Introduction
Crush syndrome (CS) occurs as a result of physical
trauma sustained during events such as earthquakes and is
associated with high mortality due to circulatory shock, kidney
failure, and systemic inflammation (1,2). CS
not only has localized effects but is also associated with systemic
failure resulting from acute respiratory distress syndrome,
systemic inflammatory response syndrome, multiple organ dysfunction
syndrome (MODS), and sepsis, following ischemia-reperfusion and
breakdown of muscle cells. The prevention of kidney failure is an
important part of treatment strategies for CS because it
contributes to worsening acute symptoms (3).
In general, kidney dysfunction is prevented by
hemodialysis and fluid therapy (i.e., kidney replacement therapy)
(4-7).
Fluid therapy is the first-line treatment for CS. Acute kidney
failure can be prevented by early fluid resuscitation using normal
saline solution containing sodium bicarbonate (8). However, the mortality rate of
patients is high despite treatment, as experienced during the
Hanshin Awaji, Marmara, and Wenchuan earthquakes (i.e., 13.4, 15.2,
and 11%, respectively) (9-11).
The high mortality rate results from the risk of inflammatory
response and infection even after treatment; that is, injury caused
by unusual and several complex pathological mechanisms, MODS, and
sepsis. Moreover, approximately 20% of patients with CS die from
heart disease-related symptoms, and over 50% die from sepsis and
systemic disease (12,13). Consequently, studies on therapeutic
effects focus on not only acute kidney and cardiac failure that
lead to death, but also inflammation and infection, highlighting
the importance of developing a therapeutic strategy for all
pathological condition phases of CS.
Salvianolic acid B
(4-[(1E)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]-3-oxo-1-propen-1-yl]-2-(3,4-dih-ydroxyphenyl)-2,3-dihydro-7-hydroxy-3-benzofurancarboxylic
acid (2S,3S)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl] ester:
SalB) is one of the components of Radix Salvia miltiorrhiza.
SalB exerts cardiac and kidney protective effects (14-17)
in an ischemia-reperfusion model by suppressing oxidative stress,
inflammation, and apoptosis (18-21)
and shows antibiotic properties (22,23).
In our previous study, we focused on not only prevent death with
cardiac failure and acute kidney failure, but also
anti-inflammatory effect associated with the endothelial damage via
ischemia-reperfusion injury, oxidative damage via mitochondrial
dysfunction, and the activities of neutrophil (NEU) during
inflammation (24-27).
The inflammation and infection often observed in patients with CS
may be related to neutrophil extracellular trapping (NET)
mechanisms mediated by the NETosis process of the NEU at the site
of injury. NETosis is neutrophil-related cell death characterized
by the secretion of large web-like structures (28). With this, the treatment of
infections prevents not only systemic dissemination of pathogens,
but also blood coagulation and endothelial damage (29). However, only a few studies have
focused on these mechanisms. In this study, we aimed to demonstrate
the survival benefit of SalB in the CS rat model.
Materials and methods
Animal CS model
Male Wistar rats weighing 250-300 g were obtained
from Japan SLC (Shizuoka, Japan) and housed in a room maintained at
a temperature of 23˚C±3˚C and relative humidity of 55±15% with a
12/12-h light/dark cycle and free access to food and water. All
animal experiments were approved by the Institutional Animal Care
and Use Committee of Josai University (approval no. JU18030).
Anesthesia induction and maintenance was performed using inhaled 2%
isoflurane. Body temperature was maintained throughout the
experiment using a heating pad. The CS model was established as
previously reported (24).
Briefly, a rubber tourniquet was applied to both the hindlimbs of
each rat, which was wrapped five times around a 2.0 kg-metal
cylinder, and the end of the band was glued. Just before
compression for 5 h, the tourniquet was removed from the limbs by
cutting the band (i.e., reperfusion 0 h).
SalB preparation
SalB (Carbosynth) dosages were determined based on
previous reports (30-32)
and were 10, 20, and 50 mg/kg. SalB was dissolved in 1% dimethyl
sulfoxide saline solution (vehicle): 100 µl.
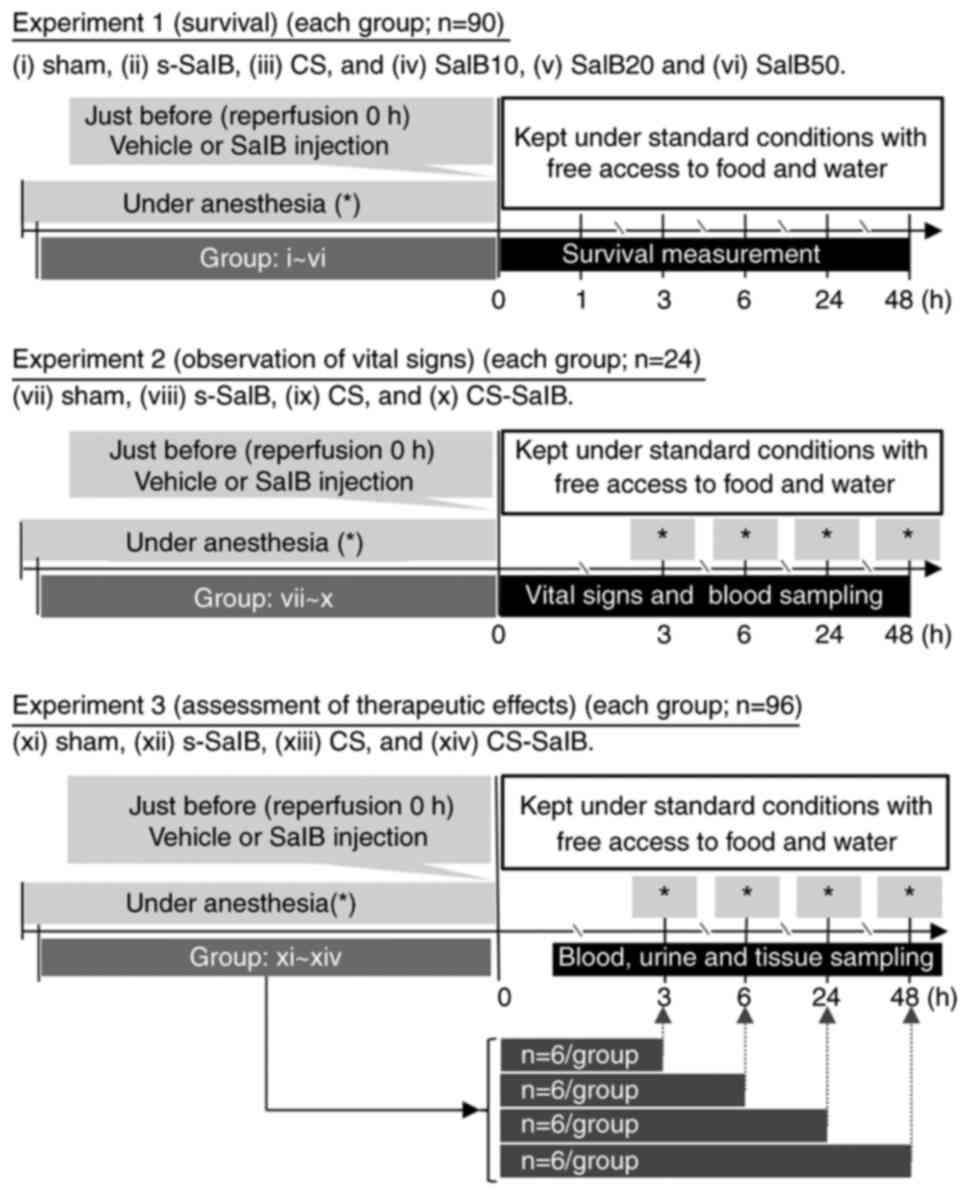
Experimental design
The experimental design is shown in Fig. 1. Experiment 1 (survival): The
anesthetized (2% isoflurane inhalation) rats were randomly divided
into six groups: i) sham with vehicle (sham groups serving as
controls, which were subjected to the same treatments as the
CS-model rats except for compression or decompression with rubber
tourniquets), ii) sham with 20 mg/kg SalB (s-SalB), iii) CS with
vehicle (CS group), and iv) CS with 10 mg/kg SalB (SalB10), v) CS
with 20 mg/kg SalB (SalB20) and vi) CS with 50 mg/kg SalB (SalB50).
Vehicle and SalB were administered via a tail vein single injection
after maintaining anesthesia for 5 h or decompression from a rubber
tourniquet. Emerge from anesthesia, rats are replaced in the cage
(free access to food and water). Survival measurement points were
0, 1, 3, 6, 24, and 48 h after reperfusion (each group; n=15).
Experiment 2 (observation of vital signs): Among the
tested SalB dosages (10, 20, and 50 mg/kg), 20 mg/kg was chosen for
this experiment as maximum survival was obtained at this dosage. To
examine the effects of SalB in CS, the animals were randomly
divided into four groups: vii) sham with vehicle, viii) s-SalB, ix)
CS group, and x) CS with 20 mg/kg SalB (CS-SalB). The anesthetized
(2% isoflurane inhalation) rats were subdivided above groups, and
cannulated a polyethylene catheter (PE-50 tubing) from a carotid
artery at 3, 6, 24 and 48 h after reperfusion for sequential
sampling (each group; n=6).
Experiment 3 (assessment of therapeutic effects): To
examine the effects of SalB in CS, the animals were randomly
divided into four groups: xi) sham with vehicle, xii) s-SalB, xiii)
CS group, and xiv) CS-SalB. Rats were subdivided at 3, 6, 24, and
48 h after reperfusion for these sampling points (each point;
n=6).
The experimental rats (n=210) were monitored for
health and behavior every hour until 6 h and every 12 h thereafter.
The rats were euthanized (confirmation by pupillary reflex to
light) when a no food and/or water intake state, dyspnea (i.e.
mouth breathing) state, and autotomy (i.e. bite) occurred in the
pressure area. All the rats used in the experiments were euthanized
(confirmation by pupillary reflex to light) by administering an
overdose of sodium pentobarbital (100 mg/kg body weight,
intravenously); Experimental 1: 48 h after reperfusion;
Experimental 2: 48 h after reperfusion; Experimental 3: just each
sampling time.
Analysis of mean arterial pressure and
blood gas levels
Experimental 2: Mean arterial pressure (MAP), heart
rate (HR), and rectal temperature (Temp) were recorded using a
PowerLab data acquisition system (AD Instruments). A carotid artery
was cannulated with PE-50 tubing connected to a pressure transducer
(AD Instruments). Arterial blood samples from each mouse were
obtained at 3, 6, 24, and 48 h after reperfusion using a carotid
artery catheter over time (24).
The arterial levels of hematocrit (Hct), potassium (K+),
blood urea nitrogen (BUN), pH, base excess (BE), anion gap, and
lactate were analyzed using the i-STAT300F blood gas analyzer
CG4+ and EC8+ cartridges (FUSO Pharmaceutical
Industries). These measurements were performed under maintained
anesthesia (2% isoflurane inhalation) for up to 6 h, after which
only the catheter was attached to the back of the rat, and then
anesthetized and measured again 1 h before 24 and 48 h (i.e., after
23 and 47 h of Reperfusion).
Analysis of biochemical parameters and
coagulation levels
Experimental 3: In each experimental group (3, 6,
24, and 48 h after reperfusion, respectively n=6), venous blood and
tissue samples from the gastrocnemius muscles were subjected to
inflammation, and tissue thiobarbituric acid reactive substance
(TBARS), myeloperoxidase (MPO) activity, an index of mitochondrial
permeability transition, and superoxide dismutase (SOD) activity
(27) were measured. The rats used
in the experiments were euthanized by administering an overdose of
sodium pentobarbital and then venous blood (from the inferior vena
cava) and tissue (kidney and muscle) was collected. Venous blood
separated into whole blood, serum, and plasma. Red blood cell
(RBC), white blood cell (WBC), NEU, and platelet (PLT) counts in
whole blood were measured using Vet Scan HM5 (ABAXIS Inc.).
Activated partial thromboplastin time (APTT) and prothrombin time
(PT) were measured using Sysmex CA-100 (Sysmex Co.). The plasma
level of creatine phosphokinase was measured using the Creatine
Kinase Assay kit, EnzyChrom (BioAssay Systems Co.), fibrinogen
(FIB) was measured using the Rat FIB ELISA kit (ASSAYPRO), von
Willebrand factor (vWF) was measured using the rat vWF ELISA kit
(USCN), and creatine phosphokinase (CPK) was measured using the
Creatine Kinase Assay kit, EnzyChrom (BioAssay Systems Co.).
Analysis of interleukin (IL)
levels
Experimental 3: The serum levels of high mobility
group box 1 (HMGB1), IL-6, IL-8, IL-10, IL-1β, and tumor necrosis
factor (TNF)-α were measured using HMGB1 ELISA kit II (Shino-Test
Co.). Rat IL-6, IL-8, IL-10, L-1β/IL-1F2, and TNF-α levels were
measured using the Quantikine® ELISA kit (RandD Systems,
Inc.), and plasminogen activator inhibitor-1 (PAI-1) was measured
using the Rat PAI1 ELISA kit (Abcam) according to the
manufacturer's instructions.
Analysis of kidney dysfunction
Experimental 3: In the blood sample, the effect of
N-acetyl-β-D-glucosaminidase (NAG) on kidney function was
determined using the β-N-acetylglucosaminidase assay kit
QuantiChrom (BioAssay Systems Co.), kidney injury marker-1 (KIM-1)
using Rat TIM-1/KIM-1/HAVCR Immunoassay (RandD Systems, Inc.),
neutrophil gelatinase-associated lipocalin (NGAL) using the Rat
Lipocalin-2 ELISA kit (Abcam), and creatinine (Cre) using the
Creatinine Assay kit, QuantiChrom (BioAssay Systems Co.). Urine
samples were then collected by bladder and centrifuged at 1,500 x g
for 5 min at 20-25˚C. Cre using the Creatinine Assay kit,
QuantiChrom (BioAssay Systems Co.), urine osmotic pressure (Osmomat
030-D; Gonotec GmbH), urine volume and glomerular filtration rate
(GFR). For histological evaluations, tissue samples were fixed in
10% formalin and embedded in paraffin, and sections were cut and
stained with hematoxylin and eosin. Microphotographs of the tissue
sections were then evaluated by a pathologist (New Histo Science
Laboratory). Renal injuries were scored by calculating the
percentage of tubules that displayed tubular dilation, cast
formation, and tubular necrosis according to a previously described
method (33). Specifically, for
each kidney, 12 cortical tubules from at least 4 different areas
(i.e., 3 cortical tubules/area) were scored, and care was taken to
avoid repeated scoring of different convolutions of the same
tubule. Higher scores represented more severe damage (the maximum
score per tubule was 7), and points were given for the presence and
extent of tubular epithelial cell flattening (1 point), brush
border loss (1 point), cell membrane bleb formation (1 point),
interstitial edema (1 point), cytoplasmic vacuolization (1 point),
cell necrosis (1 point), and tubular lumen obstruction (1
points).
Determination of reactive oxygen
species (ROS) production, MPO activity, and mitochondrial
function
ROS production in the injured gastrocnemius muscle
was determined by measuring the concentration of TBARS, MPO
activity in the blood and muscle tissue, and mitochondrial function
by cytochrome c (Cyt c) leakage into the cytoplasm.
JC-1 fluorescence intensity was used to determine mitochondrial
permeability transition (i.e., mitochondrial inner membrane
function) using the method described by Murata et al
(27). Briefly, for the JC-1
method, mitochondrial fraction was isolated using a mitochondrion
isolation kit (Sigma). Muscle tissue was homogenized and then
centrifuged at 600 x g for 5 min. The supernatant was centrifuged
at 11,000 x g for 10 min and then spun at 16,000 x g for 20 min at
4˚C to remove any residual mitochondria. The pellet (mitochondrial
fraction) was suspended with storage buffer, and then total protein
concentration was measured. In short, 90 µl of 0.2 µg/ml JC-1
staining solution was added into the wells of a 96-well plate and
then 10 µl of the isolated mitochondrial sample (0.2 µg protein)
was added. The plate was incubated at room temperature in the dark
for 7 min for uptake saturation. The absorbance was then measured
with a microplate spectrophotometer at emission and excitation
wavelengths of 540 and 570 nm (red), respectively, for apoptotic
mitochondria, and 485 and 535 nm, respectively, for healthy
mitochondria (green).
SOD activity was determined using the SOD Assay
kit-WST (Dojindo Laboratories). 1,1-diphenil-2-picryl-hydrazal
(DPPH) antioxidant assay of SalB was performed as described by
Sharma and Bhat (34). Briefly, 20
µM DPPH methanol solution and 5, 10, 25, 50, 100, 250, 500, 750,
and 1,000 µM SalB methanol solutions were mixed in a microplate at
a 1:1 volume ratio. The mixture was then incubated at 30˚C for 30
min (light shielded), and the absorbance was measured at 280 nm
(SalBabs). The absorbance of the mixture of DPPH
methanol solution/methanol (1:1) was used as DMabs, and
that of methanol as Mabs. Ascorbic acid (AA) has a high
antioxidant capacity, and it was used as an antioxidant reference
(0.018, 0.039, 0.156, 0.313, 0.625, 1.25, and 2.5 µM) for
comparison with SalB. The DPPH radical scavenging activity ratio
(AU) was calculated using the following equation:
AU=[1-(SalBabs-Mabs)/(DMabs-Mabs)]
x100, and 50% AU was calculated from the linear equation of AU and
SalB or AA.
Analysis of nitrogen oxide (NOx)and
inducible nitric oxide synthase (iNOS) levels
NOx [(total nitrite (NO2−) and
nitrate (NO3−)] concentrations in the serum
were measured using the CII and FX
NO2−/NO3− assay kits
(Dojindo Laboratories) according to the manufacturer's
instructions. Western blotting for inducible nitric oxide synthase
(iNOS) and β-actin was carried out as previously described
(26). Briefly, rat muscle tissue
was homogenized and centrifuged, and proteins in the lysate were
separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis using antibodies against iNOS (Cell Signaling
Technology) and β-actin (Cell Signaling Technology). The protein
bands were visualized using an enhanced chemiluminescence detection
system (SuperSignal West Dura Extended Duration Substrate; Pierce
Biotechnology) with horseradish peroxidase-conjugated secondary
antibodies (Pierce Biotechnology). Band intensity was quantified
using ChemiDoc XRS+ Molecular Imager with Image Lab software
(Bio-Rad Laboratories) with β-actin as a loading control.
Antimicrobial study
The minimum inhibitory concentration (MIC) of SalB
was measured using the broth microdilution method. This procedure
essentially followed the Clinical and Laboratory Standards
Institute guidelines (35). Test
bacteria used were two strains of gram-positive bacteria
(Bacillus subtilis ATCC6633 and Staphylococcus aureus
ATCC29213) and one strain of gram-negative bacteria (Escherichia
coli ATCC25922). Cation-adjusted Mueller-Hinton Broth (CAMHB;
BD Biosciences) was used as a test medium. For the test, the
solvent used to prepare the agar medium was water. SalB was added
to a concentration of 3,000 µg/ml in CAMHB. The mixture was stirred
and then diluted with CAMHB to 2,000, 1,000, 500, 300, 100, 50, 30,
10, 1, 0.5, 0.3, and 0.1 µg/ml. The test micro plates were
prepared, and an inoculum (5 µl) of the bacterium adjusted to
1.0x107 CFU/ml was spotted onto the test micro plate.
After incubating the test plates for 16-20 h at 35˚C, the growth of
each strain was observed to determine the MIC.
Statistical analysis
Results are expressed as mean ± standard error of
the mean. Survival curves were generated using the Kaplan-Meier
method, and survival was compared using the log-rank test.
Differences between groups were assessed using the one-way analysis
of variance with Tukey's honest significant difference test or
Tukey's test. Kidney injury score was assessed using the Dunn's
nonparametric comparison for post hoc testing after a
Kruskal-Wallis test. DPPH antioxidant assay was assessed using the
unpaired Student's t test between two groups. Differences were
considered significant at P<0.05 (Statcel 2, 2nd ed. OMS
Publishing Inc.).
Results
SalB treatment effects on the survival
rate in the CS rat model
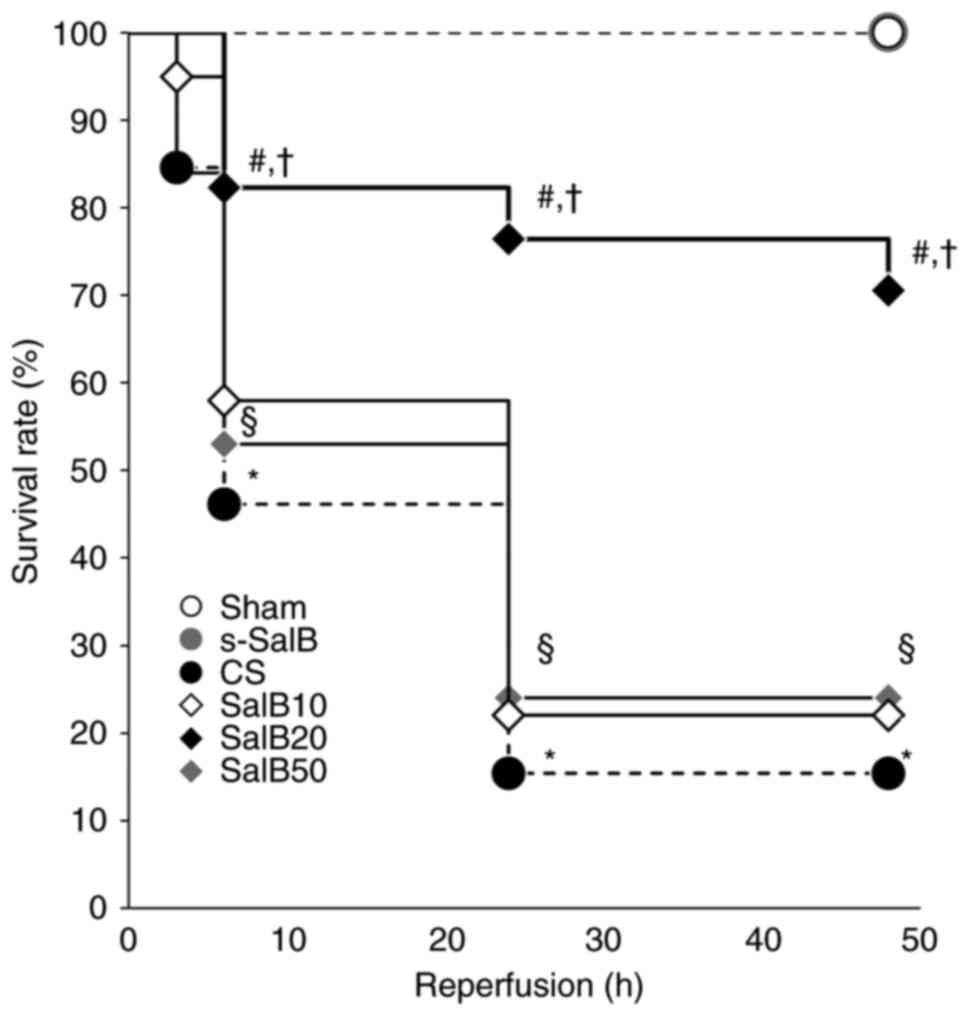
The survival rates of rats in the CS group were 92,
84, 46, 15, and 15% at 1, 3, 6, 24, and 48 h, respectively, and
they were significantly lower than those in the sham group at 6,
24, and 48 h after reperfusion (P<0.05). CS rats died of cardiac
failure and hypovolemic shock. The cause of death was related to
kidney dysfunction and systemic inflammatory response associated
with traumatic rhabdomyolysis caused by crush injury. The survival
rates of the CS-SalB group at 6, 24, and 48 h after reperfusion
(82, 76, and 71%, respectively) were significantly higher than
those of the CS group (P<0.05). No mortality was observed in the
sham and s-SalB groups (Fig. 2).
These results suggested the effectiveness of SalB in the treatment
and prevention of CS symptoms.
SalB treatment effects on kidney
disfunction in the CS rat model
The parameters of kidney functions are shown in
Fig. 3 and Table I. The SOD activity in the CS group
was significantly higher than that in the sham group at 6 and 24 h
after reperfusion, and significantly lower than that in the sham
group at 48 h after reperfusion (Fig.
3A). The NAG and KIM-1 levels in the CS group were
significantly higher than those in the sham group at 6, 24, and 48
h (Fig. 3B and C), and the NGAL level in the CS group was
significantly higher than the sham group at 3, 6, and 24 h
(Fig. 3D). In the CS-SalB group,
the kidney SOD activity was significantly higher than that in the
CS group at 48 h after reperfusion, and the NAG, KIM-1, and NGAL
levels were significantly lower than those in the CS group. In
terms of pathology, the CS group showed moderate pathological
dilation in the distal convoluted tubules, and this was improved in
the CS-SalB group after 48 h of reperfusion (Fig. 3E). These findings suggest that SalB
improves the kidney dysfunction by improving kidney tubule
epithelial damage and antioxidative effect in CS.
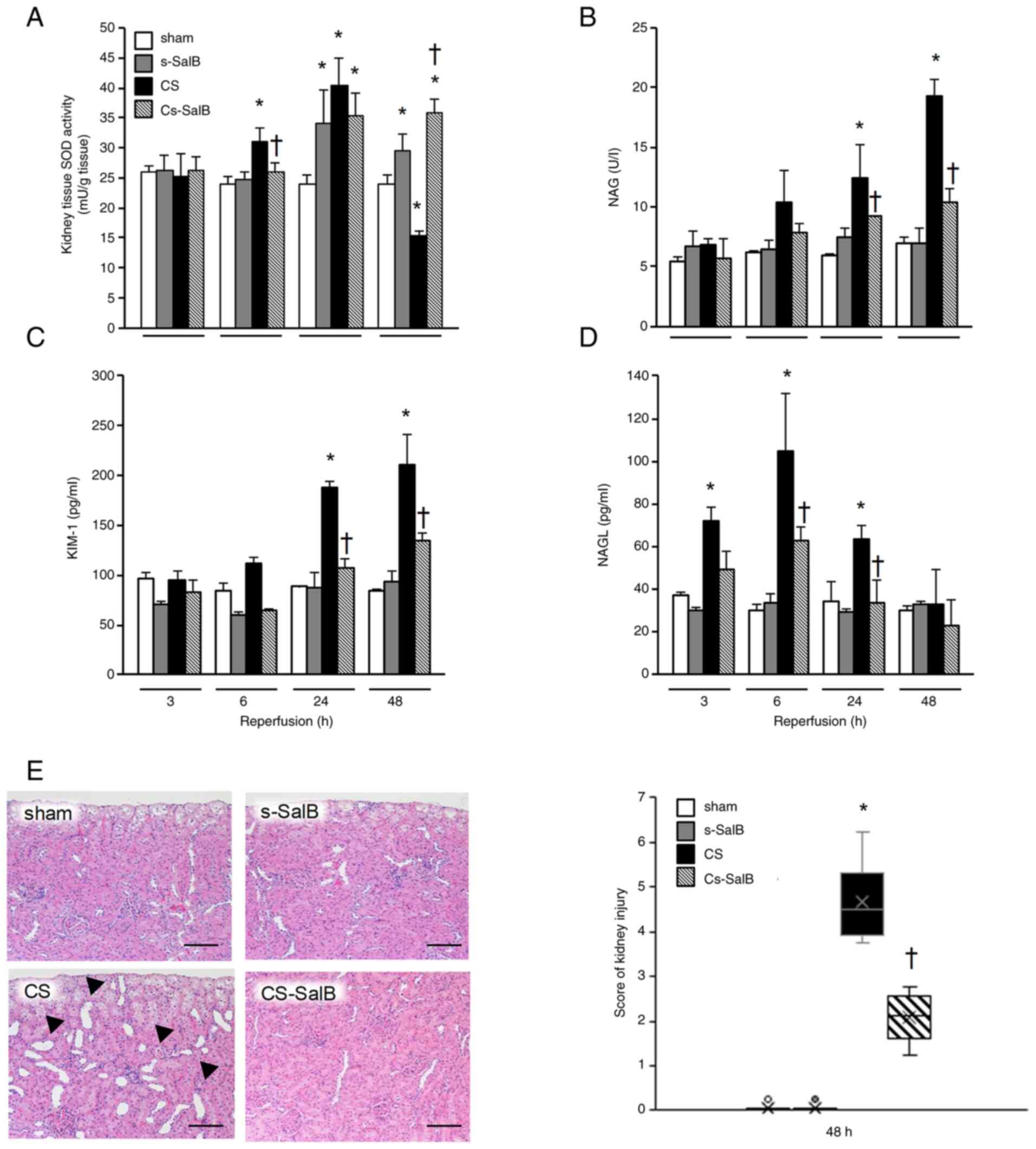 | Figure 3Effect of SalB on kidney function in
the CS rats. (A) Kidney SOD activity, (B) serum NAG level, (C)
serum KIM-1 level, (D) serum NGAL level and (E) hematoxylin and
eosin-stained kidney sections and kidney injury score following
reperfusion for 48 h. (A-D) Bar graph values are presented as the
mean ± SEM (n=6). *P<0.05 vs. sham group,
†P<0.05 vs. CS group (Tukey-Kramer test). Micrographs
are representative of three independent experiments (magnification,
x200; scale bar, 100 µm). Black arrowhead, dilated kidney tubule.
(E) Box plot for kidney injury score, *P<0.05 vs.
sham group, †P<0.05 vs. CS group (Kruskal-Wallis
test). CS, crush syndrome; SalB, salvianolic acid B; s-SalB, sham
with 20 mg/kg of SalB; CS-SalB, CS with 20 mg/kg of SalB; SOD,
superoxide dismutase; NAG, N-acetyl-β-D-glucosaminidase; KIM-1,
kidney injury marker-1; NGAL, neutrophil gelatinase-associated
lipocalin. |
 | Table IEffect of SalB on kidney function
parameters in blood and urine sample in the CS rats. |
Table I
Effect of SalB on kidney function
parameters in blood and urine sample in the CS rats.
| | Reperfusion, h |
|---|
| Parameter | Group | 3 | 6 | 24 | 48 |
|---|
| BUN, mg/dl | sham | 15.3±1.1 | 12.7±1.2 | 14.6±1.1 | 19.5±1.8 |
| | s-SalB | 14.9±1.3 |
25.7±0.6a | 24.2±10.0 | 17.3±0.2 |
| | CS |
29.3±5.6a |
41.3±5.5a |
98.5±3.5a |
88.3±2.9a |
| | C-SalB |
16.2±2.0b |
21.0±1.8b |
33.3±2.3b |
35.1±4.4b |
| Cre, mg/dl | sham | 0.3±0.3 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 |
| | s-SalB | 0.3±0.1 | 0.2±0.0 | 0.2±0.1 | 0.2±0.0 |
| | CS | 0.9±0.4 |
1.2±0.3a |
1.5±0.1a |
1.5±0.3a |
| | C-SalB | 0.4±0.2 |
0.5±0.3b |
0.5±0.2b |
0.6±0.3b |
| Urine osmotic
pressure, mOsm/kg • H20 | sham | 1.47±0.02 | 1.37±0.09 | 1.38±0.06 | 1.39±0.07 |
| | s-SalB | 1.58±0.02 | 1.38±0.35 | 1.31±0.12 | 1.25±0.17 |
| | CS |
1.12±0.02a |
0.85±0.22a |
0.66±0.15a |
0.54±0.04a |
| | C-SalB |
1.53±0.01b |
1.51±0.08b |
1.81±0.03b |
1.40±0.11b |
| Urine volume,
ml/l | sham | 0.42±0.02 | 0.40±0.05 | 0.35±0.05 | 0.50±0.06 |
| | s-SalB | 0.46±0.25 | 0.50±0.06 | 0.60±0.02 | 0.45±0.03 |
| | CS | 0.30±0.01 | 0.21±0.02 | 0.16±0.00 | 0.23±0.02 |
| | C-SalB | 0.39±0.12 |
0.56±0.02b |
0.54±0.07b |
0.45±0.07b |
| GFR, ml/min | sham | 1.69±0.22 | 1.50±0.19 | 1.45±0.10 | 1.36±0.23 |
| | s-SalB | 1.98±0.45 | 1.75±0.24 | 1.35±0.65 | 0.98±0.11 |
| | CS |
0.98±0.14a |
0.78±0.24a |
0.55±0.33a |
0.58±0.19a |
| | C-SalB |
1.78±0.45b |
1.53±0.33b |
1.75±0.57b |
1.78±0.22b |
SalB treatment effects on cardiac
failure and shock in the CS rat model
Table II shows the
parameters of the acute phase symptoms of CS. The CPK and
K+ levels in the CS group were significantly higher than
those in the sham group. In contrast, the MAP in the CS group was
significantly lower than that in the sham group. pH, BE, Hct, HR,
and Temp in the CS group were significantly lower than those in the
sham group (Table II). In
contrast, these changes in the CS-SalB group were significantly
higher than those in the CS group. In addition, the adverse effects
of SalB treatment were not observed in the groups. These finding
suggest that SalB improves shock and cardiac failure by decreasing
systemic circulation of K+ following the suppression of
muscle cell collapse in CS.
 | Table IIEffects of SalB. |
Table II
Effects of SalB.
| | Reperfusion, h |
|---|
| Parameter | Group | 3 | 6 | 24 | 48 |
|---|
| CPK, IU/l | sham | 148±20 | 193±46 | 208±40 | 206±43 |
| | s-SalB | 134±18 | 174±41 | 177±34 | 134±28 |
| | CS |
5,305±1,080a |
8,368±1,556a |
12,870±2,281a |
34,562±3,428a |
| | CS-SalB | 3,373±181 |
3,537±307b | 11,251±2,309 |
24,469±2,291b |
| K+,
mEq/l | sham | 4.4±0.3 | 4.3±0.2 | 4.6±0.2 | 3.9±0.2 |
| | s-SalB | 3.9±0.0 | 3.6±0.1 | 3.8±0.2 | 3.5±0.,1 |
| | CS | 5.5±0.2 | 5.9±0.2 |
6.4±0.3a |
7.3±0.7a |
| | CS-SalB | 4.8±0.2 | 5.7±0.2 | 6.6±0.5 |
5.6±0.2b |
| pH | sham | 7.46±0.04 | 7.48±0.01 | 7.50±0.01 | 7.45±0.01 |
| | s-SalB | 7.41±0.02 | 7.45±0.05 | 7.44±0.02 | 7.48±0.03 |
| | CS | 7.50±0.02 | 7.46±0.02 |
7.24±0.03a |
7.27±0.07a |
| | CS-SalB | 7.46±0.01 | 7.47±0.02 |
7.45±0.03b |
7.47±0.03b |
| BE, mmol/l | sham | 5.7±0.3 | 6.7±0.7 | 6.7±0.9 | 4.7±0.3 |
| | s-SalB | 1.3±1.8 | 2.0±2.3 | 2.3±2.2 | 6.3±0.7 |
| | CS | 1.3±0.9 | 0.3±1.1 |
-5.0±1.6a |
-4.4±2.7a |
| | CS-SalB | 1.0±0.8 | 1.5±1.8 | -4.5±1.5 | 2.0±0.8 |
| MAP, mmHg | sham | 131±5 | 124±5 | 112±11 | 116±9 |
| | s-SalB | 124±6 | 110±4 | 122±11 | 151±10 |
| | CS | 65±6a | 63±7a | 58±6a | 56±14a |
| | CS-SalB | 98±3b | 94±4b | 89±3b | 87±3b |
| Hct, % | sham | 46.3±0.3 | 44.7±0.3 | 43.3±1.2 | 43.7±0.9 |
| | s-SalB | 48.3±1.5 | 47.0±1.5 | 48.0±1.2 | 45.0±2.6 |
| | CS | 48.6±1.2 |
51.9±1.1a |
51.3±0.9a |
57.2±7.1a |
| | CS-SalB | 45.8±1.7 | 48.5±1.2 | 52.3±1.9 |
46.0±2.1b |
| HR, bpm | sham | 400±15 | 388±17 | 336±46 | 329±79 |
| | s-SalB | 337±20 | 309±20 | 329±5 | 385±22 |
| | CS | 307±18a | 274±26a | 344±26 | 256±38a |
| | CS-SalB | 333±5 | 374±7b | 443±26b | 416±30b |
| Temp, ˚C | sham | 36.2±0.4 | 36.4±0.1 | 36.3±0.3 | 36.3±0.6 |
| | s-SalB | 36.5±0.3 | 37.5±0.7 | 37.8±0.5 | 35.4±0.5 |
| | CS | 35.3±0.4 |
34.6±0.6a |
35.0±0.8a |
29.5±1.4a |
| | CS-SalB | 36.2±0.1 |
37.4±0.5b |
38.2±0.8b |
35.0±0.5b |
SalB treatment effects on inflammation
and coagulation disorder from endothelial cell damage
Endothelial damage with inflammation, and the levels
of IL-6, IL-10, IL-1β, TNF-α, HMGB1, and NOx levels are shown in
Fig. 4. These parameters in the CS
group were significantly higher than those in the sham group at
3-48 h. The IL-6, IL-1β, and HMGB1 levels in the CS-SalB group were
significantly lower than those in the CS group at 6-24 h. The TNF-α
and NOx levels in the CS-SalB group were significantly lower than
those in the CS group and comparable with those in the sham group.
Moreover, the IL-10 level in the s-SalB group was significantly
higher than that in the sham group. However, the IL-10 level in the
CS-SalB group was not significantly lower than that in the CS
group.
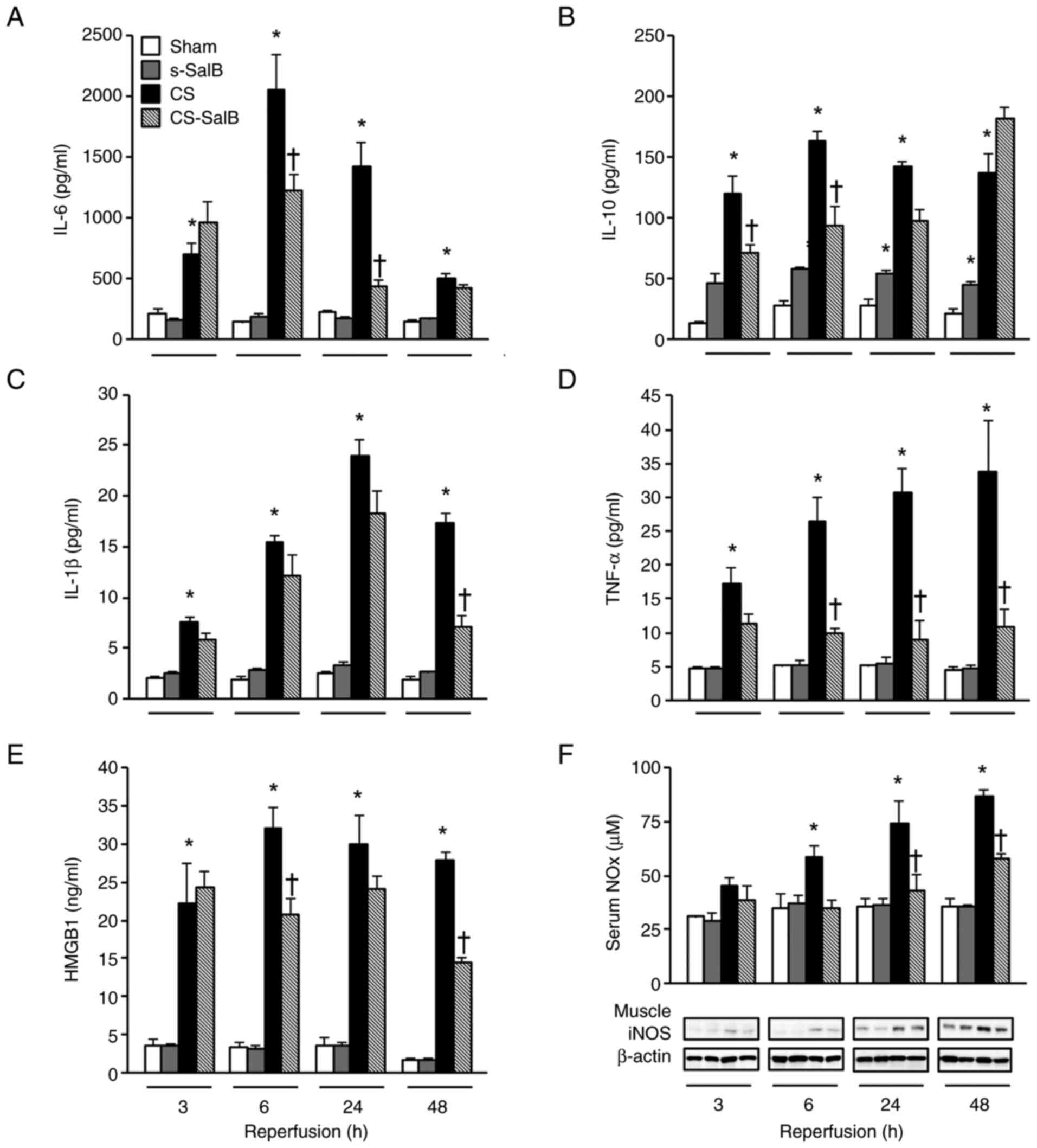 | Figure 4Effect of SalB on inflammatory
mediators in the CS rats. (A) Serum IL-6 levels, (B) serum IL-10
levels, (C) serum IL-1β levels, (D) serum TNF-α levels, (E) serum
HMGB1 levels, and (F) serum NOx levels and muscle iNOS expression.
Values are presented as the mean ± SEM (n=6). *P<0.05
vs. sham group, †P<0.05 vs. CS group (Tukey-Kramer
test). CS, crush syndrome; SalB, salvianolic acid B; s-SalB, sham
with 20 mg/kg of SalB; CS-SalB, CS with 20 mg/kg of SalB; HMGB1,
high mobility group box 1; NOx, nitrogen oxide; iNOS, inducible
nitric oxide synthase. |
Endothelial cell damage was assessed using the vWF,
PLT, FIB, PAI-1, APTT, and PT levels (Fig. 5). The vWF level in the CS group was
significantly lower than that in the sham group during the
experimental period, and temporarily inhibited in the CS-SalB group
compared with that in the CS group (Fig. 5A). The PLT and FIB levels in the
CS-SalB group were significantly higher than those in the sham
group. In particular, the PLT level in the CS-SalB group was
significantly higher than that in the CS group at 3-24 h (Fig. 5B and C). The PAI-1 level in the CS group was
significantly higher than that in the sham group, and the PAI-1
level in the CS-SalB group showed a decreasing trend, which was not
observed in the CS group (Fig.
5D). The APTT and PT levels in the CS group were significantly
higher than those in the sham group at 3-48 h, and the former's
level in the CS-SalB group was significantly lower than that in the
CS group at 3-24 h. In the s-SalB group, these were no differences
in these parameters. These finding suggested that SalB improves
coagulation disorder by suppressing the increased production of
inflammatory cytokines induced by damaged vascular endothelial
cells on CS.
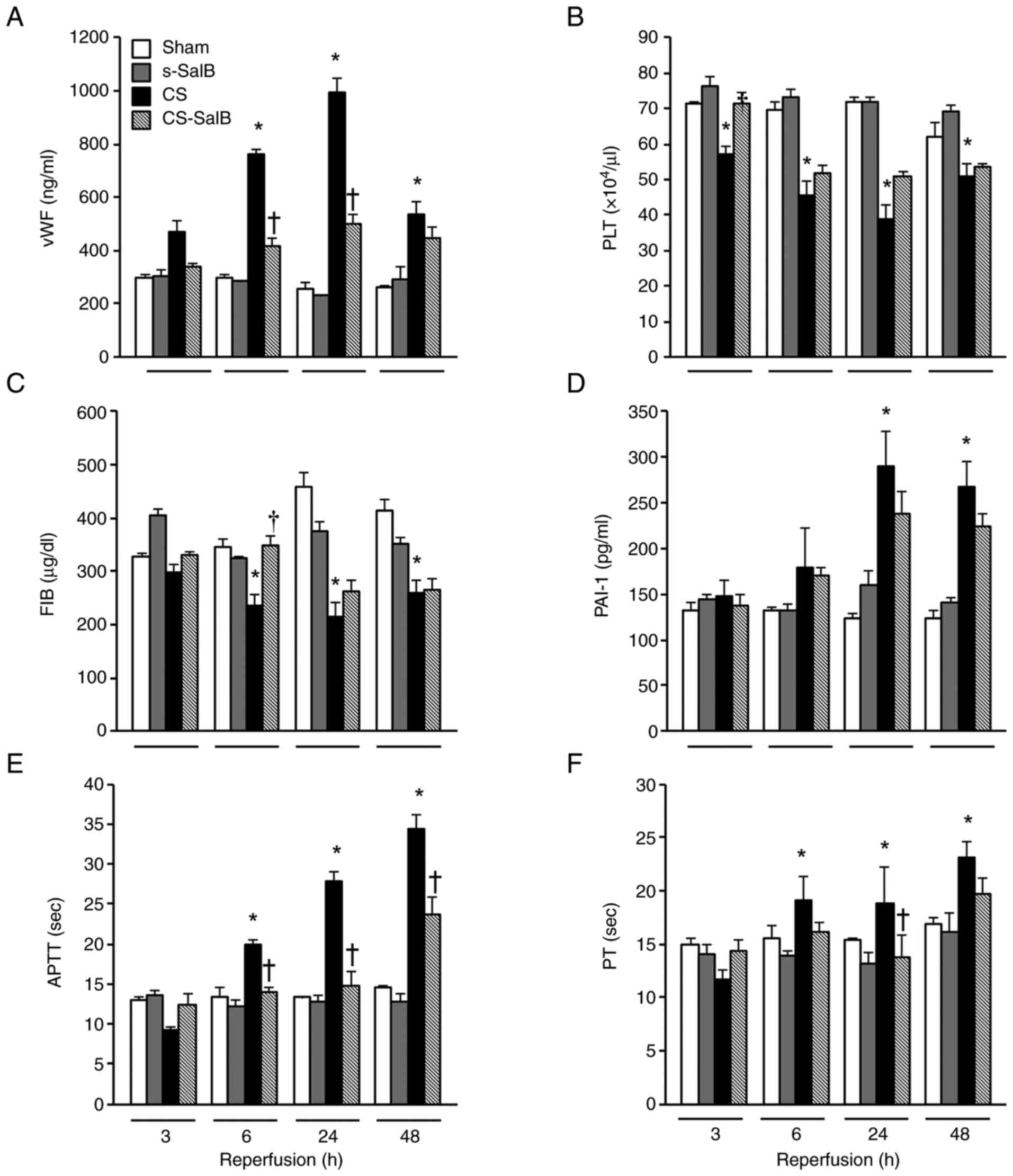 | Figure 5Effect of SalB on the coagulation
system in the CS rats. (A) Plasma vWF levels, (B) PLT levels, (C)
FIB levels, (D) serum PAI-1 levels, (E) APTT levels and (F) PT
levels. Values are presented as the mean ± SEM (n=6).
*P<0.05 vs. sham group, †P<0.05 vs. CS
group (Tukey-Kramer test). CS, crush syndrome; SalB, salvianolic
acid B; s-SalB, sham with 20 mg/kg of SalB; CS-SalB, CS with 20
mg/kg of SalB; PLT, platelet; APTT, activated partial
thromboplastin time; PT, prothrombin time; FIB, fibrinogen; vWF,
von Willebrand factor; PAI-1, plasminogen activator
inhibitor-1. |
Inhibitory effects of SalB on ROS
production and mitochondrial dysfunction in the CS rat model
ROS damage was assessed by TBARS production, and
muscle SOD activity and mitochondrial damage were assessed using
cytoplasm Cyt c and JC-1. The CS group showed significantly
higher tissue TBARS level than the sham group, and the maximum
level of tissue TBARS in the CS-SalB group was significantly lower
than that in the CS group. The muscle SOD activity in the CS group
was significantly higher than that in the sham group at 6 and 24 h
of reperfusion, and significantly lower in the CS group than in the
sham group at 48 h of reperfusion. In the CS-SalB group, the SOD
activity was significantly higher than that in the CS group at 24 h
of reperfusion (Fig. 6B). The
cytoplasmic Cyt c level in the CS group was significantly
higher than that in the sham group, and the cytoplasmic Cyt
c level in the CS-SalB group showed a tendency to decrease
compared with that in the CS group (Fig. 6C). The CS group had significantly
higher JC-1 level than the sham group at all experimental periods,
and the JC-1 level was inhibited and higher in the CS-SalB group
than in the CS group (Fig. 6D).
These finding suggested that SalB improves the antioxidation system
including the SOD activity, and ROS generation by mitochondrial
dysfunction in CS. In addition, the direct radical scavenging
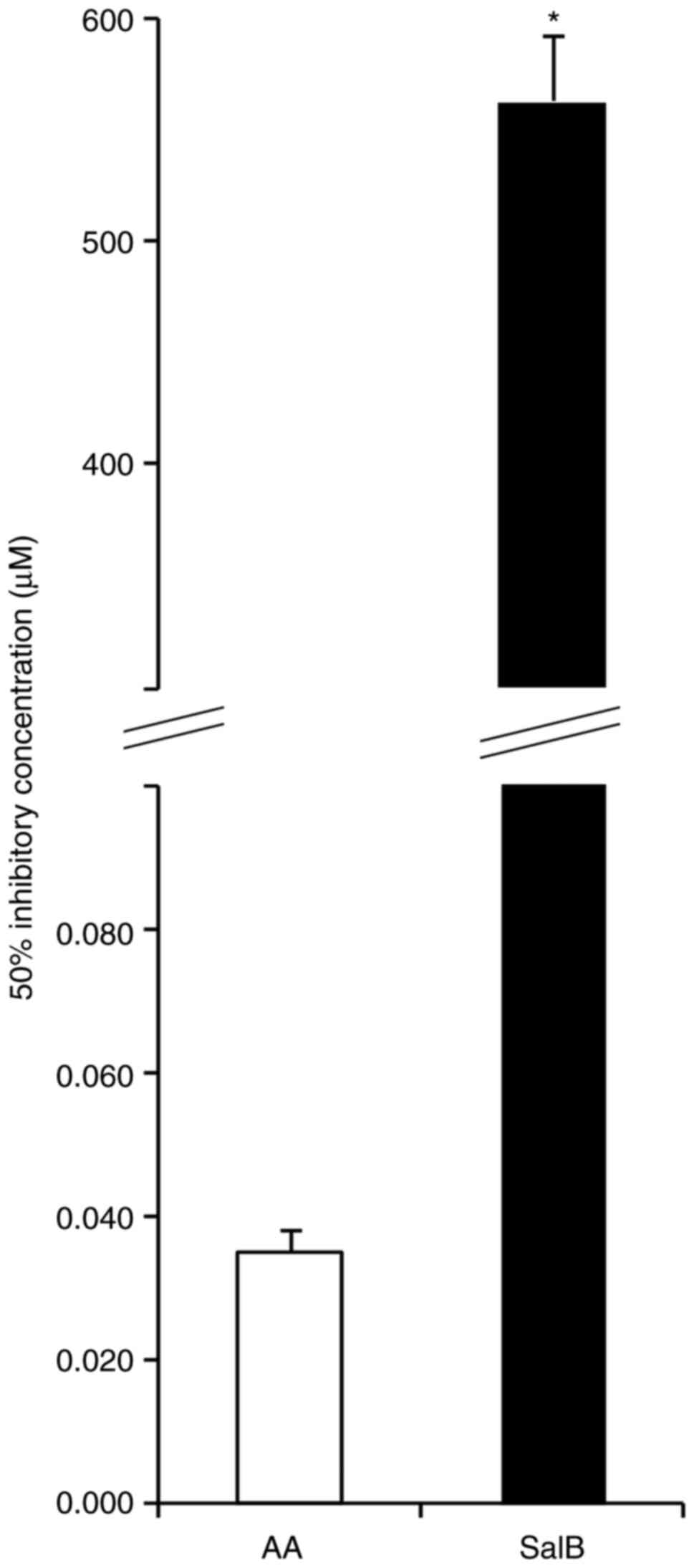
ability of the SalB group was significantly higher compared with
that in the AA group (Fig. 7).
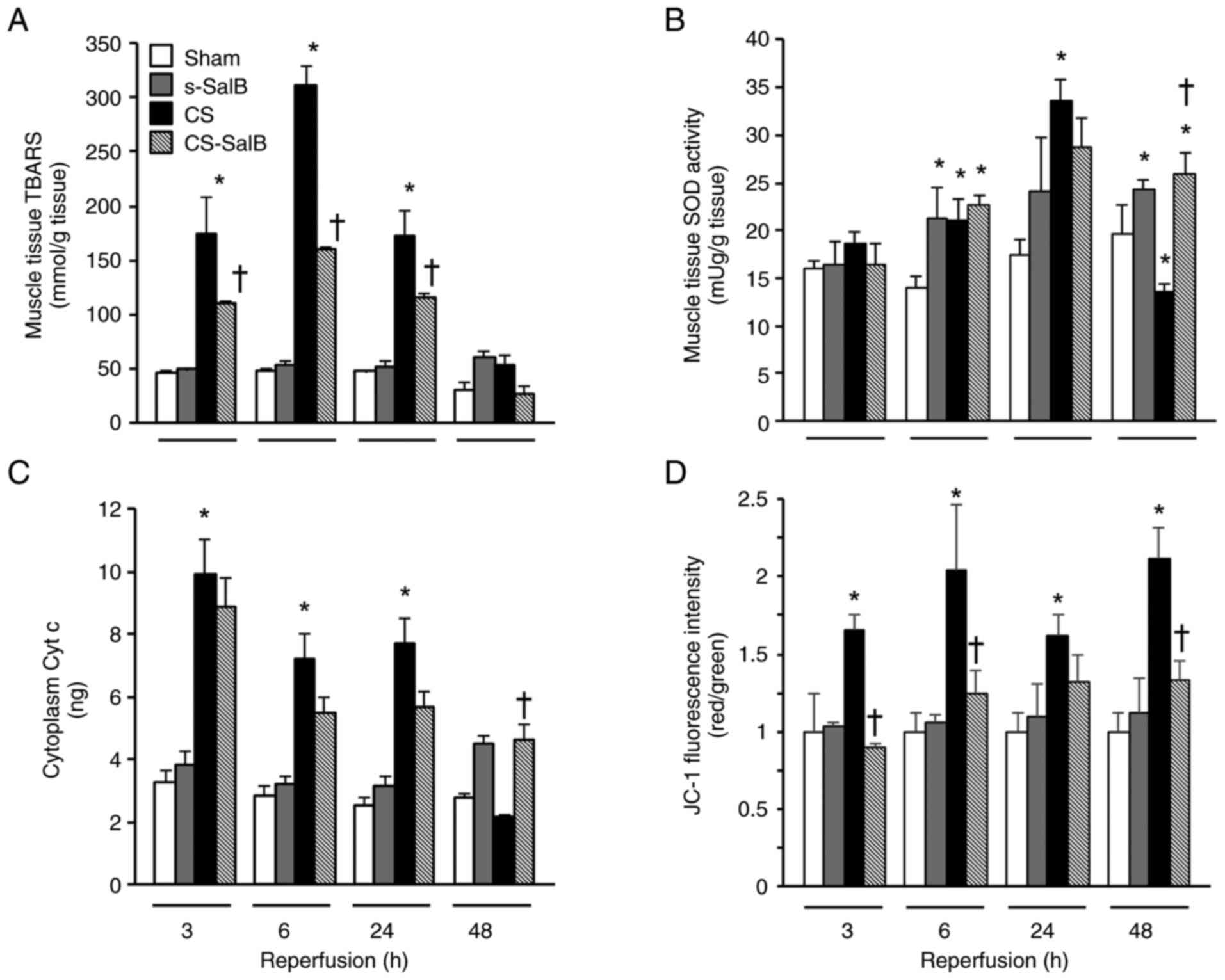 | Figure 6Effect of SalB on antioxidant action
and mitochondrial function in the CS rats. (A) Muscle TBARS levels,
(B) muscle SOD activity, (C) cytoplasm Cyt c content and (D) muscle
JC-1 fluorescence. Values are presented as the mean ± SEM (n=6).
*P<0.05 vs. sham group, †P<0.05 vs. CS
group (Tukey-Kramer test). CS, crush syndrome; SalB, salvianolic
acid B; s-SalB, sham with 20 mg/kg of SalB; CS-SalB, CS with 20
mg/kg of SalB; TBARS, thiobarbituric acid reactive substance; SOD,
superoxide dismutase; Cyt c, cytochrome. |
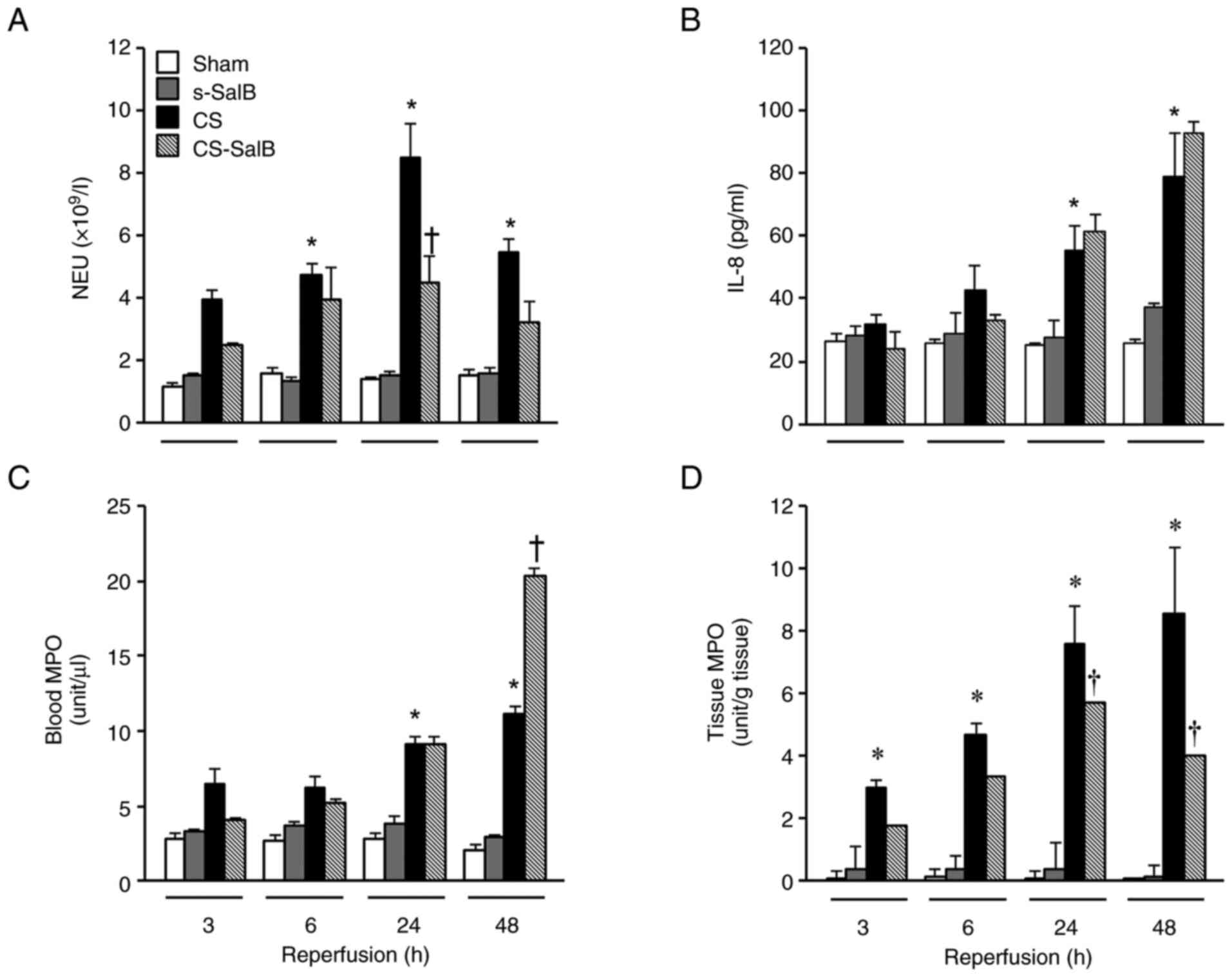
SalB induced antibacterial action via
NETosis process in the CS model
We focused in the NETosis process because it is not
only a frequent problem associated with infections such as sepsis
in patients with CS, but also related to leukocyte activation in
the CS rat model. NETosis was evaluated using NEU, IL-8, blood MPO
activity, and tissue MPO activity levels (Fig. 8). These parameters in the CS group
were significantly higher than those in the sham group during the
experimental period. In contrast, the NEU and tissue MPO activity
levels in the CS-SalB group were significantly lower than those in
the CS group, and the IL-8 level in the CS-SalB group was not
significantly different from that in the CS group. The
antibacterial effect (i.e., MIC) was not observed from 2,000 to 0.1
µg/ml (Table III). These
findings suggest that SalB exerts its antibacterial activity via
NETosis activation in CS.
 | Table IIIAntibacterial effects of SalB. |
Table III
Antibacterial effects of SalB.
| |
SalB
concentration, µg/ml |
|---|
| Test bacteria | 2000 | 1000 | 500 | 300 | 100 | 50 | 30 | 10 | 1 | 0.5 | 0.3 | 0.1 |
|---|
| Escherichia
coli | - | - | - | - | - | - | - | - | - | - | - | - |
| Staphylococcus
aureus | - | - | - | - | - | - | - | - | - | - | - | - |
| Bacillus
subtilis | - | - | - | - | - | - | - | - | - | - | - | - |
Discussion
We demonstrated that a simple therapeutic method of
SalB intravenous injection improves severe morbidity and mortality
of patients with CS. The 20 mg/kg SalB intravenous injection
presented the highest survival rate (Fig. 2), which was related to improve the
cause of death of CS rat model of kidney dysfunction and cardiac
failure. Moreover, sever systemic inflammation was related to
improve endothelial cell damage and oxidation stress. However, 50
mg/kg SalB intravenous injection did not improve the survival rate
because of metabolic and respiratory alkalosis (data not
shown).
Kidney injury leads to kidney failure, which is a
serious complication of CS that results from circulatory shock,
renal afferent arteriolar vasoconstriction (urinary concentration),
increased urinary myoglobin levels, or metabolic acidosis (urinary
acidity) (21-23).
All of these induce precipitation in distal convoluted tubules and
formation of tubular cast with the subsequent tubular obstruction.
Myoglobin accumulation has also been known to trigger oxidative
injury (24,25). We demonstrated that an improvement
in shock and acidosis by cardioprotective effect (31) for the risk of kidney disfunction
(Table I) and the protection for
kidney oxidative stress injury was the sustained activation of SOD
by SalB (Fig. 3 and Table I), hence indicated a high survival
rate. Interestingly, the SOD activity of CS model rats at 48 h was
significantly decreased compared with that at 24 h. The CS rats
showed persistently high levels of NGAL, KIM-1 and NAG,
demonstrated kidney tubule injury. These results were suggested
that the persistent injury induced excessive ROS production, and
these associated with consumption of SOD (Fig. 3A). Generally, fluid resuscitation
(kidney replacement therapy) requires an infusion preparation of 6
l/day or more for each patient with CS (3). SalB also eliminates the need to
manage such large amounts of medication. We suggest the use of SalB
to simplify the initial treatment of patients with CS who are at an
increased risk of a variety of symptoms.
Previously, we reported that survival following CS
is increased by the anti-inflammatory effects of treatment agents
that prevent systemic inflammatory response syndrome, making
systemic management difficult, even after the acute phase (24-27).
The cause of the inflammatory response in CS is not only traumatic
stress following crush injury but also reactive oxygen injury
through mitochondrial dysfunction associated with
ischemia-reperfusion injury. HMGB1 is lethally involved in
constitutive expression and vascular endothelial cell interactions
(36,37) and is a lethal inflammation mediator
(38); it is released into the
circulation from collapsed muscle cells after crush injury. In this
study, SalB significantly improved muscle damage (Table II) and suppressed the serum HMGB1
level (39). On the contrary, the
fibrinolytic system via PAI-1 generation was enhanced by
HMGB1(40), because an increase in
vWF and a decrease in PLT and FIB promoted platelet aggregation and
increased vascular endothelial interaction (Figs. 4 and 5). In addition, excessive nitric oxide
has also been implicated in the induction of endothelial damage.
According to Yang et al (41), SalB treatment reduced blood nitric
oxide level, and consequently prevented platelet aggregation. In
this study, these effected suggested, because serum NOx content and
iNOS expression were significantly enhanced toward normal range
(Fig. 4F). The inducible factor
contributing to inflammation was a mitochondrial function disorder.
First, SalB improves mitochondrial membrane potential and
mitochondrial outer membrane function (Fig. 6C and D). A direct radical scavenging ability of
SalB was only observed at a concentration of 563.4±88.3 μg/ml
(Fig. 7), which suggested that
these effects of SalB were not observed because a ~16,000-fold
higher concentration was needed in order to show a similar effect
as ascorbic acid (0.035±0.008 μg/ml). Generally, the primary
functions of mitochondria in cell apoptosis include the release of
activity factors of caspase, such as Cyt c, loss of the
mitochondrial transmembrane potential, and dysfunction of oxidative
phosphorylation of mitochondria (42). SalB affects mitochondrial function
improvement by an antiradical effect (43). In fact, the present study showed
that ROS is suppressed by improving mitochondrial membrane
potential and reducing cytoplasmic Cyt c (Fig. 6). Moreover, the anti-oxidative
stress effect was demonstrated by the low TBARS level and high SOD
activity induced by SalB treatment. It is suggested that SalB not
only suppresses HMGB1 expression and prevents the myocyte collapse
by suppressing vascular endothelial cell damage due to
anticoagulant action and anti-inflammatory action, but also
improves mitochondrial function. According to Huang et al
(44), SalB reported notably
increased SOD ability. SOD activity in CS rats was to exhausted
associated with the oxidative damage (45), therefore, SalB treated group were
higher than Sham and CS model rat.
Death due to sepsis associated with CS (i.e.,
infections) begins to occur 3 days after injury and most often
within 2 weeks (11). Therefore,
the suggested countermeasures against infection include the
prevention of nosocomial infection via antibiotic administration,
tetanus prevention, maintaining sanitary conditions in affected
hospitals (46). According to
Huttunen et al (22), SalB
induces antibacterial activity against Neisseria
meningitidis. We conducted MIC test using E. coli, S.
aureus, and B. subtilis as causative bacteria for
septicemia to simulate disaster sites. However, in our study,
0.1-2000 µg/ml SalB showed no antibacterial effect (Table III). Of note, our results showed
the potential antibacterial effect of SalB via NEU response.
Generally, the MPO level is correlated with the NEU count; however,
these levels were not matched. The heterogeneity of neutrophils is
characterized by features such as enhanced NET formation when the
state activated by DAMPs and other factors receives new stimuli
compared to the steady state. Crush injury causes neutrophils to
infiltrate the injury site via vascular endothelial cell
interaction by TNF-α, IL-1β and IL-8(47). Our CS rat model enhanced
neutrophil-mediated inflammatory responses associated with the
induction of these cytokines (Figs.
4 and 8B). SalB attenuated
vascular endothelial interaction of NEU by suppressing IL-1 and
TNF-α expression (Fig. 8C and
D) (48,49).
Nevertheless, SalB administration may have contributed to
neutrophil activation and IL-8 production rather than infiltration
by mildly inhibiting IL-1β and TNF-α production (50,51).
Generally, IL-8, also known as CXCL8, is a proinflammatory
chemokine that is produced parenchymal cells and the one produced
by monocytes and macrophages. The production of IL-8 is mainly
regulated by NF-κB transcription factors. IL-8 is a fundamental
chemokine to promote tissue infiltration by polymorphonuclear
leukocytes. And IL-8 determines in endothelial cells proangiogenic
effects that include the proliferation, survival, and migration of
vascular endothelial cells (52).
Our rat model has characteristics of neutrophil infiltration and
angiogenesis at the site of injury (27), suggested that IL-8 was involved as
a factor in the progression of these conditions. Furthermore, the
effect of NETosis was not observed in the SalB group (Figs. 4 and 8). Hence, the activated NEU released
MPO-containing NETs (i.e., NETosis), which have bactericidal action
and pathogen-capture properties via a high level of IL-8 (Fig. 8B) (53-55).
Based on these effects, SalB could be a first-choice antimicrobial
treatment for CS patients with or without acute kidney failure. A
limitation for this study is that the CS model rats were not in a
state that simulated infected patients with CS in disaster sites,
and thus, further investigations are needed to elucidate the
antibacterial efficacy of SalB and the antibacterial function via
NET process.
In conclusion, SalB administration to the CS rat
model led to a substantial improvement in survival following CS by
decreasing kidney and cardiac dysfunction, inflammation, and
endothelial dysfunction by improving the mitochondrial function and
by antibacterial effects via NETs.
Acknowledgements
The authors would like to thank Dr Hiroyuki Uchida
and Dr Junta Ito for valuable suggestions, and Ms. Shion Terada,
Ms. Shiho Morita and Ms. Chikako Murata for technical assistance
(all Faculty of Pharmaceutical Science, Josai University, Sakado,
Japan).
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IM led the project and designed and performed most
of the experiments. TS and YMu assisted with the survival and
biochemical marker analyses. YMi, JK, YI and IK conceived the
study, participated in its design and coordination, and helped
draft the manuscript. IM, TS and YMu confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Josai University
(approval no. JU18030; Sakado, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith J and Greaves I: Crush injury and
crush syndrome: A review. J Trauma. 54 (Suppl 5):S226–S230.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bosch X, Poch E and Grau JM:
Rhabdomyolysis and acute kidney injury. N Engl J Med. 361:62–72.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sever MS and Vanholder R: RDRTF of ISN
Work Group on Recommendations for the Management of Crush Victims
in Mass Disasters. Recommendation for the management of crush
victims in mass disasters. Nephrol Dial Transplant. 27 (Suppl
1):i1–i67. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Obialo CI, Okonofua EC, Nzerue MC, Tayade
AS and Riley LJ: Role of hypoalbuminemia and hypocholesterolemia as
copredictors of mortality in acute renal failure. Kidney Int.
56:1058–1063. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bullock ML, Umen AJ, Finkelstein M and
Keane WF: The assessment of risk factors in 462 patients with acute
renal failure. Am J Kidney Dis. 5:97–103. 1985.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sever MS, Erek E, Vanholder R, Koc M,
Yavuz M, Aysuna N, Ergin H, Ataman R, Yenicesu M, Canbakan B, et
al: Lessons learned from the catastrophic Marmara earthquake:
Factors influencing the final outcome of renal victims. Clin
Nephrol. 61:413–421. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Chertow GM, Christiansen CL, Cleary PD,
Munro C and Lazarus JM: Prognostic stratification in critically ill
patients with acute renal failure requiring dialysis. Arch Intern
Med. 155:1505–1511. 1995.PubMed/NCBI
|
|
8
|
Gonzalez D: Crush syndrome. Crit Care Med.
33 (Suppl 1):S34–S41. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tanaka H, Oda J, Iwai A, Kuwagata Y,
Matsuoka T, Takaoka M, Kishi M, Morimoto F, Ishikawa K, Mizushima
Y, et al: Morbidity and mortality of hospitalized patients after
the 1995 Hanshin-Awaji earthquake. Am J Emerg Med. 17:186–191.
1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vanholder R, Sever MS, Smet M, Erek E and
Lameire N: Intervention of the renal disaster relief task force in
the 1999 Marmara, Turkey earthquake. Kidney Int. 59:783–791.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang L, Fu P, Wang L, Cai G, Zhang L,
Chen D, Guo D, Sun X, Chen F, Bi W, et al: The clinical features
and outcome of crush patients with acute kidney injury after the
Wenchuan earthquake: Differences between elderly and younger
adults. Injury. 43:1470–1475. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ashkenazi I, Isakovich B, Kluger Y, Alfici
R, Kessel B and Better OS: Prehospital management of earthquake
casualties buried under rubble. Prehosp Disaster Med. 20:122–133.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Erek E, Sever MS, Serdengeçti K, Vanholder
R, Akoğlu E, Yavuz M, Ergin H, Tekçe M, Duman N and Lameire N:
Turkish Study Group of Disaster. An overview of morbidity and
mortality in patients with acute renal failure due to crush
syndrome: The Marmara earthquake experience. Nephrol Dial
Transplant. 17:33–40. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xue L, Wu Z, Ji XP, Gao XQ and Guo YH:
Effect and mechanism of salvianolic acid B on the myocardial
ischemia-reperfusion injury in rats. Asian Pac J Trop Med.
7:280–284. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kong R, Gao Y, Sun B, Chen H, Wang G, Wang
X, Zhu H, Pan S, Xue D and Jiang H: The strategy of combined
ischemia preconditioning and salvianolic acid-B pretreatment to
prevent hepatic ischemia-reperfusion injury in rats. Dig Dis Sci.
54:2568–2576. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang M, Feng W, Zhang Y, Zhong J and Zhang
J: Salvianolic acid B improves motor function after cerebral
ischemia in rats. Behav Pharmacol. 17:493–498. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pan RH, Xie FY, Chen HM, Xu LZ, Wu XC, Xu
LL and Yao G: Salvianolic acid B reverses the
epithelial-to-mesenchymal transition of HK-2 cells that is induced
by transforming growth factor-β. Arch Pharma Res. 34:477–483.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Han JY, Fan JY, Horie Y, Miura S, Cui DH,
Ishii H, Hibi T, Tsuneki H and Kimura I: Ameliorating effects of
compounds derived from Salvia miltiorrhiza root extract on
microcirculatory disturbance and target organ injury by ischemia
and reperfusion. Pharmacol Ther. 117:280–295. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Watzke A, O'Malley SJ, Bergman RG and
Ellman JA: Reassignment of the configuration of salvianolic acid B
and establishment of its identity with lithospermic acid B. J Nat
Prod. 69:1231–1233. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu Q, Shi X, Tang L, Xu W, Jiang S, Ding
W, Feng Q, Chu H, Ma Y, Li Y, et al: Salvianolic acid B attenuates
experimental pulmonary inflammation by protecting endothelial cells
against oxidative stress injury. Eur J Pharmacol. 840:9–19.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Katary MA, Abdelsayed R, Alhashim A,
Abdelhasib M and Elmarakby AA: Salvianolic acid B slows the
progression of breast cancer cell growth via enhancement of
apoptosis and reduction of oxidative stress, inflammation, and
angiogenesis. Int J Mol Sci. 20(5653)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huttunen S, Toivanen M, Liu C and
Tikkanen-Kaukanen C: . Novel anti-infective potential of
salvianolic acid B against human serious pathogen Neisseria
meningitidis. BMC Res Notes. 9(25)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xinyue W, Wang L, Tang L, Wang L, Cao S,
Wu Q, Zhang Z and Li L: Salvianolic acid B alters the gut
microbiota and mitigates colitis severity and associated
inflammation. J Funct Foods. 46:312–319. 2018.
|
|
24
|
Murata I, Ooi K, Sasaki H, Kimura S,
Ohtake K, Ueda H, Uchida H, Yasui N, Tsutsui Y, Yoshizawa N, et al:
Characterization of systemic and histologic injury after crush
syndrome and intervals of reperfusion in a small animal model. J
Trauma. 70:1453–1463. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Murata I, Ooi K, Shoji S, Motohashi Y, Kan
M, Ohtake K, Kimura S, Ueda H, Nakano N, Sonoda K, et al: Acute
lethal crush-injured rats can be successfully rescued by a single
injection of high-dose dexamethasone through a pathway involving
PI3K-Akt-eNOS signaling. J Trauma Acute Care Surg. 75:241–249.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Murata I, Miyake Y, Takahashi N, Suzuki R,
Fujiwara T, Sato Y, Inoue Y, Kobayashi J and Kanamoto I: Low-dose
sodium nitrite fluid resuscitation prevents lethality from crush
syndrome by improving nitric oxide consumption and preventing
myoglobin cytotoxicity in kidney in a rat model. Shock. 48:112–118.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Murata I, Abe Y, Yaginuma Y, Yodo K,
Kamakari Y, Miyazaki Y, Baba D, Shinoda Y, Iwasaki T, Takahashi K,
et al: Astragaloside-IV prevents acute kidney injury and
inflammation by normalizing muscular mitochondrial function
associated with a nitric oxide protective mechanism in crush
syndrome rats. Ann Intensive Care. 7(90)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Brinkmann V, Reichard U, Goosmann C,
Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y and Zychlinsky A:
Neutrophil extracellular traps kill bacteria. Science.
303:1532–1535. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fuchs TA, Bhandari AA and Wagner DD:
Histones induce rapid and profound thrombocytopenia in mice. Blood.
118:3708–3714. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin C, Liu Z, Lu Y, Yao Y, Zhang Y, Ma Z,
Kuai M, Sun X, Sun S, Jing Y, et al: Cardioprotective effect of
Salvianolic acid B on acute myocardial infarction by promoting
autophagy and neovascularization and inhibiting apoptosis. J Pharm
Pharmacol. 68:941–952. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chang PN, Mao JC, Huang SH, Ning L, Wang
ZJ, On T, Duan W and Zhu YZ: Analysis of cardioprotective effects
using purified Salvia miltiorrhiza extract on isolated rat
hearts. J Pharmacol Sci. 101:245–249. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li Y, Zhang X, Cui L, Chen R, Zhang Y,
Zhang C, Zhu X, He T, Shen Z, Dong L, et al: Salvianolic acids
enhance cerebral angiogenesis and neurological recovery by
activating JAK2/STAT3 signaling pathway after ischemic stroke in
mice. J Neurochem. 143:87–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Invest. 74:1156–1164. 1984.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sharma OP and Bhat TK: DPPH antioxidant
assay revisited. Food Chem. 113:1202–1205. 2009.
|
|
35
|
Cockerill FR, Hindler JA, Wikler MA, Patel
JB, Alder J, Powell M, Dudley MN, Swenson JM, Eliopoulos GM,
Thomson RB, et al: Methods For Dilution Antimicrobial
Susceptibility Tests For Bacteria That Grow Aerobically; Approved
Standard. CLSI M7-A9. Vol 32. 9th edition. Clinical Laboratory
Standards Institute, Wayne, PA, 2012.
|
|
36
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et
al: HMG-1 as a late mediator of endotoxin lethality in mice.
Science. 285:248–251. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Murata I, Imanari M, Komiya M, Kobayashi
J, Inoue Y and Knamoto I: Icing treatment in rats with crush
syndrome can improve survival through reduction of potassium
concentration and mitochondrial function disorder effect. Exp Ther
Med. 19:777–785. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fiuza C, Bustin M, Talwar S, Tropea M,
Gerstenberger E, Shelhamer JH and Suffredini AF:
Inflammation-promoting activity of HMGB1 on human microvascular
endothelial cells. Blood. 101:2652–2660. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang Q, Wang S, Xie Y, Wang J, Li H, Zhou
X and Liu W: Effect of salvianolic acid B and paeonol on blood
lipid metabolism and hemorrheology in myocardial ischemia rabbits
induced by pituitruin. Int J Mol Sci. 11:3696–3704. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ren M, Wang YM, Zhao J, Zhao J, Zhao ZM,
Zhang TF, He J, Ren SP and Peng SQ: Metallothioneins attenuate
paraquat-induced acute lung injury in mice through the mechanisms
of anti-oxidation and anti-apoptosis. Food Chem Toxicol.
73:140–147. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu Y, Hu Y, E Q, Zuo J, Yang L and Liu W:
Salvianolic acid B inhibits mitochondrial dysfunction by
up-regulating mortalin. Sci Rep. 7(43097)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huang M, Wang P, Xu S, Xu W, Xu W, Chu K
and Lu J: Biological activities of salvianolic acid B from
Salvia miltiorrhiza on type 2 diabetes induced by high-fat
diet and streptozotocin. Pharm Biol. 53:1058–1065. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhao DH, Wu YJ, Liu ST and Liu RY:
Salvianolic acid B attenuates lipopolysaccharide-induced acute lung
injury in rats through inhibition of apoptosis, oxidative stress
and inflammation. Exp Ther Med. 14:759–764. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li W, Qian J, Liu X, Zhang Q, Wang L, Chen
D and Lin Z: Management of severe crush injury in a front-line tent
ICU after 2008 Wenchuan earthquake in China: An experience with 32
cases. Crit Care. 13(R178)2009.PubMed/NCBI View
Article : Google Scholar
|
|
47
|
Hong CH: Current understanding in
neutrophil differentiation and heterogeneity. Immune Netw.
17:298–306. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zarbock A, Ley K, McEver RP and Hidalgo A:
Leukocyte ligands for endothelial selectins: Specialized
glycoconjugates that mediate rolling and signaling under flow.
Blood. 118:6743–6751. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lee YW, Kim DH, Jeon SJ, Park SJ, Kim JM,
Jung JM, Lee HE, Bae SG, Oh HK, Son KH and Ryu JH: Neuroprotective
effects of salvianolic acid B on an Aβ25-35 peptide-induced mouse
model of Alzheimer's disease. Eur J Pharmacol. 704:70–77.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Baggiolini M, Dewald B and Moser B:
Interleukin-8 and related chemotactic cytokines-CXC and CC
chemokines. Adv Immunol. 55:97–197. 1994.PubMed/NCBI
|
|
51
|
Mukaida N, Hishinuma A, Zachariae CO,
Oppenheim JJ and Matsushima K: Regulation of human interleukin 8
gene expression and binding of several other members of the
intercrine family to receptors for interleukin-8. Adv Exp Med Biol.
305:31–38. 1991.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gonzalez-Aparicio M and Alfaro C:
Influence of interleukin-8 and neutrophil extracellular trap (NET)
formation in the tumor microenvironment: Is there a pathogenic
role? J Immunol Res. 2019(6252138)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Parker H, Albrett AM, Kettle AJ and
Winterbourn CC: Myeloperoxidase associated with neutrophil
extracellular traps is active and mediates bacterial killing in the
presence of hydrogen peroxide. J Leukoc Biol. 91:369–376.
2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Papayannopoulos V and Zychlinsky A: NETs:
A new strategy for using old weapons. Trends Immunol. 30:513–521.
2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Fuchs TA, Abed U, Goosmann C, Hurwitz R,
Schulze I, Wahn V, Weinrauch Y, Brinkmann V and Zychlinsky A: Novel
cell death program leads to neutrophil extracellular traps. J Cell
Biol. 176:231–241. 2007.PubMed/NCBI View Article : Google Scholar
|