Introduction
Only a few types of chemotherapeutic agents are
currently approved for the treatment of advanced/recurrent
endometrial cancer and their efficacy is limited (1,2). The
anti-programmed death 1 (PD-1) monoclonal antibody, pembrolizumab,
recently became available for solid tumors with high-frequency
microsatellite instability (MSI-High). MSI-High is observed in
approximately 16% of patients with endometrial cancer (3,4) and
its response rate to pembrolizumab is approximately 57%, which is
higher than that in patients with other carcinomas (5); therefore, this agent is utilized as a
new treatment option for advanced/recurrent endometrial cancer.
Previous studies reported that the antitumor effects
of immune checkpoint inhibitors (ICIs), including pembrolizumab,
persisted even after the completion of treatment for approximately
2 years, demonstrating a durable response in patients with
malignant melanoma or lung cancer (6,7). A
durable response was also noted in patients in whom administration
was discontinued at an early stage due to adverse events in renal
pelvic cancer, pancreatic cancer, and intrahepatic
cholangiocarcinoma (8-10).
However, a similar case report has not yet been published in the
malignant gynecological tumor field.
We herein describe a patient with advanced
endometrial cancer in whom the administration of pembrolizumab was
discontinued due to renal dysfunction and its antitumor effects
persisted for more than 18 months. Notably, difficulties are
associated with selecting an appropriate duration of administration
of ICIs. The present study also discussed the optimal
administration period of ICIs.
Case report
A 79-year-old woman (gravida 3, para 3) was referred
to Jichi Medical University Hospital (Shimotsuke, Japan) with
massive vaginal bleeding in May 2019. She had a history of cerebral
infarction, dementia, interstitial pneumonia, hypertension,
hyperlipidemia, and hyperuricemia.
Colposcopy showed a fragile hemorrhagic mass exposed
from the uterine ostium. Irregular thickening of the endometrium
was detected with transvaginal ultrasonography. Endometrial biopsy
led to a diagnosis of Grade 3 endometrioid carcinoma. Hematology
showed anemia (Hb: 9.0 g/dl) and an elevated carcinoembryonic
antigen (CEA) level of 9.2 ng/dl. Carbohydrate antigen 19-9
(CA19-9) and CA-125 levels were within normal ranges. Pelvic
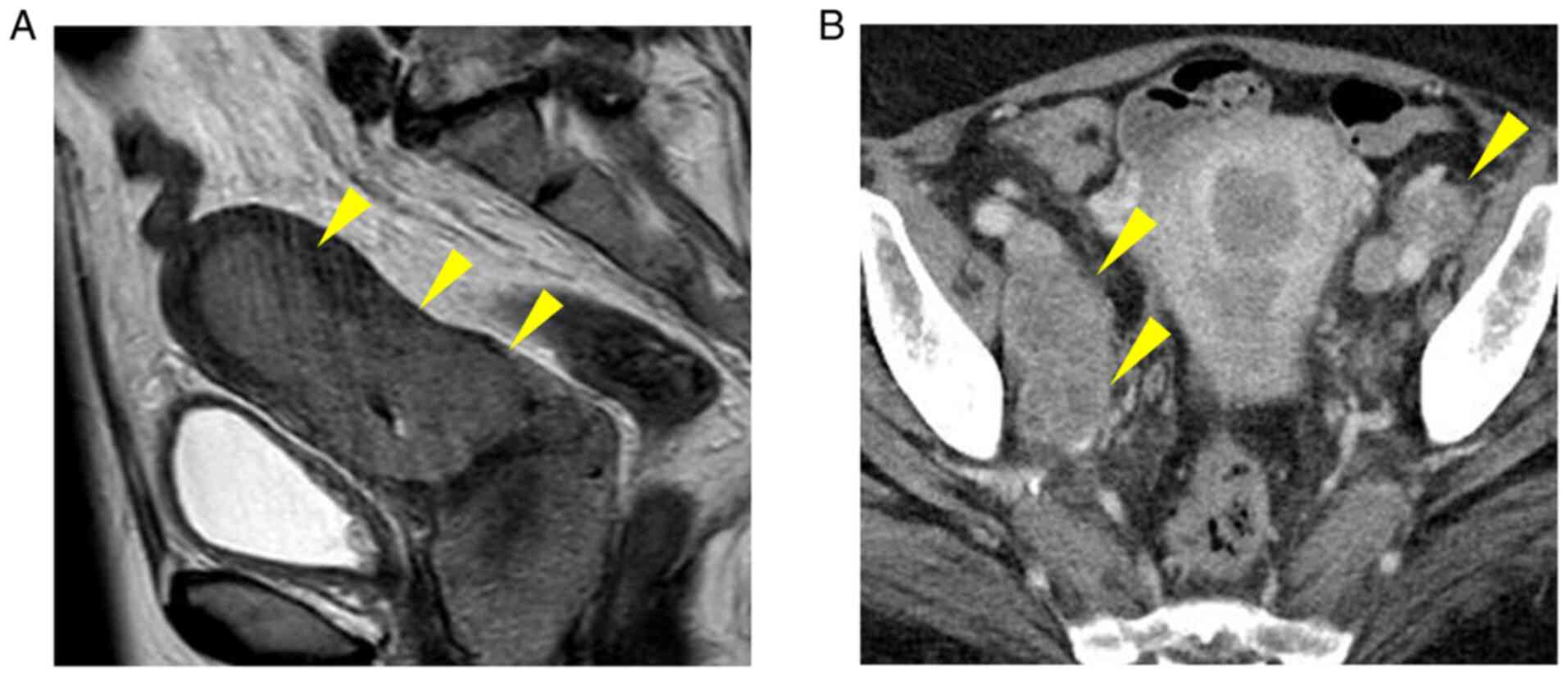
magnetic resonance imaging revealed a mass involving the uterine
body to cervix (Fig. 1A). Multiple
intrapelvic and para-aortic lymph node metastases were observed on
abdominal computed tomography (Fig.
1B). Emergency surgery (abdominal total hysterectomy, bilateral
salpingo-oophorectomy, and pelvic lymph node biopsy) was performed
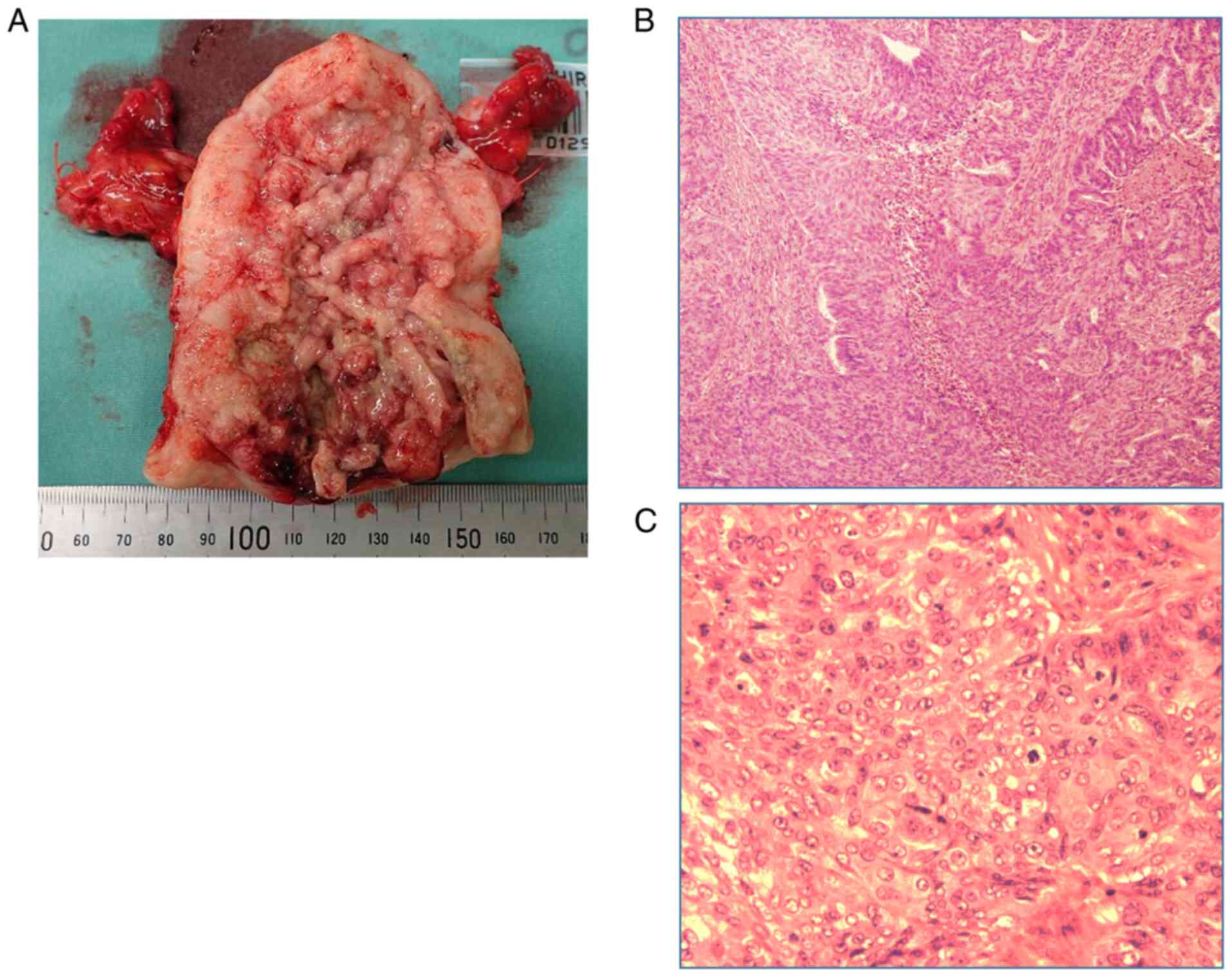
for hemorrhage control. A postoperative histopathological
examination suggested Grade 3 endometrioid carcinoma (Fig. 2A-C). According to the International
Federation of Gynecology and Obstetrics staging system, the stage
was evaluated as IIIC2 (pT2N2M0).
The TC regimen (paclitaxel, 175 mg/m2;
carboplatin, AUC6 at 3-week intervals) was introduced as
postoperative adjuvant chemotherapy. However, paclitaxel induced
anaphylactic shock and, thus, its administration was discontinued.
Three courses of the AP regimen (doxorubicin, 60 mg/m2;
cisplatin, 50 mg/m2 at 3-week intervals) were
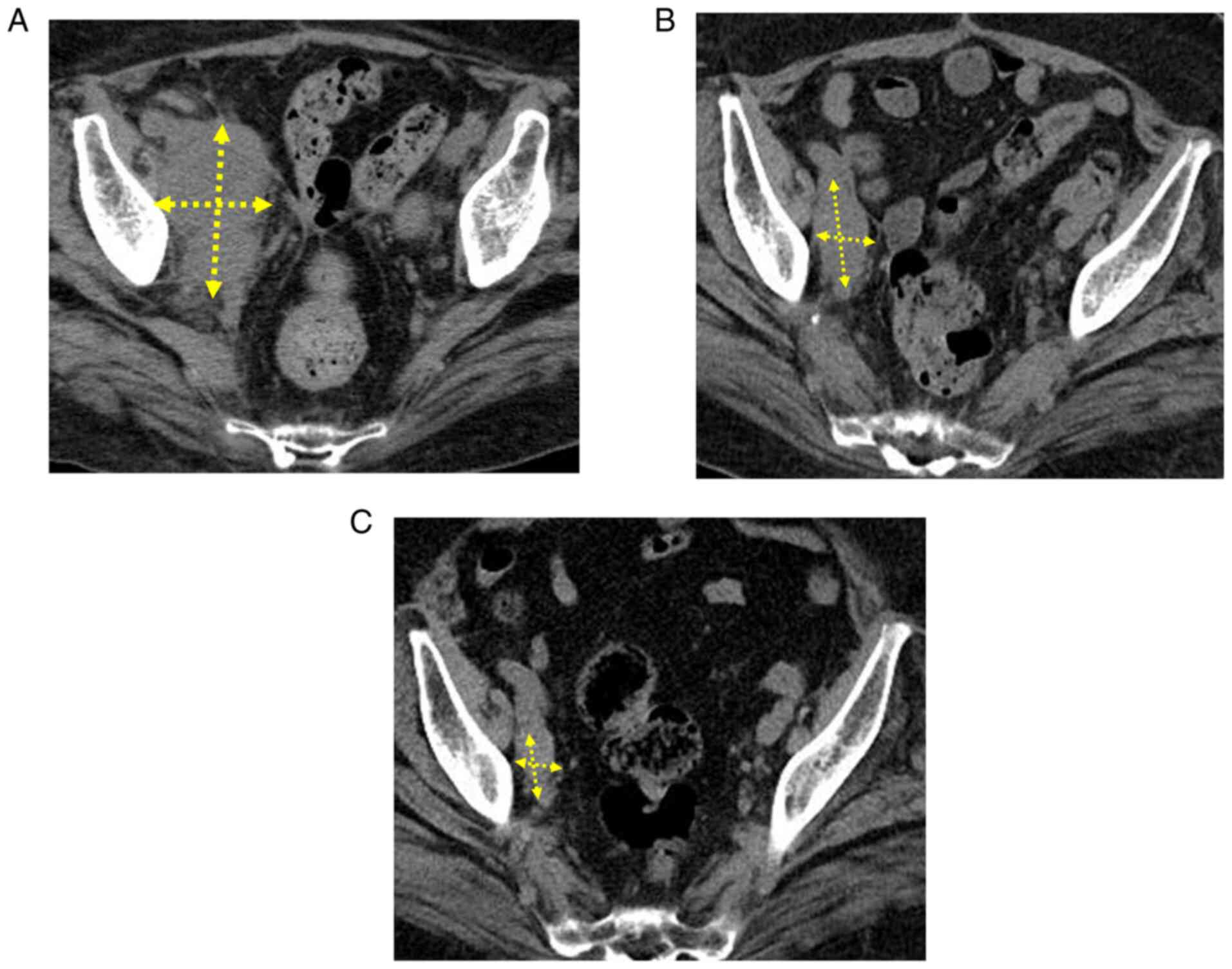
subsequently administered; however, the enlargement of multiple
metastatic lymph node foci (Fig.
3A) and an increase in the level of CEA were noted.
Furthermore, cisplatin-related renal dysfunction occurred. After
confirming MSI-High in the tumor tissue, pembrolizumab monotherapy
(200 mg/body at 3-week intervals) was initiated. Marked reductions
were observed in the size of enlarged metastatic lymph node foci
(Fig. 3B) and the level of CEA
decreased to the reference range. However, Common Terminology
Criteria for Adverse Events grade 3 renal dysfunction was
protracted and, thus, pembrolizumab therapy was discontinued after
the completion of the 7th course. After its discontinuation,
metastatic lymph node foci continued to decrease in size and the
level of CEA remained within the reference range. During the
18-month follow-up, a durable response was achieved (Fig. 3C). Since renal dysfunction remains
protracted, the patient is being followed up without the resumption
of pembrolizumab therapy in consideration of her age and
comorbidities.
Discussion
We herein report a patient with advanced endometrial
cancer in whom pembrolizumab therapy was discontinued due to renal
dysfunction; nevertheless, a durable response was achieved over a
long period.
ICIs, including pembrolizumab, inhibit
PD-1/programmed death ligand 1 (PD-L1) and exert antitumor effects
through T-cell activation; therefore, immune-related adverse events
(irAEs), such as dysthyroidism, which are not observed after the
administration of conventional cytotoxic anticancer drugs, need to
be considered. The administration of pembrolizumab was previously
discontinued at an early stage due to adrenal insufficiency in a
patient with renal pelvic cancer (8), diabetic ketoacidosis in a patient
with pancreatic cancer (9), and
bullous pemphigoid in a patient with intrahepatic
cholangiocarcinoma (10); however,
its antitumor effects persisted. To the best of our knowledge, a
similar case report has not yet been published in the malignant
gynecological tumor field. In these patients, the antitumor immune
activity of pembrolizumab may have persisted for a long period even
after its discontinuation; therefore, the onset of irAEs may be
paradoxically a clinical predictive biomarker of a durable
response.
The long-term administration of ICIs is possible in
some patients with the maintenance of antitumor effects. On the
other hand, a durable response is achieved in other patients even
though the administration of ICIs is discontinued at an early stage
due to adverse events. Therefore, difficulties are associated with
the selection of an appropriate duration of administration. The
tumor mutation burden, PD-L1 expression, mismatch-repairing
function deficiency, and MSI status have been proposed as
predictive biomarkers of ICIs (11-14).
However, a predictive biomarker with which the optimal
administration period of ICIs may be selected has yet to be
identified. In a cohort study on patients with malignant melanoma,
there was no relapse during a long-term follow-up (≥2 years) in
approximately 90% of patients who achieved a complete response (CR)
(6). Therefore, the achievement of
CR may be a clinical predictive biomarker for the completion of
administration. A durable response after the early completion of
ICIs in patients achieving CR or a partial response (PR) is
currently being examined in the Safe Stop study on patients with
malignant melanoma (15). The
findings obtained will contribute to the establishment of an
optimal administration period for ICIs.
The combination of pembrolizumab and the oral
multikinase inhibitor lenvatinib was recently approved for clinical
use (16). This combination has
achieved significantly longer progression-free survival and overall
survival than conventional chemotherapy among patients with
advanced endometrial cancer, regardless of the tumor MSI status. We
intend to use this combination therapy for similar patients in the
near future. In addition, it would be interesting to see the
expression level of several molecules such as PD-L1 and CD39;
however, we were unable to examine it because of the limited access
to preexisting materials of this patient. Due to specific adverse
reactions to ICIs and cost-effectiveness, further studies are
needed to search for a biomarker that facilitates high-accuracy
effect predictions and to establish an optimal administration
period.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SU, TK, AT and HF diagnosed, investigated and
managed the patient. SU, TK and HF determined the medical
significance of this case and wrote the manuscript. KT, YoT, TY,
ST, YS and YuT provided advice for managing the patient's treatment
and contributed to the acquisition of patient data. All authors
have read and approved the final version of this manuscript. SU and
TK confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was received from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamagami W, Mikami M, Nagase S, Tabata T,
Kobayashi Y, Kaneuchi M, Kobayashi H, Yamada H, Hasegawa K,
Fujiwara H, et al: Japan Society of Gynecologic Oncology 2018
guidelines for treatment of uterine body neoplasms. J Gynecol
Oncol. 31(e18)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brooks RA, Fleming GF, Lastra RR, Lee NK,
Moroney JW, Son CH, Tatebe K and Veneris JL: Current
recommendations and recent progress in endometrial cancer. CA
Cancer J Clin. 69:258–279. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Akagi K, Oki E, Taniguchi H, Nakatani K,
Aoki D, Kuwata T and Yoshino T: Real-world data on microsatellite
instability status in various unresectable or metastatic solid
tumors. Cancer Sci. 112:1105–1113. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Robert C, Ribas A, Hamid O, Daud A,
Wolchok JD, Joshua AM, Hwu WJ, Weber JS, Gangadhar TC, Joseph RW,
et al: Durable complete response after discontinuation of
pembrolizumab in patients with metastatic melanoma. J Clin Oncol.
36:1668–1674. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 study. J Clin Oncol. 37:2518–2527.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nagai T, Mogami T, Takeda T, Tomiyama N
and Yasui T: A case of secondary adrenocortical insufficiency due
to isolated adrenocorticotropic hormone deficiency with empty sella
syndrome after pembrolizumab treatment in a patient with metastatic
renal pelvic cancer. Urol Case Rep. 39(101766)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tsang ES, Walker EJ, Carnevale J, Fisher
GA and Ko AH: Durable response after immune checkpoint
inhibitor-related diabetes in mismatch repair-deficient pancreatic
cancer. Immunotherapy. 13:1249–1254. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eguchi S, Shinkawa H, Sato Y, Nakai K,
Takemura S, Tanaka S, Amano R, Kimura K, Ohira G, Nishio K, et al:
Durable response after discontinuation of pembrolizumab therapy for
intrahepatic cholangiocarcinoma: A case report. Clin J
Gastroenterol. 14:858–865. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Viale G, Trapani D and Curigliano G:
Mismatch repair deficiency as a predictive biomarker for
immunotherapy efficacy. Biomed Res Int.
2017(4719194)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Maleki Vareki S, Garrigós C and Duran I:
Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol
Hematol. 116:116–124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chalmers ZR, Connelly CF, Fabrizio D, Gay
L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J,
et al: Analysis of 100,000 human cancer genomes reveals the
landscape of tumor mutational burden. Genome Med.
9(34)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Duffy MJ and Crown J: Biomarkers for
predicting response to immunotherapy with immune checkpoint
inhibitors in cancer patients. Clin Chem. 65:1228–1238.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mulder EE, de Joode K, Litière S, Ten Tije
AJ, Suijkerbuijk KP, Boers-Sonderen MJ, Hospers GAP, de Groot JW,
van den Eertwegh AJ, Aarts MJ, et al: Early discontinuation of PD-1
blockade upon achieving a complete or partial response in patients
with advanced melanoma: The multicentre prospective Safe Stop
trial. BMC Cancer. 21(323)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Makker V, Colombo N, Casado Herráez A,
Santin AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay
S, Ray-Coquard I, et al: Lenvatinib plus pembrolizumab for advanced
endometrial cancer. N Engl J Med. 386:437–448. 2022.PubMed/NCBI View Article : Google Scholar
|