width)2 x length]. After visible nodules were observed,
the murine cancer model was treated with the DPP-4 inhibitor
anagliptin (20 mg/kg/day, MedChemExpress) daily by oral
administration (the usage and dosage of anagliptin was determined
based on previous experiments, Li et al, unpublished data).
The present experiments were treated with 5-fluorouracil (5-Fu,25
mg/kg/day, Selleck Chemicals) every other day intraperitoneally as
a positive control (the usage and dosage of 5-Fu was determined
based on previous experiments, Li et al, unpublished data)
(18). The murine cancer model was
treated with vehicle (saline). As soon as the volume of the
subcutaneous tumor reached 3,000 mm3, mice were
euthanized using CO2 inhalation in their home cages. The
CO2 flow rate was 30-40% of the chamber volume per min
as recommended by the Canadian Council on Animal Care guidelines on
euthanasia of animals used in science (19). Subsequently, cervical dislocation
followed to ensure death. Samples of solid tumors were harvested,
and then stored at -80˚C. Three independent experiments were
performed.
Immunohistochemistry (IHC)
Tissues were fixed in 4% paraformaldehyde for 30 min
at 4˚C, embedded in paraffin and then four sections (5-µm) were cut
at multiple levels. Tissues were dewaxed with xylene for 15 min at
room temperature, rehydrated with decreasing concentrations of
ethanol (absolute ethanol, 2 min; 95% ethanol, 2 min; 85% ethanol,
2 min; 75% ethanol, 2 min) and washed with tap water at room
temperature. Antigen retrieval was performed in 10 mM citrate
buffer (pH 6.0) for 10 min at 100˚C. Tissue sections were cooled,
blocked for endogenous peroxidase with 3%
H2O2 at room temperature for 15 min and
blocked for endogenous biotin with an avidin-biotin kit (Biocare
Medical, LLC) at room temperature for 15 min according to the
manufacturer's protocol. Tissue sections were incubated at room
temperature with 10% goat serum (cat. no. WGAR1009-5; Wuhan
Servicebio Technology Co., Ltd.) for 30 min, then incubated at room
temperature for 1 h with primary antibodies for Ki67 (1:200; cat.
no. WL01384a) and proliferating cell nuclear antigen (PCNA; 1:200;
cat. no. WL03213) (both from Wanleibio Co., Ltd.). Primary-antibody
binding was detected by biotinylated species-specific secondary
antibody (1:200; cat. no. A0277; Beyotime Institute of
Biotechnology) at 37˚C for 30 min, followed by a horseradish
peroxidase conjugate (Vectastain Elite ABC kit; Vector
Laboratories, Inc.) according to the manufacturer's instructions.
Immunoreactivity was revealed with 3,3'-diaminobenzidine (cat. no.
G1212; Wuhan Servicebio Technology Co., Ltd.). Sections were
counterstained with hematoxylin (cat. no. G1004; Wuhan Servicebio
Technology Co., Ltd.) at room temperature for 3 min. Sections were
examined microscopically with an optical microscope (Olympus
Corporation), and images were determined using digital microscopy
with SPOT Advanced software v5.3 (SPOT Imaging; Diagnostic
Instruments, Inc.).
Measurement of cell viability
CT-26 cells were seeded into 96-well plates
(5x105 cells/well) and incubated at 37˚C for 24 h. Upon
reaching 90% confluence, the cells were treated with different
concentrations of anagliptin (0.125-4 mM) at 37˚C for 24 h with or
without serum. Subsequently, 20 µl MTT (pH 4.7) was added to each
well and the cells were incubated at 37˚C for another 4 h. Then,
100 µl 10% sodium dodecyl sulfate (SDS) 0.01 M HCl was added and
the cells were incubated at 37˚C overnight to dissolve the formazan
crystals. Absorbance was measured at 570 nm.
Flow cytometric assay
After treatment with anagliptin for 24 h, CT-26
cells were harvested (centrifuged at 4˚C, 825 x g for 10 min) and
re-suspended at a density of 1x104 cells/ml in 1X
Annexin binding buffer (dilute 5X annexin-binding buffer 1:4 with
deionized water; cat. no. V13246; Invitrogen; Thermo Fisher
Scientific, Inc.). After double staining with FITC-Annexin V and
propidium iodide using the FITC Annexin V Apoptosis Detection kit
(cat. no. BB-4101; BestBio) according to the manufacturer's
protocol, cells were analyzed using a FACScan® flow
cytometer equipped with Cell Quest software (version 5.1; BD
Biosciences) according to the manufacturer's protocol to detect
early and late apoptosis of cells. All experiments were performed
in triplicate.
Cell transfection
For small interfering (si)RNA transfection, CT-26
cells were plated at 3x105 cells/ml in OPTI-MEM
serum-reduced medium (cat. no. 31985-062; Gibco; Thermo Fisher
Scientific, Inc.), and transfected with 100 pmol of targeted siRNA
or NC siRNA using 5 µl of Lipofectamine® RNAiMAX Reagent
Agent (cat. no. 13778-075; Invitrogen; Thermo Fisher Scientific,
Inc.) for 48 h in CO2 incubator at 37˚C according to the
manufacturer's protocol. Mouse MCT-4 siRNA (cat. no. sc-40120) and
control siRNA (cat. no. sc-37007) were purchased from Santa Cruz
Biotechnology, Inc. After transfection of MCT-4 for 48 h, the cells
were harvested, then the subsequence assay was performed.
Lactate concentration
The concentration of lactate in culture media was
detected using a commercial lactic acid kit (cat. no. A019-2-1;
Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's protocol.
Measurement of extracellular pH
(pHe)
Briefly, the culture medium was harvested from each
group. Experiments were conducted in room atmosphere, at 37˚C.
Extracellular pH of the media was verified using pH meter (cat. no.
ECPHWP60001, Thermo Fisher Scientific, Inc.).
Measurement of pHi
pHi was detected using a fluorescence pH indicator
[2',7'-bis(carboxyethyl)-5,6-carboxyfluorescein; BCECF] according
to the manufacturer's protocol (cat. no. BB-48121; BestBio).
Briefly, stock solutions (1 mM) of BCECF were made by dissolving in
DMSO before use. CT-26 cells (5x104 cells in 24 well)
were washed, then stained with 5 µM (final concentration) of the
cytoplasmic pH-sensitive dye BCECF in HEPES-buffer for 20 min at
37˚C in the dark. The fluorophores were loaded into the cells
through passive diffusion (to avoid compromising cell membrane
integrity) (20). For the
fluorescence measurements, the following wavelengths were set:
Excitation at 492 and 438 nm; emission at 525 nm. Fluorescence
levels were measured using a fluorescence microscope (LSM800; Carl
Zeiss AG).
JC-1 staining
According to the manufacturer's instructions, a
total of 2.5 µM JC-1 (cat. no. M8650; Beijing Solarbio Science
& Technology Co., Ltd.) was added to the media of CT-26 cells
(5x104 cells in 24 well) for 10 min at 37˚C. Cells were
then washed in HBSS media (136 mM NaCl, 3 mM KCl, 1.25 mM
CaCl2, 1.25 mM MgSO4, 10 mM HEPES and 2 mM
D-glucose), Monomeric JC-1 green fluorescence mission and aggregate
JC-1 red fluorescence emission were measured using a fluorescence
microscope at 530/590 nm (IX73; Olympus Corporation).
Western blot analysis
Frozen tissue was immersed in 600 µl lysis buffer
(containing 40% SDS, 60% RIPA (cat. no. G2002; Wanleibio Co., Ltd.)
and 1% protease inhibitor (cat. no. 539131; MilliporeSigma) and was
centrifuged at 4˚C 17,400 x g for 30 min. The supernatant was
collected and stored at -80˚C. CT-26 cells were washed with
ice-cold PBS and centrifuged at 825 x g for 10 min at 4˚C.
Subsequently, 70 µl lysis buffer containing 1% protease inhibitor
solution was added. The cell suspension was pipetted for 30 min on
ice, then centrifuged at 17,400 g for 30 min at 4˚C. The
supernatant was collected and stored at -80˚C. The protein
concentration was determined with the BCA Protein Assay kit
(Bio-Rad Laboratories, Inc.). The samples (100 µg per lane) were
separated by SDS-PAGE on 10% gels, then the separated proteins were
transferred to a nitrocellulose membrane. The membrane was blocked
in 5% non-fat milk overnight at 4˚C. Then, it was incubated with
the following primary antibodies against: Bcl-2 (cat. no. WL01556),
Bax (cat. no. WL01637), caspase-3 (cat. no. WL04004),
cleaved-caspase-3 (cat. no. WL01992), cytochrome c (cyto C;
cat. no. WL02410), MCT-4 (cat. no. 22787-1-AP) and GAPDH (cat. no.
WL01114). Antibodies against Bcl-2, Bax, caspase-3,
cleaved-caspase-3, cyto C and GAPDH were purchased from Wanleibio,
Co., Ltd. Antibodies against MCT-4 were purchased from ProteinTech
Group, Inc. All antibodies were diluted to 1:200 in PBS. After
washing with PBS-0.1% Tween-20, membranes were incubated with
fluorescence-conjugated goat anti-rabbit IgG secondary antibody
(1:10,000; cat. no. 926-32211; LI-COR Biosciences) at room
temperature for 1 h. Western blot bands were captured on the
Odyssey Infrared Imaging System (LI-COR Biosciences) and quantified
using Odyssey v3.0 software (LI-COR Biosciences) by measuring the
densitometry for each group.
Statistical analysis
Obtained data were expressed as the mean ± standard
deviation. Three independent experiments were performed. Data were
assessed with SPSS 22.0 software (IBM Corp.). One-way analysis of
variance followed by Bonferroni's correction as post hoc test was
used for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
Anagliptin reduces tumor growth
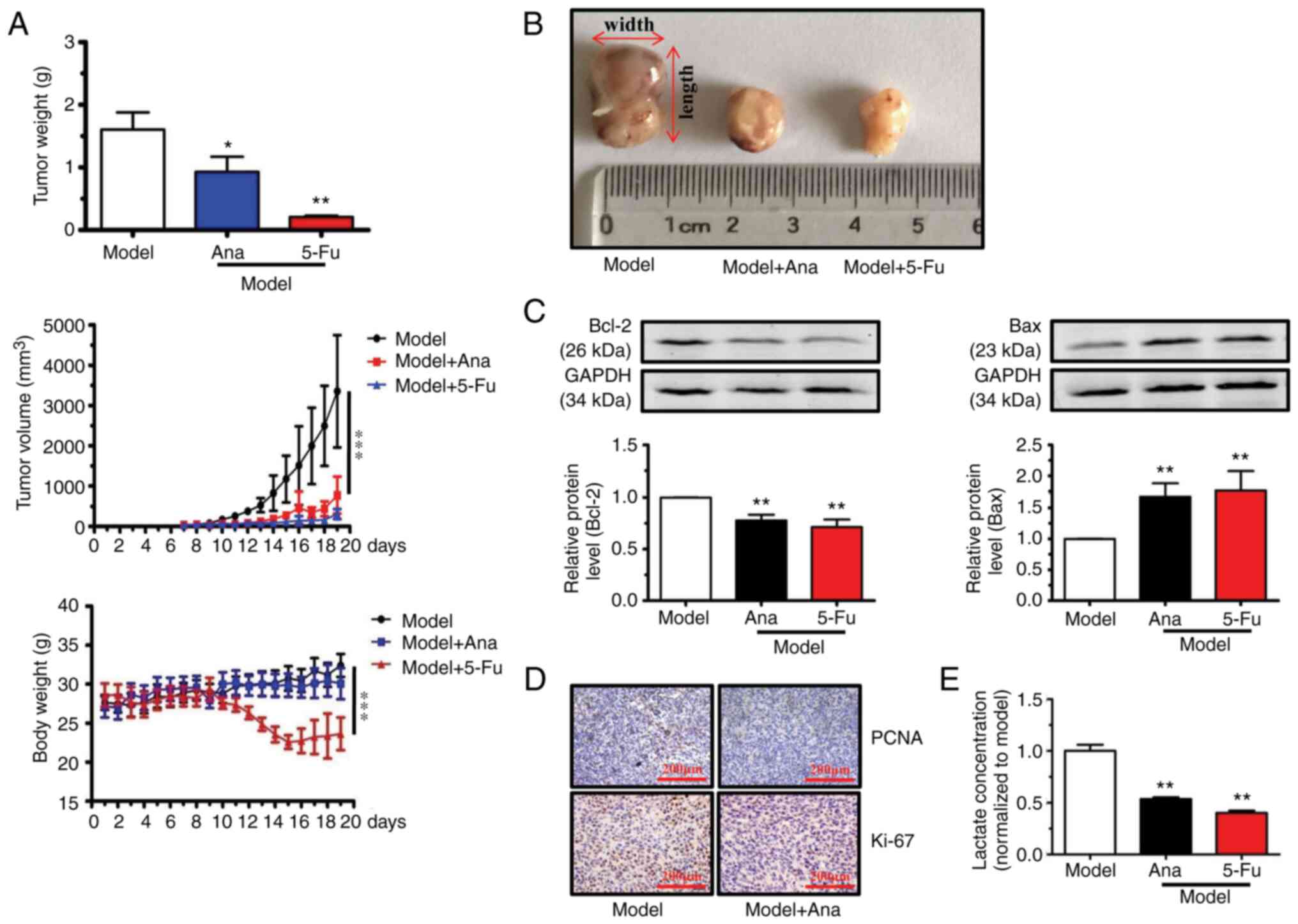
On day 7, tumor samples grew into visible nodules.
After which the growth of tumor nodule in BALB/c mice grew rapidly
to >3,000 mm3 in size and the mice were euthanized
using CO2 and sacrificed in model group (Fig. 1A). Animal models were treated with
anagliptin (20 mg/kg/day, by oral administration) and 5-Fu (25
mg/kg/day, intraperitoneally) once tumor nodules appeared (day 7).
Our pre-experiments confirmed that this dosage of anagliptin (20
mg/kg/day) was well tolerated, as no weight loss or other signs of
toxicity were observed in normal mice (Li et al, unpublished
data). In the animal experiment, 5-Fu (25 mg/kg/day,
intraperitoneally) was used as the positive control. And our
pre-experiments also indicated that the usage and dosage of 5-Fu
was also tolerated (unpublished data). As revealed in Fig. 1A, tumor nodule growth slowed from
day 7 today 10. The tumor nodule growth rapidly from day 11 to the
end of the experiment (day 19) in mice after treatment with
anagliptin and 5-Fu. After harvesting and measuring the tumor
samples at the end point of experiments, treatment with anagliptin
and 5-Fu was observed to significantly decrease the tumor volume
compared with model group (Fig. 1A
and B). Furthermore, anagliptin
administration did not influence body weight (Fig. 1A), but treatment with5-Fu decreased
the body weight from day 10 to the end of experiments.
Western blot analysis revealed that anagliptin
treatment promoted Bax and decreased Bcl-2 expression levels
(Fig. 1C). The expression levels
of Ki67 and PCNA in the animal model were next examined with IHC. A
markedly higher Ki67 and PCNA positive signal was observed in the
model group, while treatment with anagliptin caused a marked
decrease in the expression of Ki67 and PCNA (Fig. 1D). Furthermore, treatment with
anagliptin down-regulated the concentration of lactate in animal
models (Fig. 1E). These results
indicated that treatment with anagliptin had the ability to
suppress the growth of tumor.
Anagliptin induces apoptosis in CT-26
cells
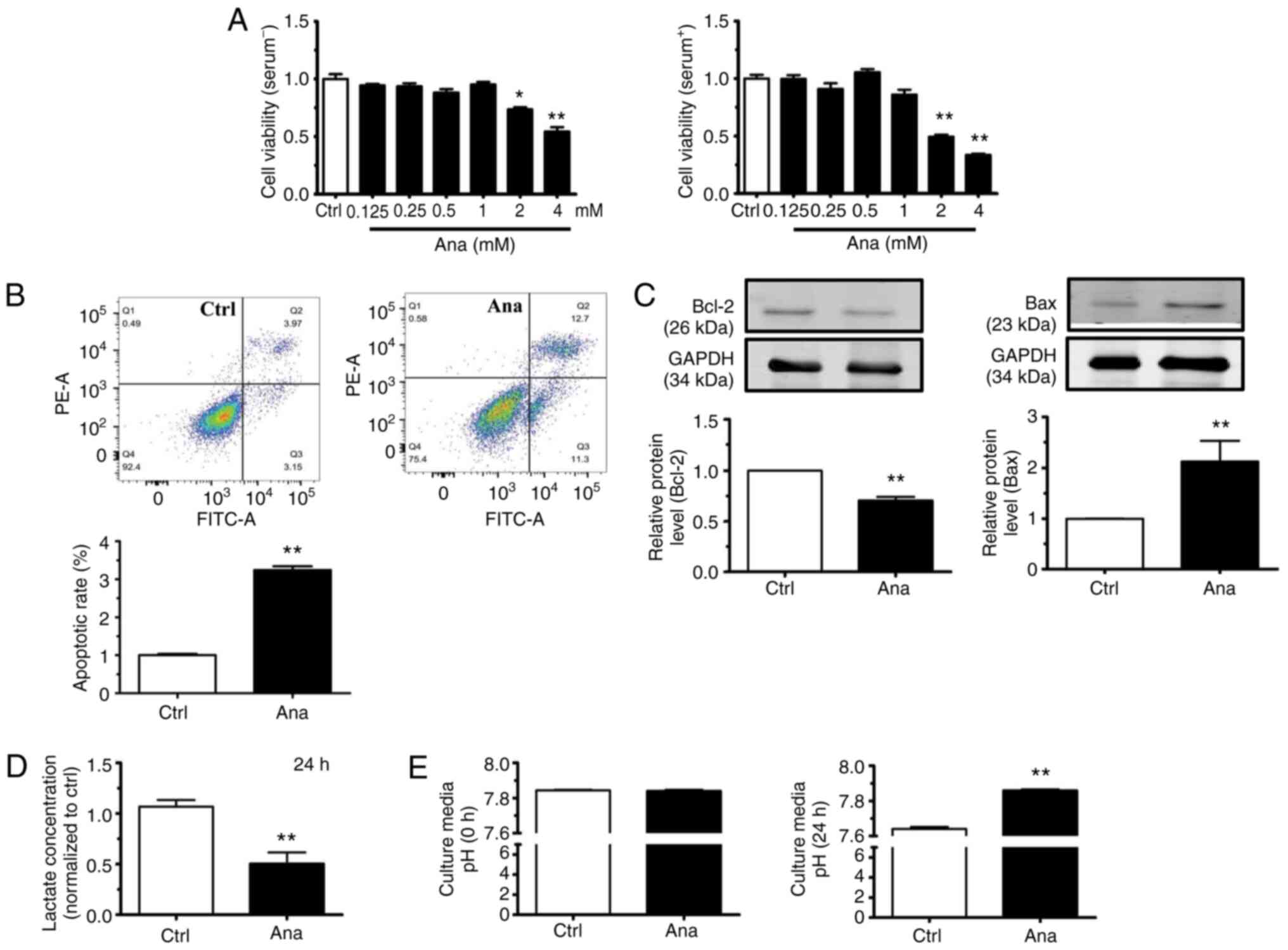
Anagliptin, at concentrations ≥2 mM, decreased the
cell viability of CT-26 cells after culturing for 24 h with or
without serum (Fig. 2A).
Therefore, 2 mM of anagliptin was then used in subsequent studies.
Flow cytometric analysis revealed that the proportion of late
apoptotic CT-26 cells was significantly increased following
treatment with anagliptin compared with in the control group
(Fig. 2B). Anagliptin treatment
also significantly reduced Bcl-2 expression levels and increased
Bax expression levels (Fig. 2C).
Those results indicated that treatment with anagliptin stimulated
the apoptosis of CT-26 cells.
In addition, anagliptin-treated CT-26 cells produced
lower levels of lactate in the cell culture medium (Fig. 2D). Meanwhile, anagliptin reversed
low extracellular pH (pHe) in cultured CT-26 cell medium after 24 h
(Fig. 2E). The results
demonstrated that, after treating with anagliptin in CT-26 cells,
the excretion of lactate was decreased which accompany with the
high extracellular pH.
Anagliptin suppresses MCT-4-mediated
lactate excretion
The present in vitro and in vivo
experiments demonstrated that anagliptin promoted CT-26 cell
apoptosis, but through an unknown mechanism. To prevent
intracellular acidification, metabolic processes within cancer
cells induce cytosolic accumulation of lactate and H+
which must be released into the extracellular space (21). A candidate protein involved in
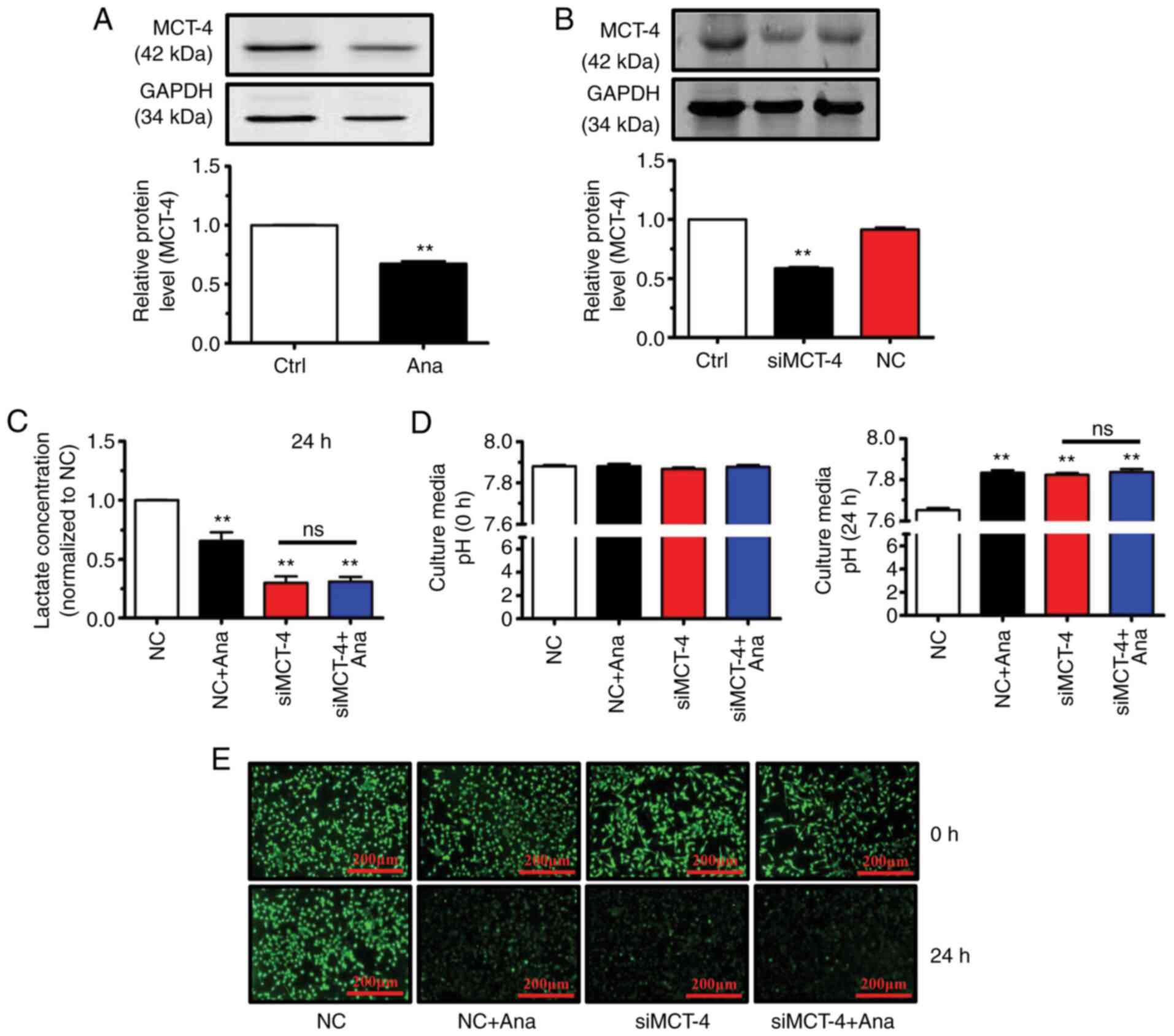
transporting lactic acid extracellularly is MCT-4(22). Anagliptin treatment decreased MCT-4
protein expression levels (Fig.
3A). It was therefore hypothesized that anagliptin affects
lactate excretion via MCT-4. MCT-4 siRNA transfection efficiency in
cultured CT-26 cells was therefore examined and it was determined
that MCT-4 levels in these cells were reduced compared within
untransfected cells (Fig. 3B).
Anagliptin-treated CT-26 cells produced lower levels
of lactate in cell culture medium compared with in the negative
control (NC) group (Fig. 3C). In
addition, MCT-4 siRNA transfection significantly reduced lactate
levels compared with in the NC group (Fig. 3C). However, co-application of MCT-4
siRNA and anagliptin produced no additive effect on lactate levels
in culture medium (Fig. 3C). Since
anagliptin inhibited lactate excretion in CT-26 cells, it was then
assessed whether anagliptin affected lactate-induced pHe
alterations. It was revealed that treatment with anagliptin
reversed low pHe (Fig. 3D) while
decreasing pHi levels after culturing for 24 h (Fig. 3E). The same results were obtained
following MCT-4 siRNA transfection (Fig. 3D and E). However, co-application of MCT-4 siRNA
and anagliptin had no further effect on the reversal of the pHi
gradient (Fig. 3D and E). The results showed that treatment with
anagliptin suppressed the excretion of lactate via MCT-4, then lead
to the reversal of the abnormal pHi and pHe.
Anagliptin reduces the mitochondrial
membrane potential (ΔΨm) via MCT-4-mediated accumulation of lactate
in CT-26 cells
Lactate strongly increases the number of reactive
oxygen species in cancer cells (23). Lactate accumulation in the
cytoplasm causes mitochondrial permeability, thus resulting in a
reduction in ΔΨm and the induction of apoptosis (24). It was therefore hypothesized that
anagliptin may induce apoptosis in CT-26 cells via MCT-4-mediated
lactate accumulation.
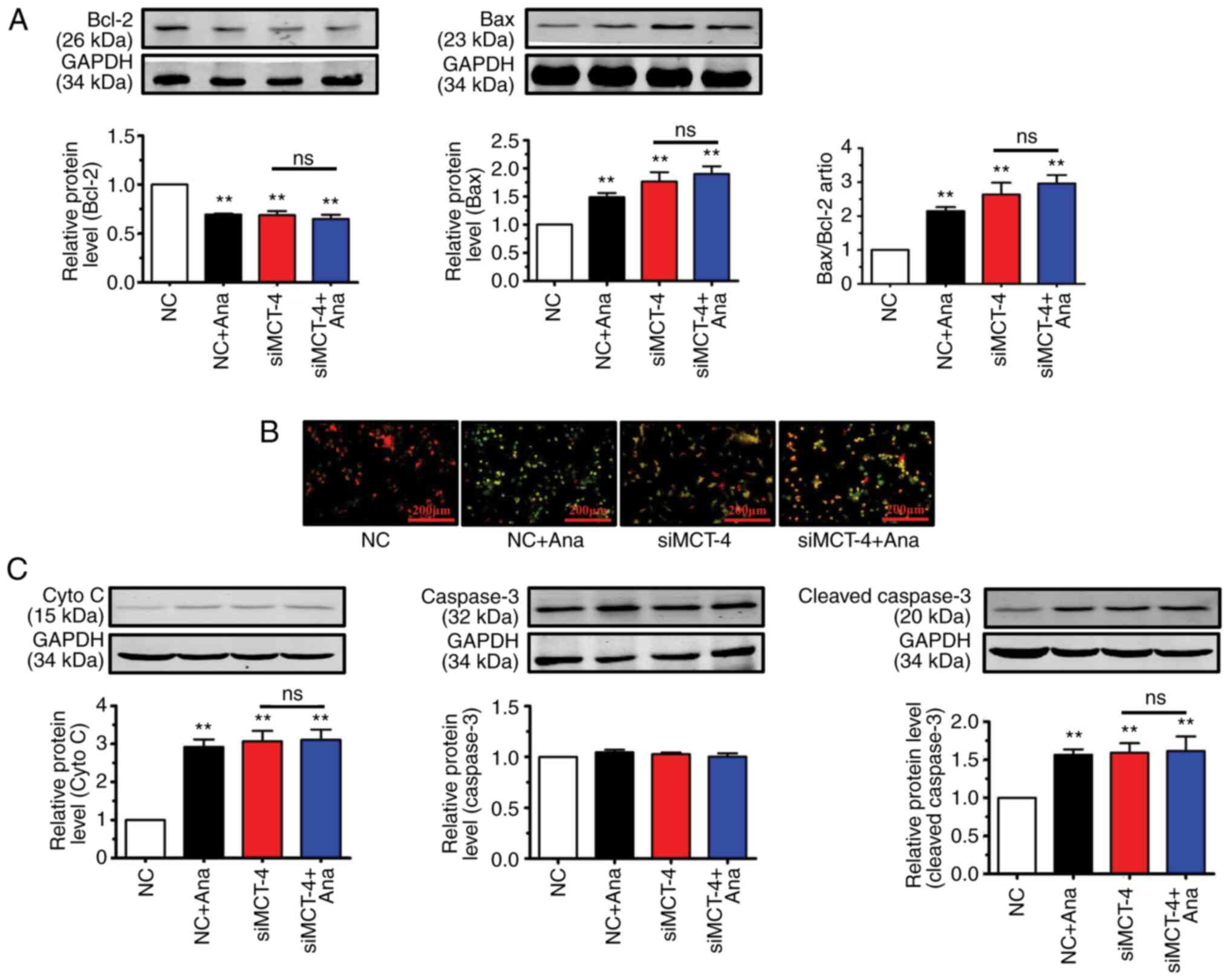
Anagliptin treatment reduced Bcl-2 expression levels
and increased Bax expression levels in CT-26 cells when compared
with the NC group (Fig. 4A).
Transfection with MCT-4 siRNA in CT-26 cells decreased the protein
level of Bcl-2 and increased Bax expression compared with NC group
(Fig. 4A). Co-application of
anagliptin and MCT-4 siRNA produced no further effect on Bcl-2 and
Bax expression. The present results showed that, after treating
CT-26 cells with anagliptin, the expression of Bcl-2 was decreased
and the expression of Bax was increased.
As demonstrated by JC-1 staining (representing the
ΔΨm), treatment with anagliptin in CT-26 cells demonstrated a
decrease in red fluorescence (red indicates aggregates with high
potential) and an increase in green fluorescence (green indicates
monomers, which have low ΔΨm potential, indicating lost membrane
potential) in the majority of cells. Transfection of CT-26 cells
with MCT-4 siRNA also led to low ΔΨm potential (decreased red
fluorescence) (Fig. 4B). The same
results was also observed after co-application MCT-4 siRNA and
anagliptin. These results showed that treatment with anagliptin
disrupted the ΔΨm potential via MCT-4.
Critical events during apoptosis are the release of
cyto C from the mitochondria and caspase-3 activation (25). Anagliptin significantly increased
cyto C and cleaved-caspase-3 expression in cultured CT-26 cells,
but not caspase-3 (Fig. 4C).
Similar results were obtained following transfection of CT-26 cells
with MCT-4 siRNA (Fig. 4C). The
same results were detected after co-application MCT-4 siRNA and
anagliptin. But, co-application of MCT-4 siRNA and anagliptin had
no further effect on cyto C and cleaved-caspase-3 expression. These
results showed that treatment with anagliptin increased the
expression levels of cyto C and cleaved-caspase-3, but not the
expression of caspase-3.
Discussion
In the present study, the mechanism by which
anagliptin induced cellular apoptosis in vivo and in
vitro was investigated, the results of which indicated that
anagliptin induced apoptosis of CT-26 cells via MCT-4-mediated
intracellular lactate accumulation which lead to intracellular
acidosis. Antagonism of lactate shuttlingmodulatesMCT-4 expression,
and is a target for predicting response to therapy. Developing
pharmaceutical therapies to block this target will be a promising
strategy in cancer therapy (26).
CD26/DPP4 plays an important role in several types
of cancer (27-31)
and DPP-4 inhibitors are being evaluated as treatments for cancer.
Certain studies have indicated that anagliptin may inhibit the
proliferation of tumor cells (32,33).
However, in those studies, the mice were fed a diet containing a
low dose of anagliptin; this was defined as ‘anagliptin mixed into
the food’ (32). This method means
artificial preparation of food (mixing the ingredients together),
which is then fed to the animals. Mixing the active pharmaceutical
ingredient with nutritional composition is simple. However,
considering the physical and chemical properties of medicine, it is
hard to ensure uniform distribution of medicine in food. Therefore,
it must be appraised before it can be used. However, it is hard to
guarantee appropriate animal intake each day. Notably, this type of
administration method cannot be practically applied due to the fact
that certain animals may intake markedly more than others, which
may lead to the heterogeneity of treatment results. Thus, in the
present study, the oral gavage method was used to guarantee
uniformity. In the present study, anagliptin was used to inhibit
the proliferation of tumor cells. Based on our pre-experiments,
different dosages of anagliptin (10-30 mg/kg) were first applied.
The results revealed that 20 and 30 mg had the same antitumor
effect, but that the effect of 10 mg was weaker than that of 20 mg
(Li et al, unpublished data). A dosage of 20 mg/kg/day
anagliptin was therefore selected for use in the present study,
which differs from previous studies (32,33).
The findings of the present study demonstrated that
anagliptin treatment promoted CT-26 apoptosis. Cancer cells control
the intracellular balance of acids and bases through mechanisms not
used by normal cells, generating a non-physiological extracellular
acidic microenvironment (34).
Therefore, the pathological reversal of the pH gradient in the
microenvironment of cancer cells is now recognized as a defining
feature of these cells (35). The
present data from cultured CT-26 cells indicated that the pHe value
was reduced after 24 h. Anagliptin treatment reversed the pHe/pHi
gradient, that is, extracellular alkalization versus intracellular
acidification. These findings suggested that anagliptin contributed
to the regulation of pH gradients and that the reversible
regulation thereof (∆pHi/∆pHe) presents a potential therapeutic
strategy against cancer.
Lactate is a metabolic byproduct of glycolysis that
contributes to extracellular acidification (36). Lactate extrusion from cancer cells
prevents intracellular acidification but also leads to
extracellular acidosis. In the present study, the aim was to
understand: i) The role of low pH in the culture medium caused by
lactate excretion, and ii) how lactate excretion is essential for
maintaining pHi homeostasis (37).
The present data demonstrated that anagliptin inhibited lactate
excretion in cultured CT-26 cells. Based on our findings, it was
concluded that anagliptin reversed the pH gradient by modulating
lactate release. These findings provided evidence that anagliptin
may suppress lactate release, neutralize acidity in the
extracellular microenvironment and decrease the pHi.
Lactate is a weak acid that cannot freely diffuse
across cell membranes. MCTs are responsible for lactate release and
may function as lactate exporters or importers (38). In the present study, it was found
that MCT-4 was a target of anagliptin and that anagliptin treatment
reduced MCT-4 protein expression levels. Notably, anagliptin
prevented the excretion of lactate from CT-26 cells via MCT-4 in
the present experiments after transfection of MCT-4 siRNA. Taken
together, it was suggested that anagliptin may reverse the pH
gradient by modulating MCT-4 expression.
In conclusion, several types of human cancer
demonstrate increased MCT-4 expression, a feature reported to be
associated with poor cancer prognosis (39). MCT-4 is able to secrete lactate
into the microenvironment (40),
which creates the ideal environment for certain acquired
characteristics of cancer cells (41). The results of the present study
suggested that anagliptin promoted the apoptosis of cancer cells
via MCT-4-mediated lactate release. The data indicated that
anagliptin reversed the abnormal pH gradient, regulating the
acid-base balance. The present study observed that treatment with
anagliptin had the ability to induce the apoptosis of CT-26 cells
via MCT-4-mediated intracellular lactate accumulation which lead to
intracellular acidosis. The function of anagliptin on the
proliferation of tumor cells in vivo and in vitro was
explored in the present study. However, only CT-26 cells were used
to study the effect of anagliptin on apoptosis; hence, in our
future studies other kinds of cancer cells will be used to detect
the anti-cancer effect of anagliptin. The
Na+/H+ exchanger (NHE) contributes to
cellular pH homeostasis by regulating the acid-base balance; this
antiporter is the predominant isoform expressed in tumors (42). Elevated NHE activity may be a major
factor in promoting extracellular/interstitial acidity from the
earliest stages of oncogene-driven neoplastic transformation
(43). Future research should
examine whether anagliptin regulates the pH gradient via
NHE-mediated H+ excretion. It was therefore proposed
that anagliptin may be a novel target for improving anticancer drug
therapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 81800242 and 81970382) and
the Top Talent of Harbin Medical University Cancer Hospital (grant
no. BJQN2019-04).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and CC conceived and designed the experiments.
XK, XQ, ZL and JL performed the experiments. XK and CC analyzed the
data. QL wrote the manuscript. CC and QL confirmed the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental procedures performed in the present
study were approved by with the Institutional Animal Care and Use
Committee of Harbin Medical University Cancer Hospital (approval
no. KY2016-16; Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ye M, Han Y, Tang J, Piao Y, Liu X, Zhou
Z, Gao J, Rao J and Shen Y: A tumor specific cascade amplification
drug release nanoparticle for overcoming multidrug resistance in
cancers. Adv Mater. 29(1702342)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Park JH, Pyun WY and Park HW: Cancer
metabolism: Phenotype, signaling and therapeutic targets. Cell.
9(2308)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Urbanska K and Orzechowski A:
Unappreciated role of LDHA and LDHB to control apoptosis and
autophagy in tumor cells. Int J Mol Sci. 20(2085)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tannock IF and Rotin D: Acid pH in tumors
and its potential for therapeutic exploitation. Cancer Res.
49:4373–4384. 1989.PubMed/NCBI
|
|
6
|
Zhang T, Suo C, Zhaeng C and Zhang H:
Hypoxia and metabolism in metastasis. Adv Exp Med Biol. 1136:87–95.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zheng T, Jaattela M and Liu B: pH gradient
reversal fuels cancer progression. Int J Biochem Cell Biol.
125(105796)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Magistretti PJ and Allaman I: Lactate in
the brain: From metabolic end-product to signaling molecule. Nat
Rev Neurosci. 19:235–249. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brooks GA: Lactate as a fulcrum of
metabolism. Redox Biol. 35(101454)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Becker HM: Carbonic anhydrase Ⅸ and acid
transport in cancer. Br J Cancer. 122:157–167. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Quade BN, Parker MD and Occhipinti R: The
therapeutic importance of acid-base balance. Biochem Pharmacol.
183(114278)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Abbott CA, Baker E, Sutherland GR and
McCaughan GW: Genomic organization, exact localization, and tissue
expression of the human CD26 (dipeptidyl peptidase IV) gene.
Immunogenetics. 40:331–338. 1994.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ropa J and Broxmeyer HE: An expanded role
for Dipeptidyl peptidase 4 in cell regulation. Curr Opin Hematol.
27:215–224. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang F, Takagaki Y, Yoshitomi Y, Ikeda T,
Li J, Kitada M, Kumagai A, Kawakita E, Shi S, Kanasaki K and Koya
D: Inhibition of dipeptidyl peptidase-4 accelerates
epithelial-mesenchymal transition and breast cancer metastasis via
the CXCL12/CXCR4/mTOR Axis. Cancer Res. 79:735–746. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li R, Zeng X, Yang M, Feng J, Xu X, Bao L,
Ye T, Wang X, Xue B and Huang Y: Antidiabetic DPP-4 inhibitors
reprogram tumor microenvironment that facilitates murine breast
cancer metastasis through interaction with cancer cells via a
ROS-NF-B-NLRP3 Axis. Front Oncol. 11(728047)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pinto LC, Rados DV, Barkan SS, Leitão CB
and Gross JL: Dipeptidyl peptidase-4 inhibitors, pancreatic cancer
and acute pancreatitis: A meta-analysis with trial sequential
analysis. Sci Rep. 8(782)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun ZG, Li ZN and Zhu HL: The research
progress of DPP-4 inhibitors. Mini Rev Med Chem. 20:1709–1718.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang C, Ma Q, Shi Y, Li X, Wang M, Wang
J, Ge J, Chen Z, Wang Z and Jiang H: A novel
5-fluorouracil-resistant human esophageal squamous cell carcinoma
cell line Eca-109/5-FU with significant drug resistance-related
characteristics. Oncol Rep. 37:2942–2954. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Canadian Council on Animal Care (CCAC):
CCAC revised guidance on euthanasia using carbon dioxide. CCAC,
Ottawa, ON, 2020. https://ccac.ca/en/news-and-events/news/2020headlines/ccac-revised-guidance-on-euthanasia-using-carbon-dioxide.html.
Accessed July 24, 2020.
|
|
20
|
Golda-VanEeckhoutte RL, Roof LT, Needoba
JA and Peterson TD: Determination of intracellular pH in
phytoplankton using the fluorescent probe, SNARF, with detection by
fluorescence spectroscopy. J Microbiol Methods. 152:109–118.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trivedi B and Danforth WH: Effect of pH on
the kinetics of frog muscle phosphofructokinase. J Biol Chem.
241:4110–4112. 1966.PubMed/NCBI
|
|
22
|
Lai SW, Lin HJ, Liu YS, Yang LY and Lu DY:
Monocarboxylate transporter 4 regulates glioblastoma motility and
monocyte binding ability. Cancer (Basel). 12(380)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu Y, Guo JZ, Liu Y, Wang K, Ding W, Wang
H, Liu X, Zhou S, Lu XC, Yang HB, et al: Nuclear lactate
dehydrogenase A senses ROS to produce α-hydroxybutyrate for
HPV-induced cervical tumor growth. Nat Commun.
9(4429)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu Y, Han XQ, Sun XJ, Yang R, Ma WQ and
Liu NF: Lactate accelerates vascular calcification through NR4A1
regulated mitochondrial fission and BNIP-3 related mitophagy.
Apoptosis. 25:321–340. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Roger C, Erkes DA, Nardone A, Aplin AE,
Fernandes-Alnemri T and Alnemri ES: Gasdermin pores permeabilize
mitochondrial to augment caspase-3 activation during apoptosis and
inflammasome activation. Nat Commun. 10(1689)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Baltazar F, Pinheiro C, Morais-Santos F,
Azevedo-Silva J, Queirós O, Preto A and Casal M: Monocarboxylate
transporters as targets and mediators in cancer therapy response.
Histol Histopathol. 29:1511–1524. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nath M, Bhattacharjee K and Choudhury Y:
Vilfagliptin, a dipeptidyl peptides-4 inhibitor, reduces betel-nut
induced carcinogenesis in femal mice. Life Sci.
266(118870)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Enz N, Vliegen G, De Meester I and
Jungraithmayr W: CD26/DPP4-a potential biomarker and target for
cancer therapy. Pharmacol Ther. 198:135–159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ali A, Fuentes A, Skelton WP IV, Wang Y,
McGorray S, Shah C, Bishnoi R, Dang LH and Dang NH: A multi-center
retrospective analysis of the effect of DPP4 inhibitors on
progression-free survival in advanced airway and colorectal
cancers. Mol Clin Oncol. 10:118–124. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
De Chiara L, Páez de la Cadena M,
Rodríguez-Berrocal J, Alvarez-Pardiñas MC, Pardiñas-Añón MC,
Varela-Calviño R and Cordero OJ: CD26-related serum biomarkers:
sCD26 protein, DPP4 activity, and anti-CD26 isotype levels in a
colorectal cancer-screening context. Dis Markers.
2020(4347936)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shah C, Hong YR, Bishnoi R, Ali A, Skelton
WP IV, Dang LH, Huo J and Dang NH: Impact of DPP4 Inhibitors in
survival of patients with prostate, pancreas, and breast cancer.
Front Oncol. 10(405)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nishina S, Yamauchi A, Kawaguchi T, Kaku
K, Goto M, Sasaki K, Hara Y, Tomiyama Y, Kuribayashi F, Torimura T
and Hino K: Dipeptidyl peptidase 4 inhibitors reduce hepatocellular
carcinoma by activating lymphocyte chemotaxis in mice. Cell Mol
Gastroenterol Hepatol. 7:115–134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nakaya K, Kubota N, Takamoto I, Kubota T,
Katsuyama H, Sato H, Tokuyama K, Hashimoto S, Goto M, Jomori T, et
al: Dipeptidyl peptidase-4 inhibitor anagliptin ameliorates
diabetes in mice with haploinsufficiency of glucokinase on a
high-fat diet. Metabolism. 62:939–951. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Toft NJ, Axelsen TV, Pedersen HL, Mele M,
Burton M, Balling E, Johansen T, Thomassen M, Christiansen PM and
Boedtkjer E: Acid-base transporters and pH dynamics in human breast
carcinomas predict proliferative activity, metastasis, and
survival. Elife. 10(e68447)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
White KA, Grillo-Hill BK and Barber DL:
Cancer cell behaviors mediated by dysregulated pH dynamics at a
glance. J Cell Sci. 130:663–669. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Berger G and Femdt SM: The metabolism of
cancer cells during metastasis. Nat Rev Cancer. 21:162–180.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu Y, Zhu L, Dong P, Liang R, Mao Y, Yang
X, Zhang Y and Luo X: Acid tolerance response of listeria
monocytogenes in various external pHs with different concentrations
of lactic acid. Foodborne Pathog Dis. 17:253–261. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Todenhofer T, Seiler R, Stewart C,
Moskalev I, Gao J, Ladhar S, Kamjabi A, Al Nakouzi N, Hayashi T,
Choi S, et al: Selective inhibition of the lactate transporter MCT4
reduces growth of invasive bladder cancer. Mol Cancer Ther.
17:2746–2755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pinheiro C, Longatto-Filho A, Scapulatempo
C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA,
Schmitt F and Baltzzar F: Increased expression of monocarboxylate
transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch.
452:139–146. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Choi SYC, Ettinger SL, Lin D, Xue H, Ci X,
Nabavi N, Bell RH, Mo F, Gout PW, Fleshner NE, et al: Targeting
MCT4 to reduce lactic acid secretion and glycolysis for treatment
of neuroendocrine prostate cancer. Cancer Med. 7:3385–3392.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Huber V, Camisaschi C, Berzi A, Ferro S,
Lugini L, Triulzi T, Tuccitto A, Tagliabue E, Castelli C and
Rivoltini L: Cancer acidity: An ultimate frontier of tumor immune
escape and a novel target of immunomodulation. Semin Cancer Biol.
43:74–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Birkeland ES, Koch LM and Dechant R:
Another consequence of the warburg effect? Metabolic regulation of
Na+/H+ exchangers may link aerobic glycolysis
to cell growth. Front Oncol. 10(1561)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rolver MG, Elingaard-Larsen LO, Andersen
AP, Counillon L and Pedersen SF: Pyrazine ring base
Na+/H+ exchanger (NHE) inhibitors potently
inhibit cancer cell growth in 3D culture, independent of NHE1. Sci
Rep. 10(5800)2020.PubMed/NCBI View Article : Google Scholar
|