Introduction
Schwannomas are rare intracranial tumors that
originate from Schwann cells and are rarely malignant. Trigeminal
schwannomas (TSs) are the second most common type of intracranial
tumor, comprising 0.2-1.0% of intracranial primary tumors and
0.8-8.0% of intracranial schwannomas (1-3).
Intracranial TSs often lead to facial numbness caused by trigeminal
nerve dysfunction, trigeminal neuralgia and paralysis of other
cranial nerves that follows tumor compression. Notably, symptoms of
TS are often related to high intracranial pressure, which can be
measured by cranial radiological imaging, such as magnetic
resonance imaging (MRI) and computed tomography (CT); however,
pathological diagnosis is the gold standard. Histologically,
schwannomas are characterized by broad interlacing ribbons of
extended spindle cells that produce a palisading pattern of nuclei
around a central mass of cytoplasm. Detection of S100 is required
to establish the neural origin of the tumor (4). Anatomically, the majority of TSs
arise from the Gasserian ganglion and spread to the intradural and
epidural cavities (5). Because of
the complicated anatomical structure, TSs located in the middle
cranial fossa are usually dissected through conventional craniotomy
surgical approaches (6). Due to
the rapid development and wide application of endoscopic
technology, minimally invasive endoscopic surgeries (2) and endoscopic-assisted surgeries
(7) have received more attention
regarding the treatment of TSs. The endoscopic transnasal maxillary
sinus approach is an operation that has been used to resect tumors
located in the pterygopalatine fossa (PPF) (8). Although rarely reported, this
approach can also be used to resect TSs located in the middle
cranial fossa (9). The present
report focused on the transnasal maxillary sinus approach in
dealing with lesions located in the middle cranial fossa, which is
worthy of more clinical application.
Case report
Case
A 59-year-old man with a 2-year history of
progressive intermittent headaches without other noteworthy medical
or family history was admitted to the Department of Neurosurgery,
Chongqing General Hospital (Chongqing, China) in May 2019. A
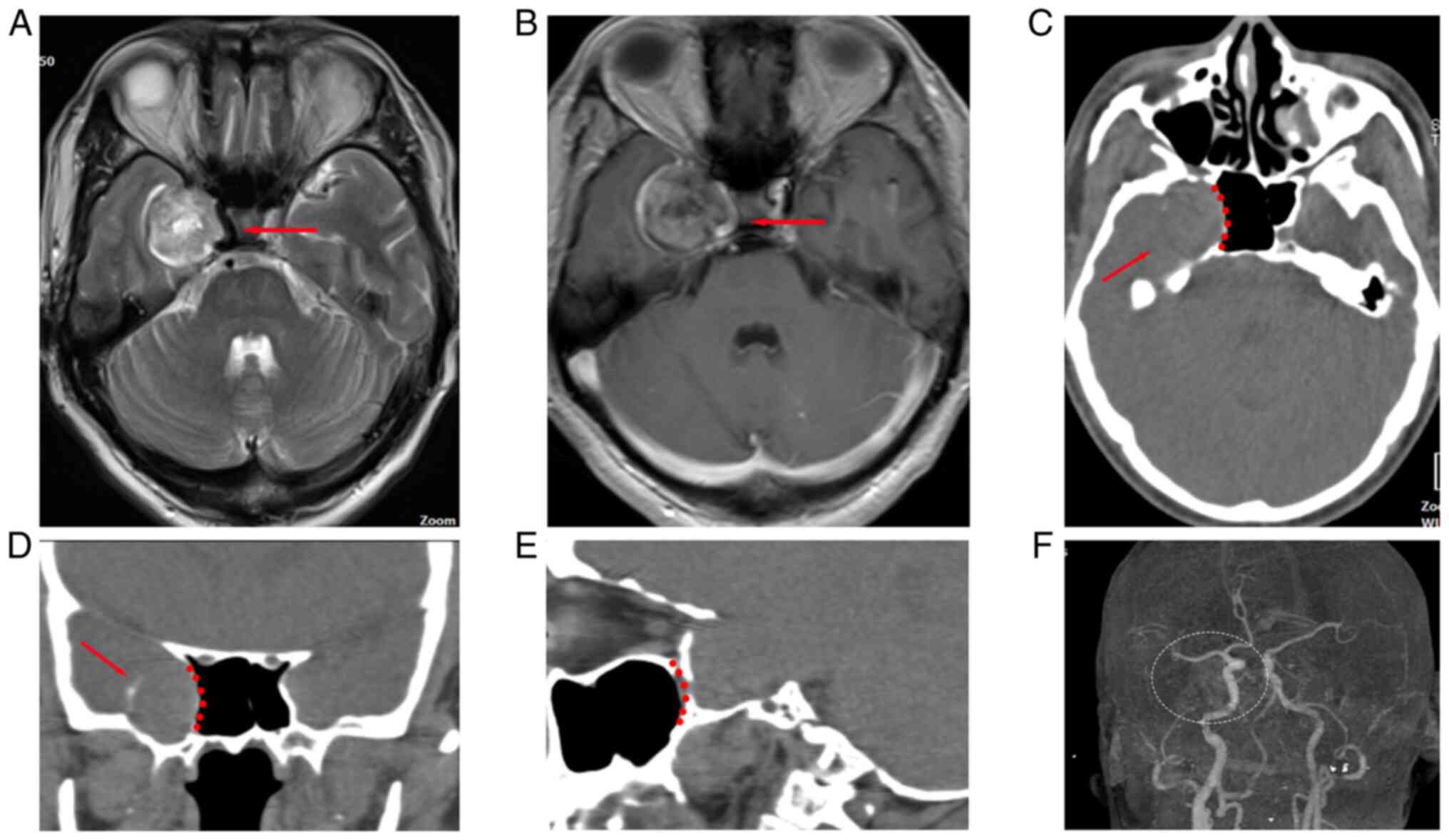
neurological test showed no abnormalities. Radiography indicated
that there was a spherical lesion located in the right middle fossa
of the cranium with eggshell calcification (Fig. 1) that was diagnosed as a TS located
in Meckel's cave. The right cavernous sinus and internal carotid
artery (ICA) that were adjacent to the lesion were compressed and
displaced. CT angiography (CTA) excluded vascular
abnormalities.
Given that the lesion was next to the lateral wall
of the sphenoid sinus and posterior wall of the maxillary sinus,
the endoscopic transnasal maxillary sinus approach was chosen to
mitigate operative injury as much as possible. After induction of
anesthesia, the patient's head was placed in a supine position,
tilted 15˚ to the right and unilateral endonasal surgery was
performed. Epinephrine (1:100,000) gauzes were plugged into the
right nasal cavity, thus decongesting the branches of the
sphenopalatine artery. The right middle turbinate was slightly
lifted and suspended. A 0˚ neuroendoscope was inserted into the
middle nasal meatus and the opening of the maxillary sinus was
visualized. To improve endoscopic vision, the openings of the
sphenoid sinus and maxillary sinus were enlarged, and the endoscope
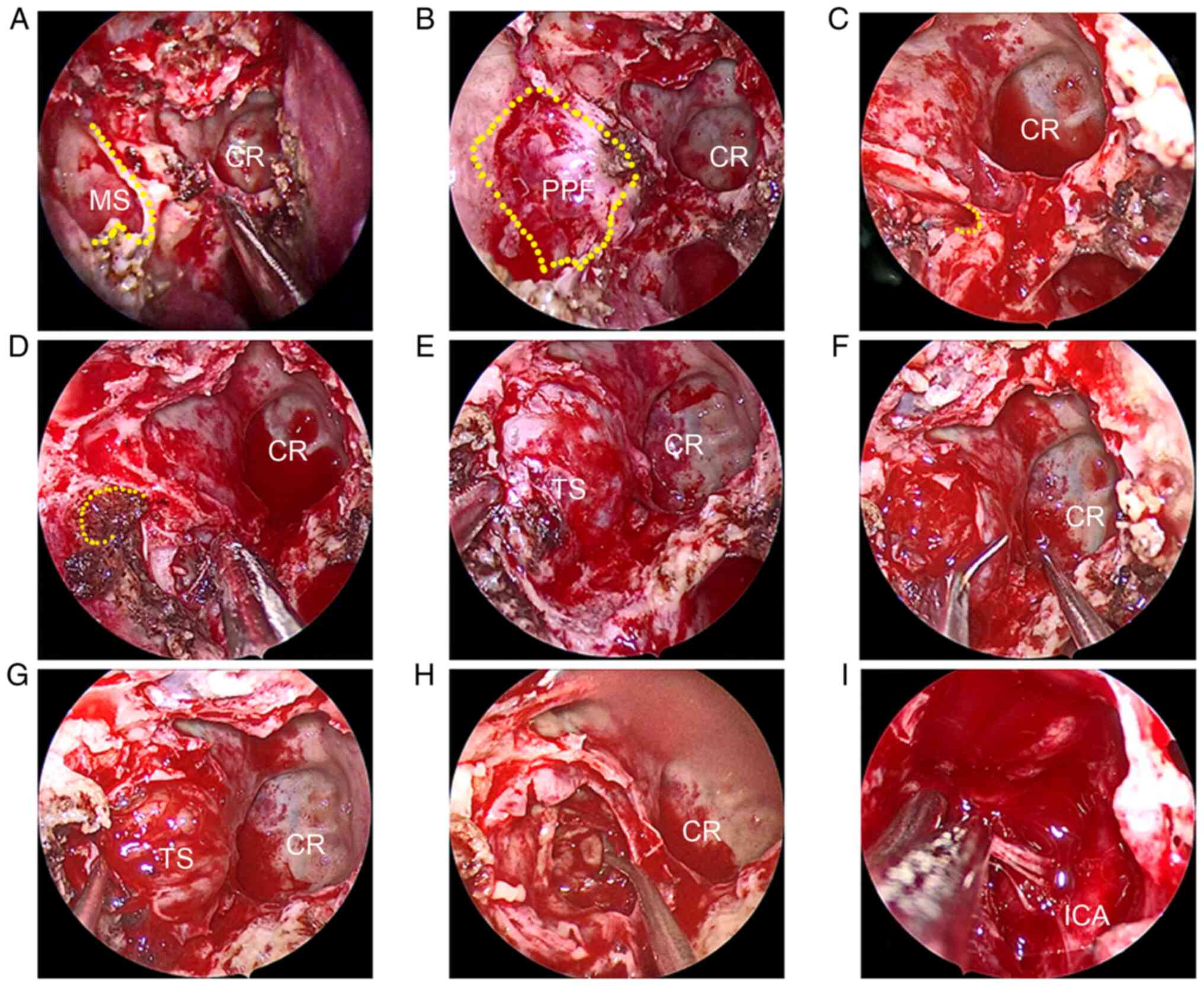
was allowed to enter the maxillary sinus. By removing the posterior
wall of the maxillary sinus and the perpendicular plate of the
palatine bone, the PPF and pterygopalatine ganglion were exposed.
The position of the Vidian canal opening following the Vidian nerve
was determined. The Vidian nerve and artery were cut off to extend
the operative space, and the foramen rotundum was exposed
supra-laterally. By removing the bone of the skull base that was
centered with the foramen rotundum, the TS was rapidly exposed.
After being decompressed intratumorally, the tumor was completely
peeled from the surrounding tissues and a piece of calcified bone
slice was removed. A vertical segment of the petrous ICA was
observed after the tumor was totally resected (Fig. 2). The residual cavity was
compressed using a gelatin sponge to meticulously prevent
hemostasis, after which, the endoscope was removed. The area
covering the expanded maxillary sinus opening was resettled and the
anatomical structure of the nasal cavity was reconstructed. Then,
the right nostril was packed tightly.
The patient who underwent TS resection via the
transnasal maxillary sinus approach experienced headache relief
without cerebrospinal fluid (CSF) leakage and ocular movement
function disorder. The patient complained of light numbness in the
distribution area of the maxillary nerve of the right side of their
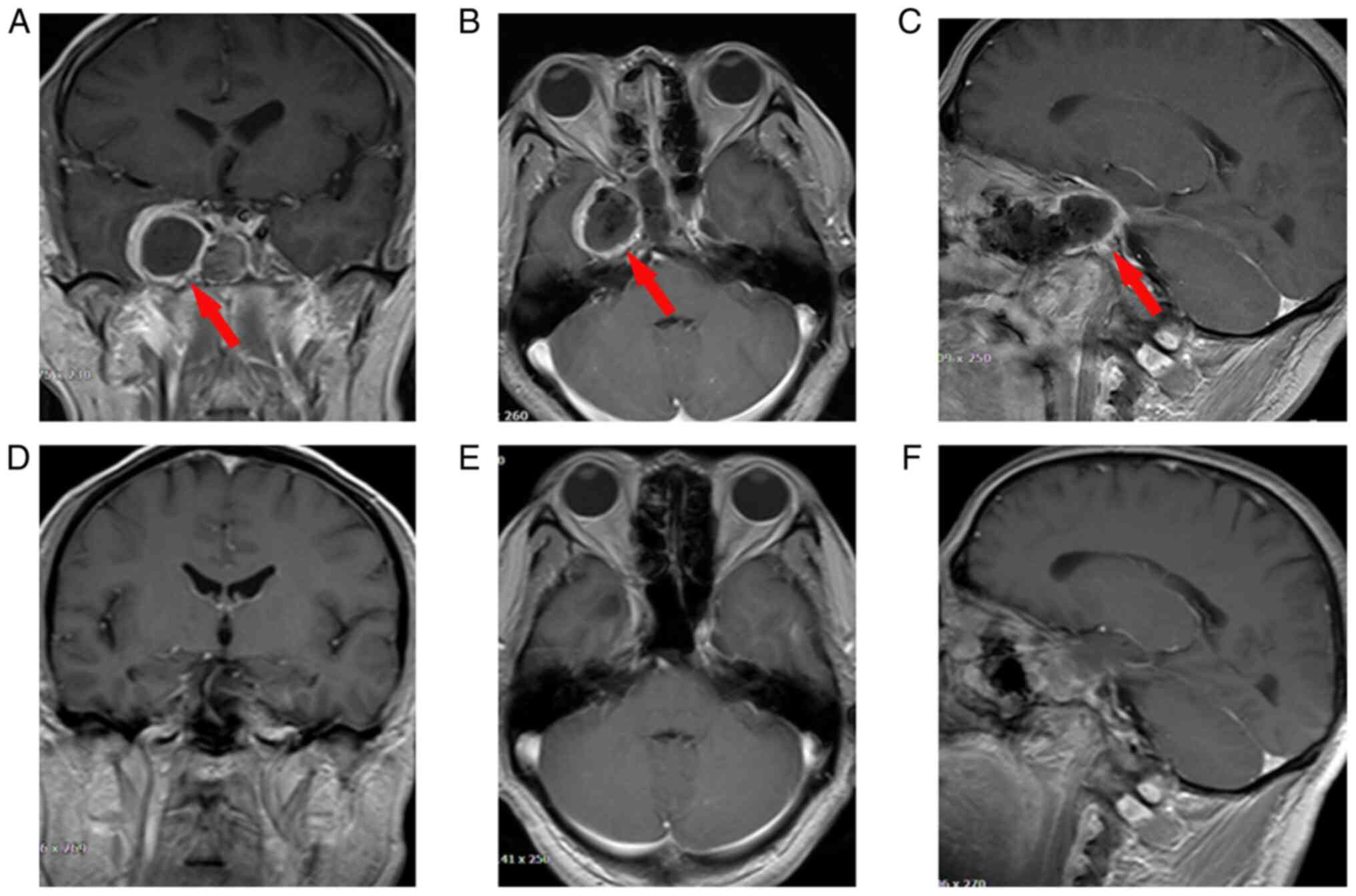
face, which was gradually relieved during the follow-up. An MRI
conducted 1 week after the operation showed that the TS was totally
resected without destruction of the sphenoid sinus and intracranial
hemorrhage. The residual cavity of the TS was covered by a layer of
edema tissue that displayed a higher T2 signal caused by
inflammatory responses. The anatomical structure of the brain
returned to normal 3 months after the operation (Fig. 3). At the last follow-up, the TS had
not relapsed. Self-healing trigeminal neuralgia and paresthesia
occasionally occurred in the patient during the follow-up; however,
the patient returned to normal life without other symptoms.
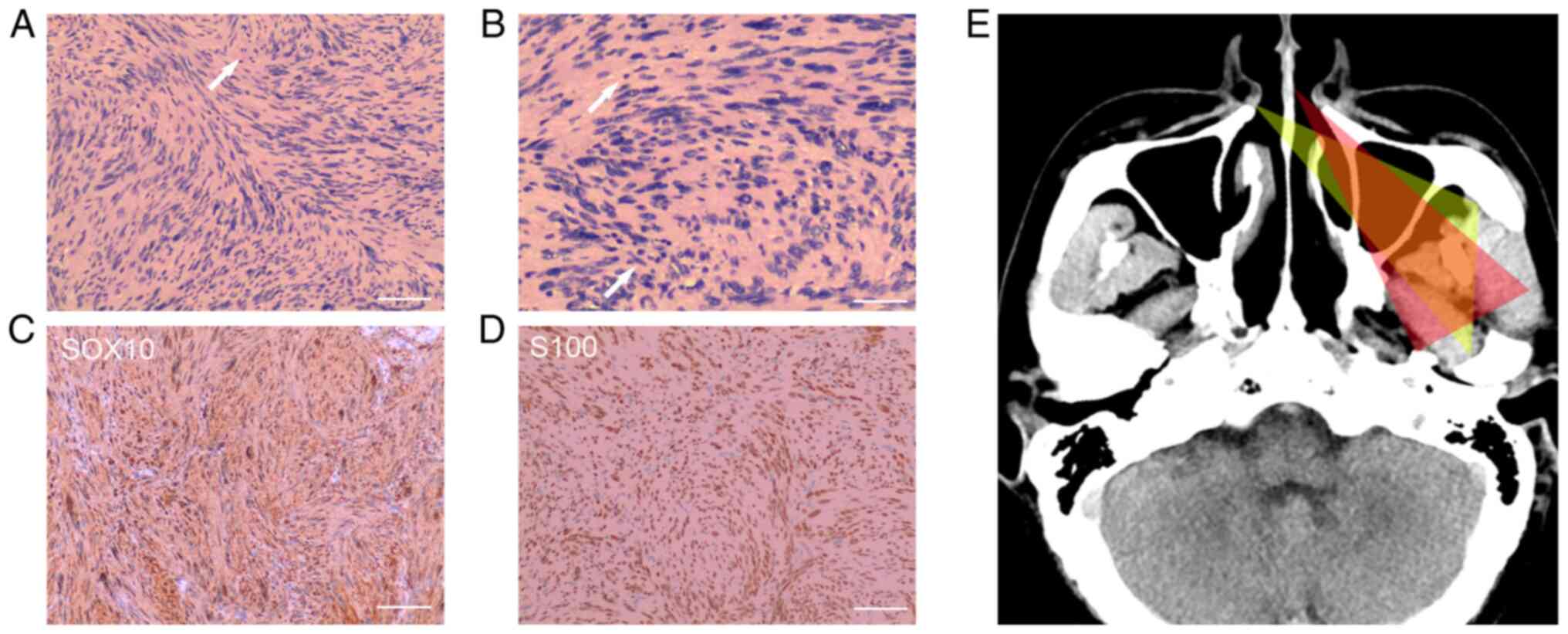
Postoperative histological analysis illustrated that the tumor was
spindle-shaped and arranged in a fence-like manner without
karyomitosis, and immunohistochemical staining showed that the TS
was positive for SOX-10 and S100, and negative for progesterone
receptor (PR). Few cells exhibited CD34 positivity. The Ki-67
labeling index of TS was 1% (data not shown). These findings
confirmed that the lesion was a cellular schwannoma that arose
around the cavernous sinus (Fig.
4A-D). Written informed consent was obtained from the patient
to publish this case report and the accompanying images.
Pathological examination
The pathological examinations were performed by the
Department of Pathology, Chongqing General Hospital. The tumor
samples were fixed in room temperature with 4% formaldehyde
solution for 24 h and embedded in paraffin, and were then cut into
4-µm sections for H&E staining and immunohistochemical
staining. For H&E staining, the sections were deparaffinized by
xylene in 60˚C for 2 h, and were stained with 0.5% hematoxylin for
3 min and 0.5% eosin for 3 min in room temperature. Subsequently,
the stained sections were dehydrated and observed with a BX51
inverted fluorescence microscope (Olympus Corporation).
Immunohistochemical staining of these sections was performed on a
BenchMark XT (Roche Diagnostics, Inc.), which is an automatic
immunohistochemical staining device. Briefly, the sections were
deparaffinization in EZ prep solution (cat. no. 950-102; Ventana
Medical Systems, Inc.) at 75˚C for 8 min and the antigen retrieval
was performed using Cell Conditioner 1 (cat. no. 950-124; Ventana
Medical Systems, Inc.) at 95˚C for 44 min. Then, the endogenous
peroxides and protein were blocked by Endogenous Biotin Blocking
kit (cat. no. 760-050; Ventana Medical Systems, Inc.) at 37˚C for 4
min. Following primary antibodies were used: Anti-S100 (cat. no.
760-2523; Ventana Medical Systems, Inc.), anti-SOX-10 (cat. no.
383A-78; Cell Marque, Millipore Sigma), anti-Ki-67 (cat. no.
790-4286; Ventana Medical Systems, Inc.), anti-PR (cat. no.
790-4296; Ventana Medical Systems, Inc.) and anti-CD34 (cat. no.
790-2927; Ventana Medical Systems, Inc.). All primary antibodies
were prediluted by the suppliers. Primary antibodies were added and
incubated for 16 min at 37˚C. Antigen-antibody reactions were
visualized using OptiView Amplification kit (cat. no. 760-080;
Ventana Medical Systems, Inc.) and OptiView Universal DAB Detection
kit (cat. no. 860-099; Ventana Medical Systems, Inc.). All was
performed in accordance with the manufacturer's protocols. Post
counterstain was incubated with Bluing Reagent (cat. no. 760-2037;
Ventana Medical Systems, Inc.) for 8 min at room temperature. The
stained sections were observed with BX51 inverted fluorescence
microscope (Olympus Corporation).
Radiological examination
The CT plain scans of coronal, sagittal, and
transverse images were gathered by Siemens Emotion 16 (Siemens,
Germany) with 100 kV. The CTA was gathered with 120 kV, and the
data were processed and analyzed via the Siemens syngo.via software
(Siemens, Germany). MRI images were obtained using a Siemens
Magnetom Trio, A Tim System 3T MRI System (Siemens, Germany).
T2-weighted images were obtained from a turbo spin echo sequence
with a repetition time (TR) of 5,000 msec, an effective echo time
(TE) of 95 msec, a field of view (FOV) of 175x230 cm, an in-plane
resolution of 256x224 and a flip angle (FA) of 150. Fluid
attenuated inversion recovery images were obtained from a turbo
inversion recovery sequence with a TR of 8,460 msec, a TE of 134
msec, a FOV of 134x250 cm, an in-plane resolution of 250x160, and a
FA of 150. The enhanced scan images were obtained from a gradient
echo sequence with a TR of 204 msec, a TE of 5 msec, a FOV of
141x250 cm, an in-plane resolution of 256x154, and a FA of 150.
Literature review
To review the cases of endoscopic endonasal
resection of middle cranial fossa TSs (including TS extended to
adjacent fossae, such as Meckel's cave, cavernous sinus, PPF, ITF),
the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) was searched and
available English literature that met the set requirements was
screened. The following terms were searched: [‘cranial fossa,
middle (MeSH)’ OR ‘meckel cave’ OR ‘cavernous sinus’] AND
[‘neurilemmoma (MeSH)’ OR ‘trigeminal schwannoma’] AND
(‘endoscopic’ OR ‘transnasal’ OR ‘endonasal’) and reviews,
irrelevant studies (which did not report endoscopic endonasal
resection of middle cranial fossa TS) and papers published in other
languages (two Chinese and one Russian) were excluded.
Based on the literature review (Table I), 15 articles reporting 106 cases
of middle cranial fossa schwannoma resection through endoscopic
endonasal approaches were identified between 2008 and 2022
(2,9-22).
Most of these cases totally dissected tumors without severe
complications. The endoscopic transnasal maxillary sinus approach
was used in five cases (9) and
obtained great efficacy.
 | Table IPreviously reported cases of
endonasal endoscopic resection of middle cranial fossa TSs. |
Table I
Previously reported cases of
endonasal endoscopic resection of middle cranial fossa TSs.
| First author,
year | Diagnosis | Number of
cases | Location | Treatment
approach | Outcome | Complications | (Refs.) |
|---|
| Kassam, 2009 | TS | 6 | MCF | Expanded endoscopic
endonasnal approach | Total/subtotal | V1 transient
deficit/V3 numbness | (10) |
| Prevedello,
2010 | TS | 1 | Meckel's cave | Endonasal
endoscopic approach with Vidian transposition | Total | None | (11) |
| Qiuhang, 2014 | TS | 4 | Cavernous
sinus | NA | Total | None | (12) |
| Battaglia,
2014 | Schwannoma | 2 | ITF | Endoscopic
endonasal transpterygoid transmaxillary approach | Total | None | (13) |
| Raza, 2014 | TS | 5 | Meckel's cave and
PCF | Endoscopic
transpterygoid approach | Total/subtotal | CN VI palsy, V1
numbness | (14) |
| Jacquesson,
2015 | TS | 1 | Cavernous
sinus | Endoscopic
transsphenoidal approach | Total | Hypoesthesia of the
maxillary nerve territory | (15) |
| Plzák, 2017 | Schwannoma | 2 | PPF/ITF/MCF | Endonasal
endoscopic approach | Total | V2 hypesthesia,
transient trismus | (16) |
| Yang, 2018 | TS | 39 | ITF/PPF/MCF/PPS/
cavernous sinus/ Meckel's cave | Endoscopic medial
maxillectomy approach/ endoscopic endonasal-assisted sublabial
transmaxillary approach/endoscopic endonasal-assisted sublabial
transmaxillary combined with septectomy | Total | Facial
numbness/facial pain/dry eyes/weakness in mastication | (17) |
| Jeon, 2018 | TS | 4 | MCF/Meckel' s
cave | Endoscopic
transorbital approach | Total | None | (18) |
| Zoli, 2018 | Neuroma | 5 | MCF/MCF and
PCF | Endoscopic
transmaxillary-pterygoid approach | Total/subtotal | None | (9) |
| Hardesty, 2018 | TS | 2 | Meckel's cave | Transpterygoid
approach | Total | None | (19) |
| Park, 2019 | TS | 25 | MCF/MCF and
PCF | Endoscopic
endonasnal approach/endoscopic transorbital approach | Total/subtotal | None | (2) |
| Almomen, 2020 | TS | 1 | ITF | Endoscopic medial
and posterior walls maxillectomies | Total | None | (20) |
| Di Somma, 2020 | TS | 1 | Meckel's cave | Endoscopic
endonasal and transorbital surgery | Total | None | (21) |
| Wu, 2021 | TS | 8 | MCF and PCF | Trans-Meckel's cave
approach and transclival approach/trans-Meckel's cave approach | Total | V1-2 numbness/VI
palsy/ dry eye/mastication weakness | (22) |
Discussion
Operations in the middle cranial fossa are
challenging due to the complicated anatomical structure and vital
contents. Conventional skull base surgical approaches, such as the
lateral approach of the middle cranial fossa or anterior
transpetrosal approach, are usually used to resect middle cranial
fossa lesions but are often too invasive. Notably, these approaches
could result in significant surgical complications with narrow
operative corridors (23),
including occlusion obstacles, temporalis muscle atrophy, facial
lesions, peripheral facial paralysis and temporal lobe retraction
(8), whereas endoscopic surgery
has an advantage over these approaches in this regard (24). Endonasal endoscopic procedures were
first used for pituitary surgery and their use has been gradually
extended to other regions; in particular, these procedures are now
the major approach for operating on lesions located in the skull
base, including the orbit (25),
parasellar space (26) and
Meckel's cave (27). These
surgeries provide access to a wide range of lesions by using the
natural surgical corridor of the nasal cavities (28), and surgeons have more space for
tumor resection and the fact that they are minimally invasive mean
they are considered beneficial for surgeons and patients.
According to the present literature review,
endoscopic surgery for dissecting middle cranial fossa TSs can be
performed through various approaches. Notably, exploiting the
optimal approach for specific lesions is important for surgeons. In
the present case report, imaging demonstrated that the tumor was
located in Meckel's cave, which is closely adjacent to the
posterior wall of the PPF and the lateral wall sphenoid sinus. The
single nostril transnasal maxillary sinus approach by two-handed
surgery, which is usually used to address lesions located in the
PPF (5,8), was adopted in tumor resection. It has
been reported that lesions in Meckel's cave could be totally
dissected through the endonasal endoscopic transpterygoid or
transsphenoidal approach (19,27);
however, this may damage the parasellar structures. In the present
case, the specific position of the TS increased the probability of
reaching the lesion through the nasal-maxillary-PPF-middle fossa
corridor. The anatomical studies illustrated the advantages of the
middle meatal transantral approach in dealing with lesions located
in the PPF and infratemporal fossa (ITF) (29,30),
revealing a new line of approach for the lateral part of the skull
base (5,8). Exploiting the maxillary sinus and PPF
provides a particularly short, direct surgical route to the
cavernous sinus and Meckel's cave. The transnasal maxillary sinus
approach is centered with PPF and reaches the anterior-lateral part
of the middle cranial based on middle meatal transantral approach.
Compared with conventional approaches, the transnasal maxillary
sinus approach is considered safer and easier to perform due to its
full exposure of the tumor at the center of the operative field and
direct visualization of the ICA during tumor removal, which extends
the application of the transpterygoid approach and transnasal
perpendicular plate palatine bone. Compared with two-nostril
surgery (operated by three or four hands), single nostril surgery
can reach deeper positions and remain closer to the midline without
injuring the contralateral nostril and nasal septum, even though it
has to sacrifice more operative space (Fig. 4E).
The recommended application of the transnasal
maxillary sinus approach is dealing with localized lesions lying on
the nasal-maxillary-PPF-middle fossa axis [e.g.,
meningoencephalocele, schwannoma and meningioma (31)] and its adjacent area, such as the
ITF and parapharyngeal space (PPS), according to our experience. As
illustrated in the present case, although benign TSs are usually
covered by membranes and separated from normal tissue, exposing the
tumor located in the middle cranial fossa through the expanded
foramen rotundum epidurally could be a reliable selection. Despite
studies suggesting that endoscopic methods maximize the chances of
complete resection and provide good postoperative surveillance of
malignancy (32,33), the prognosis is still uncertain.
For low-grade lesions, such as nasopharyngeal adenoid cystic
carcinoma (34), cribriform
cystadenocarcinomas (35) and
smooth muscle neoplasm (36),
endonasal endoscopic surgeries have been shown to acquire
satisfactory efficacy due to their slow growth and weak
invasiveness. Unfortunately, high-grade tumors always infiltrate
the surrounding tissues, such as the brain parenchyma and
vasculature, and extend to other fossae, which makes their
boundaries difficult to recognize and resect completely (37). Moreover, copiously vascularized
tumor tissues pose challenges. When the transnasal maxillary sinus
approach is performed in the dissection of tumors located in the
middle fossa, there is inadequate operative space for controlling
unpredictable bleeding that may prove fatal (37). Combined approaches might be a
better treatment option for malignancy, which warrants the highest
chances of achieving satisfactory tumor resection with a reduced
risk of complications (38).
Surgeons have adopted combined endoscopic transnasal and anterior
transmaxillary approaches in the dissection of nasopharyngeal
carcinoma that has extended to the upper PPS (39), and have used the transmaxillary
approach in combination with the endonasal endoscopic approach for
giant nasoangiofibromas and chondrosarcoma (40). The absolute contraindication of
such an approach has rarely been reported although there are still
severe complications, including skull base reconstruction failure
and intraoperative vascular lesions (38). Variations in the anatomical
structure of the maxillary sinus cannot be ignored (41), because doing so would significantly
influence surgical decision and complication risk.
Plans to perform an endonasal endoscopic surgery are
based on complete and comprehensive evaluations, including the
position, anatomical features, and pathological properties, in
order to avoid making inappropriate surgical decisions. In the
present case, the postoperative histological examination was in
accordance with the preoperative diagnosis. It has been reported
that the cells of cellular TSs are arranged predominantly in short
interlacing fascicles and the nuclei are elongated with a mixture
of plump and wavy nuclear morphologies (42,43),
which was verified in the present case. However, atypical
morphological appearance of schwannomas, such as epithelioid
schwannoma, a rare variant of nerve sheath tumor, is also reported
(44). Furthermore,
immunohistochemical examination of S100 and SOX-10 is an effective
tool for schwannoma diagnosis. It has been reported that Ki-67
labeling indices <20%, and S100, SOX-10 and neurofibromin (NF)
positivity highly indicate the diagnosis of schwannoma and can
exclude peripheral malignant nerve sheath tumors (45). Diffuse S100 positivity is the
hallmark of all schwannomas. SOX-10 is a marker of neural crest
differentiation that has also been implicated as a neural crest
stem cell marker. It has previously been reported that SOX-10 has
99% specificity and S100 has 91% specificity in schwannoma
(4,46,47).
In the present case, common markers, such as CD34 and PR, were also
tested and exhibited negativity, excluding melanoma and endothelial
tumors. Some studies have also tested p16, NF (45), glial fibrillary acidic protein,
epithelial membrane antigen and HMB45(43). These markers have been regarded as
supplementary indices for diagnosis, which have not been widely
accepted.
During the operation described in the present study,
determining the position of the foramen rotundum and Vidian canal
was necessary for the transnasal maxillary sinus approach to reach
the middle cranial fossa. Anatomically, the maxillary nerve (CN V2)
exits from the foramen rotundum traveling in the upper aspect of
the PPF and joining the pterygopalatine ganglion, and then enters
the infraorbital fissure. The Vidian nerve is inferomedial to V2,
which is made up of greater and deep petrosal nerves (48). Identification of the Vidian nerve
from the PPF can guide the surgeon to the anterior genu of the
petrous ICA and can prevent the impairment of important
neurovascular structures. Clinically, in the present case report,
the position of the foramen rotundum was determined by locating the
Vidian canal. After the opening of the Vidian canal was exposed,
the Vidian nerve and artery were cut off, as the Vidian
neurovascular bundle blocked the operative space for positioning
and exposing the foramen rotundum. Studies have reported that when
the transpterygoid approach is performed to reach the lesions in
the petrous apex, Meckel's cave or cavernous sinus, Vidian nerves
are usually dissected and the base of the pterygoid plates removed
to reveal the petrous ICA (9,14,49).
Vidian nerves are made up of sympathetic and
parasympathetic fibers, and are important for the maintenance of
lacrimal function (50). However,
a clinical study revealed that sacrificing the Vidian nerve during
endoscopic endonasal approaches would not result in severe dry eye,
although the tear volume has been shown to be reduced 1 month
post-operation (51). In the
present case, when the opening of the Vidian canal was identified,
nerves and arteries were cut off. When the Vidian neurovascular
bundle blocked the operative space for positioning and exposing the
foramen rotundum, the Vidian canal remained closed
intraoperatively; by cutting off the Vidian nerve, there was enough
space for exposure of the foramen rotundum and to enable operating
in the middle cranial fossa. The present patient did not complain
of symptoms related to lacrimal function, such as a reduction in
lacrimation or rhinitis, after the operation, which was in
accordance with the literature. However, although such symptoms
were not observed after the Vidian nerve was sacrificed, keeping it
intact is important for a better long-term prognosis. An anatomical
study reported that removing the bone of the Vidian canal and
retracting the Vidian nerve superiorly was a method to preserve the
Vidian nerve (11). Such a
technique was considered more suitable for removing lesions located
in a deeper position where surgeons need more operative space;
however, doing so can increase surgical damage and the risk of CSF
leakage.
Following tumor removal, an effective exit strategy
is essential to avoid a postoperative CSF leak and its related
complications (52,53). A vascularized nasoseptal flap and
preoperative lumbar drainage is recommended to prevent CSF leakage
(54,55). However, in the present case,
preoperative lumbar drainage or an intraoperative nasoseptal flap
were not prepared. The right middle turbinate was suspended during
the operation, and the area covering the expanded maxillary sinus
opening was resettled and the anatomical structure of the nasal
cavity was reconstructed. Benefiting from the complicated dural
structures, dissection of TS originating from the Gasserian
ganglion and their roots would not increase the risk of CSF
leakage. It has been reported that Meckel's cave is an evaginated
diverticulum of the extension of the posterior fossa dura, with
intricate relationships with the surrounding dural layers; however,
the architectural relationship among the wall of Meckel's cave,
Gasserian ganglion and roots of trigeminal nerves is still
uncertain (56). It was inferred
that resection of lesions limited to Meckel's cave through
endonasal approaches would not lead to CSF leakage because the
reflexed dura remained undamaged and the split would be covered by
multiple layers of membrane structure; however, this requires
further studies for verification. Notably, for tumors in a complex
and deep position, cooperation with the ear-nose-throat surgical
team is necessary when endonasal endoscopic surgery is performed
(38). Although endonasal
endoscopic surgeries are a mature technology, widely accepted and
used, and have a series of operative standard in neurosurgery,
otorhinolaryngologists are more familiar with the anatomical
features, have proficient operative skills and rich experience in
dealing with extracranial lesions and reconstruction of nasal
cavities, which may improve the prognosis and self-perception of
patients (57,58).
In conclusion, in the present report, a rare
application of the transnasal maxillary sinus approach in the
exposure and excision of TSs located in the middle cranial fossa
was promoted. Compared with the conventional approach, the
transnasal maxillary sinus approach is minimally invasive, provides
a more intuitive and shorter route, and has excellent surgical
vision and a safe operative space, which is worthy of further
exploration and clinical application.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CX designed the study and drafted the manuscript.
PW, JWW and WJF collected and analyzed the clinical data. NW
performed the surgery, designed the study, critically revised the
manuscript and contributed to the important intellectual content.
All authors confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were performed in in accordance with
the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. The patient provided written informed
consent to publish these features of his case, and the identity of
the patient has been protected.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this manuscript and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gering K, Marx J, Lennartz K, Fischer C,
Rajewsky M and Kindler-Röhrborn A: The interaction mode of
premalignant schwann and immune effector cells during chemically
induced carcinogenesis in the rat peripheral nervous system is
strongly influenced by genetic background. Cancer Res.
66:4708–4714. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Park H, Hong SD, Kim YH, Hong CK, Woo KI,
Yun IS and Kong DS: Endoscopic transorbital and endonasal approach
for trigeminal schwannomas: A retrospective multicenter analysis
(KOSEN-005). J Neurosurg. 133:467–476. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li M, Wang X, Chen G, Liang J, Guo H, Song
G and Bao Y: Trigeminal schwannoma: A single-center experience with
43 cases and review of literature. Br J Neurosurg. 35:49–56.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Serhrouchni KI, Chbani L, Hammas N, Kamal
D, Fatemi HE, Harmouch T, Alami NEE and Amarti A: Two rare
schwannomas of head and neck. Diagn Pathol. 9(27)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oberman D, de Almeida GC, Guasti AA and
Correa JLA: Endoscopic endonasal resection of schwannoma of
pterygopalatine fossa. World Neurosurg. 141(251)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang X, Bao Y, Chen G, Guo H, Li M, Liang
J, Bai X and Ling F: Trigeminal schwannomas in middle fossa could
breach into subdural space: Report of 4 cases and review of
literature. World Neurosurg. 127:e534–e541. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Samii M, Alimohamadi M and Gerganov V:
Endoscope-assisted retrosigmoid intradural suprameatal approach for
surgical treatment of trigeminal schwannomas. Neurosurgery.
10:565–575; discussion 575. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shi J, Chen J, Chen T, Xu X, Jia Z, Ni L,
Zhang Y and Shi W: Neuroendoscopic resection of trigeminal
schwannoma in the pterygopalatine/infratemporal fossa via the
transnasal perpendicular plate palatine bone or transnasal
maxillary sinus approach. World Neurosurg. 120:e1011–e1016.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zoli M, Ratti S, Guaraldi F, Milanese L,
Pasquini E, Frank G, Billi AM, Manzoli L, Cocco L and Mazzatenta D:
Endoscopic endonasal approach to primitive Meckel's cave tumors: A
clinical series. Acta Neurochir (Wien). 160:2349–2361.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kassam AB, Prevedello DM, Carrau RL,
Snyderman Ch, Gardner P, Osawa S, Seker A and Rhoton AL Jr: The
front door to meckel's cave: An anteromedial corridor via expanded
endoscopic endonasal approach- technical considerations and
clinical series. Neurosurgery. 64 (Suppl 3):ons71–82.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Prevedello D, Pinheiro-Neto C,
Fernandez-Miranda J, Carrau RL, Snyderman CH, Gardner PA and Kassam
AB: Vidian nerve transposition for endoscopic endonasal middle
fossa approaches. Neurosurgery. 67:478–484. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qiuhang Z, Hongchuan G, Feng K, Ge CG,
Jiantao L, Mingchu L, Yuhai B and Feng L: Resection of the
intracavernous sinus tumors using a purely endoscopic endonasal
approach. J Craniofac Surg. 25:295–302. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Battaglia P, Turri-Zanoni M, Dallan I,
Gallo S, Sica E, Padoan G and Castelnuovo P: Endoscopic endonasal
transpterygoid transmaxillary approach to the infratemporal and
upper parapharyngeal tumors. Otolaryngol Head Neck Surg.
150:696–702. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Raza SM, Donaldson AM, Mehta A, Tsiouris
AJ, Anand VK and Schwartz TH: Surgical management of trigeminal
schwannomas: Defining the role for endoscopic endonasal approaches.
Neurosurg Focus. 37(E17)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jacquesson T, Berhouma M, Picart T and
Jouanneau E: Total removal of a trigeminal schwannoma via the
expanded endoscopic endonasal approach. Technical note. Acta
Neurochir (Wien). 57:935–938; discussion 938. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Plzák J, Kratochvil V, Kešner A, Šurda P,
Vlasák A and Zvěřina E: Endoscopic endonasal approach for mass
resection of the pterygopalatine fossa. Clinics (Sao Paulo).
72:554–561. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang L, Hu L, Zhao W, Zhang H, Liu Q and
Wang D: Endoscopic endonasal approach for trigeminal schwannomas:
Our experience of 39 patients in 10 years. Eur Arch
Otorhinolaryngol. 275:735–741. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jeon C, Hong SD, Woo KI, Seol HJ, Nam DH,
Lee JII and Kong DS: Use of endoscopic transorbital and endonasal
approaches for 360˚ circumferential access to orbital tumors. J
Neurosurg. 25:1–10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hardesty DA, Montaser AS, Carrau RL and
Prevedello DM: Limits of endoscopic endonasal transpterygoid
approach to cavernous sinus and Meckel's cave. J Neurosurg Sci.
62:332–338. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Almomen A, Alyousif A, Ali Z, Yaeesh IA,
AlOmirin A, Yahya AA, AlSuqair H and Almolani F: Image-guided
endonasal endoscopic excision of Meckel's cave trigeminal
schwannoma from cavernous and petrous carotid artery. J Surg Case
Rep. 30(rjaa374)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Di Somma A, Langdon C, de Notaris M, Reyes
L, Ortiz-Perez S, Alobid I and Enseñat J: Combined and simultaneous
endoscopic endonasal and transorbital surgery for a Meckel's cave
schwannoma: Technical nuances of a mini-invasive, multiportal
approach. J Neurosurg. 134:1836–1845. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu X, Xie SH, Tang B, Yang L, Xiao LM,
Ding H, Bao YY, Tong ZG and Hong T: Single-stage endoscopic
endonasal approach for the complete removal of trigeminal
schwannomas occupying both the middle and posterior fossae.
Neurosurg Rev. 44:607–616. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Amin SM, Fathy H, Hussein A, Kamel M,
Hegazy A and Fathy M: Endoscopic endonasal approach to the lateral
wall of the cavernous sinus: A cadaveric feasibility study. Ann
Otol Rhinol Laryngol. 127:903–911. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hofstetter CP, Singh A, Anand VK, Kacker A
and Schwartz TH: The endoscopic, endonasal, transmaxillary
transpterygoid approach to the pterygopalatine fossa, infratemporal
fossa, petrous apex, and the Meckel cave. J Neurosurg. 113:967–974.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Reshef ER, Bleier BS and Freitag SK: The
endoscopic transnasal approach to orbital tumors: A review. Semin
Ophthalmol. 36:232–240. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lobo B, Zhang X, Barkhoudarian G,
Griffiths CF and Kelly DF: Endonasal endoscopic management of
parasellar and cavernous sinus meningiomas. Neurosurg Clin N Am.
26:389–401. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jouanneau E, Simon E, Jacquesson T, Sindou
M, Tringali S, Messerer M and Berhouma M: The endoscopic endonasal
approach to the meckel's cave tumors: Surgical technique and
indications. World Neurosurg. 82 (Suppl 6):S155–S161.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Verillaud B, Bresson D, Sauvaget E,
Mandonnet E, Georges B, Kania R and Herman P: Endoscopic endonasal
skull base surgery. Eur Ann Otorhinolaryngol Head Neck Dis.
129:190–196. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cavallo LM, Messina A, Gardner P, Esposito
F, Kassam AB, Cappabianca P, de Divitiis E and Tschabitscher M:
Extended endoscopic endonasal approach to the pterygopalatine
fossa: Anatomical study and clinical considerations. Neurosurg
Focus. 19(E5)2005.PubMed/NCBI
|
|
30
|
Alfieri A, Jho HD, Schettino R and
Tschabitscher M: Endoscopic endonasal approach to the
pterygopalatine fossa: Anatomic study. Neurosurgery. 52:374–378;
discussion 378-380. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kasemsiri P, Carrau RL, Filho LF,
Prevedello DM, Otto BA, Old M, de Lara D and Kassam AB: Advantages
and limitations of endoscopic endonasal approaches to the skull
base. World Neurosurg. 82 (Suppl 6):S12–S21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Oakley GM and Harvey RJ: Endoscopic
resection of pterygopalatine fossa and infratemporal fossa
malignancies. Otolaryngol Clin North Am. 50:301–313.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kashiwazaki R, Turner MT, Geltzeiler M,
Fernandez-Miranda JC, Gardner PA, Snyderman CH and Wang EW: The
endoscopic endonasal approach for sinonasal and nasopharyngeal
adenoid cystic carcinoma. Laryngoscope. 130:1414–1421.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ramjee VG, Massoth LJ, Richards JP II and
McKinney KA: Endoscopic trans-pterygoid resection of a low-grade
cribriform cystadenocarcinoma of the infratemporal fossa. World J
Otorhinolaryngol Head Neck Surg. 6:115–117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Salmasi V, Reh DD, Blitz AM, Argani P,
Ishii M and Gallia GL: Expanded endonasal endoscopic approach for
resection of a skull base low-grade smooth muscle neoplasm. Childs
Nerv Syst. 28:151–158. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Battaglia P, Lambertoni A and Castelnuovo
P: Transnasal endoscopic surgery: Surgical techniques and
complications. Adv Otorhinolaryngol. 84:46–55. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Castelnuovo P, Turri-Zanoni M, Battaglia
P, Antognoni P, Bossi P and Locatelli D: Sinonasal malignancies of
anterior skull base: Histology-driven treatment strategies.
Otolaryngol Clin North Am. 49:183–200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Martinez-Perez R, Requena LC, Carrau RL
and Prevedello DM: Modern endoscopic skull base neurosurgery. J
Neurooncol. 151:461–475. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu Q, Wang H, Zhao W, Song X, Sun X, Yu
H, Wang D, Fernandez-Miranda JC and Snyderman CH: Endoscopic
transnasal transmaxillary approach to the upper parapharyngeal
space and the skull base. Eur Arch Otorhinolaryngol. 277:801–807.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Loyo-Varela M, del Valle Robles R, Herrada
T and Coll JB: Endoscopic endonasal transmaxillary approach. World
Neurosurg. 80:502–504. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Selcuk A, Ozcan KM, Akdogan O, Bilal N and
Dere H: Variations of maxillary sinus and accompanying anatomical
and pathological structures. J Craniofac Surg. 19:159–164.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Costa FD, Dias TM, Lombardo KA,
Raghunathan A, Giannini C, Kenyon L, Saad AG, Gokden M, Burger PC,
Montgomery EA and Rodriguez FJ: Intracranial cellular schwannomas:
A clinicopathological study of 20 cases. Histopathology.
76:275–282. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Perez MT, Farkas J, Padron S, Changus JE
and Webster EL: Intrasellar and parasellar cellular schwannoma. Ann
Diagn Pathol. 8:142–150. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hart J, Gardner JM, Edgar M and Weiss SW:
Epithelioid schwannomas: An analysis of 58 cases including Atypical
variants. Am J Surg Pathol. 40:704–713. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pekmezci M, Reuss DE, Hirbe AC, Dahiya S,
Gutmann DH, von Deimling A, Horvai AE and Perry A: Morphologic and
immunohistochemical features of malignant peripheral nerve sheath
tumors and cellular schwannomas. Mod Pathol. 28:187–200.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Campero A, Baldoncini M, Román G and
Villalonga JF: Double-stage complete removal of dumbbell-shaped
trigeminal schwannoma: 3-dimensional operative video. Oper
Neurosurg (Hagerstown). 21(E51)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Karamchandani JR, Nielsen TO, van de Rijn
M and West RB: Sox10 and S100 in the diagnosis of soft-tissue
neoplasms. Appl Immunohistochem Mol Morphol. 20:445–450.
2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Daniels DL, Rauschning W, Lovas J,
Williams AL and Haughton VM: Pterygopalatine fossa: Computed
tomographic studies. Radiology. 149:511–516. 1983.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kasemsiri P, Solares C, Carrau R, Prosser
JD, Prevedello DM, Otto BA, Old M and Kassam AB: Endoscopic
endonasal transpterygoid approaches: Anatomical landmarks for
planning the surgical corridor. Laryngoscope. 123:811–815.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Osawa S, Rhoton AL Jr, Seker A, Shimizu S,
Fujii K and Kassam AB: Microsurgical and endoscopic anatomy of the
vidian canal. Neurosurgery. 64 (5 Suppl 2):S385–S411; discussion
411-382. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang EW, Gardner PA, Fraser S, Stefko ST,
Fernandez-Miranda JC and Snyderman CH: Reduced tearing with stable
quality of life after vidian neurectomy: A prospective controlled
trial. Laryngoscope. 131:1487–1491. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hardesty D, Montaser A, Kreatsoulas D,
Shah VS, VanKoevering KK, Otto BA, Carrau RL and Prevedello DM:
Complications after 1002 endoscopic endonasal approach procedures
at a single center: Lessons learned, 2010-2018. J Neurosurg.
136:393–404. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fraser S, Gardner P, Koutourousiou M,
Kubik M, Fernandez-Miranda JC, Snyderman CH and Wang EW: Risk
factors associated with postoperative cerebrospinal fluid leak
after endoscopic endonasal skull base surgery. J Neurosurg.
128:1066–1071. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Deconde A, Vira D, Thompson C, Wang M,
Bergsneider M and Suh J: Radiologic assessment of the paranasal
sinuses after endoscopic skull base surgery. J Neurol Surg Part B
Skull Base. 74:351–357. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zwagerman N, Wang E, Shin S, Chang YF,
Fernandez-Miranda JC, Snyderman CH and Gardner PA: Does lumbar
drainage reduce postoperative cerebrospinal fluid leak after
endoscopic endonasal skull base surgery? A prospective, randomized
controlled trial. J Neurosurg. 1:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bond J, Xu Z, Zhang H and Zhang M:
Meckel's cave and somatotopy of the trigeminal ganglion. World
Neurosurg. 148:178–187. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lofrese G, Vigo V, Rigante M, Grieco DL,
Maresca M, Anile C, Mangiola A and De Bonis P: Learning curve of
endoscopic pituitary surgery: Experience of a neurosurgery/ENT
collaboration. J Clin Neurosci. 47:299–303. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Noh Y, Choi JE, Lee KE, Kong DS, Nam DH,
Jung YG, Kim HY, Chung SK and Hong SD: A comparison of olfactory
and sinonasal outcomes in endoscopic pituitary surgery performed by
a single neurosurgeon or a collaborative team of surgeons. Clin Exp
Otorhinolaryngol. 13:261–267. 2020.PubMed/NCBI View Article : Google Scholar
|