Introduction
Glaucoma is a common eye disease caused by optic
nerve atrophy and visual field defect (1). Primary glaucoma comprises
angle-closure glaucoma and open-angle glaucoma (2); the most common type of glaucoma is
primary open-angle glaucoma (POAG) (3). Glaucoma is the leading cause of
irreversible vision loss and blindness (2). The condition affects >2 million
individuals annually in the United States (4). According to the World Health
Organization, the number of glaucoma patients worldwide was
predicted to reach 79.6 million by 2020, of which 11.2 million may
eventually develop blindness in both eyes (4,5). As
of 2011, 2.7 million patients in the United States suffered from
POAG alone, and the number of patients with POAG is projected to
increase to >7 million by the year 2050(5). The formation of a hypertrophic scar
(HS), which is characterized by excessive proliferation of
fibroblasts, can lead to the failure of glaucoma filtration surgery
(6,7). Currently, the major treatments for HS
include medication, surgery and physical therapy (8); however, the outcomes are not yet
satisfactory. Thus, further studies are required to identify
effective treatment strategies for HS.
Long non-coding RNAs (lncRNAs) are a class of
non-coding RNA molecules of >200 nucleotides in length that are
located in the nucleus or in the cytoplasm and have no or little
protein coding function (9).
lncRNAs are extensively involved in various signaling pathways that
influence epigenetics, cell cycle, proliferation (10). In addition, abnormal lncRNA
expression or function is closely associated with the occurrence of
human diseases, including glaucoma (11,12).
For example, Nong et al (13) reported that lncRNA COL1A2-AS1 was
highly expressed in HS and fibroblasts, and that it inhibited the
proliferation of fibroblasts by promoting Smad7 expression. In
addition, Li et al (14)
demonstrated that lncRNA8975-1 was highly expressed in HS tissues
and could inhibit fibroblast proliferation and α-smooth muscle
actin (α-SMA) expression. Zhu et al (15) reported that the lncRNA LINC01605 is
closely associated with the formation of HS and is expressed at
abnormally high levels in human dermal fibroblasts. However, the
molecular mechanism by which LINC01605 may regulate the development
of HS remains unclear.
Therefore, present study aimed to investigate the
role of LINC01605 in HS to identify novel potential treatment
strategies. To explore the effects of LINC01605 on the formation of
HS, human Tenon's capsule fibroblasts (HTFs) and corneal epithelial
cells (control cells) were collected and cultured in vitro.
For this study, CCK-8, flow cytometry and Transwell assays were
conducted to detect the viability, apoptosis and migratory of HTFs,
respectively.
Materials and methods
Specimen collection, isolation of HTFs
and cell culture
The inclusion criteria for the patients with
glaucoma included in the present study were as follows: i) Patients
diagnosed with glaucoma according to the latest diagnostic criteria
for glaucoma developed by the Cooperative Group on Fundus Diseases
of the People's Republic of China (6); and ii) patients who have undergone
glaucoma surgery. Patients were excluded based on the following
criteria: i) Patients with other diseases and currently receiving
treatment; ii) pregnant or breast-feeding women; iii) patients who
are allergic to probiotics or who have recently used/are using
antibiotics; iv) patients with alcoholism (individuals who drink ≥5
bottles of beer at a time or have a blood alcohol level of ≥0.08
g/100 ml); and v) smokers, according to a previous reference
(6).
Matched HS and normal (control/healthy) tissues were
collected from patients (n=5) with POAG who underwent glaucoma
filtration surgery at Fuyang People's Hospital (Hangzhou, China)
between November 2019 and May 2020 according to previous reports
(16,17). It has been reported that HS tissue
has more melanocytes, and that there are fewer melanocytes in
normal tissue (18). The basic
clinicopathological characteristics of the patients are listed in
Table I. Subsequently, to isolate
HTFs from HS tissues and corneal epithelial cells (control cells)
from healthy tissues. Briefly, 5x5-mm sections of Tenon's capsule
were collected, minced and placed in a 35-mm culture dish
containing Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal calf
serum (Invitrogen; Thermo Fisher Scientific, Inc.), 50 U/ml
penicillin and 50 µg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were allowed to migrate from the explanted
tissue and were then incubated at 37˚C in 5% CO2. Cells
between the third and fifth passages were used according to a
previous study (19). Next, HTFs
were maintained in DMEM supplemented with 10% FBS, 1% penicillin
and 1% streptomycin (all from Thermo Fisher Scientific, Inc.) at
37˚C with 5% CO2, as previously described (20,21).
The isolated cells were observed using fluorescent staining under a
fluorescence microscope (Olympus Corporation). The present study
was approved by the Ethics Committee of Fuyang People's Hospital
(Hangzhou, China; approval no. FPH20191011), and written informed
consent was provided by all patients prior to the study onset.
 | Table IBasic clinicopathological
characteristics of the patients. |
Table I
Basic clinicopathological
characteristics of the patients.
| Patient | Age, years | Sex | Date of hospital
admission, month/year |
|---|
| 1 | 67 | Female | 11/2019 |
| 2 | 72 | Male | 11/2019 |
| 3 | 54 | Female | 03/2020 |
| 4 | 62 | Female | 04/2020 |
| 5 | 71 | Male | 05/2020 |
Reagents
3-Methyladenine (3-MA) was obtained from
MedChemExpress (cat. no. HY-19312). Cells were treated with 1
mmol/l 3-MA for 24 h according to a previous study (22). In addition, TGF-β was provided by
Millipore Sigma (cat. no. SAB4502954). Cells were treated with 10
ng/ml TGF-β for 24 h according to a previous study (23).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from HS tissues, healthy
tissues and HTFs (5x104/ml) using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
reverse transcribed into cDNA using the EntiLink™ 1st Strand cDNA
Synthesis kit [ELK (Wuhan) Biotechnology Co., Ltd.] according to
the manufacturer's instructions. qPCR was subsequently performed
using the StepOne™ Real-Time PCR System (Thermo Fisher Scientific,
Inc.). EnTurbo™ SYBR Green PCR SuperMix kit (ELK Biotechnology Co.,
Ltd.) was used for qPCR. The thermocycling conditions were used as
follows: 3 min at 95˚C, followed by 40 cycles of 10 sec at 95˚C, 30
sec at 58˚C and 30 sec at 72˚C. mRNA expression levels were
normalized to β-actin levels. Relative expression levels were
calculated using the 2-ΔΔCq method (24). The following primer sequences were
used for qPCR: β-actin forward, 5'-GTCCACCGCAAATGCTTCTA-3' and
reverse, 5'-TGCTGTCACCTTCACCGTTC-3'; and LINC01605 forward,
5'-CAACTCATTCCCGTTACAAACA-3' and reverse,
5'-CATCTCAACTGCCTCTGTCTCC-3'.
Immunofluorescence analysis
Droplets of suspended corneal epithelial cells and
HTFs (3x105 cells/well) were cultured in a 24-well plate
at 37˚C with 5% CO2 and subsequently fixed with 4%
paraformaldehyde for 30 min at room temperature. The following
primary antibodies were diluted with 5% BSA (Beyotime
Biotechnology): Anti-vimentin (1:1,000; Abcam; cat. no. ab92547),
anti-keratin (1:1,000; Abcam; cat. no. ab8068) and anti-LC3
(1:1,000; Abcam; cat. no. ab192890) at 4˚C. Following primary
antibody incubation overnight at 4˚C, cells were incubated with
horseradish peroxidase IgG secondary antibody (1:5,000; Abcam; cat.
no. ab6728) for 1 h at room temperature. Cells were washed three
times with PBS. Cell nuclei were stained with 50-100 µl DAPI at
room temperature for 5 min. Finally, cells were observed under a
fluorescence microscope (Olympus Corporation).
Small interference (si)RNA
transfection
Three siRNAs against LINC01605 and an siRNA-negative
control (NC) were synthesized by Guangzhou RiboBio Co., Ltd. The
following sequences were used: LINC01605 siRNA1,
5'-TCTTGAAGAATAAGAAGCCACAGCT-3'; LINC01605 siRNA2,
5'-GAGTCTTGAAGAATAAGAAGCCACA-3'; LINC01605 siRNA3,
5'-TAAGAAGCCACAGCTTGTCAGGGAA-3'; and siRNA-NC,
5'-GAGGTTGAATAAGAAGAACCTCACA-3'. The siRNAs (10 nM) were
transfected into HTFs (3x105 cells/well) using
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.) for 48 h at 37˚C. After 48 h of incubation, cells were used
for subsequent experimentation. The blank group was comprised of
non-transfected cells.
Cell viability assay
The Cell Counting Kit-8 (CCK-8) assay (Beyotime
Institute of Biotechnology) was performed to assess cell viability.
HTFs were seeded into 96-well plates at a density of
5x103 cells/well. Next, siRNA-NC, LINC01605 siRNA1 and
LINC01605 siRNA2 were transfected into HTFs using
Lipofectamine® 2000 reagent for 6 h at 37˚C. The culture
medium was then changed and HTFs continued to be cultured in DMEM
for 0, 24, 48 and 72 h. Next, 10 µl CCK-8 reagent was added into
each well and cells were incubated for 2 h at 37˚C. Absorbance was
measured at a wavelength of 450 nm using a microplate reader
(Varioskan™ LUX; Thermo Fisher Scientific, Inc.).
Apoptosis analysis
Apoptosis was analyzed by flow cytometry. HTFs were
seeded into six-well plates at a density of 5x104
cells/ml. Following transfection with siRNAs, cells were stained
with Annexin V-FITC and PI (Tianjin Sungene Biotech, Co., Ltd.) for
15 min in the dark at room temperature. Subsequently, the apoptotic
rate (the sum of early and late apoptosis) was analyzed using a
FACSCanto II flow cytometer (BD Biosciences). The data was analyzed
using FlowJo software (version 10.6.2; FlowJo LLC).
Western blotting
Protein was extracted from HTFs using RIPA buffer
(Aspen Biotechnology) and concentration was determined using a BCA
kit (Aspen Biotechnology, Co., Ltd.). Proteins (30 µg/lane) were
separated by 10% SDS-PAGE, transferred onto PVDF membranes and
blocked with 5% non-fat milk diluted in TBS with 0.1% Tween-20 for
1 h at room temperature. The membranes were incubated overnight at
4˚C with primary antibodies against Bax (1:1,000; Abcam; cat. no.
ab32503), Bcl-2 (1:1,000; Abcam; cat. no. ab32124), Pro-caspase-3
(1:1,000; Abcam; cat. no. ab32150), cleaved caspase-3 (1:1,000;
Abcam; cat. no. ab2302), phosphorylated (p)-Smad2 (1:1,000; Abcam;
cat. no. ab280888), Smad2 (1:1,000; Abcam; cat. no. ab40855), α-SMA
(1:1,000; Abcam; cat. no. ab5694), MMP9 (1:1,000; Abcam; cat. no.
ab76003), autophagy-related (ATG)7 (1:1,000; Abcam; cat. no.
ab52472), p62 (1:1,000; Abcam; cat. no. ab109012), beclin 1
(1:1,000; Abcam; cat. no. ab207612), p-AMP-activated protein kinase
(AMPK) (1:1,000; Abcam; cat. no. ab133448), AMPK (1:1,000; Abcam;
cat. no. ab32047) and β-actin (1:1,000; Abcam; cat. no. ab8227).
Subsequently, the membranes were incubated with horseradish
peroxidase (HRP)-labeled goat anti-rabbit secondary antibody
(1:5,000; cat. no. ab7090; Abcam) at room temperature for 1 h.
Protein bands were visualized using the Enhanced Chemiluminescence
Reagent (Thermo Fisher Scientific, Inc.). β-actin was used as a
loading control. Finally, the density of the blots was analyzed
using AlphaEaseFC software (version 4.0; Alpha Innotech
Corporation).
Transwell migration assay
The Transwell migration assay was performed using
24-well Transwell chambers (Corning, Inc.). Firstly, HTFs during
the logarithmic growth phase were incubated overnight at 37˚C.
Next, HTFs were transfected with siRNA-NC, LINC01605 siRNA1 and
LINC01605 siRNA2 using Lipofectamine 2000 reagent for 24 h at 37˚C.
After that, HTFs (200 µl) were plated on the upper chamber
suspended with 100 µl serum-free DMEM at 37˚C, and 600 µl complete
DMEM supplemented with 10% FBS was added to the lower chamber;
cells were incubated for 24 h at 37˚C. Following incubation, the
migrated cells on the lower chamber were stained with 0.2% crystal
violet at room temperature for 30 min and observed under a light
microscope (Leica Microsystems Ltd.; cat. no. DMLB2).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (v7.0; GraphPad Software, Inc.). All experiments
were performed in triplicate. The comparison between two matched
samples was analyzed with paired Student's t-test. One-way ANOVA
followed by Tukey's post hoc test was used to compare differences
between multiple groups. All experimental data are presented as the
mean ± SD. P<0.05 was considered to indicate a statistically
significant difference.
Results
HTFs are successfully isolated and
LINC01605 knockdown decreases the viability of HTFs successfully
isolated from patients with POAG
Matching HS and healthy tissues were collected from
patients with POAG who underwent glaucoma filtration surgery at
Fuyang People's Hospital, and RT-qPCR analysis was performed to
detect LINC01605 expression levels. The results demonstrated that
LINC01605 expression was higher in HS tissues compared with that in
normal (control/healthy) tissues (Fig.
1A). Subsequently, HTFs and corneal epithelial cells were
isolated from the aforementioned tissues. In addition, it has been
reported that vimentin is a fibrocyte marker (25). Vimentin is one of the main
components of medium fiber and plays an important role in
cytoskeleton and motility (26,27).
Furthermore, keratin is the main component of cytoskeletal proteins
(28). Immunofluorescence assays
indicated that vimentin was highly expressed in HTFs and in corneal
epithelial cells, whereas keratin was expressed at a low level in
HTFs (Fig. 1B).
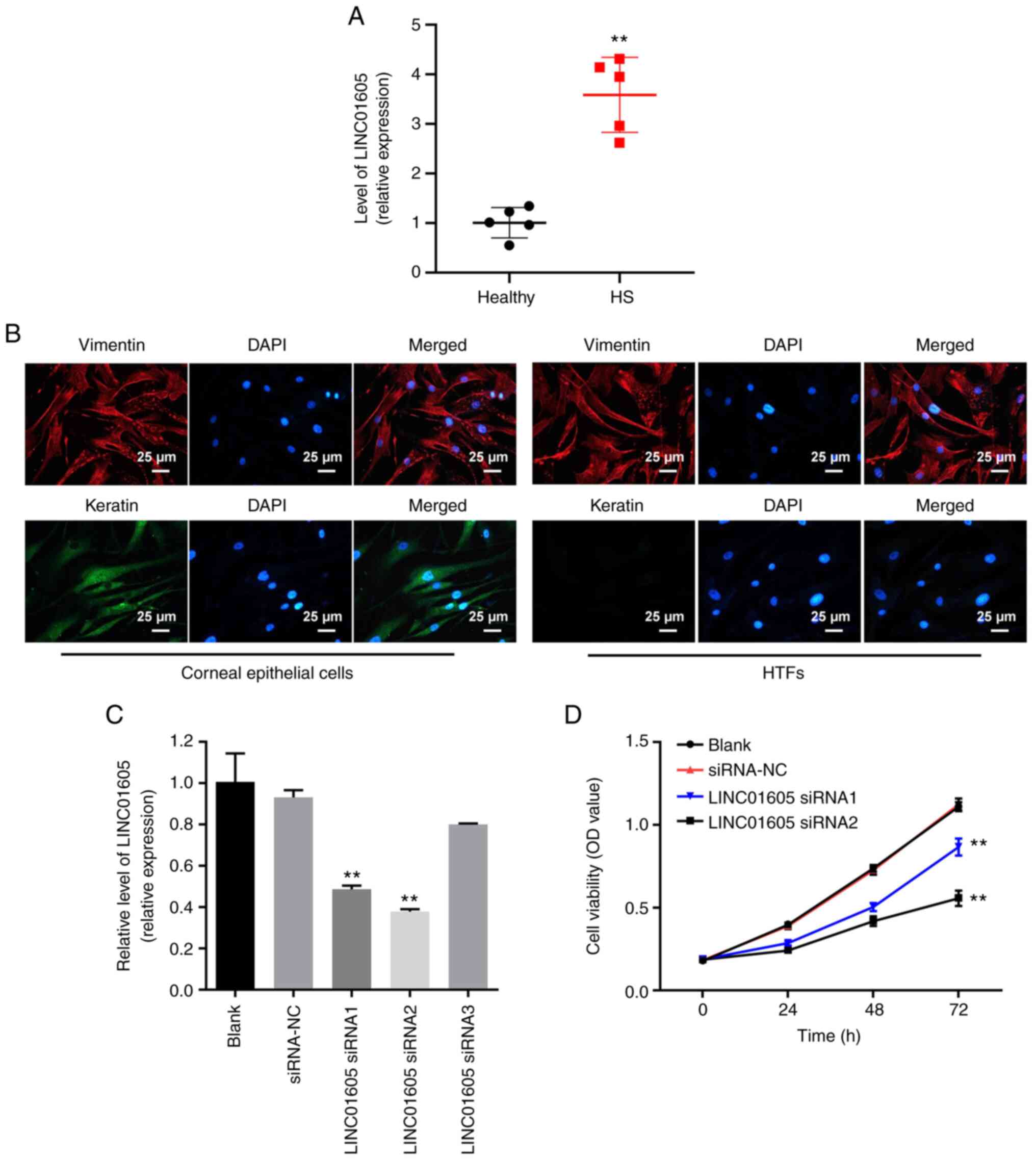 | Figure 1Successful isolation of HTFs and
LINC01605 knockdown significantly inhibits the viability of HTFs
from patients with POAG. (A) RT-qPCR was performed to detect the
expression of LINC01605 in HS or normal (control/healthy) corneal
tissues. (B) Immunofluorescence staining was performed to detect
the expression of vimentin and keratin in corneal epithelial cells
and HTF. Scale bar, 25 µm. (C) HTFs were transfected with siRNA-NC,
LINC01605 siRNA1, LINC01605 siRNA2 or LINC01605 siRNA3, and RT-qPCR
was performed to detect the expression levels of LINC01605. (D)
Cell Counting Kit-8 assay was used to detect the viability of HTFs.
n=3; **P<0.01 vs. siRNA-NC or healthy/blank group.
HS, hypertrophic scar; HTFs, human Tenon's capsule fibroblasts; NC,
negative control; OD, optical density; POAG, primary open-angle
glaucoma; RT-qPCR, reverse transcription-quantitative PCR; siRNA,
small interfering RNA. |
LINC01605 expression significantly decreased in HTFs
following transfection with LINC01605 siRNAs (Fig. 1C). The results indicated that
LINC01605 siRNA1 and LINC01605 siRNA2 had a stronger knockdown
effect compared with LINC0165 siRNA3, thus these two siRNAs were
selected for subsequent experiments (Fig. 1C). LINC01605 siRNA1 and LINC01605
siRNA2 significantly inhibited the viability of HTFs compared with
siRNA-NC (Fig. 1D). These results
suggested that HTFs were successfully isolated and LINC01605 siRNA
significantly inhibited the viability of HTFs.
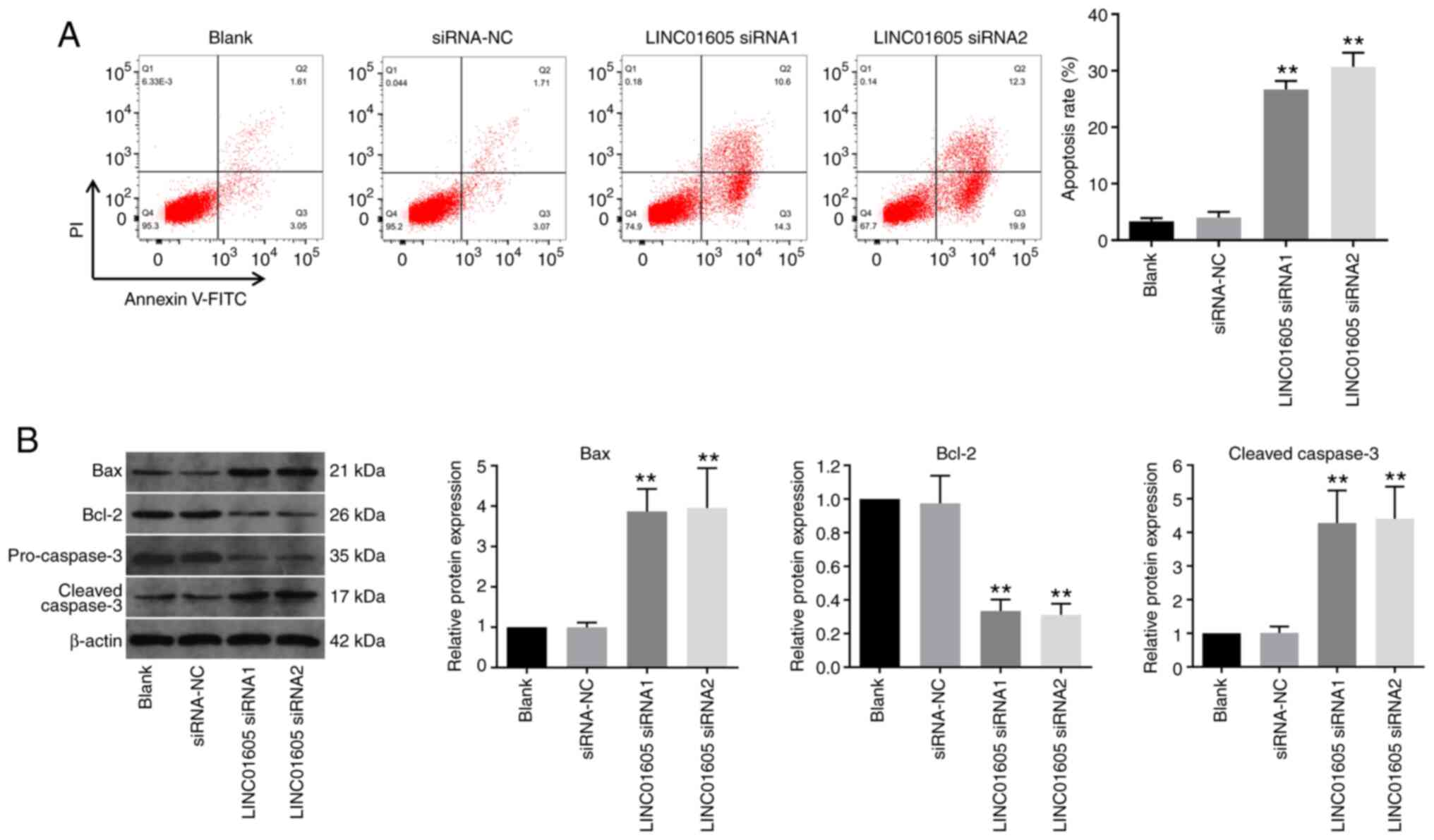
LINC01605 knockdown induces apoptosis
in HTFs
To investigate the function of LINC01605 in HTFs,
flow cytometry was performed. As presented in Fig. 2A, LINC01605 knockdown significantly
induced apoptosis in HTFs compared with the siRNA-NC or blank
group. In addition, transfection with LINC01605 siRNAs notably
increased the expression levels of apoptosis-related proteins Bax
and cleaved caspase-3, and decreased Bcl-2 expression in HTFs,
compared with the siRNA-NC or blank group (Fig. 2B). Collectively, these results
suggested that LINC01605 knockdown significantly induced apoptosis
in HTFs.
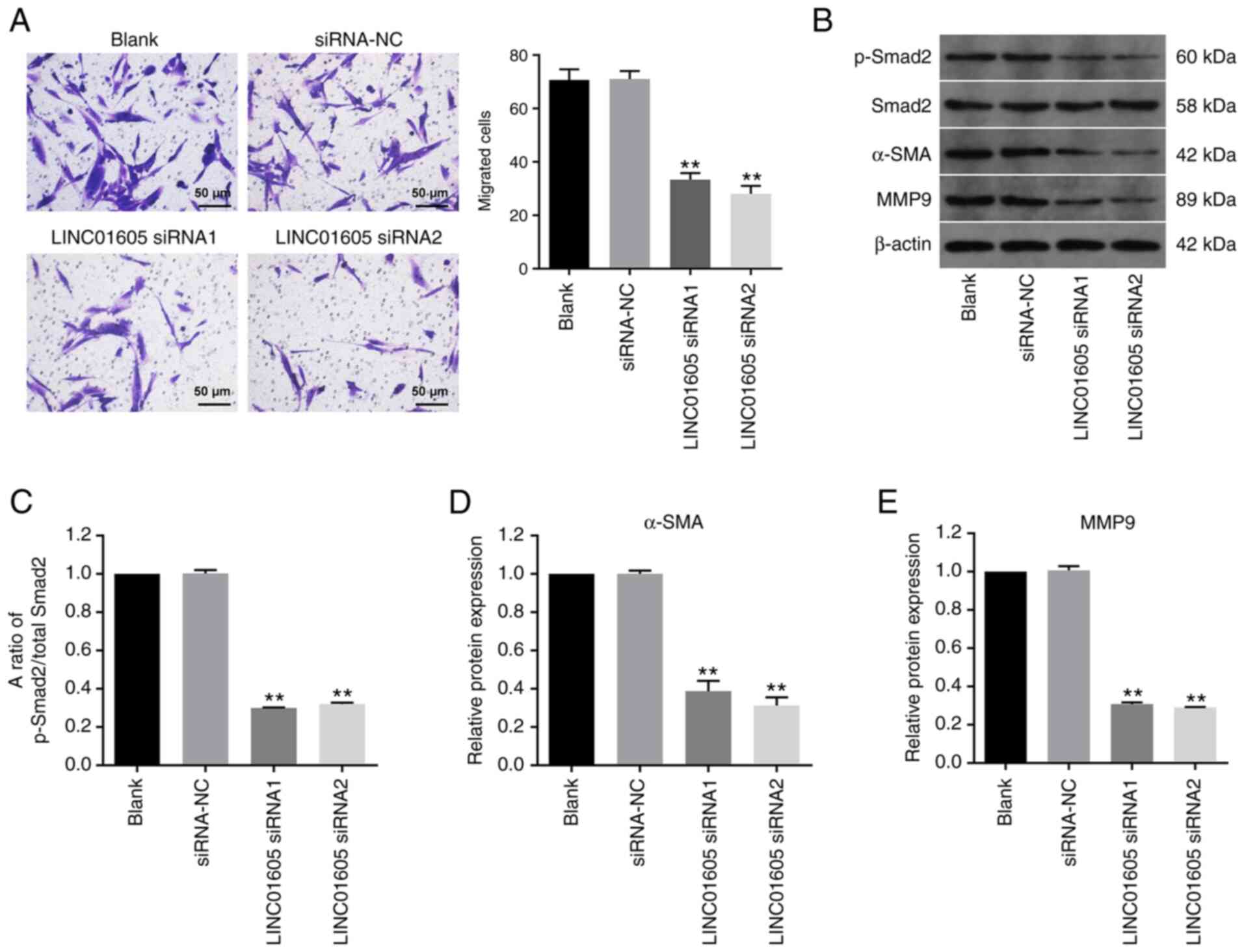
LINC01605 knockdown inhibits the
migration of HTFs
Transwell assays were performed to investigate the
effect of LINC01605 on the migratory ability of HTFs. The results
demonstrated that LINC01605 knockdown significantly inhibited the
migratory ability of HTFs compared with the siRNA-NC or blank group
(Fig. 3A). In addition, p-Smad2,
α-SMA and MMP9 are important proteins in the process of fibrosis
(29-31).
Western blot analysis demonstrated that transfection with LINC01605
siRNAs significantly downregulated the ratio of p-Smad2/total
Smad2, and the expression levels of α-SMA and MMP9 in HTFs,
compared with the siRNA-NC or blank group (Fig. 3B-E). Taken together, these results
suggested that LINC01605 knockdown inhibited the migratory ability
and the fibrosis process of HTFs.
LINC01605 knockdown inhibits autophagy
in HTFs
To determine the association between LINC01605 and
autophagy, immunofluorescence staining of LC3 was performed. The
results demonstrated that transfection with LINC01605 siRNA
significantly decreased LC3 expression in HTFs compared with the
siRNA-NC or blank group (Fig. 4A).
In addition, the expression levels of autophagy-related proteins
were examined. Transfection with LINC01605 siRNAs notably decreased
ATG7 and beclin 1 expression, and increased p62 expression in HTFs,
compared with the siRNA-NC or blank group (Fig. 4B-E). Since AMPK/mTOR has been
reported to serve a crucial role in autophagy, the function of
LINC01605 in this signaling pathway was investigated (32,33).
Western blotting results demonstrated that transfection with
LINC01605 siRNA markedly decreased p-AMPK expression in HTFs
compared with the siRNA-NC or Blank group (Fig. 4B and F), indicating that LINC01605 knockdown
markedly inhibited autophagy in HTFs. Moreover, it has been
reported that 3-methyladenine (3-MA), which is an autophagy
inhibitor, could inhibit the formation of autophagosomes (34,35).
Given that HTFs were sensitive to LINC01605 siRNA2 (Fig. 1C), it was selected for use in
subsequent experiments. LINC01605 siRNA2 significantly inhibited
the viability of HTFs compared with the siRNA-NC or blank group,
and 3-MA exacerbated this phenomenon (Fig. 4G). These results indicated that
LINC01605 knockdown inhibited autophagy in HTFs.
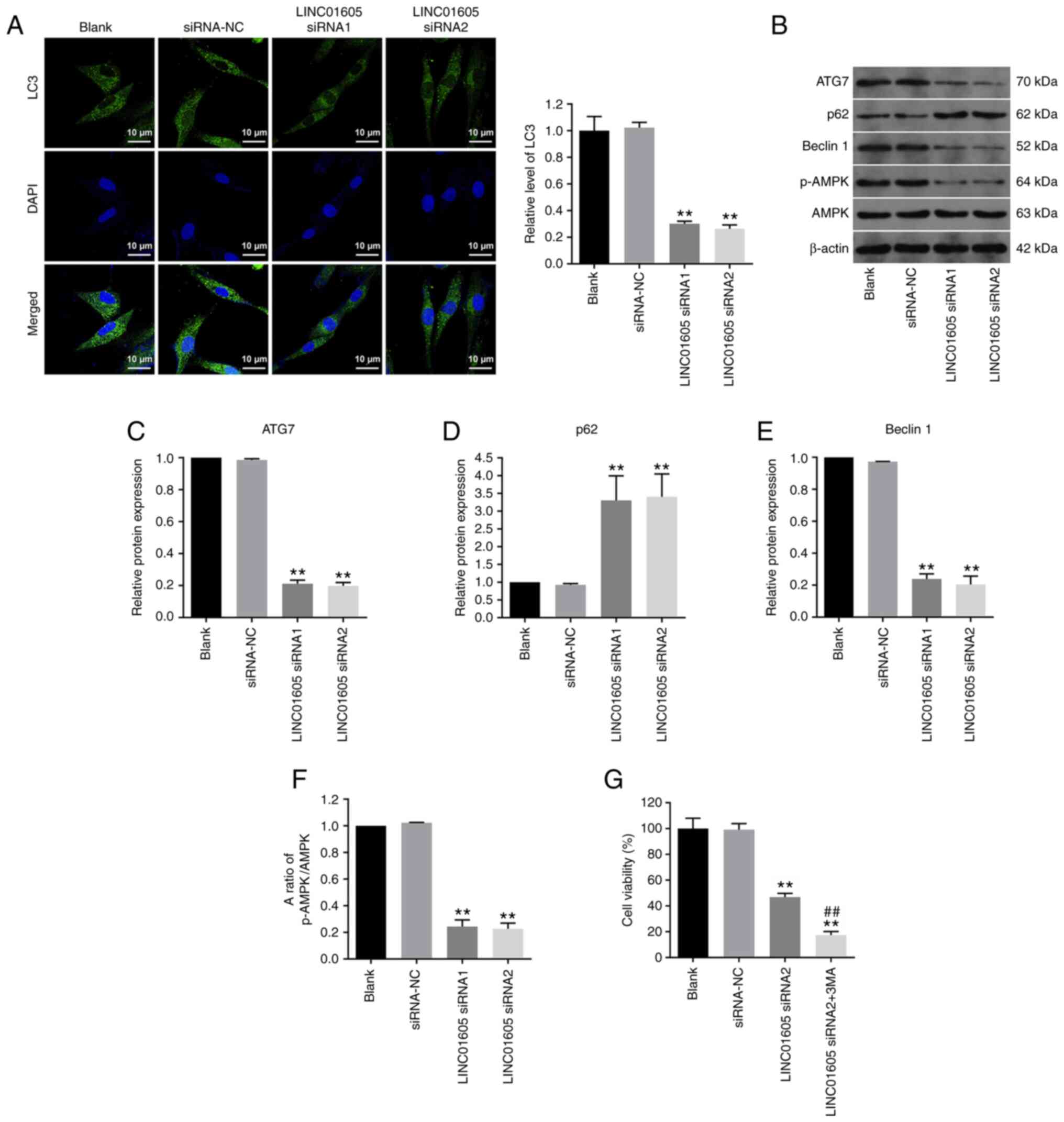 | Figure 4Knockdown of LINC01605 inhibits HTF
autophagy. (A) Immunofluorescence staining was performed to detect
the expression of LC3 in HTFs. Scale bar, 10 µm. (B) Western blot
assays were performed to detect and semi-quantify the expression
levels of (C) ATG7, (D) p62, (E) beclin 1, (F) p-AMPK and AMPK in
HTFs. (G) Cell Counting Kit-8 assay was used to detect the
viability of HTFs. n=3; **P<0.01 vs. siRNA-NC or
Blank group; ##P<0.01 vs. LINC01605 siRNA2 group.
AMPK, AMP-activated protein kinase; ATG7, autophagy-related 7;
HTFs, human Tenon's capsule fibroblasts; NC, negative control; p,
phosphorylated; siRNA, small interfering RNA. |
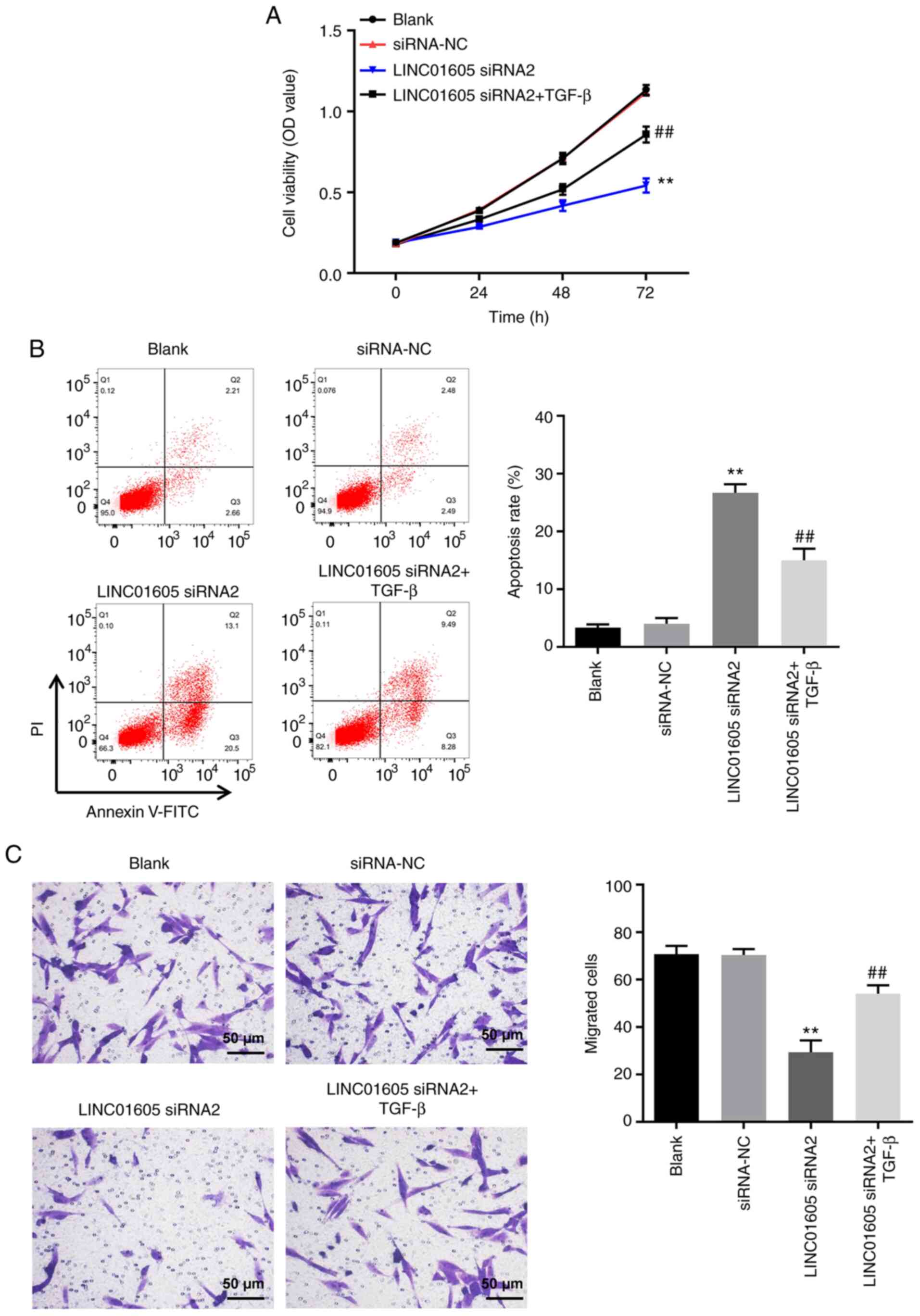
LINC01605 knockdown-induced apoptosis
in HTFs is reversed by TGF-β
It has been reported that TGF-β is closely
associated with autophagy and fibrosis (36). To further investigate the mechanism
by which LINC01605 regulates the formation of HS, rescue
experiments were performed. As presented in Fig. 5A and B, LINC01605 knockdown notably inhibited
the viability of HTFs by inducing apoptosis, compared with the
siRNA-NC or blank group, the effects of which were reversed
following treatment with TGF-β. In addition, LINC01605 knockdown
notably inhibited the migration of HTFs, compared with siRNA-NC or
Blank group, and the effects of which were reversed following
treatment with TGF-β (Fig. 5C).
Taken together, these results suggested that LINC01605
siRNA2-induced apoptosis of HTFs was reversed by TGF-β.
Discussion
It has been reported that LINC01605 serves an
important role in the formation of HS (15). The results of the present study
confirmed that LINC01605 expression was upregulated in HS tissues
compared with that in healthy tissues. This observation was similar
to that published in a previous study (37). In the present study, LINC01605 was
knocked down, which inhibited HTF migration and fibrotic phenotype,
induced apoptosis and inhibited autophagy. Overexpression of
LINC01605 has been indicated to promote the migration of bladder
cancer cells (38); moreover, Zhu
et al (15) reported that
blocking LINC01605 inhibited the M2 macrophage-induced migration,
invasion and proliferation of human dermal fibroblasts. In the
present study, it was found that knockdown of LINC01605 inhibited
HTF migration and fibrotic phenotype. The present study results
were consistent with previous reports (15).
A previous study reported that the formation of HS
was attributed to the inhibition of apoptosis in p53-deficient mice
(39). During the formation and
development of HS, cell proliferation and migration are promoted,
and apoptosis is inhibited (40).
The present study assessed Bax, Bcl-2 and cleaved caspase-3 as
apoptosis-related proteins (41-43).
The results demonstrated that LINC01605 knockdown significantly
induced the apoptosis of HTFs, which was consistent with previous
findings (39,40).
LC3 is widely used for the detection of autophagy
levels (44-46).
When autophagy increases, the number of LC3 puncta in cells
significantly increase (47). In
the present study, LINC01605 knockdown notably decreased LC3
expression in HTFs, suggesting that autophagy was inhibited in HTF.
In addition, it is well known that the association between
apoptosis and autophagy is highly complex (48-50).
For example, Chakrabarti and Ray (51) indicated that the natural flavonoid
luteolin induced glioblastoma cell apoptosis by inhibiting
autophagy. Moreover, Cao et al (52) reported that autophagy inhibitors
could promote apoptosis and thus effectively inhibit the formation
of HS. In addition, Deng et al (53) demonstrated that oxymatrine, an
alkaloid isolated from plants, was able to promote HS repair by
inhibiting autophagy and inducing apoptosis. Based on the results
of the present study, it was suggested that LINC01605 knockdown may
induce the apoptosis of HTFs by inhibiting autophagy, which is
consistent with previous research (51-53).
In addition, the present study results indicated that LINC01605
knockdown downregulated the expression levels of p-Smad2, α-SMA and
MMP9 in HTFs. Proteins (α-SMA and MMP9) that affect the Smad
pathway and induce epithelial-mesenchymal transition are involved
in fibrosis (54,55). Notably, in the present study, the
results (p-Smad2, α-SMA and MMP9 expression, characteristic of
fibrosis/fibrotic phenotype) indicated that LINC01605 knockdown
inhibited the fibrotic phenotype of HTFs.
TGF-β is closely associated with autophagy and
fibrosis (36). For example,
autophagy can downregulate TGF-β expression and inhibit renal
fibrosis (56). In addition, Wu
et al (57) indicated that
quercetin, a natural flavonoid, prevented liver fibrosis by
inhibiting autophagy. Moreover, Liu et al (58) reported that isorhamnetin, which is
a flavonol aglycone isolated from the plant Hippophae rhamnoides
L., could also inhibit liver fibrosis by reducing autophagy.
All these data suggested that TGF-β and autophagy serve an
important role in the process of fibrosis. Therefore, these results
collectively suggested that TGF-β, autophagy and fibrosis are
closely related. Thus, TGF-β was used to perform rescue experiments
in the present study.
The present study has certain limitations. For
example, the mechanism through which LINC01605 regulates the Smad
pathway remains unclear. In addition, the association between
LINC01605 and cell autophagy has not been extensively investigated.
RNA in situ hybridization of LINC01605 in the patients'
tissue sections could not be provided owing to limited experimental
conditions. Moreover, the expression levels of LINC01605 among
patients with POAG at different disease stages could not be
compared owing to the lack of adequate samples at different disease
stages.
In conclusion, results from the present study
demonstrated that LINC01605 knockdown inhibited the viability of
HTFs by inducing apoptosis and suggested that LINC01605 knockdown
may provide novel directions for the treatment of HS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QS made major contributions to the conception and
design of the study, as well as drafting and revising the
manuscript. YY and HL were responsible for data acquisition,
including conducting the experiments, data analysis, data
interpretation and manuscript revision. QS, YY and HL confirm the
authenticity of all the raw data. All authors agreed to be
accountable for all aspects of the work. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Fuyang People's Hospital (Hangzhou, China), and
written informed consent was provided by all patients prior to the
study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bluwol E: Glaucoma treatment. Rev Prat.
66:508–513. 2016.PubMed/NCBI(In French).
|
|
2
|
Weinreb RN, Aung T and Medeiros FA: The
pathophysiology and treatment of glaucoma: A review. JAMA.
311:1901–1911. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sihota R, Angmo D, Ramaswamy D and Dada T:
Simplifying ‘target’ intraocular pressure for different stages of
primary open-angle glaucoma and primary angle-closure glaucoma.
Indian J Ophthalmol. 66:495–505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Swogger J, Conner IP, Rosano M, Kemmerer
M, Happ-Smith C, Wells A, Schuman JS and Yates CC: Injected versus
sponge-applied mitomycin C (MMC) during modified trabeculectomy in
New Zealand white rabbit model. Transl Vis Sci Technol.
9(23)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vajaranant TS, Wu S, Torres M and Varma R:
The changing face of primary open-angle glaucoma in the United
States: Demographic and geographic changes from 2011 to 2050. Am J
Ophthalmol. 154:303–314.e3. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ma X and Liu L: Knockdown of FAM225B
inhibits the progression of the hypertrophic scar following
glaucoma surgery by inhibiting autophagy. Mol Med Rep.
23(204)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu X, Wang Z, Wu G, Xu X, Zhang J, Li Y,
Zhang H and Guo S: Tetramethylpyrazine induces apoptosis and
inhibits proliferation of hypertrophic scar-derived fibroblasts via
inhibiting the phosphorylation of AKT. Front Pharmacol.
11(602)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee HJ and Jang YJ: Recent understandings
of biology, prophylaxis and treatment strategies for hypertrophic
scars and keloids. Int J Mol Sci. 19(711)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang P, Luo ML, Song E, Zhou Z, Ma T, Wang
J, Jia N, Wang G, Nie S, Liu Y and Hou F: Long noncoding RNA
lnc-TSI inhibits renal fibrogenesis by negatively regulating the
TGF-β/Smad3 pathway. Sci Transl Med. 10(eaat2039)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie
Y, Wang K, Jia W, Chu WM and Sun B: The long noncoding RNA lnc-EGFR
stimulates T-regulatory cells differentiation thus promoting
hepatocellular carcinoma immune evasion. Nat Commun.
8(15129)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zheng M, Zheng Y, Gao M, Ma H, Zhang X, Li
Y, Wang F and Huang H: Expression and clinical value of lncRNA
MALAT1 and lncRNA ANRIL in glaucoma patients. Exp Ther Med.
19:1329–1335. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cissé Y, Bai L and Meng T: LncRNAs in
genetic basis of glaucoma. BMJ Open Ophthalmol.
3(e000131)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nong Q, Li S, Wu Y and Liu D: LncRNA
COL1A2-AS1 inhibits the scar fibroblasts proliferation via
regulating miR-21/Smad7 pathway. Biochem Biophys Res Commun.
495:319–324. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li J, Chen L, Cao C, Yan H, Zhou B, Gao Y,
Li Q and Li J: The long non-coding RNA LncRNA8975-1 is upregulated
in hypertrophic scar fibroblasts and controls collagen expression.
Cell Physiol Biochem. 40:326–334. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu Z, Chen B, Peng L, Gao S, Guo J and
Zhu X: Blockade of LINC01605-enriched exosome generation in M2
macrophages impairs M2 macrophage-induced proliferation, migration,
and invasion of human dermal fibroblasts. Int J Immunopathol
Pharmacol. 35(20587384211016724)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang XC, Wang T, Zhang Y, Wang LL, Zhao RY
and Tan W: Tacrolimus inhibits proliferation and induces apoptosis
by decreasing survivin in scar fibroblasts after glaucoma surgery.
Eur Rev Med Pharmacol Sci. 22:2934–2940. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jammal AA, Berchuck SI, Thompson AC, Costa
VP and Medeiros FA: The effect of age on increasing susceptibility
to retinal nerve fiber layer loss in glaucoma. Invest Ophthalmol
Vis Sci. 61(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Deflorin C, Hohenauer E, Stoop R, van
Daele U, Clijsen R and Taeymans J: Physical management of scar
tissue: A systematic review and meta-analysis. J Altern Complement
Med. 26:854–865. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Seong GJ, Hong S, Jung SA, Lee JJ, Lim E,
Kim SJ and Lee JH: TGF-beta-induced interleukin-6 participates in
transdifferentiation of human Tenon's fibroblasts to
myofibroblasts. Mol Vis. 15:2123–2128. 2009.PubMed/NCBI
|
|
20
|
Trelford CB, Denstedt JT, Armstrong JJ and
Hutnik CML: The pro-fibrotic behavior of human Tenon's capsule
fibroblasts in medically treated glaucoma patients. Clin
Ophthalmol. 14:1391–1402. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ran W, Zhu D and Feng Q: TGF-β2 stimulates
Tenon's capsule fibroblast proliferation in patients with glaucoma
via suppression of miR-29b expression regulated by Nrf2. Int J Clin
Exp Pathol. 8:4799–4806. 2015.PubMed/NCBI
|
|
22
|
Song L, Liu H, Ma L, Zhang X, Jiang Z and
Jiang C: Inhibition of autophagy by 3-MA enhances endoplasmic
reticulum stress-induced apoptosis in human nasopharyngeal
carcinoma cells. Oncol Lett. 6:1031–1038. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang J, Xiao L, Luo CH, Zhou H, Zeng L,
Zhong J, Tang Y, Zhao XH, Zhao M and Zhang Y: CD44v6 promotes
β-catenin and TGF-β expression, inducing aggression in ovarian
cancer cells. Mol Med Rep. 11:3505–3510. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang T, Gao X, Chen S, Li D, Chen S, Xie
M, Xu Z and Yang G: Genome-wide identification and expression
analysis of ethylene responsive factor family transcription factors
in Juglans regia. PeerJ. 9(e12429)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lassance L, Marino GK, Medeiros CS,
Thangavadivel S and Wilson SE: Fibrocyte migration, differentiation
and apoptosis during the corneal wound healing response to injury.
Exp Eye Res. 170:177–187. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Battaglia RA, Delic S, Herrmann H and
Snider NT: Vimentin on the move: New developments in cell
migration. F1000Res 7: F1000 Faculty Rev-1796, 2018.
|
|
27
|
Patteson AE, Carroll RJ, Iwamoto DV and
Janmey PA: The vimentin cytoskeleton: When polymer physics meets
cell biology. Phys Biol. 18(011001)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Polari L, Alam CM, Nyström JH, Heikkilä T,
Tayyab M, Baghestani S and Toivola DM: Keratin intermediate
filaments in the colon: Guardians of epithelial homeostasis. Int J
Biochem Cell Biol. 129(105878)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu Y, Lu S, Huang X, Liu Y, Huang K, Liu
Z, Xu W, Zhu W, Hou J, Liu H and Zhang X: Targeting cIAPs
attenuates CCl(4)-induced liver fibrosis by increasing MMP9
expression derived from neutrophils. Life Sci.
289(120235)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li CY, Zhang JR, Li XX, Zhao L, Xi H, Hu
WN and Li SN: Lefty1 ameliorates post-infarction fibrosis by
suppressing p-Smad2 and p-ERK1/2 signaling pathways. J Cardiovasc
Transl Res. 14:636–646. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chang J, Lan T, Li C, Ji X, Zheng L, Gou
H, Ou Y, Wu T, Qi C, Zhang Q, et al: Activation of Slit2-Robo1
signaling promotes liver fibrosis. J Hepatol. 63:1413–1420.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li MY, Zhu XL, Zhao BX, Shi L, Wang W, Hu
W, Qin SL, Chen BH, Zhou PH, Qiu B, et al: Adrenomedullin
alleviates the pyroptosis of Leydig cells by promoting autophagy
via the ROS-AMPK-mTOR axis. Cell Death Dis. 10(489)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Han D, Jiang L, Gu X, Huang S, Pang J, Wu
Y, Yin J and Wang J: SIRT3 deficiency is resistant to
autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway
and promoting GPX4 levels. J Cell Physiol. 235:8839–8851.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cao H, Jia Q, Yan L, Chen C, Xing S and
Shen D: Quercetin suppresses the progression of atherosclerosis by
regulating MST1-mediated autophagy in ox-LDL-Induced RAW264.7
macrophage foam cells. Int J Mol Sci. 20(6093)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He Z, Guo L, Shu Y, Fang Q, Zhou H, Liu Y,
Liu D, Lu L, Zhang X, Ding X, et al: Autophagy protects auditory
hair cells against neomycin-induced damage. Autophagy.
13:1884–1904. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xia Y, Li J, Chen K, Feng J and Guo C:
Bergenin attenuates hepatic fibrosis by regulating autophagy
mediated by the PPAR-γ/TGF-β pathway. PPAR Res.
2020(6694214)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang X, Song W, Zhang F and Huang R:
Dihydroartemisinin Inhibits TGF-β-induced fibrosis in human tenon
fibroblasts via inducing autophagy. Drug Des Devel Ther.
15:973–981. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Correction: High LINC01605 expression
predicts poor prognosis and promotes tumor progression via
upregulation of MMP9 in bladder cancer. Biosci Rep 40:
BSR-20180562_COR, 2020.
|
|
39
|
Aarabi S, Bhatt KA, Shi Y, Paterno J,
Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H and Gurtner GC:
Mechanical load initiates hypertrophic scar formation through
decreased cellular apoptosis. FASEB J. 21:3250–3261.
2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jiang D, Guo B, Lin F, Lin S and Tao K:
MiR-205 inhibits the development of hypertrophic scars by targeting
THBS1. Aging (Albany NY). 12:22046–22058. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang Y, Yang X, Ge X and Zhang F:
Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved
caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid
hemorrhage mice. Biomed Pharmacother. 109:726–733. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dolka I, Król M and Sapierzyński R:
Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved
caspase-3 and p53) expression in canine mammary tumors: An
immunohistochemical and prognostic study. Res Vet Sci. 105:124–133.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhao T, Fu Y, Sun H and Liu X:
Ligustrazine suppresses neuron apoptosis via the Bax/Bcl-2 and
caspase-3 pathway in PC12 cells and in rats with vascular dementia.
IUBMB Life. 70:60–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tanida I, Ueno T and Kominami E: LC3 and
autophagy. Methods Mol Biol. 445:77–88. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Schaaf MB, Keulers TG, Vooijs MA and
Rouschop KM: LC3/GABARAP family proteins: Autophagy-(un)related
functions. FASEB J. 30:3961–3978. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Runwal G, Stamatakou E, Siddiqi FH, Puri
C, Zhu Y and Rubinsztein DC: LC3-positive structures are prominent
in autophagy-deficient cells. Sci Rep. 9(10147)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752.
2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fernández A, Ordóñez R, Reiter RJ,
González-Gallego J and Mauriz JL: Melatonin and endoplasmic
reticulum stress: Relation to autophagy and apoptosis. J Pineal
Res. 59:292–307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lv W, Jiang J, Li Y, Fu L, Meng F and Li
J: MiR-302a-3p aggravates myocardial ischemia-reperfusion injury by
suppressing mitophagy via targeting FOXO3. Exp Mol Pathol.
117(104522)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chakrabarti M and Ray SK: Anti-tumor
activities of luteolin and silibinin in glioblastoma cells:
Overexpression of miR-7-1-3p augmented luteolin and silibinin to
inhibit autophagy and induce apoptosis in glioblastoma in vivo.
Apoptosis. 21:312–328. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cao C, Wang W, Lu L, Wang L, Chen X, Guo
R, Li S and Jiang J: Inactivation of Beclin-1-dependent autophagy
promotes ursolic acid-induced apoptosis in hypertrophic scar
fibroblasts. Exp Dermatol. 27:58–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Deng X, Zhao F, Zhao D, Zhang Q, Zhu Y,
Chen Q, Qiang L, Xie N, Ma J, Pan X, et al: Oxymatrine promotes
hypertrophic scar repair through reduced human scar fibroblast
viability, collagen and induced apoptosis via autophagy inhibition.
Int Wound J: Nov 8, 2021 (Epub ahead of print).
|
|
54
|
Jia L, Sun P, Gao H, Shen J, Gao Y, Meng
C, Fu S, Yao H and Zhang G: Mangiferin attenuates bleomycin-induced
pulmonary fibrosis in mice through inhibiting TLR4/p65 and
TGF-β1/Smad2/3 pathway. J Pharm Pharmacol. 71:1017–1028.
2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhang Q, Chang X, Wang H, Liu Y, Wang X,
Wu M, Zhan H, Li S and Sun Y: TGF-β1 mediated Smad signaling
pathway and EMT in hepatic fibrosis induced by Nano NiO in vivo and
in vitro. Environ Toxicol. 35:419–429. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Nam SA, Kim WY, Kim JW, Park SH, Kim HL,
Lee MS, Komatsu M, Ha H, Lim JH, Park CW, et al: Autophagy
attenuates tubulointerstital fibrosis through regulating
transforming growth factor-β and NLRP3 inflammasome signaling
pathway. Cell Death Dis. 10(78)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wu L, Zhang Q, Mo W, Feng J, Li S, Li J,
Liu T, Xu S, Wang W, Lu X, et al: Quercetin prevents hepatic
fibrosis by inhibiting hepatic stellate cell activation and
reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci
Rep. 7(9289)2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Liu N, Feng J, Lu X, Yao Z, Liu Q, Lv Y,
Han Y, Deng J and Zhou Y: Isorhamnetin inhibits liver fibrosis by
reducing autophagy and inhibiting extracellular matrix formation
via the TGF-β1/Smad3 and TGF-β1/p38 MAPK pathways. Mediators
Inflamm. 2019(6175091)2019.PubMed/NCBI View Article : Google Scholar
|