Introduction
Myocarditis is an inflammatory disorder that is
associated with an increased risk of developing dilated
cardiomyopathy (DCM) (1,2). Collagen deposition, ventricular
dilation and heart failure are indicating characteristics of
myocarditis progression to DCM. Experimental autoimmune myocarditis
(EAM) animal models are used for the investigation of the
pathophysiologic mechanism behind the transition of myocarditis to
DCM. Inflammatory cytokines, including IL-1β, IL-6 and TGF-β serve
a key role in myocardial collagen remodeling (3). A previous study reported the
potential role of inflammatory cytokines in the transition of
myocarditis to DCM via the regulation of MMP expression (4). In addition, evidence suggests that
the aldosterone receptor causes cardiac oxidative stress,
inflammation and fibrosis (5).
Spironolactone, a nonselective aldosterone receptor inhibitor, has
been reported to relieve the process of cardiac fibrosis and
remodeling following cardiac injury in numerous experimental and
clinical studies (6-8).
However, the mechanism of spironolactone in myocarditis remains to
be elucidated.
E26 transformation-specific (Ets) transcription
factor family members share a highly conserved DNA-binding domain
and are involved in cell differentiation, proliferation,
metastasis, apoptosis and tissue remodeling (9). Ets sequence-1 (Ets-1), has been
demonstrated to enhance fibrotic processes in the heart and in
other organs. In addition, Ets-1 also regulates TGF-β-induced
tissue fibrosis and participates in the tissue fibrosis process by
regulating the expression of genes encoding enzymes involved in
matrix degradation (10). Ets-1
activation regulates TNF-α-induced MMP-9(11). However, the effect of
spironolactone on Ets-1 in cardiac fibrosis has remains to be
elucidated.
The aim of the present study was to investigate the
underlying mechanisms of how spironolactone protects against
post-myocarditis remodeling. It was hypothesized that
spironolactone could improve myocardial fibrosis via the inhibition
of Ets-1 via TGF-β signaling pathways in EAM mice. Furthermore,
another aim was to identify a potential novel therapeutic approach
for patients with myocarditis/DCM.
Materials and methods
Animals
A total of 50 female BALB/c mice (age, 6-8 weeks;
weight, 18-20 g) were purchased from the Experimental Animal Center
of Shandong University (Jinan, China). All experimental procedures
were performed in accordance with animal protocols approved by The
Second Hospital of Shandong University Animal Care Committee
(approval no. KYLL-2020-(KJ)A-0134). All mice were housed in a
pathogen-free animal facility, which was maintained at 22-24˚C at
50-60% humidity with 12:12 h light-dark cycle. The mice had easy
access to food and water before the experiments. For cardiac
diameter and cardiac function assessment, mice were anesthetized
with 3% isoflurane, which was subsequently maintained at 1.3%. At
the end of study, mice were anesthetized by intraperitoneal
injection of pentobarbital sodium (100 mg/kg) and euthanized via
exsanguination.
Induction of EAM and treatment
For EAM model construction, murine cardiac α-myosin
heavy chain [α614-629 (Ac-SLKLMATLFSTYASAD-OH); GL Biochem
(Shanghai), Ltd.] was dissolved in PBS (1 mg/ml) and emulsified 1:1
with complete Freund's adjuvant (CFA; MilliporeSigma). Each mouse
was subcutaneously injected with 200 µl of emulsion (containing 200
µg of murine cardiac α-myosin) by inguinal injection or subaxillary
injection on day 0 and 7 to induce EAM (12). The control group mice were treated
with CFA mixed with PBS following the same procedure as for the EAM
group mice. To knockdown Ets-1 expression, a small interfering
(si)RNA against mouse Ets-1 was transfected into mouse hearts and a
scramble siRNA was employed as a control. The target sequence for
Ets-1 was 5'-GCUACCUUCAGUGGUUUCATT-3' and scramble siRNA sequence
was 5'-UUCUCCGAACGUGUCACGUTT-3' (Shanghai GenePharma Co., Ltd.).
Mice were randomly divided into five groups: i) Control group
(n=10); ii) non-treated EAM group (n=10); iii)
spironolactone-treated EAM group (n=10); iv) saline-treated EAM
group (n=10); and v) siRNA-Ets-1-treated EAM group (n=10).
Spironolactone treatment therapy started on day 7 following the
emulsion injection. The myocardial injection method was used to
transfect cells based on previous study (13). A total of 1x107 UT/30 µl
lentivector with siRNA-Ets-1 was injected in multiple sites in the
left ventricle of the EAM group mice at day 7. Mice were fed orally
via gastric gavage for 4 weeks from day 7 to day 36 after
immunization. The calculated daily dosage of 50 mg/kg/day
spironolactone per mouse was based on a previous study by Wehr
et al (14). Assessment and
analysis of the establishment of EAM in the mouse model were
performed on day 36. No mice succumbed throughout the duration of
the present study.
Biochemical quantification
Serum was collected for ELISA. Blood samples were
collected via eyeball removal and were stored at 4˚C overnight to
let the serum separate from the blood cells. Aldosterone was
quantified using a Aldosterone ELISA kit (cat. no. ZC-38593;
Shanghai Zhuo Cai Technology Co., Ltd.) Serum levels of IL-6 and
TNF-α were also quantified using IL-6 and TNF-α ELISA kit (cat. no.
ZC-37988 and ZC-39024, respectively; Shanghai Zhuo Cai Technology
Co., Ltd.), according to the manufacturer's protocol.
Echocardiography
Echocardiography was performed on the mice as
previously described (15,16). Wall thickness and left ventricular
(LV) dimensions, including LV internal dimensions (LVIDs) in
diastole, LVID in systole, LV posterior wall (LVPW) of diastole,
LVPW of systole and LV posterior diameter in systole, were obtained
from the short-axis view. LV ejection fraction (LVEF) was assessed
according to the American Society of Echocardiography Guidelines
(17). Pulsed-wave Doppler
echocardiography was used to measure early (E) and late (A) blood
flow velocities via the mitral valve and the E/A ratio was
determined.
Histology and
immunohistochemistry
Heart tissues were fixed using 10% formalin at room
temperature for 72 h, dehydrated with an ethanol gradient, embedded
in paraffin, sectioned (thickness, 5 µm) and stained with
hematoxylin and eosin (H&E). The main steps of H&E staining
were the following: Staining with hematoxylin for 10 min at room
temperature, washing in tap water, staining with eosin for 3 min at
room temperature, washing in distilled water and ethanol (90%),
dehydration in ethanol (95%), ethanol (100%). Masson trichome
staining was used to stain collagen fibers dark green, which were
quantified to assess fibrosis. Masson staining takes place at room
temperature and the main steps are the following: Staining with
hematoxylin for 5 min, washing with tap water, staining with
fuchsin for 5 min, rinsing in distilled water, incubating in
phosphotungstic-phophomolybdic acid for 5 min, dyeing aniline blue
for 5 min. Paraffin sections underwent immunohistochemistry using a
microwave-based antigen retrieval method. Sections were incubated
with primary antibodies for rabbit polyclonal collagen I (1:100;
cat. no. AF7001; Wuhan Servicebio Technology Co., Ltd.), collagen
III (1:500; cat. no. GB111629; Wuhan Servicebio Technology Co.,
Ltd.), TGF-β1 (1:100 cat. no. AF1027; Wuhan Servicebio Technology
Co., Ltd.) and phosphorylated (p)-Ets-1 (1:200; cat. no. CBP1153;
Assay Biotechnology Co., Inc.) overnight at 4˚C. Following the
primary antibody incubation, samples were incubated with a
HRP-conjugated secondary goat anti-rabbit antibody (cat. no.
PV9001; Zhongshan Bio-Tech Co., Ltd.) for 30 min at 37˚C. Signals
were amplified using an ABC kit (Vector Laboratories, Inc.).
Sections were imaged using a confocal FV 1000 SPD laser scanning
microscope (Olympus Corporation). Immunohistochemical staining was
analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Inc.).
Western blotting
Total protein was extracted from mice myocardium
using RIPA lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology). Subsequently, total protein was separated using
SDS-PAGE on a 10% gel. Separated protein was then transferred onto
a PVDF membrane, which was incubated overnight at 4˚C with the
following primary antibodies: total Ets-1 (1:1,000; cat. no. 6258;
Cell Signaling Technology, Inc.), p-Ets-1 (1:500; cat. no. CBP1153;
Assay Biotechnology Co., Inc.), Smad-2/3 (1:1,000; cat. no. 8685;
Cell Signaling Technology, Inc.) and p-Smad-2/3 (1:1,000 dilution;
cat. no. 8828; Cell Signaling Technology, Inc.). Following the
primary incubation membranes were incubated with an HRP-conjugated
secondary antibody for 1 h at room temperature (1:2,500 dilution;
cat. no. ZB-2306; OriGene Technologies, Inc.). Protein expression
levels were normalized to β-actin (1:1,000; cat. no. 4970; Cell
Signaling Technology, Inc.) as an internal control and p-proteins
to that of the total protein. Bands were semi-quantified using
optical densities, which were analyzed using ImageJ software (v1.8,
National Institutes of Health).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from heart tissue using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and converted into complementary DNA using a RevertAid kit
(Fermentas; Thermo Fisher Scientific, Inc.), both kits were used
according to the manufacturer's protocol. Reactions involved the
use of a real-time PCR thermocycler (IQ5 real-time PCR cycler;
Bio-Rad Laboratories) with SsoFast EvaGreen Supermix (Bio-Rad
Laboratories). The program was 30 sec at 95˚C, then 40 cycles of
96˚C for 5 sec and 56˚C for 10 sec. mRNA expression levels were
normalized to the internal reference gene GAPDH. The primer
sequences used for qPCR were as follows: MMP-2 forward F,
5'-ACAAGTGGTCCGCGTAAAGT-3' and reverse R,
5'-GTAAACAAGGCTTCATGGGGG-3'; MMP-9 F, 5'-GCCGACTTTTGTGGTCTTCC-3'
and R, 5'-GGTACAAGTATGCCTCTGCCA-3'; and GAPDH F,
5'-AGGTCGGTGTGAACGGATTTGGG-3' and R, 5'-TGTAGACCATGTAGTTGAGGTCA-3'.
Relative fold change of mRNA expression levels was calculated using
the 2-∆∆CT method (18). Data are representative of three
independent experiments.
Gelatin zymography
The enzymatic activity of MMP in myocardial tissues
was assayed using gelatin zymography. Samples were electrophoresed
via SDS-PAGE on a 10% gel containing 0.1% gelatin. After the gels
were washed twice with 2.5% Triton X-100, the gels were incubated
in activation buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10 mM
CaCl2, 1 µM ZnCl2] at 37˚C overnight.
Subsequently, gels were stained with Coomassie brilliant blue R-250
solution for 3 h at room temperature. Gels were de-stained with 45%
methanol and 10% acetic acid until the bands of lysis become clear.
Lytic bands of gelatin digestion were represented by MMP-2 (72 kDa)
and MMP-9 (92 kDa) activity.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA) and data are presented as
the mean ± SD. Data were analyzed by one-way ANOVA followed by
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
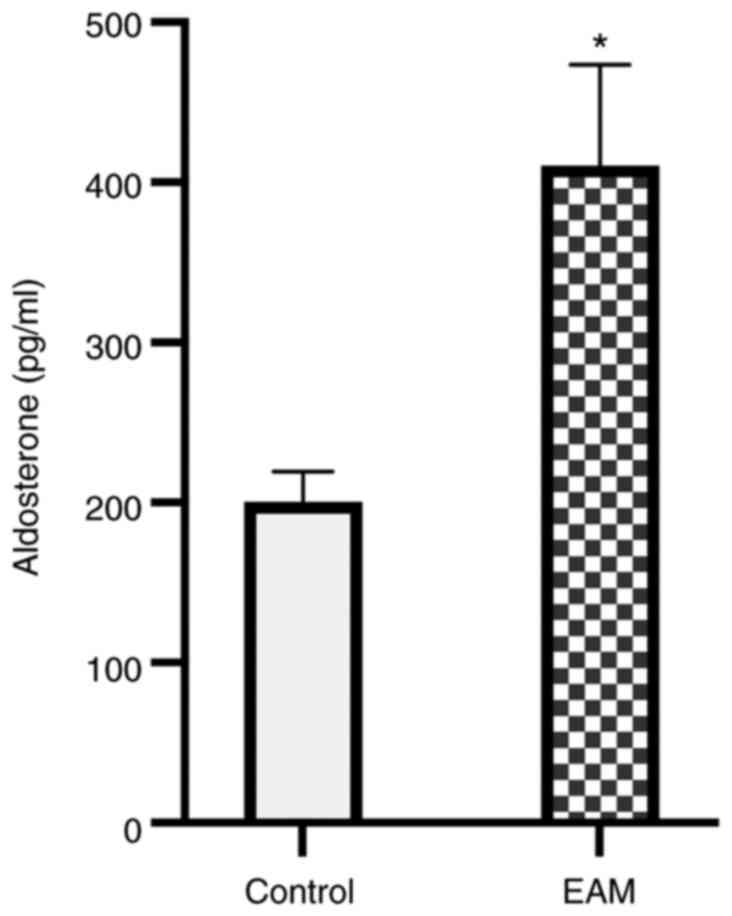
Aldosterone serum concentration
increases in EAM mice
Aldosterone synthase expression levels are high in
human myocardium during acute myocarditis (5). Aldosterone serves an important role
in the pathophysiology of cardiac remodeling (19). Therefore, aldosterone concentration
in EAM mice myocardium was assessed using ELISA. Compared with the
controls, aldosterone serum concentrations were significantly
increased in EAM mice on day 36 (P<0.05; Fig. 1).
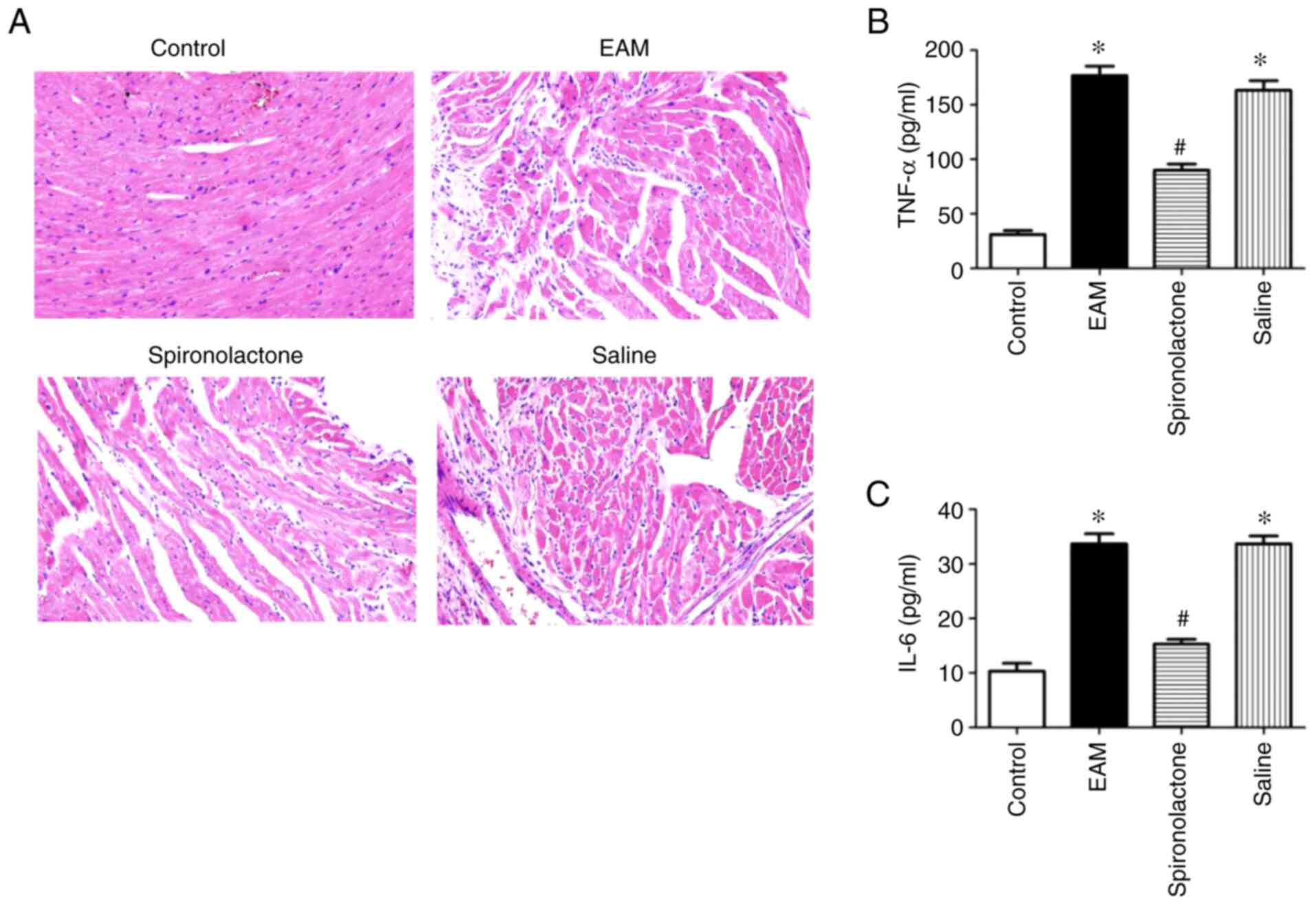
Spironolactone decreases inflammatory
cytokine levels in EAM myocardial tissue
Previous results have demonstrated that inflammatory
cytokines serve an important role in the progression of
post-myocarditis cardiac remodeling (20). On day 36, H&E staining
demonstrated that inflammatory cell infiltration was present in the
EAM group compared with the control groups. However, spironolactone
treatment significantly reduced cell infiltration in the EAM group
(Fig. 2A). To evaluate the levels
of inflammatory cytokines in the EAM myocardium, TNF-α and IL-6
serum concentrations were quantified using ELISA. Compared with the
control groups, the serum concentrations of TNF-α and IL-6
significantly increased in the EAM group (P<0.05) but
significantly decreased in the EAM model with spironolactone
treatment (P<0.05; Fig. 2B and
C).
Spironolactone ameliorates
myocarditis-induced myocardium hypertrophy and diastolic
dysfunction
Echocardiography was used to assess cardiac function
on day 36 of the experiment. It was determined that the LVEF, E/A
ratio, LV chamber size and wall thickness did not differ between
animals in each group. At day 36, compared with the control groups,
a significantly lower E/A ratio was observed in EAM mice
(P<0.05), which was accompanied by a significant increase in the
LVPW (P<0.05). Spironolactone treatment significantly
ameliorated the reduced E/A ratio and LVPW in EAM mice (P<0.05).
However, there was no statistically significant difference in LVEF
or LVID between the four groups investigated (P>0.05). These
results indicated that EAM mice may exhibit LV hypertrophy and
diastolic dysfunction at day 36, but there was no evidence of LV
dilatation and systolic dysfunction (Table I).
 | Table IEchocardiographic parameters. |
Table I
Echocardiographic parameters.
| | Control | EAM | Spironolactone | Saline |
|---|
| LVPWd (mm) | 0.48±0.07 |
0.71±0.11a |
0.57±0.09b |
0.73±0.10a |
| LVPWs (mm) | 0.83±0.17 |
1.31±0.29a |
1.02±0.18b |
1.26±0.22a |
| LVIDd (mm) | 4.51±0.24 | 4.32±0.36 | 4.59±0.35 | 4.40±0.28 |
| LVIDs (mm) | 3.70±0.38 | 3.55±0.31 | 3.74±0.24 | 3.54±0.25 |
| LVEF (%) | 64.65±11.25 | 68.37±9.81 | 71.50±13.18 | 69.38±11.65 |
| E/A | 1.40±0.18 |
0.69±0.25a |
1.10±0.36b |
0.79±0.27a |
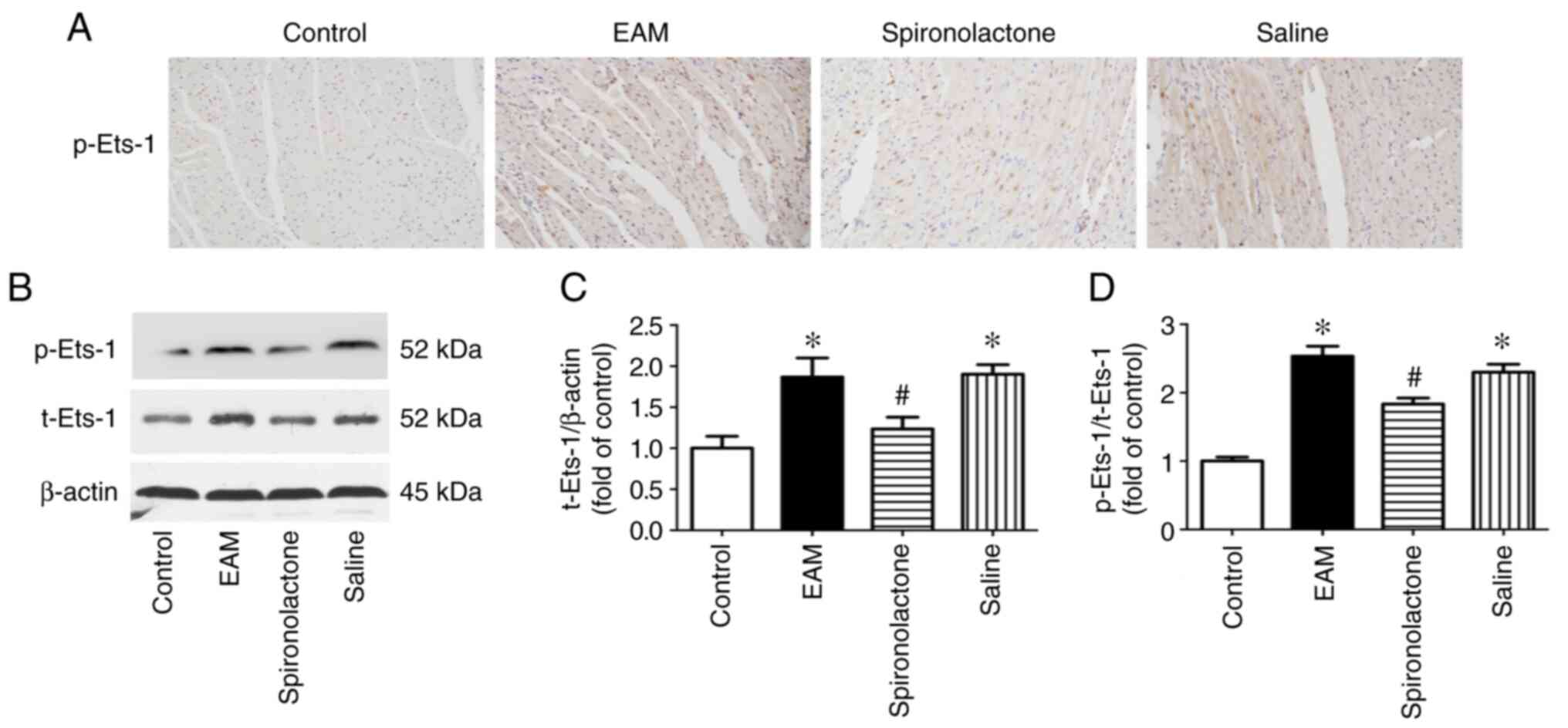
Ets-1 activation is downregulated in
spironolactone-treated EAM mice
Subsequently, whether Ets-1 participated in
EAM-induced cardiac fibrosis was investigated. Ets-1 protein
expression levels were determined using immunohistochemistry
(Fig. 3A) and western blotting
(Fig. 3B). The results
demonstrated that total Ets-1 and p-Ets-1 protein expression levels
were significantly increased (P<0.05; Fig. 3C and D) in EAM mice myocardium. Subsequently,
the effect of spironolactone on myocarditis-induced Ets-1
activation was investigated. Compared with the myocarditis mice
groups, Ets-1 protein expression levels and phosphorylation were
significantly reduced by spironolactone treatment (P<0.05).
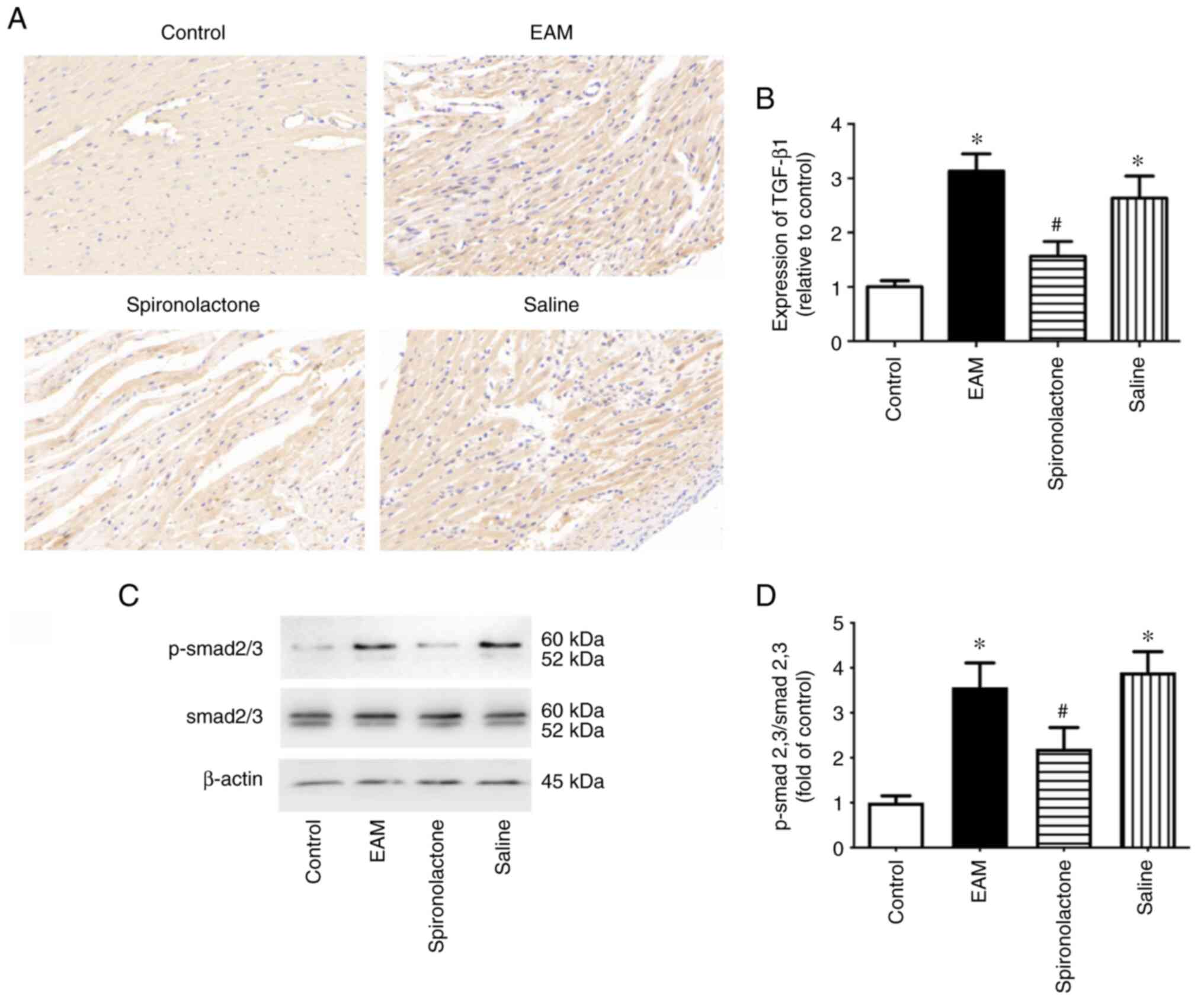
Spironolactone inhibits
TGF-β1/Smad-2/3 signaling pathway activation in EAM mice
TGF-β1 performs a crucial role in cardiac fibrosis,
whereby Ets-1 participates in matrix remodeling in response to
TGF-β1(21). In the present study,
the effect of spironolactone on the TGF-β1/Smad-2/3 signaling
pathway was explored. The protein expression level of TGF-β1 in the
myocardium was determined by immunohistochemistry. The results
demonstrated that TGF-β1 protein expression levels were
significantly increased in EAM mice compared with the control
groups (P<0.05). However, these increased protein expression
levels were significantly reduced with spironolactone treatment
(P<0.05; Fig. 4A and B). Smad proteins are the downstream
signaling molecules of the TGF family and are also involved in
myocardial remodeling (22). The
effect of spironolactone on p-Smad-2/3 protein expression levels in
EAM mice was assessed. Western blotting demonstrated that
spironolactone significantly inhibited the myocarditis-induced
increase of p-Smad-2/3 in myocardial tissue (P<0.05; Fig. 4C and D). These results indicated that
spironolactone may inhibit Ets-1 activation via the TGF-β1/Smad-2/3
signaling pathway in EAM mice.
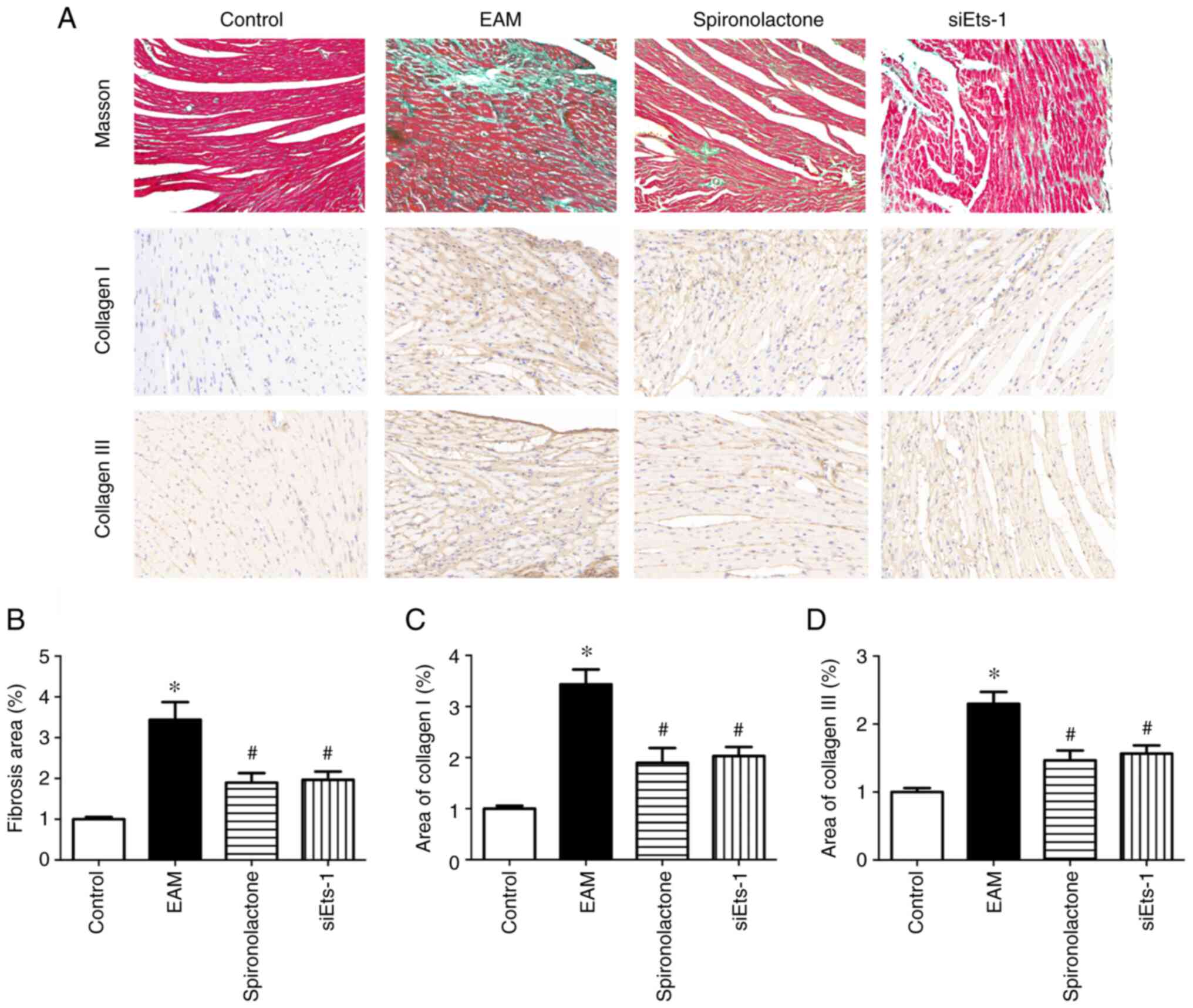
Ets-1 knockdown attenuates myocardial
fibrosis in EAM mice
Aldosterone antagonists are established therapeutics
for patients with heart failure (23). The Masson's trichrome staining
method was used to assess the degree of myocardial fibrosis. The
results demonstrated that in the EAM group fibrosis was
significantly increased (P<0.05; Fig. 5A and B). As reported in previous studies, the
present study demonstrated that spironolactone significantly
inhibited myocardium fibrosis in EAM mice (P<0.05). Furthermore,
in EAM mice, significantly increased protein expression levels of
collagens I and III were observed in the myocardium (P<0.05;
Fig. 5C and D). Spironolactone treatment significantly
decreased the protein expression levels of collagen I and III in
the EAM group (P<0.05). Ets factors are important mediators of
ECM remodeling (24). To further
examine the effects of Ets-1 on cardiac fibrosis induced by
myocarditis, Ets-1 expression was silenced using siRNA. Compared
with the control, Ets-1 protein expression levels were
significantly reduced by siRNA transfection (P<0.05; Fig. S1). Immunohistochemistry analysis
demonstrated that the quantities of collagen I and collagen III
significantly decreased in the siEts-1 group compared with EAM
group (P<0.05, Fig. 5). These
results indicated that spironolactone may limit myocarditis-induced
fibrosis that is mediated by the inhibition of Ets-1.
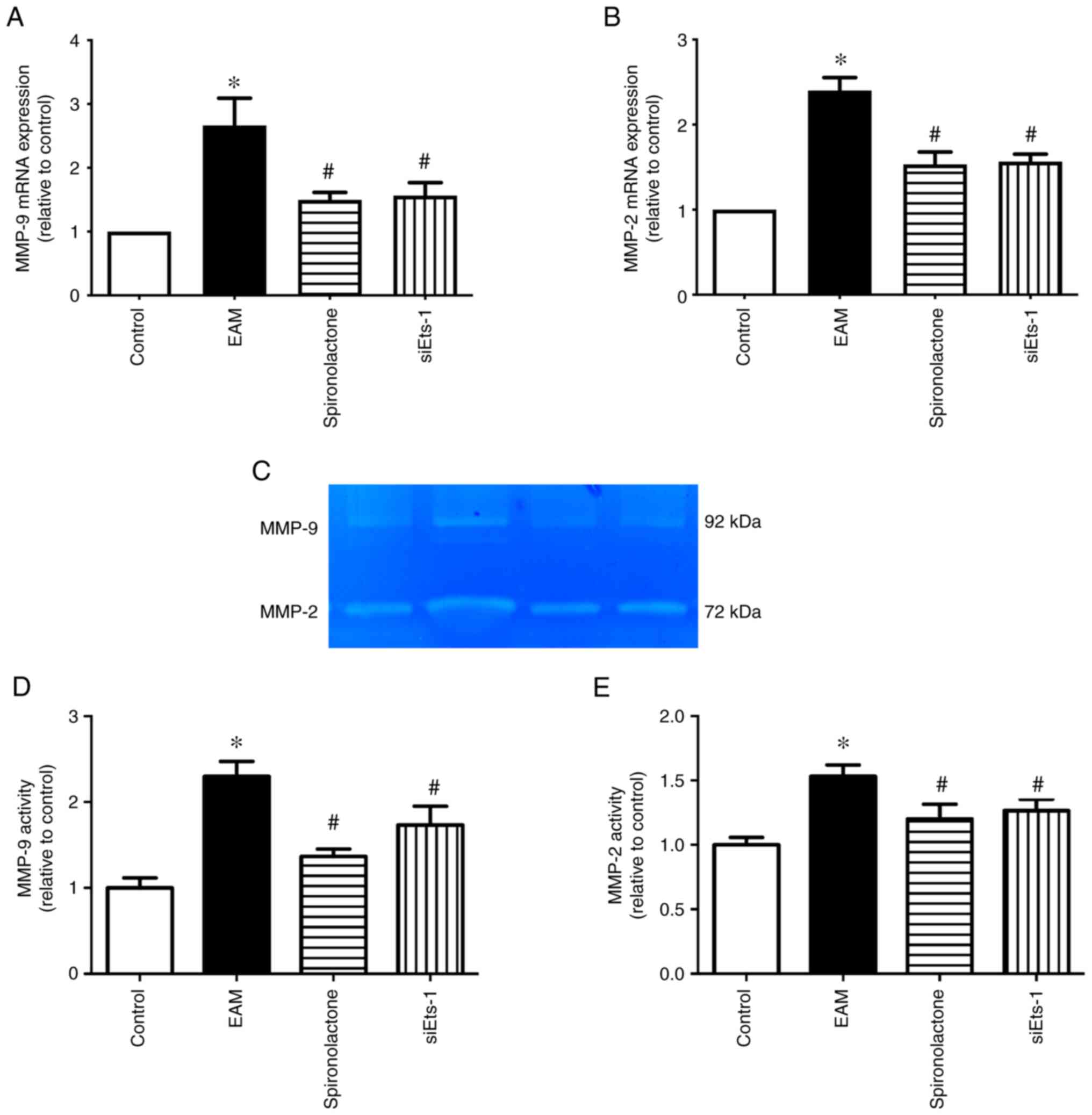
Ets-1 inhibition decreases MMP-2 and
MMP-9 expression and activity levels in EAM mice
MMPs may also serve a central role in ECM remodeling
(25). RT-qPCR and gelatin
zymography demonstrated that the mRNA expression levels and the
activity of MMP-2 and MMP-9 increased significantly in the EAM
group compared with the controls, whereas spironolactone treatment
significantly attenuated this effect (P<0.05; Fig. 6). Furthermore, the role of Ets-1 in
MMP mRNA expression and activity in EAM mice was explored. The
results demonstrated that compared with EAM mice, the increased
mRNA expression levels and activity of MMP-2 and MMP-9 were
significantly downregulated by Ets-1-siRNA (P<0.05). These
results therefore indicated that spironolactone may inhibit the
expression and activation of MMP-2 and MMP-9 via Ets-1 activation
suppression.
Discussion
Myocarditis is a suspected common precursor of DCM
(26). Previous studies
demonstrate that increased aldosterone causes oxidative stress,
inflammation and fibrosis (27).
Spironolactone is a widely used antagonist of aldosterone that is
used to treat chronic heart failure. Numerous clinical studies have
demonstrated that the administration of an aldosterone antagonist
improves LV remodeling in patients with heart failure (28,29).
However, the underlying mechanism of the protective effects of
spironolactone on myocarditis remains to be fully elucidated. In
the present study, the results demonstrated that aldosterone serum
concentrations significantly increased in EAM mice. Spironolactone
was demonstrated to significantly reduce inflammation and fibrosis
and significantly improved LV diastolic functions in EAM mice.
Furthermore, the results indicated that the protective effect of
spironolactone via downregulated Ets-1 expression levels may be via
the TGF-β1/Smad-2/3 signaling pathway in EAM mice.
Inflammation is a key pathophysiologic factor in the
EAM and is closely associated with myocardial fibrosis (1,30).
Following coxsackievirus B3 infection, inflammatory cytokines, such
as TNF-α, IL-1β, IL-4 and TGF-β1 are increased in the myocardium
(31). Inflammatory cytokines may
stimulate the expression of profibrotic factors like TGF-β and
plasminogen activator inhibitor-1. Biomarkers of inflammation are
associated with a risk of developing DCM. Li et al (32) report that TNF-α overexpression in
the myocardium causes myocardial remodeling and LV dysfunction
associated with increased MMP expression. In vitro, IL-1β
and IL-6 promote the remodeling of interstitial collagen by
increasing total MMP activity in cardiac fibroblasts (33). These aforementioned studies
indicate that inflammatory induction of IL-β1, IL-6 and TNF-α may
contribute to myocardial collagen remodeling mediated via the
MMP/tissue inhibitors of metalloproteinases system. Consistent with
these findings, the results of the present study demonstrated a
significant increase in the TNF-α and IL-6 concentrations in EAM
mice. However, spironolactone significantly reduced these
levels.
Aldosterone is a multifunctional molecule that
serves a significant role in heart failure. Spironolactone slows
the progression of heart failure by decreasing the serum markers of
fibrosis or type I and/or Ⅲ collagen metabolism, which reverses
changes to cardiovascular structure and function in patients at
high risk of developing heart failure (34). Spironolactone prevents
perivascular/interstitial fibrosis in experimental models of
hypertension (35). Furthermore,
spironolactone significantly reduces cardiac fibrosis and
inflammation in streptozotocin-induced diabetic rats (36). In addition, the aldosterone
antagonist eplerenone is an anti-inflammatory and protects viral
myocarditis mice from heart remodeling (6). In the present study, the results
demonstrated that EAM mice exhibited significant LV hypertrophy and
diastolic dysfunction at day 36, whereas no LV dilatation or
systolic dysfunction were observed. Spironolactone significantly
reduced abnormal interstitial collagen accumulation and improved LV
hypertrophy and diastolic dysfunction. In addition, this protective
effect was attributed to the significantly decreased levels of
proinflammatory cytokines such as TNF-α, IL-6 and TGF-β1, as well
as the significant inhibition of MMP-2 and MMP-9 mRNA expression
and activity levels in EAM mice. Even though experimental studies
and clinical trials have indicated that spironolactone slows the
progression of cardiac fibrosis, the precise mechanisms have
remained uncertain. To the best of the authors' knowledge, the
present study was the first to demonstrate that spironolactone
significantly downregulated the expression of Ets-1 in EAM mice.
This result indicated that spironolactone may improve EAM-induced
cardiac dysfunction via inhibition of Ets-1 activation.
The transcription factor Ets-1 is a critical
mediator of ECM remodeling. Ets-1 governs a wide spectrum of
ECM-related target genes, including matrix proteins and enzymes
(21). Previous studies have
demonstrated that Ets-1 is a transcriptional regulator of numerous
proteinases, including MMP-1, MMP-3 and urokinase-type plasminogen
activator (u-PA) (21,37,38).
MMPs not only modulate the degradation of matrix components but
also effect collagen synthesis. Previous studies have demonstrated
that increased MMP expression accompanies severe fibrosis in
myocardial tissue (39).
Furthermore, abnormal MMP activity results in excessive collagen
deposition, which contributes to the development of myocardial
fibrosis and cardiac dysfunction (40). The role of Ets-1 as a regulator of
ECM in tumors and autoimmune diseases has also been well
characterized (38,41), whereas the role of Ets-1 in
myocardial fibrosis has rarely been reported. It has previously
been reported that the inhibition of Ets-1 could diminish
angiotensin II-induced cardiac fibrosis via the inhibition of the
endothelial-to-mesenchymal transition (42). However, to the best of the authors'
knowledge, the effect of Ets-1 on cardiac fibrosis induced by
myocarditis has not previously been explored. In the present study,
myocardial fibrosis and MMP-2 and MMP-9 mRNA expression levels and
activity were significantly upregulated in EAM mice and these
enhanced effects were significantly attenuated by the inhibition of
Ets-1.
Myocardial fibrosis is a typical characteristic
observed during the transition of myocarditis to DCM (43). A previous study reported the
contribution of Smad-dependent signaling pathways to TGF-β-induced
cardiac fibrosis (44). TGF-β1
signaling via Smad-2/3 phosphorylation contributes to the binding
of angiotensin II to the angiotensin type 1 receptor, which induces
cardiac fibrosis (45). TGF-β1
mediates MMP expression via its inhibitory element in the promoter
region of the MMP genes (46). The
results of the present study demonstrated that the increased TGF-β1
and p-Smad2/3 protein expression levels were significantly
inhibited by spironolactone treatment in EAM mice. Therefore, the
protective effect of spironolactone may be associated with the
TGF-β1/Smad2/3 signaling pathway. A previous study also reports
that Ets-1 could mediate TGF-β induced tissue fibrosis (47). Therefore, it was hypothesized that
the effect of spironolactone on Ets-1 was mediated by
TGF-β1/Smad-2/3 signaling pathway in EAM mice.
In conclusion, the present study demonstrated that
Ets-1 inhibition may attenuate myocarditis-induced cardiac
fibrosis. The results indicated that the protective effect of
spironolactone on post-myocarditis remodeling may be a result of
inflammation and fibrosis suppression via the inhibition of Ets-1.
Furthermore, the results indicated that Ets-1 activity induced by
myocarditis may be associated with the TGF-β/Smad-2/3 signaling
pathway. The present study may have helped identify a novel
therapeutic approach for the treatment of myocarditis-induced
DCM.
Supplementary Material
Efficacy of Ets-1 siRNA was determined
using western blotting. *P<0.05 vs. control. Ets-1,
E26 transformation-specific sequence-1; si, short interfering; t-,
total; N.C, negative control.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Natural Science
Foundation of Shandong Province (grant no. ZR2021MH064) and the
National Natural Science Foundation of China (grant nos. 81500217
and 81502050).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WKW designed the study, performed the experiments
and wrote the manuscript. BW designed the study and analyzed the
data. XHC performed the experiments and analyzed the data. YSL
designed the study and wrote the manuscript. WKW and YSL confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of the Second Hospital of Shandong University (approval
no. KYLL-2020-(KJ)A-0134; Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Watanabe K, Sukumaran V, Veeraveedu PT,
Thandavarayan RA, Gurusamy N, Ma M, Arozal W, Sari FR, Lakshmanan
AP, Arumugam S, et al: Regulation of inflammation and myocardial
fibrosis in experimental autoimmune myocarditis. Inflamm Allergy
Drug Targets. 10:218–225. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Blyszczuk P, Muller-Edenborn B, Valenta T,
Osto E, Stellato M, Behnke S, Glatz K, Basler K, Luscher TF,
Distler O, et al: Transforming growth factor-β-dependent Wnt
secretion controls myofibroblast formation and myocardial fibrosis
progression in experimental autoimmune myocarditis. Eur Heart J.
38:1413–1425. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siwik DA, Chang DL and Colucci WS:
Interleukin-1beta and tumor necrosis factor-alpha decrease collagen
synthesis and increase matrix metalloproteinase activity in cardiac
fibroblasts in vitro. Circ Res. 86:1259–1265. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ono K, Matsumori A, Shioi T, Furukawa Y
and Sasayama S: Cytokine gene expression after myocardial
infarction in rat hearts: Possible implication in left ventricular
remodeling. Circulation. 98:149–156. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cardona A, Baker P, Kahwash R, Smart S,
Phay JE, Basso C and Raman SV: Evidence of aldosterone synthesis in
human myocardium in acute myocarditis. Int J Cardiol. 275:114–119.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xiao J, Shimada M, Liu W, Hu D and
Matsumori A: Anti-inflammatory effects of eplerenone on viral
myocarditis. Eur J Heart Fail. 11:349–353. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Leader CJ, Moharram M, Coffey S, Sammut
IA, Wilkins GW and Walker RJ: Myocardial global longitudinal
strain: An early indicator of cardiac interstitial fibrosis
modified by spironolactone, in a unique hypertensive rat model.
PLoS One. 14(e0220837)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cohen JB, Schrauben SJ, Zhao L, Basso MD,
Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, et
al: Clinical phenogroups in heart failure with preserved ejection
fraction: Detailed phenotypes, prognosis, and response to
spironolactone. JACC Heart Fail. 8:172–184. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tanaka H, Sagisaka A, Suzuki N and
Yamakawa M: Bombyx mori E26 transformation-specific 2 (BmEts2), an
Ets family protein, represses Bombyx mori Rels (BmRels)-mediated
promoter activation of antimicrobial peptide genes in the silkworm
Bombyx mori. Insect Mol Biol. 25:566–579. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gum R, Lengyel E, Juarez J, Chen JH, Sato
H, Seiki M and Boyd D: Stimulation of 92-kDa gelatinase B promoter
activity by ras is mitogen-activated protein kinase kinase
1-independent and requires multiple transcription factor binding
sites including closely spaced PEA3/ets and AP-1 sequences. J Biol
Chem. 271:10672–10680. 1996.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nakamura Y, Esnault S, Maeda T, Kelly EA,
Malter JS and Jarjour NN: Ets-1 regulates TNF-alpha-induced matrix
metalloproteinase-9 and tenascin expression in primary bronchial
fibroblasts. J Immunol. 172:1945–1952. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cihakova D, Sharma RB, Fairweather D,
Afanasyeva M and Rose NR: Animal models for autoimmune myocarditis
and autoimmune thyroiditis. Methods Mol Med. 102:175–193.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang WK, Wang B, Lu QH, Zhang W, Qin WD,
Liu XJ, Liu XQ, An FS, Zhang Y and Zhang MX: Inhibition of
high-mobility group box 1 improves myocardial fibrosis and
dysfunction in diabetic cardiomyopathy. Int J Cardiol. 172:202–212.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wehr MC, Hinrichs W, Brzozka MM,
Unterbarnscheidt T, Herholt A, Wintgens JP, Papiol S,
Soto-Bernardini MC, Kravchenko M, Zhang M, et al: Spironolactone is
an antagonist of NRG1-ERBB4 signaling and schizophrenia-relevant
endophenotypes in mice. EMBO Mol Med. 9:1448–1462. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Perera P, Lobo V, Williams SR and
Gharahbaghian L: Cardiac echocardiography. Crit Care Clin.
30:47–92, v. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pan XC, Li ZX, Wu DZ, Li SY, Xiang HB and
Song YT: Mapping changes of whole brain blood flow in rats with
myocardial ischemia/reperfusion injury assessed by positron
emission tomography. Curr Med Sci. 39:653–657. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rudski LG, Lai WW, Afilalo J, Hua L,
Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and
Schiller NB: Guidelines for the echocardiographic assessment of the
right heart in adults: A report from the American Society of
Echocardiography endorsed by the European Association of
Echocardiography, a registered branch of the European Society of
Cardiology, and the Canadian Society of Echocardiography. J Am Soc
Echocardiogr. 23:685–713; quiz 786-8. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Weber KT: Aldosterone in congestive heart
failure. N Engl J Med. 345:1689–1697. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tschope C, Ammirati E, Bozkurt B, Caforio
ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hubner
N, et al: Myocarditis and inflammatory cardiomyopathy: Current
evidence and future directions. Nat Rev Cardiol. 18:169–193.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trojanowska M: Ets factors and regulation
of the extracellular matrix. Oncogene. 19:6464–6471.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hanna A, Humeres C and Frangogiannis NG:
The role of Smad signaling cascades in cardiac fibrosis. Cell
Signal. 77(109826)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC,
Givertz MM, et al: 2017 ACC/AHA/HFSA Focused Update of the 2013
ACCF/AHA Guideline for the management of heart failure: A report of
the American college of cardiology/American heart association task
force on clinical practice guidelines and the heart failure society
of America. Circulation. 136:e137–e161. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Iwasaka C, Tanaka K, Abe M and Sato Y:
Ets-1 regulates angiogenesis by inducing the expression of
urokinase-type plasminogen activator and matrix metalloproteinase-1
and the migration of vascular endothelial cells. J Cell Physiol.
169:522–531. 1996.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kukacka J, Prusa R, Kotaska K and Pelouch
V: Matrix metalloproteinases and their function in myocardium.
Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 149:225–236.
2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Staudt A, Staudt Y, Dorr M, Bohm M, Knebel
F, Hummel A, Wunderle L, Tiburcy M, Wernecke KD, Baumann G and
Felix SB: Potential role of humoral immunity in cardiac dysfunction
of patients suffering from dilated cardiomyopathy. J Am Coll
Cardiol. 44:829–836. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Keidar S, Kaplan M, Pavlotzky E, Coleman
R, Hayek T, Hamoud S and Aviram M: Aldosterone administration to
mice stimulates macrophage NADPH oxidase and increases
atherosclerosis development: A possible role for
angiotensin-converting enzyme and the receptors for angiotensin II
and aldosterone. Circulation. 109:2213–2220. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu LJ, Chen YQ, Deng SB, Du JL and She Q:
Additional use of an aldosterone antagonist in patients with mild
to moderate chronic heart failure: A systematic review and
meta-analysis. Br J Clin Pharmacol. 75:1202–1212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tsutamoto T: Mineralocorticoid receptor
antagonist spironolactone improves left ventricular remodeling in
patients with congestive heart failure and acute myocardial
infarction. Nihon Yakurigaku Zasshi. 124:90–100. 2004.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
30
|
Liu X, Zhang X, Ye L and Yuan H:
Protective mechanisms of berberine against experimental autoimmune
myocarditis in a rat model. Biomed Pharmacother. 79:222–230.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li J, Schwimmbeck PL, Tschope C, Leschka
S, Husmann L, Rutschow S, Reichenbach F, Noutsias M, Kobalz U,
Poller W, et al: Collagen degradation in a murine myocarditis
model: Relevance of matrix metalloproteinase in association with
inflammatory induction. Cardiovasc Res. 56:235–247. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li YY, Kadokami T, Wang P, McTiernan CF
and Feldman AM: MMP inhibition modulates TNF-alpha transgenic mouse
phenotype early in the development of heart failure. Am J Physiol
Heart Circ Physiol. 282:H983–H989. 2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Huber SA, Polgar J, Schultheiss P and
Schwimmbeck P: Augmentation of pathogenesis of coxsackievirus B3
infections in mice by exogenous administration of interleukin-1 and
interleukin-2. J Virol. 68:195–206. 1994.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cleland JGF, Ferreira JP, Mariottoni B,
Pellicori P, Cuthbert J, Verdonschot JAJ, Petutschnigg J, Ahmed FZ,
Cosmi F, Brunner La Rocca HP, et al: The effect of spironolactone
on cardiovascular function and markers of fibrosis in people at
increased risk of developing heart failure: The heart ‘OMics’ in
AGEing (HOMAGE) randomized clinical trial. Eur Heart J. 42:684–696.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Brilla CG and Weber KT: Reactive and
reparative myocardial fibrosis in arterial hypertension in the rat.
Cardiovasc Res. 26:671–677. 1992.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu W, Gong W, He M, Liu Y, Yang Y, Wang
M, Wu M, Guo S, Yu Y, Wang X, et al: Spironolactone protects
against diabetic cardiomyopathy in streptozotocin-induced diabetic
rats. J Diabetes Res. 2018(9232065)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Okuducu AF, Zils U, Michaelis SA,
Michaelides S and von Deimling A: Ets-1 is up-regulated together
with its target gene products matrix metalloproteinase-2 and matrix
metalloproteinase-9 in atypical and anaplastic meningiomas.
Histopathology. 48:836–845. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hahne JC, Okuducu AF, Fuchs T, Florin A
and Wernert N: Identification of ETS-1 target genes in human
fibroblasts. Int J Oncol. 38:1645–1652. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hori Y, Kashimoto T, Yonezawa T, Sano N,
Saitoh R, Igarashi S, Chikazawa S, Kanai K, Hoshi F, Itoh N and
Higuchi S: Matrix metalloproteinase-2 stimulates collagen-I
expression through phosphorylation of focal adhesion kinase in rat
cardiac fibroblasts. Am J Physiol Cell Physiol. 303:C947–C953.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li Y, Ma J, Zhu H, Singh M, Hill D, Greer
PA, Arnold JM, Abel ED and Peng T: Targeted inhibition of calpain
reduces myocardial hypertrophy and fibrosis in mouse models of type
1 diabetes. Diabetes. 60:2985–2994. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao N, Zou H, Qin J, Fan C, Liu Y, Wang
S, Shan Z, Teng W and Li Y: MicroRNA-326 contributes to autoimmune
thyroiditis by targeting the Ets-1 protein. Endocrine. 59:120–129.
2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xu L, Fu M, Chen D, Han W, Ostrowski MC,
Grossfeld P, Gao P and Ye M: Endothelial-specific deletion of Ets-1
attenuates Angiotensin II-induced cardiac fibrosis via suppression
of endothelial-to-mesenchymal transition. BMB Rep. 52:595–600.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Steinl DC, Xu L, Ochoa-Espinosa A, Punjabi
M and Kaufmann BA: Non-invasive contrast enhanced ultrasound
molecular imaging of inflammation in autoimmune myocarditis for
prediction of left ventricular fibrosis and remodeling. PLoS One.
14(e0224377)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-beta/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yue Y, Meng K, Pu Y and Zhang X:
Transforming growth factor beta (TGF-β) mediates cardiac fibrosis
and induces diabetic cardiomyopathy. Diabetes Res Clin Pract.
133:124–130. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Duivenvoorden WC, Hirte HW and Singh G:
Transforming growth factor beta1 acts as an inducer of matrix
metalloproteinase expression and activity in human
bone-metastasizing cancer cells. Clin Exp Metastasis. 17:27–34.
1999.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Okano K, Hibi A, Miyaoka T, Inoue T,
Sugimoto H, Tsuchiya K, Akiba T and Nitta K: Inhibitory effects of
the transcription factor Ets-1 on the expression of type I collagen
in TGF-beta1-stimulated renal epithelial cells. Mol Cell Biochem.
369:247–254. 2012.PubMed/NCBI View Article : Google Scholar
|