Introduction
Osteoporosis is a systemic skeletal disease
characterized by low bone mass that increases bone fragility and
the risk of fractures in patients (1). It increases due to estrogen
deficiency after menopause, even though bone density in women is
originally lower than in men (2).
Various bone resorption inhibitors have been used to treat
osteoporosis to prevent its progression (3). Because of the nature of the disease
and the prolonged drug administration, osteoporosis drugs have a
high bone resorption inhibitory effect and a high safety value for
other tissues. The therapeutic agents frequently used in clinical
practice, such as bisphosphonates and anti-receptor activator of
nuclear factor-κB ligand (RANKL) monoclonal antibodies, can reduce
the risk of fragility fractures. However, they have marked side
effects, such as inducing jaw osteonecrosis and increasing the risk
of cancer and heart disease (4-6).
Therefore, the development of safer and more effective therapeutic
agents is an ongoing research effort.
Mesenchymal stem cells (MSCs) in the bone marrow and
connective tissue reportedly exhibit immunosuppression in addition
to pluripotency (7). For example,
transplantation of bone marrow-derived MSCs in patients with
autoimmune diseases, such as systemic lupus erythematosus and
multiple sclerosis, has been shown to cause tolerance and alleviate
symptoms in the recipients (8,9). On
the other hand, stem cells from human exfoliated deciduous teeth
(SHED), which are present in the pulp tissue of deciduous teeth,
are unique stem cells identified as a highly proliferative clonal
cell population that can differentiate into a variety of cell types
including neurons, adipocytes, osteoblasts, and endothelial cells
(10). SHED are considered to be
derived from the cranial neural crest and express early markers of
both mesenchymal and neuroectodermal stem cells (11,12).
Furthermore, SHED have been reported to perform more potent
immunomodulatory functions compared to bone marrow-derived MSCs
(13). Currently, therapies that
use the properties of MSCs, such as their pluripotency and immune
control ability, are being tested for various diseases. For
example, Yang et al (14)
reported that SHED transplantation ameliorated glandular
inflammation and dryness via soluble programmed cell death ligand 1
released from the transplanted SHED in mice exhibiting Sjögren's
syndrome-induced dryness. Kitase et al (15) reported that SHED administration
protected against cortical damage caused by hypoxic-ischemic
encephalopathy and ameliorated behavioral deficits such as
tetraplegia caused by cerebral ischemia in rats. Li et al
(16) administered three
intravenous doses of SHED over a six-week period in a cohort of 24
patients with type 2 diabetes on insulin therapy and followed these
patients for 12 months. The authors reported that glycated serum
albumin and glycated hemoglobin were normalized in 19 and 15
patients, respectively, following SHED transplantation.
Liu et al (17) reported that SHED transplantation
improved the osteopenia phenotype in osteoporotic mice. The
mechanism underlying suppressed osteoporosis included the induction
of apoptosis of inflammatory by SHED transplantation via the Fas
ligand (FasL)-mediated Fas pathway, with consequent suppression of
osteoclast induction. The transplantation of SHED using their
immunoregulatory ability is effective for the treatment of
osteoporosis, although concerns remain regarding the safety of the
transplanted cells, including tumor formation, which should be
resolved before the implementation of SHED transplantation in
clinical use. On the other hand, SEHD have been reported to release
a variety of biologically active secreted factors, and studies in
mice and rats have shown that the factors secreted from SHED are
also highly effective in the treatment of various diseases such as
neurodegenerative and autoimmune disorders, diabetes, and liver
cirrhosis (18-21).
These studies suggest that these factors secreted from SHED are
effective for the treatment of many diseases. However, it remains
unclear whether these factors secreted from SHED are effective in
the treatment of osteoporosis. Therefore, we hypothesized that
factors released from SHED would be effective in preventing
osteoporosis. In the present study, we administered
SHED-conditioned medium (CM) to ovariectomized (OVX) mice to
determine its ability to prevent OVX-induced early osteoporosis
phenotype.
Materials and methods
Cell culture
SHED were isolated and cultured from deciduous teeth
(maxillary deciduous central incisors) donated by the Department of
Pediatrics and Disabled Dentistry, Hokkaido University Hospital,
under the approval of the Institutional Voluntary Clinical Research
Review Board (approval no. 010-116). They were cultured with a
minimum essential medium (a-MEM) (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 20% fetal bovine serum (FBS)
(Roche Diagnostics) in gas phase at 37˚C with 5% CO2
using a standardized protocol (10). Each cell was subcultured and
replaced with serum-free a-MEM at 80% confluency, and culture
supernatants were harvested after another 24 h of culture. The
harvested culture supernatant was then centrifuged to remove cell
debris.
Animal studies
The Hokkaido University Animal Experiment Committee
approved this study (approval no. 15-0015). The animal experiments
adhered to the Hokkaido University Animal Experiment Guidelines. We
used 11-week-old female C3H/HeJ mice (Sankyo Labo Service
Corporation, Tokyo, Japan). We divided them into two groups: the
OVX group, which underwent ovariectomy, and the Sham group, which
underwent sham open surgery (17).
Mice were anesthetized with sodium pentobarbital (40 mg/kg,
intraperitoneal injection). The OVX procedures were performed to
generate osteoporotic model mice. The OVX mice were further
classified into three groups: OVX mice, the OVX serum-free medium
(SF-MEM) mice that received 200 µl of SF-MEM
intraperitoneally, and the OVX SHED-CM mice that received 200
µl of SHED-CM intraperitoneally. Both subgroups were
administered a total of eight doses over 4 weeks, starting
immediately after ovariectomy and proceeding twice weekly. At 4
weeks post-OVX, the mice were sacrificed using CO2 gas
(flow rate was 30% displacement of the cage volume per min) for
further examination.
MicroCT (µCT) imaging
Femur and lumbar vertebrae underwent immersion
fixation with 4% paraformaldehyde for 2 days after harvest. Fixed
samples were subjected to µCT imaging using Latheta LCT200
(Hitachi, Japan) at a tube voltage of 50 kV and a pixel size of
24.0 µm according to a previously published protocol
(18). Measurements in the
photographed samples were taken using the analysis software
included in the Latheta LCT200 system following the manufacturer's
standards.
Histological analyses
After µCT imaging, femurs underwent demineralization
with 10% ethylenediaminetetraacetic acid solution for 10 days,
followed by alcoholic dehydration and paraffin embedding according
to the flow cytometry method. Paraffin-embedded materials produced
5-µm sections that were subjected to hematoxylin and eosin
(H&E) staining and tartrate-resistant acid phosphatase staining
according to a conventional protocol (17). Image-Pro Premier (Media
Cybernetics) was used for the uptake and analysis of section
images. The lumbar spine was cut in the central region in the
frontal transection orientation using a micro-cutting machine,
BS-300CP (Meiwafosis, Japan), and the trabecular bone status was
observed by stereomicroscopy.
Flow cytometry analysis
Peripheral blood was drawn from the fundus venous
plexus using heparin-coated glass capillaries. The peripheral blood
mononuclear cells (PBMNCs) were fixed and permeabilized using Cell
Fixation and Permeabilization Kit (Abcam). Next, the PBMNCs were
stained with PE/Cy7-labeled anti-IFN-γ antibody (Biolegend) and
PerCP/Cy5.5-labeled anti-IL-17A antibody (Biolegend). Peritoneal
macrophages were collected via peritoneal lavage. Furthermore, 10
ml of sterile phosphate-buffered saline was injected into the upper
part of the abdominal cavity using a syringe. The abdomen of the
mouse was massaged several times, and peritoneal lavage was
performed. The cells were collected via centrifugation at 400 x g
for 10 min and then stained with PerCP/Cy5.5-labeled anti-CD80
(Biolegend) and APC-labeled anti-F4/80 antibody (Biolegend).
Appropriate immunoglobulin (Ig) G-conjugated antibodies were used
for isotype controls. Flow cytometry was performed on FACSVerse (BD
Biosciences) and analyzed using FlowJo ver. 7.6 (BD Biosciences)
using a standardized protocol (22).
Enzyme-linked immunosorbent assay
(ELISA)
Interferon-γ (IFN-γ), interleukin-17 (IL-17), and
osteoprotegerin (OPG) levels in the blood serum samples were
assayed using the Quantikine ELISA Kit (R&D Systems) according
to a standardized protocol (23).
Administration of IL-4-neutralizing
antibody
For inhibiting the polarization of M2 macrophages, a
neutralizing antibody (nAb) for IL-4 was injected. The OVX mice
were intraperitoneally administered 25 µg of either
anti-mouse IL-4 antibody (U-CyTech Biosciences) or anti-rat
IgG1 antibody as isotype control (U-CyTech) on the day
after OVX. At 4 weeks post-OVX, the mice were sacrificed using
CO2 gas for further examination.
Statistical analysis
The data are represented as mean ± standard error of
the mean. Statistical analyses of the results were performed using
one-way analysis of variance (ANOVA) and post-hoc Tukey test and
two-way repeated-measures ANOVA and post-hoc Bonferroni's test
using the StatView ver. 5.0 (SAS Institute) software package.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Administration of SHED-CM prevents
ovariectomy-induced bone loss
The present study investigated whether SHED-CM
improved the osteoporotic phenotype by injecting OVX mice with
SHED-CM and analyzing the treatment efficacy 4 weeks after OVX
(Fig. 1A). µCT analysis revealed
that the femur's trabecular bone volume fraction (BV/TV), bone
mineral density (BMD), and average cortical thickness (Ct.Th) were
increased in the OVX SHED-CM group compared with the OVX group
(Fig. 1B-E). There was no
significant difference between the OVX and the OVX SF-MEM groups.
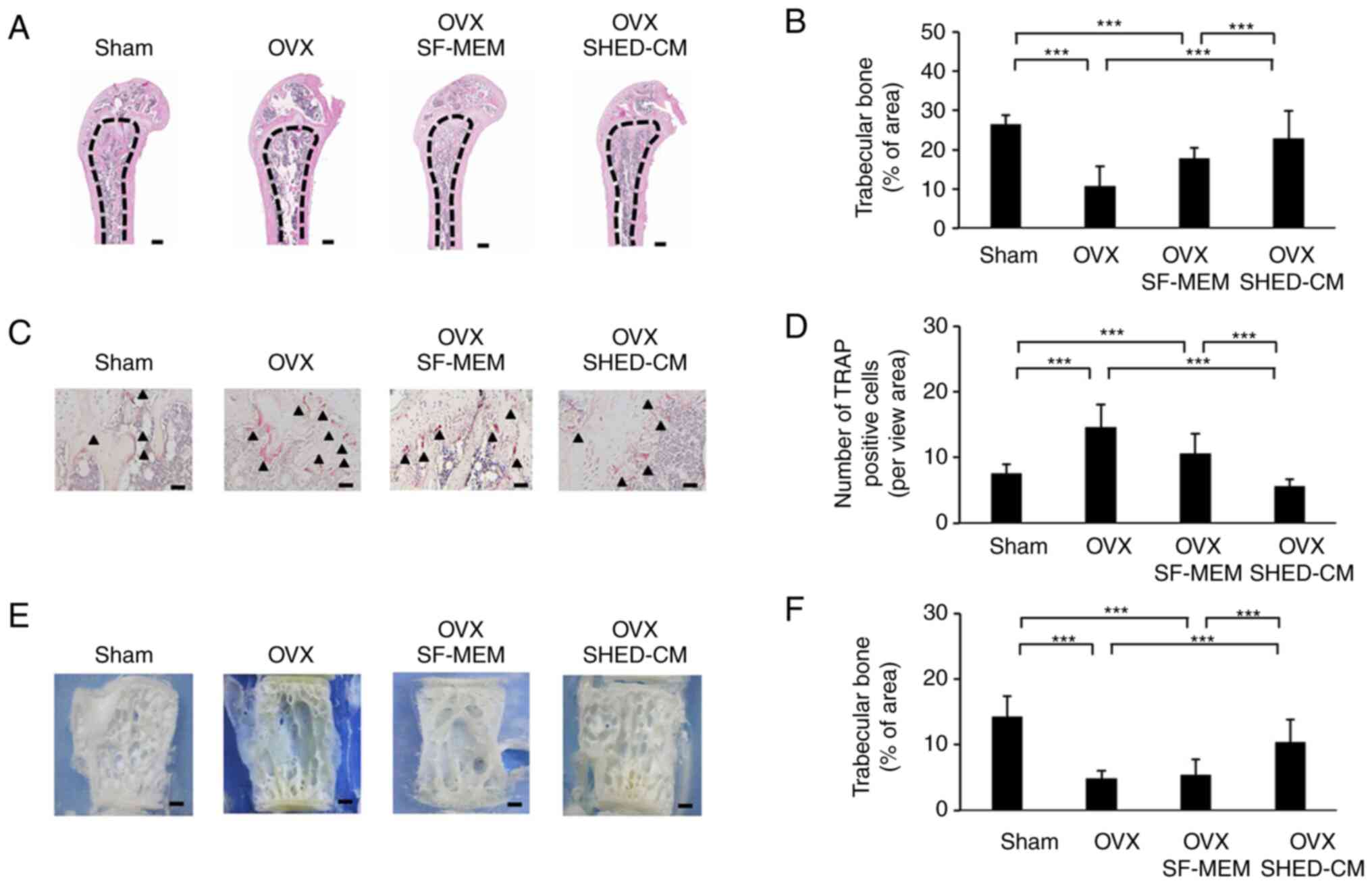
Histological analysis showed that the trabecular bone area in the
OVX SHED-CM group was markedly elevated compared with that in the
OVX group (Fig. 2A and B). Furthermore, the OVX SHED-CM group
showed a significantly reduced number of tartrate-resistant acid
phosphatase-positive cells in the distal femoral diaphysis
compared with that in the OVX group (Fig. 2C and D). Observations on the lumbar spine's
trabecular architecture showed that trabecular bone resorption in
the central region was prominent in the OVX group (Fig. 2E and F). Despite performing OVX in the OVX
SHED-CM group, their trabecular architecture was maintained in the
same way as that in the Sham group.
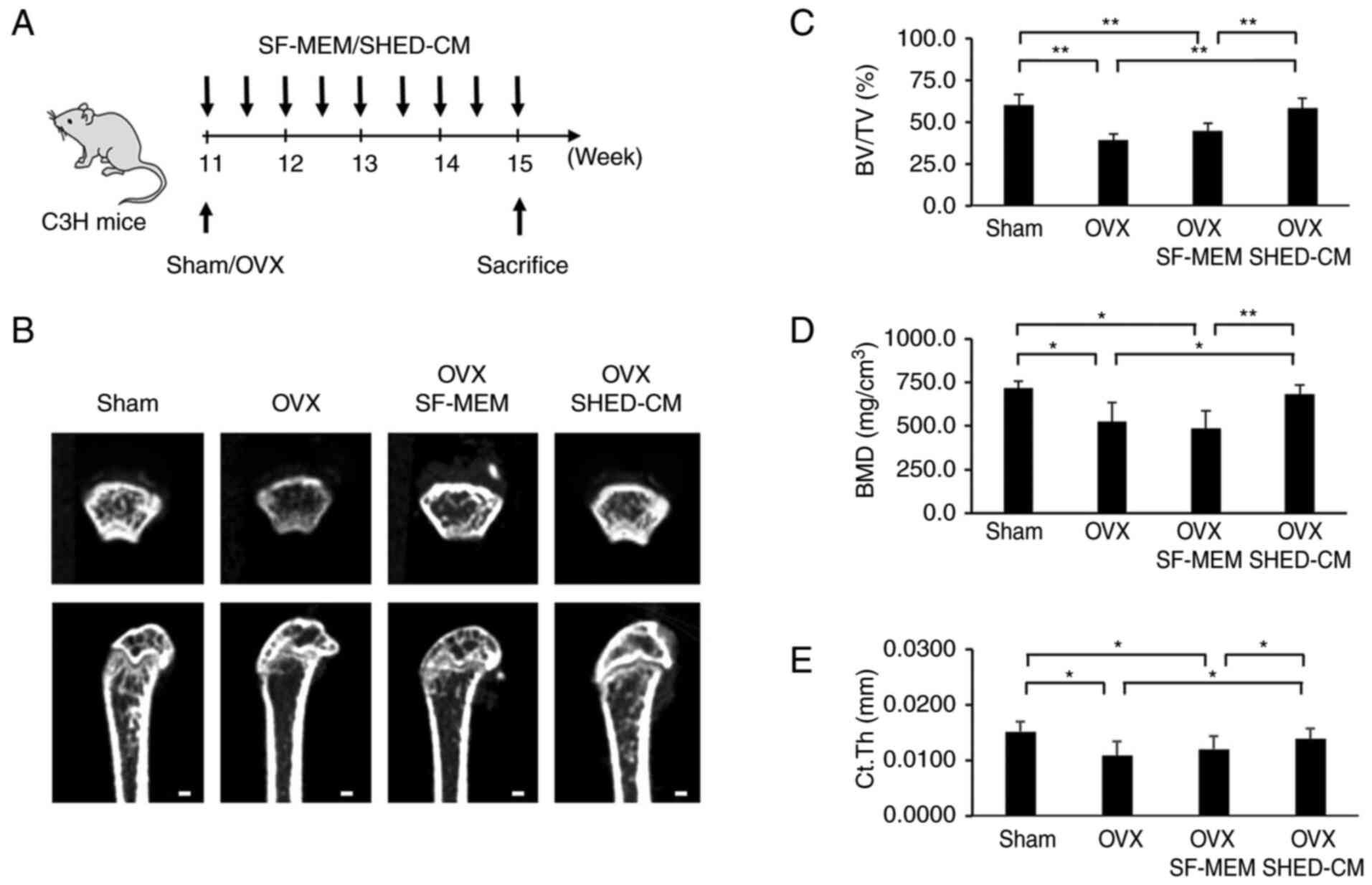 | Figure 1Experimental protocol and analysis of
the femur with microCT (µCT) imaging. SHED transplantation
prevented the development of the osteoporotic phenotype in the OVX
mice. (A) Schema indicating the experimental design for
administering SHED-CM. (B) µCT images of the cancellous bone
architecture in the femur of the Sham, OVX, OVX SF-MEM and OVX
SHED-CM mice. The cancellous bone volume in the OVX mice was
observed to be decreased compared with that in the sham mice. The
reduction in the cancellous bone volume induced by OVX in the OVX
SHED-CM mice was partially restored. Scale bar, 100 µm. (C-E) µCT
analysis revealed that BV/TV (C), BMD (D) and Ct.Th (E) after OVX
were decreased. Administration of SHED-CM increased femoral BV/TV,
BMD, and Ct.Th in the OVX mice. Data are expressed as mean ± SD,
n=5. *P<0.05, **P<0.01. SHED, stem
cells from human exfoliated deciduous teeth; OVX, ovariectomized;
SF-MEM, serum-free medium; SHED-CM, SHED-conditioned medium; BV/TV,
trabecular bone volume percentage; BMD, bone mineral density;
Ct.Th, cortical thickness. |
SHED-CM inhibits osteopenia by
suppressing T cell activation via M2 macrophages
The numbers of IFN-γ-producing cells and that of
IL-17-producing cells in the peripheral blood were significantly
increased in the OVX group and OVX SF-MEM group compared with the
Sham group. The number of IFN-γ-producing cells and IL-17-producing
cells in the OVX SHED-CM group were decreased compared with that in
the OVX and the OVX SF-MEM group due to the administration of
SHED-CM (Fig. 3A-C). Furthermore,
the analysis of the blood serum concentration of IFN-γ and IL-17
using ELISA assay revealed that the IFN-γ and IL-17 concentrations
in the OVX group were significantly higher than those in the Sham
group. Interestingly, the blood serum concentrations of IFN-γ and
IL-17 in the OVX SHED-CM group was markedly reduced compared with
those in the OVX and the OVX SF-MEM group even though OVX was
performed (Fig. 3D and E). Osteoprotegerin (OPG) concentrations
in the blood serum of the OVX group mice and the OVX SHED-CM group
were significantly decreased compared with those in the Sham group.
Conversely, there was no significant difference between the OVX
group and the OVX SHED-CM group in terms of OPG concentration
(Fig. 3F). Regarding the
distribution of macrophages in the peritoneal cavity, the
expression rate of M1 macrophages
[F4/80+CD80+ mononuclear cells (MNCs)] was
not significantly different between the Sham (49.4±4.3%) and the
OVX (70.3±4.9%) groups. In contrast, the expression rate was
markedly decreased in the OVX SHED-CM (40.5±10.5%) group compared
with the OVX (70.3±4.9%) and the SF-MEM (66.8±8.3%) groups
(Fig. 3G and I). The expression rate of M2 macrophages
(F4/80+CD206+ MNCs) was not significantly
different between the Sham (38.9±4.8%) and the OVX (25.1±3.8%)
groups, whereas the expression rate was increased significantly in
the OVX SHED-CM (34.3±7.4%) compared with the OVX (25.1±3.8%) and
the OVX SF-MEM (25.8±5.1%) (Fig.
3G-J).
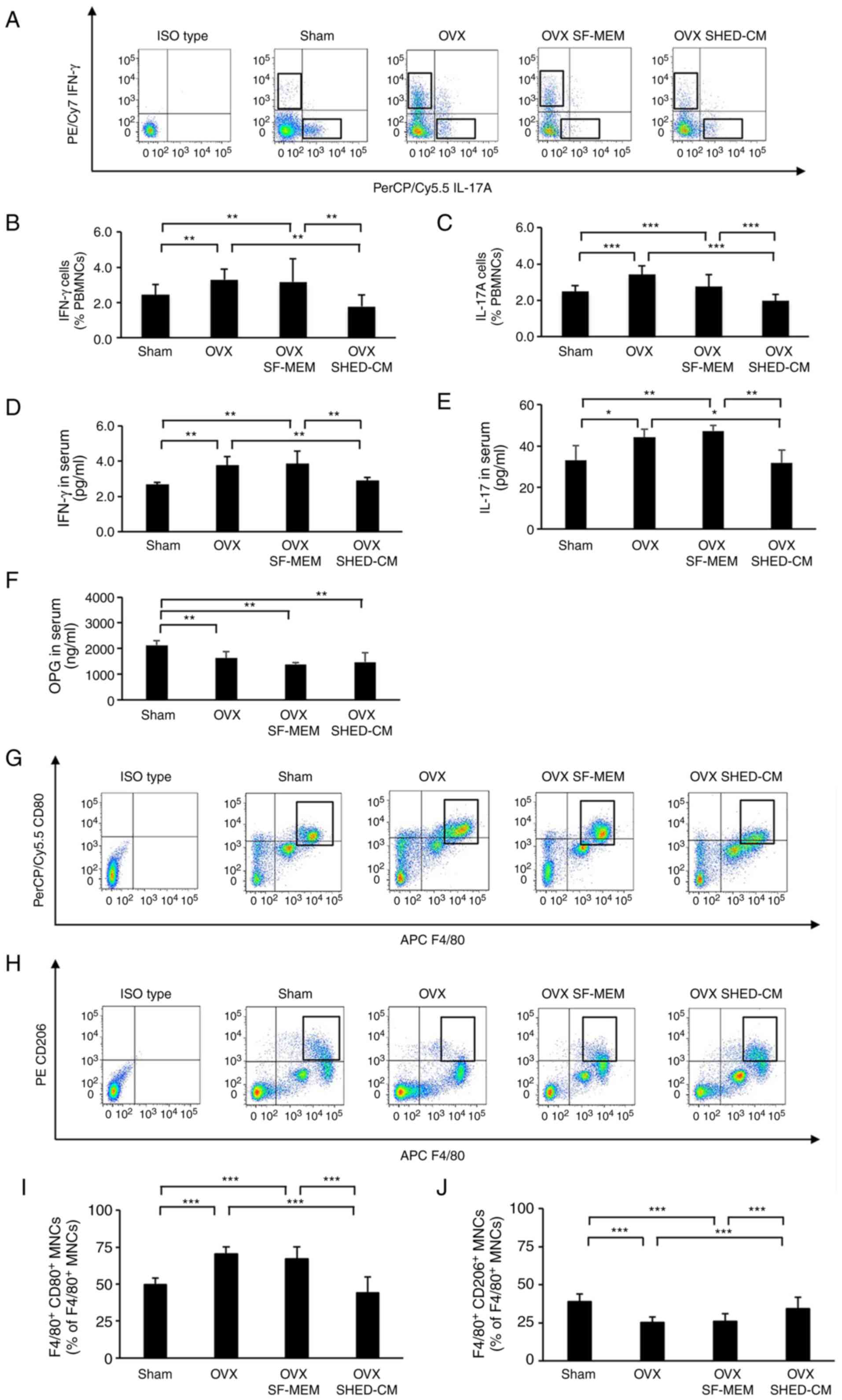 | Figure 3SHED-CM improved the balance between
T cell subsets in the OVX mice and M1/M2 macrophages in the
peritoneal cavity. (A-C) Flow cytometric analysis showed the
percentages of IFN-γ and IL-17 cells increased in the peripheral
blood of the OVX mice. Administration of SHED-CM significantly
decreased the percentages of IFN-γ and IL-17 cells compared with
OVX and the OVX SF-MEM mice. (D and E) ELSA showed increased
concentrations of IFN-γ and IL-17 in the serum of the OVX mice
compared with those in the serum of the sham mice. After SHED-CM
administration, the concentrations of IFN-γ and IL-17 decreased
markedly. (F) ELISA showed increased OPG concentrations in the
serum of OVX mice compared with those in the serum of sham mice.
After OVX, OPG concentrations decreased markedly. (G-J) Flow
cytometric analysis showed that the percentage of M1 and M2
macrophages increased in the peritoneal cavity of the OVX mice.
Administration of SHED-CM resulted in a reduction in the percentage
of M1 macrophages. Data are expressed as mean ± SD, n=5;
*P<0.05, **P<0.01,
***P<0.001. SF-MEM, serum-free medium; SHED-CM,
conditioned medium of stem cells from human exfoliated deciduous
teeth; OVX, ovariectomized; PBMNCs, peripheral blood mononuclear
cells; ELISA, enzyme-linked immunosorbent assay analysis; IFN-γ,
interferon-γ; IL-17, interleukin-17; OPG, osteoprotegerin. |
Knockdown of M2 macrophages using IL-4
nAb
As per µCT images, the OVX anti-IL-4 nAb mice as
well as the OVX anti-IL-4 nAb SHED-CM mice showed considerable
trabecular loss just below the growth plate (Fig. 4A and B). Analysis revealed that femur's
trabecular bone volume fraction (BV/TV), bone mineral density
(BMD), and average cortical thickness (Ct.Th) in the OVX IgG, the
OVX IL-4 nAb and the OVX IL-4 nAb SHED-CM groups were significantly
lower than those in the Sham group (Fig. 4C and D). BV/TV in the OVX IL-4 nAb, OVX IL-4
nAb SF-MEM and OVX IL-4 nAb SHED-CM groups was seen to
significantly decrease compared that in the OVX IgG group (Fig. 4C). However, there was no
significant difference in terms of BV/TV between the OVX IL-4 nAb,
OVX IL-4 nAb SF-MEM and OVX IL-4 nAb SHED-CM groups (Fig. 4C). BMD was not significantly
different between the OVX IgG, the OVX IL-4 nAb, and the OVX IL-4
nAb SHED-CM groups (Fig. 4D).
Similarly, Ct.Th was not significantly different between the OVX
IgG, the OVX IL-4 nAb SF-MEM, and the OVX IL-4 nAb SHED-CM groups
(Fig. 4E).
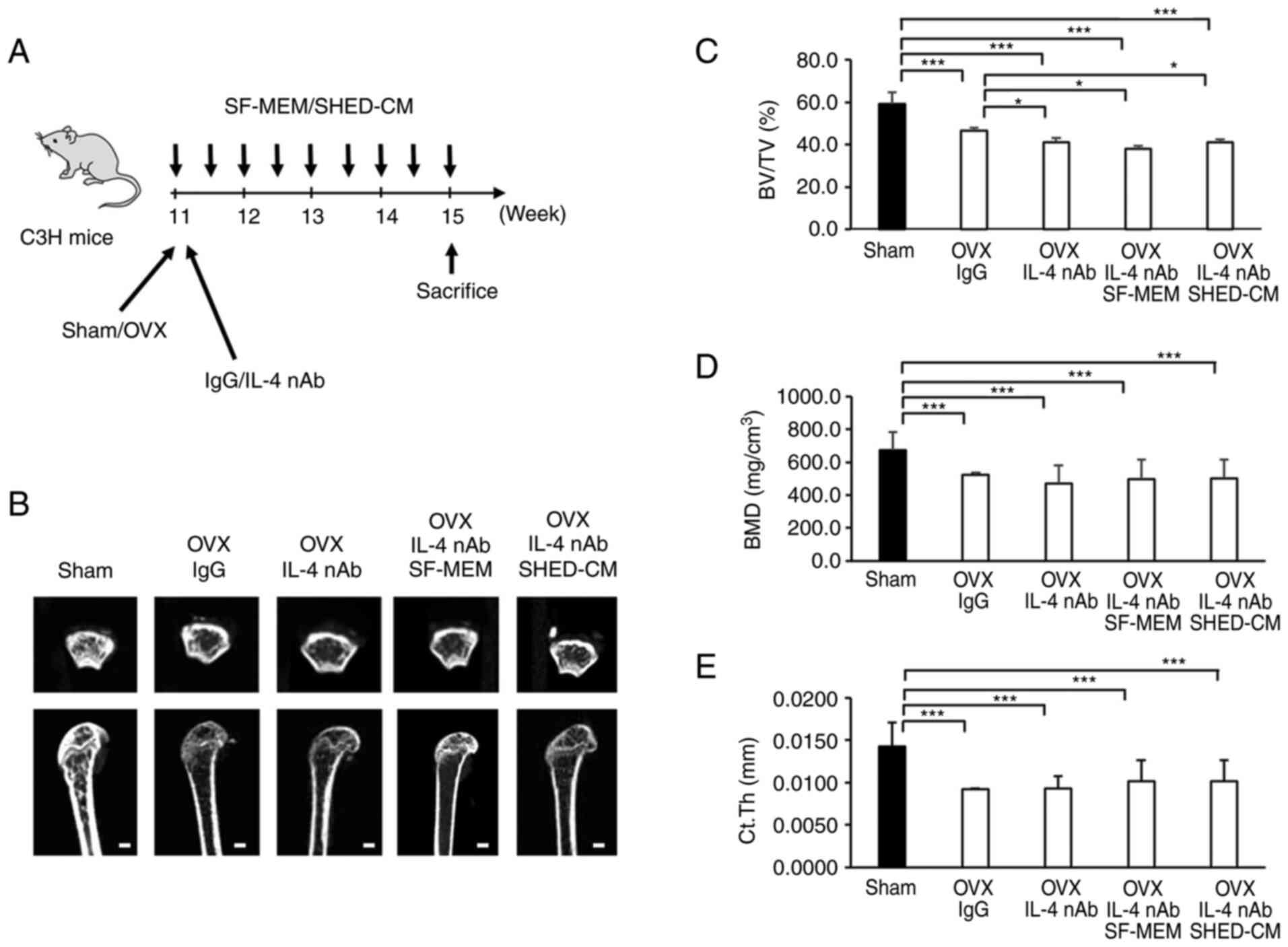 | Figure 4SHED-CM did not ameliorate the
osteoporotic phenotype in the OVX mice with the knockdown of M2
macrophages using IL-4 nAb. (A) Schema indicating the experimental
design for administering IL-4 nAb. (B) microCT (µCT) imaging of
cancellous bone architecture in the femur. (C-E) µCT analysis of
BV/TV, BMD, and Ct.Th. Scale bar, 100 µm. Data are expressed as
mean ± SD, n=5; *P<0.05, ***P<0.001.
OVX, ovariectomized; IL-4 nAb, anti-interleukin-4-neutralizing
antibody; IgG, anti-rat immunoglobulin G1 antibody;
SF-MEM, serum-free medium; SHED-CM, conditioned medium of the stem
cells from human exfoliated deciduous teeth; BV/TV, bone volume
fraction; BMD, bone mineral density; Ct.Th, cortical thickness. |
Effect of IL-4 nAb on the expression
rate of M2 macrophages
The expression rate of M1 macrophage was
significantly increased in the OVX IgG, the OVX IL-4 nAb, and the
OVX IL-4 nAb SHED-CM groups compared with the Sham group (Fig. 5A).
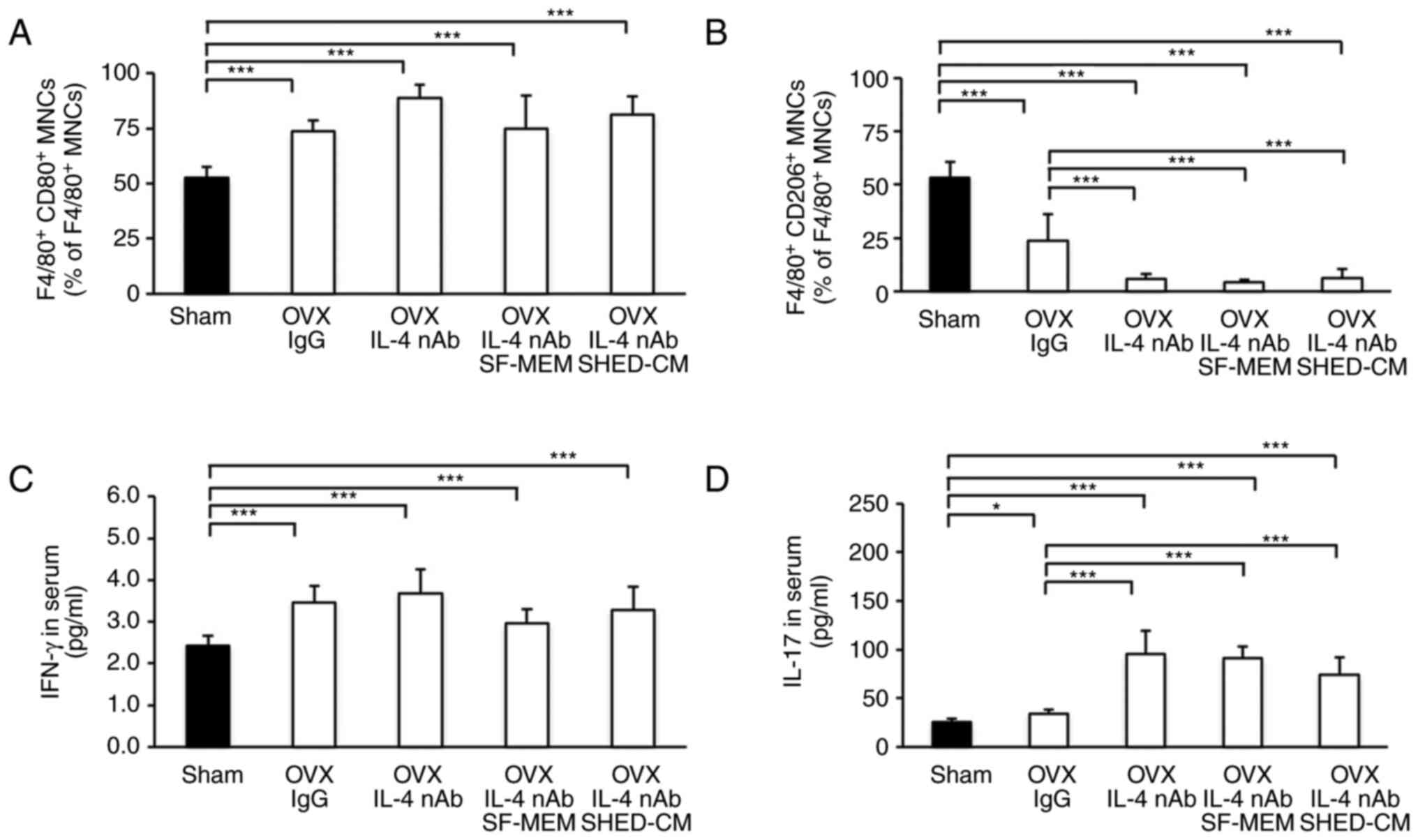 | Figure 5Effect of IL-4 nAb on the expression
rate of M2 macrophages (F4/80+CD80- MNCs) and
IFN-γ and IL-17 serum concentrations. (A and B) The percentages of
M1 and M2 macrophages according to flow cytometric analysis. (C and
D) Concentrations of IFN-γ and IL-17 in the serum. Data are
expressed as mean ± SD, n=5; *P<0.05,
***P<0.001. OVX, ovariectomized; IL-4-nAb,
anti-interleukin-4-neutralizing antibody; IgG, anti-rat
immunoglobulin G1; SF-MEM, serum-free medium; SHED-CM,
conditioned medium of the stem cells from human exfoliated
deciduous teeth; PBMNCs, peripheral blood mononuclear cells; ELISA,
enzyme-linked immunosorbent assay analysis; IFN-γ, interferon-γ;
IL-17, interleukin-17. |
The expression rate of M1 macrophages in the OVX
IgG, the OVX IL-4 nAb, the OVX IL-4 nAb SF-MEM and the OVX IL-4 nAB
SHED-CM were increased in the Sham group (Fig. 5A). However, there was no
significant difference in the expression rate of M1 macrophages
between the OVX IL-4 nAb group and OVX IL-4 nAb SHED-CM group
(Fig. 5A). Furthermore, the
expression rate of M2 macrophages
(F4/80+CD206+MNCs) was significantly
decreased in the OVX IgG, the OVX IL-4 nAb, and the OVX IL-4 nAb
SHED-CM groups compared with the Sham group (Fig. 5B). The expression rate of M2
macrophages in the OVX IL-4-nAb and the OVX IL-4 nAb SHED-CM groups
were observed to significantly decrease compared with that in the
OVX IgG group (Fig. 5B). However,
there was no significant difference in the expression rate of M2
macrophages between the OVX IL-4 nAb and the OVX IL-4 nAb SHED-CM
groups (Fig. 5B).
Effect of IL-4 nAb on IFN-γ and IL-17
serum concentrations
The serum concentration of IFN-γ in the OVX IgG, the
OVX IL-4-nAb, and the OVX IL-4 nAb SHED-CM groups were
significantly higher than that in the Sham group (Fig. 5C). However, the serum concentration
of IFN-γ was not significantly different between OVX IgG, the OVX
IL-4 nAb, and the OVX IL-4 nAb SHED-CM groups (Fig. 5C). The serum concentration of IL-17
in the OVX IgG, the OVX IL-4-nAb, and the OVX IL-4 nAb SHED groups
were significantly higher than that in the Sham group (Fig. 5D). Additionally, the serum
concentration of IL-17 was significantly higher in the OVX IL-4 nAb
and the OVX IL-4 nAb SHED-CM groups compared with the OVX IgG group
(Fig. 5D).
Discussion
In the present study, human exfoliated deciduous
teeth conditioned medium (SHED-CM) administration improved the
early osteoporotic phenotype in ovariectomized (OVX) mice and
exerted an inhibitory effect on bone resorption, in agreement with
the findings of a previous study using systemic SHED injection
(17). Animal models provide
important insights in the study of the therapeutic modality of
osteoporosis. Therefore, OVX mice have been considered the most
important model for elucidating the cause of osteoporosis in humans
and for the development of therapeutic agents (24). The cause of osteoporosis due to
estrogen deficiency is not fully understood, but several mechanisms
have been proposed to date. For example, estrogen deficiency causes
chronic T cell activation and promotes bone resorption by
osteoclasts (25,26). In addition, murine studies have
shown that increased concentrations of IFN-g and T helper 17 (Th17)
produced by activated T cells are primarily associated with the
development of osteoporosis (27-30).
In the presence of estrogen deficiency, IFN-γ promoted the
expression of class II transactivator in immune cells to enhance
antigen presentation between macrophages and T cells, which could
upregulate tumor necrosis factor (TNF)-α and receptor activator for
nuclear factor-κB ligand (RANKL) and promote bone resorption
(27). Furthermore, estrogen
deficiency-induced increase in interferon-γ (IFN-γ) has been
demonstrated to promote bone loss by regulating osteoclastogenesis
in mice (28). Tyagi et al
(31) reported that estrogen
deficiency led to increased differentiation of Th17 cells with
related upregulation of signal transducer and activator of
transcription 3 (STAT3), RAR-related orphan receptor (ROR)-γt, and
ROR-α and induced bone loss by increasing the release of
proosteoclastogenic cytokines including TNFα, IL-6, and RANKL from
osteoblasts. Using flow cytometric analysis of T-cell subtypes in
blood samples of women, Bhadricha et al (32) showed that Th17 cell frequency and
IL-17 levels increased after menopause. In a previous study, SHED
transplantation completely suppressed the osteopenia phenotype
after OVX (17). The present study
showed that cell-to-cell contacts between transplanted SHED and T
cells activated FasL/Fas signaling and induced the apoptosis of T
cells, which inhibited osteoclast differentiation by suppressing
the increase in the plasma concentrations of IFN-γ and IL-17. On
the other hand, research has shown that inhibiting FasL/Fas
signaling by SHED transplantation is very effective against
osteoporosis as ovariectomy-induced osteopenia is not induced in
Fas gene knockout mice (33).
Interestingly, the mice treated with SHED in this study showed no
progression of the osteopenia phenotype. SHED also suppressed the
increase in plasma concentrations of IFN-γ and IL-17 after
ovariectomy. Thus, these data indicate that some secretions in the
culture medium of SHED indirectly inhibited the increase in plasma
concentrations of IFN-γ and IL-17 after ovariectomy. These findings
are consistent with the experimental findings of the previous study
but suggest that a mechanism of action that is distinct from the
FasL/Fas signaling pathway between transplanted SHED and T cells
exists.
Although macrophages play a pivotal role in innate
immunity, macrophages of various subtypes have been clearly shown
to be involved in disease development and tissue healing (34). M1 macrophages activated during
bacterial, viral, and allergic responses express several
inflammatory cytokines and induce a phenotypic immune response
(35,36). M2 macrophages, which have
anti-inflammatory and immunosuppressive functions, produce
anti-inflammatory mediators to promote regression of the injury
response and tissue repair (37-39).
Therefore, we investigated how the administration of SHED-CM
altered the expression ratio of M1 macrophages to M2 macrophages.
M2 macrophages, which were only scarcely present in the sham and
OVX mice, were significantly increased in the SHED-CM mice. In
addition, to clarify whether M2 macrophages were involved in
improving the osteopenia phenotype induced by OVX, we administered
the nAb of IL-4 required for the polarization of M2 macrophages and
evaluated the expression of M2 macrophages. In the OVX anti-IL-4
nAb SHED-CM mice, the osteopenia phenotype induced by ovariectomy
was not improved, even though SHED-CM was administered.
The polarization of M2 macrophages was suppressed in
the mice treated with a neutralizing antibody (nAb), as their ratio
of M2 macrophages to M1 macrophages decreased. In addition, the
serum concentration of IFN-γ and that of Th17 were increased
significantly in the mice treated with IL-4 nAb compared with the
sham mice. The increases in IFN-γ and Th17 may be due to the
inhibition of M2 macrophage polarization by the effects of the
nAb.
In the present study, we did not identify the active
factor that mitigates bone resorption in SHED-CM. However, Hiraki
et al (40) reported that
SHED-CM contains bone metabolism-related factors,
angiogenesis-related factors, and neurotrophic factors. Moreover,
MSC-CM contains insulin-like growth factor-1, transforming growth
factor (TGF)-β1, and vascular endothelial growth factor (VEGF), and
these factors affect the regeneration of bone cells (41). It is possible that these cytokines
in SHED-CM may have acted to improve osteoporosis in this
experiment. In addition, several other factors may have cooperated
to activate various signaling pathways; those affecting the
improvement of osteoporosis need further investigation.
We determined the therapeutic efficacy of SHED-CM on
osteoporosis caused by estrogen deficiency in OVX mice. Our results
showed that SHED-CM inhibited osteoclast differentiation by
suppressing IFN-γ and IL-17 cells by inducing M2 macrophage
polarization, thereby improving the pathology of OVX-induced
osteoporosis. In conclusion, we suggest that SHED-CM has the
potential to be used as a therapeutic agent targeting the
inhibition of bone resorption, which is an important therapeutic
strategy for osteoporosis.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported in part by KAKENHI
(Grant-in-Aid for Scientific Research from the Japan Society for
the Promotion of Science: 20K10219, 19K10280, 21K10172).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AM, TK, and YYo performed the animal experiments,
histological analyses and flow cytometry analysis. TK and YYo
conducted the statistical analysis. YYa and TS performed the μCT
study. AM and TK wrote the manuscript. AM, TK, and YYa conceived
the study and participated in its design and coordination. All
authors confirm the authenticity of all the raw data. All authors
read and approved the final manuscript for publication.
Ethics approval and consent to
participate
SHED were isolated and cultured from deciduous teeth
donated by the Department of Pediatrics and Disabled Dentistry,
Hokkaido University Hospital (Kita-ku, Sapporo, Japan) under the
approval of the Institutional Voluntary Clinical Research Review
Board (approval no. 010-116). The study protocols for the animal
experiments were approved by The Hokkaido University Animal
Experiment Committee. All animal experiments adhered to Hokkaido
University Animal Experiment Guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Kanis JA, McCloskey EV, Harvey NC,
Johansson H and Leslie WD: Intervention thresholds and the
diagnosis of osteoporosis. J Bone Miner Res. 30:1747–1753.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khosla S, Atkinson EJ, Melton LJ III and
Riggs BL: Effects of age and estrogen status on serum parathyroid
hormone levels and biochemical markers of bone turnover in women: A
population-based study. J Clin Endocrinol Metab. 82:1522–1527.
1997.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vidal M, Thibodaux RJ, Neira LFV and
Messina OD: Osteoporosis: A clinical and pharmacological update.
Clin Rheumatol. 38:385–395. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Marx RE: Pamidronate (Aredia) and
zoledronate (Zometa) induced avascular necrosis of the jaws: A
growing epidemic. J Oral Maxillofac Surg. 61:1115–1117.
2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McClung M, Harris ST, Miller PD, Bauer DC,
Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK and Lewiecki EM:
Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug
holiday. Am J Med. 126:13–20. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Deligiorgi MV and Trafalis DT: The safety
profile of denosumab in oncology beyond the safety of denosumab as
an anti-osteoporotic agent: Still more to learn. Expert Opin Drug
Saf. 20:191–213. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Akiyama K, Chen C, Wang D, Xu X, Qu C,
Yamaza T, Cai T, Chen W, Sun L and Shi S:
Mesenchymal-stem-cell-induced immunoregulation involves
FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell.
10:544–555. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tang WY, Liu JH, Peng CJ, Liao Y, Luo JS,
Sun X, Tang YL and Luo XQ: Functional characteristics and
application of mesenchymal stem cells in systemic lupus
erythematosus. Arch Immunol Ther Exp (Warsz). 69(7)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barati S, Tahmasebi F and Faghihi F:
Effects of mesenchymal stem cells transplantation on multiple
sclerosis patients. Neuropeptides. 84(102095)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Miura M, Gronthos S, Zhao M, Lu B, Fisher
LW, Robey PG and Shi S: SHED: Stem cells from human exfoliated
deciduous teeth. Proc Natl Acad Sci USA. 100:5807–5812.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sakai VT, Zhang Z, Dong Z, Neiva KG,
Machado MA, Shi S, Santos CF and Nör JE: SHED differentiate into
functional odontoblasts and endothelium. J Dent Res. 89:791–796.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chadipiralla K, Yochim JM, Bahuleyan B,
Huang CY, Garcia-Godoy F, Murray PE and Stelnicki EJ: Osteogenic
differentiation of stem cells derived from human periodontal
ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue
Res. 340:323–333. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y,
Gronthos S, Wong S and Shi S: Immunomodulatory properties of stem
cells from human exfoliated deciduous teeth. Stem Cell Res Ther.
1(5)2010.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Yang N, Liu X, Chen X, Yu S, Yang W and
Liu Y: Stem cells from exfoliated deciduous teeth transplantation
ameliorates Sjögren's syndrome by secreting soluble PD-L1. J Leukoc
Biol: Oct 8, 2021 (Epub ahead of print).
|
|
15
|
Kitase Y, Sato Y, Ueda K, Suzuki T,
Mikrogeorgiou A, Sugiyama Y, Matsubara K, Tsukagoshi Okabe Y,
Shimizu S, Hirata H, et al: A novel treatment with stem cells from
human exfoliated deciduous teeth for hypoxic-ischemic
encephalopathy in neonatal rats. Stem Cells Dev. 29:63–74.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li W, Jiao X, Song J, Sui B, Guo Z, Zhao
Y, Li J, Shi S and Huang Q: Therapeutic potential of stem cells
from human exfoliated deciduous teeth infusion into patients with
type 2 diabetes depends on basal lipid levels and islet function.
Stem Cells Transl Med. 10:956–967. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu Y, Wang L, Liu S, Liu D, Chen C, Xu X,
Chen X and Shi S: Transplantation of SHED prevents bone loss in the
early phase of ovariectomy-induced osteoporosis. J Dent Res.
93:1124–1132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mita T, Furukawa-Hibi Y, Takeuchi H,
Hattori H, Yamada K, Hibi H, Ueda M and Yamamoto A: Conditioned
medium from the stem cells of human dental pulp improves cognitive
function in a mouse model of Alzheimer's disease. Behav Brain Res.
293:189–197. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bartholomew A, Sturgeon C, Siatskas M,
Ferrer K, McIntosyu K, Patil S, Hardy W, Devine S, Ucker D, Deans
R, et al: Mesenchymal stem cells suppress lymphocyte proliferation
in vitro and prolong skin graft survival in vivo. Exp Hematol.
30:42–48. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He Q, Wang L, Zhao R, Yan F, Sha S, Cui C,
Song J, Hu H, Guo X, Yang M, et al: Mesenchymal stem cell-derived
exosomes exert ameliorative effects in type 2 diabetes by improving
hepatic glucose and lipid metabolism via enhancing autophagy. Stem
Cell Res Ther. 11(223)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen
L, Wang M, Zhou Y, Zhu W, Li W and Xu W: Exosomes derived from
human umbilical cord mesenchymal stem cells alleviate liver
fibrosis. Stem Cells Dev. 22:845–854. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lubura M, Hesse D, Neumann N, Scherneck S,
Wiedmer P and Schürmann A: Non-invasive quantification of white and
brown adipose tissues and liver fat content by computed tomography
in mice. PLoS One. 7(e37026)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fathi E, Farahzadi R and Valipour B:
Alginate/gelatin encapsulation promotes NK cells differentiation
potential of bone marrow resident C-kit+ hematopoietic
stem cells. Int J Biol Macromol. 177:317–327. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Komori T: Animal models for osteoporosis.
Eur J Pharmacol. 759:287–294. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fathi E, Farahzadi R, Vietor I and
Javanmardi S: Cardiac differentiation of bone-marrow-resident
c-kit+ stem cells by L-carnitine increases through
secretion of VEGF, IL6, IGF-1, and TGF-β as clinical agents in
cardiac regeneration. J Biosci. 45(92)2020.PubMed/NCBI
|
|
26
|
Pacifici R: Estrogen deficiency, T cells
and bone loss. Cell Immunol. 252:68–80. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fischer V and Haffner-Luntzer M:
Interaction between bone and immune cells: Implications for
postmenopausal osteoporosis. Semin Cell Dev Biol: May 20, 2021
(Epub ahead of print).
|
|
28
|
Cenci S, Toraldo G, Weitzmann MN, Roggia
C, Gao Y, Qian WP, Sierra O and Pacifici R: Estrogen deficiency
induces bone loss by increasing T cell proliferation and lifespan
through IFN-gamma-induced class II transactivator. Proc Natl Acad
Sci USA. 100:10405–10410. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gao Y, Grassi F, Ryan MR, Terauchi M, Page
K, Yang X, Weitzmann MN and Pacifici R: IFN-gamma stimulates
osteoclast formation and bone loss in vivo via antigen-driven T
cell activation. J Clin Invest. 117:122–132. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Zhao R: Immune regulation of bone loss by
Th17 cells in oestrogen-deficient osteoporosis. Eur J Clin Invest.
43:1195–1202. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tyagi AM, Srivastava K, Mansoori MN,
Trivedi R, Chattopadhyay N and Singh D: Estrogen deficiency induces
the differentiation of IL-17 secreting Th17 cells: A new candidate
in the pathogenesis of osteoporosis. PLoS One.
7(e44552)2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bhadricha H, Patel V, Singh AK, Savardekar
L, Patil A, Surve S and Desai M: Increased frequency of Th17 cells
and IL-17 levels are associated with low bone mineral density in
postmenopausal women. Sci Rep. 11(16155)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kovacic N, Grcevic D, Katavic V, Lukic IK,
Grubisic V, Mihovilovic K, Cvija H, Croucher PI and Marusic A: Fas
receptor is required for estrogen deficiency-induced bone loss in
mice. Lab Invest. 90:402–413. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Murray PJ and Wynn TA: Protective and
pathogenic function of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Lu LY, Loi F, Nathan K, Lin TH, Pajarinen
J, Gibon E, Nabeshima A, Cordova L, Jämsen E, Yao Z and Goodman SB:
Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal
stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res.
35:2378–2385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu Q, Tian Y, Zhao X, Jing H, Xie Q, Li
P, Li D, Yan D and Zhu X: NMAAP1 expressed in BCG-activated
macrophage promotes M1 macrophage polarization. Mol Cells.
38:886–894. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
van Dalen FJ, van Stevendaal MHME,
Fennemann FL, Verdoes M and Ilina O: Molecular repolarisation of
tumour-associated macrophages. Molecules. 24(9)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mills CD: M1 and M2 macrophages: Oracles
of health and disease. Crit Rev Immunol. 32:463–488.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hiraki T, Kunimatsu R, Nakajima K, Abe T,
Yamada S, Rikitake K and Tanimoto K: Stem cell-derived conditioned
media from human exfoliated deciduous teeth promote bone
regeneration. Oral Dis. 26:381–390. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ando Y, Matsubara K, Ishikawa J, Fujio M,
Shohara R, Hibi H, Ueda M and Yamamoto A: Stem cell-conditioned
medium accelerates distraction osteogenesis through multiple
regenerative mechanisms. Bone. 61:82–90. 2014.PubMed/NCBI View Article : Google Scholar
|