Introduction
Colorectal cancer (CRC) is a serious disease that is
caused by malignant changes of the colorectal mucosa as a result of
various environmental and genetic factors (1). The incidence of CRC is increasing
annually and is occurring in younger patients (2). CRC is more commonly observed in
developed countries (3), possibly
due to the population aging, poor dietary habits, and higher rates
of smoking, physical inactivity and obesity in Western countries
(3). The current treatments for
CRC mainly include screening, surgical treatment and chemotherapy
(4). However, despite advances in
surgery and screening, little change in patient survival has been
observed in recent years. As regards chemotherapy, oxaliplatin is a
third-generation platinum drug that is used as first-line
chemotherapy for CRC (5).
Oxaliplatin acts on DNA by producing hydrated derivatives that form
intra- and inter-strand cross-links, thereby inhibiting DNA
synthesis and exerting cytotoxic antitumor effects (6). Unfortunately, only <40% of
patients with advanced CRC benefit from oxaliplatin due to tumor
resistance and the toxic effects arising from long-term use
(7). Oxaliplatin is therefore
unable to further control tumor progression. Improving the impact
of oxaliplatin on the survival of patients with advanced disease
remains a major challenge in CRC treatment.
Ferroptosis is an iron-dependent and reactive oxygen
species (ROS)-dependent type of cell death (8). Ferroptosis is genetically,
biochemically and morphologically different from cell necrosis,
autophagy and apoptosis (9).
Recently, ferroptosis has emerged as a popular research area for
reversing tumor drug resistance (10), as it can inhibit phospholipid
glutathione peroxidase 4 (GPX4) and the accumulation of lipid ROS
in cells (11) to trigger
drug-resistant cancer cell death. Interestingly, cancer cells that
exhibit drug resistance are more likely to undergo death via
ferroptosis inducers compared with cells exhibiting no drug
resistance (12). Moreover,
ferroptosis has been revealed to induce oxidative stress and death
of CRC cells (13), suggesting
that ferroptosis induction may represent a valuable method of CRC
treatment. However, there are relatively few reports on the
interactions between oxaliplatin and ferroptosis in CRC.
It has been demonstrated that oxaliplatin inhibits
the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling
pathway (14), and that inhibition
of Nrf2 enhances the sensitivity of CRC cells to oxaliplatin
(15). This suggests that the Nrf2
signaling pathway serves a key role in the anticancer mechanism of
oxaliplatin. P62-Keap1-Nrf2 protect HCC cells against ferroptosis
via upregulation of multiple genes involved in iron and ROS
metabolism (16). Additionally,
Nrf2 downregulation enhances the sensitivity of cancer cells to
ferroptosis-promoting agents (17). Therefore, Nrf2 inhibition is an
important regulatory pathway that leads to ferroptosis (16). Consequently, the present study was
undertaken to investigate whether the anticancer effects of
oxaliplatin in CRC may be enhanced via the inhibition of Nrf2
signaling, resulting in oxidative stress and ferroptosis.
Materials and methods
Cell culture and treatment
The base medium used for the culture of the human
CRC cell line, HT29 (American Type Culture Collection; ATCC), was
ATCC-formulated McCoy's 5a (Modified) Medium containing a final
concentration of 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
Cells were cultured at 37°C in an incubator containing
5% CO2.
HT29 cells were cultured in serum-free medium
overnight prior to treatment, after which time insulin was added to
the medium for 15 min. Oxaliplatin at various concentrations (0.5,
1, 2 and 3 µM) was subsequently added to the medium for 72 h. A
total of 200 µM Nrf2 activator, NK-252 (MedChemExpress) was then
added to the medium to activate the Nrf2 pathway. A total of 10 µM
erastin (Shanghai Rechem Science Co., Ltd.) was prepared in DMSO
for the induction of ferroptosis.
Cell viability assay
HT29 cells (2x104) in untreated control,
Erastin, Oxaliplatin and Erastin + Oxaliplatin (3 µM) and
maintained in 96-well plates at 37°C for 24 h. After
incubation, 10 µl Cell Counting Kit-8 (CCK-8) reagent (Shanghai
Yeasen Biotechnology Co., Ltd.) was added to each well for 2 h.
Subsequently, absorbance at 450 nm was measured using a microplate
reader.
TUNEL assay
HT29 cell apoptosis was detected using One Step
TUNEL Apoptosis Assay kit (Beyotime Institute of Biotechnology) in
accordance with the manufacturer's protocol. Cells were fixed with
4% paraformaldehyde for 15 min at room temperature, after which
time samples were washed with PBS once for 5 min. Adherent cell
monolayers were permeabilized using 0.1% Triton X-100 and incubated
with the TUNEL kit for 1 h at 37°C. Then, 0.5 µg/ml DAPI
was used to stain the nuclei of HT29 cells for 5 min at room
temperature. After washing in triplicate with PBS, the antifade
mounting medium was added into the cells and then a fluorescence
microscope (Leica Microsystems GmbH) was employed to observe
TUNEL-positive cells in five randomly selected views.
Western blotting
Protein was extracted from HT29 cells using ice-cold
RIPA buffer (Elabscience Biotechnology, Inc.) and determined by
using a BCA protein assay kit (Phygene). Protein samples were
subjected to separation via 10% SDS-PAGE (DetaiBio Tech) and
transferred onto PVDF membranes (Roche Diagnostics), after which
time the membranes were blocked with 5% non-fat milk for 2 h at
room temperature. The membranes were incubated at 4˚C overnight
with the following primary antibodies (all Abcam): Nrf2 (1:1,000,
cat. no. ab137550), heme oxygenase-1 (HO-1; 1:2,000, cat. no.
ab52947), NADPH dehydrogenase quinone 1 (NQO1; 1:10,000, cat. no.
ab80588), GPX4 (1:1,000, cat. no. ab125066), ferritin heavy chain 1
(FTH1; 1:1,000, cat. no. ab183781), transferrin (1:1,000, cat. no.
ab277635) and GAPDH (1:1,000, cat. no. ab8245). Following primary
antibody incubation, the membranes were incubated with
HRP-conjugated secondary antibodies (goat anti-mouse IgG H&L,
1:2,000, cat. no. ab6789; or goat anti-rabbit IgG H&L, 1:2,000,
cat. no. ab6721) for 2 h at room temperature. An ECL Western
Blotting Substrate kit (AmyJet Scientific, Inc.) was applied to
visualize protein bands. The resultant images were analyzed using
ImageJ software (version 1.8.0; National Institutes of Health) and
the quantification of each group was performed in triplicate.
Detection of oxidative stress
The levels of malondialdehyde (MDA) and glutathione
(GSH) were detected by using ELISA kits for MDA (cat. no. ab118970;
Abcam) and GSH (cat. no. ab239727; Abcam). HT29 cells were
collected and lysed with 300 µl lysis solution per well. Samples
were centrifuged at 13,000 x g for 10 min at 4˚C and the
supernatant was subsequently collected. A total of 600 µl
thiobarbituric acid was added to 200 µl supernatant and the mixture
was incubated at 95˚C for 60 min, and cooled in an ice bath for 10
min. Subsequently, the absorbance at 532 and 450 nm was detected
immediately on a microplate reader (Thermo Fisher Scientific,
Inc.). The levels of ROS in HT29 cells were measured with a
commercially available kit (cat. no. ab139476, Abcam) according to
the manufacturers' instructions. Briefly, the control cells were
pretreated with a ROS inhibitor (N-acetyl-l-cysteine) for 30 min at
room temperature. HT29 cells treated with oxaliplatin (3 µM) with
or without NK-252 (200 µM) or erastin (10 µm) added to 100 µl/well
of ROS/superoxide detection solution and incubated for 2 h at 37˚C
in the dark. The fluorescence was detected at an excitation
wavelength of 490 nm and at an emission wavelength of 520 nm by
using a SpectraMax i3x microplate reader (Molecular Devices,
LLC).
Iron measurement
An iron Assay kit (BioAssay Systems) was utilized to
evaluate the concentration of Fe2+ in HT29 cells
following treatment with oxaliplatin and the ferroptosis inducer,
erastin. The experiment was performed in accordance with the
recommendations of the manufacturer, and the absorbance of cells at
593 nm was measured by using a spectrophotometer (Shanghai Mapada
Instruments Co., Ltd.).
Statistical analysis
Experimental data are expressed as the mean ± SD and
were analyzed using SPSS 19.0 software (IBM Corp.). All experiments
were repeated independently at least 3 times. One-way ANOVA with a
post hoc Bonferroni multiple comparisons test was used to compare
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Oxaliplatin inhibits HT29 cell
viability
The viability of HT29 cells was assessed following
treatment with different concentrations of oxaliplatin using a
CCK-8 assay. The results revealed that, when compared with the
control group, HT29 cell viability was decreased following
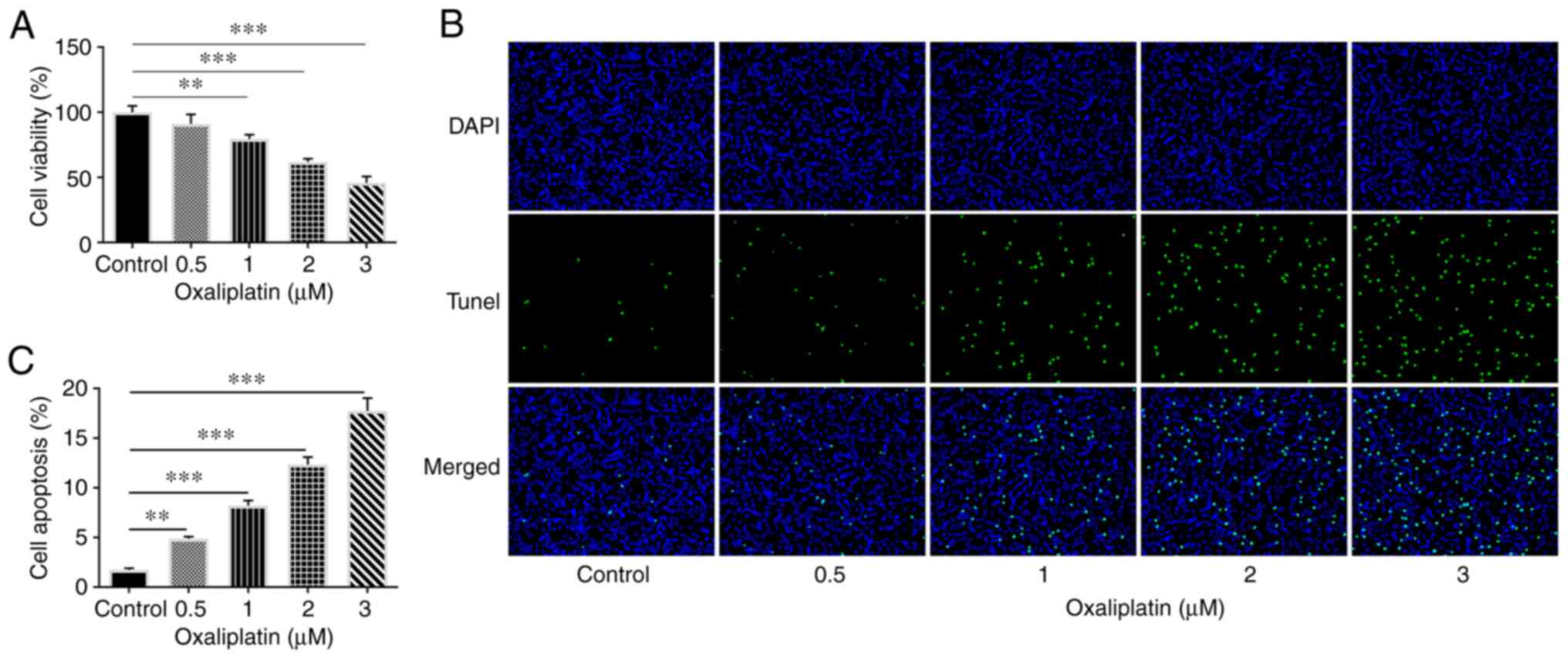
oxaliplatin treatment in a concentration-dependent manner (Fig. 1A). Subsequently, a TUNEL assay was
performed to measure apoptosis. As presented in Fig. 1B and C, oxaliplatin increased the apoptosis
rate of HT29 cells in a dose-dependent manner. The results
suggested that oxaliplatin exerted an inhibitory and
concentration-dependent effect on HT29 cell viability.
Additionally, since the effects of 0.5 µM oxaliplatin were less
prominent, concentrations of 1, 2 and 3 µM oxaliplatin were
selected for subsequent experimentation.
Oxaliplatin suppresses the Nrf2
signaling pathway in HT29 cells
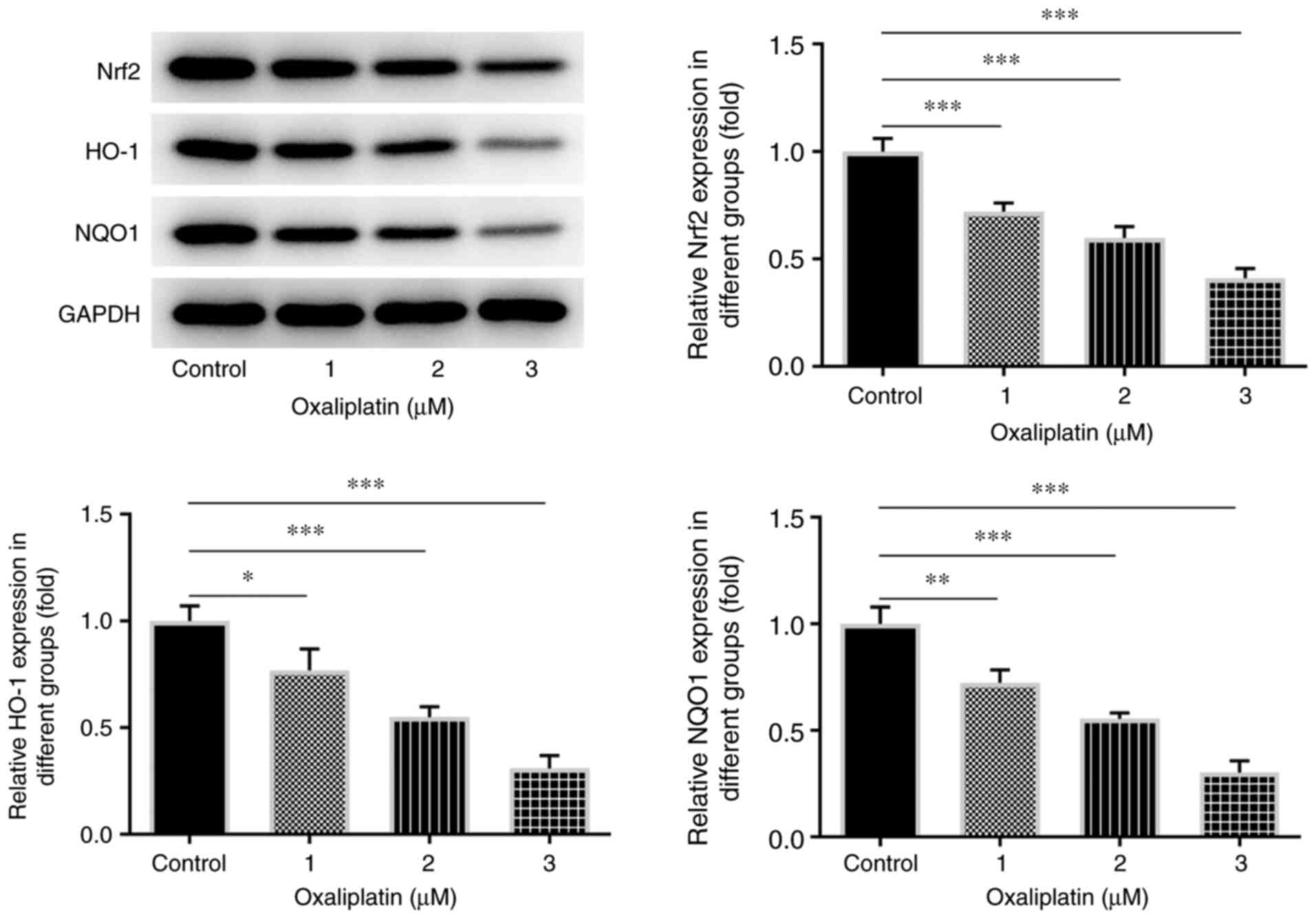
The results of western blotting revealed that the
expression levels of certain Nrf2 pathway-associated proteins,
including Nrf2, HO-1 and NQO1, were decreased compared with the
control group (Fig. 2).
Additionally, the greatest decrease in Nrf2, HO-1 and NQO1
expression levels was observed in cells treated with 3 µM
oxaliplatin. Accordingly, 3 µM oxaliplatin was selected for use in
follow-up experiments. These results indicated that oxaliplatin
could notably inhibit the Nrf2 signaling pathway in CRC.
Oxaliplatin promotes HT29 cell
ferroptosis and oxidative stress through the Nrf2 signaling
pathway
To determine whether the effects of oxaliplatin on
HT29 cell ferroptosis and oxidative stress were mediated through
the Nrf2 signaling pathway, relative total iron and Fe2+
levels were measured using an iron testing kit. As shown in
Fig. 3A and B, relative total iron and Fe2+
levels were increased in 3 µM oxaliplatin-treated HT29 cells
compared with the control group. However, these effects were
reduced following treatment with the Nrf2 activator, NK-252. The
results of western blotting revealed that, when compared with the
control group, a marked decrease was observed in certain
ferroptosis-related proteins, including GPX4 and FTH1, and a marked
increase was observed in transferrin expression in HT29 cells
treated with oxaliplatin (Fig. 3C
and D). However, subsequent NK-252
treatment significantly increased GPX4 and FTH1 expression levels,
and decreased transferrin expression levels.
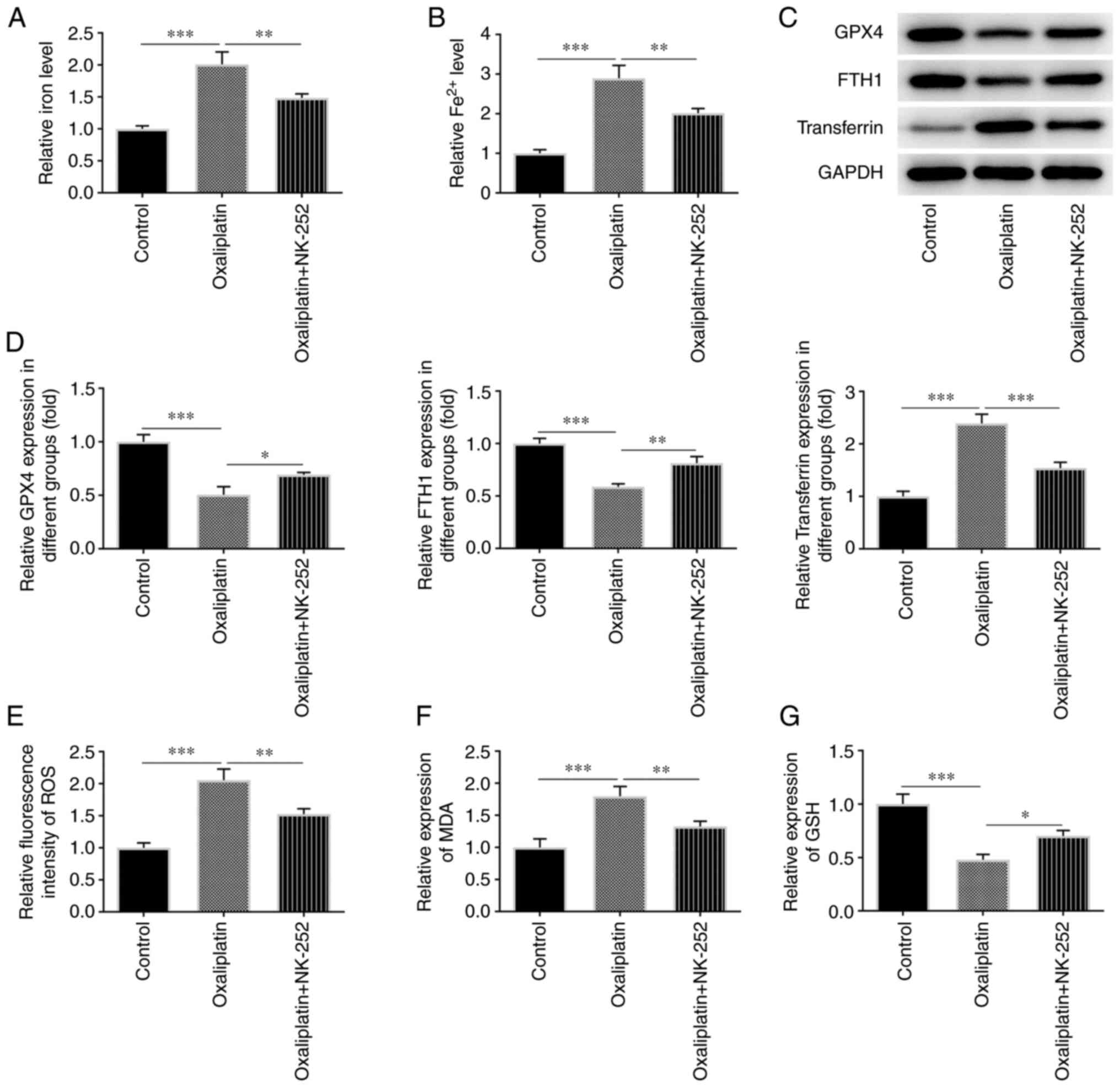 | Figure 3Oxaliplatin promotes ferroptosis and
oxidative stress in colorectal cancer cells through inhibiting the
Nrf2 signaling pathway. (A and B) Detection of relative total iron
level and Fe2+ level in HT29 cells was performed using
an iron ion test kit in the control group and the groups treated
with 3 µM of oxaliplatin as well as oxaliplatin (3 µM) + NK-252. (C
and D) Western blotting was used to detect the levels of the
ferroptosis-related proteins GPX4, FTH1 and transferrin in the
control group and the groups treated with 3 µM of oxaliplatin as
well as oxaliplatin (3 µM) + NK-252. (E-G) ELISA was used to detect
the levels of the oxidative stress-related factors ROS, MDA and GSH
in the control group and the groups treated with 3 µM of
oxaliplatin as well as oxaliplatin (3 µM) + NK-252. Data are
expressed as mean ± SD. *P<0.05,
**P<0.01, ***P<0.001. Nrf2, nuclear
factor erythroid 2-related factor 2; GPX4, glutathione peroxidase
4; FTH1, ferritin heavy chain 1; ROS, reactive oxygen species; MDA,
malondialdehyde; GSH, glutathione. |
ELISA kits were used to measure the levels of
various oxidative stress markers, including ROS (Fig. 3E), MDA (Fig. 3F) and GSH (Fig. 3G), in oxaliplatin-treated HT29
cells. When compared with the control group, ROS and MDA levels
decreased, while GSH levels increased in HT29 cells treated with
oxaliplatin and NK-252. These results suggested that oxaliplatin
could promote HT29 cell ferroptosis and oxidative stress via the
Nrf2 signaling pathway.
Oxaliplatin enhances the effects of
erastin on HT29 cell ferroptosis and oxidative stress
Thus far, the results of the current study have
demonstrated that oxaliplatin can promote HT29 cell ferroptosis and
oxidative stress by means of Fe2+ detection, ELISA and
western blotting. The same experiments were subsequently performed,
with the addition of the ferroptosis inducer, erastin. As indicated
in Fig. 4A and B, the relative total iron and
Fe2+ levels were increased in the erastin group compared
with the control group. Additionally, oxaliplatin treatment largely
increased the levels of relative total iron and Fe2+ in
the erastin + oxaliplatin group when compared with the erastin
group. Compared with the control group, erastin treatment markedly
reduced GPX4 and FTH1, and increased transferrin protein expression
levels (Fig. 4C and D). However, subsequent erastin +
oxaliplatin treatment resulted in lower levels of GPX4 and FTH1,
and higher levels of transferrin. Furthermore, erastin treatment
led to increased levels of ROS and MDA (Fig. 4E and F), as well as a decreased levels of GSH
(Fig. 4G) when compared with the
control group. When erastin treatment was subsequently combined
with oxaliplatin, the opposite effects to those described above
were observed. These results indicated that oxaliplatin enhanced
the promotive effects of erastin on HT29 cell ferroptosis and
oxidative stress.
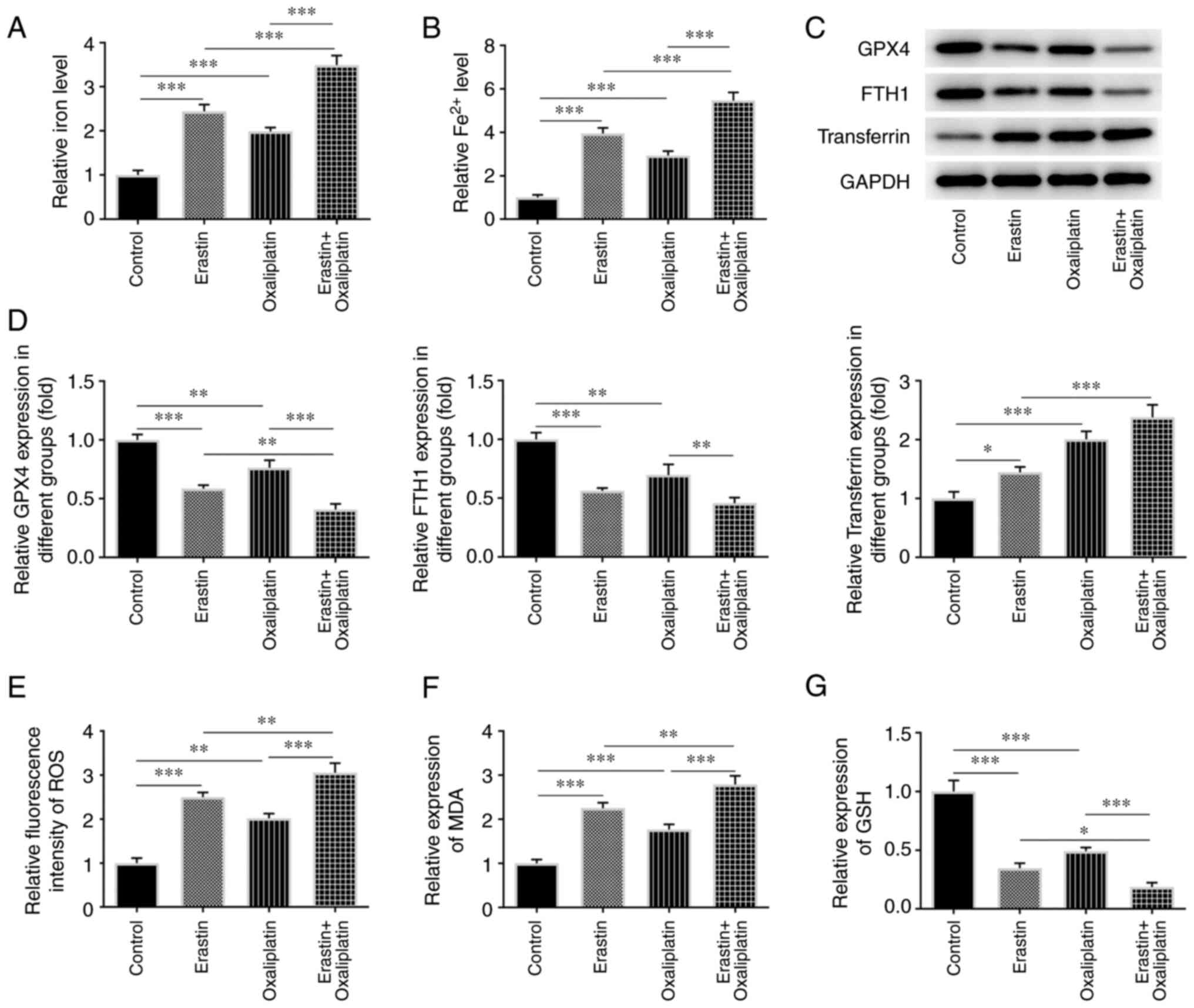 | Figure 4Oxaliplatin enhances the promotive
role of erastin in ferroptosis and oxidative stress of colorectal
cancer cells. (A and B) Detection of relative total iron level and
Fe2+ level in HT29 cells was carried out using an iron
ion test kit in the groups of control, erastin, oxaliplatin as well
as oxaliplatin (3 µM) + erastin. (C and D) Western blotting was
used to detect the levels of the ferroptosis-related proteins GPX4,
FTH1 and transferrin in the control, erastin and oxaliplatin
groups, as well as in the oxaliplatin (3 µM) + erastin group. (E-G)
ELISA was used to detect the levels of the oxidative stress-related
factors ROS, MDA and GSH in the control, erastin and oxaliplatin
groups, as well as in the oxaliplatin (3 µM) + erastin group. Data
are expressed as mean ± SD. *P<0.05,
**P<0.01, ***P<0.001. GPX4, glutathione
peroxidase 4; FTH1, ferritin heavy chain 1; ROS, reactive oxygen
species; MDA, malondialdehyde; GSH, glutathione. |
Oxaliplatin enhances the suppressive
effects of erastin on HT29 cell viability
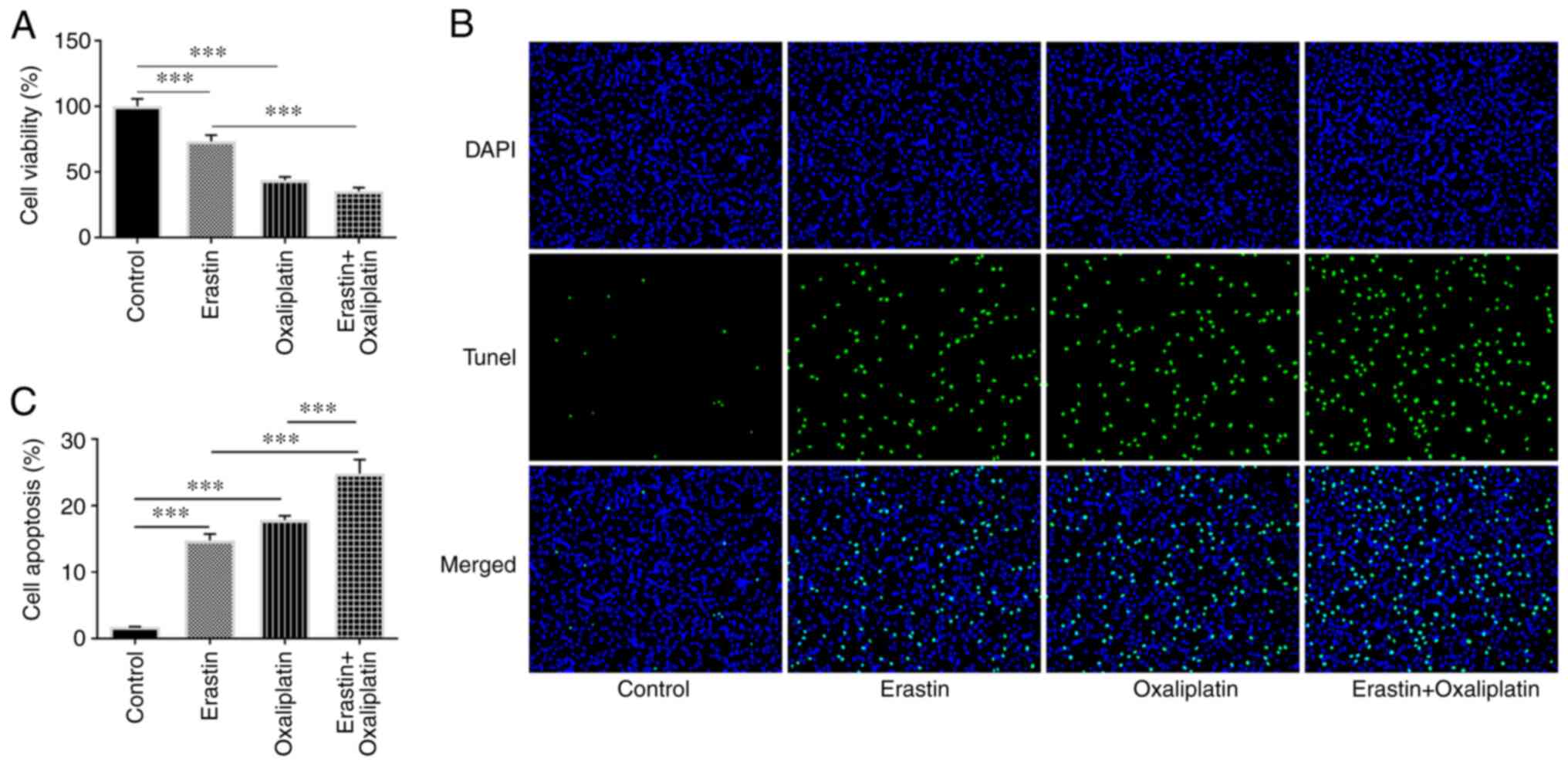
According to the aforementioned results, oxaliplatin
markedly suppressed HT29 cell viability. Erastin treatment was
subsequently applied to further assess the function of oxaliplatin.
As presented in Fig. 5A, cell
viability was markedly decreased in the erastin group compared with
the control group. Furthermore, oxaliplatin treatment applied in
combination with erastin resulted in enhanced suppression of cell
viability. Erastin treatment also led to increased levels of HT29
cell apoptosis when compared with the control group. However, HT29
cell apoptosis was higher in the erastin + oxaliplatin group
compared with either the erastin alone and oxaliplatin alone groups
(Fig. 5B and C). These results demonstrated that
oxaliplatin enhanced the inhibitory effect of erastin on the
viability of HT29 cells.
Discussion
CRC usually develops from benign tumors or serrated
polyps (18) and is a common type
of cancer with a worldwide prevalence. Although current treatments
have improved patient survival, a significant proportion of
patients with CRC still experience relapse. The HT29 cell line is a
human CRC cell line that is commonly used in physiological and
pathological studies of CRC (19-21).
In the current study, this cell line was used to study the effects
of oxaliplatin on ferroptosis and oxidative stress in CRC cells and
elucidate the underlying mechanism. It was observed that
oxaliplatin significantly inhibited the proliferation and promoted
the apoptosis of HT29 cells. Mechanistic investigations revealed
that oxaliplatin accelerated the process of ferroptosis and
oxidative stress, which may involve the blockade of the Nrf2
signaling pathway.
Oxaliplatin is a first-line chemotherapeutic CRC
agent that primarily exerts its effects by combining with and
damaging DNA, thereby inhibiting DNA replication (22). It was previously revealed that
oxaliplatin exerts an inhibitory effect on cell viability and
promotes CRC cell apoptosis (23).
In addition, Limagne et al (24) reported that the combination of
trifluridine/tipiracil and oxaliplatin improved PD-1 blockade in
CRC via the induction of immunogenic cell death and depletion of
macrophages. The CCK-8 and TUNEL assays in the present study
revealed that HT29 cell viability was decreased and apoptosis was
increased, suggesting that oxaliplatin inhibited the proliferation
of CRC cells.
Nrf2 functions as a key promoter of chemoresistance
by regulating antioxidants and detoxifying enzymes (25). It was previously determined that
oxaliplatin inhibits the Nrf2 signaling pathway (14). For example, the suppression of Nrf2
signaling in patients with CRC provided an essential strategy for
overcoming oxaliplatin resistance (25). The results of the present study
found that oxaliplatin significantly suppressed the protein
expressions of Nrf2, HO-1 and NQ in the Nrf2 signaling pathway in a
dose-dependent manner, which was in agreement with previous
results.
Due to its toxic side effects, oxaliplatin has poor
long-term efficacy (26).
Furthermore, CRC cell resistance to oxaliplatin also contributes to
the poor prognosis of patients with CRC. Therefore, reducing cell
resistance to oxaliplatin may be an effective method for improving
patient survival. It has been reported that induction of
ferroptosis may be applied to treat aggressive malignancies that
are resistant to traditional therapies (27). This is due to the fact that
ferroptosis can inhibit the activity of GSX4(28), which is highly expressed in CRC
cells. Furthermore, the upregulation of GSX4 can suppress the
therapeutic effects of drugs, leading to tumor cell resistance
(29). In the present study,
Fe2+ content was decreased and the expression levels of
ferroptosis-related proteins GPX4 and FTH1 were increased,
suggesting that oxaliplatin promoted ferroptosis in CRC cells.
Additionally, elevated levels of oxidative stress
act as markers of cancer (30),
and are closely associated with CRC development and progression due
to ROS and nitrogen species overproduction (31). Research has indicated that
oxidative stress can be induced by ferroptosis in CRC cells
(13,32). In addition, it has been revealed
that oxaliplatin enhanced oxidative stress in gastric cancer
(33). The present study,
therefore, aimed to assess whether oxaliplatin exerted its effects
through inducing oxidative stress in CRC cells. The data indicated
that the levels of oxidative stress-related factors, including ROS
and MDA, were decreased, while GSH levels were increased. Thus,
oxaliplatin promoted oxidative stress through the Nrf2 signaling
pathway in CRC cells.
Erastin acts as an inducer of ferroptosis. In
addition, the combination of erastin and sulfasalazine inhibits
system Xc- in cancer cells, which leads to an unusual
iron-dependent cell death known as ferroptosis (34). Therefore, CRC cells were treated
with erastin in the present study. The results revealed a marked
increase in Fe2+ content and a decrease in the
expression levels of ferroptosis-associated proteins in CRC cells.
It was suggested that oxaliplatin enhanced the ferroptosis-inducing
effect of erastin on CRC cells. Similarly, elevated levels of ROS
and MDA, and reduced levels of GSH, indicated that oxaliplatin
enhanced the role of erastin in inducing CRC cell oxidative stress.
In addition, the marked decrease of CRC cell viability detected via
CCK-8 and TUNEL assays also demonstrated that the inhibitory effect
of erastin was enhanced by oxaliplatin treatment. However, there
were certain limitations to the present study. The utilization of
multiple cell lines may better demonstrate the anticancer effects
of oxaliplatin. However, the main focus of the present study was
the effects and mechanism through which oxaliplatin inhibits CRC.
Thus, the representative CRC cell line HT29 was selected and the
effects of oxaliplatin in promoting ferroptosis and oxidative
stress in HT29 cells were demonstrated. Functional experiments will
also be performed in other CRC cell lines to verify the present
results, and the effects and potential mechanism of oxaliplatin on
other CRC cell lines will be more extensively investigated in
future research.
In conclusion, oxaliplatin effectively suppresses
CRC cell viability and promotes ferroptosis and oxidative stress
via the Nrf2 signaling pathway. Treatment with oxaliplatin can also
significantly enhance the ferroptosis and oxidative stress-inducing
effects of erastin in CRC cells. Oxaliplatin treatment was also
shown to enhance the inhibitory effects of erastin on the
proliferation of CRC cells. Therefore, the anticancer effects of
oxaliplatin may be increased by inhibiting the Nrf2 signaling
pathway, resulting in CRC cell ferroptosis and oxidative
stress.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and HW designed the study, performed the
experiments, drafted and revised the manuscript. BL analyzed the
data and searched the literature. BL and HW confirm the
authenticity of all the raw data. Both authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weitz J, Koch M, Debus J, Höhler T, Galle
PR and Büchler MW: Colorectal cancer. Lancet. 365:153–165.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pilonis ND, Bugajski M, Wieszczy P,
Franczyk R, Didkowska J, Wojciechowska U, Pisera M, Rupinski M,
Regula J and Kaminski MF: Long-term colorectal cancer incidence and
mortality after a single negative screening colonoscopy. Ann Intern
Med. 173:81–91. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Kuipers EJ, Grady WM, Lieberman D,
Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ and Watanabe T:
Colorectal cancer. Nat Rev Dis Primers. 1(15065)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Binefa G, Rodríguez-Moranta F, Teule A and
Medina-Hayas M: Colorectal cancer: From prevention to personalized
medicine. World J Gastroenterol. 20:6786–6808. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hsu HH, Chen MC, Baskaran R, Lin YM, Day
CH, Lin YJ, Tu CC, Vijaya Padma V, Kuo WW and Huang CY: Oxaliplatin
resistance in colorectal cancer cells is mediated via activation of
ABCG2 to alleviate ER stress induced apoptosis. J Cell Physiol.
233:5458–5467. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Arango D, Wilson AJ, Shi Q, Corner GA,
Arañes MJ, Nicholas C, Lesser M, Mariadason JM and Augenlicht LH:
Molecular mechanisms of action and prediction of response to
oxaliplatin in colorectal cancer cells. Br J Cancer. 91:1931–1946.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun X, Wang X, Feng W, Guo H, Tang C, Lu
Y, Xiang X and Bao Y: Gene signatures associated with drug
resistance to irinotecan and oxaliplatin predict a poor prognosis
in patients with colorectal cancer. Oncol Lett. 13:2089–2096.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang F, Li F, Lu GH, Nie W, Zhang L, Lv
Y, Bao W, Gao X, Wei W, Pu K and Xie HY: Engineering magnetosomes
for ferroptosis/immunomodulation synergism in cancer. ACS Nano.
13:5662–5673. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zou Y, Palte MJ, Deik AA, Li H, Eaton JK,
Wang W, Tseng YY, Deasy R, Kost-Alimova M, Dančík V, et al: A
GPX4-dependent cancer cell state underlies the clear-cell
morphology and confers sensitivity to ferroptosis. Nat Commun.
10(1617)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shin D, Kim EH, Lee J and Roh JL: Nrf2
inhibition reverses resistance to GPX4 inhibitor-induced
ferroptosis in head and neck cancer. Free Radic Biol Med.
129:454–462. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sang M, Luo R, Bai Y, Dou J, Zhang Z, Liu
F, Feng F, Xu J and Liu W: Mitochondrial membrane anchored
photosensitive nano-device for lipid hydroperoxides burst and
inducing ferroptosis to surmount therapy-resistant cancer.
Theranostics. 9:6209–6223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sui X, Zhang R, Liu S, Duan T, Zhai L,
Zhang M, Han X, Xiang Y, Huang X, Lin H and Xie T: RSL3 drives
ferroptosis through GPX4 inactivation and ROS production in
colorectal cancer. Front Pharmacol. 9(1371)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lu Y, Wu S, Xiang B, Li L and Lin Y:
Curcumin attenuates oxaliplatin-induced liver injury and oxidative
stress by activating the Nrf2 pathway. Drug Des Devel Ther.
14:73–85. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chian S, Li YY, Wang XJ and Tang XW:
Luteolin sensitizes two oxaliplatin-resistant colorectal cancer
cell lines to chemotherapeutic drugs via inhibition of the Nrf2
pathway. Asian Pac J Cancer Prev. 15:2911–2916. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23(101107)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Singh R, Zorrón Cheng Tao Pu L, Koay D and
Burt A: Sessile serrated adenoma/polyps: Where are we at in 2016?
World J Gastroenterol. 22:7754–7759. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xiao ZM, Wang AM, Wang XY and Shen SR:
Effects of ethanol extract of radix sophorae flavescentis on
activity of colon cancer HT29 cells. Afr J Tradit Complement Altern
Med. 10:352–355. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Przygodzka P, Papiewska-Pająk I,
Bogusz-Koziarska H, Sochacka E, Boncela J and Kowalska MA:
Regulation of miRNAs by Snail during epithelial-to-mesenchymal
transition in HT29 colon cancer cells. Sci Rep.
9(2165)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mittag A, Schneider T, Westermann M and
Glei M: Toxicological assessment of magnesium oxide nanoparticles
in HT29 intestinal cells. Arch Toxicol. 93:1491–1500.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Riddell IA: Cisplatin and oxaliplatin: Our
current understanding of their actions. Met Ions Life Sci.
18:2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang W, Wang M, Xu J, Long F and Zhan X:
Overexpressed GATA3 enhances the sensitivity of colorectal cancer
cells to oxaliplatin through regulating MiR-29b. Cancer Cell Int.
20(339)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Limagne E, Thibaudin M, Nuttin L, Spill A,
Derangère V, Fumet JD, Amellal N, Peranzoni E, Cattan V and
Ghiringhelli F: Trifluridine/tipiracil plus oxaliplatin improves
PD-1 blockade in colorectal cancer by inducing immunogenic cell
death and depleting macrophages. Cancer Immunol Res. 7:1958–1969.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pirpour Tazehkand A, Akbarzadeh M, Velaie
K, Sadeghi MR and Samadi N: The role of Her2-Nrf2 axis in induction
of oxaliplatin resistance in colon cancer cells. Biomed
Pharmacother. 103:755–766. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang C, Zhang Y, Lin S, Liu Y and Li W:
Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces
ferroptosis and enhances the sensitivity of colorectal cancer to
oxaliplatin. Aging (Albany NY). 13:13515–13534. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu T, Shi L, Yu C, Dong Y, Qiu F, Shen L,
Qian Q, Zhou G and Zhu X: Ferroptosis promotes photodynamic
therapy: Supramolecular photosensitizer-inducer nanodrug for
enhanced cancer treatment. Theranostics. 9:3293–3307.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Roh JL, Kim EH, Jang H and Shin D: Nrf2
inhibition reverses the resistance of cisplatin-resistant head and
neck cancer cells to artesunate-induced ferroptosis. Redox Biol.
11:254–262. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shi Y, Wang Y, Huang W, Wang Y, Wang R and
Yuan Y: Integration of metabolomics and transcriptomics to reveal
metabolic characteristics and key targets associated with cisplatin
resistance in nonsmall cell lung cancer. J Proteome Res.
18:3259–3267. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhu J, Xiong Y, Zhang Y, Wen J, Cai N,
Cheng K, Liang H and Zhang W: The molecular mechanisms of
regulating oxidative stress-induced ferroptosis and therapeutic
strategy in tumors. Oxid Med Cell Longev.
2020(8810785)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Basak D, Uddin MN and Hancock J: The role
of oxidative stress and its counteractive utility in colorectal
cancer (CRC). Cancers (Basel). 12(3336)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shen LD, Qi WH, Bai JJ, Zuo CY, Bai DL,
Gao WD, Zong XL, Hao TT, Ma Y and Cao GC: Resibufogenin inhibited
colorectal cancer cell growth and tumorigenesis through triggering
ferroptosis and ROS production mediated by GPX4 inactivation. Anat
Rec (Hoboken). 304:313–322. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Wang J, Sun Y, Zhang X, Cai H, Zhang C, Qu
H, Liu L, Zhang M, Fu J, Zhang J, et al: Oxidative stress activates
NORAD expression by H3K27ac and promotes oxaliplatin resistance in
gastric cancer by enhancing autophagy flux via targeting the
miR-433-3p. Cell Death Dis. 12(90)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3(e02523)2014.PubMed/NCBI View Article : Google Scholar
|