Introduction
After ~2 years of coronavirus disease 2019
(COVID-19) pandemic caused by severe acute respiratory syndrome
coronavirus 2 which was first identified in 2019(1), most countries have faced multiple
surges of newly diagnosed patients with COVID-19(2). Patients with COVID-19 are treated
with existing and newly developed therapeutic approaches according
to the severity of the disease (3,4).
Most patients without symptoms are treated in primary care
facilities with supportive care, including oxygen inhalation and
vital sign monitoring. Even symptomatic patients whose status
requires inpatient care are sometimes forced to stay at home due to
the limitations of medical resources (5). An increased risk of cardiac arrest at
home has been reported among COVID-19 patients (6). Exacerbation of symptoms is common in
inpatient care (7), and 15-30% of
hospitalized patients develop acute respiratory distress syndrome
(8). Therefore, the establishment
of practical and concise criteria to triage COVID-19 patients is
necessary to prepare for another wave of the pandemic surge when
medical resources are limited.
Specifically, in the field of radiology, there is a
high demand for diagnostic imaging, including chest radiography and
computed tomography (CT), to assess the severity and future risk of
exacerbation in COVID-19 patients (9-11).
However, the resources available for diagnostic imaging are
limited, especially for at home medical care or in local community
hospitals; therefore, unnecessary imaging studies should be
avoided. Thus, the establishments of practical triage systems will
help reduce the burden of radiologic examinations under pandemic
surges.
The advanced lung cancer inflammation index (ALI)
was initially developed as a prognostic indicator for metastatic
non-small cell lung cancer (12).
ALI is currently used for other types of neoplasm, including
colorectal cancer, lymphoma, and pancreatic cancer (13-16).
as well as for non-neoplasmic diseases such as Crohn's disease
(17). ALI is calculated using
body mass index (BMI), serum albumin level, neutrophil count, and
lymphocyte count (The forumula is described in Material and Method
section), which are measured and readily available at primary care
facilities (18). Because the
variables used in the ALI calculation are known to be correlated
with the severity of COVID-19 patients, the ALI is presumed to be
related to the prognosis of these patients. For instance, patients
with both hypoalbuminemia and lymphopenia have a high risk of
severe COVID-19(19), and obesity
increases the risk of hospitalization, intensive care unit
admission, and death (20,21).
This study aimed to assess the feasibility of
applying the ALI in COVID-19 patients; evaluate the correlation
among the ALI, imaging studies (radiography and CT), and prognosis;
and establish concise triage criteria to mitigate workload in the
radiology service under a pandemic surge.
Materials and methods
Patients
This retrospective study was approved by the IRB of
National Hospital Organization Nishisaitama-Chuo National Hospital
(IRB no. 2020-18), and written informed consent was waived. We
enrolled patients admitted to National Hospital Organization
Nishisaitama-Chuo National Hospital from March to October 2020 due
to diagnosis of COVID-19 infection via polymerase chain
reaction test of respiratory tract specimen. Patients without
clinical information and/or chest CT upon admission were excluded
(13 patients). A total of 79 patients (age: 43±19 years,
male/female: 45:34) were enrolled.
Clinical parameters
A pulmonologist (Y.H.) reviewed the patients'
medical records to abstract age, sex, body weight (kg), height
(cm), BMI (kg/m2), white blood cell (WBC) count (/µl),
neutrophil/lymphocyte ratio (NLR), albumin level (g/dl), clinical
severity at admission, and clinical course. Clinical severity upon
admission was classified into two categories: mild, percutaneous
oxygen saturation (SpO2) ≥93%; severe, SpO2
<93% or requiring oxygen inhalation. One of our authors (Y.I.)
archived the CT images upon admission and the chest radiographs
obtained within 24 hs of the CT examinations. Radiographs were
available in 72 of 79 patients. The patient outcome was classified
in terms of exacerbation during the short-term clinical course; the
exacerbated group required a ventilator and was transferred to the
intensive care unit in another institution, and the improved group
was discharged without being transferred to another institution for
ventilator support. The ALI equation is as follows: ALI = BMI x
Alb/NLR
We also assessed each patient's risk of developing
critical illness using an established predictive scoring system
reported by Liang et al (22). Liang's risk score predictors
include abnormality on chest radiography, age, hemoptysis, dyspnea,
unconsciousness, number of comorbidities, cancer history, NLR,
lactate dehydrogenase, and direct bilirubin (calculator is
available in the following website: https://reference.medscape.com/calculator/750/covid-19-critical-illness-prediction-tool-covid-gram).
Image evaluation
A radiologist (A.I., with 12 years of experience in
imaging diagnosis) evaluated the chest radiographs (positive or
negative for pulmonary opacity). As a semiquantitative approach,
another radiologist (H.T., with 7 years of experience in imaging
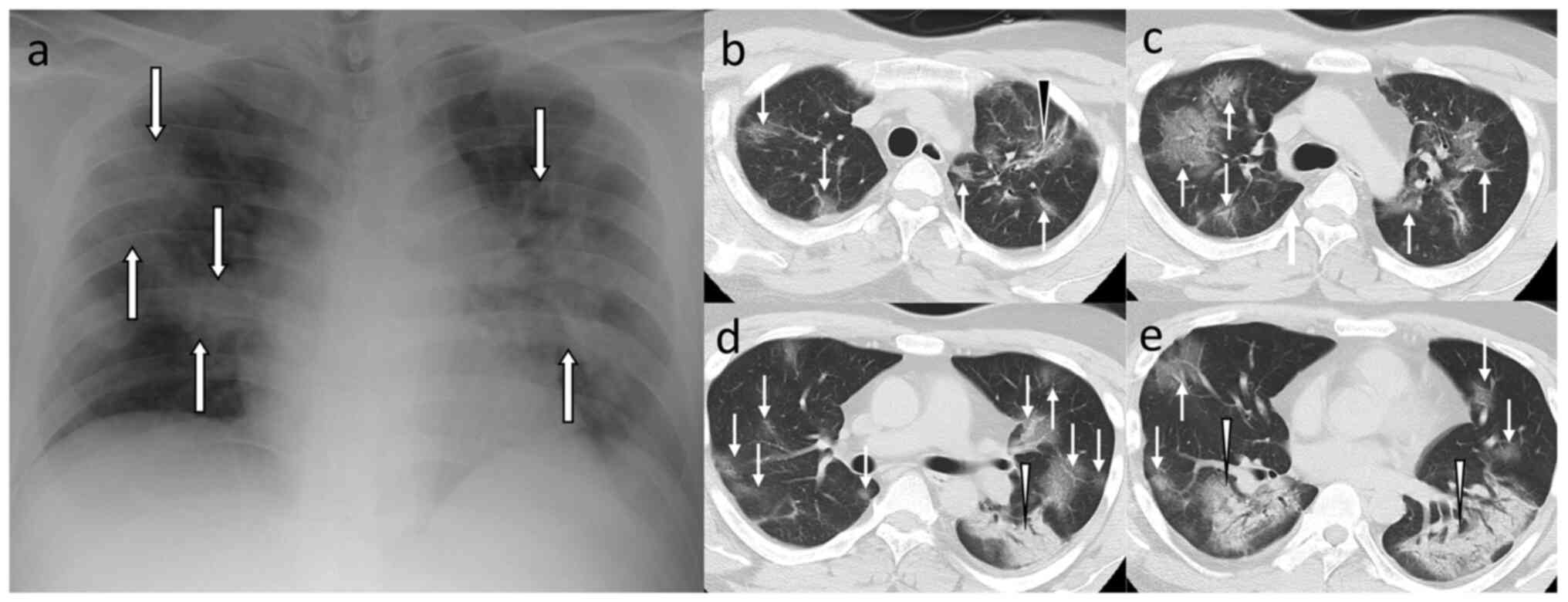
diagnosis) rated the scores using the chest CT score (CCTS;
Fig. 1) (23). We used the CCTS as it has been
demonstrated to show the best performance among the three
semiquantitative CT scoring systems, namely, CCTS (23), total CT score (24), and CT severity score (25,26).
The radiologist reviewed the chest CTs with the lung window setting
and evaluated the extent of disease involvement using a five-point
scale (0: 0%, 1: 0-4%, 2: 5-25%, 3: 26-49%, 4: 50-75%, and 5:
76-100%) for five lung lobes. The patient-level score (0-25) was
calculated by summing up the five lobe-level score.
Statistical analysis
Descriptive statistics demonstrated the frequency
(%) or median and interquartile range for each parameter. We
employed Fisher's exact test or Mann-Whitney U test to
compare each parameter between the groups. The combined ALI and
radiologic risk prediction model was constructed via random
forest classification using variables that have significant
difference between the improved and exacerbated groups by
univariate analysis (age, ALI, severity at admission, chest
radiograph abnormality, and CCTS). The accuracy of predicting
exacerbation using the ALI, CCTS, and Liang's clinical risk score
was evaluated using the receiver-operating characteristic (ROC)
curve analysis. A P value <0.05 was considered to indicate a
statistically significant difference. All statistical tests were
conducted using the R software (version 4.0.2; R Foundation for
Statistical Computing, Vienna, Austria).
Results
Among 79 patients, 72 experienced improvement, and 7
experienced exacerbation after admission. The univariate analysis
revealed a significant difference between the improved and
exacerbated groups in age (34 vs. 62 years; P=0.032), WBC (median:
4,550 vs. 7,300/µl, P=0.027), NLR (median: 2.2 vs. 7.2, P=0.012),
albumin level (median: 4.4 vs. 3.7 mg/dl, P=0.004), ALI (47.6 vs.
13.2; P=0.011), frequency of chest radiograph abnormality (24.7 vs.
83.3%; P<0.001), CCTS (1 vs. 9, P<0.001), Liang score (49.1
vs. 110.6; P<0.001), and severity at admission (rate of severe
case: 5.6 vs. 85.7%; P<0.001) (Table I).
 | Table IPatient characteristics and results of
the univariate analysis. |
Table I
Patient characteristics and results of
the univariate analysis.
| Variables | Total (n=79) | Improved group
(n=72) | Exacerbated group
(n=7) | P-value |
|---|
| Patient
demographics | | | | |
|
Median age,
years (IQR) | 35 (26, 58) | 34 (25, 56) | 62 (42, 76) | 0.032a |
|
Female
(%) | 34 (43.0) | 33 (45.8) | 1 (14.3) | 0.229 |
|
Body weight,
kg (IQR) | 62.0 (53.6,
77.7) | 61.9 (53.4,
77.2) | 63.0 (62.1,
89.3) | 0.259 |
|
Height, cm
(IQR) | 163.8 (159.1,
170.2) | 163.7 (158.7,
170.9) | 169.0 (163.0,
169.4) | 0.227 |
|
Body mass
index (IQR) | 23.4 (21.4,
27.0) | 23.3 (21.2,
26.8) | 24.1 (22.8,
28.7) | 0.422 |
| Laboratory
results | | | | |
|
WBC (/µl),
(IQR) | 4,700 (3,700,
6,350) | 4,550 (3,700,
6,025) | 7,300 (4,800,
8,350) | 0.027a |
|
Neut/Lymph
ratio (IQR) | 2.30 (1.40,
4.00) | 2.20 (1.38,
3.70) | 7.20 (4.95,
9.40) | 0.012a |
|
Albumin,
g/dl (IQR) | 4.40 (4.00,
4.60) | 4.40 (4.10,
4.60) | 3.70 (3.65,
3.90) | 0.004a |
| Scoring | | | | |
|
ALI
(IQR) | 46.5 (23.6,
75.9) | 47.6 (27.3,
80.0) | 13.2 (9.9,
16.9) | 0.011a |
|
Liang score
(IQR) | 51.5 (32.0,
81.4) | 49.1 (31.7,
71.6) | 110.6 (102.2,
121.9) | <0.001 |
| Imaging
examination | | | | |
|
Chest
radiograph abnormality (%)b | 24 (32.9) | 18 (24.7) | 6 (83.3) |
<0.001a |
|
Chest CT
score (IQR) | 2 (0, 7) | 1 (0, 6) | 9 (9, 17.5) |
<0.001a |
| Severity at
admission | | | | |
|
Mild | 69 (87.3) | 68 (94.4) | 1 (14.3) |
<0.001a |
|
Severe | 10 (12.7) | 4 (5.6) | 6 (85.7) | |
| Treatment, n
(%) | | | | |
|
Oxygen
inhalation | 12 (15.2) | 6 (8.3) | 6 (85.7) |
<0.001a |
|
Active
treatment | 30 (40.0) | 25 (34.7) | 5 (71.4) | 1.000 |
|
Favipiravir | 13 (16.5) | 10 (13.9) | 3 (42.9) | 0.083a |
|
Nafamostat | 7 (8.9) | 5 (6.9) | 2 (28.6) | 0.115 |
|
Systemic
steroid | 17 (21.5) | 13 (18.1) | 4 (57.1) | 0.035a |
|
Inhaled
steroid | 12 (15.2) | 12 (16.7) | 0 (0) | 0.587 |
|
Anticoagulants | 2 (2.5) | 1 (1.4) | 1 (14.3) | 0.170 |
|
Antibiotics | 7 (8.9) | 3 (4.2) | 4 (57.1) |
<0.001a |
During the entire clinical course, 15.2% (12/79) of
patients inhaled oxygen, 21.5% (17/79) of patients were
administered with systemic steroid therapy, and 15.2% (12/79) of
patients were given inhaled steroid therapy. Favipiravir and
nafamostat tocilizumab were used in 16.5% (13/79) and 8.8% (7/79)
of patients, respectively. Supplemental antibiotics were
administered in 8.8% (7/79) of patients (Table I).
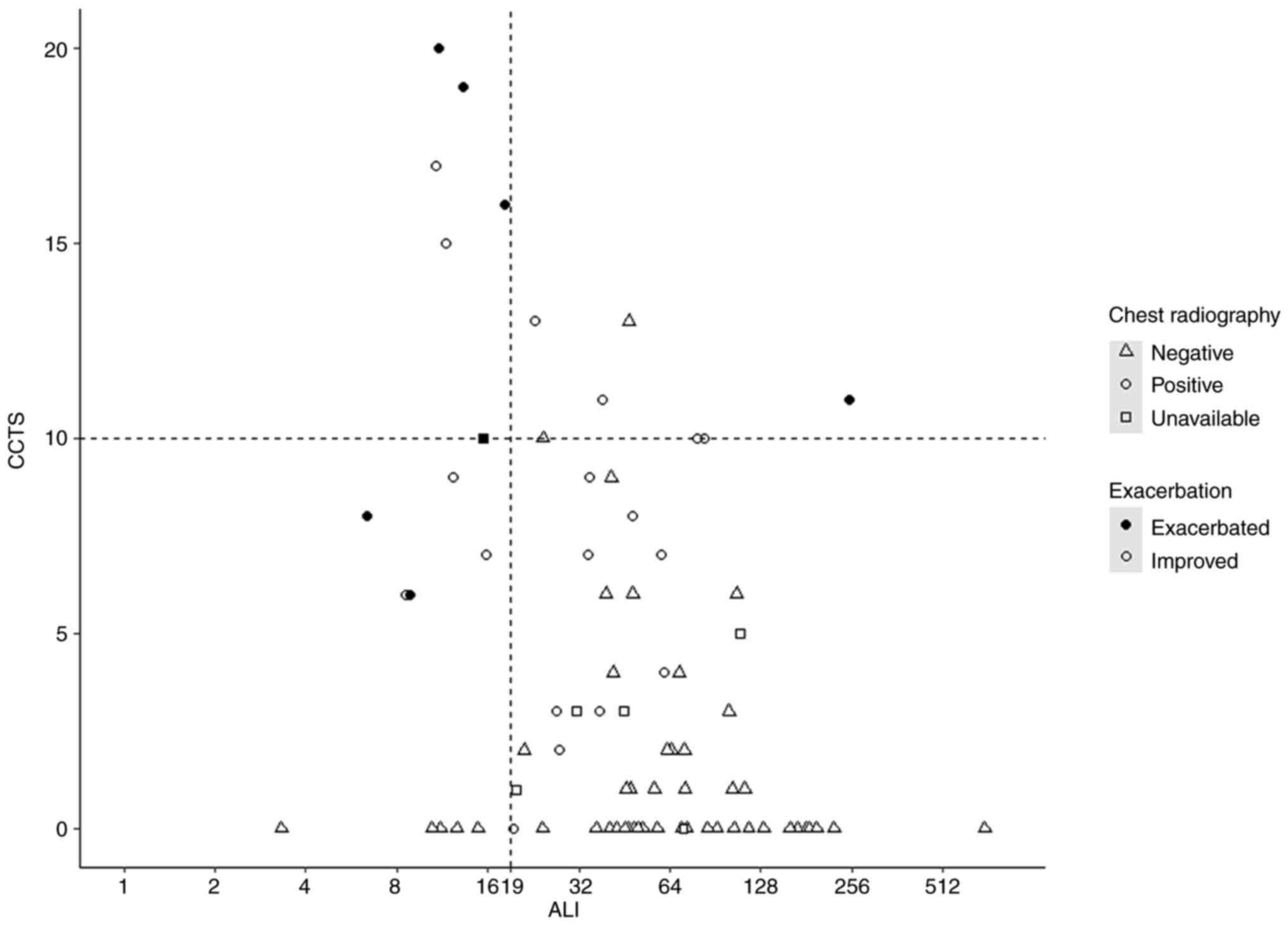
Fig. 2 presents the
relationship between the ALI and CCTS, and Tables II and III summarizes the detailed results of
the ALI and imaging findings, including CCTS and radiograph. Six of
seven exacerbated cases had an ALI less than 19 (Table II), with a BMI ranging from 18.7
to 31.7 kg/m2, NLR ranging from 3.5 to 12.7, and albumin
ranging from 2.9 to 4.0 g/dl. One exacerbated case had an outlier
ALI score of 250.6, with a BMI of 36.6 kg/m2, NLR of
0.7, and albumin of 4.7 g/dl (Fig.
1). Among the exacerbated cases, the CCTS ranged from 6 to 20.
Among the 72 patients who underwent chest radiography, all patients
who experienced exacerbation (n=6) exhibited a positive abnormality
on chest radiograph (Table III).
Liang's clinical risk score was available in 89.8% (71 of 79) of
patients due to the lack of chest radiograph (n=7) and laboratory
data (n=1).
 | Table IIRelationship between the ALI and
chest CT score. |
Table II
Relationship between the ALI and
chest CT score.
| ALI (n=79) | ALI ≥19 (n=63) | ALI <19
(n=16) | P-value |
|---|
| Exacerbation
(%) | 1 (15.9) | 6 (37.5) |
<0.001a |
| CCTS (IQR) | 1 (0, 4.5) | 7.5 (0, 15.25) | 0.014a |
| CCTS ≥10 (%) | 7 (11.1) | 6 (37.5) | 0.021a |
 | Table IIIRelationship between chest radiograph
abnormality and other parameters. |
Table III
Relationship between chest radiograph
abnormality and other parameters.
| Chest radiograph
(n=72) | Negative
(n=48) | Positive
(n=24) | P-value |
|---|
| Exacerbation
(%) | 0 (0) | 6 (25.0) |
<0.001a |
| ALI (IQR) | 56.9 (41.0,
103.1) | 25.1 (12.1,
40.8) |
<0.001a |
| ALI <19 (%) | 5 (10.4) | 10 (41.7) | 0.004a |
| CCTS (IQR) | 0 (0, 2) | 8.5 (6, 11.5) |
<0.001a |
| CCTS ≥10 (%) | 2 (4.2) | 10 (41.7) |
<0.001a |
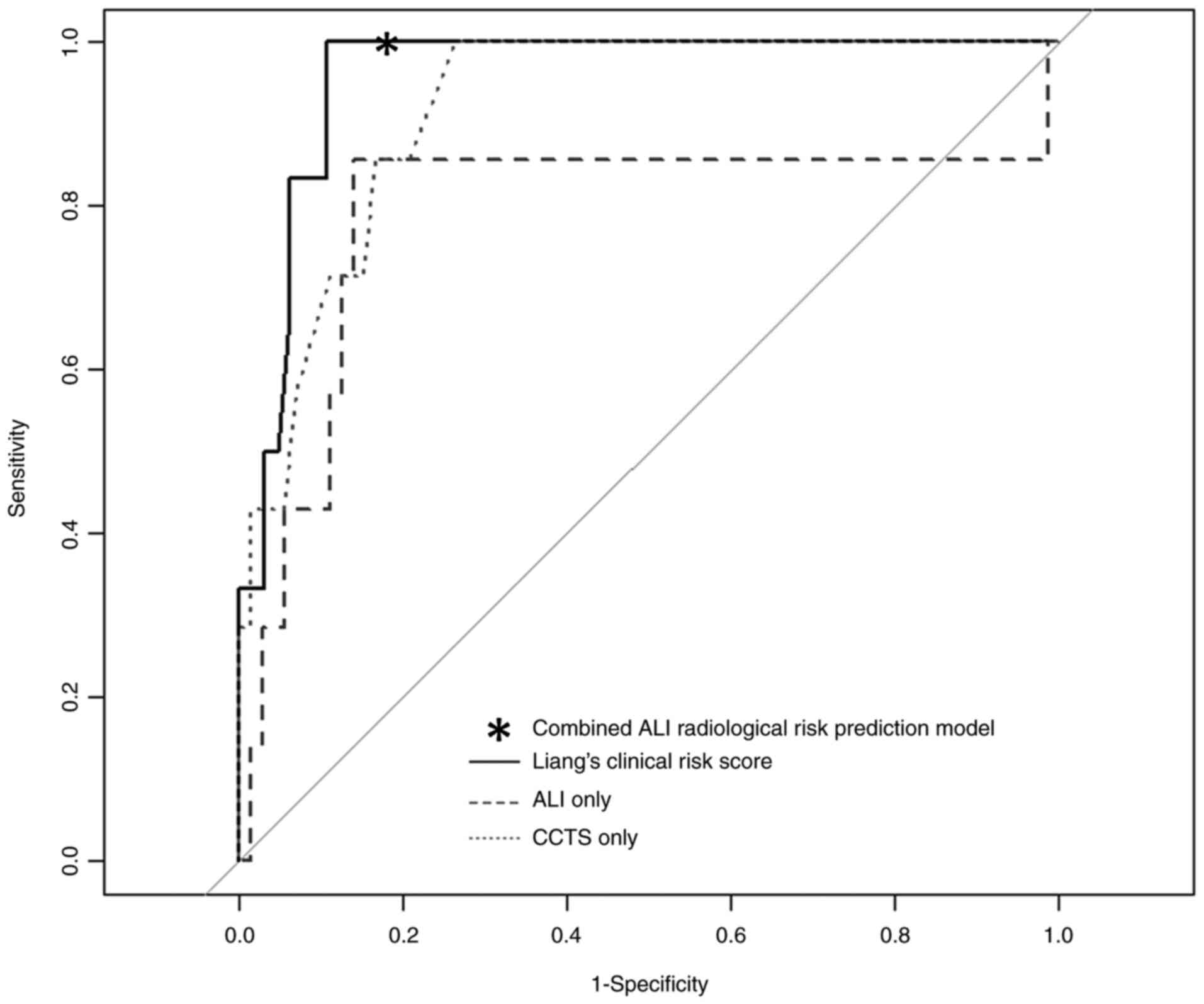
For the accuracy of predicting exacerbation, the ROC
analysis demonstrated AUCs of 0.79, 0.92, and 0.96 for the ALI,
CCTS, and Liang's clinical risk scores, respectively (Fig. 3). With a cutoff value of 19, the
ALI alone had a sensitivity of 0.86 and specificity of 0.86. With a
cutoff value of 10, the CCTS alone had a sensitivity of 0.72 and a
specificity of 0.89. With a cutoff value of 88.4 calculated using
the Youden index, Liang's clinical risk score had a sensitivity of
1.00 and a specificity of 0.89.
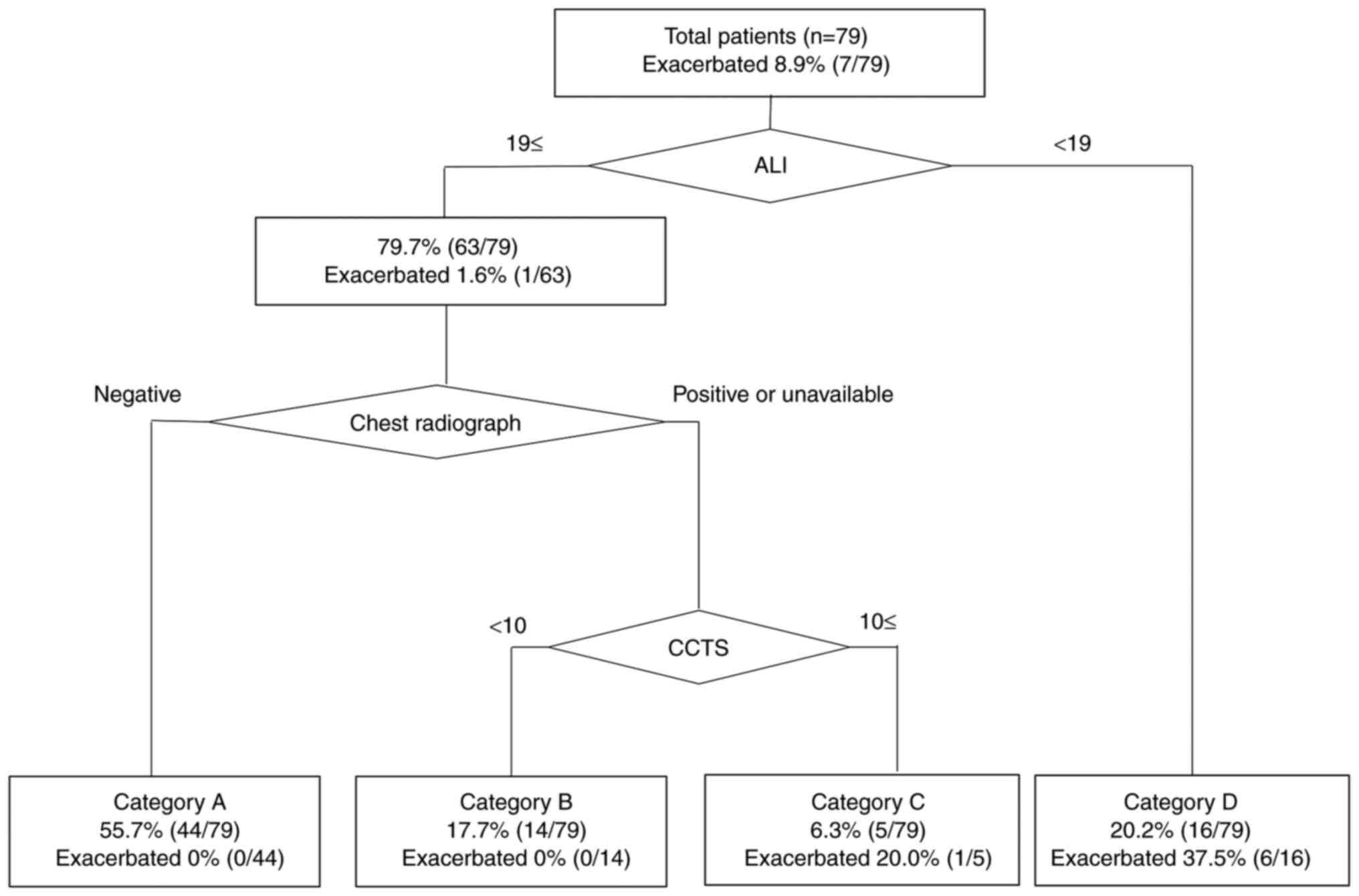
The combined ALI and radiologic risk prediction
model (Fig. 4) was developed using
the ALI, chest radiograph abnormality, and CCTS as the first-,
second-, and third-step significant classifiers, respectively. The
result of random forest classification itself adopted only two
classifiers (ALI and CCTS), and therefore we manually inserted
chest radiograph as the second classifier to fit our clinical
practice. This combined model categorized patients into four
groups: category A (ALI ≥19 and negative chest radiograph
abnormality: 55.7% [44/79]), category B (ALI ≥19 and positive or
nonavailable chest radiograph and CCTS <10: 17.7% [14/79]),
category C (ALI ≥19 and positive or nonavailable chest radiograph
and CCTS ≥10: 6.3% [5/79]), and category D (ALI <19: 20.2%
[16/79]). Exacerbation was observed in one patient in category C
and in six patients in category D. The combined ALI and radiologic
risk prediction model had a sensitivity of 1.00 and a specificity
of 0.81 when categories A and B were considered as negative and
categories C and D as positive.
Discussion
Our findings indicate that the ALI score was
significantly higher in the improved group (47.6) than in the
exacerbated group (13.2; P=0.011), and patients with a lower ALI
score had a greater probability of CT or chest radiograph
abnormalities. The ALI alone and CCTS alone had a modest AUC for
predicting exacerbation of COVID-19 (0.79 and 0.92). The combined
ALI and radiologic risk prediction model had decent sensitivity
(1.00) and specificity (0.81), which was almost similar to the
established clinical risk score (Liang's clinical risk score:
sensitivity of 1.00 and specificity of 0.89).
Our combined ALI and radiologic risk prediction
model adopted an ALI threshold of 19, which was included within the
range of the previously documented ALI thresholds (15.5-43.5) for
other diseases (12,16,17,27-33).
The ALI is directly proportional to the BMI and albumin and
inversely proportional to the NLR. In our study, exacerbated cases
exhibited a significantly higher NLR (7.2 vs. 2.2) and lower
albumin (3.7 vs. 4.4) compared with improved cases, which should
result in a significantly lower ALI in exacerbated cases. Six of
the seven exacerbated cases had an ALI <19. One exacerbated case
had an outlier ALI of 250.6, which could be explained by obesity
(i.e., high BMI of 36.6) and low NLR of 0.7. This case suggested
that the ALI might not necessarily reflect the actual risk in
patients with high BMI. If we assume that there are two patients
with similar values of albumin and NLR, a patient with a lower BMI
should be categorized as a higher risk (i.e., lower ALI). This
could be true in the setting of evaluating patients with cachexia
but not for patients with obesity (high BMI), which is known to be
an important risk factor for COVID-19 exacerbation (20). Thus, the ALI may underestimate the
potential risk of COVID-19 exacerbation in obese patients. The
modest accuracy of the ALI in predicting COVID-19 exacerbation in
our study might be due to the lower proportion of obese patients in
our patient cohort (BMI >30: 14% [11/78]) compared with the
previous study (34,35). Another potential factor that could
influence the accuracy of ALI risk prediction is NLR. The ALI is
inversely proportional to NLR, and therefore, patients with a low
NLR value could have an extraordinarily high ALI value. The one
exacerbated case with outlier ALI had a lower NLR (0.7) compared
with other exacerbated cases (3.5-12.7). Therefore, the application
of the ALI should be avoided in patients with neutropenia.
The current guidelines do not recommend routine
chest radiography and CT for all COVID-19 patients (36,37).
The avoidance of unnecessary imaging examinations is important for
the reduction of radiation exposure for patients, viral exposure
for medical staff, and medical costs (38). Our combined ALI and radiologic risk
prediction model helps avoid performing unnecessary imaging for
patients with a low risk of exacerbation. In our study cohort,
55.7% (44/79) of patients were categorized as category A, and they
can waive CT scans based on the negativity of chest radiograph and
ALI ≥19. CT scan was suggested only for patients with a positive
radiograph, and a positive chest radiograph in patients with
COVID-19 was correlated with higher CCTS scores. Of the 48 patients
in our study, 2 with a negative radiograph (4.2%) had a CCTS ≥10,
whereas 10 of the 24 patients with a positive radiograph (42.0%)
had a CCTS ≥10. We must be aware of the probability of a
false-negative result on radiography. In our study, the BMI of the
two patients with a negative radiograph and a CCTS ≥10 was more
than 30 kg/m2. We considered that a large body size
could degrade the image quality of radiograph, which could result
in false-negative results.
Despite its high accuracy in predicting COVID-19
exacerbation, the major drawback of Liang's clinical risk score is
that it requires us to input a fairly large number of clinical
data, including patient's history, chest radiograph, and lab values
(lactate dehydrogenase and direct bilirubin) to obtain the
estimated risk results. On the contrary, the ALI can be calculated
using only the BMI, albumin level, and NLR and is therefore
available in home medical care settings or primary care clinics. We
can now expect the application of our combined ALI and radiologic
risk prediction model in the primary care facility. The patients in
category D (i.e., ALI <19) are at a high risk and therefore
should be evaluated further, treated intensively, or referred to a
hospital. The patients in other categories (i.e., ALI ≥19) should
be evaluated with additional chest radiograph at primary care
facilities. In our model, negativity of chest radiograph could
waive CT scans in a large amount of patients with low risk of
exacerbation (category A). However, patients with a positive chest
radiograph should be subsequently assessed with CT scans to
distinguish those at a high risk (category C) who should be treated
intensively or referred to a hospital from those at a low risk
(category B) who may be followed-up at a primary care facility. We
adopted a CCTS threshold of 10 to distinguish between groups B and
C, which is slightly higher than the previously reported threshold
(CCTS of 7) used to identify the critical disease of COVID-19
pneumonia upon admission with a sensitivity of 0.80 and a
specificity of 0.83(23). This
means that our model's threshold has presumably higher specificity
and lower sensitivity for distinguishing group C from group B. This
is reasonable because we used the CCTS as the third classifier, and
most low risk patients were already classified into group A using
the ALI and radiograph. Our threshold was lower than another
threshold (CCTS of 18) used to predict mortality in a short-term
follow-up (39), indicating that
our model could include less severe cases in group C than those at
a high risk of short-term death. As such, our combined ALI and
radiologic risk prediction model could be helpful in the rapid
decision making for patient triage during the COVID-19 pandemic,
when medical resources are limited.
This study has several limitations. First, the study
design was retrospective in nature with a relatively small number
of patients experiencing exacerbation. The survival rate was not
determined because the patients with exacerbation were transferred
to the tertiary referral institution equipped with an intensive
care unit. Second, we did not conduct an external validation of our
model. Third, our patient cohort consisted of admitted patients and
did not include outpatients. Further analysis is necessary to apply
our model in an external patient cohort that includes both
inpatients and outpatients. Forth, we didn't include COVID-19
negative patients in our study cohort. The purpose of our study is
to evaluate the usefulness of applying our combined model to
patients with known diagnosis of COVID-19 confirmed by polymerase
chain reaction test, and we didn't intend to apply this model to
patients without COVID-19 infection. Therefore, no control group
enrollment in this study does not affect our result. Finally, the
patients were enrolled in this study before the vaccine was
released. The COVID-19 vaccine obviously prevents severe disease
and exacerbation (40); therefore,
it is unknown if the results can be similarly applied to the
vaccinated population.
In conclusion, the ALI could be applicable in
evaluating the risk of COVID-19 infection. Patients with COVID-19
infection who have a lower ALI score tend to have a higher
probability of CT or chest radiograph abnormalities. The ALI alone
and CCTS alone modestly predict the exacerbation of COVID-19, and
the combined ALI and radiologic risk prediction model exhibit
decent sensitivity and specificity. However, prediction using the
ALI may not be accurate in patients with obesity or
neutropenia.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AI, HT and YH conceptualized the study. HT designed
the methodology. AI and HT performed the formal analysis. TI, HI
and YK performed the experiments. YI acquired and collected data.
YI and BB summarized data. AI, HT and BB interpreted data. AI wrote
the original draft. HT and YH wrote the review and edited the
manuscript. YH supervised the study. All authors have read and
approved the final manuscript. HT and YH confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
This retrospective study was approved by National
Hospital Organization Nishisaitama-Chuo National Hospital (approval
no. 2020-18; Tokorozawa, Japan), and written informed consent was
waived for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Akitoshi Inoue (ORCID: 0000-0002-8610-2571); Dr
Hiroaki Takahashi (ORCID: 0000-0002-8731-0081); Dr Yoichiro
Hamamoto (ORCID: 0000-0002-2165-9475).
References
|
1
|
Chauhan S: Comprehensive review of
coronavirus disease 2019 (COVID-19). Biomed J. 43:334–340.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arima Y, Kanou K, Arashiro T, K Ko Y,
Otani K, Tsuchihashi Y, Takahashi T, Miyahara R, Sunagawa T and
Suzuki M: Epidemiology of coronavirus disease 2019 in Japan:
Descriptive findings and lessons learned through surveillance
during the first three waves. JMA J. 4:198–206. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nitulescu GM, Paunescu H, Moschos SA,
Petrakis D, Nitulescu G, Ion GND, Spandidos DA, Nikolouzakis TK,
Drakoulis N and Tsatsakis A: Comprehensive analysis of drugs to
treat SARS-CoV-2 infection: Mechanistic insights into current
COVID-19 therapies (Review). Int J Mol Med. 46:467–488.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dehelean CA, Lazureanu V, Coricovac D,
Mioc M, Oancea R, Marcovici I, Pinzaru I, Soica C, Tsatsakis AM and
Cretu O: SARS-CoV-2: Repurposed drugs and novel therapeutic
approaches-insights into chemical structure-biological activity and
toxicological screening J Clin. Med. 9(2084)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou W, Wang A, Wang X, Cheke RA, Xiao Y
and Tang S: Impact of hospital bed shortages on the containment of
COVID-19 in Wuhan. Int J Environ Res Public Health.
17(8560)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Baldi E, Sechi GM, Mare C, Canevari F,
Brancaglione A, Primi R, Klersy C, Palo A, Contri E, Ronchi V, et
al: COVID-19 kills at home: The close relationship between the
epidemic and the increase of out-of-hospital cardiac arrests. Eur
Heart J. 41:3045–3054. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Attaway AH, Scheraga RG, Bhimraj A, Biehl
M and Hatipoğlu U: Severe covid-19 pneumonia: Pathogenesis and
clinical management. BMJ. 372(n436)2021.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Al-Smadi AS, Bhatnagar A, Ali R, Lewis N
and Johnson S: Correlation of chest radiography findings with the
severity and progression of COVID-19 pneumonia. Clin Imaging.
71:17–23. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Berrill M, Karaj J, Zamfir G, Colman J,
Mason R, Akbar S, Sharma S, Sheehan F, Dhamija K, Saltissi F, et
al: Chest radiographs may assist in predicting the outcome in the
early phase of Covid-19. UK district general hospital experience of
Covid-19 first wave. Expert Rev Respir Med. 15:537–541.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bellos I, Tavernaraki K, Stefanidis K,
Michalopoulou O, Lourida G, Korompoki E, Thanou I, Thanos L,
Pefanis A and Argyraki A: Chest CT severity score and radiological
patterns as predictors of disease severity, ICU admission, and
viral positivity in COVID-19 patients. Respir Investig. 59:436–445.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jafri SH, Shi R and Mills G: Advance lung
cancer inflammation index (ALI) at diagnosis is a prognostic marker
in patients with metastatic non-small cell lung cancer (NSCLC): A
retrospective review. BMC Cancer. 13(158)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hua X, Chen J, Wu Y, Sha J, Han S and Zhu
X: Prognostic role of the advanced lung cancer inflammation index
in cancer patients: A meta-analysis. World J Surg Oncol.
17(177)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pian G, Hong SY and Oh SY: Prognostic
value of advanced lung cancer inflammation index in patients with
colorectal cancer liver metastases undergoing surgery. Tumori.
108:56–62. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie H, Huang S, Yuan G, Kuang J, Yan L,
Wei L, Tang S and Gan J: The advanced lung cancer inflammation
index predicts short and long-term outcomes in patients with
colorectal cancer following surgical resection: A retrospective
study. PeerJ. 8(e10100)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Barth DA, Brenner C, Riedl JM, Prinz F,
Klocker EV, Schlick K, Kornprat P, Lackner K, Stöger H, Stotz M, et
al: External validation of the prognostic relevance of the advanced
lung cancer inflammation index (ALI) in pancreatic cancer patients.
Cancer Med. 9:5473–5479. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kusunoki K, Toiyama Y, Okugawa Y, Yamamoto
A, Omura Y, Kusunoki Y, Yin C, Kondo S, Okita Y, Ohi M, et al: The
advanced lung cancer inflammation index predicts outcomes in
patients with Crohn's disease after surgical resection. Colorectal
Dis. 23:84–93. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hamamoto Y, Ibe T, Kodama H, Mouri A and
Mineshita M: Retrospective prognostic study of death at home or
hospice versus at a hospital among patients with advanced non-small
cell lung cancer. Am J Hosp Palliat Care. 37:129–135.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yoo EH, Chang SH, Song DY, Lee CH, Cheong
GY, Park S, Lee JH, Lee S, Kwak SG, Jeon CH and Song KE:
Comprehensive laboratory data analysis to predict the clinical
severity of coronavirus disease 2019 in 1,952 patients in Daegu,
Korea. Ann Lab Med. 4:24–35. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang Y, Lu Y, Huang YM, Wang M, Ling W,
Sui Y and Zhao HL: Obesity in patients with COVID-19: A systematic
review and meta-analysis. Metabolism. 113(154378)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sattar N, Ho FK, Gill JM, Ghouri N, Gray
SR, Celis-Morales CA, Katikireddi SV, Berry C, Pell JP, McMurray JJ
and Welsh P: BMI and future risk for COVID-19 infection and death
across sex, age and ethnicity: Preliminary findings from UK
Biobank. Diabetes Metab Syndr. 14:1149–1151. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liang W, Liang H, Ou L, Chen B, Chen A, Li
C, Li Y, Guan W, Sang L, Lu J, et al: Development and validation of
a clinical risk score to predict the occurrence of critical illness
in hospitalized patients with COVID-19. JAMA Intern Med.
180:1081–1089. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li K, Wu J, Wu F, Guo D, Chen L, Fang Z
and Li C: The clinical and chest CT features associated with severe
and critical COVID-19 pneumonia. Invest Radiol. 55:327–331.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y,
Liu X, Huang M, Liao Y and Li S: CT image visual quantitative
evaluation and clinical classification of coronavirus disease
(COVID-19). Eur Radiol. 30:4407–4416. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang R, Li X, Liu H, Zhen Y, Zhang X,
Xiong Q, Luo Y, Gao C and Zeng W: Chest CT severity score: An
imaging tool for assessing severe COVID-19. Radiol Cardiothorac
Imaging. 2(e200047)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Inoue A, Takahashi H, Ibe T, Ishii H,
Kurata Y, Ishizuka Y and Hamamoto Y: Comparison of semiquantitative
chest CT scoring systems to estimate severity in coronavirus
disease 2019 (COVID-19) pneumonia. Eur Radiol: Jan 12, 2022 (Epub
ahead of print).
|
|
27
|
He X, Zhou T, Yang Y, Hong S, Zhan J, Hu
Z, Fang W, Qin T, Ma Y, Zhao Y, et al: Advanced lung cancer
inflammation index, a new prognostic score, predicts outcome in
patients with small-cell lung cancer. Clin Lung Cancer.
16:e165–e171. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim EY, Kim N, Kim YS, Seo JY, Park I, Ahn
HK, Jeong YM and Kim JH: Prognostic significance of modified
advanced lung cancer inflammation index (ALI) in patients with
small cell lung cancer_ Comparison with Original ALI. PLoS One.
11(e0164056)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kobayashi S, Karube Y, Inoue T, Araki O,
Maeda S, Matsumura Y and Chida M: Advanced lung cancer inflammation
index predicts outcomes of patients with pathological stage IA lung
adenocarcinoma following surgical resection. Ann Thorac Cardiovasc
Surg. 25:87–94. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tomita M, Ayabe T, Maeda R and Nakamura K:
Comparison of inflammation-based prognostic scores in patients
undergoing curative resection for non-small cell lung cancer. World
J Oncol. 9:85–90. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Park YH, Yi HG, Lee MH, Kim CS and Lim JH:
Prognostic value of the pretreatment advanced lung cancer
inflammation index (ALI) in diffuse large B cell lymphoma patients
treated with R-CHOP chemotherapy. Acta Haematol. 137:76–85.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shibutani M, Maeda K, Nagahara H, Fukuoka
T, Matsutani S, Kimura K, Amano R, Hirakawa K and Ohira M: The
prognostic significance of the advanced lung cancer inflammation
index in patients with unresectable metastatic colorectal cancer: A
retrospective study. BMC Cancer. 19(241)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jank BJ, Kadletz L, Schnöll J, Selzer E,
Perisanidis C and Heiduschka G: Prognostic value of advanced lung
cancer inflammation index in head and neck squamous cell carcinoma.
Eur Arch Otorhinolaryngol. 276:1487–1492. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Petrilli CM, Jones SA, Yang J, Rajagopalan
H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F and
Horwitz LI: Factors associated with hospital admission and critical
illness among 5279 people with coronavirus disease 2019 in New York
City: Prospective cohort study. BMJ. 369(m1966)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Klang E, Kassim G, Soffer S, Freeman R,
Levin MA and Reich DL: Severe obesity as an Independent risk factor
for COVID-19 mortality in hospitalized patients younger than 50.
Obesity (Silver Spring). 28:1595–1599. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Homayounieh F, Holmberg O, Umairi RA, Aly
S, Basevičius A, Costa PR, Darweesh A, Gershan V, Ilves P,
Kostova-Lefterova D, et al: Variations in CT utilization,
protocols, and radiation doses in COVID-19 pneumonia: Results from
28 countries in the IAEA Study. Radiology. 298:E141–E151.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dennie C, Hague C, Lim RS, Manos D,
Memauri BF, Nguyen ET and Taylor J: Canadian Society of Thoracic
Radiology/Canadian Association of Radiologists consensus statement
Regarding chest imaging in suspected and confirmed COVID-19. Can
Assoc Radiol J. 71:470–481. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chia AQX, Cheng LT, Wijaya L, Png MA, Sim
WY, Hong WL and Chen RC: Chest radiographs and CTs in the era of
COVID-19: Indications, operational safety considerations and
alternative imaging practices. Acad Radiol. 27:1193–1203.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Francone M, Iafrate F, Masci GM, Coco S,
Cilia F, Manganaro L, Panebianco V, Andreoli C, Colaiacomo MC,
Zingaropoli MA, et al: Chest CT score in COVID-19 patients:
Correlation with disease severity and short-term prognosis. Eur
Radiol. 30:6808–6817. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rosenberg ES, Dorabawila V, Easton D,
Bauer UE, Kumar J, Hoen R, Hoefer D, Wu M, Lutterloh E, Conroy MB,
et al: Covid-19 vaccine effectiveness in New York State. N Engl J
Med. 386:116–127. 2022.PubMed/NCBI View Article : Google Scholar
|