Introduction
Lichen sclerosus (LS) is a chronic inflammatory skin
disease with a predilection for the anogenital area. The estimated
prevalence of the disease is 1:300-1:1,000 and it is primarily seen
in postmenopausal women, although men and children also can be
affected (1). In men, LS occurs
mainly between the ages of 30 to 50 years (2,3). LS
is presented clinically with hypopigmentated areas, petechiae, and
in males also with preputial and meatal constriction (4). In females, LS has been associated
with an increased risk of vulvar squamous cell carcinoma (SCC),
estimated at 2.6-6.7% (5). Also,
in males an increased rate of SCC has been shown with a prevalence
of 1-6% (3,4,6,7).
The diagnosis of LS is often based on the
aforementioned, typical clinical criteria. Dermoscopy can give
additional information and thereby assisting the diagnosis
(8). Nevertheless, in most
patients, a skin biopsy is required to confirm the diagnosis of LS
and, in some cases, to rule out SCC. The typical histological
features of LS are a thinned epidermis, a dermal hyaline sclerosis
and below this a band-like chronic inflammation (9). The genital LS can also lack epidermal
atrophy and in some cases show spongiosis. The use of invasive
biopsies in the genital area is not always uncomplicated. This
sensitive area has a dense network of blood vessels that can cause
bleeding and aesthetic problems, like scars, can be seen after a
skin biopsy. Furthermore, the skin biopsy is associated with
diagnosis delay and laboratory costs. Therefore, there is a need
for finding a fast, accurate, and non-invasive diagnostic procedure
for LS.
Non-invasive imaging techniques for medical
diagnostics have evolved over the last decade. Among optical
microscopy techniques, laser scanning microscopy has been
particularly promising because it allows for a three-dimensional,
non-invasive visualization of biological tissue with high
resolution (10,11).
Reflectance confocal microscopy (RCM) is a
well-established laser scanning microscopy technique for imaging
skin in vivo. RCM achieves contrast by utilizing the
inherent refractive index properties of various cellular
microstructures. Commercially available RCM devices create images
with a resolution comparable to histological examination (12). RCM is an emerging tool for skin
cancer diagnostics (13,14). Moreover, several studies have shown
that RCM can be used to diagnose psoriasiform and interface
dermatitis (15-17).
The epidermal layer of the prepuce and glans which is approximately
140 µm (18,19) could theoretically be appropriate
for investigation with RCM, which has an image depth of
approximately 150-200 µm, reaching the papillary dermis. There are
preliminary reports that RCM has been used as a complementary
diagnostic and monitoring tool for LS (20,21).
Nevertheless, these studies contain a small number of patients and
they lack comparison to the healthy skin. More studies are needed
to confirm existing data. Multiphoton microscopy (MPM) is a related
technology to RCM (22). However,
its translation into the clinics has so far not proceeded to the
same extent as RCM.
Thus, in this descriptive study we aimed to
investigate the potential for laser scanning microscopy, and RCM in
particular, as a diagnostic tool for LS in comparison to normal
penile skin and other penile inflammatory disorders. Furthermore,
we used MPM ex vivo in one skin biopsy from one LS patient
to compare the findings to those of the RCM. In addition to
assessing the diagnostic potential of the approach, the recruited
patients were asked to assess their experience of diagnostic
procedure experience.
Materials and methods
Participants in the study
All the participants signed an informed consent
form. The study was approved by the regional ethical review board
of Gothenburg (Dnr 415-17), and institutional rules for the
clinical investigation of human subjects were followed. The
inclusion criteria were ≥18 years of age, histopathologically
confirmed LS. As controls, clinically diagnosed nonspecific
balanoposthitis, plasma cell balanitis, psoriasis and healthy
individuals were included. The participants were not allowed to
apply any topical treatment on the genital area 14 days prior to
the inclusion in the study. Patients were recruited at the
Department of Dermatology and Venereology, at the Sahlgrenska
University Hospital, in Gothenburg, Sweden, from 2018 to 2020. In
total, 30 male patients were included of which 17 patients were
diagnosed with LS, five patients with nonspecific balanoposthitis,
three with plasma cell balanitis, one patient with psoriasis and
four were healthy individuals. The date of the obtained skin biopsy
confirming the LS diagnosis varied from eight years prior to the
inclusion up to the same day of the inclusion. Asymptomatic
patients visiting the clinic to exclude sexually transmitted
diseases, and patients evaluated for extragenital skin disorders,
were recruited as controls with healthy penile skin. All patients
were subject to RCM investigation, as described below. The prepuce
was investigated with RCM in all cases. All the participants were
asked if they had oral LS and all of them denied it. The oral
cavity was not examined since oral LS very rarely occurs in this
location. In addition, the patients diagnosed with LS answered a
questionnaire that contained inquiries related to LS, circumcision,
treatment, and experiences from the biopsy procedure.
Reflectance confocal laser scanning
microscopy
All patients were examined using an in vivo
RCM (VivaScope 1500™, MAVIG GmbH,), using an adopted protocol based
on an established clinical routine for dermatological
investigations. Oil was applied to an adhesive window attached to a
stainless-steel tissue ring. The window was placed onto the
affected or healthy penile skin. Ultrasound gel was applied to the
center of the adhesive window. Then, the laser tube of the RCM was
affixed to the tissue ring. To be oriented during imaging, a
dermoscopic image was obtained with the VivaCam, which is
incorporated into the VivaScope system. A standardized
image-capturing process was applied in each investigation. The
instrument was equipped with an 830 nm continuous wave laser and a
customized objective lens (P/N 04288, NA=0.9, Photon Gear),
resulting in an imaging resolution corresponding to ~1.8 µm lateral
and ~3 µm axial. Both Vivastacks and Vivablocks were acquired.
Vivastacks were obtained by performing a series of 70-80 images in
3 µm steps to a depth of approximately 200 µm. Vivablocks up to 8x8
mm were composed of sequential RCM images at 500x500 µm each. In
one LS patient, images were first acquired with RCM in vivo
and then a skin biopsy was obtained from the area investigated with
RCM. This biopsy was then complementary investigated using MPM
technology ex vivo (Data
S1).
Data analysis
The RCM images were acquired using the VivaScan
software and exported as TIFF interface using mD4 (MAVIG GmbH). The
acquired raw-Data images were subject to brightness and contrast
enhancement using Photoshop (Adobe Systems Inc.) before assessment.
All RCM images were evaluated by the same dermatologist (the first
author). The complementary H&E-stained slides of the LS and
plasma cell balanitis patients were evaluated by a pathologist
specializing in dermatopathology (the second author). Then, the
first and the second author together performed a more detailed
comparison of the findings from RCM and the correspondence with
histology, which accounts for the results presented.
Results
Patients and histopathology
In total, 30 males were included (17 with LS, 3 with
plasma cell balanitis, 5 with nonspecific balanoposthitis, 1 with
psoriasis and 4 healthy individuals). The clinical Data of the LS
patients are presented in Table
SI in the Data S1. All the LS
patients had clinically active lesions. The cases with LS and
plasma cell balanitis diagnosis were verified histopathologically.
Furthermore, one patient with LS was histopathologically verified
to have PeIN. In one patient with LS, an additional skin biopsy was
taken after the examination with RCM, which was examined ex
vivo with MPM. The MPM findings are presented in the Data S1.
All 17 biopsies from LS patient showed sclerosis
histologically. In 12/17 samples the sclerosis was obvious and in
5/17 samples the sclerosis was mild.
RCM findings
The morphological features observed from the RCM
investigation and the correlation with their histopathological
counterparts are summarized in Table
I.
 | Table IOverview of the features observed by
RCM and a comparison with their histopathological counterparts. |
Table I
Overview of the features observed by
RCM and a comparison with their histopathological counterparts.
| Histopathological
features | RCM features |
|---|
| Normal epidermal
architecture | Typical honeycomb
pattern |
| Parakeratosis | Parakeratosis |
|
Spongiosis/exocytosis | Exocytosis |
| Inflammatory cells
in the dermis | Bright cells in the
superficial dermis |
| Inflammatory cells
inside the vessels in the dermis | Bright cells
flowing inside the black lumen in the dermis |
| Irregular
papillae | Irregular
papillae |
| Normal papillary
architecture | Edged papillae |
| Sclerosis in
dermis | Prominent,
fiber-like structures in dermis |
A quantitative assessment of the characteristic RCM
features observed is presented in Table II, comparing the Data acquired
from the LS patients and the healthy group. The most significant
features observed in LS were prominent fiber structures in the
dermis, typical honeycomb pattern, irregular papillae and bright
cells in the superficial dermis, while edged papillae, typical
honeycomb pattern and irregular papillae were observed in all the
health individuals.
 | Table IIOverview and incidence of the
features observed by RCM in LS and healthy penile skin. |
Table II
Overview and incidence of the
features observed by RCM in LS and healthy penile skin.
| RCM features | LS (incidence) | Healthy penile skin
(incidence) |
|---|
| Typical honeycomb
pattern | 13/17 | 4/4 |
| Parakeratosis | 4/17 | 0/4 |
| Spongiosis | 9/17 | 0/4 |
| Bright cells in
basal layer | 1/17 | 0/4 |
| Bright cells in the
superficial dermis | 12/17 | 0/4 |
| Bright cells
flowing inside black lumen in the dermis | 4/17 | 0/4 |
| Irregular
papillae | 12/17 | 4/4 |
| Dilated
papillae | 12/17 | 0/4 |
| Edged papillae | 2/17 | 4/4 |
| Elongated
papillae | 5/17 | 0/4 |
| Prominent fiber
structures in dermis | 8/17 | 0/4 |
The prominent fiber structures corresponding to
hyaline sclerosis were observed in almost half (8/17) of the
patients with LS (Table II). This
feature is illustrated by Fig. 1.
The cases in which RCM did not show sclerosis represented only mild
sclerosis in histopathological examination. Interestingly, RCM
could identify prominent fiber structures in one case of LS with
mild sclerosis histopathologically. It should be noted that
prominent fiber structures were observed in one of the plasma cell
balanitis cases (Data S2,
Table SII); however, this
observation was done in a penile area where the patient had a scar
and therefore deemed unrelated to the plasma cell balanitis.
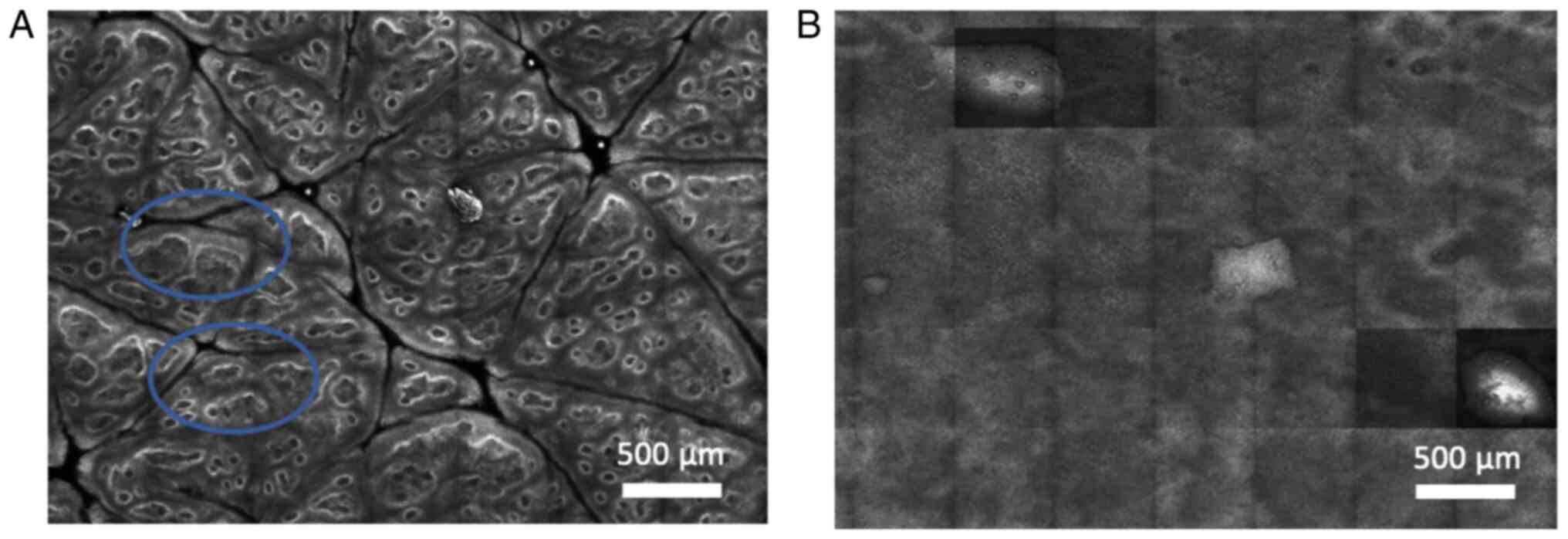
The typical normal tissue architecture of the
stratum spinosum observed as a honeycomb pattern in RCM (for
illustration, Data S1, Fig. S1) was found in almost all patients
including LS, in healthy individuals, nonspecific balanoposthitis,
and plasma cell balanitis. Interestingly, in the LS patient who had
histopathologically confirmed PeIN, the RCM investigation revealed
an atypical honeycomb pattern and scattered, small, bright cells in
the basal layer, probably corresponding to the cell dysplasia
(Data S1, Figs. S1E and S2).
When investigating the dermo-epidermal junction,
illustrated in Fig. 2, the healthy
penile skin revealed edged papillae, representing rims of bright
basal cells around the dermal papillae, corresponding to the normal
papillary architecture, whereas in LS the edged papillae were
absent or obscured.
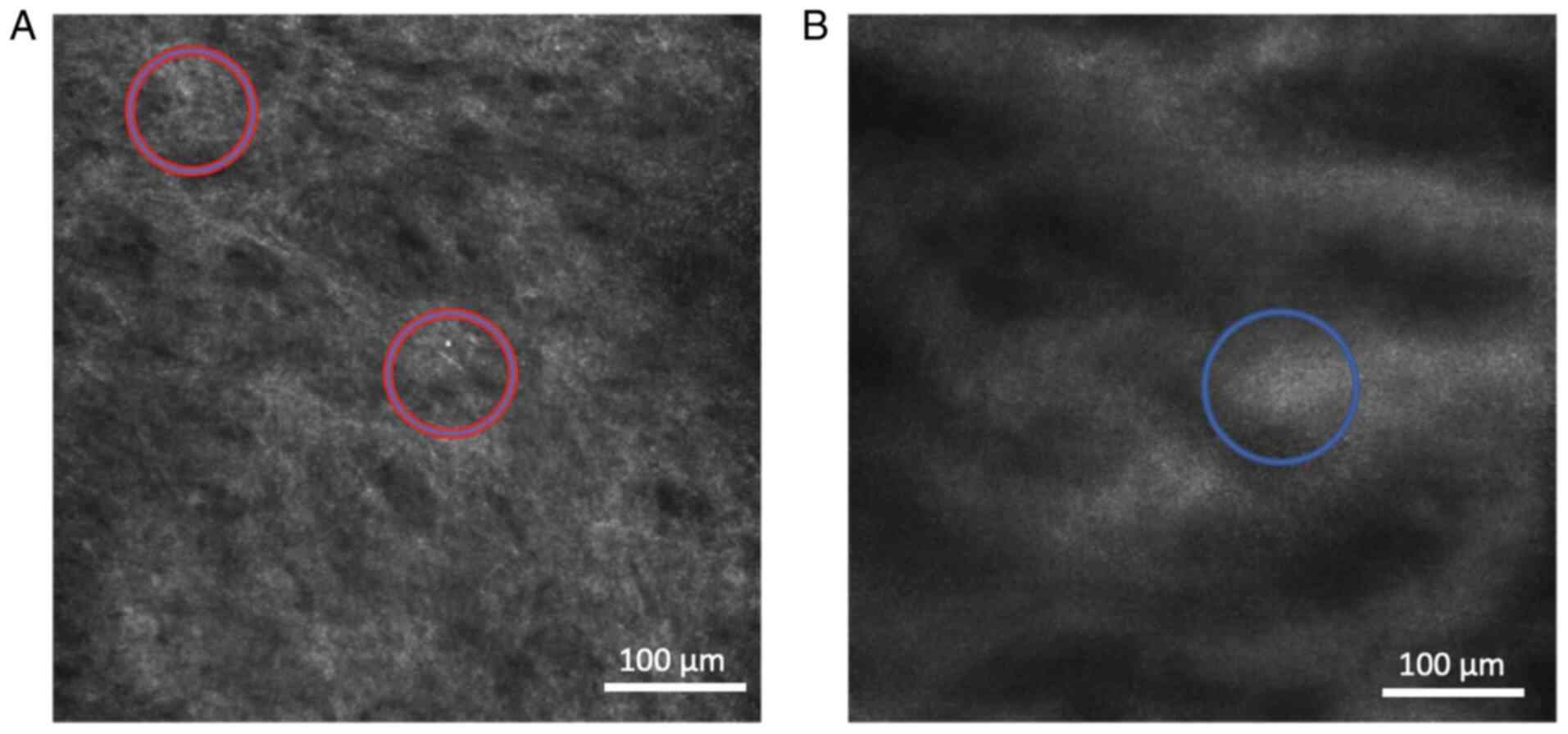
Another feature observed using RCM was dermal
inflammatory infiltrate observed as the presence of abundant bright
cells in the dermis (Data S1,
Fig. S3). This feature was
observed in a majority of LS cases (12/17, Table II), while it was not found in
healthy penile skin; however, it should be noted the feature was
common feature in nonspecific balanoposthitis patients, why its
diagnostic specificity for LS was low. In addition, RCM was also
able to visualize bright cells flowing inside linear, canalicular
structures in black lumen of the papillae in the dermis possibly
representing the dilated vessels in the papillae (Data S1, Fig. S4). This was a common feature in
patients with nonspecific balanoposthitis and in patients with
plasma cell balanitis (Data S2,
Table SII). Interestingly, this
feature was uncommon in LS patients (Table II).
Among patients with plasma cell balanitis the most
common feature was mildly refractive cells seen in the
intercellular spaces (exocytosis) between keratinocytes, associated
with spongiosis (Data S1,
Fig. S5). Irregularity of the
papillae in terms of their shape (Data S1, Fig. S6) was found in all groups,
including the one with healthy individuals. An overview of the
observed features in nonspecific balanoposthitis, plasma cell
balanitis and psoriasis are shown in Table SII in the Data S2.
Complementary to RCM imaging, a tissue biopsy
acquired from one of the patients was also investigated using MPM
ex vivo (Fig. S7, Data S1). This is, to the best of our
knowledge, the first time a case of LS has been investigated by
MPM. The underlying principles of MPM is based on non-linear
optical processes, makes it ideal to study collagen fibers.
Consistent with histopathology, MPM revealed bright collagen fibers
referring to sclerosis, but with greater contrast than the
corresponding RCM image. Since sclerosis is a prominent feature
signifying LS, this result implies that MPM would be an interesting
complementary technique to visualize this feature in LS.
Questionnaire
In order to assess the patients' experience of the
diagnostic procedure, the patients were asked to respond to a
simple questionnaire. All but one patient answered that in order to
receive a diagnosis, they preferred to be evaluated with RCM in
vivo instead of undergoing a skin biopsy. RCM was experienced
as painless, caused no discomfort and did not require local
anesthesia. A majority, i.e., 12/17 LS patients, experienced the
skin biopsy procedure as uncomfortable and unpleasant. The only
reported disadvantage with RCM was that the procedure was time
consuming (average time of investigation was ~30 min). This means
that RCM investigation was overall well appreciated by the
patients. According to the questionnaire, four LS patients had
undergone complete circumcision and two LS patients had undergone
partial circumcision before the examination with RCM.
Discussion
To date, RCM is a widely used technique in
dermatology as a diagnostic tool for both tumors and inflammatory
diseases (23,24). This study demonstrates the
potential of RCM in vivo to visualize the characteristic
histopathological features of LS. The acquired RCM images were
evaluated by a dermatologist and all the H&E-stained slides of
the LS and plasma cell balanitis patients were evaluated by a
pathologist specializing in dermatopathology. Complementary to
earlier studies on the topic (20,21),
this study includes an important comparison with images acquired
from healthy penile skin, nonspecific balanoposthitis and plasma
cell balanitis. In addition, the patient experience of the
procedure was assessed by a questionnaire. Most of the LS patients
described the skin biopsy procedure as unpleasant and preferred the
non-invasive and painless RCM examination, supporting the clinical
relevance of the study that there is a need of finding non-invasive
diagnostic tools for the genital area and that RCM could fulfill
this need.
Hyaline sclerosis is the key feature in the
histopathological diagnosis of LS. In this investigation, this
feature was observed as prominent, thick, fiber-like structures
using RCM, and was found in almost half of the LS patients. Our
results regarding sclerosis in LS patients was in line with other
reports on genital and extragenital LS investigated with RCM
(20,25). Similar, coarse, fiber-like
structures in the dermis were noticed in a patient with plasma cell
balanitis, however these represented a typical scar that the
patient had in the area affected by plasma cell balanitis.
The most common feature identified in LS patients by
RCM investigation was the typical honeycomb pattern along with the
dilated and irregular papillae and bright cells in the dermis.
These results agreed with those found by Lacarrubba et al
(20). The comparison of the
dermo-epidermal junction in the images obtained from LS and healthy
penile skin, revealed edged papillae in the latter group. However,
this feature was absent or obscured in the LS cases. The
irregularity of the papillae and absence of edged papillae or
non-rimmed papillae could indicate basal hydropic degeneration and
loss of the melanogenesis of the basal cells, which are
histological features found in LS. Reports support the fact that
tumor necrosis factor-α and interleukin 17 act synergistically in
inhibiting melanogenesis, thereby leading to the loss of melanin
around the papillae causing the loss of the rims in inflammatory
disorders such as psoriasis (26)
and it could also explain the melanin loss in LS, which can be seen
clinically as hypopigmentation. Moreover, the loss of edged
papillae can be explained in certain cases due to the atrophic
epidermis and the flattening of the junctional zone.
An inflammatory infiltrate in the dermis was
identified in the majority of LS patients. Interestingly, bright
cells flowing inside linear, canalicular structures in the black
lumen of the papillae in the dermis was found more often in
patients with nonspecific balanoposthitis than in LS. This feature
may correspond to the dilated vessels found more commonly in
balanitis than in LS. Nevertheless, neither the inflammatory
infiltrate nor the dilated vessels seen with RCM are pathognomonic
for nonspecific balanoposthitis or LS. Thus, these features cannot
be used as diagnostic criteria for these disorders.
The investigation of one of the LS patients revealed
an atypical honeycomb pattern in the epidermis and scattered round,
nucleated, bright cells in the basal layer. These features are
commonly found in squamous cell carcinoma in situ (20,27).
The histopathological analysis of a skin biopsy obtained from the
same area confirmed the diagnosis of PeIN. It is a common practice
that LS patients are followed up regularly for signs of penile
malignancy. To rule out cell dysplasia, skin biopsies are obtained
from the already sensitive penile skin affected by LS. RCM is a
diagnostic tool that can be used to evaluate nonmelanocytic skin
tumors (28). More specifically,
it has been used in order to differentiate between balanoposthitis
and squamous cell carcinoma in situ (29), as well as to improve the diagnosis
of oral carcinoma and its precursors (30). This study supports its use as a
noninvasive monitoring tool for LS in risk of penile cancer and as
a diagnostic tool for genital dysplasia.
In addition to RCM imaging, a tissue biopsy acquired
from one of the patients was also investigated using multiphoton
laser microscopy (MPM) ex vivo (Fig. S7, Data S1). This is, to the best of our
knowledge, the first time a case of LS has been investigated by
MPM. Complementary to RCM, MPM enables label-free imaging based on
two-photon excitation and non-linear optical scattering (also known
as second harmonic generation), making it ideal to study collagen
fibers. Consistent with histopathology, MPM imaging revealed bright
collagen fibers referring to sclerosis, but with greater contrast
than the corresponding RCM image. In vivo MPM microscope is
now commercially available, and it has been used to study skin
tumors (31), although the
technology has not yet been as clinically established as RCM. Based
on the preliminary result in this study, an in vivo handheld
MPM device could potentially be a more effective tool for
visualizing sclerosis in the dermis and should be subject to
further investigation in LS diagnostics.
The main limitation of this study is that sclerosis,
which is the main characteristic of LS, was not identified in all
LS patients examined by RCM. Several reasons account for this
drawback. In three cases where RCM failed to detect the sclerosis,
only mild sclerosis was seen histopathologically, thus making it
difficult to be observed using RCM. However, the RCM was able to
identify one case of mild sclerosis. Moreover, in some cases where
the RCM investigation was performed several months or years after
the skin biopsy was obtained, the patients received local treatment
with potent steroid cream (5/17); the latter could have altered the
typical histopathological features of LS and diminished the
sclerosis making it more difficult to observe. In these cases, the
histology is not directly comparable to the RCM findings. However,
the participants were not allowed to use topical treatment 14 days
prior to the RCM investigation. In addition, all the LS patients
had clinical signs of active disease. Another factor that could
have attributed to the absence of sclerosis features in RCM images
obtained from LS patients could be the limitation of RCM to reach a
skin depth of more than 150-200 µm. This limitation could be
overcome by scanning the tissue with optical coherence tomography
that has previously been used to study collagen fibers in LS and
other conditions with excessive collagen deposition (32). In this study we did not have access
to a hand-held RCM device, which could have simplified and hastened
the imaging process. The image assessment was performed in an
unblinded way that might have led to interpretation bias.
Nevertheless, the images were evaluated in a standardized way at
defined layers of the skin i.e., stratum corneum, stratum spinosum,
stratum basale and dermis. The investigation was time consuming,
and therefore it was not possible to examine all the affected area.
Evaluating the produced images was also time consuming, in average
three to four hours for every participant, making it difficult to
implement RCM as a diagnostic tool in everyday clinical practice
today. In the future focus should be given in the application of
machine learning-based image analysis on RCM and MPM Data to
provide more quantitative and objective results (33). In addition, the images obtained
using RCM present a horizontal view of the skin layers, thus making
it difficult to directly correlate them with the histopathological
images showing a vertical view of the skin. Furthermore, The RCM
counterparts to skin atrophy and follicular hyperkeratosis were not
evaluated in this study as they less commonly are present in
genital LS. Another drawback of this study is the small number of
healthy individuals included. Nevertheless, the RCM features
observed on healthy penile skin were consistent, enabling us to
clearly differentiate it from LS and nonspecific
balanoposthitis.
In summary, our study showed that RCM could
visualize the thick fiber-like structures corresponding to
sclerosis in the dermis, confirming the previously reported
findings on genital LS. In addition, we clearly showed the
differences between healthy penile skin and LS by identifying the
edged papillae on healthy skin and their absence or obscureness in
LS patients. Importantly, RCM revealed a precursor of penile cancer
in one LS patient.
In conclusion, RCM is a promising tool for
diagnosing LS in a non-invasive manner. It can help discriminate LS
from nonspecific balanoposthitis and plasma cell balanitis if
sclerosis is present. Moreover, RCM could be a valuable tool for
monitoring LS patients for dysplasia, reducing the number of
follow-up biopsies and thereby eliminating potential
complications.
Potentially, lasers scanning microscopy can become
an important, and by patients well tolerated, non-invasive tool to
detecting and mapping cell dysplasia, reducing the need for
obtaining multiple biopsies form the penile area, thereby
accelerating the treatment process by directly referring the
patient to urologists for surgery.
Supplementary Material
eMPM findings. In one LS
patient, images were first acquired with RCM in vivo and
then a skin biopsy was obtained from the area investigated with
RCM. This biopsy was then investigated with MPM ex vivo. An
LSM 710 NLO microscope system (Carl Zeiss MicroImaging GmbH,
Germany) was used for MPM imaging. The optical resolution was 0.5
μm lateral and 1.5 μm axial. An InSight x3 laser (Spectra-Physics,
Newport Corporation, USA) was used for excitation. The excitation
wavelength was set to 780 nm to target autofluorescence, and the
power setting was optimized to yield a similar fluorescence signal.
Fluorescence from the tissue was collected in the emission range of
410-690 nm using a non-descanned, highly sensitive GaAsP detector.
The fiber-like structures in the papillary dermis representing
hyaline sclerosis and the typical honeycomb pattern were visualized
with both techniques (Fig. S7).
Nevertheless, the fiber structures were brighter and sharper in the
MPM. Thus, the data from this patient support that hyaline
sclerosis can be visualized better with MPM ex vivo than
with RCM in vivo.
Reflectance confocal microscopy data
acquired from (A) 1 patient with LS, (B) 1 individual with healthy
penile skin, (C) 1 patient with nonspecific balanoposthitis, (D) 1
patient with plasma cell balanitis and (E) 1 patient with PeIN in
the level of stratum spinosum. A typical honeycomb pattern (blue
circles) was observed in (A) LS, (B) healthy penile skin, (C)
nonspecific balanoposthitis and (D) plasma cell balanitis, (E)
whereas an atypical honeycomb pattern (red circles) was seen in
PeIN. The typical honeycomb pattern corresponds to the normal
epidermal architecture, whereas the atypical honeycomb pattern
corresponds to the disarrayed epidermis architecture found in
squamous cell carcinoma in situ. Size of images, 0.5x0.5 mm.
Scale bar, 100 μm. LS, lichen sclerosus; PeIN, penile
intraepitelial neoplasia.
Reflectance confocal microscopy data
acquired from 1 patient with lichen sclerosus with simultaneous
penile intraepitelial neoplasia. Scattered, small, bright cells
(blue arrow) are seen in the stratum basale, which may correspond
to the cell atypia and hyperchromacy. Size of image, 0.5x0.5 mm.
Scale bar, 100 μm.
Reflectance confocal microscopy data
acquired at the level of the papillary dermis from (A) 1 patient
with LS, (B) 1 patient with nonspecific balanoposthitis and (C) 1
individual with healthy penile skin. As is illustrated by the
figure, bright areas (blue circles) corresponding to the dermal
inflammatory infiltrate were found in LS and nonspecific balanitis.
However, this feature was not seen in the healthy skin. Size of
images, 0.5x0.5 mm. Scale bar, 100 μm. LS, lichen sclerosus.
Reflectance confocal microscopy data
acquired from a patient with nonspecific balanoposthitis at the
level of stratum basale. Refractive cells were seen (red asterisks)
and bright cells flowing inside linear, canalicular structures in
the black lumen of the papillae (blue arrow). The refractive cells
represent inflammatory cells, and the linear structures represent
dilated vessels. Image size, 0.5x0.5 mm. Scale bar, 100 μm.
Reflectance confocal microscopy data
acquired from a patient with plasma cell balanitis taken at the
stratum spinosum layer. As can be seen, mildly refractive cells
were observed in the intercellular spaces (exocytosis) between
keratinocytes (blue arrows). This feature is associated with
spongiosis. Image size, 0.5x0.5 mm. Scale bar, 100 μm.
Reflectance confocal microscopy data
acquired at the level of the papillary dermis from (A) 1 patient
with lichen sclerosus, (B) 1 patient with balanoposthitis, (C) 1
patient with plasma cell balanitis and (D) 1 individual with
healthy penile skin. Irregular papillae in terms of their shape
(blue arrows) were observed in all images. Image size, 0.5x0.5 mm.
Scale bar, 100 μm.
(A) Reflectance confocal microscopy
data compared with (B) MPM Data acquired from the same area in 1
patient with lichen sclerosus at the level of the papillary dermis.
As can be seen, the fiber-like structures representing the
sclerosis were visualized with both microscopes (blue arrows).
However, they were brighter and sharper for MPM. Size of images,
0.5x0.5 mm. Scale bar, 100 μm. MPM, multiphoton microscopy.
Clinical data of patients with
LS.
Overview of the features observed by
RCM for nonspecific balanoposthitis, plasma cell balanitis and
psoriasis.
Acknowledgements
The authors would like to thank Marica Ericson,
Professor at the Biomedical Photonics Group, Department of
Chemistry and Molecular Biology, University of Gothenburg
(Gothenburg, Sweden) for contributing to writing of the manuscript
and her valuable input in the interpretation of the data.
Funding
Funding: The present study was financed by a grant from the
health care committee of the region Västra Götaland (grant no.
VGFOUREG-653511).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DK interviewed all the participants in order to be
included in the study, performed the microscopy imaging, obtained
the skin biopsies, analyzed and interpreted the data, and wrote the
manuscript. NN helped analyze and interpret the data, and
contributed to the writing of the manuscript. KM helped to find
patients with LS diagnosis to be included, interpreted the data and
commented on the manuscript. AMWL interpreted the data and
commented on the manuscript. PT contributed to analyzing and
interpreting the data, as well as in compiling and writing the
manuscript. DK and PT confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Regional Ethics Review
Board in Gothenburg (Dnr 415-17; Gothenburg, Sweden), and
institutional rules for the clinical investigation of human
subjects were followed. All participants provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wallace HJ: Lichen sclerosus et
atrophicus. Trans St Johns Hosp Dermatol Soc. 57:9–30.
1971.PubMed/NCBI
|
|
2
|
Tasker GL and Wojnarowska F: Lichen
sclerosus. Clin Exp Dermatol. 28:128–133. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lipscombe TK, Wayte J, Wojnarowska F,
Marren P and Luzzi G: A study of clinical and aetiological factors
and possible associations of lichen sclerosus in males. Australas J
Dermatol. 38:132–136. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kantere D, Löwhagen GB, Alvengren G,
Månesköld A, Gillstedt M and Tunbäck P: The clinical spectrum of
lichen sclerosus in male patients-a retrospective study. Acta Derm
Venereol. 94:542–546. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bleeker MC, Visser PJ, Overbeek LI, van
Beurden M and Berkhof J: Lichen sclerosus: Incidence and risk of
vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev.
25:1224–1230. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nasca MR, Innocenzi D and Micali G: Penile
cancer among patients with genital lichen sclerosus. J Am Acad
Dermatol. 41:911–914. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lewis FM, Tatnall FM, Velangi SS, Bunker
CB, Kumar A, Brackenbury F, Mohd Mustapa MF and Exton LS: British
association of dermatologists guidelines for the management of
lichen sclerosus, 2018. Br J Dermatol. 178:839–853. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lacarrubba F, Borghi A, Verzi AE, Corazza
M, Stinco G and Micali G: Dermoscopy of genital diseases: A review.
J Eur Acad Dermatol Venereol. 34:2198–2207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Larre Borges A, Tiodorovic-Zivkovic D,
Lallas A, Moscarella E, Gurgitano S, Capurro M, Apalla Z, Bruno J,
Popovic D, Nicoletti S, et al: Clinical, dermoscopic and
histopathologic features of genital and extragenital lichen
sclerosus. J Eur Acad Dermatol Venereol. 27:1433–1439.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pan ZY, Lin JR, Cheng TT, Wu JQ and Wu WY:
In vivo reflectance confocal microscopy of Basal cell carcinoma:
Feasibility of preoperative mapping of cancer margins. Dermatol
Surg. 38:1945–1950. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jain M, Robinson BD, Shevchuk MM, Aggarwal
A, Salamoon B, Dubin JM, Scherr DS and Mukherjee S: Multiphoton
microscopy: A potential intraoperative tool for the detection of
carcinoma in situ in human bladder. Arch Pathol Lab Med.
139:796–804. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Calzavara-Pinton P, Longo C, Venturini M,
Sala R and Pellacani G: Reflectance confocal microscopy for in vivo
skin imaging. Photochem Photobiol. 84:1421–1430. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gerger A, Koller S, Kern T, Massone C,
Steiger K, Richtig E, Kerl H and Smolle J: Diagnostic applicability
of in vivo confocal laser scanning microscopy in melanocytic skin
tumors. J Invest Dermatol. 124:493–498. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Balu M, Kelly KM, Zachary CB, Harris RM,
Krasieva TB, König K, Durkin AJ and Tromberg BJ: Distinguishing
between benign and malignant melanocytic nevi by in vivo
multiphoton microscopy. Cancer Res. 74:2688–2697. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ardigo M, Cota C, Berardesca E and
González S: Concordance between in vivo reflectance confocal
microscopy and histology in the evaluation of plaque psoriasis. J
Eur Acad Dermatol Venereol. 23:660–667. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ardigò M, Maliszewski I, Cota C, Scope A,
Sacerdoti G, Gonzalez S and Berardesca E: Preliminary evaluation of
in vivo reflectance confocal microscopy features of Discoid lupus
erythematosus. Br J Dermatol. 156:1196–1203. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Contaldo M, Di Stasio D, Petruzzi M,
Serpico R and Lucchese A: In vivo reflectance confocal microscopy
of oral lichen planus. Int J Dermatol. 58:940–945. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mills SE (ed): Histology for pathologists.
4th edition. Lippincott Williams & Wilkins, Philadelphia, PA,
2012.
|
|
19
|
Watt FM and Green H: Involucrin synthesis
is correlated with cell size in human epidermal cultures. J Cell
Biol. 90:738–742. 1981.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lacarrubba F, Verzì AE, Ardigò M and
Micali G: Handheld reflectance confocal microscopy, dermatoscopy
and histopathological correlation of common inflammatory balanitis.
Skin Res Technol. 24:499–503. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mazzilli S, Giunta A, Galluzzo M, Garofalo
V, Campione E, DI Prete M, Orlandi A, Ardigò M and Bianchi L:
Therapeutic monitoring of male genital lichen sclerosus: Usefulness
of reflectance confocal microscopy. Ital J Dermatol Venerol.
156:718–719. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zipfel WR, Williams RM and Webb WW:
Nonlinear magic: Multiphoton microscopy in the biosciences. Nat
Biotechnol. 21:1369–1377. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Hoogedoorn L, Peppelman M, van de Kerkhof
PC, van Erp PE and Gerritsen MJ: The value of in vivo reflectance
confocal microscopy in the diagnosis and monitoring of inflammatory
and infectious skin diseases: A systematic review. Br J Dermatol.
172:1222–1248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Edwards SJ, Mavranezouli I, Osei-Assibey
G, Marceniuk G, Wakefield V and Karner C: VivaScope®
1500 and 3000 systems for detecting and monitoring skin lesions: A
systematic review and economic evaluation. Health Technol Assess.
20:1–260. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Jacquemus J, Debarbieux S, Depaepe L,
Amini M, Balme B and Thomas L: Reflectance confocal microscopy of
extra-genital lichen sclerosus atrophicus. Skin Res Technol.
22:255–258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang CQF, Akalu YT, Suarez-Farinas M,
Gonzalez J, Mitsui H, Lowes MA, Orlow SJ, Manga P and Krueger JG:
IL-17 and TNF synergistically modulate cytokine expression while
suppressing melanogenesis: Potential relevance to psoriasis. J
Invest Dermatol. 133:2741–2752. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rishpon A, Kim N, Scope A, Porges L,
Oliviero MC, Braun RP, Marghoob AA, Fox CA and Rabinovitz HS:
Reflectance confocal microscopy criteria for squamous cell
carcinomas and actinic keratoses. Arch Dermatol. 145:766–772.
2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ahlgrimm-Siess V, Cao T, Oliviero M,
Hofmann-Wellenhof R, Rabinovitz HS and Scope A: The vasculature of
nonmelanocytic skin tumors on reflectance confocal microscopy:
Vascular features of squamous cell carcinoma in situ. Arch
Dermatol. 147(264)2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Arzberger E, Komericki P, Ahlgrimm-Siess
V, Massone C, Chubisov D and Hofmann-Wellenhof R: Differentiation
between balanitis and carcinoma in situ using reflectance confocal
microscopy. JAMA Dermatol. 149:440–445. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Romano A, Di Stasio D, Petruzzi M, Fiori
F, Lajolo C, Santarelli A, Lucchese A, Serpico R and Contaldo M:
Noninvasive imaging methods to improve the diagnosis of oral
carcinoma and its precursors: State of the art and proposal of a
three-step diagnostic process. Cancers (Basel).
13(2864)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Balu M, Zachary CB, Harris RM, Krasieva
TB, König K, Tromberg BJ and Kelly KM: In vivo multiphoton
microscopy of basal cell carcinoma. JAMA Dermatol. 151:1068–1074.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ring HC, Mogensen M, Hussain AA, Steadman
N, Banzhaf C, Themstrup L and Jemec GB: Imaging of collagen
deposition disorders using optical coherence tomography. J Eur Acad
Dermatol Venereol. 29:890–898. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kose K, Bozkurt A, Alessi-Fox C, Brooks
DH, Dy JG, Rajadhyaksha M and Gill M: Utilizing machine learning
for image quality assessment for reflectance confocal microscopy. J
Invest Dermatol. 140:1214–1222. 2020.PubMed/NCBI View Article : Google Scholar
|