Introduction
Cardiovascular disease and cancer are two of the
most important contributors of morbidity and mortality in the
European Union and worldwide (1).
Although the heart may be affected by cancer therapy, it has been
suggested that cancer itself is responsible for a subclinical
degree of cardiovascular damage in certain patients (2).
High levels of troponin typically indicate acute
coronary syndrome (ACS). Lower (relative to ACS), but above-normal
levels of troponin, may point to another diagnosis, such as heart
failure, myocarditis, pulmonary embolism, sepsis or kidney failure
(3). This biochemical abnormality
has also been associated with left ventricular hypertrophy (LVH)
(4) and is more common in the
elderly (5,6). Similarly, N-terminal-pro B-type
natriuretic peptide (NT-proBNP) is a widely recognized biomarker of
heart failure, although slightly abnormal values have also been
associated with increased age and body mass index (BMI) (7,8).
Moreover, other clinical conditions are associated with
significantly increased levels of NT-proBNP (8,9).
Previous studies have reported elevated levels of
hs-TnT (10-15)
and NT-proBNP in patients with cancer (2,14).
Although there is no evidence of cardiac involvement, these
abnormalities are important because they could indicate a
subsequent risk for cardiac complications during cancer
treatment.
A recent statement of multiple medical societies
involved in Cardio-Oncology proposed a baseline proforma
assessment, including cardiovascular biomarkers, hs-TnT and
NT-proBNP, as easy-to-use tools for oncologists to stratify
cardiovascular risk in patients with cancer, prior to the start of
treatment (16).
The aim of the present study was to investigate the
hypothesis that in patients with newly diagnosed colon cancer,
before the start of any cancer treatment, myocardial involvement
may already be present. Moreover, regarding cardiovascular
biomarkers, another aim of the current study was to establish
cut-off values for this hypothetical low-intensity injury.
Materials and methods
Study design and setting
The present prospective, cross-sectional study was
conducted between February 2016 and May 2019 in the Clinical
Municipal Hospital of Cluj-Napoca (Romania) and included patients
with newly diagnosed colon cancer before starting any cancer
treatment. The patients were enrolled during hospitalization in
internal medicine or gastroenterology departments. Detailed patient
history was recorded, and patients were excluded if any previous
heart disease or electrocardiogram abnormalities were identified.
Age, sex and common risk factors, such as hypertension, diabetes
mellitus and smoking status were recorded. To define the baseline
levels for each variable, a control group consisting of volunteer
medical staff was also similarly analyzed. Patients were diagnosed
with colon cancer if they met the criteria for this diagnosis
according to the European Society for Medical Oncology guidelines
for diagnosis of colorectal cancer (17). Patients <18 years, those with
more than one oncological disease and those who underwent any
oncological treatment were excluded. Written informed consent was
obtained from all participants before their inclusion in the study.
The Ethics Committee of The Clinical Municipal Hospital of
Cluj-Napoca approved the study.
Anthropometric data
Height, weight and waist circumference (WC) were
recorded. BMI was calculated based on height and weight
(kg/m2). The assessment of metabolic syndrome (MetS) was
performed according to the Joint Interim Statement of The
International Diabetes Federation Task Force on Epidemiology and
Prevention 2009(18). MetS entails
the presence of any three of the following five features: i)
Elevated WC (≥94 cm for males and ≥80 cm for females) and
triglyceride levels (>150 mg/dl); ii) reduced high-density
lipoprotein (HDL)-cholesterol (<40 mg/dl in males and <50
mg/dl in females); iii) raised blood pressure (systolic pressure
≥130 mmHg; or diastolic pressure ≥85 mmHg); iv) raised fasting
plasma glucose (≥100 mg/dl); and v) previously diagnosed type-2
diabetes.
Laboratory
Venous blood samples were obtained and analyzed in
the Clinical Municipal Hospital of Cluj-Napoca, according to our
local laboratory standard procedures. In addition to routine
measurements [risk factors and MetS evaluation, such as fasting
blood sugar, cholesterol, HDL-cholesterol, low-density lipoprotein
(LDL)-cholesterol, triglycerides], high-sensitivity C-reactive
protein (hs-CRP) and cardiovascular biomarker levels [hs-TnT,
creatine kinase-MB (CK-MB) and NT-proBNP] were determined. hs-TnT
and NT-proBNP levels were measured using the Elecsys TnT-hs (cat.
no. 05092728190) and the Elecsys NT-proBNP (cat. no. 04842464190)
kits, respectively, on the Cobas platform (Roche Diagnostics).
Normal values for these parameters in our laboratory are 14 ng/l
for ACS and 125 pg/ml for heart failure.
Ultrasonography
All patients included in the present study were
evaluated using echocardiograms with the ProSound Alpha 7 (Hitachi
Aloka Medical Ltd.) ultrasound system and a phased-array transducer
(1-15 MHz range). The examinations were performed during the same
hospital stay by two qualified physicians.
For the cardiac examinations, the patients were
placed in left lateral decubitus. The left atrium (LA),
end-diastolic interventricular septum, end-diastolic posterior-wall
left ventricle (LV), as well as the LV end-systolic diameter
(LVESD) and LV end-diastolic diameter (LVEDD) were measured (in mm)
in the parasternal long axis incidence. LV ejection fraction (LVEF)
was calculated using LVESD and LVEDD measurements using the
Teicholz formula (Volume=7D3/(2.4 + D, where D
represents LV diameter) (19). The
data regarding the diastolic function were not uniform considering
the data collection by two different examiners and were therefore
not subsequently analyzed.
Cardiovascular risk
stratification
Baseline proforma cardiovascular risk assessment was
carried out using the current recommendations for the treatment of
patients with colon cancer (16),
which included the following: History of prior cardiovascular
disease, cardiovascular biomarkers, demographic and cardiovascular
risk factors, previous cardiotoxic cancer treatment and lifestyle
risk factors. Each class of risk included several variables
identified as contributing to cardiovascular risk for patients
receiving the specific cancer therapy according to the evidence
available and expert opinion. Once completed, a risk level was
calculated, and the patients were classified as being at low,
medium, high or very high cardiovascular risk (16).
Statistical analysis
Continuous data are presented either as the median
with a 95% confidence interval for skewed variables or as mean ± SD
for normally distributed variables. Skewed variables were compared
using Mann-Whitney U-test, while Student's t-test was used for
comparing normally distributed variables. When >2 variables were
compared, Kruskal-Wallis test was used instead (in conjunction with
Dunn's test). Normally distributed variables were compared using
the independent two-sample t-test. Discrete variables are expressed
as n (%). Comparison of the discrete variables was performed using
χ2 test. Variables with P<0.05 following univariate
analysis (carried out using regression method) were included in the
multivariate analysis. For multivariate analysis, a binary logistic
regression using the backward logistic regression model was used in
order to determine the cardiac markers independently associated
with the presence of cancer. Receiver operating characteristic
(ROC) analysis was used to evaluate the diagnostic accuracy of
cardiac biomarkers and ultrasound modifications for the subclinical
lesions induced by oncological disease. The closest value to the
maximum sensitivity and specificity was selected as the optimal
cut-off value. Statistical analysis was performed using the SPSS
software version 27 (IBM Corp). P<0.05 was considered to
indicate a statistically significant difference.
Results
Initially, 78 patients with colon cancer, naïve of
any cancer treatment, were included in the analysis. After
echocardiography, due to the presence of LVH, which represents a
confounding factor, 27 patients were excluded.
The mean age in the final patient group (n=51) was
63.94±11.83 years, with a slight predominance of female sex
(54.91%). Most of the patients had normal weight [BMI, 23.80
(23.62-25.96)], normal blood pressure [130 (124.35-130.56)/80
(70.78-75.59) mmHg] and normal fasting plasma glucose [(98
(96.20-105.8) mg/dl], although 32.72% of them met the criteria for
MetS. In terms of metabolic risk factors, when comparing the
patient group with controls, a significant difference was observed
only for systolic arterial blood pressure [130 (124.35-130.56) vs.
120 (115.14-127.71) mmHg; P=0.004], with the values in the normal
range. Triglyceride levels were also significantly higher in the
study group [98 (100-141.61) vs. 68 (62.87-93.34) mg/dl;
P<0.001]. Inflammation was also observed in the patient group,
as evidenced by high hs-CRP levels [1.81 (2.16-8.27) vs. 0.09
(0.48-2.24) mg/l; P<0.001]. The other variables measured, namely
BMI, smoking, sAP, dAP, heart rate, fasting plasma glucose,
cholesterol, HDL-cholesterol, LDL-cholesterol and the presence of
metabolic syndrome, were not significantly different between the
two groups The general metabolic and demographic data are presented
in Table I.
 | Table IDescriptive presentation of the
demographic and metabolic features of the patient and control
groups. |
Table I
Descriptive presentation of the
demographic and metabolic features of the patient and control
groups.
| Variable | Patient group
(n=51) | Control group
(n=28) | P-value | Normal range |
|---|
| Age, years | 63 (59.27-66.26) | 33.5
(34.12-46.24) | <0.001 | NA |
| Number of males, n
(%) | 23 (45.09) | 18 (64.28) | 0.144 | NA |
| BMI,
kg/m2 | 23.8
(23.62-25.96) | 22.64
(21.52-25.37) | 0.088 | 18.50-24.90 |
| Smoking, n (%) | 13 (25.49) | 7 (25.00) | 0.891 | NA |
| sAP, mmHg | 130
(124.35-130.56) | 120
(115.14-127.71) | 0.004 | <120 |
| dAP, mmHg | 80 (70.78-75.59) | 70 (66.24-74.83) | 0.108 | <80 |
| Heart rate, bpm | 80 (77.25-83.11) | 80 (74.70-82.44) | 0.472 | 60-100 |
| Fasting blood
glucose, mg/dl | 98 (96.20-105.8) | 101 (98-111.12) | 0.118 | 70-99 |
| Cholesterol,
mg/dl | 188.41±37.38 | 166.11±51.50 | 0.053 | <200 |
| HDL-cholesterol,
mg/dl | 42 (40.71-48.09) | 45 (42.09-54.05) | 0.404 | >40 |
| LDL-cholesterol,
mg/dl | 116.51±27.78 | 101.71±39.94 | 0.023 | <100 |
| Triglycerides,
mg/dl | 98
(100-141.61) | 68
(62.87-93.34) | <0.001 | <150 |
| Metabolic syndrome,
n (%) | 17 (32.72) | 7(25) | 0.470 | NA |
| hs-CRP (mg/l) | 1.81
(2.16-8.27) | 0.09
(0.48-2.24) | <0.001 | 0.8-1 |
In terms of biochemical analysis, minimal myocardial
damage (as evidenced by high values of CK-MB and hs-TnT) was
observed in the patient group, with significantly higher values
than in healthy subjects, although not indicative of a potential
coronary syndrome. Minimal myocardial damage (high values of
hs-TnT) and myocardial strain (high values of NT pro-BNP) were
observed in the patient group. It should be noted that the
myocardial injury did not reach a significant threshold for a
possible ACS, while the myocardial strain is compatible with heart
failure. Regarding the cardiac morphological modifications detected
by ultrasound, larger LV dimensions were observed in the patients
in comparison with the control group [(29.50 (28.76-31.84) vs.
26.00 (25.22-27.55) mm; P<0.001]. By contrast, similar LVEF
values were observed in both groups [61 (58.59-62.71) vs. 61
(60.31-61.69) mm; P<0.001] (Table
II).
 | Table IIComparison of biological and
ultrasound variables demonstrating myocardial microlesions in the
patient and control groups. |
Table II
Comparison of biological and
ultrasound variables demonstrating myocardial microlesions in the
patient and control groups.
| Variable | Patient group
(n=51) | Control group
(n=28) | P-value |
|---|
| CK-MB, IU/l | 17.00
(6.78-82.78) | 11.00
(9.94-14.11) | <0.001 |
| hs-TnT, ng/l | 8.20
(9.00-17.89) | 3.00
(3.29-8.57) | <0.001 |
| NT-proBNP,
pg/ml | 155.40
(268.71-718.28) | 48.50
(37.95-727.03) | 0.001 |
| LA, mm | 35
(34.35-38.25) | 30
(28.91-31.47) | <0.001 |
| IVS, mm | 10
(9.72-10.58) | 9.50
(9.35-9.95) | 0.115 |
| PWLV, mm | 10
(9.34-10.06) | 9 (8.94-9.45) | 0.003 |
| LVs, mm | 29.50
(28.76-31.84) | 26.00
(25.22-27.55) | <0.001 |
| LVd, mm | 44.50
(44.37-47.38) | 38.00
(37.13-39.72) | <0.001 |
| LVEF, % | 61
(58.59-62.71) | 61
(60.31-61.69) | 0.938 |
In the multivariate analysis (Table III), CK-MB and hs-TNT retained
statistical significance (P=0.004 and P=0.045, respectively) for
biological variables, while neither NT-proBNP and none of the
ultrasound features (LV dimensions and LVEF; data not shown)
reached statistical significance.
 | Table IIIMultivariate analysis of cardiac
biomarkers. |
Table III
Multivariate analysis of cardiac
biomarkers.
| Variable | B | SE | Wald | df | P-value | Exp (B) |
|---|
| CK-MB, IU/l | 0.172 | 0.061 | 8.105 | 1 | 0.004 | 1.188 |
| NT-proBNP,
pg/ml | -0.001 | 0.001 | 1.186 | 1 | 0.276 | 0.999 |
| hs-TnT, ng/l | 0.100 | 0.058 | 2.976 | 1 | 0.045 | 1.105 |
As the cardiac biomarkers were indicative of minimal
myocardial damage, the diagnostic accuracy of hs-TnT, CK-MB and
NT-proBNP for the detection of myocardial lesion was then evaluated
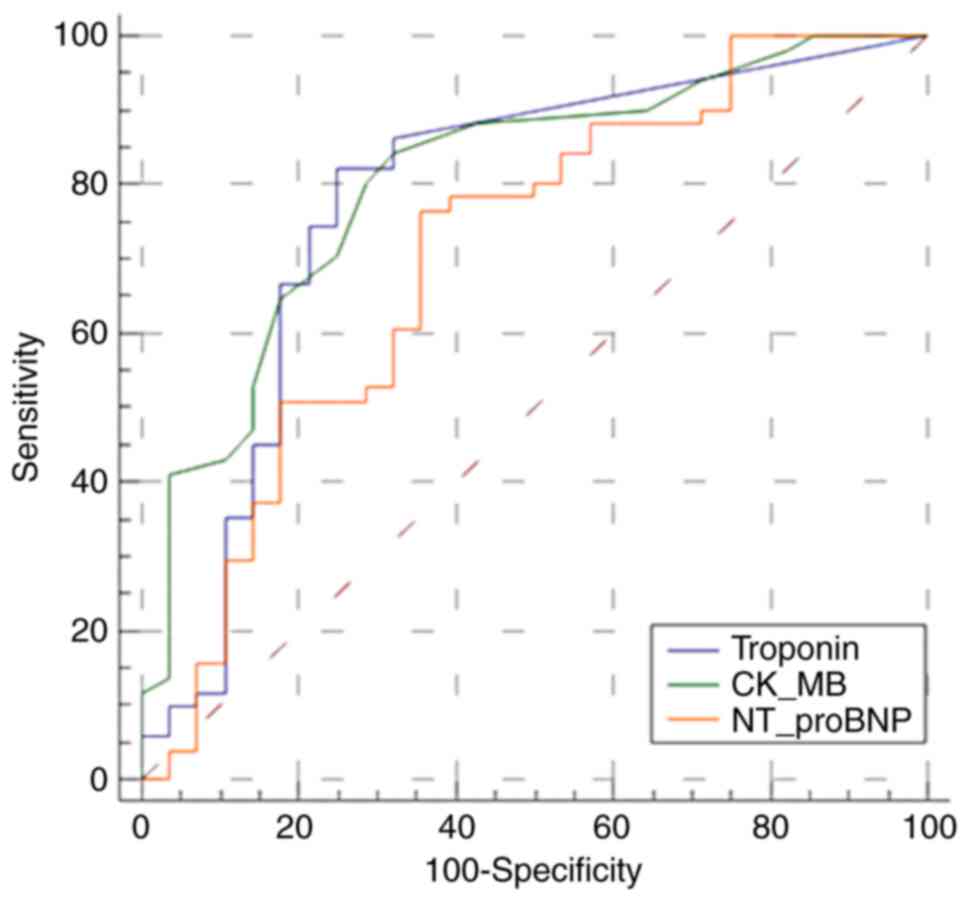
in the entire patient group (n=78 patients) (Fig. 1). hs-TnT had an area under the ROC
curve (AUROC) 0.791. For CK-MB, the AUROC was 0.804, whereas
NT-proBNP had the lowest value (0.721).
One of the major aims of the current study was to
find and introduce specific cut-offs for hs-TnT that would suggest
cardiac microdamage. In this respect, the patients were divided
into two age groups: <65 and ≥65 years old. The levels of hs-TnT
varied significantly between these two groups [5.36 (5.07-17.89)
vs. 16.79 (11.14-22.07) ng/l; P<0.001]. Although not
significantly different from those related to ACS, the levels of
NT-proBNP were significantly higher in patients ≥65 years old
[75.69 (92.27-313.44) vs. 252.70 (249.23-1162.39) pg/ml;
P<0.001]. In addition, the median hs-TnT levels in each age
group were then compared with those of the control group. A
significant difference was observed both between patients <65
years old and controls and between patients ≥65 years old and
controls [5.36 (5.07-17.89) vs. 16.79 (11.14-22.07); P<0.001 and
3.00 (2.88-5.84), respectively; P<0.001] (Table IV).
 | Table IVComparison of cardiac biomarkers
between age patient subgroups and controls. |
Table IV
Comparison of cardiac biomarkers
between age patient subgroups and controls.
| Variable | Patients <65
years (n=21) | Patients ≥65 years
(n=30) | Controls
(n=28) |
P-valuea |
P-valueb |
P-valuec |
|---|
| CK-MB, IU/l | 17.00
(16.27-23.57) | 17.00
(13.88-31.27) | 11.00
(9.71-12.99) | 0.82 | <0.001 | <0.001 |
| hs-TNT, ng/l | 5.36
(5.07-17.89) | 16.79
(11.14-22.07) | 3.00
(2.88-5.84) | <0.001 | <0.001 | <0.001 |
| NT-proBNP,
pg/ml | 75.69
(92.27-313.44) | 252.70
(249.23-1,162.39) | 44.45
(126.26-603.92) | <0.001 | 0.002 | <0.001 |
Moreover, the cut-off hs-TnT value that could
identify patients >65 years old with cardiac damage was
calculated using Receiver Operating Characteristic (ROC) curve
analysis. A cut-off value of 8 ng/l (AUROC, 0.865) and 220.00 pg/ml
(AUROC, 0.833) was obtained for hs-TnT and NT-proBNP,
respectively.
Using the recently described criteria dividing
patients according to their risk of developing cardiac disease
after chemotherapy (16), 32
patients in the study group met the criteria for low risk, while
the rest were at medium risk. No patient met the criteria for high
or very high risk. In the analysis of the low- and medium-risk
subgroups, high hs-TnT and NT-proBNP levels were observed in
patients at medium risk of developing cardiac disease. The results
were as follows when comparing the low-risk with the medium-risk
group: i) CK-MB, 17.00 (15.86-22.52) vs. 17.00 (14.46-33.75) IU/l,
P=0.49; ii) NT-proBNP, 82.13 (80.61-242.09) vs. 358.30
(334.43-1322.90) pg/ml, P<0.001; and iii) hs-TnT, 5.70
(5.83-8.09) vs. 18.10 (11.21-30.98) ng/l, P<0.001 (Table V).
 | Table VComparison of cardiac biomarker
levels in patients at low or medium risk of developing cardiac
disease following cancer treatment. |
Table V
Comparison of cardiac biomarker
levels in patients at low or medium risk of developing cardiac
disease following cancer treatment.
| Variable | Low risk
(n=32) | Medium risk
(n=21) | P-value |
|---|
| CK-MB, IU/l | 17.00
(15.86-22.52) | 17.00
(14.46-33.75) | 0.49 |
| hs-TnT, ng/l | 5.70
(5.83-8.09) | 18.10
(11.21-30.98) | <0.001 |
| NT-proBNP,
pg/ml | 82.13
(80.61-242.09) | 358.30
(334.43-1322.90) | <0.001 |
Discussion
Cardiovascular disease and cancer share several
common pathophysiological mechanisms for disease incidence and
progression (14,15,20).
Furthermore, the prognosis of patients with cancer is associated
with cardiovascular status prior or during cancer therapy. The
hypothesis that cancer itself may damage the heart muscle
irrespectively of exposure to cancer therapy has been previously
studied using different echocardiographic techniques and/or
cardiovascular biomarkers (2,21).
Cardiovascular vulnerability due to cancer or
treatment regimen differs depending on tumor type and localization
(12-14,20).
For this reason, the present study only included patients with
colon tumors. In the recruited population, due to the inclusion and
exclusion criteria, baseline cardiovascular risk assessment
(16) identified only low- and
moderate-risk patients.
In an echocardiographic study, patients with cancer,
whether treated or not, had similarly reduced strain measurements,
indicating impaired heart function, compared with healthy
individuals (22). Another study
used combined speckle tracking echocardiography and hs-TnT for
early detection and prediction of future cardiac dysfunction
(21). In serial analyses, global
longitudinal strain and hs-TnT provided a reliable and non-invasive
method for the prediction of cardiac dysfunction in patients
receiving anthracycline-based chemotherapy (20).
In the present study, impaired systolic heart
function was observed using basic techniques (the Teicholz formula
for LVEF). However, patients with colon cancer presented higher
left cardiac chamber dimensions compared with healthy individuals,
suggesting adaptative remodeling compatible with LV dysfunction.
Due to its complex function, LA size is considered an important
risk identifier in preclinical cardiovascular disease (23). In addition, in a cohort study
(Multi-Ethnic Study of Atherosclerosis) involving asymptomatic
adults without cardiovascular disease, LV dilation predicted heart
failure during a 12-year follow-up period (24). In a study regarding the association
between hs-TnT elevation and metabolic syndrome in a general
population sample, the prevalence of MetS was higher in those with
detectable and elevated levels of hs-TnT. The number of MetS
components and presence of MetS were markedly associated with an
increased risk for detectable hs-TnT levels (18,25).
In the present study, although LDL-cholesterol and triglyceride
levels were significantly different between patients and controls,
the incidence of MetS was similar in both groups. Therefore, it may
be concluded that lipid levels did not significantly result in
detectable differences in hs-TnT levels.
Elevated levels of cardiovascular biomarkers, such
as hs-TNT or NT-proBNP are encountered mostly in solid cancer
types. Slightly elevated hs-TnT levels are frequent findings among
hospitalized patients, mostly unrelated to ACS (1). Heart failure, myocarditis, pulmonary
embolism, sepsis or kidney failure are some of the most diagnosed
clinical conditions (3,5,6). Age
and LVH are also associated with high concentrations of hs-TnT
(4-6).
Several studies have analyzed different
cardiovascular biomarkers at baseline before starting cancer
therapies. One study on cardiac involvement using myocardial
troponins demonstrated frequent subclinical damage in patients with
gynecological cancers. Whether using conventional or
high-sensitivity assays, a considerable percentage of untreated
patients with ovarian cancer presented higher values of TnT I,
compared with other surgical patients with non-malignant masses or
endometriosis (10). In search for
better tools to predict the cardiac complications of anthracycline
chemotherapy, a previous study investigated the utility of hs-TnT,
NT-proBNP, cardiac TnT and troponin I and CK-MB in patients with
different types of solid and hematological cancer before and during
therapy (9). Upregulated baseline
hs-TnT levels identified a patient subgroup at high risk of
developing cardiac complications following chemotherapy. NT-proBNP
levels were indicated to be elevated in previous studies in
patients with cancer (8,9). Markedly elevated NT-proBNP may
indicate volume overload in the cancer population (8). One possible explanation is that a
number of abnormalities, more frequently observed in older
patients, seem to be significantly associated with the risk of
increased levels of hs-TnT, CK-MB and/or NT-proBNP.
Another study published in 2015 analyzed several
circulating cardiovascular hormones along with hs-TnT levels,
including NT-proBNP, prior to cancer therapy (2). The study revealed elevated
cardiovascular biomarkers in patients with untreated cancer.
Furthermore, cardiac biomarker levels increased concomitantly with
tumor stage and were strongly associated with all-cause mortality
during follow-up. The underlying mechanism remained unclear since
no clinical manifestation of a cardiac disease could be
confirmed.
Using the recently proposed baseline assessment to
stratify cardiovascular risk in cancer patients (16) prior to treatment start, the present
study identified groups of patients with low and moderate
cardiovascular risk, with significant differences between groups
when comparing cardiovascular biomarker levels. In the recruited
population with newly diagnosed colon neoplasia prior to any cancer
therapy, patients with elevated hs-TnT and NT-proBNP levels were
identified, contributing to significantly higher values overall
compared with the healthy group. The values remained higher also
when the group was divided according to age, compared with the
control group. Interestingly, although higher in patients with
colon cancer, hs-TnT and NT-proBNP had different behaviors compared
to traditional levels of ACS and heart failure, respectively they
reported a degree of myocardial strain without myocardial injury.
These results are consistent with the hypothesis that hs-TnT and
NT-proBNP baseline cut-off values for cardiac involvement in cancer
are different than the accepted value for ischemic disease and
heart failure, with different thresholds according to age.
The attempted selection in order to obtain a group
of patients without cardiovascular disease, subclinical or
clinically manifest, led us to a significantly younger age in the
control group when compared to the study group. Even when rigorous
exclusions of people with subclinical heart disease are performed,
different age-related troponin concentrations are real (26,27).
In this respect, significant differences between groups could bias
our results. For this reason, a comparison between cardiac
biomarkers in the control group and age subgroups in the study
group was added.
The main limitations of the present study are the
cross-sectional design and the limited availability of advanced
echocardiographic techniques for LV function analysis. In addition,
probably with a limited impact, the differences in the prevalence
of certain risk factors for coronary artery disease, such as age,
systolic blood pressure and LDL-cholesterol, could have partially
resulted in the differences in cardiac biomarker levels and LV size
between the two groups.
In conclusion, our study demonstrated the presence
of a certain degree of cardiovascular injury in treatment-naïve
patients with colon cancer, regardless of age or comorbidities.
This was evidenced by enlarged left chambers on echocardiography
and elevated levels of cardiovascular biomarkers, compared with
healthy subjects. Due to slight differences at baseline,
appropriate cut-off values of cardiovascular biomarkers adapted for
patients with cancer were proposed, although further studies on a
larger population are required to confirm these values.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LR, LA and EB contributed to the conception, design
of the study and writing of the manuscript. AG, CG, LS, VD, SC and
VM contributed to the design of the study and writing of the
manuscript. LR, LA, DC and EB performed the data analysis and
interpretation. DR and AB contributed to the conception, design of
the study, and final proofreading. DC and EB confirm the
authenticity of all raw data. All authors critically revised the
manuscript, approved the final version to be published and agree to
be accountable for all aspects of the work.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The Clinical Municipal Hospital in Cluj-Napoca,
Romania. Written informed consent was obtained from all
participants before their inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gong FF, Cascino GJ, Murtagh G and Akhter
N: Circulating biomarkers for cardiotoxicity risk prediction. Curr
Treat Options Oncol. 22(46)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pavo N, Raderer M, Hülsmann M, Neuhold S,
Adlbrecht C, Strunk G, Goliasch G, Gisslinger H, Steger GG, Hejna
M, et al: Cardiovascular biomarkers in patients with cancer and
their association with all-cause mortality. Heart. 101:1874–1880.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stein GY, Alon D, Korenfeld R and Fuchs S:
Clinical implications of high-sensitivity cardiac troponin
measurements in hospitalized medical patients. PLoS One.
10(e0117162)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lyngbakken MN, Aagaard EN, Kvisvik B,
Berge T, Pervez MO, Brynildsen J, Tveit A, Steine K, Røsjø H and
Omland T: Cardiac troponin I and T are associated with left
ventricular function and structure: Data from the akershus cardiac
examination 1950 study. Clin Chem. 66:567–578. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Normann J, Mueller M, Biener M, Vafaie M,
Katus HA and Giannitsis E: Effect of older age on diagnostic and
prognostic performance of high-sensitivity troponin T in patients
presenting to an emergency department. Am Heart J. 164:698–705.e4.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Masson S, Latini R, Mureddu GF, Agabiti N,
Miceli M, Cesaroni G, Forastiere F, Wienhues-Thelen UH, Block D,
Zaugg C, et al: High-sensitivity cardiac troponin T for detection
of subtle abnormalities of cardiac phenotype in a general
population of elderly individuals. J Intern Med. 273:306–317.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bernstein LH, Zions MY, Alam ME, Haq SA,
Heitner JF, Zarich S, Seamonds B and Berger S: What is the best
approximation of reference normal for NT-proBNP? Clinical levels
for enhanced assessment of NT-proBNP (CLEAN). J Med Lab Diag.
2:16–21. 2011.
|
|
8
|
Burjonroppa SC, Tong AT, Xiao LC, Johnson
MM, Wamique YS and Lenihan DJ: Cancer patients with markedly
elevated B-type natriuretic peptide may not have volume overload.
Am J Clin Oncol. 30:287–293. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Popat J, Rivero A, Pratap P and Guglin M:
What is causing extremely elevated amino terminal brain natriuretic
peptide in cancer patients? Congest Heart Fail. 19:143–148.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cardinale D, Sandri MT, Colombo A, Colombo
N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G,
Fiorentini C and Cipolla CM: Prognostic value of troponin I in
cardiac risk stratification of cancer patients undergoing high-dose
chemotherapy. Circulation. 109:2749–2754. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Danese E, Montagnana M, Giudici S, Aloe R,
Franchi M, Guidi GC and Lippi G: Highly-sensitive troponin I is
increased in patients with gynecological cancers. Clin Biochem.
46:1135–1138. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Blaes AH, Rehman A, Vock DM, Luo X, Menge
M, Yee D, Missov E and Duprez D: Utility of high-sensitivity
cardiac troponin T in patients receiving anthracycline
chemotherapy. Vasc Health Risk Manag. 11:591–594. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lim E, Li Choy L, Flaks L, Mussa S, Van
Tornout F, Van Leuven M and Parry GW: Detected troponin elevation
is associated with high early mortality after lung resection for
cancer. J Cardiothorac Surg. 1(37)2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Narayan V, Thompson EW, Demissei B, Ho JE,
Januzzi JL Jr and Ky B: Mechanistic Biomarkers Informative of Both
Cancer and Cardiovascular Disease: JACC State-of-the-art review. J
Am Coll Cardiol. 75:2726–2737. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Narayan V and Ky B: Common cardiovascular
complications of cancer therapy: Epidemiology, risk prediction, and
prevention. Ann Rev Med. 69:97–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lyon AR, Dent S, Stanway S, Earl H,
Brezden-Masley C, Cohen-Solal A, Tocchetti CG, Moslehi JJ, Groarke
JD, Bergler-Klein J, et al: Baseline cardiovascular risk assessment
in cancer patients scheduled to receive cardiotoxic cancer
therapies: A position statement and new risk assessment tools from
the cardio-oncology study group of the heart failure association of
the European society of cardiology in collaboration with the
international cardio-oncology society. Eur J Heart Fail.
22:1945–1960. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Alberti KG, Eckel RH, Grundy SM, Zimmet
PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith
SC Jr, et al: Harmonizing the metabolic syndrome: A joint interim
statement of the international diabetes federation task force on
epidemiology and prevention; national heart, lung, and blood
institute; American heart association; world heart federation;
international atherosclerosis society; and international
association for the study of obesity. Circulation. 120:1640–1645.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Arora G, Morss AM, Piazza G, Ryan JW,
Dinwoodey DL, Rofsky NM, Manning WJ and Chuang ML: Differences in
left ventricular ejection fraction using teichholz formula and
volumetric methods by cmr: Implications for patient stratification
and selection of therapy. J Cardiovasc Magn Reson.
12(P202)2010.
|
|
20
|
Demissei BG, Hubbard RA, Zhang L, Smith
AM, Sheline K, McDonald C, Narayan V, Domchek SM, DeMichele A, Shah
P, et al: Changes in cardiovascular biomarkers with breast cancer
therapy and associations with cardiac dysfunction. J Am Heart
Assoc. 9(e014708)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kang Y, Xu X, Cheng L, Li L, Sun M, Chen
H, Pan C and Shu X: Two-dimensional speckle tracking
echocardiography combined with high-sensitive cardiac troponin T in
early detection and prediction of cardiotoxicity during
epirubicine-based chemotherapy. Eur J Heart Fail. 16:300–308.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Daher IN, Kim C, Saleh RR, Plana JC, Yusuf
SW and Banchs J: Prevalence of abnormal echocardiographic findings
in cancer patients: A retrospective evaluation of echocardiography
for identifying cardiac abnormalities in cancer patients.
Echocardiography. 28:1061–1067. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Patel DA, Lavie CJ, Milani RV, Shah S and
Gilliland Y: Clinical implications of left atrial enlargement: A
review. Ochsner J. 9:191–196. 2009.PubMed/NCBI
|
|
24
|
Yeboah J, Bluemke DA, Hundley WG,
Rodriguez CJ, Lima JA and Herrington DM: Left ventricular dilation
and incident congestive heart failure in asymptomatic adults
without cardiovascular disease: Multi-ethnic study of
atherosclerosis (MESA). J Card Fail. 20:905–911. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Milwidsky A, Fisher E, Brzezinski RY,
Ehrenwald M, Shefer G, Stern N, Shapira I, Zeltser D, Rosenbaum Z,
Greidinger D, et al: Metabolic syndrome is associated to
high-sensitivity cardiac troponin T elevation. Biomarkers.
24:153–158. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hickman PE, Abhayaratna WP, Potter JM and
Koerbin G: Age-related differences in hs-cTnI concentration in
healthy adults. Clin Biochem. 69:26–29. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gore MO, Seliger SL, Defilippi CR, Nambi
V, Christenson RH, Hashim IA, Hoogeveen RC, Ayers CR, Sun W,
McGuire DK, et al: Age- and sex-dependent upper reference limits
for the high-sensitivity cardiac troponin T assay. J Am Coll
Cardiol. 63:1441–1448. 2014.PubMed/NCBI View Article : Google Scholar
|