Introduction
Schizophrenia (SCZ) is a complex neurodevelopmental
condition influenced by a range of environmental and genetic
variables. According to global burden of disease studies, the
global age-standardized point prevalence of SCZ is estimated to be
~0.28% (95% CI, 0.24-0.31) (1).
Positive symptoms (such as delusions, hallucinations and behavioral
abnormalities) and negative symptoms (such as depression, anxiety
and aphasia), as well as cognitive impairments (such as attention
deficit, impaired learning and memory ability), are the clinical
manifestations associated with SCZ (2,3). To
date, the pathogenesis of SCZ remains poorly understood. Numerous
studies have demonstrated that the function of myelin and
oligodendrocytes (OLs) is critical in the pathophysiology of SCZ.
Therefore, a better understanding of OLs may aid in the development
of SCZ treatment strategies (4,5).
Postmortem studies demonstrated a strong association between
aberrant OL development and function, as well as demyelination and
the pathogenesis of SCZ (6,7).
Moreover, histological examinations of the brain revealed a
decrease in the number of hippocampal OLs and neurons in patients
with SCZ, and the interaction between OLs and neurons was abnormal
(8,9). Additionally, cellular ultrastructural
examinations indicated significant pathological changes in SCZ,
including OL edema, vacuolation, paucity of ribosomes and
mitochondria, and an accumulation of lipofuscin granules in
prefrontal white matter (10).
Given the apparent role of OLs and related molecules in the
pathogenesis of SCZ, it is critical to develop an effective
strategy for the early prevention and management of SCZ.
Adenosine is widely distributed and can exert its
neuromodulatory effects in the central nervous system (CNS) at low
concentrations (30-300 nM) through its receptors (11). The adenosine receptors (ARs) belong
to the G protein-coupled receptor family, which includes four kinds
of receptors, A1, A2A, A2B and
A3. A2BAR was initially cloned from a human
brain in 1992(12).
A2BAR was demonstrated to be broadly distributed in all
rat tissues (13), but mainly on
neurons and glia in the CNS (14).
Additionally, A2BAR is known as a low-affinity receptor
due to its modest-to-negligible affinity for adenosine and
prototypic agonists (15).
Nevertheless, it has been demonstrated that A2BAR
expression is selectively upregulated under pathological
conditions, such as tissue hypoxia and inflammation, as well as
when the adenosine concentration in the tissue increases rapidly
(16,17), implying that A2BAR may
play a critical role under pathological conditions. Previous
studies have demonstrated that the A2BAR plays a crucial
role in a variety of neurological diseases, including sensorineural
hearing, neurogenic bladder spontaneous activity following spinal
cord injury and midazolam-induced cognitive dysfunction (18-20).
Furthermore, it was previously demonstrated that A2BAR
inhibition could facilitate the remyelination process after
hypoxic-ischemic injury (21);
however, it is unknown whether A2BAR exerts a role in
myelin repair in SCZ. Therefore, it is critical to elucidate the
role of A2BAR in SCZ pathophysiology.
Animal models of SCZ are valuable for elucidating
the etiology of the disease and for establishing novel therapeutic
options. Based on the limited understanding of SCZ, there are
mainly three types of animal models available: Developmental
models, drug-induced models and genetic models (22). The N-methyl-D-aspartic acid (NMDA)
receptor is an ionotropic glutamate receptor (23) whose expression in cerebral cortex
is usually lower (24). It has
been shown that repeated injection of a high dose of the
noncompetitive NMDA receptor antagonist, dizocilpine maleate
(MK-801), could be used to induce SCZ in animal models (25). In the present study, behavioral,
morphological and biochemical experiments were performed to explore
the effect of A2BAR on learning and memory abilities, as
well as the myelin sheath.
Materials and methods
Animals and ethics statement
According to the experimental requirements, 40
specific pathogen-free ICR male mice (6-weeks-old; weight, 18-22 g)
were obtained from the Experimental Animal Center of Ningxia
Medical University (Yinchuan, China). Experimental procedures were
conducted following the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (NIH Publications no. 8023,
revised 1978) and were approved by the Ethics Committee of Ningxia
Medical University (approval no. 2018-006). All the animals were
housed at room temperature (22±1˚C), with 40-60% humidity, using a
12 h light-dark cycle and allowed free access to water and food
with constant air renewal.
Preparation and grouping of the
MK-801-induced SCZ mouse model
A total of 40 mice were randomly divided into four
groups (10 mice per group) as follows: Control group (Control),
MK-801 group (MK-801), MK-801 plus A2BAR agonist group
(MK-801 + BAY) and MK-801 plus A2B AR antagonist group
(MK-801 + PSB). The SCZ model was established according to a
previously described method (26).
Briefly, mice were injected intraperitoneally with MK-801 (0.6
mg/kg/day; cat. no. M107; MilliporeSigma) once a day for 14
consecutive days, and the intervention was performed between 3:00
and 4:00 p.m. every day. The control group was treated with the
same volume of normal saline. The optimal dose of A2BAR
selective agonist BAY 60-6583 (21,27,28)
and A2B AR selective antagonist PSB 603 (21,29)
was determined according to previous studies. Intraperitoneal
injection of BAY 60-6583 (80 µg/kg/day; cat. no. 910487-58-0;
Tocris Bioscience) and PSB 603 (25 µg/kg/day; cat. no.
1092351-10-40; Tocris Bioscience) was administered to the MK-801 +
BAY and MK-801+ PSB groups, respectively, every 2 days between day
8 and day 14 of the MK-801 treatment period. The mice in the
control and MK-801 groups were intraperitoneally injected with an
equal volume of normal saline.
Morris water maze (MWM)
experiment
The MWM was mainly used for testing the spatial
learning and memory of the rodents. Briefly, a circular swimming
pool (80 cm in diameter and 40 cm in depth) was used, and the
position of the round escape platform (6 cm in diameter) was fixed
at the center of one quadrant of the pool. The water, which was
kept at 22±2˚C, was dyed black with ink and mixed evenly to ensure
that the platform, which was 1 cm below the surface of the water,
was hidden from the mice. Each mouse underwent swimming training
for 4 days, four times daily with at least 15 min intervals
between, and the directional cruise and spatial probe tests were
performed as follows. Mice were gently placed into the water facing
the wall at one of the artificially designed four quadrants, and
four quadrant tests were completed daily, a timer of 1 min was then
started. If the mice found the platform they were kept there for 10
sec and the experiment would end. If the mice failed to find the
platform in 1 min, they were guided to find the platform and were
allowed to stay on the platform for 15 sec. In the directional
cruise test, the mice were allowed to enter the water once in each
of the four quadrants to find the platform and were allowed to stay
there for 10 sec. In the spatial probe test, the underwater
platform was removed, and the number of times the mice crossed the
location of the original platform over a period of 1 min was
recorded. The small animal behavior records analysis system (Smart
3.0 Premium; Panlab) was simultaneously used to record the trial.
Statistical analysis was performed on the obtained data.
Mouse brain tissue preparation
After animal behavior testing and analysis, the mice
were decapitated. The cerebral cortex was rapidly dissected and
half of the samples were instantly frozen and kept at -80˚C for
western blotting. The remaining tissues were fixed in 4%
paraformaldehyde for 24 h at 4˚C. Paraffin-embedded tissue sections
were used for immunohistochemistry and immunofluorescence
staining.
Western blot analysis
Freshly taken brain tissues were weighed and
cortical proteins were extracted using lysis buffer (Nanjing KeyGen
Biotech Co., Ltd.). Total protein was quantified using a
bicinchoninic acid protein assay (Nanjing KeyGen Biotech Co.,
Ltd.). Western blot analyses were performed as previously described
(30). The membranes were
incubated with primary antibodies against chondroitin sulphate
proteoglycan 4 (NG2; 1:1,000; cat. no. ab12905; Abcam), G
protein-coupled receptor 17 (GPR17; 1:1,000; cat. no. 10136; Cayman
Chemical Company), myelin basic protein (MBP; 1:1,000; cat. no.
ab7349; Abcam), β-tubulin (1:2,000; cat. no. ab009-100; Multi
Sciences Biotech) and β-actin (1:5,000; cat. no. TA-09; Origene
Technologies, Inc.) overnight at 4˚C. β-tubulin or β-actin was used
as an internal loading control. After overnight incubation at 4˚C,
HRP-labeled secondary antibodies, goat anti-rabbit IgG (1:5,000;
cat. no. A21020), goat anti-rat IgG (1:5,000; cat. no. A21040) and
goat anti-mouse IgG (1:5,000; cat. no. A21010) (all Abbkine
Scientific Co., Ltd.), were applied to the membranes for a 1-h
incubation period at room temperature. Subsequently, the membranes
were exposed to a luminescent solution (Omni-ECL Pico Light
Chemiluminescence kit; cat. no. SQ202L; Epizyme, Inc.), and the
protein bands were quantified using Image-J software (National
Institutes of Health; version 1.53). All assays were performed
independently and in triplicate.
Immunohistochemical staining
Paraffin brain sections 5 µm in thickness were
heated for 1 h at 60˚C and then dewaxed with xylene at room
temperature. The sections were subsequently rehydrated with a
descending alcohol series at room temperature (anhydrous ethanol I,
5 min; anhydrous ethanol II, 5 min; 95% alcohol, 5 min; 85%
alcohol, 5 min; and 75% alcohol, 5 min). The sections were hydrated
with PBS for 3 min. Next, the sections were placed in a microwave
for antigen retrieval. After incubation in 0.3% hydrogen peroxide
to deactivate endogenous peroxidase for 20 min at 37˚C, sections
were blocked with 1% goat serum (MilliporeSigma)and 0.3% Triton
X-100 (MilliporeSigma) for 30 min at 37˚C. Subsequently, the brain
sections were incubated with primary antibodies against MBP (1:200;
cat. no. ab7349; Abcam) and NG2 (1:200; cat. no. ab129051; Abcam)
overnight at 4˚C. The next day, sections were incubated with
HRP-conjugated goat anti-rabbit IgG (1:400; cat. no. A21020;
Abbkine Scientific Co., Ltd.) and goat anti-rat IgG (1:400; cat.
no. A21040; Abbkine Scientific Co., Ltd.) secondary antibodies for
1 h at room temperature. Following three washes using PBS, the
sections were counterstained with DAB for 5 min and hematoxylin for
2 min at room temperature for observation. The cerebral cortex was
identified and images were captured after observation by
brightfield microscopy. The observers were blinded to the
experimental groups. All assays were performed independently and in
triplicate.
Immunofluorescence staining
The brain slices were heated for 1 h at 60˚C and
then dewaxed with xylene at room temperature. The sections were
subsequently rehydrated with a descending alcohol series at room
temperature (anhydrous ethanol I, 5 min; anhydrous ethanol II, 5
min; 95% alcohol, 5 min; 85% alcohol, 5 min; and 75% alcohol, 5
min). The sections were hydrated with PBS for 3 min. Following
antigen retrieval using a microwave for 10 min, sections were
blocked with 1% goat serum in 0.3% Triton X-100 for 30 min at 37˚C.
Subsequently, the brain sections were incubated with primary
antibodies against the anti-adenomatous polyposis coli clone CC-1
(CC-1; 1:100; cat. no. OP80-100UG; Merck KGaA) and OL transcription
factor 2 (Olig2; 1:100; cat. no. ab109186; Abcam) overnight at 4˚C.
Following three washes using PBS, the sections were incubated with
a fluorescent secondary antibody mixture of goat anti-rabbit IgG
(Dylight 594; 1:300; cat. no. A23420; Abbkine Scientific Co., Ltd.)
and goat anti-mouse IgG (Dylight 488; 1:300; cat. no. A23210;
Abbkine Scientific Co., Ltd.) for 1 h at room temperature. Finally,
all brain sections were counterstained with DAPI for 5 min at room
temperature. Images of the cerebral cortex were captured using a
fluorescence microscope. An observer blind to the present study
counted the number of co-labeled positive cells by using ImageJ
software (National Institutes of Health, version 1.53). All assays
were performed independently and in triplicate.
Statistical methods
SPSS (version 22.0; IBM Corp.) and GraphPad software
(version 8.0; GraphPad Software, Inc.) were used to perform all
statistical analyses. The data are presented as the mean ± standard
error. The differences between groups were analyzed using one-way
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Protective effect of BAY 60-6583 on
animal learning and memory ability in an MK-801-induced SCZ mouse
model
MK-801 (0.6 mg/kg/day) was intraperitoneally
injected for 14 consecutive days to establish the SCZ model, BAY
60-6583 or PSB 603 was administered every 2 days between day 8 and
day 14 of the MK-801 treatment period (Fig. 1A). The results showed that the body
weight of the MK-801 group significantly decreased (P<0.05),
with BAY 60-6583 and PSB 603 treatment failing to rescue the slow
body weight gain (P>0.05) (Fig.
1B). Next, the MWM test was utilized, and as shown by the
trajectory chart (Fig. 1C), the
escape latency of the SCZ mice in the directional cruise test was
notably prolonged (P<0.05) (Fig.
1D). In comparison, BAY 60-6583 treatment significantly reduced
the escape latency of the mouse model (P<0.05) (Fig. 1D). Notably, in the spatial probe
test, the number of crossings of the MK-801 group was markedly
lower compared with that of the control group (P<0.05) (Fig. 1E), while subsequent BAY 60-6583
treatment notably increased the number of crossings compared with
the MK-801 group (P<0.05) (Fig.
1E). Furthermore, there were no significant differences in
terms of crossings and escape latency between the MK-801 + PSB and
the MK-801 groups (P>0.05) (Fig.
1D and E).
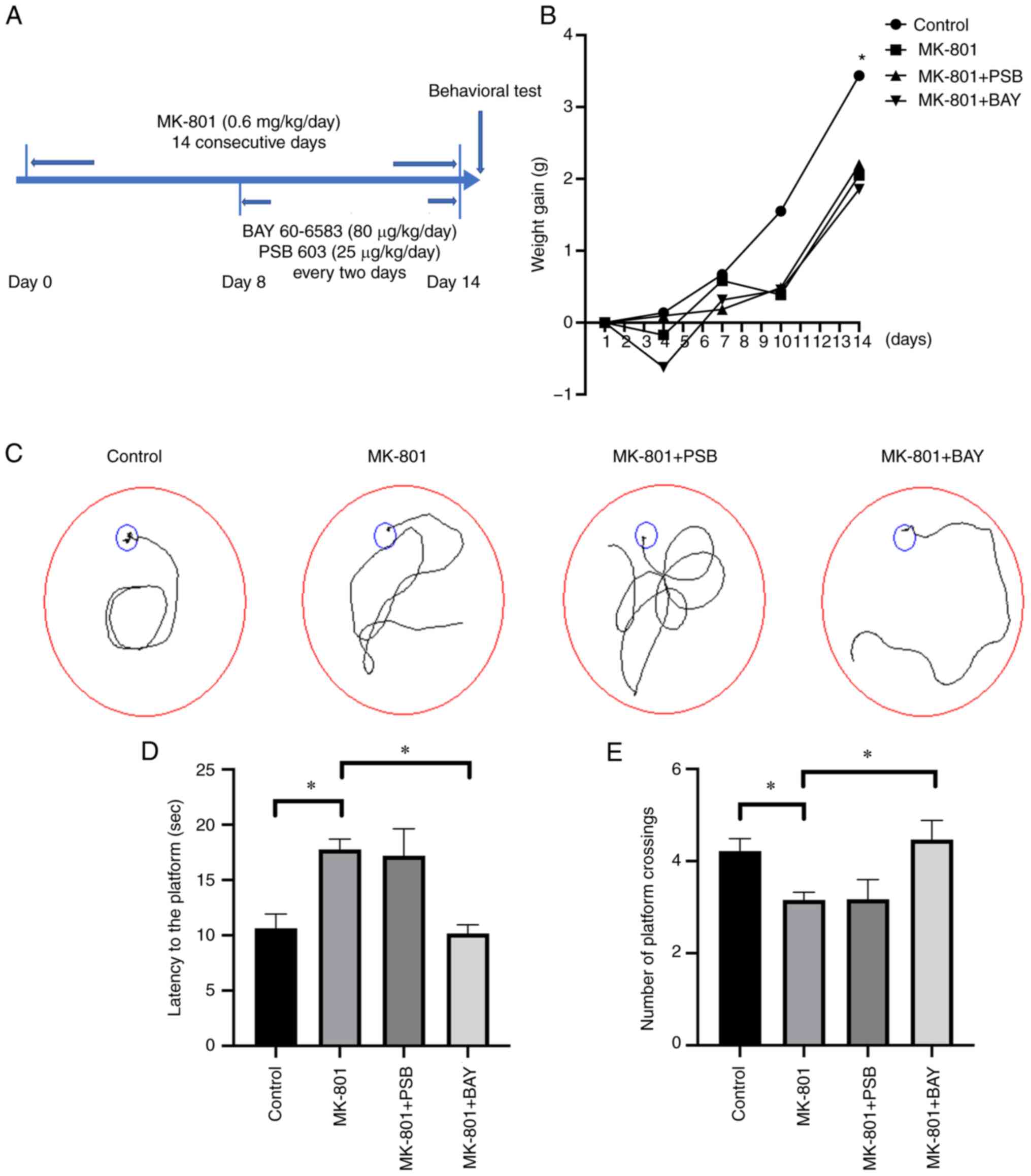 | Figure 1Impaired learning and memory ability
is restored following BAY 60-6583 treatment in SCZ mice. (A) MK-801
was administered via intraperitoneal injection for 14 consecutive
days to establish the SCZ mouse model. On the 8th day, BAY 60-6583
(80 µg/kg/day) or PSB 603 (25 µg/kg/day) was administered via
intraperitoneal injection every 2 days. (B) Changes in body weight
during MK-801 treatment. The MK-801 group, the MK-801 + PSB group,
the MK-801 + BAY group were compared with the control group as
indicated by the asterisk. (C) A representative trace diagram of
mice in the MWM test. (D) The mice in the MK-801 group had a
notably longer escape latency (time taken to locate the submerged
platform) than the control mice, and this was reversed by BAY
60-6583 administration. (E) The number of platform crossings in the
MWM test were reduced in the MK-801 group. BAY 60-6583 treatment
significantly increased the number of platform crossings. Data are
presented as the mean ± SEM. *P<0.05. SCZ,
schizophrenia; MK-801, dizocilpine maleate; A2BAR,
A2B adenosine receptor; BAY 60-6583, A2BAR
agonist; PSB 603, A2BAR antagonist; MWM, Morris water
maze. |
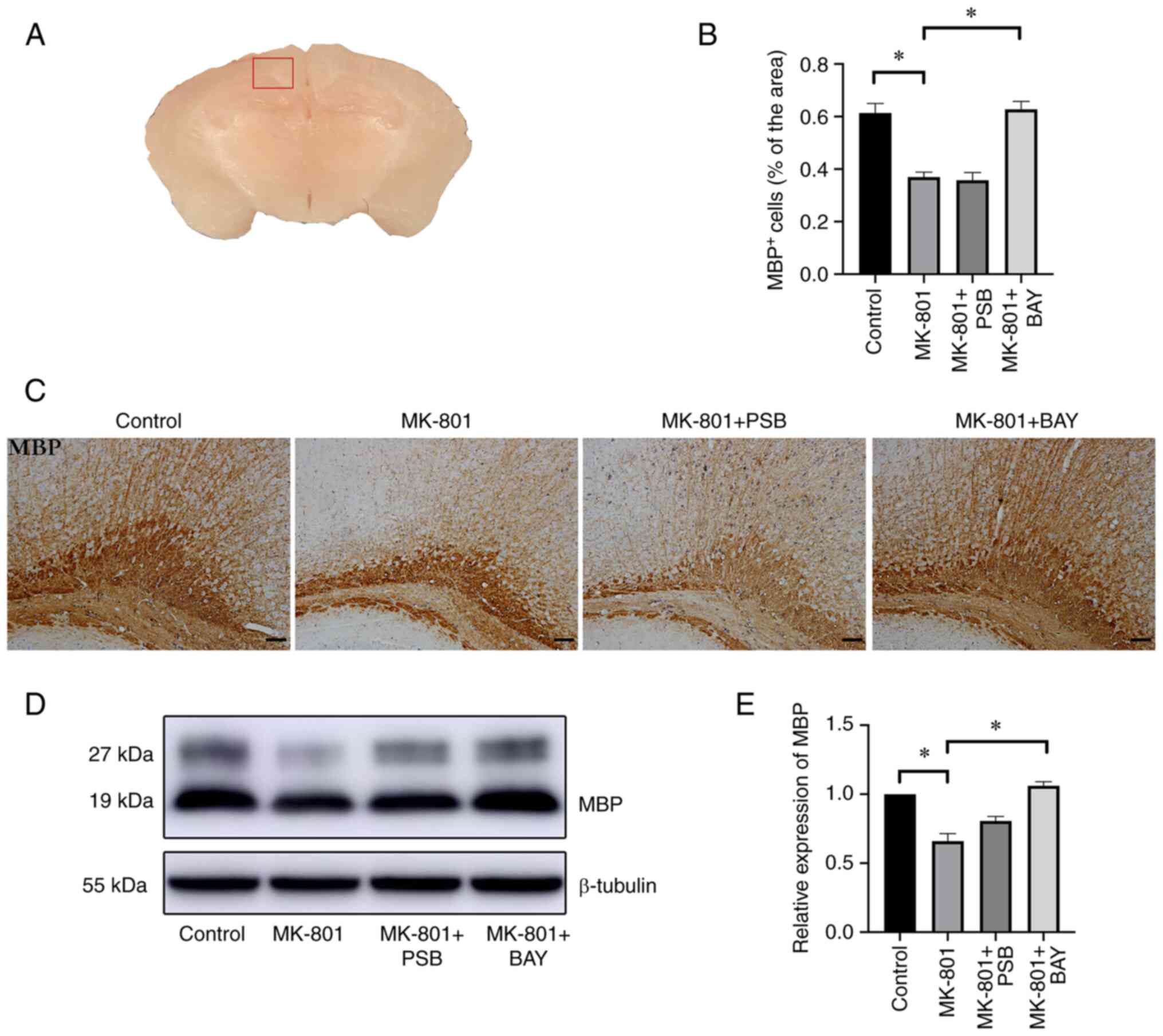
Effect of BAY 60-6583 on the
maturation of OLs in the SCZ mouse model
Immunohistochemical analysis demonstrated that the
area stained for MBP, a specific marker for myelin sheath (31,32),
in the cerebral cortex (Fig. 2A)
of the MK-801 group was lower than that in the control group
(P<0.05) (Fig. 2B and C). Moreover, BAY 60-6583 administration
significantly alleviated the decrease in MBP in the cerebral cortex
of the mouse model, displaying higher neurite density and
arborization in the cortex, with better shape and a dense
distribution (Fig. 2B and C), while no changes in MBP staining were
found in the MK-801 + PSB group compared with that in the MK-801
group (P>0.05) (Fig. 2B and
C). Consistent with the
immunohistochemistry results, western blotting revealed that the
expression levels of MBP in the brain of the MK-801 group were
markedly decreased compared with those in the control group
(P<0.05) (Fig. 2D and E). By contrast, a significant increase in
MBP expression levels was observed in the MK-801 + BAY group
compared with the model group (P<0.05) (Fig. 2D and E), whereas there was no difference in MBP
expression levels in the MK-801 + PSB group compared with those in
the MK-801 group (P>0.05) (Fig.
2D and E).
BAY 60-6583 protects the survival of
OL precursor cells (OPCs) and the differentiation of OLs in the SCZ
mouse model
Immunohistochemical analysis further revealed that
the number of OPCs that were positive for NG2 in the cerebral
cortex was markedly decreased in the MK-801 group compared with
that in the control group (Fig. 3A
and B) (P<0.05). Subsequent BAY
60-6583 administration notably increased the number of
NG2+ cells in the cerebral cortex compared with that in
the MK-801 group (P<0.05) (Fig.
3A and B). Consistently,
western blot analysis showed that the protein expression levels of
NG2 were reduced, while those of GPR17 were increased in the MK-801
group compared with those in the control group (P<0.05)
(Fig. 3C-F). Activation of
A2BAR (BAY 60-6583 treatment) resulted in an increase in
the protein expression levels of NG2 in the MK-801+BAY group
(P<0.05) (Fig. 3C and D), while the protein expression levels of
GPR17 were markedly decreased (P<0.05) (Fig. 3E and F). CC-1 is a specific marker of mature
OLs that is often co-labeled with Olig2, representing mature OLs
(33). Immunofluorescent double
staining for CC-1/Olig2 revealed low levels in the cerebral cortex
of the MK-801 group, and a lower number of mature OLs compared with
that in the control group (P<0.05) (Fig. 3G and H). Meanwhile, immunofluorescence revealed
that A2BAR activation resulted in increased CC-1/Olig2
levels in the cerebral cortex region of the MK-801 + BAY group
(P<0.05) (Fig. 3G and H). Similarly, there was no change in the
number of cells positive for CC-1/Olig2 in the cerebral cortex of
the MK-801 + PSB group (P>0.05) (Fig. 3G and H).
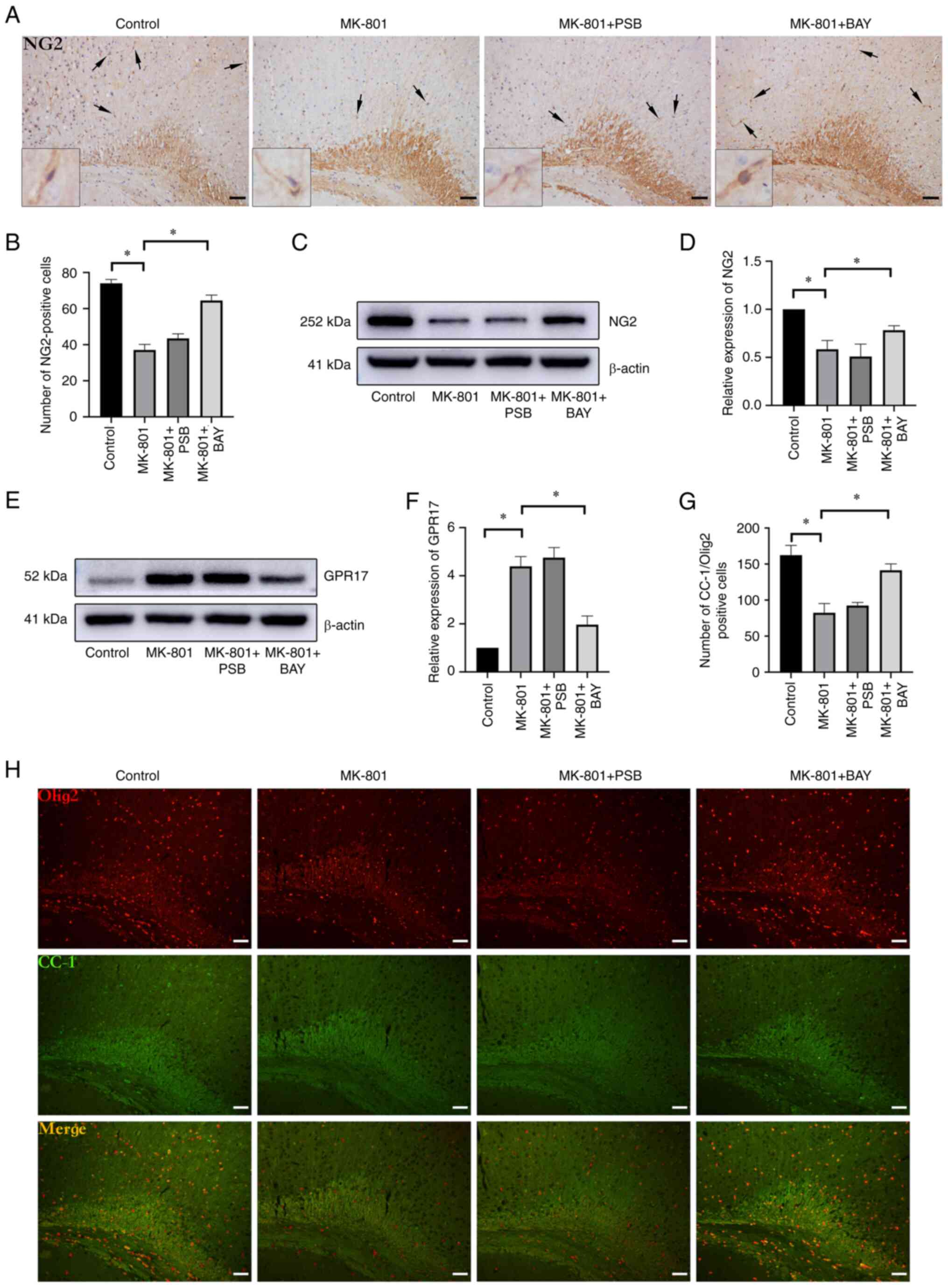 | Figure 3Survival and maturation of OLs are
modified by A2BAR activation in SCZ mice. (A)
Immunohistochemical images of NG2+ OPC staining in the
cerebral cortex (scale bar, 50 µm) The arrows and insert box
indicate the NG2+ cells; the inset boxes are at x8
magnification. (B) MK-801 treatment markedly reduced the number of
NG2+ OPCs in the cerebral cortex. Administration of BAY
60-6583 in the SCZ mice notably increased the number of
NG2+ OPCs in the cerebral cortex. (C) Representative
immunoblots of the NG2 protein levels. (D) MK-801-treated mice
showed a significant reduction in NG2, while BAY 60-6583
administration increased NG2 protein levels in the cerebral cortex.
(E) Representative immunoblots of GPR17 protein expression levels.
(F) GPR17 protein expression levels were significantly increased in
MK-801-treated mice and were restored in MK-801 + BAY-treated mice.
(G) The number of CC-1+/Olig2+ cells was
reduced in the MK-801 group, while a significant increase was
observed in the MK-801 + BAY group. (H) Immunofluorescence staining
was performed for CC-1/Olig2 in the cerebral cortex (scale bar, 50
µm). Data are presented as the mean ± SEM. *P<0.05.
OLs, oligodendrocytes; A2BAR, A2B adenosine
receptor; SCZ, schizophrenia; NG2, chondroitin sulfate glycoprotein
4; OPCs, OL precursor cells; MK-801, dizocilpine maleate; GPR17, G
protein-coupled receptor 17; CC-1, anti-adenomatous polyposis coli
clone CC-1; Olig2, OL transcription factor 2; BAY 60-6583,
A2BAR agonist; PSB 603; A2BAR antagonist. |
Discussion
The present study aimed to investigate the
regulatory effects of A2BAR on the cognitive behavior
and myelin sheath degeneration in mice, for which a SCZ mouse model
was established. The glutamatergic system in SCZ is usually
abnormal, characterized by the glutamatergic signal transduction
dysfunction of the NMDA receptor (34-36).
The delivery of NMDA receptor antagonist not only elicits an
SCZ-like behavioral change in healthy animals, but also causes
further SCZ-like brain pathological changes, such as OL injury,
demyelination, and GABAergic and dopaminergic system disorders
(37). This is why SCZ animal
models treated with NMDA receptor antagonists have been widely used
to investigate SCZ pathogenesis. The present study established an
SCZ mouse model by intraperitoneal injection of MK-801, an NMDA
receptor antagonist, for 14 consecutive days. As a result, the mice
exhibited hyperlocomotion, ataxia, hindlimb abduction, flat body
posture, stereotyped head-rotation behavior, spontaneous locomotor
activity and gradual weight gain. These findings were consistent
with other previous findings (38,39).
In addition, a significant decrease in learning and memory
abilities was observed in the SCZ mice. Notably, selective
activation of A2BAR could alleviate the learning and
spatial memory impairment in the SCZ mice.
Previous studies have demonstrated the following: i)
Demyelination is closely associated with the cognitive and learning
dysfunction in SCZ (40-42);
ii) OL and myelin dysfunction are considered primary changes in
SCZ, not purely as secondary consequences of the illness or
treatment (43); and iii)
enhancing remyelination could alleviate SCZ-like symptoms (30). Based on this theory, the changes in
MBP levels in the cerebral cortex of SCZ mice were initially
investigated in the present study. Unexpectedly, a marked decrease
in MBP expression levels was detected using both western blot and
immunostaining analysis. This finding implies that the development
of SCZ is most likely related to aberrant myelination. Xiu et
al (44) also showed that
MK-801 could cause loss of myelin fibers in the cerebral cortex of
SCZ mice. Likewise, in the present study, the administration of BAY
60-6583 significantly elevated MBP expression levels in SCZ mice,
suggesting that BAY 60-6583 could facilitate myelin repair, leading
to the improvement of cognitive function in SCZ.
Myelin regulation is a dynamic process in which both
freshly generated and pre-existing OLs remyelinate to allow
learning and plasticity, or respond to activity (45). In the CNS, OLs are derived from
mitotically active and migratory OPCs, which undergo stepwise
genotypic and phenotypic differentiation processes before myelin
formation. Accordingly, we hypothesized that the abnormal
myelination was mainly due to myelin loss or abnormal development
of OPCs. As expected, the delivery of MK-801 decreased the number
of NG2+ cells and decreased NG2 protein expression
levels in the cerebral cortex. Although previous studies have shown
an increase in NG2+ cells migrating to the lesion site
after myelin damage (46), MK-801
has also been shown to have a general cytotoxic impact on brain
tissues (47,48), with OLs appearing to be more
affected by MK-801 treatment than other cell types (49). Myelin injury in the brains of
MK-801-induced SCZ mice is considered to be caused by aberrant
OPCs. Additional experiments in the present study revealed that BAY
60-6583 treatment could markedly increase the number of
NG2+ cells and boost NG2 protein expression in the SCZ
model mice. Due to the limited number of OPCs in the brain,
increased OPC numbers are necessary for myelin repair. Meanwhile,
the demyelination or myelin destruction caused by various factors
may be incurable without replenishing the OLs derived from OPCs. To
that end, the differentiation of OPCs after treatment with BAY
60-6583 or PSB 603 was further investigated and the number of
CC-1+/Olig2+ cells was found to be markedly
reduced in SCZ mice. BAY 60-6583 could significantly increase the
number of CC-1+/Olig2+ cells in the SCZ mice,
implying that BAY 60-6583 may promote the differentiation and
maturation of OLs. These findings were supported by the change in
GPR17 expression levels between the two groups. That is, BAY
60-6583 significantly reduced the increase in GPR17 expression
caused by MK-801. GPR17 acts as a suppressor of OL differentiation
and myelination (50). GPR17
knockout in the CNS can facilitate OPC differentiation and
maturation (51,52). GPR17 inhibition can enhance the
maturation of primary rat and mouse OLs, efficiently favor human OL
differentiation (53) and
accelerate the remyelination process after lysolecithin-induced
demyelinating injury (54). This
suggests that the impaired differentiation and maturation of OLs is
closely associated with MK-801-mediated brain demyelination in SCZ
mice and that the A2BAR agonist, BAY 60-6583, likely
promotes the differentiation and maturation of OLs.
Moreover, the present study showed that cognitive
behavior and brain demyelination did not deteriorate in SCZ mice
treated with PSB 603. Theoretically, A2BAR is an AR with
a low affinity for adenosine that is activated by a high quantity
of adenosine. The concentration of adenosine produced by the brains
of SCZ mice is insufficient to activate A2BAR.
In conclusion, the present study established
A2BAR as a possible therapeutic target for reversing
demyelination in a SCZ mouse model. The data from this study
corroborated prior findings by demonstrating that A2BAR
activation could alleviate SCZ symptoms caused by myelin
degeneration and reverse learning and memory impairments in SCZ
mice. Notably, the present study revealed that GPR17 might be
involved in A2BAR-mediated differentiation and
maturation of OLs and protection of the myelin sheath, thus
providing new targets for the treatment of SCZ. However, the
present study is limited by the absence of further behavioral tests
for SCZ-like symptoms, and additional mechanisms require
investigation. Therefore, further in-depth studies are warranted in
the future.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the CAS ‘Light of West
China’ Program (grant no. XAB2017AW05), the National Natural
Science Foundation of China (grant nos. 31360240 and 82060238), the
Ningxia Natural Science Foundation (grant nos. NZ17072 and
2022AAC03155) and the ‘Chunhui Plan’ of the Ministry of Education
(grant no. Z2016063).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QM and DW confirm the authenticity of all the raw
data. DW, YiL, JW and JLi performed the experiments. QM, JLiu and
JS contributed to the conception of the study and supervised the
graduate students. DW, YuL and HY contributed to the acquisition
and interpretation of data. HY and JS contributed to the revision
of the manuscript. QM, JS and HY contributed to obtaining funding,
designed the project and gave final approval of the version to be
published. DW and YuL wrote the manuscript and analyzed the data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures and protocols used in
this study were reviewed and approved by the Ethics Committee for
the Use of Experimental animals at Ningxia Medical University
(Yinchuan, China; approval no. 2018-006).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Charlson FJ, Ferrari AJ, Santomauro DF,
Diminic S, Stockings E, Scott JG, McGrath JJ and Whiteford HA:
Global epidemiology and burden of schizophrenia: Findings from the
global burden of disease study 2016. Schizophr Bull. 44:1195–1203.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hilker R, Helenius D, Fagerlund B, Skytthe
A, Christensen K, Werge TM, Nordentoft M and Glenthøj B:
Heritability of schizophrenia and schizophrenia spectrum based on
the nationwide danish twin register. Biol Psychiatry. 83:492–498.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lesh TA, Niendam TA, Minzenberg MJ and
Carter CS: Cognitive control deficits in schizophrenia: Mechanisms
and meaning. Neuropsychopharmacology. 36:316–338. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mighdoll MI, Tao R, Kleinman JE and Hyde
TM: Myelin, myelin-related disorders, and psychosis. Schizophr Res.
161:85–93. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gouvêa-Junqueira D, Falvella ACB, Antunes
ASLM, Seabra G, Brandão-Teles C, Martins-de-Souza D and Crunfli F:
Novel treatment strategies targeting myelin and oligodendrocyte
dysfunction in schizophrenia. Front Psychiatry.
11(379)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Raabe FJ, Galinski S, Papiol S, Falkai PG,
Schmitt A and Rossner MJ: Studying and modulating
schizophrenia-associated dysfunctions of oligodendrocytes with
patient-specific cell systems. NPJ Schizophr. 4(23)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
He J, Zu Q, Wen C, Liu Q, You P, Li X and
Wang W: Quetiapine attenuates schizophrenia-like behaviors and
demyelination in a MK-801-induced mouse model of schizophrenia.
Front Psychiatry. 11(843)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Falkai P, Malchow B, Wetzestein K,
Nowastowski V, Bernstein HG, Steiner J, Schneider-Axmann T, Kraus
T, Hasan A, Bogerts B, et al: Decreased oligodendrocyte and neuron
number in anterior hippocampal areas and the entire hippocampus in
schizophrenia: A stereological postmortem study. Schizophr Bull. 42
(Suppl 1):S4–S12. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Falkai P, Steiner J, Malchow B, Shariati
J, Knaus A, Bernstein HG, Schneider-Axmann T, Kraus T, Hasan A,
Bogerts B and Schmitt A: Oligodendrocyte and interneuron density in
hippocampal subfields in schizophrenia and association of
oligodendrocyte number with cognitive deficits. Front Cell
Neurosci. 10(78)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vikhreva OV, Rakhmanova VI, Orlovskaya DD
and Uranova NA: Ultrastructural alterations of oligodendrocytes in
prefrontal white matter in schizophrenia: A post-mortem
morphometric study. Schizophr Res. 177:28–36. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Choudhury H, Chellappan DK, Sengupta P,
Pandey M and Gorain B: Adenosine receptors in modulation of central
nervous system disorders. Curr Pharm Des. 25:2808–2827.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pierce KD, Furlong TJ, Selbie LA and Shine
J: Molecular cloning and expression of an adenosine A2b receptor
from human brain. Biochem Biophys Res Commun. 187:86–93.
1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dixon AK, Gubitz AK, Sirinathsinghji DJ,
Richardson PJ and Freeman TC: Tissue distribution of adenosine
receptor mRNAs in the rat. Br J Pharmacol. 118:1461–1468.
1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu YJ, Chen J, Li X, Zhou X, Hu YM, Chu
SF, Peng Y and Chen NH: Research progress on adenosine in central
nervous system diseases. CNS Neurosci Ther. 25:899–910.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Beukers MW, den Dulk H, van Tilburg EW,
Brouwer J and Ijzerman AP: Why are A(2B) receptors low-affinity
adenosine receptors? Mutation of Asn273 to Tyr increases affinity
of human A(2B) receptor for 2-(1-Hexynyl)adenosine. Mol Pharmacol.
58:1349–1356. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Haskó G, Csóka B, Németh ZH, Vizi ES and
Pacher P: A(2B) adenosine receptors in immunity and inflammation.
Trends Immunol. 30:263–270. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kong T, Westerman KA, Faigle M, Eltzschig
HK and Colgan SP: HIF-dependent induction of adenosine A2B receptor
in hypoxia. FASEB J. 20:2242–2250. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Manalo JM, Liu H, Ding D, Hicks J, Sun H,
Salvi R, Kellems RE, Pereira FA and Xia Y: Adenosine A2B receptor:
A pathogenic factor and a therapeutic target for sensorineural
hearing loss. FASEB J. 34:15771–15787. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Doyle C, Cristofaro V, Sack BS, Lukianov
SN, Schäfer M, Chung YG, Sullivan MP and Adam RM: Inosine
attenuates spontaneous activity in the rat neurogenic bladder
through an A2B pathway. Sci Rep. 7(44416)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gile J, Oyama Y, Shuff S and Eckle T: A
role for the adenosine ADORA2B receptor in midazolam induced
cognitive dysfunction. Curr Pharm Des. 26:4330–4337.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang J, Wang D, Zheng X, Li Y, Li Y, Ma T,
Li J, Sun J, Wang Y and Ma Q: A2B adenosine receptor
inhibition ameliorates hypoxic-ischemic injury in neonatal mice via
PKC/Erk/Creb/HIF-1α signaling pathway. Brain Res.
1782(147837)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Winship IR, Dursun SM, Baker GB, Balista
PA, Kandratavicius L, Maia-de-Oliveira JP, Hallak J and Howland JG:
An overview of animal models related to schizophrenia. Can J
Psychiatry. 64:5–17. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Adell A: Brain NMDA receptors in
schizophrenia and depression. Biomolecules. 10(947)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Catts VS, Lai YL, Weickert CS, Weickert TW
and Catts SV: A quantitative review of the postmortem evidence for
decreased cortical N-methyl-D-aspartate receptor expression levels
in schizophrenia: How can we link molecular abnormalities to
mismatch negativity deficits? Biol Psychol. 116:57–67.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eyjolfsson EM, Brenner E, Kondziella D and
Sonnewald U: Repeated injection of MK801: An animal model of
schizophrenia? Neurochem Int. 48:541–546. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ding J, Zhou HH, Ma QR, He ZY, Ma JB, Liu
YM, Zhang YW, He YQ and Liu J: Expression of NR1 and apoptosis
levels in the hippocampal cells of mice treated with MK-801. Mol
Med Rep. 16:8359–8364. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wen J, Wang B, Du C, Xu G, Zhang Z, Li Y
and Zhang N: A2B adenosine receptor agonist improves erectile
function in diabetic rats. Tohoku J Exp Med. 237:141–148.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tian Y, Piras BA, Kron IL, French BA and
Yang Z: Adenosine 2B receptor activation reduces myocardial
reperfusion injury by promoting anti-inflammatory macrophages
differentiation via PI3K/Akt pathway. Oxid Med Cell Longev.
2015(585297)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kaji W, Tanaka S, Tsukimoto M and Kojima
S: Adenosine A(2B) receptor antagonist PSB603 suppresses tumor
growth and metastasis by inhibiting induction of regulatory T
cells. J Toxicol Sci. 39:191–198. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shao Y, Ding J, He QX, Ma QR, Liu Q, Zhang
C, Lv HW and Liu J: Effect of Sox10 on remyelination of the
hippocampus in cuprizone-induced demyelinated mice. Brain Behav.
10(e01623)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kuhn S, Gritti L, Crooks D and Dombrowski
Y: Oligodendrocytes in development, myelin generation and beyond.
Cells. 8(1424)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cherchi F, Pugliese AM and Coppi E:
Oligodendrocyte precursor cell maturation: Role of adenosine
receptors. Neural Regen Res. 16:1686–1692. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yazdankhah M, Ghosh S, Shang P, Stepicheva
N, Hose S, Liu H, Chamling X, Tian S, Sullivan MLG, Calderon MJ, et
al: BNIP3L-mediated mitophagy is required for mitochondrial
remodeling during the differentiation of optic nerve
oligodendrocytes. Autophagy. 17:3140–3159. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Coyle JT: The glutamatergic dysfunction
hypothesis for schizophrenia. Harv Rev Psychiatry. 3:241–253.
1996.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Deakin JF, Slater P, Simpson MD, Gilchrist
AC, Skan WJ, Royston MC, Reynolds GP and Cross AJ: Frontal cortical
and left temporal glutamatergic dysfunction in schizophrenia. J
Neurochem. 52:1781–1786. 1989.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mohn AR, Gainetdinov RR, Caron MG and
Koller BH: Mice with reduced NMDA receptor expression display
behaviors related to schizophrenia. Cell. 98:427–436.
1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sheri AT and Stephen RS: A review of NMDA
receptors and the phencyclidine model of schizophrenia.
Pharmacotherapy. 16:82–93. 1996.PubMed/NCBI
|
|
38
|
Park SJ, Lee Y, Oh HK, Lee HE, Lee Y, Ko
SY, Kim B, Cheong JH, Shin CY and Ryu JH: Oleanolic acid attenuates
MK-801-induced schizophrenia-like behaviors in mice.
Neuropharmacology. 86:49–56. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Missault S, Van den Eynde K, Vanden Berghe
W, Fransen E, Weeren A, Timmermans JP, Kumar-Singh S and
Dedeurwaerdere S: The risk for behavioural deficits is determined
by the maternal immune response to prenatal immune challenge in a
neurodevelopmental model. Brain Behav Immun. 42:138–146.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kiljan S, Prins M, Baselmans BM, Bol JGJM,
Schenk GJ and van Dam AM: Enhanced GABAergic immunoreactivity in
hippocampal neurons and astroglia of multiple sclerosis patients. J
Neuropathol Exp Neurol. 78:480–491. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Deoni S, Dean D III, Joelson S, O'Regan J
and Schneider N: Early nutrition influences developmental
myelination and cognition in infants and young children.
Neuroimage. 178:649–659. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang Y, Zhang Y, Luo F and Li B: Chronic
stress regulates NG2+ cell maturation and myelination in the
prefrontal cortex through induction of death receptor 6. Exp
Neurol. 277:202–214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Takahashi N, Sakurai T, Davis KL and
Buxbaum JD: Linking oligodendrocyte and myelin dysfunction to
neurocircuitry abnormalities in schizophrenia. Prog Neurobiol.
93:13–24. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xiu Y, Kong XR, Zhang L, Qiu X, Gao Y,
Huang CX, Chao FL, Wang SR and Tang Y: The myelinated fiber loss in
the corpus callosum of mouse model of schizophrenia induced by
MK-801. J Psychiatr Res. 63:132–140. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sampaio-Baptista C and Johansen-Berg H:
White matter plasticity in the adult brain. Neuron. 96:1239–1251.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Valny M, Honsa P, Kriska J and Anderova M:
Multipotency and therapeutic potential of NG2 cells. Biochem
Pharmacol. 141:42–55. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Coleman LG Jr, Jarskog LF, Moy SS and
Crews FT: Deficits in adult prefrontal cortex neurons and behavior
following early post-natal NMDA antagonist treatment. Pharmacol
Biochem Behav. 93:322–330. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kawabe K and Miyamoto E: Effects of
neonatal repeated MK-801 treatment on delayed
nonmatching-to-position responses in rats. Neuroreport. 19:969–973.
2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Guest PC, Iwata K, Kato TA, Steiner J,
Schmitt A, Turck CW and Martins-de-Souza D: MK-801 treatment
affects glycolysis in oligodendrocytes more than in astrocytes and
neuronal cells: Insights for schizophrenia. Front Cell Neurosci.
9(180)2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Fratangeli A, Parmigiani E, Fumagalli M,
Lecca D, Benfante R, Passafaro M, Buffo A, Abbracchio MP and Rosa
P: The regulated expression, intracellular trafficking, and
membrane recycling of the P2Y-like receptor GPR17 in Oli-neu
oligodendroglial cells. J Biol Chem. 288:5241–5256. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang J, He X, Meng H, Li Y, Dmitriev P,
Tian F, Page JC, Lu QR and He Z: Robust myelination of regenerated
axons induced by combined manipulations of GPR17 and microglia.
Neuron. 108:876–886.e4. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fumagalli M, Daniele S, Lecca D, Lee PR,
Parravicini C, Fields RD, Rosa P, Antonucci F, Verderio C,
Trincavelli ML, et al: Phenotypic changes, signaling pathway, and
functional correlates of GPR17-expressing neural precursor cells
during oligodendrocyte differentiation. J Biol Chem.
286:10593–10604. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Merten N, Fischer J, Simon K, Zhang L,
Schröder R, Peters L, Letombe AG, Hennen S, Schrage R, Bödefeld T,
et al: Repurposing HAMI3379 to block GPR17 and promote rodent and
human oligodendrocyte differentiation. Cell Chem Biol.
25:775–786.e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ou Z, Sun Y, Lin L, You N, Liu X, Li H, Ma
Y, Cao L, Han Y, Liu M, et al: Olig2-targeted G-protein-coupled
receptor Gpr17 regulates oligodendrocyte survival in response to
lysolecithin-induced demyelination. J Neurosci. 36:10560–10573.
2016.PubMed/NCBI View Article : Google Scholar
|