Introduction
Alzheimer's disease (AD) is a neurodegenerative
disorder contributing to 60-70% of cases of dementia globally,
based on the estimations of the World Health Organization (WHO)
(1). Vitamin D is an established
molecule which is crucial for the development of neural cells and
the nervous system (2,3). A number of studies have reported that
vitamin D deficiency is associated with a higher risk of developing
dementia and neurodegenerative disorders, such as AD (4-8).
In parallel, the beneficial effects of vitamin D in neural cells
have been reported in a number of studies that have investigated
its functions in relation to nerve growth factor, inducible nitric
oxide synthase, amyloid β-peptide clearance and calcium homeostasis
(9-14).
Vitamin D receptor (VDR) is the critical protein involved in the
mediation of the functions of vitamin D (15).
Over the past few years, a number of studies have
investigated the potential association of vitamin D with the
development of late-onset AD (LOAD) in various populations,
focusing on the VDR gene single nucleotide polymorphisms
(SNPs) (16-22).
One reason for which the VDR gene has received increasing
attention is that it is included in the risk locus in chromosome
12, which was previously reported by genetic studies to be linked
to LOAD (23,24). Vitamin D functions as a
transcription factor after binding to VDR and the large number of
transcription binding sites, that have been estimated up to 10,000
per cell, are another good basis for researching the potential
effects of VDR variants in association with the functions of
vitamin D in the nervous system and in the development of LOAD
(6,25). However, the initial indication for
a potential association of the disease with VDR was
described in an older study, which reported lower VDR mRNA
levels in hippocampal and pyramidal cells of patients with AD
(26).
In addition, to date, only a limited number of
studies have been able to provide knowledge regarding the molecular
mechanisms or pathways through which vitamin D and VDR affect the
development of AD. A previous study concluded that vitamin D and
VDR regulated the production of amyloid β, and therefore the
development of the disease through the expression of the proteins
involved in secretases (25).
Information regarding the potential pathways and genes affected by
vitamin D and VDR was also provided through an in vivo
animal study, which concluded that there was a close connection
between vitamin D molecular pathways and Alzheimer's molecular
pathways through certain groups of genes affecting amyloid β
production, neuroinflammation or other AD-related molecules
(27,28). The molecular pathways involved in
the mechanisms of action of vitamin D affect proteins that play a
key role in the development of a diverse group of diseases,
including diabetes, autoimmune, cardiovascular and malignant
diseases, Parkinson's disease and AD. One indicative example can be
described for the protein kinase B (Akt) pathway. Akt
phosphorylation is decreased by PTEN in AD, leading to the
progression of the disease (29).
In parallel, it has been reported that vitamin D through VDR,
stimulates the Akt signaling pathway, leading to a potential
attenuation of the progression of the disease (29,30).
Overall, the molecular pathways of vitamin D and VDR associated
with the development of LOAD have not yet been fully investigated.
Moreover, the potential effects of the VDR gene SNPs on
these pathways have not yet been fully described. VDR
polymorphisms have been also investigated in specific neurological
diseases and conditions, such as multiple sclerosis, Parkinson's
disease and cluster headaches, with the results indicating possible
associations (31-33).
Thus far, the results from studies investigating
VDR SNPs and LOAD in various populations have been
contradictory. One of the most extensively studied VDR SNP
is TaqI (rs731236, c.1056T>C, ATT>ATC, p.Ile, NM_000376.3)
which is located in exon 9 and is included in the ligand binding
site of the gene. The TaqI polymorphism does not result in an amino
acid change of the receptor protein, and has been proposed as a
genetic biomarker for AD; however, the results differ according to
the ethnic population reported in each study (16,34).
The aim of the present study was to investigate
potential associations of the single nucleotide variation TaqI of
the VDR gene with the development of LOAD in a Southeastern
European Caucasian (SEC) cohort. The present study also wished to
compare the observed results with the data from other populations
and to evaluate the potential association of this variation with
neuropsychology mini-mental state examination (MMSE) and frontal
assessment battery (FAB) assessments.
Subjects and methods
Study subjects
The study sample included 90 patients with
well-ascertained LOAD (median age, 74 years; range, 51-92 years;
males, 48.9%; females, 51.1%; median MMSE score of 21; median FAB
score of 10) and 103 healthy controls (median age, 57 years; range,
51-90 years; males, 49.5%; females, 50.5%). LOAD diagnosis was
based on current diagnostic criteria for AD, including a physical
examination, MMSE/FAB score, a brain CT/MRI scan and amyloid β, tau
and p-tau levels in the cerebrospinal fluid. Patients or healthy
control subjects not belonging to the SEC population were not
included in the study. Patients were recruited from the Outpatient
Clinic of the Cognitive Disorder-Dementia Unit of the Second
Department of Neurology at the University General Hospital
‘ATTIKON’ (Athens, Greece). Sample collection took place from
January, 2018 to February, 2019. The demographic data of the
patient and control groups are presented in Table I. The present study has been
approved by the Scientific Council and Bioethics Committee of the
University General Hospital ‘ATTIKON’ (Reg. no. 312; December 21,
2017). Written informed consent for participation in the study and
the use of their genetic data was obtained from all
participants.
 | Table IDemographic data of the study
groups. |
Table I
Demographic data of the study
groups.
| Parameter | Patients | Controls |
|---|
| Number (n) | 90 | 103 |
| Age, median
(years) | 74 | 57 |
| Age, mean
(years) | 73 | 60 |
| Max value
(years) | 92 | 90 |
| Min value
(years) | 51 | 51 |
| Range | 41 | 39 |
| Males, n (%) | 44 (48.9) | 51 (49.5) |
| Females, n (%) | 46 (51.1) | 52 (50.5) |
| MMSE score,
median | 21 | - |
| FAB score,
mean | 10 | - |
DNA isolation and quantitative PCR
(qPCR)
Blood samples from the patients and controls were
analyzed to determine the genotypes of the SNP TaqI (rs731236) of
the VDR gene. DNA extraction was performed from 200 µl whole
blood samples using the NucleoSpin® Genomic DNA from
Tissue kit (Macherey-Nagel GmbH & Co. KG). Genotypes of the
TaqI (rs731236) polymorphism were determined using qPCR (using the
Light Cycler® 480 system; Roche Diagnostics) with the
LightSNiP (SimpleProbe®) assay (TIB Molbiol). For qPCR,
an initial polymerase activation and denaturation step at 95˚C for
10 min was followed by 45 amplification cycles for each sample in
the LightCycler instrument. Cycles included denaturation (95˚C for
10 sec), annealing (60˚C for 10 sec) and extension (72˚C for 15
sec). Fluorescence was measured at the end of the annealing period
of each cycle to monitor the progress of amplification. Following
amplification, melting curves were created by cooling/holding
temperature at 40˚C for 30 sec and gradually increasing the
temperature up to 95˚C. Melting peaks were produced accordingly
based on the fluorescence signal using LightCycler® 480
software, version 1.5. Differences in the hybridization between the
SimpleProbe® oligonucleotide and the DNA sequence (due
to the presence of the polymorphism) result in different melting
temperatures, allowing the detection of the alleles. In rs731236,
the C allele with three hydrogen bonds is detected at higher
temperatures than the T allele with two hydrogen bonds. Homozygous
samples (TT or CC) were detected, providing a single melting peak,
while heterozygous samples (TC) provided two melting peaks each one
corresponding to each allele.
Statistical analysis
Data from the genotyping results were analyzed using
SNPstats web-based software, developed by the Catalan Institute of
Oncology, 2006 and SPPS® 17.0.0 software (SPPS, Inc.).
Logistic regression analysis was applied to analyze the association
of the genotypes in each heredity model with the disease and odds
ratios (ORs) with 95% confidence intervals (CIs) were calculated.
Fisher's exact test was performed to compare the genotyping results
with the MMSE and FAB score within the group of patients. A value
of P<0.05 was considered to indicate a statistically significant
difference. The results were consistent with the Hardy-Weinberg
equilibrium.
Results
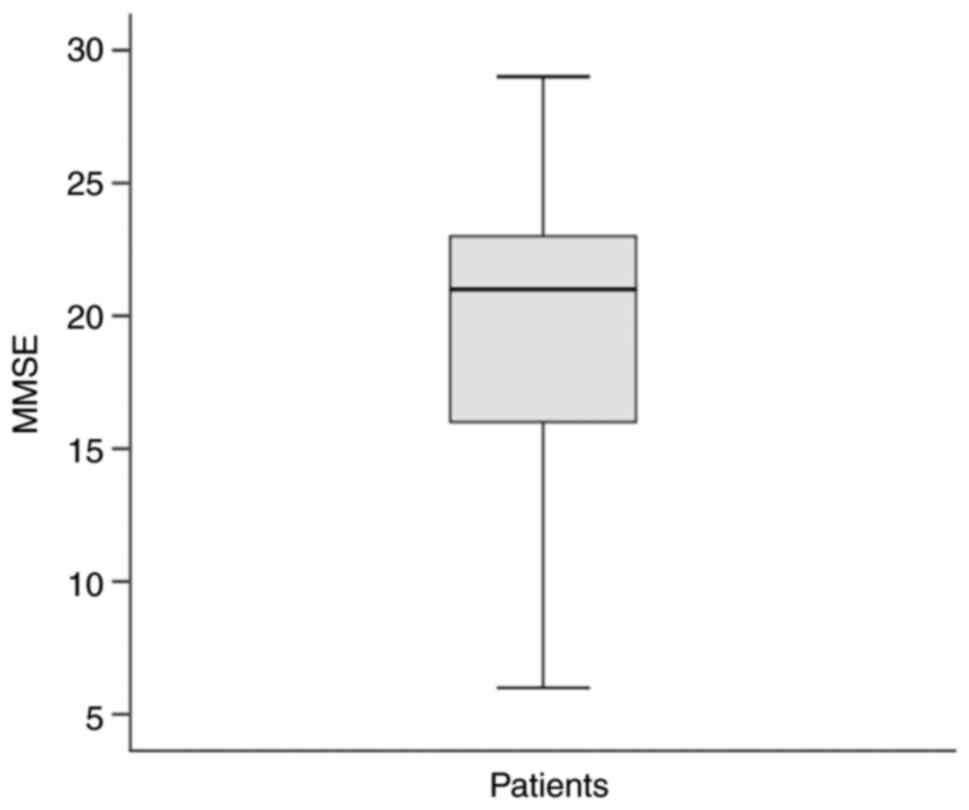
The patient group had a median MMSE score of 21
(Fig. 1) and a FAB score of 10.
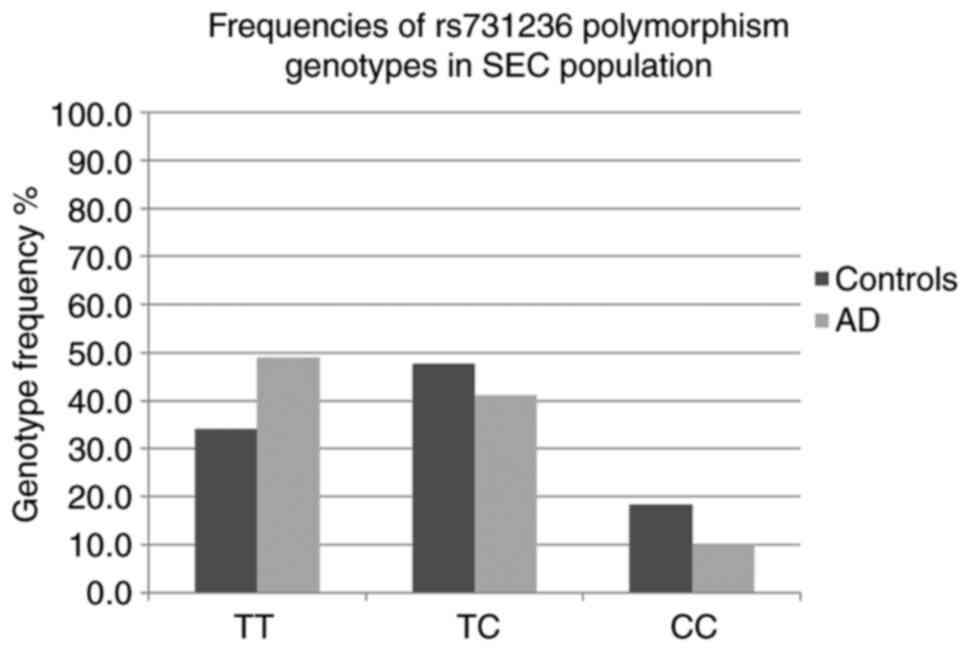
The frequencies (%) of the TaqI TT, TC and CC genotypes in the
controls/patients were 34.0/48.9, 47.6/41.1 and 18.4/10.0,
respectively (Fig. 2). A
statistically significant difference was observed for the TaqI C
allele in the dominant model of inheritance TT vs. CT + CC (OR,
0.54; 95% CI, 0.30-0.96; P=0.035) (Table II). On the other hand, a
statistically significant difference was observed for the TT
genotype in the recessive model of inheritance CC + TC vs. TT (OR,
1.86; 95% CI, 1.04-3.32; P=0.035) (Table II). In the patient group, when
analyzing TaqI genotypes in relation to the median value of the
MMSE score (21), a statistically
significant difference was observed for the TaqI CC genotype and
MMSE score <21, CC vs. TC + TT (OR, 0.119; 95% CI, 0.014-0.995;
P=0.032) (Table III). No
statistically significant difference was observed when analyzing
TaqI genotype frequencies and FAB score in the patient group (data
not shown).
 | Table IIFrequencies of TaqI genotypes in the
different inheritance models. |
Table II
Frequencies of TaqI genotypes in the
different inheritance models.
| | TaqI rs731236
association with AD (n=193) | |
|---|
| Model | Genotype | Controls, n
(%) | AD, n (%) | OR (95% CI) | P-value |
|---|
| Codominant | TT | 35 (34%) | 44 (48.9) | 1.00 | 0.064 |
| | TC | 49 (47.6) | 37 (41.1) | 0.60
(0.32-1.11) | |
| | CC | 19 (18.4) | 9(10) | 0.38
(0.15-0.94) | |
| Dominant | TT | 35(34) | 44 (48.9) | 1.00 | |
| | TC/CC | 68(66) | 46 (51.1) | 0.54
(0.30-0.96)a | 0.035 |
| Recessive | TT/TC | 84 (81.5) | 81(90) | 1.00 | |
| | CC | 19 (18.4) | 9(10) | 0.49
(0.21-1.15) | 0.092 |
| Recessive (for T
allele) | CC/TC | 68 (66%) | 46 (51.1%) | 1.00 | |
| | TT | 35 (34%) | 44 (48.9%) | 1.86
(1.04-3.32)a | 0.035 |
 | Table IIITaqI CC genotype vs. MMSE score
<21 crosstabulation in the group of patients. |
Table III
TaqI CC genotype vs. MMSE score
<21 crosstabulation in the group of patients.
| | TaqI | |
|---|
| MMSE score | CT + TT (%) | CC (%) | OR | 95% CI | P-value |
|---|
| >21 | 38 (48.7) | 8 (88.9) | 0.119 | 0.014-0.995 | 0.032 |
| <21 | 40 (51.3) | 1 (11.1) | | | |
Discussion
In the SEC cohort examined in the present study, the
TaqI TT genotype was associated with a higher risk of developing AD
by 1.8-fold. In addition, the TaqI C allele was associated with a
potential protective effect against the disease, since it was
calculated that TaqI C carriers had a 46% less likelihood of
developing AD (Table II).
Moreover, in the patient group, the TaqI CC genotype was associated
with a 88% less likelihood of developing severe cognitive
impairment measured using the MMSE score (Table III). One of the assuming effects
of the VDR polymorphism on the molecular level is the
affinity of the ligand site of the receptor to vitamin D. It has
been previously reported that VDR polymorphisms may decrease
the affinity of VDR to vitamin D and may therefore affect the
beneficial effects of vitamin D in neural cells as regards calcium
homeostasis, neurotrophin levels and inflammation (16,17).
Another potential effect of the TaqI polymorphism is on the
stability of VDR mRNA expression (17). It can be assumed that the
insufficient effects of vitamin D, associated with the TaqI TT
genotype, either by decreased affinity to the VDR or reduced mRNA
levels in the nervous system may lead to neurodegeneration and
cognitive impairment in SECs. On the contrary, the TaqI C allele
appears to be associated with enhanced effects of vitamin D in the
nervous system in the specific population studied.
The results of the present study in the SEC cohort
are not in agreement with those produced by a previous study in the
Turkish population, in which the TaqI TT genotype was more frequent
in the control group with a statistically significant difference
(16). However, another study in
the Asian population reported an association of the TaqI TC
genotype with AD with an increased risk of 2.8-fold for the
heterozygous carriers (35). By
contrast, the TaqI TC frequency in the present study was higher in
the control group and no statistically significant association with
the disease was observed (Table
II). In another study on Northwestern European Caucasians, it
was reported that the presence of TaqI C allele in adults <75
years of age resulted in a 3-fold higher risk of developing AD in
comparison to the control group with a statistically significant
difference (17). On the contrary,
the TaqI C allele in the SEC cohort of the present study was
associated with a potential protective effect against the disease
and a lower likelihood of developing AD (Table II). The VDR TaqI
polymorphism has also been investigated in the Iranian population;
however, the results of that study did not reveal any statistically
significant difference for TaqI genotypes between the patient and
control group, and the polymorphism was not associated with the
disease (18,20). The results of the present study
differ from those reported for the Iranian population in which no
risk or protective TaqI allele for AD was detected. The TaqI
polymorphism was also previously investigated in a Northeastern
European Caucasian cohort and no association with AD was reported
(21). Another study on the
Spanish population investigated the TaqI polymorphism in relation
to serum vitamin D levels (22).
However, it was reported that TaqI did not result in any
statistically significant difference in the serum vitamin D levels
nor was it associated with a higher risk of developing AD (22). These data provide from different
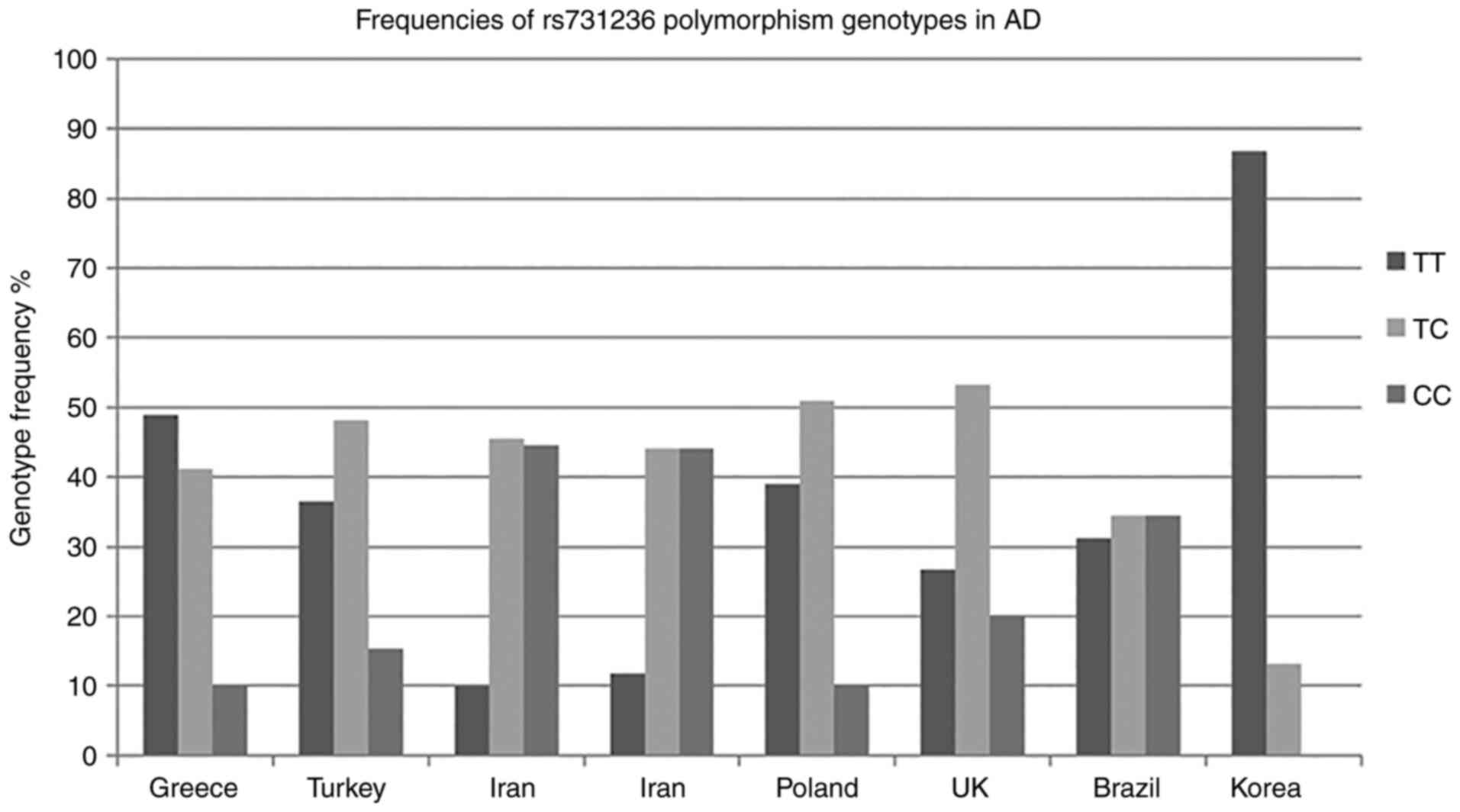
studies are presented in the graph depicted in Fig. 3 (16-18,20-22,35).
Overall, the results from published studies for each
population are contradictory and do not allow for a definite
conclusion regarding the association of TaqI with AD (Table IV). The TaqI C allele that appears
to increase the risk of developing AD in Northwestern European
Caucasians (17) has been shown to
exert a protective effect in SECs from the present study. In
another study on Northwestern European Caucasians, the TaqI C
allele was reported to be associated with the decline in the
scoring of tests measuring cognitive performance (36). However, that study did not include
patients with AD and therefore a direct comparison to the studies
with patients with AD is not applicable. However, the indication of
the TaqI C allele that increases the risk of developing cognitive
impairment is not confirmed by the results of the present study, in
which the TaqI C allele appeared to decrease the risk of developing
AD (Table II); homozygous TaqI CC
patients also had higher MMSE scores (Table III). The interpretation of the
results from different populations should take into account a
number of factors, two of which are sun exposure and vitamin D
intake. A previous study on Northwestern European Caucasians
reported that the activity of VDR may be related to the level of
sun exposure of each population (21). That study concluded that in
subjects with a lower sun exposure or lower vitamin D intake, the
amount of VDR produced was the crucial factor associated with the
development of AD (21). In
addition, the same study described that VDR polymorphisms
(TaqI and ApaI) were not associated with AD in populations with a
high sun exposure due to the increased endogenous vitamin D
production, which renders the activity of VDR less dependent to its
amount (21). The results of the
present study on the SEC population cannot confirm the
aforementioned assumption, since sun exposure can be considered
high in the geographical area of Greece and the TaqI TT genotype
was associated with AD.
 | Table IVTaqI genotype frequencies of
published studies. |
Table IV
TaqI genotype frequencies of
published studies.
| | TaqI TT % | TaqI TC % | TaqI CC % | |
|---|
| Country | Population | Author |
nAD/nCO | Mean age (years)
±SD AD/control group | AD | Control group | AD | Control group | AD | Control group | (Refs.) |
|---|
| Turkey | Turkish | Gezen-Ak et
al | 104/109 |
75.1±5.7/73.6±7.3 | 36.5 | 48.6 | 48.1 | 35.8 | 15.4 | 15.6 | (16) |
| Iran | Iranian | Esfehani et
al | 101/109 | >65/>65 | 9.9 | 11.9 | 45.5 | 42.2 | 44.6 | 45.9 | (18) |
| Iran | Iranian | Khorshid et
al | 145/162 | 78±8/77±7 | 11.8 | 13 | 44.1 | 40.1 | 44.1 | 46.9 | (20) |
| Poland | Northwestern
European Caucasian | Łaczmański et
al | 108/77 |
73.3±8.6/64.5±7.8 | 38.9 | 40.2 | 50.9 | 49.4 | 10.2 | 10.4 | (21) |
| UK | Northwestern
European Caucasian | Lehmann et
al | 255/260 |
78.8±8.5/78.1±8.8 | 26.7 | 38.8 | 53.3 | 45 | 20 | 16.2 | (17) |
| Brazil | Spanish | Oliveira et
al | 32/24 | 69.8±9/74± 7.2 | 31.2 | 54.2 | 34.4 | 25 | 34.4 | 20.8 | (22) |
| Korea | Asian | Mun et
al | 144/335 |
79.82±7.02/68.94±6.10 | 86.8 | 90.0 | 13.2 | 9.7 | 0 | 0.3 | (35) |
The potentially protective effects of the TaqI C
allele or the higher MMSE scores of the TaqI genotype CC in
patients with AD that have been described in the present study in
the SEC population is an interesting finding that creates further
questions to be answered regarding the molecular pathways affected
by this polymorphism. It appears that the TaqI C allele is
associated with the more efficient utilization of vitamin D and
therefore, in a more potent effect as regards neurotrophic factor
production or amyloid β clearance (6).
The present study concluded that the TaqI
polymorphism is associated with AD in the SEC population, both as a
risk and protective factor, and may thus be considered a potential
biomarker. However, further studies on larger SEC sample sizes are
required to confirm these results. Of note, the present study has
provided an initial indication as regards the association of the
VDR TaqI polymorphism with AD in the SEC population.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ND conceived study and provided the control samples
and data. MSK and ML performed the sample analysis. ED obtained the
ethics committee study approval, performed the literature review
and the statistical and data analyses, and was responsible for the
manuscript composition under the supervision of ND, CK and KA. DAS
and AT contributed to the editing of the final manuscript. KA and
CK reviewed and edited the statistical analysis. DAS, AT, SP, VP
and ES contributed to the collection of the clinical data and
patient scores. SP, PM and CK also provided the patient samples.
All authors discussed the results and agreed on the conclusions of
the study and all authors have read and approved the final
manuscript. All authors confirm the authenticity of the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Scientific
Council and Bioethics Committee of the University General Hospital
‘ATTIKON’ (Reg. No 312; December 21, 2017). Written informed
consent for participation in the study and use of their genetic
data was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
All the other authors declare that they have no competing
interests.
References
|
1
|
Global Action Plan on the Public Health
Response to Dementia 2017-2025. World Health Organization, Geneva,
2017.
|
|
2
|
Orme RP, Middleditch C, Waite L and
Fricker RA: The Role of Vitamin D3 in the development
and neuroprotection of midbrain dopamine neurons. Vitam Horm.
100:273–297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wrzosek M, Łukaszkiewicz J, Wrzosek M,
Jakubczyk A, Matsumoto H, Piątkiewicz P, Radziwoń-Zaleska M, Wojnar
M and Nowicka G: Vitamin D and the central nervous system.
Pharmacol Rep. 65:271–278. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Littlejohns TJ, Henley WE, Lang IA,
Annweiler C, Beauchet O, Chaves PH, Fried L, Kestenbaum BR, Kuller
LH, Langa KM, et al: Vitamin D and the risk of dementia and
Alzheimer disease. Neurology. 83:920–928. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Afzal S, Bojesen SE and Nordestgaard BG:
Reduced 25-hydroxyvitamin D and risk of Alzheimer's disease and
vascular dementia. Alzheimers Dement. 10:296–302. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gezen-Ak D, Yilmazer S and Dursun E: Why
Vitamin D in Alzheimer's Disease? The Hypothesis. J Alzheimers Dis.
40:257–269. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Landel V, Annweiler C, Millet P, Morello M
and Féron F: Vitamin D, Cognition and Alzheimer's Disease: The
Therapeutic benefit is in the D-Tails. J Alzheimers Dis.
53:419–444. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mavraki E, Ioannidis P, Tripsianis G,
Gioka T, Kolousi M and Vadikolias K: Vitamin D in mild cognitive
impairment and Alzheimer's disease. A study in older Greek adults.
Hippokratia. 24:120–126. 2020.PubMed/NCBI
|
|
9
|
Gezen-Ak D, Dursun E and Yilmazer S: The
effect of Vitamin D treament On Nerve Growth Factor (NGF) Release
from Hippocampal Neurons. Noro Psikiyatr Ars. 51:157–162.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dursun E, Gezen-Ak D and Yilmazer S: The
Influence of Vitamin D treatment on the Inducible Nitric Oxide
Synthase (INOS) Expression in Primary Hippocampal Neurons. Noro
Psikiyatr Ars. 51:163–168. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gradinaru D, Borsa C, Ionescu C, Margina
D, Prada GI and Jansen E: Vitamin D status and oxidative stress
markers in the elderly with impaired fasting glucose and type 2
diabetes mellitus. Aging Clin Exp Res. 24:595–602. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Mizwicki MT, Menegaz D, Zhang J,
Barrientos-Durán A, Tse S, Cashman JR, Griffin PR and Fiala M:
Genomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3
Promotes the Recovery of Amyloid-β Phagocytosis by Alzheimer's
Disease Macrophages. J Alzheimers Dis. 29:51–62. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mizwicki MT, Liu G, Fiala M, Magpantay L,
Sayre J, Siani A, Mahanian M, Weitzman R, Hayden EY, Rosenthal MJ,
et al: 1α,25-dihydroxyvitamin D3 and resolvin D1 retune the balance
between amyloid-β phagocytosis and inflammation in Alzheimer's
disease patients. J Alzheimers Dis. 34:155–170. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gezen-Ak D, Dursun E and Yilmazer S: The
effects of vitamin D receptor silencing on the expression of
LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons.
PLoS One. 6(e17553)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cui X, Gooch H, Petty A, McGrath JJ and
Eyles D: Vitamin D and the brain: Genomic and non-genomic actions.
Mol Cell Endocrinol. 453:131–143. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gezen-Ak D, Dursun E, Ertan T, Hanagasi H,
Gurvit H, Emre M, Eker E, Ozturk M, Engin F and Yilmazer S:
Association between Vitamin D receptor gene polymorphism and
Alzheimer's disease. Tohoku J Exp Med. 212:275–282. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lehmann DJ, Refsum H, Warden DR, Medway C,
Wilcock GK and Smith AD: The vitamin D receptor gene is associated
with Alzheimer's disease. Neurosci Lett. 504:79–82. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Esfehani NT, Rahgozar M and Biglarian A:
Identification of genetic polymorphism interactions in sporadic
Alzheimer's disease using logic regression. Iran Rehabil J.
9:45–50. 2011.
|
|
19
|
Gezen-Ak D, Dursun E, Bilgic B, Hanagasi
H, Ertan T, Gurvit H, Emre M, Eker E, Ulutin T, Uysal O and
Yilmazer S: Vitamin D receptor gene haplotype is associated with
late onset Alzheimer's disease. Tohoku J Exp Med. 228:189–196.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Khorram Khorshid HR, Gozalpour E,
Saliminejad K, Karimloo M, Ohadi M and Kamali K: Vitamin D receptor
(VDR) polymorphisms and late-onset Alzheimer's disease: An
association study. Iran J Publ Health. 42:1253–1258.
2013.PubMed/NCBI
|
|
21
|
Łaczmański L, Jakubik M,
Bednarek-Tupikowska G, Rymaszewska J, Słoka N and Lwow F: Vitamin D
receptor gene polymorphisms in Alzheimer's disease patients. Exp
Gerontol. 69:142–147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Oliveira ACR, Magalhães CA, Loures CMG,
Fraga VG, de Souza LC, Guimarães HC, Cintra MTG, Bicalho MA, Sousa
MCR, Silveira JN, et al: BsmI polymorphism in the vitamin D
receptor gene is associated with 25-hydroxy vitamin D levels in
individuals with cognitive decline. Arq Neuropsiquiatr. 76:760–766.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Poduslo SE and Yin X: Chromosome 12 and
late-onset Alzheimer's disease. Neurosci Lett. 310:188–190.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gezen-Ak D and Dursun E: Molecular basis
of vitamin D action in neurodegeneration: The story of a team
perspective. Hormones (Athens). 18:17–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gezen-Ak D, Atasoy IL, Candaş E,
Alaylioglu M, Yılmazer S and Dursun E: Vitamin D receptor regulates
amyloid beta 1-42 production with protein disulfide isomerase A3.
ACS Chem Neurosci. 8:2335–2346. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sutherland MK, Somerville MJ, Yoong LK,
Bergeron C, Haussler MR and McLachlan DR: Reduction of vitamin D
hormone receptor mRNA levels in Alzheimer as compared to huntington
hippocampus: Correlation with calbindin-28k mRNA levels. Brain Res
Mol Brain Res. 13:239–250. 1992.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Landel V, Millet P, Baranger K, Loriod B
and Féron F: Vitamin D interacts with Esr1 and Igf1 to regulate
molecular pathways relevant to Alzheimer's disease. Mol
Neurodegener. 11(22)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gubandru M, Margina D, Tsitsimpikou C,
Goutzourelas N, Tsarouhas K, Ilie M, Tsatsakis AM and Kouretas D:
Alzheimer's disease treated patients showed different patterns for
oxidative stress and inflammation markers. Food Chem Toxicol.
61:209–214. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nitulescu GM, Van De Venter M, Nitulescu
G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Gradinaru D, Tsatsakis
A, Tsoukalas D, et al: The Akt pathway in oncology therapy and
beyond (Review). Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zaulkffali AS, Md Razip NN, Syed Alwi SS,
Abd Jalil A, Abd Mutalib MS, Gopalsamy B, Chang SK, Zainal Z,
Ibrahim NN, Zakaria ZA and Khaza'ai H: Vitamins D and E stimulate
the PI3K-AKT signalling pathway in insulin-resistant SK-N-SH
neuronal cells. Nutrients. 11(2525)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang D, Wang L, Zhang R and Li S:
Association of vitamin D receptor gene polymorphisms and the risk
of multiple sclerosis: A meta-analysis. Arch Med Res. 50:350–361.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Niu MY, Wang L and Xie AM: ApaI, BsmI,
FokI, and TaqI polymorphisms in the vitamin D receptor gene and
Parkinson's disease. Chin Med J (Engl). 128:1809–1814.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Papasavva M, Vikelis M, Siokas V, Katsarou
MS, Dermitzakis E, Raptis A, Dardiotis E and Drakoulis N: VDR gene
polymorphisms and cluster headache susceptibility: Case-control
study in a Southeastern European caucasian population. J Mol
Neurosci. 72:382–392. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Geng J, Zhang J, Yao F, Liu X, Liu J and
Huang Y: A systematic review and meta-analysis of the associations
of vitamin D receptor genetic variants with two types of most
common neurodegenerative disorders. Aging Clin Exp Res. 32:21–27.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mun MJ, Kim MS, Kim JH and Jang WC: A TaqI
polymorphism of vitamin D receptor is associated with Alzheimer's
disease in Korean population: A case-control study. Int J Clin Exp
Med. 9:19268–19279. 2016.
|
|
36
|
Kuningas M, Mooijaart SP, Jolles J,
Slagboom PE, Westendorp RG and Van Heemst D: VDR gene variants
associate with cognitive function and depressive symptoms in old
age. Neurobiol Aging. 30:466–473. 2009.PubMed/NCBI View Article : Google Scholar
|