Introduction
Sepsis is a life-threatening organ dysfunction
caused by a dysregulated host response to an infection. It is
currently the major cause of morbidity and mortality in intensive
care units worldwide (1). The
heart is one of the important target organs in sepsis and a large
number of studies have demonstrated that ~50% of patients with
sepsis exhibited cardiac dysfunction (2,3). It
has been reported that septic patients with cardiac dysfunction had
significantly higher mortality rates compared with those without
cardiac dysfunction (4). The
degree of myocardial structural damage and functional impairment is
associated with the clinical adverse outcomes of the illness.
Although much remains unknown, over the past decades, substantial
research has improved the understanding of its underlying
pathophysiologic mechanisms, including an excessive inflammatory
response, autonomic nervous system dysregulation, endothelial
dysfunction, cardiac autophagy and apoptosis, calcium regulation
disorders and metabolic reprogramming (5-10).
Among these factors, nitric oxide (NO) synthesis impairment serves
a crucial role in sepsis-induced cardiac dysfunction (11). NO is endogenously produced from
L-arginine by nitric oxide synthase (NOS), an enzyme with three
isoforms; ineuronal, endothelial and inducible NOS (nNOS, eNOS and
iNOS, respectively). In addition, it serves a wide range of
physiological and pathophysiological roles in the cardiovascular
system (12). Some studies have
demonstrated that sepsis-induced cardiac dysfunction is caused by
enhanced iNOS activity and resultant NO (13,14).
Conversely, another study revealed that the brain-derived
neurotrophic factor attenuated cardiac dysfunction by increasing
myocardial eNOS expression in sepsis models (15). In view of this, it is particularly
important to understand the role that NOS serves in sepsis-induced
cardiac dysfunction. Thus, despite improvements in antibiotic
therapies and supportive care, but due to the lack of specific
treatment, the expected results in clinical applications have not
been achieved and new therapeutic interventions need to be
explored.
Berberine is a natural pentacyclic isoquinoline
alkaloid that is the principal bioactive ingredient of Rhizoma
coptidis (also named Huang Lian in Chinese). It is also the
principal component of many other medicinal herbs. Berberine
exhibits a wide spectrum of pharmacological effects and is widely
used in clinical conditions for the treatment of different
diseases, including hypertension, stroke, diabetes, cancers,
atherosclerosis and viral infections (16-18).
It has been demonstrated that berberine functions as a negative
regulator in lipopolysaccharide (LPS)-induced sepsis and reduces
sepsis-related organ damage by suppressing inflammatory responses
(19-21).
However, the cardioprotective effect of berberine on sepsis-induced
cardiac dysfunction has not been fully understood and the
underlying mechanisms remain unclear.
Thus, the present study aimed to investigate the
cardioprotective role of berberine in sepsis-induced cardiac
dysfunction. It aimed to determine whether the cardioprotective
effects of berberine were mediated by upregulating the Akt/eNOS
pathway.
Materials and methods
Animals and treatment
All animal experimental procedures were conducted in
accordance with the Guidelines for the Care and Use of Laboratory
Animals (8th edition; pubmed.ncbi.nlm.nih.gov/21595115/) and were approved
by the ethics committee of Bengbu Medical College [approval no.
(2020) 211]. Male C57BL/6J 8-12-week-old mice were fed a standard
laboratory diet and tap water ad libitum and were housed in
plastic cages in a room with a 12-h light/dark cycle, temperature
of 22-24˚C and humidity of 60%. After 1 week of acclimation, the
mice were randomly divided into the following four groups (n=6 in
each group): Control, LPS, LPS + berberine and LPS +
Nω-nitro-L-arginine methyl ester (L-NAME) + berberine.
To develop a mouse septic cardiac dysfunction model, a single dose
(10-mg/kg body weight) of LPS was administered intraperitoneally
(22) and the Control group was
intraperitoneally administered with the equivalent volume of
saline. In the LPS + berberine and LPS + L-NAME + berberine groups,
berberine (10-mg/kg body weight) (23) dissolved in hot water was
administered intraperitoneally 30 min after the LPS treatment. In
the LPS + L-NAME + berberine group, L-NAME (100-mg/kg body weight)
(24) dissolved in saline was
administered intraperitoneally 30 min before the LPS treatment.
Berberine and LPS were purchased from Beijing Solarbio Science
& Technology Co., Ltd. L-NAME was purchased from
MilliporeSigma.
Echocardiographic study
After 6 h of LPS treatment, the mice were
anesthetized with 1.5-2% of isoflurane and echocardiography was
performed to assess cardiac function using a Vevo 2100 ultrasound
device (FUJIFILM VisualSonics Inc.). In total, three consecutive
cardiac cycle measurements were averaged and the left ventricular
ejection fraction (LVEF) and left ventricular fractional shortening
(LVFS) were measured to evaluate heart function. Subsequently, mice
were euthanized by an overdose of sodium pentobarbital (250 mg/kg;
intraperitoneal injection). Blood was collected from the eyelids of
the mice and placed into tubes containing 4% sodium citrate. The
plasma was then centrifuged for 10 min at 1,000 x g at 4˚C and the
heart tissues were obtained and frozen at -80˚C.
Plasma biochemical and inflammatory
cytokine analysis
The plasma levels of lactate dehydrogenase (LDH),
creatine kinase (CK) and creatine kinase-MB (CK-MB) were determined
using corresponding assay kits (cat. nos. A020-2-2, A032-1-1 and
H197-1-2 for CK-MB, Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's protocols. The plasma inflammatory
cytokines, tumor necrosis factor (TNF)-α and interleukin (IL)-1β
were measured using ELISA kits (cat. nos. MTA00B for TNF-α, MLB00C
for IL-1β, R&D Systems, Inc.) according to the manufacturer's
protocols.
Measurement of oxidative stress
The heart tissues were homogenized with 50-mmol/l
potassium phosphate buffer. Following centrifugation for 5 min at
12,000 g at 4˚C, the supernatant was used to measure the oxidative
stress levels. The levels of cardiac hydrogen peroxide
(H2O2), malondialdehyde (MDA) and glutathione
(GSH) were determined using the corresponding assay kits (S0038 for
H2O2, S0131S for MDA, S0053 for GSH, Beyotime
Institute of Biotechnology) and the activity of cardiac superoxide
dismutase (SOD) was measured using a total SOD assay kit with WST-8
(S0101S, Beyotime Institute of Biotechnology) according to the
manufacturer's protocols. The above values were standardized by
protein content, determined using a bicinchoninic acid (BCA)
protein assay kit (P0010S, Beyotime Institute of
Biotechnology).
Measurement of the heart NOS
activity
The heart NOS activity was measured using the Griess
method with the corresponding assay kits (Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
protocols. The heart NOS activity was standardized by protein
content that was determined using the BCA protein assay kit.
Western blot analysis
Protein used for western blot was extracted from the
myocardial tissue using RIPA lysis buffer containing phosphatase
and a protease inhibitor cocktail (Beyotime Institute of
Biotechnology) and then quantified using a BCA protein assay kit.
Equal amounts of protein (80 µg/lane) were separated with 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred onto polyvinylidene fluoride
membranes (MilliporeSigma). After blocking with 5% non-fat milk at
4˚C for 1 h, the membranes were incubated with primary antibodies,
namely, phosphorylated (p)-Akt (Ser473, 1:1,000, ProteinTech Group,
Inc.), Akt (1:1,000, ProteinTech Group, Inc.), p-eNOS (Ser1177,
1:1,000, Abcam) and eNOS (1:1,000, Abcam) at 4˚C overnight. GAPDH
(1:1,000, ProteinTech Group, Inc.) was used as the loading control.
After washing with TBST three times, the membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies (cat.
no. SA00001-2;1:2,000; ProteinTech Group, Inc.) at room temperature
for 1 h and the target signal was visualized using the ECL exposure
system. The intensity of the bands was quantified using an image
analysis system (ImageJ V1.8.0; National Institutes of Health).
Statistical analysis
All statistical analyses were conducted using SPSS
software, version 21.0 (IBM Corp.). The data were expressed as mean
± SEM and the differences between ≥ groups were assessed using
one-way analysis of variance followed by Tukey post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Berberine improved cardiac function in
LPS-induced septic mice, but it was inhibited by L-NAME
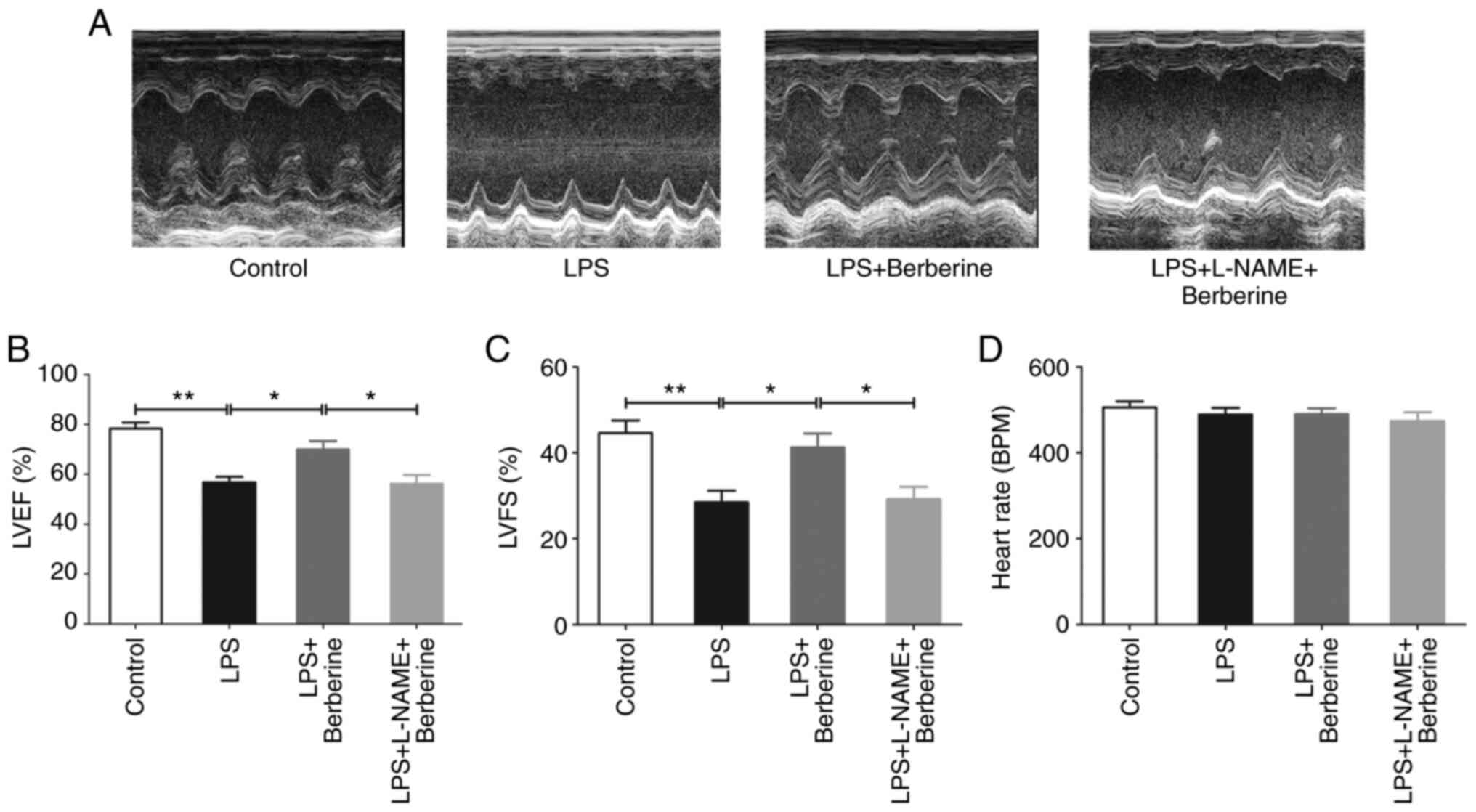
As presented in Fig.
1A-C, echocardiography revealed that there was a significant
decrease in the LVEF and LVFS after LPS injection compared with the
Control group. Berberine treatment increased LVEF and LVFS as
compared with the LPS group. However, LVEF and LVFS were
significantly decreased in LPS + L-NAME + berberine group as
compared with LPS + berberine group. There were no significant
differences in heart rate among the four groups (Fig. 1D).
Berberine alleviated cardiac injury in
LPS-induced septic mice, but it was inhibited by L-NAME
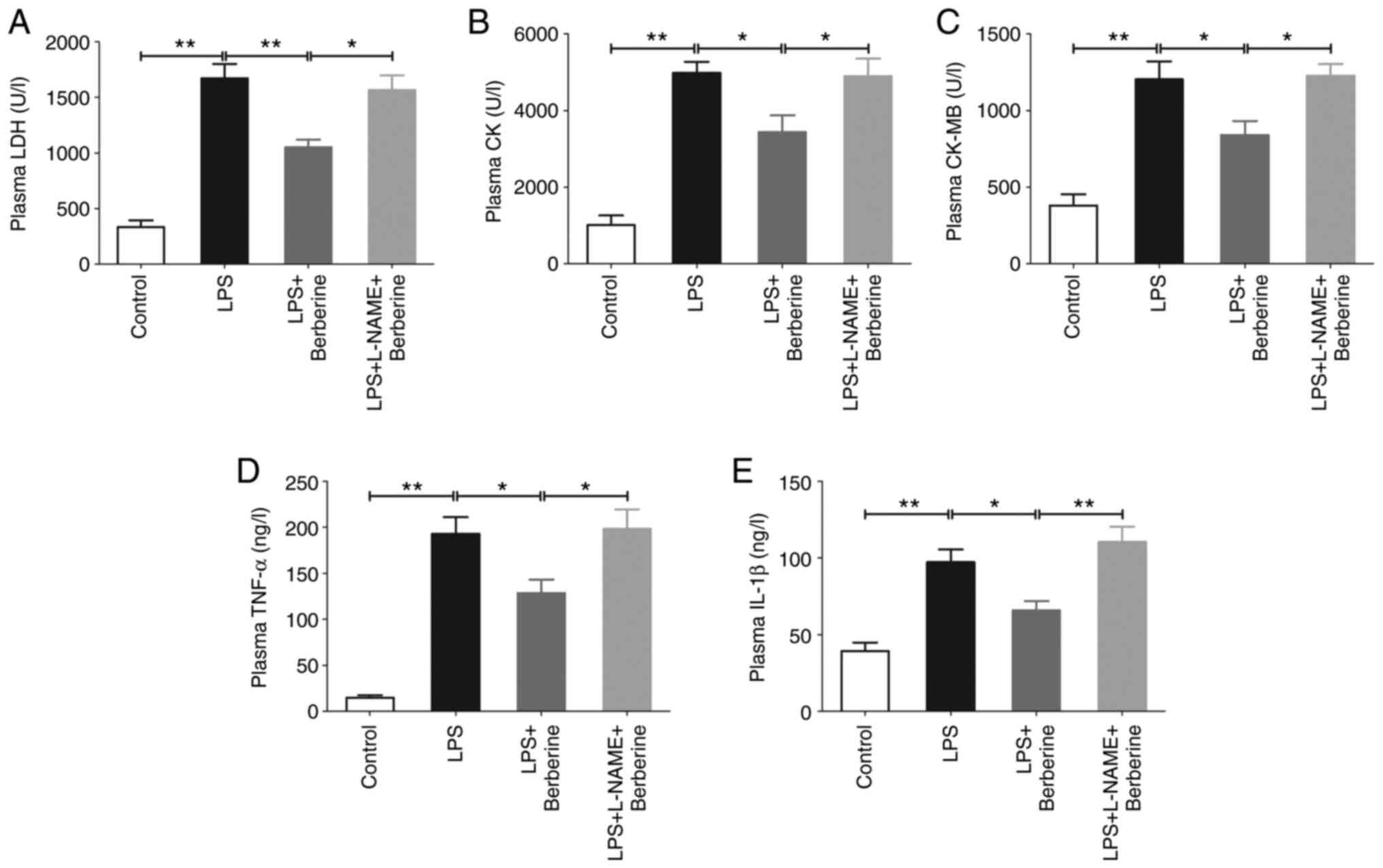
The levels of plasma LDH, CK and CK-MB, which are
myocardial injury markers, were significantly increased in the LPS
group compared with the Control group (Fig. 2A-C). The inflammatory factors TNF-α
and IL-1β in plasma were also significantly increased in the LPS
group(Fig. 2D and E). However, berberine treatment decreased
plasma LDH, CK, CK-MB, TNF-α and IL-1β levels as compared with the
LPS group, which was inhibited by L-NAME pre-treatment.
Berberine reduced oxidative stress in
LPS-induced cardiac dysfunction, but it was inhibited by
L-NAME
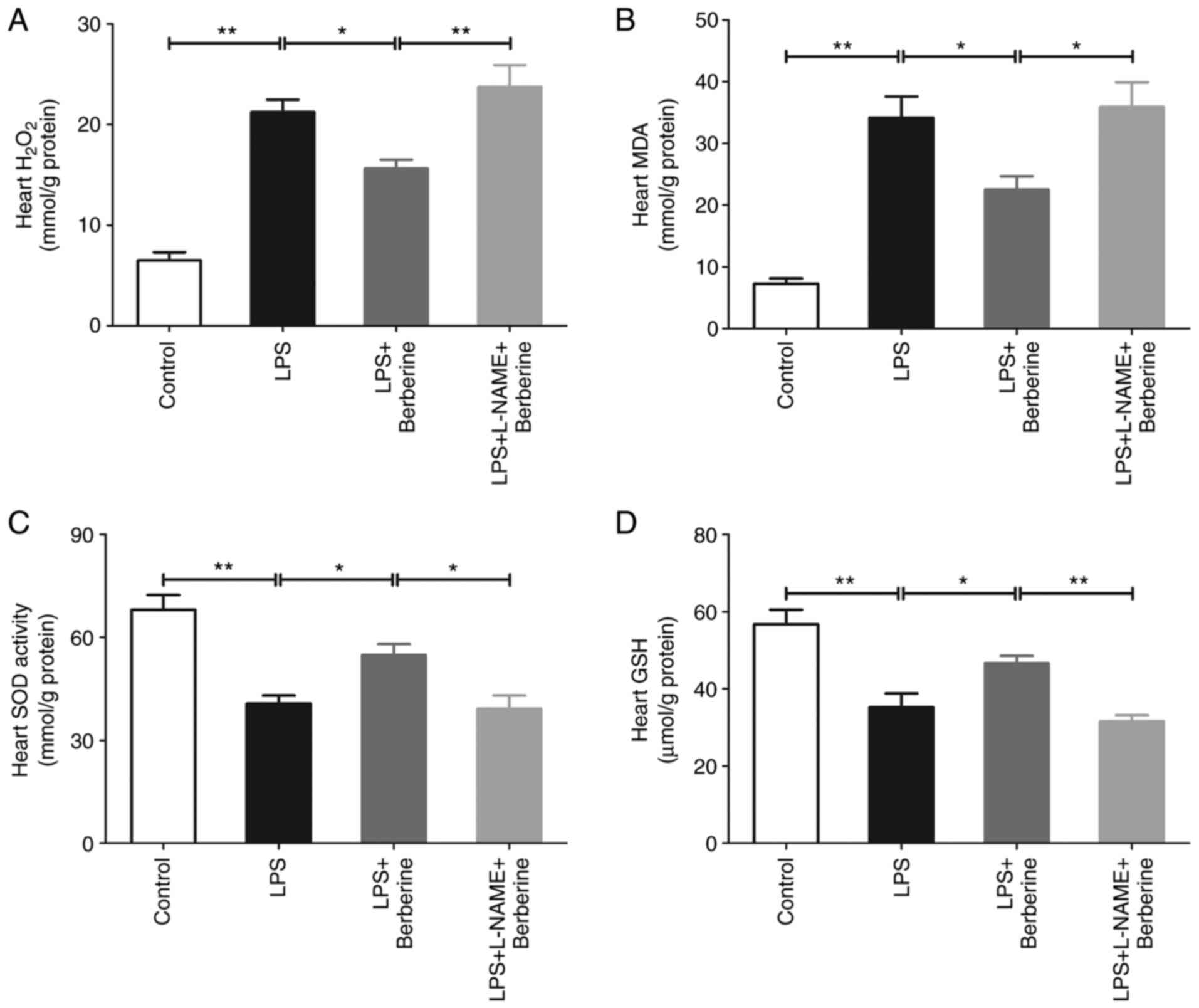
Sepsis-induced cardiac dysfunction was associated
with oxidative stress injury. As presented in Fig. 3A and B, the heart H2O2
and MDA levels significantly increased after the LPS injection
compared with the Control group, whereas the berberine treatment
markedly attenuated the elevation of H2O2 and
MDA in the hearts of the LPS-induced septic mice. There was also a
significant decrease in the activity of SOD and levels of GSH after
the LPS injection as compared with the Control group and berberine
treatment significantly enhanced the activity of SOD and level of
GSH in the hearts of the LPS-induced septic mice (Fig. 3C and D). L-NAME pre-treatment increased heart
H2O2 and MDA levels in the LPS + berberine
group, but decreased SOD activity and GSH levels.
Berberine upregulated the Akt/eNOS
pathway in LPS-induced cardiac dysfunction
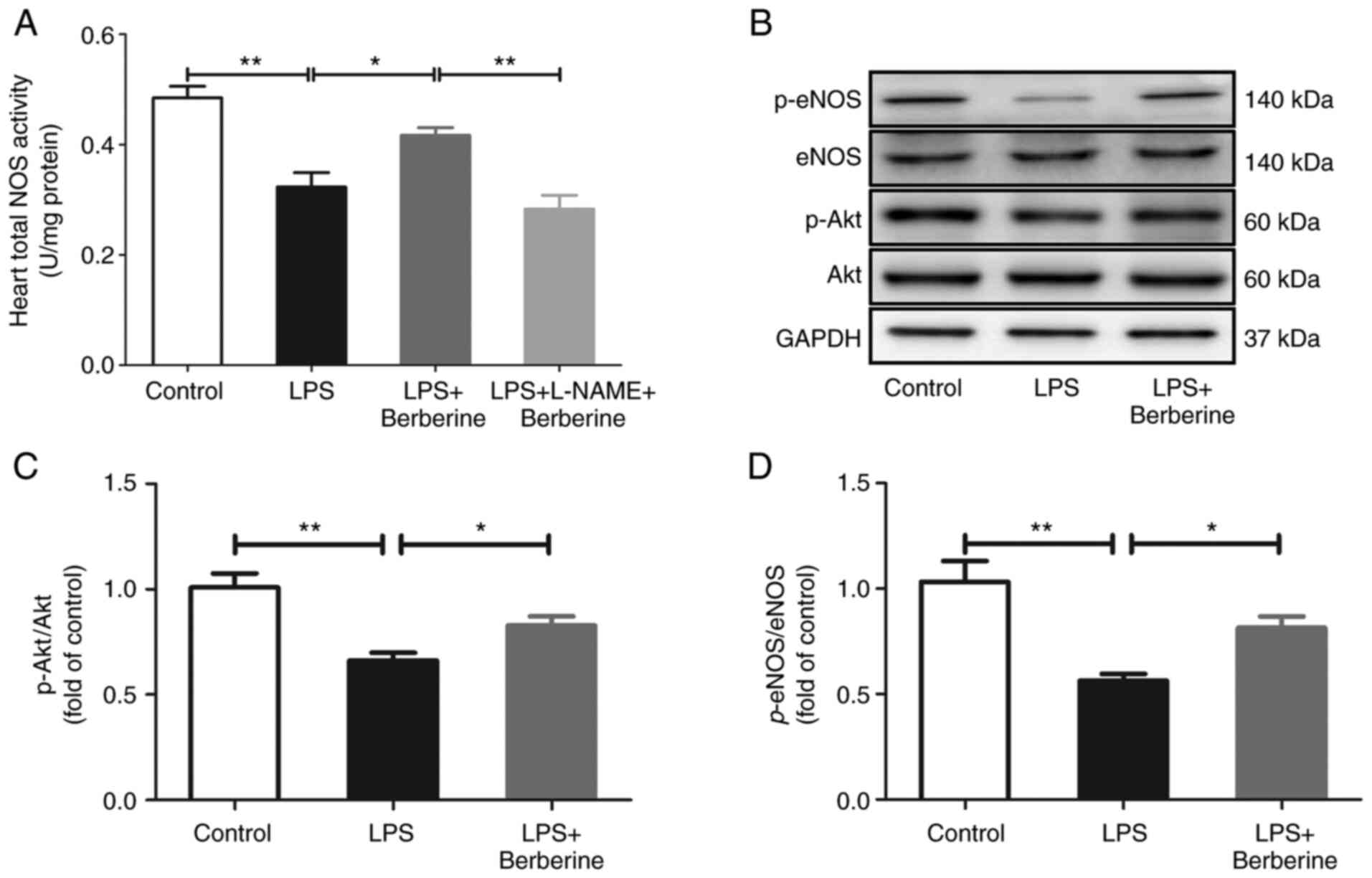
As presented in Fig.
4A, the heart total NOS activity was decreased after the LPS
injection as compared with the Control group. There was also a
significant decrease in the protein levels of p-Akt at Ser473
(Fig. 4C) and p-eNOS at Ser1177
(Fig. 4D). The berberine treatment
increased the heart total NOS activity which was inhibited by
L-NAME pre-treatment and berberine treatment also upregulated the
protein expressions of p-Akt and p-eNOS.
Discussion
The present study found that berberine had a
protective effect on sepsis-induced cardiac dysfunction and this
was accompanied by a decrease in myocyte injury marker enzymes,
which reduced cardiac inflammation and oxidative stress.
Mechanistically, it provided evidence showing that berberine
upregulated the Akt/eNOS pathway by which it ameliorated
sepsis-induced cardiac dysfunction.
Sepsis-induced cardiac dysfunction, which is defined
as a global but reversible dysfunction (systolic and diastolic) of
the heart induced by sepsis, is extremely common and contributes to
the adverse clinical outcomes of patients with sepsis (25). As a bioactive alkaloid in
traditional Chinese medicine, berberine has been used in critical
care medicine to treat sepsis and sepsis-related organ failure
according to its various pharmacological properties (26). Although it is shown to have
beneficial effects (27,28), the therapeutic effects of berberine
for sepsis-induced cardiac dysfunction have not been studied in
detail to date. In the present study, mice were intraperitoneally
injected with 10-mg/kg body LPS to induce cardiac dysfunction,
which is a widely accepted noninvasive reliable model (29,30).
After 6 h of LPS treatment, cardiac function was severely impaired,
as evidenced by the decreases in LVEF and LVFS and the increase in
myocardial injury markers. Berberine administration could
significantly increase LVEF and LVFS and decrease myocardial injury
markers. The above results were in line with a recent report that
found that berberine attenuated septic cardiomyopathy by inhibiting
TLR4/NF-κB signaling in rats (31).
It is well known that inflammation and oxidative
stress serve a key role in cardiac dysfunction induced by sepsis.
After activation by LPS, proinflammatory cytokines, such as TNF-α
and IL-1β, are released to promote inflammatory reaction and then,
they induce oxidative stress injury. However, the inhibition of
inflammation and oxidative stress injury can ameliorate
sepsis-induced cardiac dysfunction (32,33).
The present study found that berberine suppressed inflammatory
responses and ameliorated oxidative stress injury, as evidenced by
the significant decrease in the levels of
H2O2 and MDA and enhancement of the activity
of SOD and levels of GSH in the heart. A growing body of evidence
has suggested that NO is an antioxidant and serves an
anti-inflammatory role and that the downregulation and uncoupling
of the NOS protein leads to inflammation and increased oxidative
stress (34,35). In addition, NO serves a crucial
role in sepsis-induced cardiac dysfunction, although this is still
controversial. It has been reported that cardiac dysfunction in
sepsis is caused by the induction of NO production via iNOS
hyperactivity (36) and that the
inhibition of iNOS reversed cardiac dysfunction (10). On the other hand, other studies
demonstrate that high doses of L-N-monomethyl arginine, a
nonselective NOS inhibitor, increases mortality in patients with
septic shock and cardiomyocyte-specific overexpression of eNOS
decreases systemic inflammation and prevents cardiac dysfunction in
the murine models of septic shock (37,38).
In the present study, the heart total NOS activity and the protein
levels of p-eNOS at Ser1177 were decreased following the LPS
injection, whereas the berberine treatment increased the total NOS
activity in the heart and upregulated the protein expressions of
p-eNOS, which indicated that the eNOS activity was increased by
berberine. The results were in agreement with the study by Wang
et al (39), who found that
berberine prevents hyperglycemia-induced endothelial injury and
enhances vasodilatation by enhancing phosphorylation of eNOS at
Ser1177. It is well known that phosphorylation of the amino acids
Ser1177 in eNOS is facilitated by the Akt pathway activation
(40). The results of the present
study indicated that there was a significant decrease in the
protein levels of p-Akt at Ser473 following LPS treatment, whereas
berberine upregulated the protein expressions of p-Akt. Berberine
is reported to exert an anti-apoptotic effect and improve cardiac
functional recovery following myocardial ischemia/reperfusion by
activating PI3K-Akt-eNOS signaling in diabetic rats (41). It is also reported that berberine
protects endothelial progenitor cells from TNF-α damage via the
PI3K/AKT/eNOS signaling pathway (42). L-NAME is used to inhibit the
activity of eNOS (43). The
results of the present study indicated that cardiac function was
significantly decreased in the LPS + L-NAME +berberine group
compared with the LPS + berberine group, which was accompanied by a
significant decrease in the heart total NOS activity. The
myocardial injury markers, inflammatory factors and heart oxidative
stress levels were significantly increased after the L-NAME
pre-treatment. These data were in line with the report of Bougaki
et al (44), who found that
eNOS knockout mice exhibit shorter survival times in murine
polymicrobial sepsis and that eNOS deficiency worsens systemic
inflammation and exaggerated myocardial dysfunction.
There are several limitations to the present study.
One limitation is that L-NAME is just a non-specific NOS inhibitor.
So eNOS knockout mice should be used for the further studies and
p-eNOS and p-Akt expression changes should be detected after L-NAME
treatment or eNOS knockout. Another limitation is that the present
study was designed as pre-clinical animal experiment. Whether it
can be applied direct to clinical practice, requires further
work.
In conclusion, the present study suggested that
berberine could attenuate sepsis-induced cardiac dysfunction by
upregulating the Akt/eNOS pathway. Therefore, berberine has the
potential to become a therapeutic medicine for septic patients with
cardiac dysfunction.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by fund of Bengbu
Medical College (grant no. BYKY2019053ZD).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and GYL designed the experiments and wrote the
manuscript. HZ and YYT performed the experiments. HZ and XFW
analyzed and interpreted the data. XFW and GYL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were conducted in
accordance with the Guidelines for the Care and Use of Laboratory
Animals (8th edition, pubmed.ncbi.nlm.nih.gov/21595115/) and were approved
by the ethics committee of Bengbu Medical College [approval no.
(2020) 211].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
L'Heureux M, Sternberg M, Brath L,
Turlington J and Kashiouris MG: Sepsis-induced cardiomyopathy: A
comprehensive review. Curr Cardiol Rep. 22(35)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang J, Wang XT, Liu DW, Zhang HM and Su
LX: Induction and deduction in sepsis-induced cardiomyopathy: Five
typical categories. Chin Med J (Engl). 133:2205–2211.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Landesberg G, Gilon D, Meroz Y, Georgieva
M, Levin PD, Goodman S, Avidan A, Beeri R, Weissman C, Jaffe AS and
Sprung CL: Diastolic dysfunction and mortality in severe sepsis and
septic shock. Eur Heart J. 33:895–903. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W
and Tang Q: STING-IRF3 contributes to lipopolysaccharide-induced
cardiac dysfunction, inflammation, apoptosis and pyroptosis by
activating NLRP3. Redox Biol. 24(101215)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Carrara M, Ferrario M, Bollen Pinto B and
Herpain A: The autonomic nervous system in septic shock and its
role as a future therapeutic target: A narrative review. Ann
Intensive Care. 11(80)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Winkler MS, Nierhaus A, Poppe A, Greiwe G,
Gräler MH and Daum G: Sphingosine-1-Phosphate: A potential
biomarker and therapeutic target for endothelial dysfunction and
sepsis? Shock. 47:666–672. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci
B, Xie Y, Carlson D, Rothermel BA, Sun Y, et al: Beclin-1-dependent
autophagy protects the heart during sepsis. Circulation.
138:2247–2262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sepúlveda M, Gonano LA, Viotti M, Morell
M, Blanco P, López Alarcón M, Peroba Ramos I, Bastos Carvalho A,
Medei E and Vila Petroff M: Calcium/calmodulin protein kinase
ii-dependent ryanodine receptor phosphorylation mediates cardiac
contractile dysfunction associated with sepsis. Crit Care Med.
45:e399–e408. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hollenberg SM and Singer M:
Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol.
18:424–434. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kawaguchi S, Okada M, Ijiri E, Koga D,
Watanabe T, Hayashi K, Kashiwagi Y, Fujita S and Hasebe N:
β3-Adrenergic receptor blockade reduces mortality in
endotoxin-induced heart failure by suppressing induced nitric oxide
synthase and saving cardiac metabolism. Am J Physiol Heart Circ
Physiol. 318:H283–H294. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Farah C, Michel LYM and Balligand JL:
Nitric oxide signalling in cardiovascular health and disease. Nat
Rev Cardiol. 15:292–316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ndongson-Dongmo B, Lang GP, Mece O,
Hechaichi N, Lajqi T, Hoyer D, Brodhun M, Heller R, Wetzker R,
Franz M, et al: Reduced ambient temperature exacerbates
SIRS-induced cardiac autonomic dysregulation and myocardial
dysfunction in mice. Basic Res Cardiol. 114(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ndongson-Dongmo B, Heller R, Hoyer D,
Brodhun M, Bauer M, Winning J, Hirsch E, Wetzker R, Schlattmann P
and Bauer R: Phosphoinositide 3-kinase gamma controls
inflammation-induced myocardial depression via sequential cAMP and
iNOS signalling. Cardiovasc Res. 108:243–253. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zeng N, Xu J, Yao W, Li S, Ruan W and Xiao
F: Brain-derived neurotrophic factor attenuates septic myocardial
dysfunction via eNOS/NO pathway in rats. Oxid Med Cell Longev.
2017(1721434)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cai Y, Xin Q, Lu J, Miao Y, Lin Q, Cong W
and Chen K: A new therapeutic candidate for cardiovascular
diseases: Berberine. Front Pharmacol. 12(631100)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang S, Li D, Yu Z, Li Y and Wu M:
Multi-pharmacology of berberine in atherosclerosis and metabolic
diseases: Potential contribution of gut microbiota. Front
Pharmacol. 12(709629)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Šudomová M, Berchová-Bímová K, Marzocco S,
Liskova A, Kubatka P and Hassan STS: Berberine in human oncogenic
herpesvirus infections and their linked cancers. Viruses.
13(1014)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan C, Wu M, Xiao Q, Zhao W, Li H, Zhong
Y, Zhao M, Li C, Li Y and Yang X: Blocking Msr1 by berberine
alkaloids inhibits caspase-11-dependent coagulation in bacterial
sepsis. Signal Transduct Target Ther. 6(92)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Du P and Jiang D: Berberine
functions as a negative regulator in lipopolysaccharide-induced
sepsis by suppressing NF-κB and IL-6 mediated STAT3 activation.
Pathog Dis. 78(ftaa047)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
He Y, Yuan X, Zuo H, Li X, Sun Y and Feng
A: Berberine induces ZIP14 expression and modulates zinc
redistribution to protect intestinal mucosal barrier during
polymicrobial sepsis. Life Sci. 233(116697)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen YH, Teng X, Hu ZJ, Tian DY, Jin S and
Wu YM: Hydrogen sulfide attenuated sepsis-induced myocardial
dysfunction through TLR4 pathway and endoplasmic reticulum stress.
Front Physiol. 12(653601)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xu L, Zheng X, Wang Y, Fan Q, Zhang M, Li
R, Ye J, Wu X, Zhao W and Zhang Y: Berberine protects acute liver
failure in mice through inhibiting inflammation and
mitochondria-dependent apoptosis. Eur J Pharmacol. 819:161–168.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bülbül M and Sinen O: Dual autonomic
inhibitory action of central Apelin on gastric motor functions in
rats. Auton Neurosci. 212:17–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Antonucci E, Fiaccadori E, Donadello K,
Taccone FS, Franchi F and Scolletta S: Myocardial depression in
sepsis: From pathogenesis to clinical manifestations and treatment.
J Crit Care. 29:500–511. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fan TT, Cheng BL, Fang XM, Chen YC and Su
F: Application of Chinese medicine in the management of critical
conditions: A review on sepsis. Am J Chin Med. 48:1315–1330.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Y, Zhou J, Qiu J, Huang Z, Wang W, Wu P
and Feng A: Berberine reduces gut-vascular barrier permeability via
modulation of ApoM/S1P pathway in a model of polymicrobial sepsis.
Life Sci. 261(118460)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
He Y, Yuan X, Zuo H, Sun Y and Feng A:
Berberine exerts a protective effect on Gut-vascular barrier via
the modulation of the Wnt/beta-catenin signaling pathway during
sepsis. Cell Physiol Biochem. 49:1342–1351. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mou SQ, Zhou ZY, Feng H, Zhang N, Lin Z,
Aiyasiding X, Li WJ, Ding W, Liao HH, Bian ZY and Tang QZ:
Liquiritin attenuates lipopolysaccharides-induced cardiomyocyte
injury via an AMP-Activated protein kinase-dependent signaling
pathway. Front Pharmacol. 12(648688)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dai S, Ye B, Zhong L, Chen Y, Hong G, Zhao
G, Su L and Lu Z: GSDMD Mediates LPS-induced septic myocardial
dysfunction by regulating ROS-dependent NLRP3 inflammasome
activation. Front Cell Dev Biol. 9(779432)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen H, Liu Q, Liu X and Jin J: Berberine
attenuates septic cardiomyopathy by inhibiting TLR4/NF-κB
signalling in rats. Pharm Biol. 59:121–128. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu Y, Yang W, Sun X, Xie L, Yang Y, Sang
M and Jiao R: SS31 ameliorates sepsis-induced heart injury by
inhibiting oxidative stress and inflammation. Inflammation.
42:2170–2180. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Giustina AD, Bonfante S, Zarbato GF,
Danielski LG, Mathias K, de Oliveira AN Jr, Garbossa L, Cardoso T,
Fileti ME, De Carli RJ, et al: Dimethyl fumarate modulates
oxidative stress and inflammation in organs after sepsis in rats.
Inflammation. 41:315–327. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Daiber A, Kröller-Schön S, Oelze M, Hahad
O, Li H, Schulz R, Steven S and Münzel T: Oxidative stress and
inflammation contribute to traffic noise-induced vascular and
cerebral dysfunction via uncoupling of nitric oxide synthases.
Redox Biol. 34(101506)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ilari S, Dagostino C, Malafoglia V, Lauro
F, Giancotti LA, Spila A, Proietti S, Ventrice D, Rizzo M, Gliozzi
M, et al: protective effect of antioxidants in nitric Oxide/COX-2
interaction during inflammatory pain: The role of nitration.
Antioxidants (Basel). 9(1284)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dal-Secco D, DalBó S, Lautherbach NES,
Gava FN, Celes MRN, Benedet PO, Souza AH, Akinaga J, Lima V, Silva
KP, et al: Cardiac hyporesponsiveness in severe sepsis is
associated with nitric oxide-dependent activation of G protein
receptor kinase. Am J Physiol Heart Circ Physiol. 313:H149–H163.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
López A, Lorente JA, Steingrub J, Bakker
J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L,
Breen D, et al: Multiple-center, randomized, placebo-controlled,
double-blind study of the nitric oxide synthase inhibitor 546C88:
Effect on survival in patients with septic shock. Crit Care Med.
32:21–30. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ichinose F, Buys ES, Neilan TG, Furutani
EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki
M, et al: Cardiomyocyte-specific overexpression of nitric oxide
synthase 3 prevents myocardial dysfunction in murine models of
septic shock. Circ Res. 100:130–139. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye
H, Lau CW, Vanhoutte PM and Xu A: Berberine prevents
hyperglycemia-induced endothelial injury and enhances
vasodilatation via adenosine monophosphate-activated protein kinase
and endothelial nitric oxide synthase. Cardiovasc Res. 82:484–492.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim HJ, Yoo HY, Jang JH, Lin HY, Seo EY,
Zhang YH and Kim SJ: Wall stretch and thromboxane A2
activate NO synthase (eNOS) in pulmonary arterial smooth muscle
cells via H2O2 and Akt-dependent
phosphorylation. Pflugers Arch. 468:705–716. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen K, Li G, Geng F, Zhang Z, Li J, Yang
M, Dong L and Gao F: Berberine reduces ischemia/reperfusion-induced
myocardial apoptosis via activating AMPK and PI3K-Akt signaling in
diabetic rats. Apoptosis. 19:946–957. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xiao M, Men LN, Xu MG, Wang GB, Lv HT and
Liu C: Berberine protects endothelial progenitor cell from damage
of TNF-α via the PI3K/AKT/eNOS signaling pathway. Eur J Pharmacol.
743:11–16. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Aremu OO, Oyedeji AO, Oyedeji OO,
Nkeh-Chungag BN and Rusike CRS: In vitro and in vivo antioxidant
properties of taraxacum officinale in
Nω-Nitro-l-Arginine Methyl ester (L-NAME)-induced
hypertensive rats. Antioxidants (Basel). 8(309)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bougaki M, Searles RJ, Kida K, Yu J, Buys
ES and Ichinose F: Nos3 protects against systemic inflammation and
myocardial dysfunction in murine polymicrobial sepsis. Shock.
34:281–290. 2010.PubMed/NCBI View Article : Google Scholar
|