Introduction
Extended culture leading to embryo transfer at the
blastocyst stage is considered a major advance in in vitro
fertilization (IVF) as it has been shown to result in higher live
birth rates in comparison to cleavage-stage embryo transfer
(1,2). Transferring blastocysts is therefore
perceived as the best option for elective single-embryo transfer
(eSET), by reducing the risk of multiple pregnancies (3,4)
without compromising live birth rates. With advances in
cryopreservation techniques, improved implantation rates have been
achieved, and pregnancy rates after frozen embryo transfer (FET)
are at least comparable with those after the transfer of fresh IVF
embryos (5). How to select
single-blastocyst transfer for FET to reduce the time to successful
pregnancy is very important. Thus, it is crucial to determine
whether blastocyst developmental stage [day 5 (D5)/day 6 (D6)] or
blastocyst quality is more important for a successful
pregnancy.
The comparison of pregnancy outcomes between D5 and
D6 blastocysts remains controversial. Some reports have concluded
that the blastocyst development time is crucial and suggest that D5
is more superior than D6 in terms of implantation rate and live
birth rate (6,7). However, other reports have shown that
the clinical outcomes between D5 and D6 cryopreserved blastocyst
transfers did not significantly differ (8,9),
while some studies recommend that blastocyst quality is an
important factor that affects the pregnancy outcomes of the
vitrified-warmed cycles (10,11).
Previous relevant studies only performed simple
statistical analyses of the clinical outcomes of vitrified-warmed
cycles (6-11).
However, all frozen D5/D6 blastocysts came from IVF and
intracytoplasmic sperm injection (ICSI) fresh cycles. The present
study was the first to consider the D5/D6 high-quality blastocyst
rate of fresh cycle without embryo transfer and the ratio if only
D6 have blastocysts. The present study also aimed to analyze the
pregnancy outcomes of D5/D6 blastocysts with respect to the
blastocyst quality in programmed single vitrified-warmed blastocyst
transfer (SVBT).
Patients and methods
Patients
This was a retrospective study carried out at The
Center for Reproductive Medicine and Infertility, The Fourth
Hospital of Shijiazhuang, China from March 2017 to May 2020. In
total, 1,560 (IVF, 1,100 and ICSI 460) all blastocyst frozen cycles
were analyzed and 1,161 SVBT cycles (D5 975 and D6 186) were
included in the study. The criteria for allocating patients to IVF
and ICSI were male semen factors. The women were given IVF protocol
in the first assisted reproductive technology (ART) treatment cycle
unless accompanied by severe male-factor infertility. Otherwise
ICSI treatment was performed. Patients included in the analysis
were <35 years at the oocyte collection in their first fresh
cycle without fresh embryo transfer, and were undergoing their
first autologous FET cycle. The Fourth Hospital of Shijiazhuang
Ethics Committee approved (approval no. 20170063; approval date,
January 5, 2017) this study.
Blastocyst culture and scoring
Cumulus-oocyte-complexes (COC) were isolated from
follicular fluid, rinsed in G-IVF™ medium (VitroLife,
Sweden) transferred to 0.5 ml G-IVF™ medium and cultured
in an incubator under 5% O2, 6% CO2, and 89%
N2. Sperm was used for either routine IVF insemination
or ICSI procedure using a standard method as described by Jiang
et al (12). Insemination
were performed 38-40 h after trigger. Fertilization was identified
by the presence of two pronuclei approximately 16-19 h after
insemination or microinjection. On day 3, the embryos were
transferred into G-2 culture medium in group culture (Vitrolife).
In the morning of D5 or D6, blastocysts were scored by two
experienced embryologist using the system of Gardner and
Schoolcraft (13). On day 5,
embryos at the morula or early blastocyst stage were left in
culture for 1 day more. For blastocysts graded as 3-6 (i.e., full
blastocysts onward), the development of the inner cell mass (ICM)
was assessed as follows: A, tightly packed, many cells; B, loosely
grouped, several cells; or C, very few cells. The trophectoderm
(TE) was assessed as follows: A, many cells forming a cohesive
epithelium; B, few cells forming a loose epithelium; or C, very few
large cells. Blastocysts with a score ≥3, including those with
grades BC, CB were selected on day 5 and day 6 for vitrification.
High-quality blastocysts (HBs) were defined as blastocysts ≥3 BB
(AA, AB, BA, BB). Low-quality blastocysts (LBs) were defined as
vitrified blastocysts, excluding those HBs.
Blastocyst vitrification and warming
procedures
The procedure was always performed using one
blastocyst for each straw. An artificial shrinkage (AS), using a
laser pulse was performed before vitrification. The blastocyst was
then moved at room temperature (22-25˚C) to Kitazato (Japan)
equilibration solution (ES). After 6-8 min, the blastocyst was
quickly washed in vitrification solution (VS) for 45-60 sec and
transferred onto the straw (Kitazato Japan) using a micropipette
and immersed vertically into liquid nitrogen.
A Kitazato Thaw Kit Kitazato) was used for warming.
The carrier containing the embryo was removed from the straw and
placed quickly into the dish containing the thawing medium (thawing
solution) preheated at 37˚C. The blastocysts immediately fell from
the device and could be easily identified in the medium. After 1
min, the blastocysts were transferred to the DS medium (dilution
solution) for 3 min at room temperature (22-25˚C). In the last two
step, the blastocysts were placed for 5 min, in the WS1 medium and
WS2 (washing solution). The embryo was then returned to G-2 medium
for culture until transfer. At this stage, an assessment was
performed on an inverted microscope to establish if the embryo
survived based on morphological integrity of the ICM and
trophectoderm. After 1 or 2 h of culture, the embryo was reassessed
again and often the re-expansion of the blastocoel was reported;
this indicated that the embryo physiologically survived the warming
procedure. Embryo transfer was normally performed within 2 or 3 h.
All programmed warmed cycles, both at D5 and D6, were transferred
in D5 endometrium.
If the patient had both D5 and D6 blastocysts, the
best quality embryo was warmed first. If blastocyst quality was the
same, the D5 blastocyst was given priority to transfer. If the
embryo did not survive, another embryo was warmed if the patient
had any in storage, otherwise the transfer was canceled. Some
patient blastocysts were not thawed, did not survived, or two
blastocysts transferred were not included in this study.
Endometrial programming and
observational indicators
All vitrified-warmed cycles of endometrium
preparation were natural cycle (NC) or artificial cycle [hormone
replacement therapy (HRT)] based on the implantation programs. NC
was applicable for patients with a regular menstrual cycle.
Follicular development was monitored using B ultrasound on days
8-10 of menstruation. The follicular and endometrial development
conditions were assessed and combined with the estradiol
(E2) and luteinizing hormone (LH) levels to confirm the
ovulation time. Embryo transfer was performed on D5 of ovulation.
HRT was applicable for patients with an irregular menstrual cycle,
ovulation disorder, or poor endometrial and follicular development
in NC. Starting from days 2-3 of menstruation, 2-6 mg/day of
estradiol valerate (Progynova, Bayer) was administered, and the
endometrial thickness and serum E2 levels were monitored
using B ultrasound. When the endometrial thickness was at least 8
mm, progesterone 60 mg/day was additionally administered. Embryo
transfer was performed on day 6 of progesterone injection. All
warmed blastocysts, both vitrified on D5 or D6 were replaced in the
D5 endometrium. All embryo transfers were performed using
transabdominal ultrasound guidance.
Observation of the gestational sac and fetal heart
by B ultrasound at 35 days after implantation was diagnosed as
clinical pregnancy. The implantation rate was defined as the ratio
between the number of gestational sacs and fetal heart observed
under B ultrasound and the number of transferred blastocysts.
Implantation rates, pregnancy rates, and twinning of D5/D6 SVBT
were analyzed.
Statistical analyses
Statistical analyses were performed using SPSS 19.0
statistical software (SPSS Inc.). The data are presented as the
mean ± standard deviation (SD). The mean values of two groups were
compared using the independent samples t-test. Percentages were
compared using the χ2 test and P<0.05 was considered
statistically significant.
Results
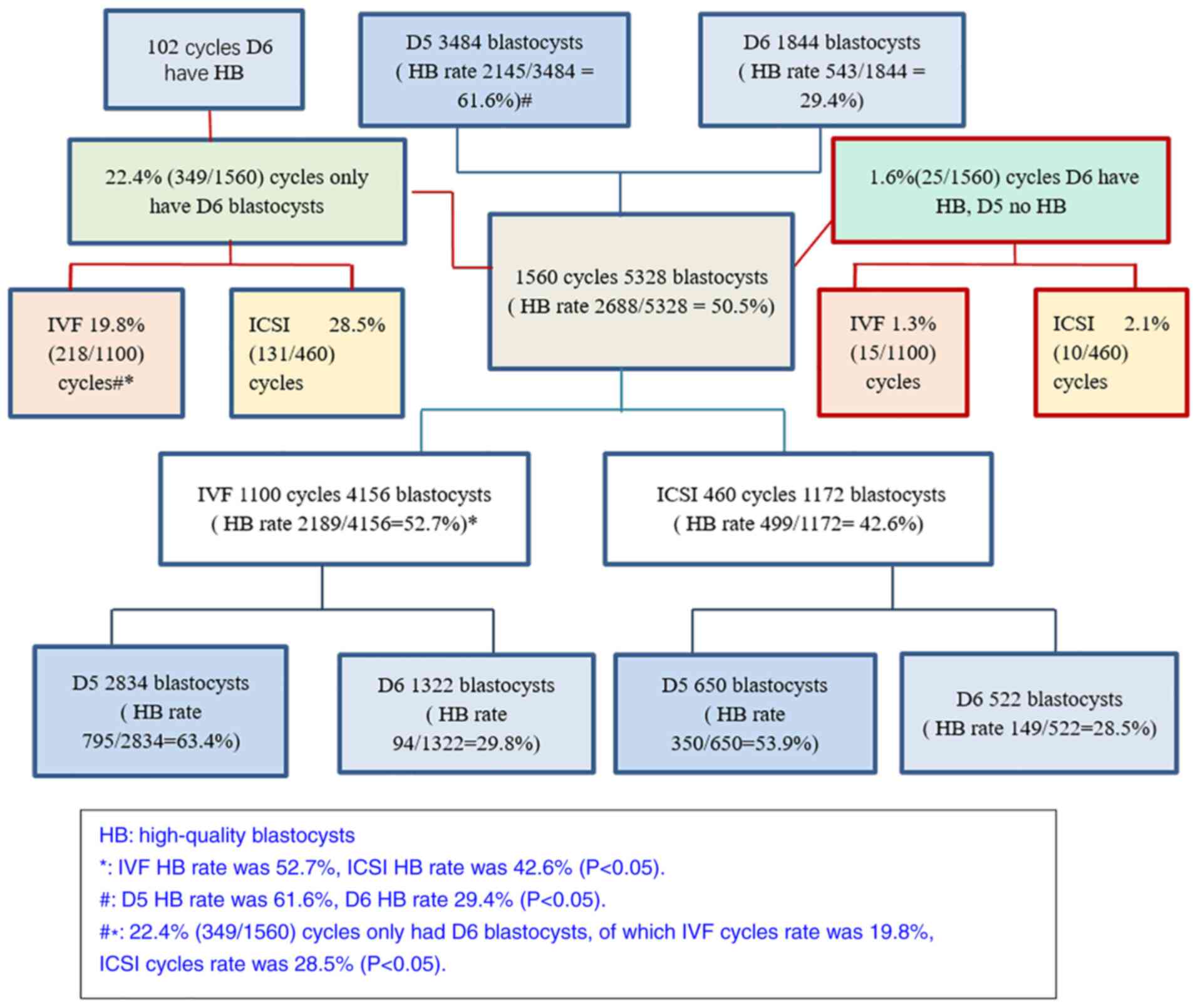
D5/D6 HB blastocyst rate in 1,560
fresh IVF/ICSI cycles
The total HB rate was 50.5% (2,688/5,328) for which
IVF was higher than ICSI (52.7% vs. 42.6%; P<0.05). The D5 HB
rate was much higher than the D6 HB rate (61.6% vs. 29.4%;
P<0.05). There were 22.4% (349/1560) cycles of only cultured D6
blastocysts, in which IVF was lower than ICSI (19.8% vs. 28.5%;
P<0.05) (Fig. 1).
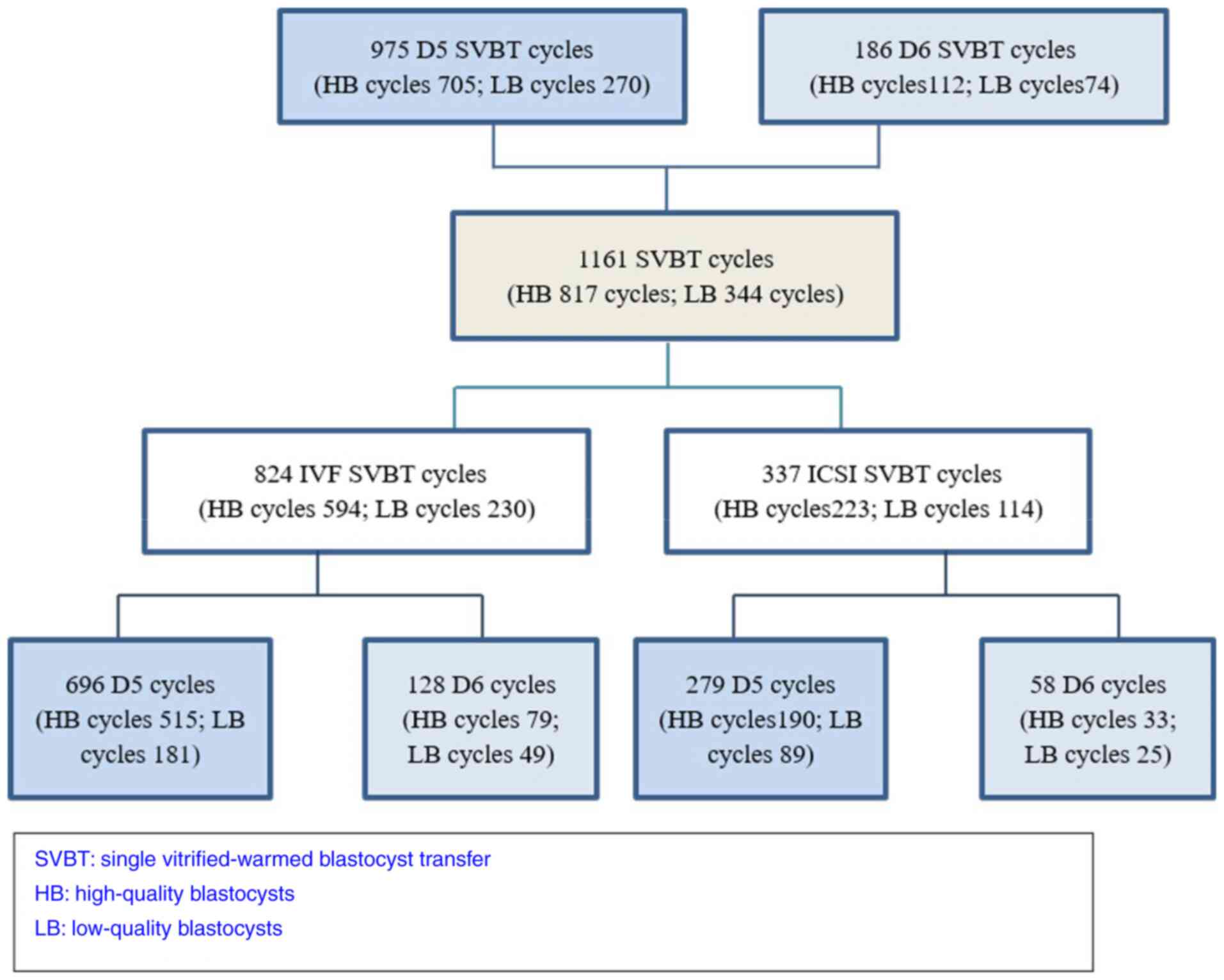
Clinical pregnancy rate of HB/LB SVBT
on D5 and D6 in IVF/ICSI
In total, 1,161 SVBT cycles (D5 975 and D6 186) were
analyzed (Fig. 2). The mean age of
the women in the D5 group and D6 group was not different (31.8
years vs. 31.9 years). The clinical pregnancy rate and implantation
rate in the D5 group were significantly higher than these rates in
the D6 group (57.4 vs. 46.2%, 58.9 vs. 47.3%; P<0.05). However,
the clinical pregnancy rate and implantation rate of D5 HB were not
significantly different from those of D6 HB (60 vs. 54.5%, 62 vs.
56.3%; P>0.05). The clinical pregnancy rate and implantation
rate of D5 LB were higher than those of D6 LB (49.6 vs. 33.8%, 50.7
vs. 33.8%; P<0.05) (Table I).
The clinical pregnancy rate and implantation rate were similar in
IVF and ICSI groups (56.3 vs. 54.0%, 57.0 vs. 56.1%; P>0.05)
(Table II). The multiple
pregnancy rate was similar in the D5/D6 groups, and ICSI was higher
than IVF, but not statistically significant (1.44 vs. 1.08%, 2.08
vs. 1.09%; P>0.05). The D5 male rate was higher than the D6 male
rate (54.0 vs. 48.4%), but was not statistically significant
(Table I). The male rate was
similar in the IVF/ICSI groups (52.2 vs. 56.4%; P>0.05)
(Table II).
 | Table IHB/LB pregnancy data between the day 5
(D5) and day 6 (D6) groups. |
Table I
HB/LB pregnancy data between the day 5
(D5) and day 6 (D6) groups.
| Variables | Groups | Day 5 | Day 6 | χ2 | P-value |
|---|
| No. of patients | | 975 | 186 | | |
| No. of high-quality
blastocyst (HBs) | | 705 | 112 | | |
| No. of low-quality
blastocysts (LBs) | | 270 | 74 | | |
| Clinical pregnancy
rate (%) | Total | 57.4 (560/975) | 46.2
(86/186)a | 7.94 | 0.0045 |
| | HBs | 60 (426/705) | 54.5 (61/112) | 1.43 | 0.2320 |
| | LBs | 49.6 (134/270) | 33.8
(25/74)a | 5.87 | 0.0150 |
| Implantation rate
(%) | Total | 58.9 (574/975) | 47.3
(88/186)a | 8.52 | 0.0025 |
| | HBs | 62.0 (437/705) | 56.3 (63/112) | 1.34 | 0.2470 |
| | LBs | 50.7(137/270) | 33.8
(25/74)a | 6.70 | 0.0100 |
| Multiple pregnancy
rate (%) | Total | 1.44 (14/975) | 1.08 (2/186) | 0.149 | 0.7150 |
| | HBs | 1.56 (11/705) | 1.78 (2/112) | - | 0.6960 |
| | LBs | 1.11 (3/270) | (0/74) | - | - |
| Male rate (%) | Total | 54.0 (233/431) | 48.4 (31/64) | 0.708 | 0.3500 |
| | HBs | 53.9 (181/336) | 50.0 (24/48) | 0.253 | 0.6150 |
| | LBs | 54.7 (52/95) | 43.8 (7/16) | 0.664 | 0.4150 |
 | Table IIIVF/ICSI pregnancy data between the
day 5 (D5) and day 6 (D6) groups of SVBT. |
Table II
IVF/ICSI pregnancy data between the
day 5 (D5) and day 6 (D6) groups of SVBT.
| Variables | Groups | IVF | ICSI | χ2 | P-value |
|---|
| No. of patients | | 824 | 337 | | |
| No. of D5
blastocysts | | 696 | 279 | | |
| No. of D6
blastocysts | | 128 | 58 | | |
| Clinical pregnancy
rate (%) | Total | 56.3 (464/824) | 54.0 (182/337) | 0.515 | 0.470 |
| | D5 | 58.0 (404/696) | 55.9 (156/279) | 0.370 | 0.543 |
| | D6 | 46.8 (60/128) | 44.8 (26/58) | 0.067 | 0.795 |
| Implantation rate
(%) | Total | 57.0 (473/824) | 56.1 (189/337) | 0.035 | 0.860 |
| | D5 | 59.2 (412/696) | 58.1 (162/279) | 0.105 | 0.746 |
| | D6 | 47.7 (61/128) | 46.6 (27/58) | 0.020 | 0.889 |
| Multiple pregnancy
rate (%) | Total | 1.09 (9/824) | 2.08 (7/337) | 1.71 | 0.175 |
| | D5 | 1.15 (8/696) | 2.15 (6/279) | 0.792 | 0.374 |
| | D6 | 0.78 (1/128) | 1.72 (1/58) | - | 0.528 |
| Male rate (%) | Total | 52.2 (189/362) | 56.4 (75/133) | 0.68 | 0.430 |
| | D5 | 52.8 (167/316) | 57.4 (66/115) | 0.701 | 0.403 |
| | D6 | 47.8 (22/46) | 50.0 (9/18) | 0.024 | 0.876 |
Discussion
Extending embryo culture to the blastocyst stage has
become a routine in many in vitro fertilization (IVF)
laboratories. The most widely used grading system is that
originally proposed by Gardner and Schoolcraft (13). Although the system does not cover
all aspects of blastocyst morphology it has been very effective in
classifying the appearance and compactness of the inner cell mass
(ICM), the cohesiveness and number of trophectoderm (TE) and degree
of expansion of the blastocoel cavity.
Whether there are differences in the pregnancy
outcomes of blastocysts cryopreserved during different
developmental stages remains under debate because the results among
studies are inconsistent. A meta-analysis of clinical outcomes
showed that in day 5 (D5) vs. day 6 (D6) blastocyst transfers,
clinical pregnancy rate and live birth rates were significantly
higher following D5 compared to D6 blastocyst transfers (14). Therefore, ART practitioners should
preferably transfer D5 rather than D6 blastocysts in both fresh and
frozen cycles (14). Single embryo
transfers of D6 vitrified/warmed blastocysts were found to result
in a lower implantation and clinical pregnancy rate compared to D5
embryos (15). The effect of
delayed blastulation may be responsible for implantation failures
and negatively affects outcomes (4). However, in their studies, Behr et
al (16) and El-Toukhy et
al (9) did not observe a
significant difference in the implantation and pregnancy rates
between D5 and D6 blastocysts.
In addition, blastocyst grade plays an important
role in pregnancy outcomes. Blastocysts with trophectoderm grades
A, B, and C were found to have euploidy rates of 71.43, 60.00 and
19.67%, respectively (P<0.05) (17). Yang et al (11) reported that high-quality D6
blastocysts in vitrified-warmed cycles had similar developmental
potential and pregnancy outcomes compared to those of high-quality
D5 blastocysts, while Irani et al (18) observed that embryos reaching
good-quality blastocysts on day 5 yielded significantly higher
implantation rate (77.7% vs. 58.7%) compared with those reaching
similar quality blastocysts on day 6. Similarly, D5 average-quality
embryos conveyed a significantly higher implantation rate compared
with D6 embryos of the same quality (64.4% vs. 53.4%) (18).
In previous research, patients who underwent single
vitrified-warmed blastocyst transfer (SVBT) cycles were able to
obtain optimal pregnancy outcomes, especially in the <35 year
age group (19), while those older
than 35 years may have a higher probability of pregnancy failure
due to chromosomal abnormalities, age or other factors (20). This is why our research
selected patients age <35 years and without biopsy. The multiple
pregnancy rate was 1.08% (0.98% for D5 vs. 1.3% for D6) (15), which was similar to our
results.
While the previous studies focused on warming embryo
of D5/D6 frozen embryo transfer (FET) cycles, the present study was
the first to consider the high-quality blastocyst (HB) rate in
fresh in vitro fertilization/intracytoplasmic sperm
injection (IVF/ICSI) cycles, and the rate of D6 had blastocysts
whereas D5 had none. The present results showed that the D5 HB rate
was twice higher than the D6 HB rate in fresh cycles (61.6% vs.
29.4%; P<0.05), and this was probably the reason why the
clinical pregnancy rate and implantation rate in the D5 group were
significantly higher than these rates in the D6 group (57.4% vs.
46.2%, 58.9% vs. 47.3%; P<0.05).
From this research, we know that in IVF, the
cultured blastocysts and HBs per cycle were more than these
parameters in ICSI, and cultured blastocysts in IVF were earlier
than ICSI. We concluded that the fertilization method directly
influenced HB and blastocyst development rates. Therefore, the
IVF/ICSI ratio needs to be considered when analyzing D5/D6 SVBT. In
the present SVBT study, D5 (IVF 71.4% and ICSI 28.6%) cycles and D6
(IVF 68.8% and ICSI 31.2%) IVF/ICSI ratios were not significantly
difference. Speyer et al (21) also showed that IVF-derived embryos
developed to the blastocyst stage at a significantly faster rate
than ICSI-derived embryos. A previous study using time-lapse showed
the different developmental time between IVF and ICSI embryos.
During the early cleavage stages there was a statistically
significant delay (+1.5 to +1.1 h) among the IVF-fertilized
embryos, and at the blastocyst stage IVF-fertilized embryos showed
faster development (22). IVF/ICSI
sibling oocyte split design demonstrated a higher-quality
blastulation rate in the IVF group compared to the ICSI group when
calculated per 2PN, but not per oocyte allocated to each
insemination procedure (23).
Most patients prefer to use D5 HB in their first FET
cycle, and finally choose D6 blastocysts when none of the thawed D5
blastocysts have resulted in successful pregnancy. Therefore, the
inclusion criteria were patients who were in their first fresh
cycle without fresh embryo transfer and who were undergoing their
first SVBT cycle.
In conclusion, following control of patient age,
transfer frequency, and endometrium on day 5, it is not the
development stage (D5/D6) but the transfer blastocyst quality that
plays an important role in achieving the optimal pregnancy
outcomes. The D5 HB rate was found to be 2-times higher than D6,
and the IVF HB rate was also higher than ICSI, which may be the
reason for the current debate in the literature regarding the
pregnancy outcomes of D5/D6 SVBT.
Acknowledgements
Not applicable.
Funding
Funding: The present research study was funded by the
Shijiazhuang Science and Technology Research and Development Plan
Project (grant no. 191200853).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ conceived the study and wrote the paper. YJ and
GS performed the experiments and analyzed the data. YJ and XHZ
contributed to design and conception. SBM and XHW contributed to
acquisition and interpretation of data. GS, XHZ, SBM and XHW
confirmed the authenticity of all of the data. XHW supervised the
study. All authors read and approved the final manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The Fourth Hospital of Shijiazhuang Ethics Committee
approved (approval no. 20170063; approval date, January 5, 2017)
this study. The procedures used in this study adhered to the tenets
of the Declaration of Helsinki. Informed consent was obtained from
all individual participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Revelli A, Canosa S, Carosso A, Filippini
C, Paschero C, Gennarelli G, Delle Piane L and Benedetto C: Impact
of the addition of early embryo viability assessment to
morphological evaluation on the accuracy of embryo selection on day
3 or day 5: A retrospective analysis. J Ovarian Res.
12(73)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Glujovsky D, Farquhar C, Quinteiro Retamar
AM, Alvarez Sedo CR and Blake D: Cleavage stage versus blastocyst
stage embryo transfer in assisted reproductive technology. Cochrane
Database Syst Rev. 2016(CD002118)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Freeman MR, Hinds MS, Howard KG, Howard JM
and Hill GA: Guidance for elective single-embryo transfer should be
applied to frozen embryo transfer cycles. J Assist Reprod Genet.
36:939–946. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vega M, Zaghi S, Buyuk E and Jindal S: Not
all twins are monozygotic after elective single embryo transfer:
Analysis of 32,600 elective single embryo transfer cycles as
reported to the society for assisted reproductive technology.
Fertil Steril. 109:118–122. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Reljič M and Porović A: Maternal serum
levels of angiogenic markers and markers of placentation in
pregnancies conceived with fresh and vitrified-warmed blastocyst
transfer. J Assist Reprod Gene. 36:1489–1495. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sciorio R, Thong KJ and Pickering SJ:
Increased pregnancy outcome after day 5 versus day 6 transfers of
human vitrified-warmed blastocysts. Zygote. 27:279–284.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kovalevsky G, Carney SM, Morrison LS,
Boylan CF, Neithardt AB and Feinberg RF: Should embryos developing
to blastocysts on day 7 be cryopreserved and transferred: An
analysis of pregnancy and implantation rates. Fertil Steril.
100:1008–1012. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hiraoka K, Hiraoka K, Miyazaki M, Fukunaga
E, Horiuchi T, Kusuda T, Okano S, Kinutani M and Kinutani K:
Perinatal outcomes following transfer of human blastocysts
vitrified at day 5, 6 and 7. J Exp Clin Assist Reprod.
6(4)2009.PubMed/NCBI
|
|
9
|
El-Toukhy T, Wharf E, Walavalkar R, Singh
A, Bolton V, Khalaf Y and Braude P: Delayed blastocyst development
does not influence the outcome of frozen-thawed transfer cycles.
BJOG. 118:1551–1556. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gardner DK, Lane M, Stevens J, Schlenker T
and Schoolcraft WB: Reprint of: Blastocyst score affects
implantation and pregnancy outcome: Towards a single blastocyst
transfer. Fertil Steril. 112 (4 Suppl 1):e81–e84. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang H, Yang Q, Dai S, Li G, Jin H, Yao G
and Sun Y: Comparison of differences in development potentials
between frozen-warmed D5 and D6 blastocysts and their relationship
with pregnancy outcomes. J Assist Reprod Genet. 33:865–872.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiang Y, Cao Q, Zhao X, Li L, Li S and Gao
F: Percutaneous epididymal sperm aspiration and short time
insemination in the treatment of men with obstructive azoospermia.
J Assist Reprod Genet. 30:1175–1179. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gardner DK and Schoolcraft WB: Culture and
transfer of human blastocysts. Curr Opin Obstet Gynecol.
11:307–311. 1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bourdon M, Pocate-Cheriet K, Finet de
Bantel A, Grzegorczyk-Martin V, Amar Hoffet A, Arbo E, Poulain M
and Santulli P: Day 5 versus day 6 blastocyst transfers: A
systematic review and meta-analysis of clinical outcomes. Hum
Reprod. 34:1948–1964. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sciorio R, Thong KJ and Pickering SJ:
Single blastocyst transfer (SET) and pregnancy outcome of day 5 and
day 6 human blastocysts vitrified using a closed device.
Cryobiology. 84:40–45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Behr B, Gebhardt J, Lyon J and Milki AA:
Factors relating to a successful cryopreserved blastocyst transfer
program. Fertil Steril. 77:697–699. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoshida IH, Santos M, Berton CZ, Chiarella
CL, Tanada MS, BCordts E, Carvalho WP and Barbosa CP: Can
trophectoderm morphology act as a predictor for euploidy? JBRA
Assist Reprod. 22:113–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Irani M, O'Neill C, Palermo GD, Xu K,
Zhang C, Qin X, Zhan Q, Clarke RN, Ye Z, Zaninovic N and Rosenwaks
Z: Blastocyst development rate influences implantation and live
birth rates of similarly graded euploid blastocysts. Fertil Steril.
110:95–102.e1. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ueno S, Berntsen J, Ito M, Uchiyama K,
Okimura T, Yabuuchi A and Kato K: Pregnancy prediction performance
of an annotation-free embryo scoring system on the basis of deep
learning after single vitrified-warmed blastocyst transfer: A
single-center large cohort retrospective study. Fertil Steril.
116:1172–1180. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ozgur K, Berkkanoglu M, Bulut H, Yoruk
GDA, Candurmaz NN and Coetzee K: Single best euploid versus single
best unknown-ploidy blastocyst frozen embryo transfers: A
randomized controlled trial. J Assist Reprod Genet. 36:629–636.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Speyer B, O'Neill H, Saab W, Seshadri S,
Cawood S, Heath C, Gaunt M and Serhal P: In assisted reproduction
by IVF or ICSI, the rate at which embryos develop to the blastocyst
stage is influenced by the fertilization method used: A plit
IVF/ICSI study. J Assist Reprod Genet. 36:647–654. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cruz M, Garrido N, Gadea B, Muñoz M,
Pérez-Cano I and Meseguer M: Oocyte insemination techniques are
related to alterations of embryo developmental timing in an oocyte
donation model. Reprod Biomed Online. 27:367–375. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sauerbrun-Cutler MT, Huber WJ III, Has P,
Shen C, Hackett R, Alvero R and Wang S: Is intracytoplasmic sperm
(ICSI) better than traditional in vitro fertilization (IVF):
Confirmation of higher blastocyst rates per oocyte using a split
insemination design. J Assist Reprod Genet. 37:1661–1667.
2020.PubMed/NCBI View Article : Google Scholar
|