Introduction
Ankylosing spondylitis (AS) is a progressive and
debilitating form of arthritis with predominant onset before the
age of 40, characterized by lower back pain and morning stiffness
(1,2). Statistics indicate that one in 200
individuals may suffer from AS; however, a conclusive diagnosis is
often made several years after the onset of symptoms (3). The average prevalence of AS is
approximately 0.1-1.4% with slight male patient predominance
(4). The pathogenesis of AS has
been associated with several genetic factors and histocompatibility
leukocyte antigen (HLA)-B27(5). AS
presents with numerous complications such as impaired spinal
mobility, postural abnormalities, buttock pain, hip pain,
peripheral arthritis, enthesitis and dactylitis (6). Under poor treatment, AS may progress
to severe disability and impair the quality of life (7). The pathogenesis of AS is not fully
determined yet and effective treatment methods should be
investigated. Furthermore, the development of novel treatment
approaches is crucial.
MicroRNAs (miRNAs) are small non-coding endogenously
expressed RNAs that can serve as regulators of gene expression
(8). Essentially, miRNAs can
modulate cellular differentiation, inflammation and immune
responses (9). miRNAs can also
modulate the interaction between fibroblasts and osteoclasts in AS
progression (10). An existing
study determined the ability of miR-148a to serve as a potential
disease-modifying agent in osteoarthritis (11). Moreover, an elevated expression of
miR-148a was identified during the osteogenic differentiation of
rat bone marrow-derived mesenchymal stem cells (BMSCs) (12). Moreover, miR-148a-3p could regulate
adipocyte and osteoblast differentiation (13). miR-148a-3p in extracellular
vesicles derived from BMSCs alleviates osteonecrosis of the femoral
head (14). Fibroblasts have been
implicated as vital components in the ossification and ankylosis of
ligament tissues (15).
Accumulating research has established an association between the
excessive proliferation of fibroblasts and heterotopic ossification
with AS (16,17). However, the role of miR-148a-3p in
osteogenic differentiation of human AS fibroblasts remains to be
elucidated.
Dickkopf homologue 1 (DKK1) serves as a crucial
component in the osteogenic differentiation of fibroblast in AS
(18). DKK1 plays a fundamental
role in the pathogenesis of rheumatoid arthritis (19). Increasing evidence has elicited the
regulation of DKK1 by different miRNAs in the osteogenic
differentiation of fibroblasts in AS (20,21).
The hypothesis is that miR-148a-3p participates in the osteogenic
differentiation of fibroblasts in AS by regulating DKK1 expression.
The aim of the present study was to determine miR-148a-3p
expression in AS fibroblasts and its functional mechanism and to
identify new therapeutic targets for osteogenic differentiation of
AS fibroblasts.
Materials and methods
Ethic statement
The present study was performed in accordance with
the Helsinki Declaration (22) and
the experiment procedures were conducted with approval of the
Ethics Committee of Seventh People's Hospital of Shanghai
University of Traditional Chinese Medicine (Shanghai, China;
approval no. 2017-IRBQYYS-057). All patients signed the informed
consent.
Human samples
A total of 20 AS patients hospitalized in the
Seventh People's Hospital of Shanghai University of Traditional
Chinese Medicine from May 2017 to May 2019 were chosen for sample
collection. The 20 patients had undergone surgical intervention of
total hip replacement due to severe and persistent pain worsening
quality of life due to involvement of the hip joint on the basis of
the 1984 modified New York criteria of American Rheumatism
Association (23). The patients
presented with notable findings such as inflammatory low backache,
ossification of ankle joint, positive HLA-B27, and increased
C-reactive protein (CRP) level and erythrocyte sedimentation rate
(ESR). The samples used in the control group were provided by 20
non-AS patients who required hip replacement due to fracture of the
femoral neck (excluding other types of osteoarthritis) caused by
blunt trauma. The capsular ligament tissues were isolated during
surgical intervention. Fibroblasts dissociated from ligament
tissues were cultured in Dulbecco's modified Eagle's medium (DMEM)
with 10% fetal bovine serum (FBS; Zhejiang Tianhang Biotechnology
Co., Ltd.).
Cell isolation and culture
Fibroblasts were isolated from the capsular ligament
tissues of the enrolled patients. The ligament tissues were
sectioned at 0.5 mm3 and rinsed twice with
phosphate-buffered saline (PBS). The ligament sections were placed
in plates containing 5 ml of serum-free DMEM and 0.2 µg/ml Collagen
I (Thermo Fisher Scientific, Inc.). The collagen fibers were
removed using a 0.22-µm filter (MilliporeSigma) at 120 g. The
precipitated cells were cultured in DMEM containing 20% serum and
1% streptomycin in 5% CO2 for 72 h at 37˚C. The ligament
sections were removed after observable growth of the fibroblasts
from tissue fragments and adhered to the plate. The DMEM was
replaced every 3 days. Fibroblasts were divided at the ratio of 1:3
and cultured to 80-90% confluence. The 3rd generation fibroblasts
were used for subsequent experimentation. Briefly, the fibroblasts
were cultured in normal medium supplemented containing a
combination of 0.1 µl/l dexamethasone, 10 mmol/l β-glycerophosphate
and 50 µl/l ascorbic acid to induce osteogenic differentiation
(24).
Cell staining
Alizarin red staining was performed to detect the
degree of calcification during heterotopic ossification. After a
PBS rinse, the cells in 24-well plates were fixed with 95% ethanol
for 30 min at 37˚C. Following fixation, the cells stained with
Alizarin red solution (Sigma-Aldrich) were incubated for 30 min at
37˚C. Immunohistochemical staining (IHC) was performed on
fibroblast with vimentin antibody (at a dilution ratio of 1:250;
catalog no. ab92547; Abcam) using an IHC kit (Wuhan Boster
Biological Technology Co., Ltd.) in strict accordance with the
provided instructions. Hematoxylin and eosin (H&E) and BCIP/NBT
staining were conducted based on the provided instructions of the
corresponding kits (Nanjing Jiancheng Bioengineering
Institute).
Cell transfection
Lipofectamine® 2000 (cat. no. 11668-019,
Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect
the inhibitor negative control (NC), miR-148a-3p inhibitor, si-NC,
and si-DKK1 (Shanghai GeneChem Co., Ltd.) (miRNA-inhibitor 50 nM,
miRNA-mimic 30 nM, si-RNA 40-100 nM) into the experimental or 293T
cells. The sequence of miR-148a-3p inhibitor, inhibitor NC, si-DKK1
and si-NC are presented in Table
I. Briefly, miR-148a-3p inhibitor, si-DKK1, and corresponding
controls were delivered into target cells using Lipofectamine
RNAIMAX Transfection kit (Invitrogen; Thermo Fisher Scientific,
Inc.) as per the protocol. Cells were seeded in 6-well plates
(1x106 cells/well) one day prior to transfection. On the
day of transfection, Lipofectamine RNAIMAX (1 µl) reagent was
thoroughly mixed with 100 µl opti-MEM culture medium (Thermo Fisher
Scientific) and the transfection complex at room temperature for 10
min and added in the 6-well plates. After 48-h transfection, the
cells were detached with trypsin (Thermo Fisher Scientific) and
washed once with PBS for subsequent experimentation. Cell grouping
was as follows: the control group (fibroblasts of non-AS patients),
the AS group (fibroblasts of AS patients), the AS + inhibitor-NC
group (AS fibroblasts transfected with inhibitor NC), the AS +
inhibitor-miR group (AS fibroblasts transfected with miR-148a-3p
inhibitor), the AS + inhibitor-miR + si-NC group (AS fibroblasts
transfected with miR-148a-3p and si-NC), and the AS + inhibitor-miR
+ si-DKK1 group (AS fibroblasts transfected with miR-148a-3p
inhibitor and si-DKK1).
 | Table ISequences of miR-148a-3p inhibitor,
inhibitor NC, si-DKK-1 and si-NC. |
Table I
Sequences of miR-148a-3p inhibitor,
inhibitor NC, si-DKK-1 and si-NC.
| Name of primer | Sequences
(5'-3') |
|---|
| miR-148a-3p
inhibitor |
ACAAAGTTCTGTAGTGCACTGA |
| Inhibitor NC |
TCTATGTGAAGTCACGAAGTCA |
| si-DKK1 |
AAAAUGACCGUCACUUUGCAA |
| si-NC |
UGAACCGAAAUCAAUUCCAUG |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA content was extracted from the
ligament tissues or cells using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA was reverse transcribed into cDNA using the
cDNA reverse transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Quantitative PCR was amplified using the
SYBR® Premix Ex Taq™ kit (Takara Bio, Inc.). U6 or GAPDH
served as the internal control. The reaction conditions were:
Pre-denaturation at 95˚C for 120 sec, and 40 cycles of denaturation
at 95˚C for 15 sec and extension at 60˚C for 60 sec. Relative
expression was calculated based on the 2-ΔΔCq method
(25). The experiments on each
sample were conducted three times independently. PCR primers are
presented in Table II.
 | Table IIRT-qPCR primer sequences. |
Table II
RT-qPCR primer sequences.
| Name of primer | Sequences |
|---|
| miR-148a-3p-F |
TCAGTGCACTACAGAACTTTGT |
| miR-148a-3p-R |
GAATACCTCGGACCCTGC |
| DKK1-F |
GGTATTCCAGAAGAACCACCTTG |
| DKK1-R |
CTTGGACCAGAAGTGTCTAGCAC |
| GAPDH-F |
CATCACCATCTTCCAGGAGCG |
| GAPDH-R |
TGACCTTGCCCACAGCCTTG |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
Western blot analysis
The total protein content was isolated from the
ligament tissues or cells by radio immunoprecipitation assay lysis
buffer and centrifuged at 4˚C, 12,000 x g for 10 min. Protein
concentration was determined using the bicinchoninic acid kit. The
protein sample (30 µg) was separated by 8% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes. A membrane blockade was
conducted using PBS containing 5% skimmed milk for 2 h at room
temperature. Subsequently, the primary antibodies were added for
incubation at 4˚C overnight. The membranes were co-incubated with
the HRP-conjugated goat anti-rabbit IgG (at a dilution ratio of
1:2,000, ab97051, Abcam) secondary antibody for 2 h. Protein bands
were detected using an enhanced chemiluminescence kit (Thermo
Fisher Scientific) and estimated using the Image J software.
Primary antibodies included in the present study were: runt-related
gene 2 (RUNX2; at a dilution ratio of 1:5,000, ab76956, Abcam),
Osteocalcin (at a dilution ratio of 1:1,000, ab133612, Abcam), DKK1
(at a dilution ratio of 1:1,000, ab109416, Abcam), Wnt1 (at a
dilution ratio of 1:1,000, ab15251, Abcam), β-catenin (1:5,000,
ab32572, Abcam), and p-β-catenin (at a dilution ratio of 1:5,000,
ab75777, Abcam).
Dual-luciferase reporter assay
The binding sites of miR-148a-3p and DKK1 were
predicted using the Starbase database (http://starbase.sysu.edu.cn/), RNAInter (http://www.rna-society.org/raid/search.html),
Jefferson (https://cm.jefferson.edu/rna22/Precomputed/), and
miRDB (http://mirdb.org/) websites. The binding and
mutation sequences were cloned to the luciferase vector pGL3
(Promega Corporation) to construct the wild-type (DKK1-wt) and
mutation-type (DKK1-mut) luciferase plasmids. The 293T cells (ATCC)
were seeded in 6-well plates (2x105 cells/well) and
subsequently incubated for 24 h. The constructed luciferase vectors
and mimic NC or miR-148a-3p mimic (5'-UCAGUGCACUACAGAACUUUGU-3')
(Shanghai GeneChem) (miRNA-mimic 30 nM) were co-transfected into
the 293T cells using Lipofectamine 2000 in strict accordance with
the provided instructions. Luciferase activity was detected using
the dual-luciferase reporter assay kit (Beijing Solarbio Science
& Technology Co., Ltd.) after 24 h of transfection. The cell
experiments were conducted three times independently.
Statistical analysis
Statistical data were processed using the SPSS 21.0
statistical software (IBM Corp.). Data were all measurement data.
The experimental data are presented as mean ± standard deviation
(SD). GraphPad Prism 8.0 (GraphPad Software Inc.) was utilized for
graphing. Normal distribution of data was assessed using the
Shapiro-Wilk test. Data comparisons between two groups were
analyzed using an unpaired t-test, and data comparisons among
multiple groups were analyzed using one-way analysis of variance
(ANOVA), followed by Tukey's multiple comparisons test. The P-value
was obtained through two-sided test. In all statistical references,
a value of P<0.05 was considered to indicate statistical
significance.
Results
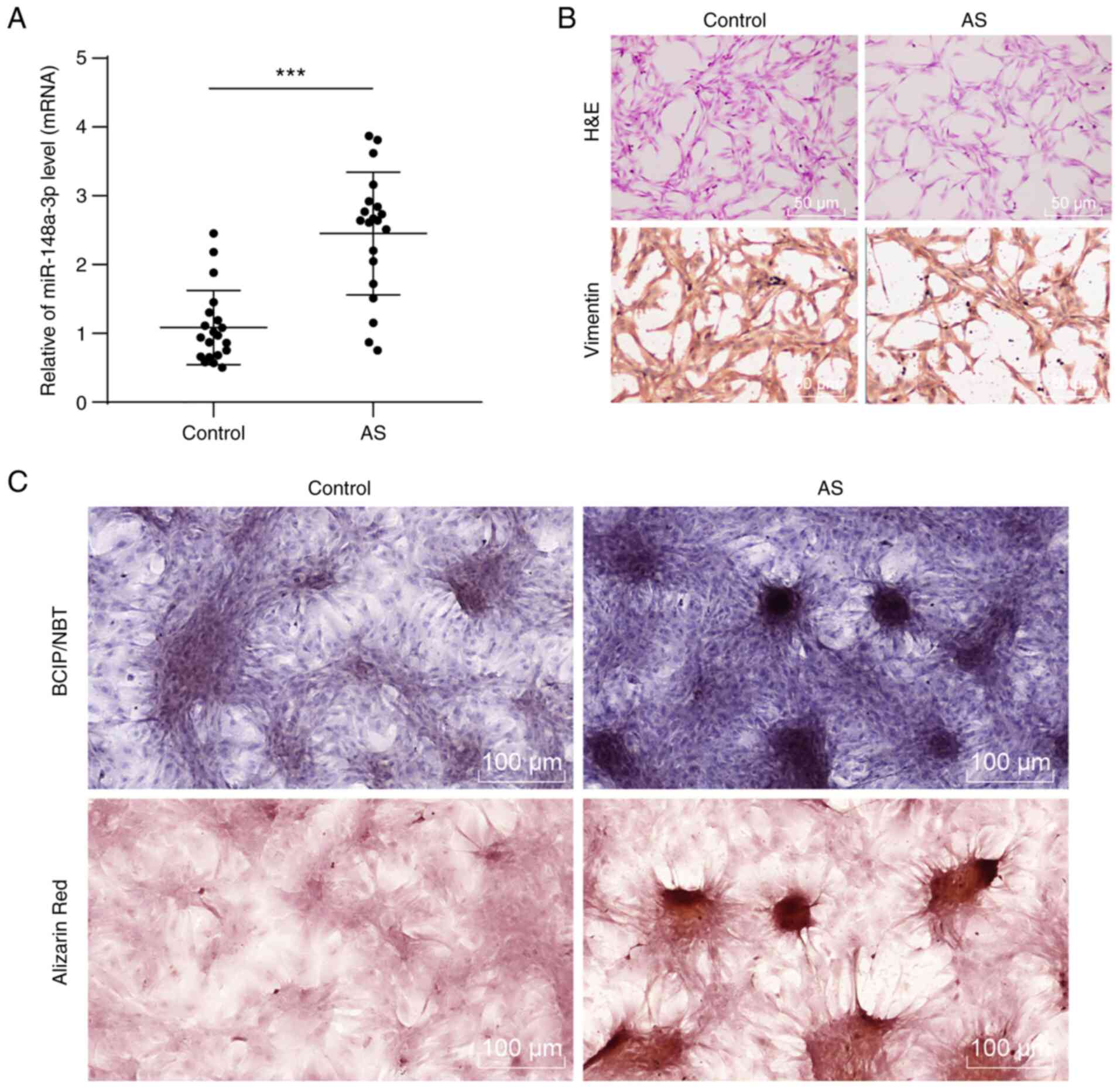
miR-148a-3p was highly expressed in
fibroblasts of AS patients
Initially, the miR-148a-3p expression pattern in the
harvested ligament tissues was detected by RT-qPCR, with results
revealing that the miR-148a-3p expression pattern in the ligament
tissues of AS patients had increased relative to the non-AS
patients (Fig. 1A; P<0.001).
Fibroblasts were identified by H&E staining and IHC staining
(Fig. 1B). Fusiform fibroblasts
with regular ellipsical nucleus in the control group were
identified while long fusiform or flat star-shaped fibroblasts were
observed in the AS group (P<0.001). IHC staining showed a
positive expression pattern of the specific fibroblast marker
vimentin (18) in normal
fibroblasts and a decreased expression pattern of fibroblast marker
vimentin (in dark brown) in AS group compared to the control group
(P<0.001). Following induction of osteogenic differentiation,
the results of BCIP/NBT and Alizarin red staining showed an
increase in calcified nodules with mineralization degree in
fibroblasts of the AS group relative to the control group (Fig. 1C; P<0.001). These results
indicated that miR-148a-3p may participate in the osteogenic
differentiation of fibroblasts.
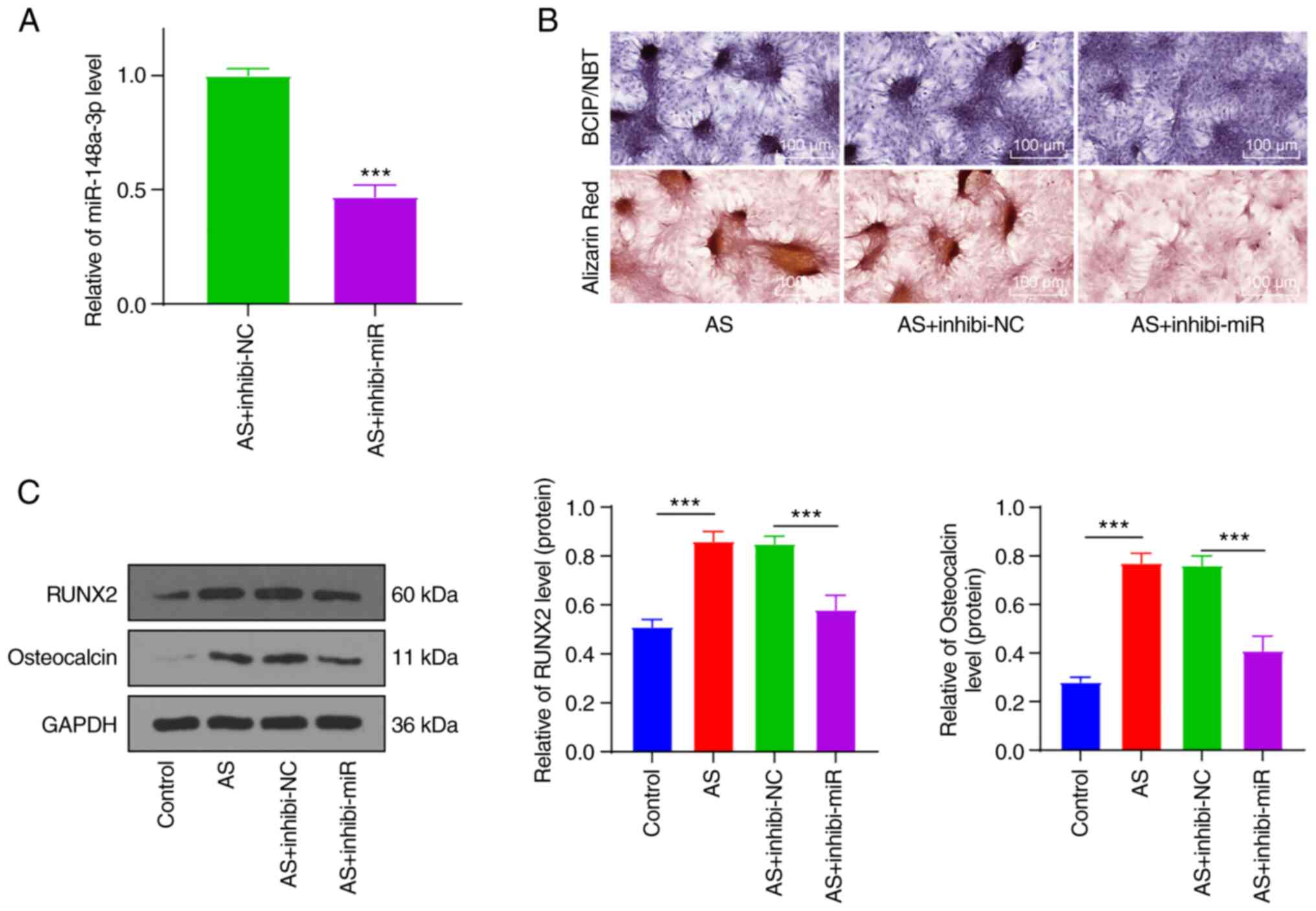
Silencing miR-148a-3p inhibited the
osteogenic differentiation of AS fibroblasts
To further investigate the function of miR-148a-3p,
the miR-148a-3p inhibitor was transfected into the AS fibroblasts
(Fig. 2A). BCIP/NBT and Alizarin
red staining showed that silencing miR-148a-3p reversed the degree
of increased calcified nodules and mineralization (Fig. 2B). Protein levels of osteogenic
proteins RUNX2(26) and
Osteocalcin (27) were detected by
western blot analysis (Fig. 2C).
The result showed that levels of RUNX2 and Osteocalcin were
significantly increased in AS fibroblasts (P<0.001), but were
reduced in AS fibroblasts transfected with the miR-148a-3p
inhibitor (P<0.001). These results demonstrated that silencing
miR-148-3p could inhibit the osteogenic differentiation of AS
fibroblasts.
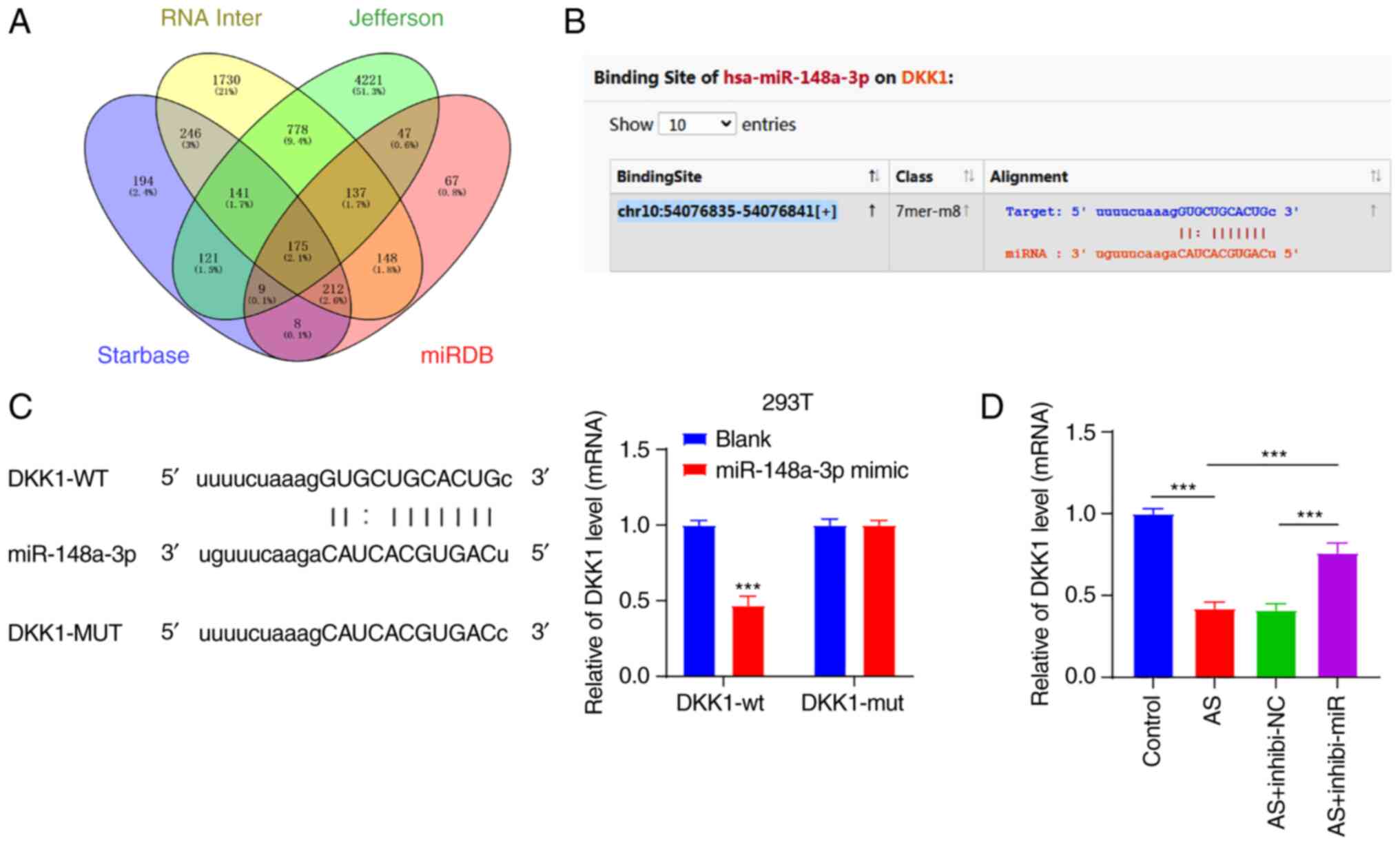
miR-148a-3p targeted DKK1
To examine the functional mechanism of miR-148a-3p
in AS fibroblasts, the downstream target genes of miR-148a-3p were
predicted through a combination of the Starbase database
(http://starbase.sysu.edu.cn/), RNAInter
(http://www.rna-society.org/raid/search.html),
Jefferson (https://cm.jefferson.edu/rna22/Precomputed/) and miRDB
(http://mirdb.org/) websites, and intersections were
determined (Fig. 3A). DKK1 was
identified among the intersections; the significance of DKK1 in the
osteogenic differentiation of AS fibroblasts was previously
indicated (18). Thus, miR-148a-3p
affected AS fibroblasts via DKK1. To verify our hypothesis, the
binding sites of miR-148a-3p and DKK1 were initially predicted
through the Starbase website (Fig.
3B), and their binding relation was verified by a
dual-luciferase reporter assay in the 293T cells (Fig. 3C). Subsequently, the DKK1
expression pattern was detected in fibroblasts by RT-qPCR (Fig. 3D). The result showed that the DKK1
expression pattern was downregulated in AS fibroblasts
(P<0.001), while silencing miR-148a-3p inverted the
downregulation of DKK1 (P<0.001). These results suggested that
miR-148a-3p targeted DKK1.
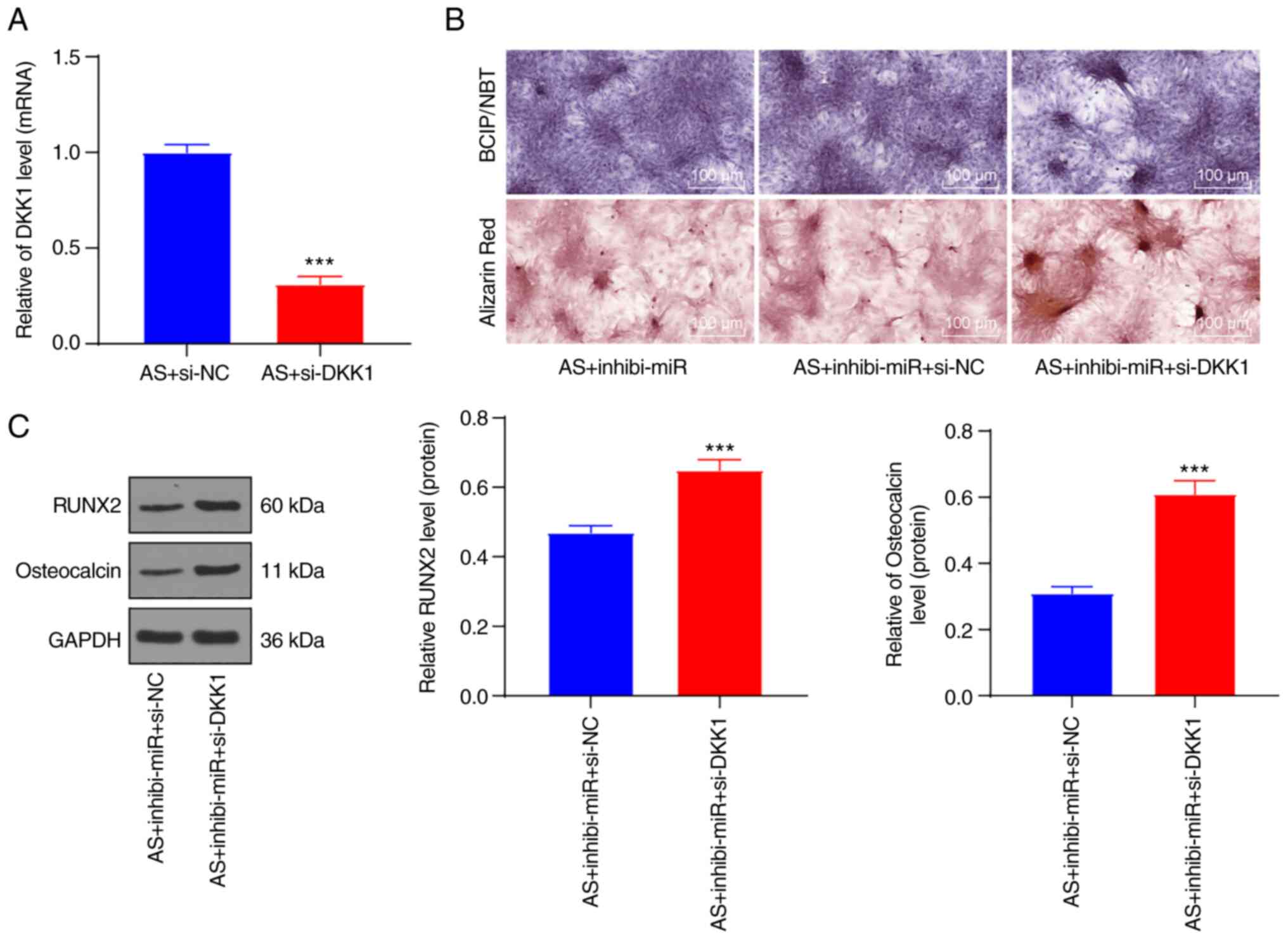
DKK1 knockdown reversed the inhibitory
effect of miR-148a-3p knockdown on the osteogenic differentiation
of AS fibroblasts
To verify the preceding results, si-DKK1 was
transfected into the AS fibroblasts treated with silencing
miR-148a-3p to observe the effect on AS fibroblasts. First of all,
the transfection efficiency of si-DKK1 was verified by means of
RT-qPCR (Fig. 4A). Subsequently,
BCIP/NBT and Alizarin red staining showed that DKK1 knockdown in AS
fibroblasts with silencing miR-148a-3p could increase calcified
nodules and the mineralization degree (Fig. 4B). Western blot analysis showed
that the downregulation of RUNX2 and Osteocalcin induced by
miR-148a-3p inhibitor was reversed after DKK1 knockdown (Fig. 4C; P<0.001). Briefly, DKK1
knockdown could invert the inhibition of silencing miR-148a-3p on
the osteogenic differentiation of AS fibroblasts.
miR-148a-3p affected the osteogenic
differentiation of AS fibroblasts by modulating Wnt/β-catenin
pathway via DKK1 regulation
The participation of Wnt proteins has been
identified in the osteogenic differentiation of AS fibroblasts.
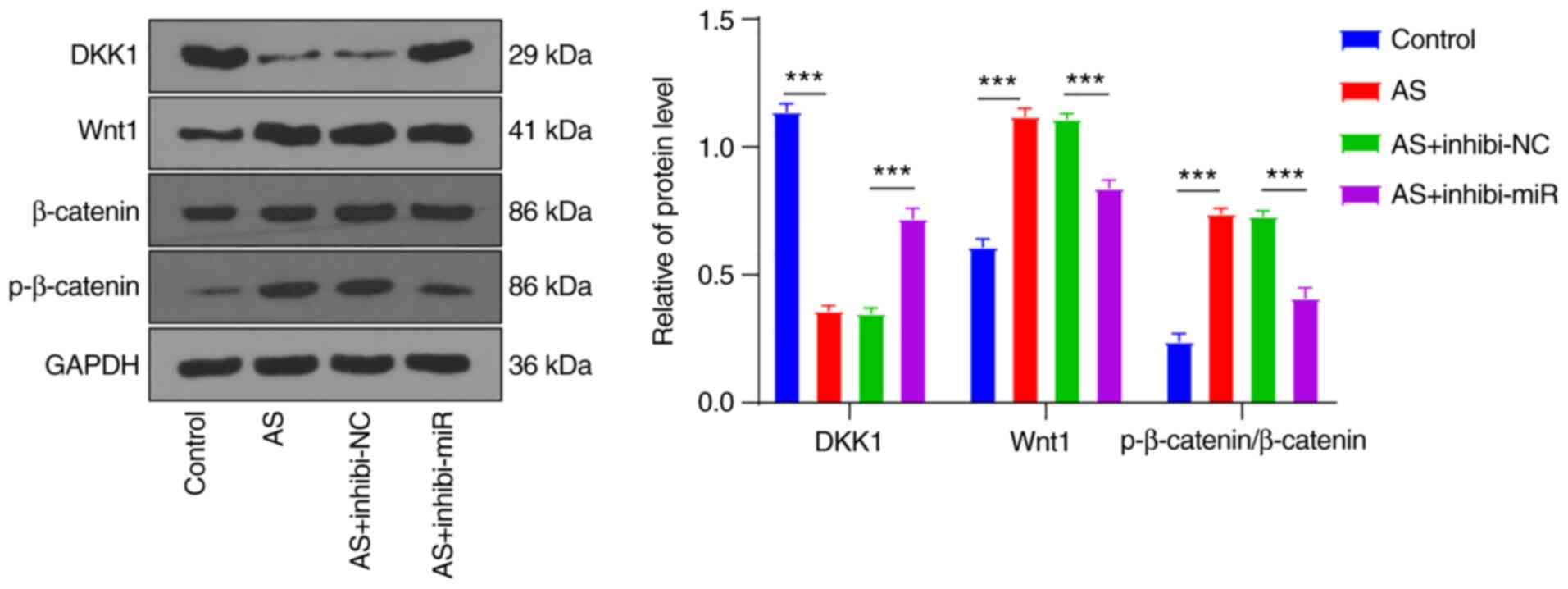
Therefore, the levels of DKK1, Wnt protein Wnt1, and
p-β-catenin/β-catenin in AS fibroblasts with silenced miR-148a-3p
were determined by western blot analysis with controls in AS
fibroblasts revealing overexpression of DKK1 (Fig. 5). The result showed that the
protein changes of DKK1 were consistent with the RT-qPCR result.
Protein levels of Wnt1 and p-β-catenin/β-catenin were elevated in
AS fibroblasts (all P<0.001), while silencing miR-148a-3p
reversed the upregulation of Wnt1 and p-β-catenin/β-catenin
proteins (all P<0.001), which was consistent with the result of
overexpressing DKK1 in AS fibroblasts. The aforementioned results
revealed that miR-148a-5p suppressed the DKK1 expression pattern
and thus activated the Wnt/β-catenin pathway for participation in
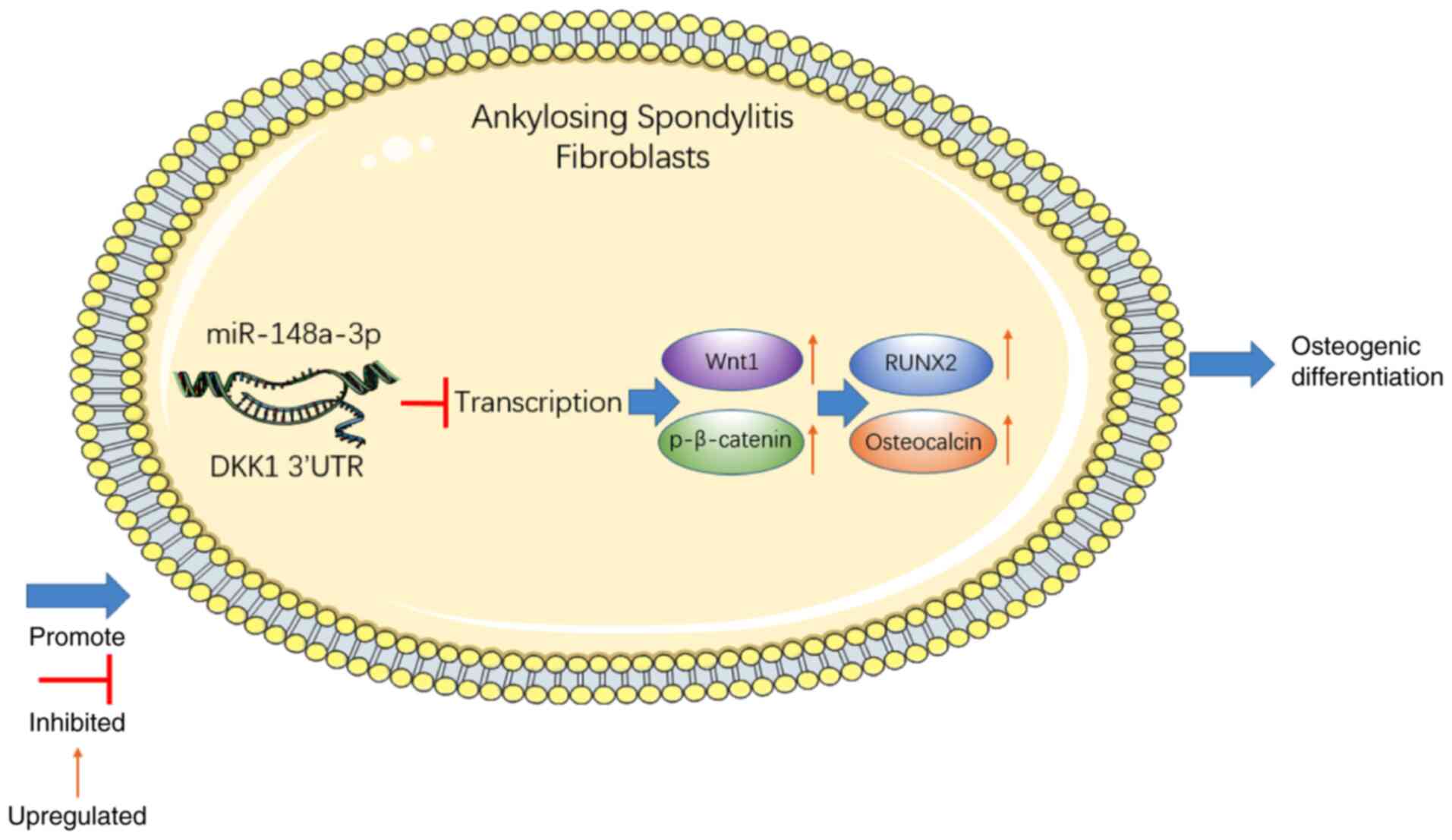
the osteogenic differentiation of AS fibroblasts (Fig. 6).
Discussion
AS may manifest as a multifocal disorder with
numerous symptoms, affecting skeletal and extra skeletal organs and
can radically increase the risk of multiple diseases (6). Several miRNAs, endogenous, non-coding
small RNAs which can regulate mRNA gene expression, have been
suggested to serve as definitive markers or therapeutic targets of
AS (8). Numerous miRNAs have been
implicated in the fundamental functionality of AS physiological and
pathological processes (10). In
the present study, the effect of miR-148a-3p on AS was evaluated,
and the results highlighted that miR-148a-3p stimulated the
osteogenic differentiation of AS fibroblasts by inhibiting the DKK1
expression and activating the Wnt pathway. Heo et al
observed a weakly positive osteogenic differentiation of
fibroblasts during identification of the differentiation capability
of bone marrow mesenchymal stem cells with fibroblasts as negative
controls (28). According to Ding
et al, osteogenic differentiation of fibroblasts occurs in
AS patients (24). The present
findings identified the property of osteogenic differentiation of
fibroblasts in AS patients, and therefore the molecular mechanism
of osteogenic differentiation with fibroblasts in AS patients was
examined.
As a common and genetically heterozygous
inflammatory rheumatic disease, AS is characterized by progressive
ankylosis and inflammation of hip, sacroiliac joints and spine, and
new bone formation; miRNAs also show different expression patterns
with the development of AS (8).
miR-148a was upregulated in rheumatoid arthritis (10). Moreover, previous findings
determined the ability of miR-148a-3p to regulate adipocyte or
osteoblast differentiation by targeting lysine-specific demethylase
6b (13). Newly initiated
ossification of ligaments is characteristic of AS with progression
of pathological bone formation leading to loss of joint function
and disability (29).
Additionally, miR-148a-3p is a potential contributor to heterotopic
ossification in AS in light of preceding literature (30). In the present study, an elevated
miR-148a-3p expression was identified in the AS ligament tissues.
Osteogenic differentiation of fibroblasts is the primary cause of
osteophyte formation and ankylosis in AS (31). Previous results validated the
functionality of vimentin fragments as potential markers of
rheumatoid synovial fibroblasts (32). Findings of the present study
denoted a reduced expression of vimentin in AS fibroblasts, while
the concentration of calcified nodules and mineralization degree
were increased. Moreover, the miR-148a-3p from BMSCs-derived
extracellular vesicles can facilitate the osteogenic
differentiation in osteonecrosis of the femoral head (14). The current results validated the
participation of miR-148a-3p in the osteogenic differentiation of
fibroblasts in AS. To further verify the effect of miR-148a-3p on
AS fibroblasts, the miR-148a-3p inhibitor was transfected into AS
fibroblasts, the result of which suggested that the calcified
nodules and mineralization degree were increased. Research has
implicated RUNX2 and Osteocalcin as vital factors in osteoblast
differentiation (33,34). The results of this study
demonstrated markedly elevated expressions of RUNX2 and Osteocalcin
in AS fibroblasts. However, the upregulation was reversed by
silencing miR-148a-3p in AS fibroblasts. Similarly, miR-148a-3p
downregulation could impede the differentiation of rabbit
preadipocytes (35). The
aforementioned results suggested that miR-148a-3p knockdown
radically inhibited the osteogenic differentiation of AS
fibroblasts.
To examine the downstream mechanism of miR-148a-3p
in AS fibroblasts, the downstream target genes of miR-148a-3p were
predicted. A negative correlation was identified between the DKK1
level and AS severity (36).
Therefore, miR-148a-3p could impact AS fibroblasts via DKK1. The
binding sites of miR-148a-3p and DKK1 were predicted using the
Starbase website. The target association of miR-148a-3p and DKK1
was verified by the dual-luciferase reporter assay. The results of
the present study identified the downregulation of DKK1 in AS
fibroblasts; however; the downregulation could be abolished after
silencing miR-148a-3p. Briefly, miR-148a-3p could target DKK1. The
effects of the downregulation of DKK1 were determined in a previous
study, where this downregulation exacerbated fibroblast
proliferation and enhanced the osteogenesis of fibroblasts in AS
(37). In the present study, the
concentration of calcified nodules and mineralization degree were
increased after DKK1 knockdown. The results showed that the
downregulation of RUNX2 and Osteocalcin mediated by silencing
miR-148a-3p was inverted after DKK1 knockdown. Pathologically, the
downregulation of DKK1 can facilitate the osteogenic
differentiation of human adipose-derived MSCs in the progression of
bone repair and reverse the suppression of primary human osteoblast
differentiation (38,39). Conjointly, findings of this study
denoted that DKK1 knockdown reversed the inhibition of silencing
miR-148a-3p in the osteogenic differentiation of AS
fibroblasts.
DKK1 is a natural inhibitor of the Wnt pathway
(40,41). Wnt proteins are essential for
normal bone homeostasis, especially in osteoblastic new bone
formation (42-44).
Wnt1 is a protein that can activate the Wnt pathway with β-catenin
serving as a mediator (45). In
the current study, miR-148a-3p in the fibroblasts of AS patients
regulated DKK1 transcription, evidenced by deviations in the
expressions of Wnt1 and p-β-catenin proteins while silencing
miR-148a-3p reversed the downregulation. Thus, a fundamental role
of DKK1 in AS fibroblasts as an endogenous inhibitor of the Wnt
pathway was indicated. Wnt1 in the canonical Wnt signaling pathway
is predicted as the target gene of hsa-miR-148a-3p with significant
regulation in osteoporosis (46).
The ability of human recombinant DKK1 to facilitate the
differentiation of adipose-derived stem cells via the Wnt signaling
pathway was determined (47).
Moreover, miR-148a can modulate adipocyte differentiation of MSCs
via the Wnt signaling (48). DKK1
has been reported to serve as an endogenous inhibitor of the Wnt
pathway in vivo (49). The
aforementioned results suggest that miR-148a-3p in fibroblasts of
AS patients regulated DKK1 transcription, accompanied by expression
alterations in Wnt1 and p-β-catenin proteins. Thus, that DKK1 plays
an essential role in AS fibroblasts as an endogenous inhibitor of
the Wnt pathway. Collectively, results of the present study
identified the participation of miR-148a-3p in osteogenic
differentiation by inhibiting DKK1 expression and activating the
Wnt/β-catenin pathway.
To conclude, the current study demonstrated that the
high expression of miR-148a-3p promoted the osteogenic
differentiation of AS fibroblasts by downregulating DKK1 and
activating the Wnt/β-catenin pathway. Novel insight has been
provided for the management of osteogenic differentiation of AS
fibroblasts. Nevertheless, the participation of miR-148a-3p in the
osteogenic differentiation of AS fibroblasts was not verified in an
animal experiment, and therefore requires subsequent validation.
Moreover, the osteogenic differentiation of AS fibroblasts may be
modulated by multiple pathways including the Wnt/β-catenin pathway.
Whether miR-148a-3p affects the osteogenic differentiation of AS
fibroblasts by modulating other pathways requires further
investigation. Lastly, the effect of AS on patients comes mainly
from the ankylosis of spine, which is often caused by heterotopic
ossification. The molecular mechanism in AS from the perspective of
heterotopic ossification was also examined. Fibroblasts were
isolated from the ligaments of AS and non-AS patients, cultured
with the same medium, and osteogenically induced with the same
method, followed by observation of the effect of AS on osteogenic
differentiation of fibroblasts. However, apoptosis-related changes
were not detected in this study. ANKH, a multichannel transmembrane
protein, has been reported to affect the metabolism of AS
fibroblasts and inhibit fibroblast viability, ossification and
mineralization (50). Recent
findings have elucidated that ANKH plays a regulatory role in
fibroblast viability, ossification and mineralization (50). In the current study, the Starbase
database predicted ANKH as a downstream target gene of miR-148a-3p.
miR-148a-3p might modulate fibroblasts via ANKH. We predicted that
ANKH is the downstream target gene of miR-148a-3p on Starbase
database, and further exploration should be conducted. Future
studies should focus on exploring whether miR-148a-3p can serve as
the new target for the clinical treatment for osteogenic
differentiation of AS fibroblasts, whether miR-148-3p can regulate
the osteogenic differentiation of AS fibroblasts through any other
mechanisms and the molecular mechanism of miR-148a-3p in
fibroblasts via targeting ANKH.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by Shanghai Municipal Health
Commission as a prospective study comparing the efficacy of
trans-paravertebral muscle space with percutaneous pedicle screw
internal fixation for thoracolumbar fracture (grant no.
20194Y0211).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS, SL and HJ substantially contributed to the
conception and the design of the study. WS and HY were responsible
for the acquisition, analysis and interpretation of the data. HJ,
SL and HY contributed to manuscript drafting or critical revisions
of the intellectual content. WS and HJ approved the final
manuscript to be published, and SL agreed to be accountable for all
aspects of the work, so that any questions relating to research
integrity or scientific accuracy in any part of the study are
appropriately investigated and resolved. WS and HJ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Helsinki Declaration and the experiment procedures were
conducted with approval of the Ethics Committee of Seventh People's
Hospital of Shanghai University of Traditional Chinese Medicine
(Shanghai, China; approval no. 2017-IRBQYYS-057). All the patients
signed the informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith JA: Update on ankylosing
spondylitis: Current concepts in pathogenesis. Curr Allergy Asthma
Rep. 15(489)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang L, Zhang YJ, Chen J, Huang XL, Fang
GS, Yang LJ, Duan Y and Wang J: The association of HLA-B27 and
Klebsiella pneumoniae in ankylosing spondylitis: A systematic
review. Microb Pathog. 117:49–54. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Golder V and Schachna L: Ankylosing
spondylitis: An update. Aust Fam Physician. 42:780–784.
2013.PubMed/NCBI
|
|
4
|
Pecourneau V, Degboe Y, Barnetche T,
Cantagrel A, Constantin A and Ruyssen-Witrand A: Effectiveness of
exercise programs in ankylosing spondylitis: A meta-analysis of
randomized controlled trials. Arch Phys Med Rehabil. 99:383–389.e1.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ranganathan V, Gracey E, Brown MA, Inman
RD and Haroon N: Pathogenesis of ankylosing spondylitis-recent
advances and future directions. Nat Rev Rheumatol. 13:359–367.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wenker KJ and Quint JM: Ankylosing
Spondylitis. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2021.
|
|
7
|
Blair HA: Secukinumab: A review in
ankylosing spondylitis. Drugs. 79:433–443. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Z, Wong SH, Shen J, Chan MTV and Wu
WKK: The role of MicroRNAS in ankylosing spondylitis. Medicine
(Baltimore). 95(e3325)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yan L, Liang M, Hou X, Zhang Y, Zhang H,
Guo Z, Jinyu J, Feng Z and Mei Z: The role of microRNA-16 in the
pathogenesis of autoimmune diseases: A comprehensive review. Biomed
Pharmacother. 112(108583)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Motta F, Carena MC, Selmi C and Vecellio
M: MicroRNAs in ankylosing spondylitis: Function, potential and
challenges. J Transl Autoimmun. 3(100050)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vonk LA, Kragten AH, Dhert WJ, Saris DB
and Creemers LB: Overexpression of hsa-miR-148a promotes cartilage
production and inhibits cartilage degradation by osteoarthritic
chondrocytes. Osteoarthritis Cartilage. 22:145–153. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu H, Su H, Wang X and Hao W: MiR-148a
regulates bone marrow mesenchymal stem cells-mediated fracture
healing by targeting insulin-like growth factor 1. J Cell Biochem:
Oct 18, 2018 (Epub ahead of print). doi: 10.1002/jcb.27121.
|
|
13
|
Tian L, Zheng F, Li Z, Wang H, Yuan H,
Zhang X, Ma Z, Li X, Gao X and Wang B: miR-148a-3p regulates
adipocyte and osteoblast differentiation by targeting
lysine-specific demethylase 6b. Gene. 627:32–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang S, Li Y, Wu P, Xiao Y, Duan N, Quan
J and Du W: microRNA-148a-3p in extracellular vesicles derived from
bone marrow mesenchymal stem cells suppresses SMURF1 to prevent
osteonecrosis of femoral head. J Cell Mol Med. 24:11512–11523.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma C, Wen B, Zhang Q, Shao PP, Gu W, Qu K,
Shi Y and Wang B: Emodin induces apoptosis and autophagy of
fibroblasts obtained from patient with ankylosing spondylitis. Drug
Des Devel Ther. 13:601–609. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hamdi W, Chelli-Bouaziz M, Ahmed MS,
Ghannouchi MM, Kaffel D, Ladeb MF and Kchir MM: Correlations among
clinical, radiographic, and sonographic scores for enthesitis in
ankylosing spondylitis. Joint Bone Spine. 78:270–274.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li DH, He CR, Liu FP, Li J, Gao JW, Li Y
and Xu WD: Annexin A2, up-regulated by IL-6, promotes the
ossification of ligament fibroblasts from ankylosing spondylitis
patients. Biomed Pharmacother. 84:674–679. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qin X, Zhu B, Jiang T, Tan J, Wu Z, Yuan
Z, Zheng L and Zhao J: miR-17-5p regulates heterotopic ossification
by targeting ANKH in ankylosing spondylitis. Mol Ther Nucleic
Acids. 18:696–707. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu L, Zuo Y, Xu Y, Zhang Z, Li Y and Pang
J: MiR-613 inhibits proliferation and invasion and induces
apoptosis of rheumatoid arthritis synovial fibroblasts by direct
down-regulation of DKK1. Cell Mol Biol Lett. 24(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang J, Song G, Yin Z, Fu Z and Zhang L:
Altered expression of microRNAs targeting Dkk-1 in peripheral blood
mononuclear cells of patients with ankylosing spondylitis. Cent Eur
J Immunol. 44:59–64. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ma S, Wang DD, Ma CY and Zhang YD:
microRNA-96 promotes osteoblast differentiation and bone formation
in ankylosing spondylitis mice through activating the Wnt signaling
pathway by binding to SOST. J Cell Biochem. 120:15429–15442.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
World Medical Association. World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Raychaudhuri SP and Deodhar A: The
classification and diagnostic criteria of ankylosing spondylitis. J
Autoimmun. 48-49:128–133. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ding L, Yin Y, Hou Y, Jiang H, Zhang J,
Dai Z and Zhang G: microRNA-214-3p suppresses ankylosing
spondylitis fibroblast osteogenesis via BMP-TGF β axis and
BMP2. Front Endocrinol (Lausanne). 11(609753)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu Z, Yao X, Yan G, Xu Y, Yan J, Zou W
and Wang G: Mediator MED23 cooperates with RUNX2 to drive
osteoblast differentiation and bone development. Nat Commun.
7(11149)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Franck H and Keck E: Serum osteocalcin and
vitamin D metabolites in patients with ankylosing spondylitis. Ann
Rheum Dis. 52:343–346. 1993.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Heo JS, Choi Y, Kim HS and Kim HO:
Comparison of molecular profiles of human mesenchymal stem cells
derived from bone marrow, umbilical cord blood, placenta and
adipose tissue. Int J Mol Med. 37:115–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shao F, Liu Q, Zhu Y, Fan Z, Chen W, Liu
S, Li X, Guo W, Feng GS, Yu H, et al: Targeting chondrocytes for
arresting bony fusion in ankylosing spondylitis. Nat Commun.
12(6540)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tan H, Ren R, Zhang J, Huang Z, Niu Q and
Yang B: Analysis of inflammation-related microRNA expression in
patients with ankylosing spondylitis. Immunol Res. 70:23–32.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao J, Zhang Y and Liu B: MicroRNA2045p
inhibits the osteogenic differentiation of ankylosing spondylitis
fibroblasts by regulating the Notch2 signaling pathway. Mol Med
Rep. 22:2537–2544. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Vasko R, Streich JH, Blaschke S, Muller
GA, Mai B, Kostrzewa M, Sparbier K, Korsten P, Bohr S and Dihazi H:
Vimentin fragments are potential markers of rheumatoid synovial
fibroblasts. Clin Exp Rheumatol. 34:513–520. 2016.PubMed/NCBI
|
|
33
|
Kook SH, Heo JS and Lee JC: Crucial roles
of canonical Runx2-dependent pathway on Wnt1-induced osteoblastic
differentiation of human periodontal ligament fibroblasts. Mol Cell
Biochem. 402:213–223. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tsao YT, Huang YJ, Wu HH, Liu YA, Liu YS
and Lee OK: Osteocalcin mediates biomineralization during
osteogenic maturation in human mesenchymal stromal cells. Int J Mol
Sci. 18(159)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He H, Cai M, Zhu J, Xiao W, Liu B, Shi Y,
Yang X, Liang X, Zheng T, Hu S, et al: miR-148a-3p promotes rabbit
preadipocyte differentiation by targeting PTEN. In Vitro Cell Dev
Biol Anim. 54:241–249. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yucong Z, Lu L, Shengfa L, Yongliang Y,
Ruguo S and Yikai L: Serum functional dickkopf-1 levels are
inversely correlated with radiographic severity of ankylosing
spondylitis. Clin Lab. 60:1527–1531. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zou YC, Yang XW, Yuan SG, Zhang P, Ye YL
and Li YK: Downregulation of dickkopf-1 enhances the proliferation
and osteogenic potential of fibroblasts isolated from ankylosing
spondylitis patients via the Wnt/β-catenin signaling pathway in
vitro. Connect Tissue Res. 57:200–211. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lin L, Qiu Q, Zhou N, Dong W, Shen J,
Jiang W, Fang J, Hao J and Hu Z: Dickkopf-1 is involved in
BMP9-induced osteoblast differentiation of C3H10T1/2 mesenchymal
stem cells. BMB Rep. 49:179–184. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Negri S, Wang Y, Sono T, Qin Q, Hsu GC,
Cherief M, Xu J, Lee S, Tower RJ, Yu V, et al: Systemic DKK1
neutralization enhances human adipose-derived stem cell mediated
bone repair. Stem Cells Transl Med. 10:610–622. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bafico A, Liu G, Yaniv A, Gazit A and
Aaronson SA: Novel mechanism of Wnt signalling inhibition mediated
by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol.
3:683–686. 2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Glinka A, Wu W, Delius H, Monaghan AP,
Blumenstock C and Niehrs C: Dickkopf-1 is a member of a new family
of secreted proteins and functions in head induction. Nature.
391:357–362. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
42
|
Baron R and Rawadi G: Targeting the
Wnt/beta-catenin pathway to regulate bone formation in the adult
skeleton. Endocrinology. 148:2635–2643. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Goldring SR and Goldring MB: Eating bone
or adding it: The Wnt pathway decides. Nat Med. 13:133–134.
2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Johnson ML and Kamel MA: The Wnt signaling
pathway and bone metabolism. Curr Opin Rheumatol. 19:376–382.
2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jin L, Cao Y, Yu G, Wang J, Lin X, Ge L,
Du J, Wang L, Diao S, Lian X, et al: SFRP2 enhances the osteogenic
differentiation of apical papilla stem cells by antagonizing the
canonical WNT pathway. Cell Mol Biol Lett. 22(14)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gu H, Wu L, Chen H, Huang Z, Xu J, Zhou K,
Zhang Y, Chen J, Xia J and Yin X: Identification of differentially
expressed microRNAs in the bone marrow of osteoporosis patients. Am
J Transl Res. 11:2940–2954. 2019.PubMed/NCBI
|
|
47
|
Lu H, Li X, Mu P, Qian B, Jiang W and Zeng
L: Dickkopf-1 promotes the differentiation and adipocytokines
secretion via canonical Wnt signaling pathway in primary cultured
human preadipocytes. Obes Res Clin Pract. 10:454–464.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shi C, Zhang M, Tong M, Yang L, Pang L,
Chen L, Xu G, Chi X, Hong Q, Ni Y, et al: miR-148a is associated
with obesity and modulates adipocyte differentiation of mesenchymal
stem cells through Wnt signaling. Sci Rep. 5(9930)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Llorente I, García-Castañeda N, Valero C,
González-Álvaro I and Castañeda S: Osteoporosis in rheumatoid
arthritis: Dangerous liaisons. Front Med (Lausanne).
7(601618)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
He X and Dong Y: Ankylosis progressive
homolog upregulation inhibits cell viability and mineralization
during fibroblast ossification by regulating the Wnt/β-catenin
signaling pathway. Mol Med Rep. 22:4551–4560. 2020.PubMed/NCBI View Article : Google Scholar
|