Introduction
Ovarian cancer is the most lethal gynecological
malignancy; >70% patients diagnosed with stage III and IV cancer
show poor survival rate due to treatment failure, worldwide
(1,2). Cytoreductive surgery is the main
surgical method for patients with advanced stage ovarian cancer,
followed by combined chemotherapy with paclitaxel and platinum, and
is considered to be the standard treatment for ovarian cancer
(2-4).
However, one common cause for treatment failure is acquired drug
resistance due to post-surgery chemotherapy (5-7).
Therefore, it is crucial to identify the mechanism of acquired drug
resistance in patients with advanced ovarian cancer and to develop
novel targets for the treatment of drug-resistant ovarian
cancer.
MicroRNAs (miRNAs or miRs) are small non-coding RNAs
that negatively regulate target genes at the post-transcriptional
level (8). Numerous studies have
demonstrated the regulatory role of miRNAs in multiple biological
processes, including tumorigenesis (9-11).
Specific miRNA expression patterns are involved in tumor
progression, which includes processes such as cell proliferation,
differentiation, migration, immune suppression and drug resistance
(12,13). miR-let-7b belongs to the let-7
family and functions as a tumor suppressor gene (14-16).
Previous studies have reported significant downregulation of
miR-let-7b in several types of cancer, such as multiple myeloma,
glioma and osteosarcoma (16-18).
However, the impact of miR-let-7b on carcinogenesis of ovarian
cancer remains unclear.
In traditional Chinese medicine, radix Ranunculus
ternati is applied in the treatment of numerous types of
disease, including scrofula, tuberculosis and pharyngitis (19), and comprises saponins,
polysaccharides and certain fatty substances (20,21).
Radix ranunculus temate saponins (RRTS) has shown satisfactory
in vitro ability to suppress gastric cancer cells growth and
proliferation (22,23). However, its underlying molecular
mechanisms need to be elucidated.
In the present study, the roles of miR-let-7b in
ovarian cancer were investigated. We further explored the effect of
RRTS on ovarian cancer and discovered its role suppressing ovarian
cancer cells.
Materials and methods
Patient samples and clinical data
A total of 57 females (age, 32-75 years; stage I to
IV) were enrolled and pathologically diagnosed with high-grade
serous ovarian cancer at Sun Yat-sen University Cancer Center
(SYSUCC) in Guangzhou, Guangdong, China, from October 2008 to May
2016. Written informed consent was provided by all patients. The
present study was approved by the Research and Ethical Committee of
Guangdong Provincial People's Hospital and complied with all
relevant ethical regulations. All specimens were confirmed as
primary ovarian cancer by pathological examination. None of the
patients had received chemotherapy before the surgery. Fresh
samples were collected <30 min following surgical removal during
routine surgery and stored at -80˚C in the cancer resource bank of
SYSUCC. Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.).
To compare the expression of miR-let-7b in tumor and
normal tissues, fresh high-grade ovarian serous cancer and matched
normal fallopian tube tissue were collected from six female
patients (age, 39-67 years, stage I and II) who received
comprehensive surgical staging (which is the main surgical method
for patients with early-stage ovarian cancer), or cytoreductive
surgery at SYSUCC, between November 2010 to August 2015. Matched
normal fallopian tissues were used as healthy tissues.
In addition, fresh high-grade ovarian serous cancer
tissue samples were collected from 12 female patients (age, 44-71
years, stage III and IV) who received cytoreductive surgery and
subsequent chemotherapy at SYSUCC, between October 2008 and April
2011 to identify the expression levels of miR-let-7b in
chemo-sensitive and -resistant ovarian cancer. Patients with
recurrent tumor <6 months following chemotherapy were considered
TR.
High-grade ovarian serous cancer tissue samples were
collected from 39 female patients (age, 32-75 years, stage III and
IV) who received comprehensive staging or cytoreductive surgery at
SYSUCC, between October 2008 and May 2016, to investigate the
association between miR-let-7b and COL3A1 in ovarian cancer.
Cell lines and culture
Ovarian cancer cell lines (A2780, A2780/TR, OVCAR3,
SK-OV-3 and SK-OV-3/TR) were acquired from American Type Culture
Collection. A2780, A2780/TR and OVCAR3 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) in an incubator at 37˚C with 5%
CO2. SK-OV-3 and SK-OV-3/TR cells were cultured in
Roswell Park Memorial Institute (RPMI)-1640 (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS in an incubator at 37˚C with
5% CO2.
Transient miRNA transfection
miRNA-let-7b mimic (5'-UGAGGUAGUAGGUUGUGUGGUU-3')
and negative control (5'-UUUGUACUACACAAAAGUACUG-3') were
synthesized by Ribobio. A2780, OVCAR3 and SK-OV-3 cells
(1x105 cells/well) were divided into groups, namely the
control group (Mock), the negative control (NC) group and the
miR-let-7b group. Cells in the miR-let-7b group were transfected
with miRNA-let-7b mimic (50 nM) using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Cells in the NC group were transfected
with negative control. The control group was left untreated. After
48 h transfection, cells were used for subsequent
experimentation.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from tissues or cells
(A2780, OVCAR3 and SK-OV-3) using TRIzol® (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instruction,
followed by determination of the RNA concentration. PrimeScript RT
reagent kit (Takara Biotechnology Co., Ltd.) was used to synthesize
the complementary DNA. Mature miRNAs in the sample were
reverse-transcribed to cDNA using TaqMan Advanced miRNA cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.). RT-qPCR was
performed using SYBR Green (Takara Biotechnology Co., Ltd.). GAPDH
and U6 were used to normalize mRNA and miRNA levels, respectively.
The PCR process was: Initial denaturing 10 min at 95˚C;
denaturation 10 sec at 95˚C; annealing 20 sec at 60˚C; and
extension 15 sec at 72˚C; for 40 cycles. The relative expression
levels of genes were calculated using the 2-ΔΔCq method
(24). All assays were performed
in triplicate. Each test was repeated three times. The primer
sequences were as follows: miR-let-7b forward,
5'-GGGTGAGGTAGTAGGTTGTGTG-3' and reverse,
5'-CAGGGAAGGCAGTAGGTTGT-3'; U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and
reverse, 5'-AACGCTTCACGAATTTGCGT-3'; COL3A1 forward,
5'-GCCAAATATGTGTCTGTGACTCA-3' and reverse,
5'-GGGCGAGTAGGAGCAGTTG-3' and GAPDH forward,
5'-GTCTCCTCTGACTTCAACAGCG-3' and reverse,
5'-ACCACCCTGTTGCTGTAGCCAA-3'.
Cell Counting Kit (CCK)-8 assay
CCK-8 assay was used to detect the viability of
cells.
To identify the role of miR-let-7b in ovarian cancer
cells, A2780 cells overexpressing miR-let-7b or control miRNA were
seeded into a 96-well culture plate at a density of
1x104 cells/well and grown in DMEM supplemented with 10%
FBS at 37˚C. The cells were treated with Taxol (0.00, 6.25, 12.50,
25.00, 50.00, 100.00 nM) for 16 h, then were detected by CCK-8
assay.
To investigate the treatment effect of RRTS, A2780
and SK-OV-3 cells were seeded into a 96-well culture plate at a
density of 1x104 cells/well and grown in DMEM or
RMPI-1640 supplemented with 10% FBS at 37˚C. These cells were
treated with RRTS (0.00, 6.25, 12.50, 25.00, 50.00, 100.00, 200.00
µg/ml) for 48 h, then were detected by CCK-8 assay.
To investigate whether RRTS sensitizes ovarian
cancer cells to Taxol, A2780, A2780/TR, SK-OV-3 and SK-OV-3/TR
cells were seeded into a 96-well culture plate at a density of
1x104 cells/well and grown in DMEM or RMPI-1640
supplemented with 10% FBS at 37˚C. These cells were pre-treated
with 25 µg/ml RRTS for 24 h, followed by Taxol (0.000, 3.125,
6.250, 12.500, 25.000, 50.000 and 100.000 nM) for a further 24 h,
then were detected by CCK-8 assay.
For CCK-8 assay, 20 µl CCK-8 reagent (Shanghai
Yeasen Biotechnology Co., Ltd.) was added to each well. The plate
was incubated for 2 h, followed by measurement of the optical
density at 450 nm.
MTT assay
MTT assay was used to detect the metabolic activity
of cells. A2780, OVCAR3 and SK-OV-3 cells overexpressing miR-let-7b
or control miRNA were seeded into a 96-well culture plate at a
density of 500 cells/well and grown in DMEM or RMPI-1640
supplemented with 10% FBS. MTT reagent (Sigma-Aldrich; Merck KGaA)
was dissolved in PBS (5 mg/ml). Medium was replaced with fresh DMEM
or RMPI-1640 + 10% FBS + 10% MTT and incubated for 4 h at 37˚C.
After removing the incubation medium, formazan crystals were
dissolved in 200 µl DMSO. Optical density was measured at 570
nm.
Wound healing assay
Wound healing assay was used to detect cell
migration ability. A2780, OVCAR3 and SK-OV-3 cells overexpressing
miR-let-7b or control miRNA were seeded into 6-well culture plates
and grown in DMEM or RMPI-1640 supplemented with 10% FBS until 90%
confluence. Confluent cells were scratched with a pipette tip.
Culture medium was removed and cells were rinsed with PBS, then
incubated in DMEM or RMPI-1640 without FBS. Images were captured
using a Nikon Coolpix camera at 0 and 24 h. The scratch area (SA)
was measured using Image-Pro Plus 6.0 (NIH) and wound healing rate
was calculated as follows: Wound healing rate=(initial SA-final
SA)/initial SA.
Matrigel invasion assay
Cell invasion was determined using Matrigel-coated
Transwell cell culture chambers (Corning, Inc.). Matrigel (BD
Biosciences) was thawed on ice overnight and diluted in serum
free-cold DMEM or RMPI-1640 (2 mg/ml). Diluted Matrigel (100 µl)
was placed into upper chamber of 24-well Transwell and incubated at
37˚C for 4 h for gelling. A2780, OVCAR3 and SK-OV-3 cells
overexpressing miR-let-7b or control miRNA were serum starved for
12 h at 37˚C, then trypsinized, resuspended in serum-free DMEM or
RMPI-1640. Cells (1x105) were seeded in the upper
chamber of the Transwell insert coated with Matrigel. The lower
chamber was filled with DMEM or RMPI-1640 containing 10% FBS as a
chemoattractant. Following incubation for 24 h at 37˚C, cells were
fixed with methanol and stained with crystal violet for 30 min at
room temperature. Noninvaded cells on the top of the Transwell were
scraped off with a cotton swab. The number of cells in five
randomly selected fields of view were imaged under a
photomicroscope (light) and counted using Image-Pro Plus 6.0 (Media
Cybernetic, Inc.).
Western blotting
A2780 cells overexpressing miR-let-7b or control
miRNA were lysed in RIPA buffer (25.0 mM Tris-HCl, pH 7.6, 150.0 mM
NaCl, 1.0 NP-40, 1.0 sodium deoxycholate and 0.1% SDS) containing
protease and phosphatase inhibitors. Protein quantification was
performed by BCA Protein Assay (Pierce; Thermo Fisher Scientific,
Inc.). Then 20 µg of soluble protein were loaded onto each lane of
10% Bis-Tris gel. The proteins were transferred to polyvinylidene
fluoride (PVDF) membrane. For the immunoblot, the membranes were
blocked with 5% skimmed milk (Bio-Rad Laboratories, Inc.) in TBST
(0.5% Tween) for 1 h, at room temperature. Primary antibodies
(1:1,000 dilution) in 5% bovine serum albumin (BSA) were added and
incubated overnight in 4˚C on a shaker. The membranes then were
washed with TBST and incubated with secondary antibody (1:5,000
dilution) in 5% skimmed milk at room temperature for 1 h. The
membranes then were washed with TBST and incubated with ECL mix
(Epizyme; cat. no. SQ202). The membrane was removed from the ECL
mix and placed between layers of plastic. The membrane was then
exposed to autoradiography film (Kodak) using an OPTIMAX X-Ray Film
Processor (Protec GmbH) in a dark room. Rabbit antibody against
COL3A1 (cat. no. 22734), mouse antibody against GAPDH (cat. no.
60004) and horseradish peroxidase-conjugated secondary antibodies
(cat. no. SA00001-1 and SA00001-2) were obtained from ProteinTech
Group, Inc.
Flow cytometry assay
Apoptotic rate was detected by flow cytometry using
an Annexin V apoptosis detection kit (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. A2780
cells overexpressing miR-let-7b or control miRNA were treated with
Taxol (0, 6.25, 12.5, 25, 50, 100 nM) for 16 h, then were collected
for detection. A2780, A2780/TR, SK-OV-3 and SK-OV-3/TR cells were
pre-treated with 25 µg/ml of RRTS for 24 h, followed by 25 nM of
Taxol for a further 24 h, then were collected for detection.
In this flow cytometry assay, A2780, A2780/TR,
SK-OV-3 and SK-OV-3/TR cells were rinsed with ice-cold PBS,
followed by rinsing with binding buffer and adjusted to
1x105 cells/ml. Next, 100 µl of cell suspension was
stained with 5 µl of Annexin V-APC for 15 min in the dark at room
temperature. Following another rinse with binding buffer, 5 µl of
PI was added to the cells and incubated on ice for 5 min. Following
filtration, the cell mixture was analyzed using FASCcan Flow
Cytometer (BD Biosciences) and data were analyzed using FlowJo_V10
(FlowJo LLC).
Isobolographic analysis
Isobolographic analysis was performed to
characterize the extent of the interaction between RRTS and Taxol
in A2780 cells. The IC50 (half maximal inhibitory
concentration) of Taxol (32.5 nM) and RRTS (42 µg/ml) were plotted
on the x and y axes in a two-coordinate plot. In order to connect
the IC50 value of each drug alone and that of the combination, a
nonlinear regression analysis (equation y=(top-bottom) x exp(-kx) +
bottom) has been carried out by GraphPad Prism 5 (GraphPad
Software, Inc.) (25,26). The line connecting these two points
represented an additive interaction. The concentrations of drugs
used in combination were placed in the same plot.
The effect was considered synergistic when the plot
was located below the line and antagonistic when the plot was above
the line.
In vivo ovarian cancer xenograft
model
Animal protocols were approved by the animal care
committee of Guangdong Provincial People's Hospital (approval no.
GDREC2019582A). This study was approved in 2019. A total of 36 mice
were used in this study. At the start of the study, the body weight
ranged from 18.5-20 g. Throughout the entire project, the mice were
housed in standard polypropylene cages, at optimum density and in
standard laboratory conditions (temperature 25 ± 1˚C, relative
humidity 55 ± 5%, and 12 h light/dark cycle). They were allowed
free access to standard granular diet and water. A2780 cells
(1x106) were injected subcutaneously into the back of
six-week-old BALB/c nu/nu female mice. At 3 weeks post-inoculation,
tumor formation was observed and nude mice were randomly divided
into 4 groups (n=9) as follows: Control (saline); RRTS (25 mg/kg);
Taxol (15 mg/kg) and 25 mg/kg RRTS + 15 mg/kg Taxol. Nude mice were
intraperitoneally injected once/week for 6 weeks. Tumor volume was
recorded weekly for duration of experiment. Tumor volume was
estimated as follows: Volume=(longest diameter x shortest
diameter2)/2. The mice were euthanized humanely by
CO2 asphyxiation at the end of the experiment or when
tumor growth was excessive (diameter ≥20 mm). A displacement rate
of 25% chamber volume/min was used for euthanasia.
Statistical analysis
SPSS 16.0 (SPSS, Inc.) and GraphPad Prism 5
(GraphPad Software, Inc.) were used for statistical analysis.
Paired t-test was used to compare ovarian cancer and adjacent
healthy tissue. An unpaired t-test was utilized to analyze data
conforming to normal distribution and homogeneity of variance
between two groups. The association between miR-let-7b and COL3A1
expression in tumor tissues was analyzed using Spearman's
correlation analysis. All experiments were repeated three times
unless stated otherwise. Data are presented as the mean ± SD.
Two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-let-7b modulates sensitivity of
ovarian cancer to Taxol
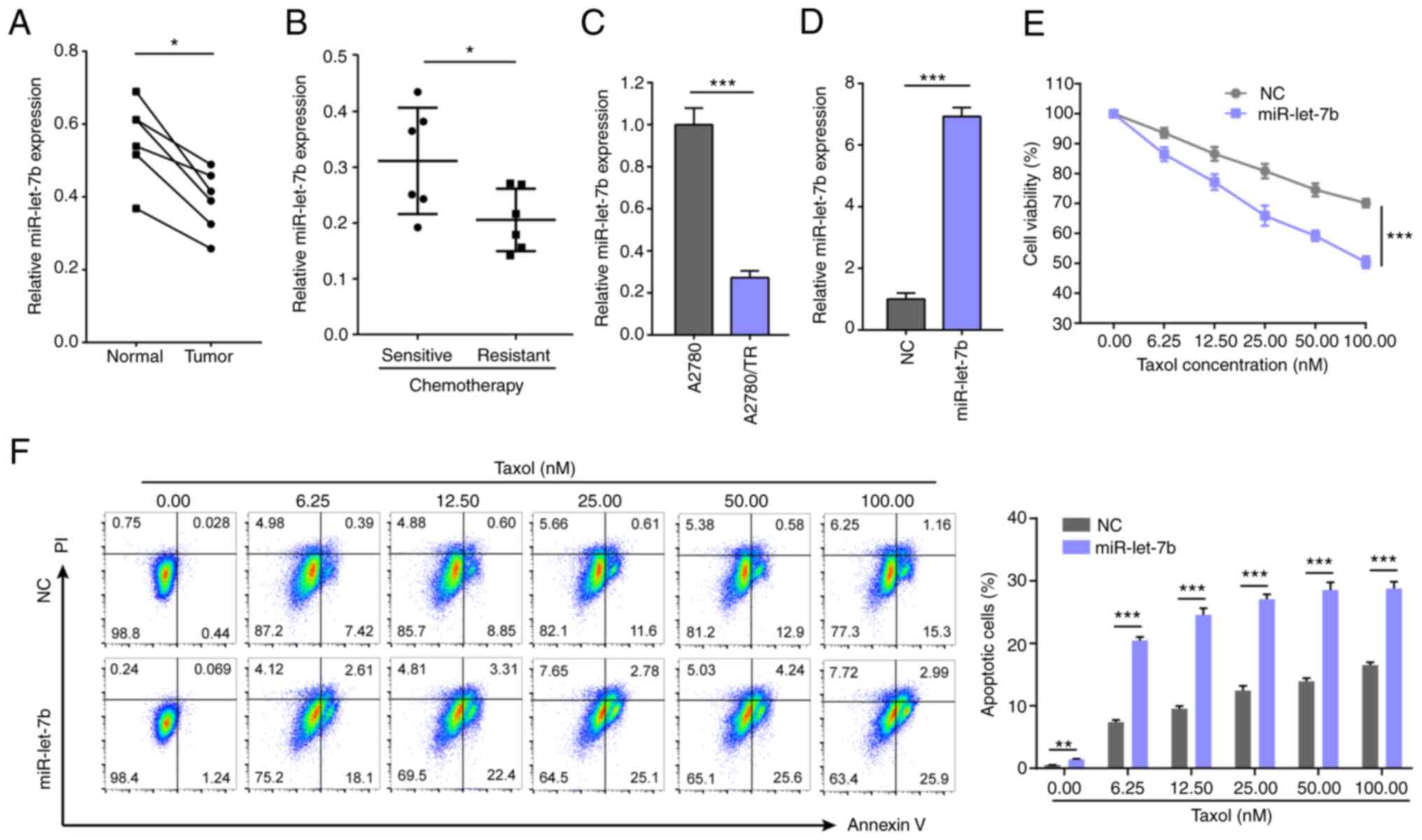
In the present study, miR-let-7b was detected in six
pairs of primary ovarian cancer and corresponding healthy tissue.
miR-let-7b was downregulated in tumors (Fig. 1A). Further comparison between 12
chemo-sensitive and -resistant ovarian cancer tissue found that
miR-let-7b expression levels were lower in patients with
chemoresistance (Fig. 1B).
miR-let-7b expression was also decreased in A2780/TR cells
(Fig. 1C). These results suggested
that miR-let-7b downregulation may be involved in Taxol resistance
of ovarian cancer. miR-let-7b mimic or control miRNA was
transfected into A2780 cells (Fig.
1D) to confirm these results. Following treatment with Taxol,
viability and apoptosis of A2780 cells overexpressing miR-let-7b
were compared with control miRNA-transfected cells. Cell viability
decreased and apoptosis increased in a dose-dependent manner when
cells were exposed to Taxol. Cells with miR-let-7b overexpression
were more sensitive to Taxol. Following treatment with 100 nM Taxol
for 24 h, the viability of control miRNA-transfected A2780 cells
was decreased to 70.1%, while that of miR-let-7b overexpressing
A2780 cells was decreased to 50.4% (Fig. 1E). Meanwhile, apoptosis rate was
increased to 16.46% in control miRNA-transfected A2780 cells and
28.89% in miR-let-7b overexpressing A2780 cells (Fig. 1F). These results indicated that
upregulated miR-let-7b expression sensitized ovarian cancer cells
to Taxol.
miR-let-7b suppresses the
aggressiveness of ovarian cancer cells
To assess the biological role of miR-let-7b in
ovarian cancer, the proliferation ability of ovarian cancers cells
overexpressing miR-let-7b or control miRNA was determined (Fig. 2A). Overexpression of miR-let-7b
significantly decreased the proliferation (Fig. 2B) of A2780, OVCAR3 and SK-OV-3
cells, which indicated an anti-tumor effect of miR-let-7b in
ovarian cancer. Subsequently, the effect of miR-let-7b expression
on migration and invasion of ovarian cancer cells was assessed
in vitro. miR-let-7b overexpression significantly suppressed
migration and invasion of ovarian cancer cells (Fig. 2C and D). These results demonstrated the role of
miR-let-7b in blocking progression of ovarian cancer in
vitro.
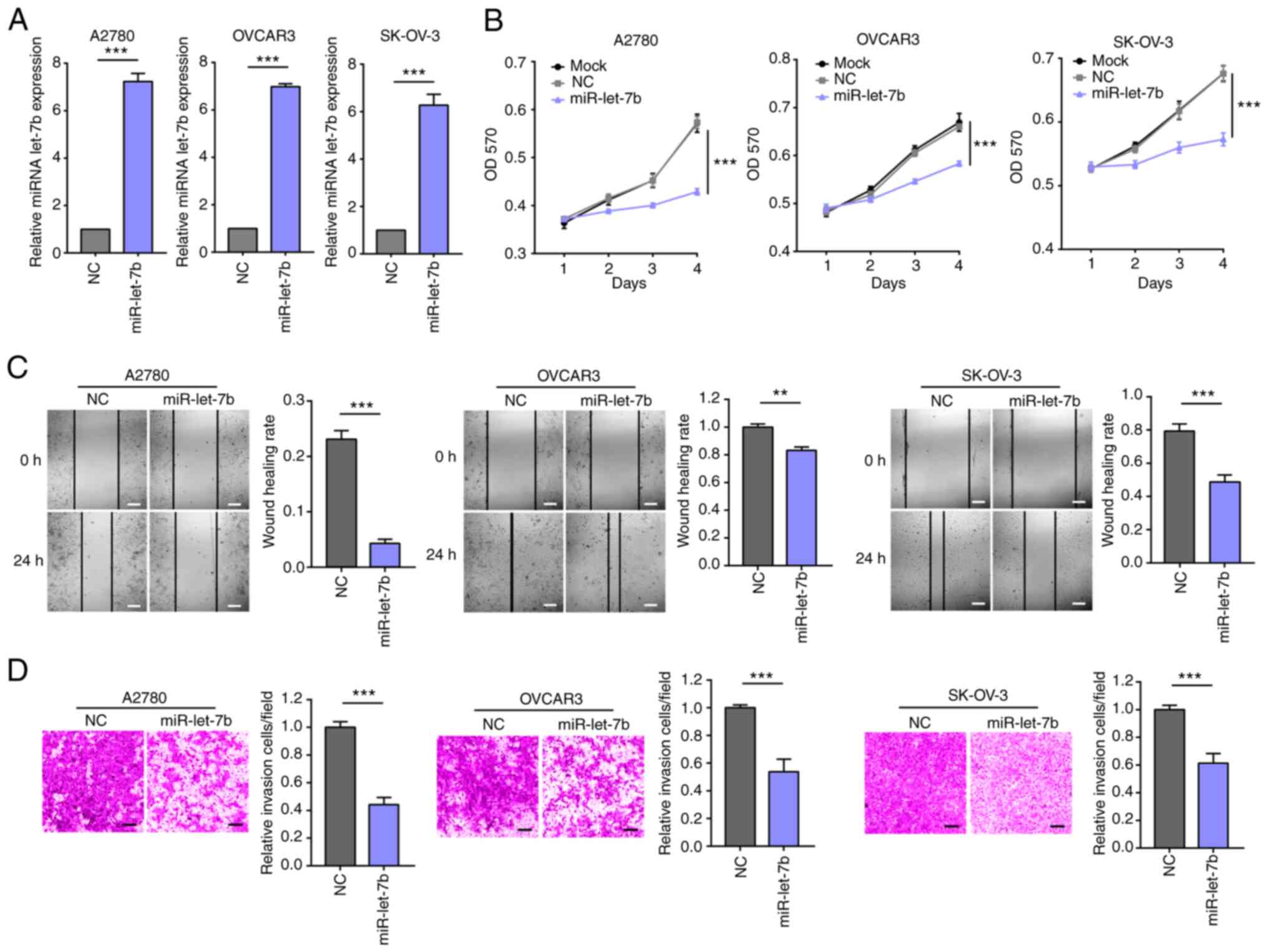 | Figure 2miR-let-7b regulates proliferation,
colony formation, migration and invasion of ovarian cancer cells.
(A) RT-qPCR assay verified overexpression of miR-let-7b in A2780,
OVCAR3 and SK-OV-3 cells. (B) Viability of A2780, OVCAR3 and
SK-OV-3 cells transfected with either miR-let-7b or control miRNA
was evaluated by MTT assay. (C) Migration ability of A2780, OVCAR3
and SK-OV-3 cells transfected with miR-let-7b or control miRNA was
evaluated by wound healing assay. Would healing rate=(initial
SA-final SA)/initial SA. Scale bar, 100 µm. (D) Invaded ability of
A2780, OVCAR3 and SK-OV-3 cells transfected with either miR-let-7b
or control miRNA was evaluated by Matrigel assay. Scale bar, 100
µm. **P<0.01 and ***P<0.001. miR,
microRNA; let, lethal; OD, optical density; SA, scratch area. |
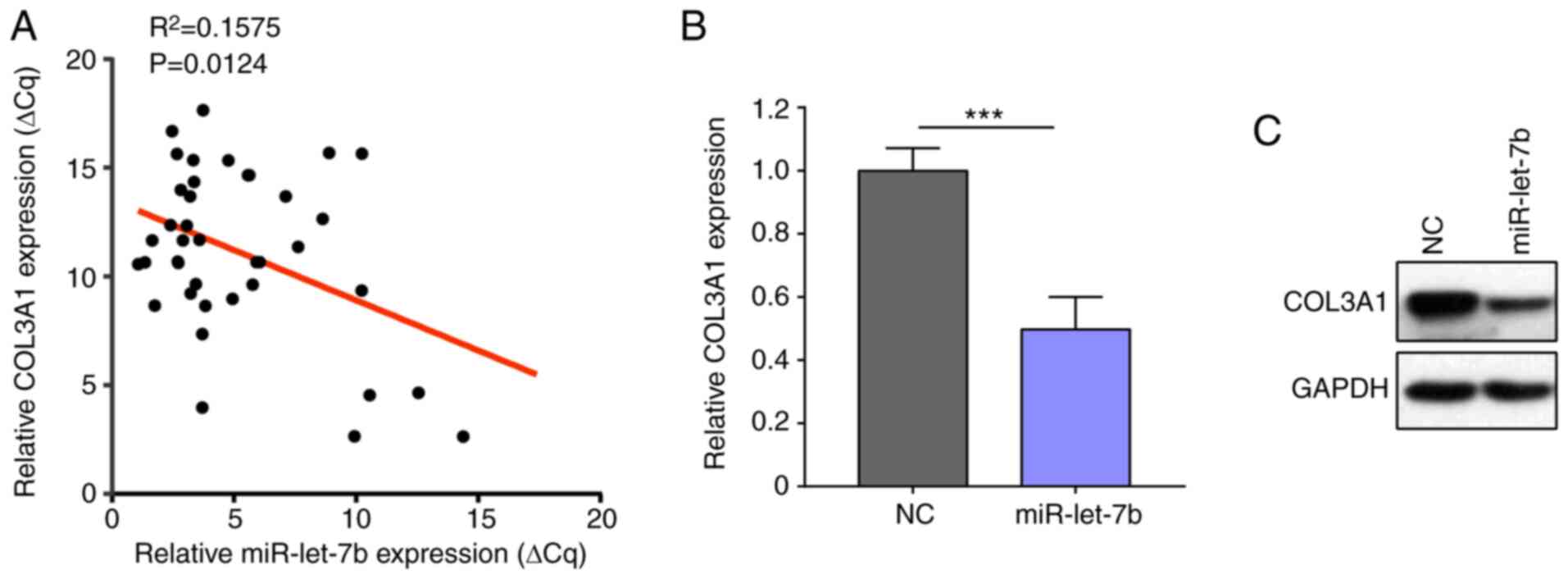
miR-let-7b supresses expression of
COL3A1 in ovarian cancer
To test whether the regulating effect miR-let-7b on
COL3A1 is conserved in vivo, the present study analyzed the
association between expression of miR-let-7b and COL3A1 in samples
from 39 patients with primary ovarian cancer. COL3A1 expression was
inversely associated with miR-let-7b expression (Fig. 3A). Ovarian cancer cell line was
used to confirm that miR-let-7b overexpression downregulated COL3A1
expression (Fig. 3B and C).
RRTS sensitizes ovarian cancer cells
to Taxol treatment
The present study evaluated the cytotoxic effect of
RRTS on A2780 and SK-OV-3 cells. RRTS displayed a notable cytotoxic
effect on both cell lines; the inhibitory effect on cell
proliferation was dose-dependent, with an inhibition rate of 82.6%
(IC50=37.22±3.54 µg/ml) in A2780 and 64.8% at 200 µg/ml
(IC50=45.58±4.76 µg/ml) in SK-OV-3 cells (Fig. 4A). These data suggested that RRTS
killed tumor cells effectively. RRTS treatment induced miR-let-7b
expression in A2780 and SK-OV-3 cells (Fig. 4B) and decreased COL3A1 expression
(Fig. 4C). Subsequently, it was
investigated whether RRTS sensitizes ovarian cancer cells to Taxol.
A2780, A2780/TR, SK-OV-3 and SK-OV-3/TR cells were pre-treated with
25 µg/ml RRTS for 24 h, followed by Taxol (0.000, 3.125, 6.250,
12.50, 25.000, 50.000 and 100.000 nM) for a further 24 h. Cell
viability was decreased following sequential treatment (Fig. 4D and E). In addition, cell apoptosis was
increased following sequential treatment with 25 µg/ml RRTS and 25
nM Taxol (Fig. 4F and G). To determine the interaction between
RRTS and Taxol, a detailed isobolographic analysis was performed.
The combined treatment showed synergistic effect in A2780 cells
(Fig. 4H). The effect of combined
treatment was also assessed in vivo. A2780 cells were
subcutaneously injected into BALB/c nude mice. The mice were
randomly assigned into saline, RRTS, Taxol or combined treatment
groups when they developed palpable tumors (>5 mm diameter; 3
weeks). RRTS (25 mg/kg), Taxol (15 mg/kg) or an equal volume of
saline were intraperitoneally injected once/week for 6 weeks. Taxol
inhibited tumor growth. Although RRTS treatment showed no notable
inhibitory effect, the combination of RRTS and Taxol induced a
synergistic significant decrease in tumor growth (Fig. 4I). These data indicated that RRTS +
Taxol synergistically killed ovarian cancer cells.
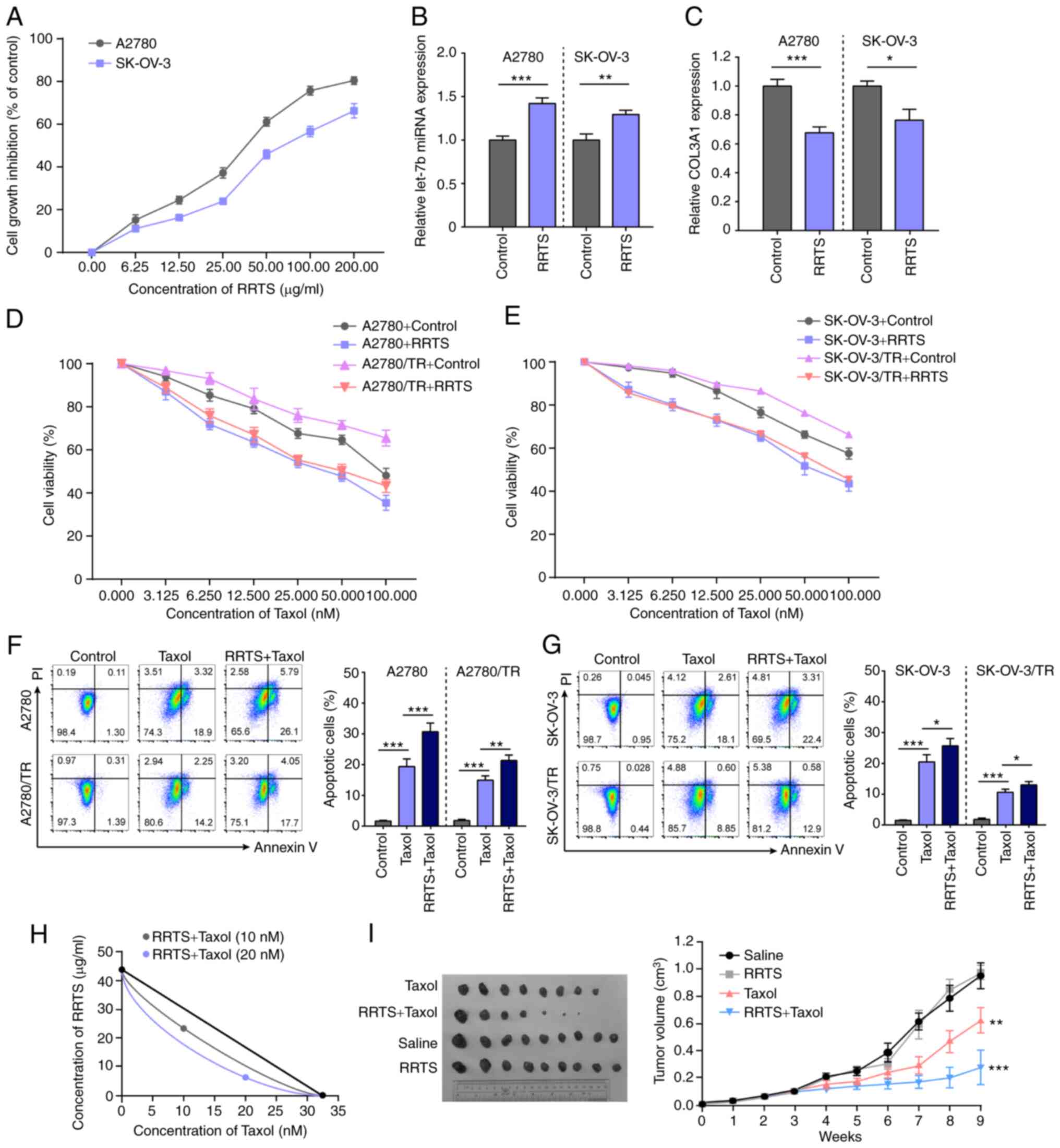 | Figure 4RRTS treatment sensitizes ovarian
cancer to Taxol. (A) RRTS exerted an inhibitory effect on
proliferation of A2780 and SK-OV-3 cells. A2780 and SK-OV-3 cells
were treated with RRTS (0.00, 6.25, 12.50, 25.00, 50.00, 100.00 and
200.00 µg/ml) for 24 h. Cell viability was evaluated by Cell
Counting Kit-8 assay. (B) RRTS treatment induced miR-let-7b
expression in A2780 and SK-OV-3 cells. (C) RRTS treatment decreased
COL3A1 expression in A2780 and SK-OV-3 cells. RRTS (25 µg/ml)
pre-treatment increased the sensitivity of (D) SK-OV-3 and (E)
SK-OV-3/TR cells to Taxol. RRTS (25 µg/ml) pre-treatment increased
apoptosis of Taxol-treated (25 nM) (F) A2780 and A2780/TR and (G)
SK-OV-3 and SK-OV-3/TR cells. Cell apoptosis was assessed by flow
cytometry using Annexin V-PI dual-staining. (H) Isobolographic
analysis of the cytotoxic effect obtained with the combination of
RRTS and Taxol at the concentrations of 10 and 20 nM in A2780
cells. The same level of apoptosis can be induced by 32.5 nM Taxol
and 42 µg/ml RRTS. (I) A2780 cells were injected into nude mice.
RRTS, Taxol or saline was intraperitoneally injected once/week for
6 weeks. Tumors were measured with Vernier Calipers.
*P<0.05, **P<0.01,
***P<0.001. COL3A1, collagen type II α1 chain; miR,
microRNA; let, lethal; NC, negative control; RRTS, Radix ranunculus
temate saponins; TR, Taxol-resistant. |
Discussion
Ovarian cancer is the leading cause of mortality in
gynecological malignancy (27).
The front-line treatment for ovarian cancer is primarily surgery,
supplemented by chemotherapy (28). Certain patients are treated with
targeted therapy (28). However,
eventual tumor recurrence and development of chemotherapy
resistance shorten the long-term survival of patients. Paclitaxel
is one of the most widely used chemotherapeutic agents in cancer
treatment and acts by blocking the cell cycle and inducing cell
apoptosis (29). Taxol resistance
occurs during treatment and limits the therapeutic effect, thus
negatively affecting the prognosis of patients with advanced
ovarian cancer (30). Therefore,
it is essential to decrease chemoresistance in these patients. The
mechanism of Taxol resistance has been described before (31). Tumor-specific cell cycle
deregulation and alterations to tubulin structure are associated
with Taxol resistance (31). Taxol
is a substrate for ABC transporter, which functions as a
drug-efflux pump; overexpression of this transport system in breast
cancer is associated with resistance to Taxol (32). However, only a small percentage of
ovarian cancers show high level of ABCB1 expression after Taxok
treatment (33), the underlying
mechanism for the development of Taxol resistance in ovarian cancer
remain to be elucidated.
miRNAs are small non-coding RNAs that regulate the
expression of target genes, which take participate physiological
and pathological processes. Due to the specific expression patterns
that are associated with prognosis, miRNAs are potential tumor
markers (34,35). For example, high expression of
miR-221 and miR-let-7 is associated with good prognosis, as opposed
to elevated miR-137, miR-372 and miR-182, which is associated with
poor prognosis in patients with lung cancer (36). Plasma miR-10b and miR-373 are
potential prognostic biomarkers for breast cancer (37). Additionally, expression levels of
miR-410 and miR-645 are negatively associated with overall survival
in advanced serous ovarian cancer (38). The let-7 miRNA family is considered
to be a tumor suppressor gene based on its effects on decreasing
cancer aggressiveness, chemoresistance and radioresistance
(39). In multiple types of human
cancer, such as multiple myeloma, glioma and osteosarcoma,
miR-let-7b expression is downregulated and associated with tumor
progression (16-18).
The present study found that miR-let-7b expression was
significantly downregulated in ovarian cancer tissue, particularly
in patients with chemoresistance. Additionally, overexpression of
miR-let-7b increased the sensitivity of ovarian cancer cell lines
to Taxol.
Collagen is a primary component of the tumor
microenvironment that favors tumor progression (40). In solid tumors, increased collagen
content is associated with chemotherapy resistance via integrins,
discoidin domain and tyrosine kinase receptors and other signaling
pathways (40). Collagen serves as
a barrier to limit diffusion of therapeutic agents into tumor
tissue (41,42). The diffusion speed of molecules is
inversely associated with levels of fibrillar collagen in
extracellular matrix (43,44). Certain cytostatic drugs, such as
methotrexate, vinblastine and paclitaxel, bind to collagen,
limiting their availability to tumor tissue (42). Knowledge of collagen regulation may
provide options for overcoming chemoresistance.
Multiple types of collagen are highly expressed in
ovarian cancer (45,46). COL3A1 is the most abundantly
expressed collagen in ovarian cancer cell lines (46). High expression of COL3A1 is
observed in paclitaxel-, topotecan- and cisplatin-resistant cell
lines, suggesting that COL3A1 is associated with resistance of
ovarian cancer to chemotherapy (46). Previous studies also confirmed that
COL3A1 is associated with shortened overall survival of patients
with ovarian carcinoma (47,48).
Collagen biosynthesis is regulated by tumor cells via numerous
signaling pathways, including mutated genes, transcription factors,
signaling pathways and receptors (40). miRNAs are associated with collagen
in cancer. Several collagens, such as COL1A1, COL1A2 and COL3A1,
are targets of miR-let-7b (49-51).
The present results were consistent with a previous study (49): COL3A1 mRNA was inversely correlated
with miR-let-7b levels in ovarian cancer clinical specimens.
Overexpression of miR-let-7b downregulated COL3A1 in ovarian cancer
cell lines. These results partially identified the role of
miR-let-7b in chemotherapy resistance of patients with ovarian
cancer. The present findings also suggested that the
miR-let-7b/COL3A1 regulatory pathway served a role in ovarian
cancer aggressiveness and chemotherapy resistance.
Radix Ranunculus ternati, a traditional
Chinese herbal medicine, has been used to treat numerous types of
disease and as an adjuvant therapy for cancer many years (19). The pharmacology of Radix R.
ternate depend on saponins and polysaccharides (20,21).
However, further evidence is needed to prove their anti-tumor
effect. The present study demonstrated that RRTS induced miR-let-7b
expression to suppress the aggressiveness of tumor cells. No
notable side effects of RRTS (25 mg/kg) were observed in animal
experiments. Although RRTS exhibited no notable effect on tumor
growth, it enhanced the inhibitory effect of Taxol. The present
study aimed to evaluate the theraputic effect of RRTS on human
ovarian cancer cells with Taxol resistance and demonstrated that
RRTS may be a novel adjuvant for Taxol chemotherapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Scientific Research
Projects of Guangdong Traditional Chinese Medicine Bureau (grant
nos. 20191011 and 20201002) and the Project of Guangzhou Science
and Technology Plan (grant nos. 201904010031 and 202102080046).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL, KY and HY conceived and designed the
experiments, revised the manuscript and confirm the authenticity of
all the raw data. WL, KY, YL and LC performed the experiments. WL
analyzed and interpreted the data and wrote the manuscript. All the
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients. The study protocol was approved by the Ethics Committee
of Guangdong Provincial People's Hospital [approval nos.
GDREC2019582A and GDREC2019582H(R1)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2(16061)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lheureux S, Gourley C, Vergote I and Oza
AM: Epithelial ovarian cancer. Lancet. 393:1240–1253.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gorodnova TV, Sokolenko AP, Kuligina E,
Berlev IV and Imyanitov EN: Principles of clinical management of
ovarian cancer. Chin Clin Oncol. 7(56)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee JM, Minasian L and Kohn EC: New
strategies in ovarian cancer treatment. Cancer. 125 (Suppl
24):S4623–S4629. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Christie EL and Bowtell DDL: Acquired
chemotherapy resistance in ovarian cancer. Ann Oncol. 28 (Suppl
8):viii13–viii15. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Freimund AE, Beach JA, Christie EL and
Bowtell DDL: Mechanisms of drug resistance in high-grade serous
ovarian cancer. Hematol Oncol Clin North Am. 32:983–996.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Patch AM, Christie EL, Etemadmoghadam D,
Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey
P, et al: Whole-genome characterization of chemoresistant ovarian
cancer. Nature. 521:489–494. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gebert LFR and MacRae IJ: Regulation of
microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dragomir MP, Knutsen E and Calin GA:
Classical and noncanonical functions of miRNAs in cancers. Trends
Genet: Oct 30, 2021 (Epub ahead of print).
|
|
10
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Goodall GJ and Wickramasinghe VO: RNA in
cancer. Nat Rev Cancer. 21:22–36. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther.
1(15004)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ayers D and Vandesompele J: Influence of
microRNAs and Long Non-Coding RNAs in Cancer Chemoresistance. Genes
(Basel). 8(95)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fu TY, Chang CC, Lin CT, Lai CH, Peng SY,
Ko YJ and Tang PC: Let-7b-mediated suppression of basigin
expression and metastasis in mouse melanoma cells. Exp Cell Res.
317:445–451. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao Y, Deng C, Lu W, Xiao J, Ma D, Guo M,
Recker RR, Gatalica Z, Wang Z and Xiao GG: Let-7 microRNAs induce
tamoxifen sensitivity by downregulation of estrogen receptor alpha
signaling in breast cancer. Mol Med. 17:1233–1241. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu H, Liu C, Zhang Y, Guo X, Liu Z, Luo Z,
Chang Y, Liu S, Sun Z and Wang X: Let-7b-5p regulates proliferation
and apoptosis in multiple myeloma by targeting IGF1R. Acta Biochim
Biophys Sin (Shanghai). 46:965–972. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang W, Zhao W, Ge C, Li X, Yang X, Xiang
Y and Sun Z: Decreased let-7b is associated with poor prognosis in
glioma. Medicine (Baltimore). 98(e15784)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen F, Chen C, Yang S, Gong W, Wang Y,
Cianflone K, Tang J and Wang DW: Let-7b inhibits human cancer
phenotype by targeting cytochrome P450 epoxygenase 2J2. PLoS One.
7(e39197)2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
China Pharmacopoeia Committee:
Pharmacopoeia of the People's Republic of China. China Chemical
Industry Press, Beijing, pp223-224, 2005.
|
|
20
|
Tian JK, Sun F and Cheng YY: Chemical
constituents from the roots of Ranunculus ternatus. J Asian Nat
Prod Res. 8:35–39. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Deng KZ, Xiong Y, Zhou B, Guan YM and Luo
YM: Chemical constituents from the roots of Ranunculus ternatus and
their inhibitory effects on Mycobacterium tuberculosis. Molecules.
18:11859–11865. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li ML, Gu HM, Hang HY, Jiang YL, Jiang J,
Gu QN and WU WY: Radix ranunculus temate saponins induces apoptosis
via the death receptor and mitochondrial pathways in SGC-7901
cells. Mol Cell Toxicol. 11:449–455. 2015.
|
|
23
|
Niu L, Zhou Y, Sun B, Hu J, Kong L and
Duan S: Inhibitory effect of saponins and polysaccharides from
Radix ranunculi ternati on human gastric cancer BGC823 cells. Afr J
Tradit Complement Altern Med. 10:561–566. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Di Sotto A, Irannejad H, Eufemi M,
Mancinelli R, Abete L, Mammola CL, Altieri F, Mazzanti G and Di
Giacomo S: Potentiation of Low-Dose Doxorubicin Cytotoxicity by
Affecting P-Glycoprotein through Caryophyllane Sesquiterpenes in
HepG2 Cells: An in vitro and in silico study. Int J Mol Sci.
21(633)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
DI Giacomo S, DI Sotto A, Mazzanti G and
Wink M: Chemosensitizing properties of β-caryophyllene and
β-caryophyllene oxide in combination with doxorubicin in human
cancer cells. Anticancer Res. 37:1191–1196. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Redondo A, Guerra E, Manso L,
Martin-Lorente C, Martinez-Garcia J, Perez-Fidalgo JA, Varela MQ,
Rubio MJ, Barretina-Ginesta MP and Gonzalez-Martin A: SEOM clinical
guideline in ovarian cancer (2020). Clin Transl Oncol. 23:961–968.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marth C, Reimer D and Zeimet AG:
Front-line therapy of advanced epithelial ovarian cancer: Standard
treatment. Ann Oncol. 28 (Suppl 8):viii36–viii39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pokhriyal R, Hariprasad R, Kumar L and
Hariprasad G: Chemotherapy resistance in advanced ovarian cancer
patients. Biomark Cancer. 11(1179299X19860815)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kavallaris M: Microtubules and resistance
to tubulin-binding agents. Nat Rev Cancer. 10:194–204.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Robey RW, Pluchino KM, Hall MD, Fojo AT,
Bates SE and Gottesman MM: Revisiting the role of ABC transporters
in multidrug-resistant cancer. Nat Rev Cancer. 18:452–464.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gillet JP, Wang J, Calcagno AM, Green LJ,
Varma S, Bunkholt Elstrand M, Trope CG, Ambudkar SV, Davidson B and
Gottesman MM: Clinical relevance of multidrug resistance gene
expression in ovarian serous carcinoma effusions. Mol Pharm.
8:2080–2088. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lan H, Lu H, Wang X and Jin H: MicroRNAs
as potential biomarkers in cancer: Opportunities and challenges.
Biomed Res Int. 2015(125094)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Markou A, Liang Y and Lianidou E:
Prognostic, therapeutic and diagnostic potential of microRNAs in
non-small cell lung cancer. Clin Chem Lab Med. 49:1591–1603.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen W, Cai F, Zhang B, Barekati Z and
Zhong XY: The level of circulating miRNA-10b and miRNA-373 in
detecting lymph node metastasis of breast cancer: Potential
biomarkers. Tumour Biol. 34:455–462. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shih KK, Qin LX, Tanner EJ, Zhou Q,
Bisogna M, Dao F, Olvera N, Viale A, Barakat RR and Levine DA: A
microRNA survival signature (MiSS) for advanced ovarian cancer.
Gynecol Oncol. 121:444–450. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chirshev E, Oberg KC, Ioffe YJ and
Unternaehrer JJ: Let-7 as biomarker, prognostic indicator, and
therapy for precision medicine in cancer. Clin Trans Med.
8(24)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu S, Xu H, Wang W, Li S, Li H, Li T,
Zhang W, Yu X and Liu L: The role of collagen in cancer: From bench
to bedside. J Transl Med. 17(309)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chauhan VP, Stylianopoulos T, Boucher Y
and Jain RK: Delivery of molecular and nanoscale medicine to
tumors: Transport barriers and strategies. Annu Rev Chem Biomol
Eng. 2:281–298. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Di Paolo A and Bocci G: Drug distribution
in tumors: Mechanisms, role in drug resistance, and methods for
modification. Curr Oncol Rep. 9:109–114. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ramanujan S, Pluen A, McKee TD, Brown EB,
Boucher Y and Jain RK: Diffusion and convection in collagen gels:
Implications for transport in the tumor interstitiumv. Biophys J.
83:1650–1660. 2002.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Brown E, McKee T, diTomaso E, Pluen A,
Seed B, Boucher Y and Jain RK: Dynamic imaging of collagen and its
modulation in tumors in vivo using second-harmonic generation. Nat
Med. 9:796–800. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
45
|
Cho A, Howell VM and Colvin EK: The
extracellular matrix in epithelial ovarian cancer-A piece of a
puzzle. Front Oncol. 5(245)2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Januchowski R, Swierczewska M, Sterzynska
K, Wojtowicz K, Nowicki M and Zabel M: Increased expression of
several collagen genes is associated with drug resistance in
ovarian cancer cell lines. J Cancer. 7:1295–1310. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Engqvist H, Parris TZ, Kovacs A, Nemes S,
Werner Rönnerman E, De Lara S, Biermann J, Sundfeldt K, Karlsson P
and Helou K: Immunohistochemical validation of COL3A1, GPR158 and
PITHD1 as prognostic biomarkers in early-stage ovarian carcinomas.
BMC Cancer. 19(928)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sun Q, Zhao H, Zhang C, Hu T, Wu J, Lin X,
Luo D, Wang C, Meng L, Xi L, et al: Gene co-expression network
reveals shared modules predictive of stage and grade in serous
ovarian cancers. Oncotarget. 8:42983–42996. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang Q, She Y, Bi X, Zhao B, Ruan X and
Tan Y: Dexmedetomidine protects PC12 cells from lidocaine-induced
cytotoxicity through downregulation of COL3A1 mediated by
miR-let-7b. DNA Cell Biol. 36:518–528. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yu DH, Ruan XL, Huang JY, Liu XP, Ma HL,
Chen C, Hu WD and Li S: Analysis of the interaction network of Hub
miRNAs-Hub genes, being involved in idiopathic pulmonary fibers and
its emerging role in non-small cell lung cancer. Front Genet.
11(302)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Liu J, Luo C, Yin Z, Li P, Wang S, Chen J,
He Q and Zhou J: Downregulation of let-7b promotes COL1A1 and
COL1A2 expression in dermis and skin fibroblasts during heat wound
repair. Mol Med Rep. 13:2683–2688. 2016.PubMed/NCBI View Article : Google Scholar
|