Introduction
Periodontitis is a chronic inflammatory disease and
is the sixth most common human disease, with a prevalence of 45-50%
worldwide (1). Although this
disease can manifest either during childhood or adolescence, it
most commonly occurs early adulthood, with a small number of cases
also occurring in later life (2).
Periodontitis occurs due to the accumulation of microbial plaque,
such as that caused by Porphyromonas gingivalis and
Tannerella forsythia, which have cell walls containing
lipopolysaccharide (LPS), a major cause of the disease (3). The main features of periodontitis
include clinical attachment loss, which is defined by the loss of
the connective tissues and bone that support teeth, loss of
alveolar bone (which can be assessed using radiology), periodontal
pockets and bleeding gums (4).
There is evidence to suggest an independent association between
severe periodontitis and a number of different non-communicable
diseases, including diabetes, atherosclerotic cardiovascular
disease, chronic obstructive pulmonary disease and chronic kidney
disease (1). The current treatment
methods of periodontitis including scaling, surgery, and systemic
antibiotics have made great progress, but the treatment effect
remains unsatisfactory (2). If
left untreated or addressed adequately, periodontitis can lead to
the loss of the supporting tissues and teeth whilst also adversely
obstruct chewing function, severely impairing the quality of life
and significantly increasing the cost of dental treatment (5,6).
Therefore, an effective treatment for periodontitis is in urgent
demand.
Lin-28 homolog A (LIN28A) is an RNA-binding protein
that has been found to regulate multiple aspects of cellular
activity, including stem cell self-renewal, metabolism and cell
proliferation (7). Additionally,
LIN28A serves an important role in the maintenance of pluripotency
in embryonic stem cells (8).
LIN28A has been indicated to promote the expression of stem cell
markers CD133, CD44, Oct4 and Nanog homeobox in ovarian cancer
(9). In addition, LIN28A has been
reported to enhance the therapeutic possibilities of cultured
neural stem cells in a rat model of Parkinson's disease (10). Overexpression of LIN28A can also
potentiate osteogenic differentiation and its expression is
downregulated during human embryonic stem cell differentiation
(11). LIN28A mainly acts by
inhibiting the maturation of lethal (Let)-7 microRNA (miRNA)
precursors (12). Let-7a and
Let-7b expression has been revealed to be regulated by LPS
(13). LPS, a major component of
the Gram-negative cell wall, induces inflammation and oxidative
stress to inhibit osteogenic differentiation and stimulate
mineralization (14-18).
Additionally, a number of studies have demonstrated that the
expression of LIN28A is decreased in periodontal tissues affected
by periodontitis, whereby following the inhibition of LIN28A
expression, the osteogenic differentiation of human periodontal
ligament stem cells (hPDLSCs) was also suppressed (3,19).
However, the precise mechanistic role of LIN28A in periodontitis
and osteoblast physiology remains poorly understood.
Therefore, the present study aimed to determine the
expression profile of LIN28A and its potential effects on the
inflammatory damage, oxidative stress, osteoblast differentiation
and mineralization in LPS-induced hPDLSCs.
Materials and methods
Tissue sample collection and isolation
of hPDLSCs
All the experimental protocols required for the
present study were approved by the Ethics Committee of Meizhou
People's Hospital, Meizhou Academy of Medical Sciences (Meizhou,
China). In total, eight patients who were diagnosed with
periodontitis (between the ages of 24 and 30, with a mean of
27.1±2.9 years; four male and four female) and eight healthy
individuals (between the ages of 12 and 16, with a mean of 14.1±1.9
years; four male and four female) who wanted to receive
orthodontics were selected for the present study and enrolled at
the Meizhou People's Hospital between January 2016 and January
2018. All the patients had no history of chemotherapy and did not
have other cancers, infectious diseases, or autoimmune diseases.
All participants and their guardians signed written informed
consent to take part in the study. The periodontal ligament tissues
were extracted from the aforementioned patients and stored at -80˚C
until further processing.
The teeth were extracted for orthodontic reasons
from eight healthy individuals before being washed using PBS
supplemented with 10% penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). Subsequently, periodontal ligament tissues were
scraped from the middle third of the tooth root and sectioned,
because the periodontal ligament tissues in middle third were the
thinnest, making it easier to digest to obtain cell suspensions and
the rest, including coronal and apical portion of the periodontal
ligament fibers, were discarded. These slices were then digested
using collagenase/trypsin (3 mg/ml; MilliporeSigma) for 1 h at
37˚C. hPDLSCs were isolated from third-passage periodontal ligament
cells using a cluster of differentiation (CD)146 microbead kit
(Miltenyi Biotec GmbH) according to the manufacturer's
instructions. The hPDLSCs were cultured in α-modified Eagle's
medium (α-MEM; Gibco; Thermo Fisher Scientific, Inc.) containing
10% fetal bovine serum (FBS; Beyotime Institute of Biotechnology)
and 100 mg/ml streptomycin and 100 U/ml penicillin in an incubator
at 37˚C and 5% CO2. Morphological changes of hPDLSCs
were observed under a Labomed Inc. LB-601 metallurgical microscope
(cat. no. 50-193-8116; Thermo Fisher Scientific, Inc.) at days 7,
10 and 14 during the cell culture. The hPDLSCs from passages 3-5
were used for the subsequent experiments.
Cell transfection
The full length of the LIN28A sequence was cloned
into the pcDNA3.1 vector by Shanghai GenePharma Co., Ltd. to
construct LIN28A overexpression (Ov-LIN28A) and negative control
(Ov-NC) plasmids and cells were transferred into six-well plates.
After 24 h, the LIN28A overexpression and negative control plasmids
at a concentration of 20 µM were stably transfected into the
hPDLSCs using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Cells were incubated with 5% CO2 at 37˚C for 8 h and
were used in subsequent experiments 48 h post-transfection.
Cell treatment
The hPDLSCs were treated with different
concentrations (0.1, 1 and 10 µg/ml) of LPS (cat. no. ST1470;
Beyotime Institute of Biotechnology) at 37˚C for 3 days. In another
experimental protocol, hPDLSCs were treated with LPS at 10 µg/ml at
37˚C for 3 days 48 h post-transfection. Each experiment was
repeated three times.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from periodontal ligament
tissues and hPDLSCs using TRIzol® (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
extracted RNA was reverse transcribed into complementary DNA using
a PrimeScript RT Reagent kit (Takara Bio, Inc.) following the
manufacturer's protocol as follows: 25˚C for 5 min, 42˚C for 60 min
and 70˚C for 5 min. mRNA expression quantification was performed
via qPCR using One step SYBR Green RT-qPCR with MMLV and hot-start
Taq DNA Polymerase (cat. no. QR0100; MilliporeSigma). The
thermocycling conditions were: 95˚C for 5 min, followed by 40
cycles at 95˚C for 10 sec, 60˚C for 30 sec and 72˚C for 30 sec. All
mRNA expression was normalized to that of GAPDH. The relative
expression levels of the genes were calculated using the
2-ΔΔCq method (20).
The experiments performed were performed in triplicate and repeated
twice. The following primers were used for qPCR: LIN28A forward,
5'-TGCGGGCATCTGTAAGTGG-3' and reverse, 5'-GGAACCCTTCCATGTGCAG-3';
runt-related transcription factor 2 (RUNX2) forward,
5'-CCTGAACTCTGCACCAAGTC-3' and reverse, 5'-GAGGTGGCAGTGTCATCATC-3';
IL-8 forward, 5'-ATGACTTCCAAGCTGGCCGTGGCT-3' and reverse,
5'-TCTCAGCCCTCTTCAAAAACTTCTC-3'; IL-1β forward,
5'-GCGGCCAGGATATAACTGACTTC-3' and reverse,
5'-TCCACATTCAGCACAGGACTCTC-3'; IL-6 forward,
5'-ATGAACTCCTTCTCCACAAGCGC-3' and reverse,
5'-GAAGAGCCCTCAGGCTGGACT-3'; alkaline phosphatase (ALP) forward,
5'-ACTGGTACTCAGACAACGAGAT-3' and reverse,
5'-ACGTCAATGTCCCTGATGTTATG-3'; osteopontin (OPN) forward,
5'-GAAGTTTCGCAGACCTGACAT-3' and reverse,
5'-GTATGCACCATTCAACTCCTCG-3'; osterix (OSX) forward,
5'-GGCGTCCTCCCTGCTTGA-3' and reverse, 5'-TGCTTTGCCCAGAGTTGTTG-3';
osteocalcin (OCN) forward, 5'-CCCAGGCGCTACCTGTATCAA-3' and reverse,
5'-GGTCAGCCAACTCGTCACAGTC-3' and β-actin forward,
5'-GACCTCTATGCCAACACAGT-3' and reverse,
5'-AGTACTTGCGCTCAGGAGGA-3'.
Western blot analysis
The protein samples were extracted from periodontal
ligament tissues using RIPA buffer (Beyotime Institute of
Biotechnology) containing 40 mM NaF, 20 mM Tris, 2.5 mM EDTA, 1%
deoxycholate, 1% Triton X-100, 0.1% SDS, 10 mM
Na4P2O7 and 1 mM
phenylmethylsulfonyl fluoride on ice and centrifuged at 14,000 x g
for 30 min at 4˚C. Protein concentration was measured using a BCA
Protein Assay kit. Equal amounts of protein (40 µg per lane) were
loaded into each well of an 8% SDS-PAGE gel and separated using
electrophoresis. The Separated proteins were then transferred onto
PVDF membranes (MilliporeSigma). After blocking with 5% skimmed
milk for 1 h at room temperature, the membranes were incubated with
primary antibodies LIN28A (cat. no. ab279647; dilution, 1:1,000;
Abcam), RUNX2 (cat. no. ab236639; dilution, 1:1,000; Abcam),
phosphorylated (p-)-NF-κB p65 (cat. no. ab76302; dilution, 1:1,000;
Abcam), NF-κB p65 (cat. no. ab32536; dilution, 1:1,000; Abcam),
Lamin B1 (cat. no. ab16048; dilution, 1:1,000; Abcam), OPN (cat.
no. ab214050; dilution, 1:1,000; Abcam), OSX (cat. no. ab209484;
dilution, 1:1,000; Abcam), OCN (cat. no. ab133612; dilution,
1:1,000; Abcam) and GAPDH (cat. no. ab9485; dilution, 1:2,500;
Abcam) overnight at 4˚C. These membranes were then washed with PBS
three times and probed with the HRP-conjugated goat anti-rabbit IgG
antibody (cat. no. ab6721; dilution, 1:2,000; Abcam) for 1 h at
room temperature. The protein bands were visualized using enhanced
chemiluminescent (ECL) substrate (cat. no. WBKLS0050;
MilliporeSigma) and imaged using a ChemiDoc MP imager (Bio-Rad
Laboratories, Inc.). Protein expression was quantified using the
Image J 1.51 software (National Institutes of Health).
Measurement of reactive oxygen species
(ROS), superoxide dismutase (SOD) and glutathione (GSH)
The levels of ROS, SOD and GSH were measured to
assess the extent of oxidative stress in hPDLSCs. A
2',7'-Dichlorofluorescin diacetate (DCFH-DA) measurement kit
(Shanghai Enzyme-linked Biotechnology Co., Ltd.) was used to detect
ROS generation. Briefly, hPDLSCs cells at 1x106 cells
per well were washed twice with pre-warmed serum-free α-MEM and
then stained with 5 µM DCFH-DA in serum-free medium at 37˚C for 20
min in the dark according to the manufacturer's protocol. SOD
activity was evaluated using a SOD assay kit-WST (cat. no. S311-10;
Dojindo Molecular Technologies, Inc.) according to the
manufacturer's protocol. GSH activity was assessed using a GSH
assay kit (cat. no. KA3779; Abnova) following the manufacturer's
protocol.
Induction of osteogenic
differentiation in hPDLSCs
hPDLSCs were maintained at 37˚C in six-well plates
loaded with osteogenic differentiation medium (Cyagen Biosciences,
Inc.) containing 50 µg/ml ascorbic acid, 1 µM dexamethasone, 3 mM
β-glycerophosphate from Guangzhou Saiguo Biotech Co., Ltd. and 5%
FBS when they reached 80% confluence. The medium was refreshed
every 3 days. For the control group, standard culture medium was
loaded into the plates. The differentiation of hPDLSCs was observed
through subsequent ALP activity assay and Alizarin red staining
after culturing the cells for 21 days.
Alkaline phosphatase (ALP) activity
assay
ALP activity was evaluated using an ALP assay kit
(cat. no. P0321S; Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's protocol. Cells were washed with
PBS and homogenized using 50 µl ALP assay buffer on ice for 5 min
and centrifuged (2,000 x g) at room temperature for another 5 min
following 14 days of osteoblastic induction. Subsequently, 30 µl
cell lysate, 50 µl assay buffer and 50 µl p-nitrophenyl phosphate
were added into a 96-well plate. After incubation for 1 h at 25˚C,
20 µl stop solution was added into each well. Finally, the
absorbance was measured at 450 nm using a microplate reader (cat.
no. 11-120-533; Thermo Fisher Scientific, Inc.).
Alizarin red staining
The mineralization capacity of osteoblasts was
assessed using Alizarin red staining. Cells were seeded into
24-well plates at a density of 1x105 cells. Cells were
grown in the osteogenic differentiation medium (Cyagen Biosciences,
Inc.) for 21 days at 37˚C. Subsequently, cells were fixed with 4%
cold methanol for 15 min at room temperature and stained with
Alizarin red (Beijing Solarbio Science & Technology Co., Ltd.)
for 30 min at 37˚C. Subsequently, the mineralization capacity of
hPDLSCs was imaged using an inverted light microscope
(magnification, x200) in five fields.
Statistical analysis
All experimental data are presented as the mean ± SD
and all experiments were repeated at least three times. Statistical
analysis was performed using the SPSS 13.0 Statistics Software
(SPSS, Inc.). An unpaired Student's t-test was used to analyze
results between two groups and one-way analysis of variance
followed by a Tukey's post hoc test was applied to analyze results
among ≥ three groups. Pearson's correlation analysis was utilized
to confirm the correlation between LIN28A and RUNX2. P<0.05 was
considered to indicate a statistically significant difference.
Results
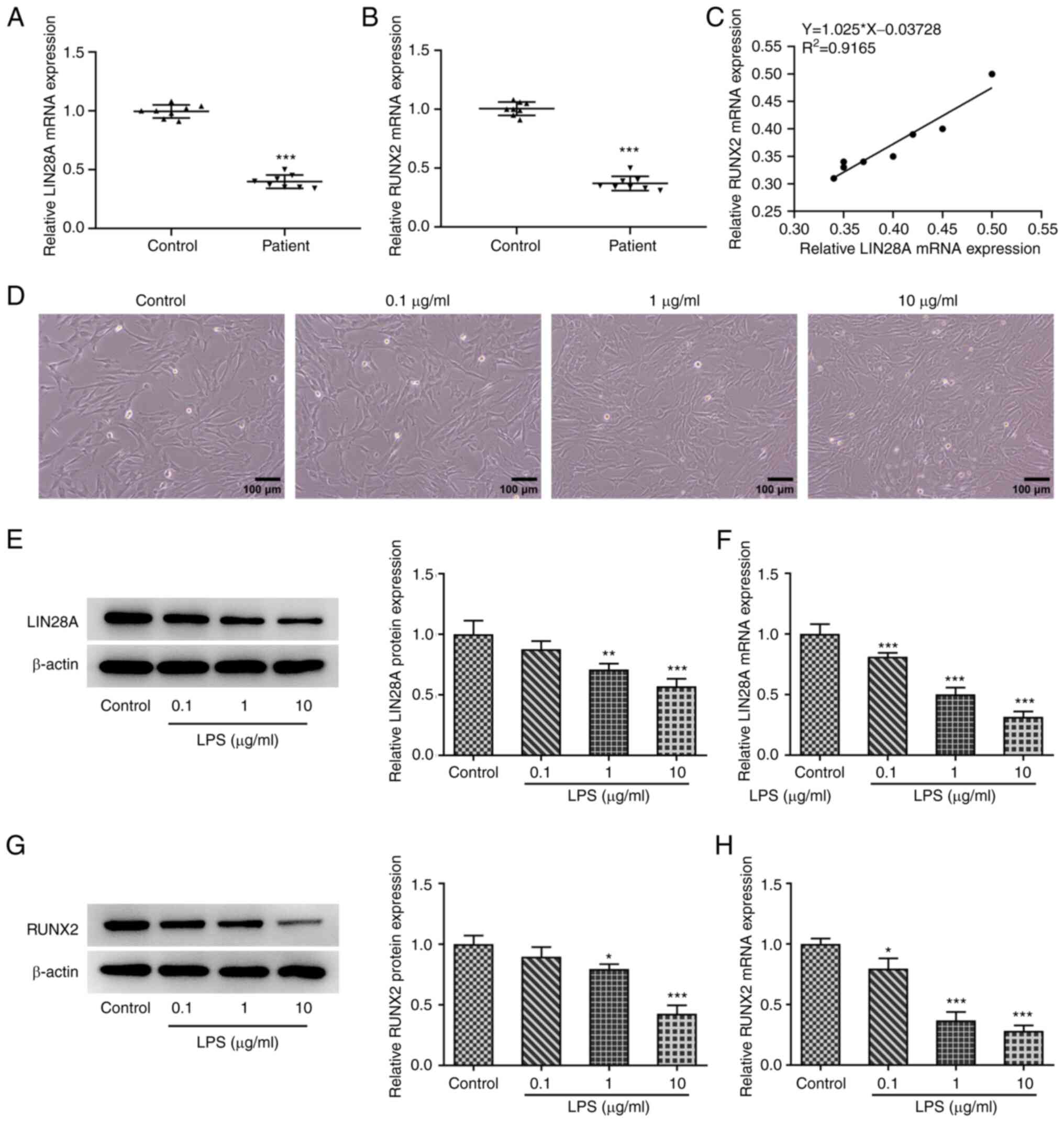
LIN28A expression is reduced in
periodontal biopsy tissues and is positively associated with RUNX2
expression
To determine the expression levels of LIN28A and
RUNX2, samples were divided into two groups of control and
pathological tissues. The measurements were subsequently performed
using RT-qPCR. Fig. 1A indicates a
significant decrease in LIN28A expression in the periodontitis
group compared with that in the control group. Similarly, a
significant decrease in RUNX2 expression in the periodontitis group
was found compared with that in the control group (Fig. 1B). In addition, the expression of
LIN28A and RUNX2 was demonstrated to be positively associated in
the periodontal biopsy tissues (Fig.
1C).
LIN28A expression is reduced in
LPS-induced hPDLSCs
The expression of LIN28A and RUNX2 were assessed in
hPDLSCs using RT-qPCR and western blotting after 3 days of LPS
treatment at concentrations of 0.1, 1 and 10 µg/ml. A marked
increase in the number of hPDLSCs was observed following LPS
induction (Fig. 1D). The
expression of LIN28A was revealed to be significantly decreased in
hPDLSCs with increasing concentration of LPS (Fig. 1E and F). The results also demonstrated
progressively decreasing RUNX2 expression with increasing LPS
concentrations (Fig. 1G and
H). These results suggest that
LIN28A expression was decreased in LPS-induced hPDLSCs.
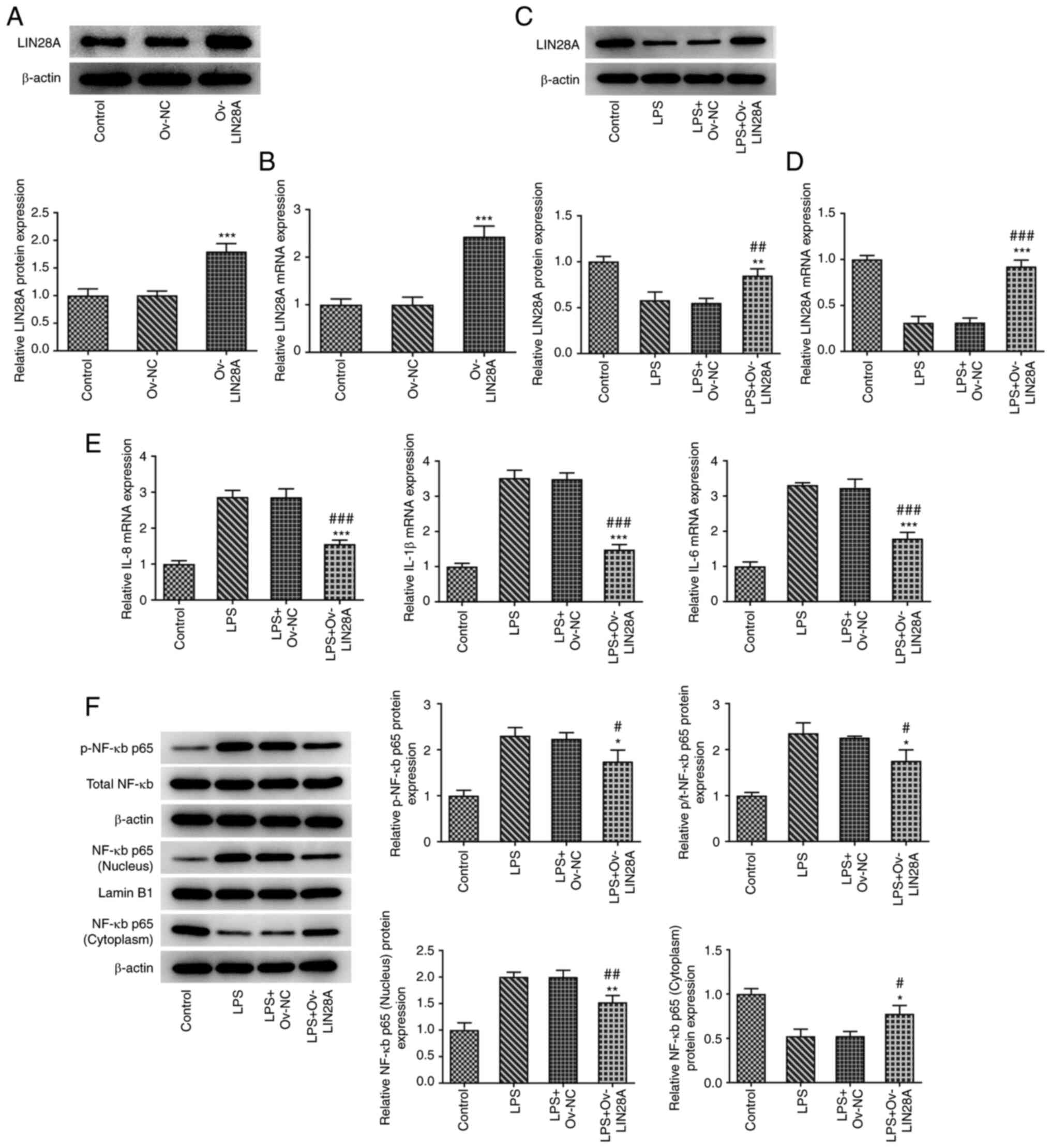
LIN28A attenuates LPS-induced
inflammatory damage in hPDLSCs
To assess the potential effects of LIN28A on
inflammatory damage in LPS-induced hPDLSCs, the expression of
LIN28A, IL-8, IL-1β and IL-6, the protein levels of NF-κB p65 in
the nucleus and cytoplasm and total NF-κB p65 phosphorylation in
both the cytoplasm and nucleus were measured using RT-qPCR and
western blotting. After the hPDLSCs were transfected with
Ov-LIN28A, the expression of LIN28A was significantly increased
compared with the Ov-NC group (Fig.
2A and B). LIN28A expression
was discovered to be downregulated following LPS treatment and
there was also a significant increase in the expression of LIN28A
in LPS-induced hPDLSCs transfected with Ov-LIN28A compared with
that in LPS-induced hPDLSCs transfected with Ov-NC (Fig. 2C and D). In addition, LPS treatment elevated
the levels of IL-8, IL-1β and IL-6. The expression of IL-8, IL-1β
and IL-6 exhibited a significant decrease following overexpression
of LIN28A in comparison with the LPS+Ov-NC group (Fig. 2E). LPS treatment increased the
protein levels of NF-κB p65 in the nucleus and p-NF-κB p65 and
while decreased the protein level of NF-κB p65 in the cytoplasm.
Furthermore, following the overexpression of LIN28A, there was a
significant decrease in the protein levels of NF-κB p65 in the
nucleus and p-NF-κB p65, in addition to a significant rise in the
protein levels of NF-κB p65 in the cytoplasm compared with the
LPS+Ov-NC group (Fig. 2F). Results
from the present study revealed that the levels of inflammatory
factors IL-8, IL-1β and IL-6, NF-κB p65 in the nucleus and p-NF-κB
p65 were decreased following the overexpression of LIN28A. This
suggests that LIN28A may serve a role in reducing inflammatory
damage in LPS-induced hPDLSCs.
LIN28A alleviates oxidative stress
damage in LPS-induced hPDLSCs
To investigate further if LIN28A can reduce
oxidative stress injury induced by LPS in hPDLSCs, the levels of
ROS, SOD and GSH were examined using corresponding commercial kits.
ROS level was noted to be enhanced following LPS treatment and a
marked decrease in ROS levels was found following LIN28A
overexpression compared with those in the LPS+Ov-NC group (Fig. 3A). However, the levels of
anti-oxidants SOD and GSH were decreased following LPS induction
but were significantly elevated after transfection with LIN28A
compared with the LPS+Ov-NC group (Fig. 3B and C). These data suggest that LIN28A
overexpression can mitigate oxidative stress injury in LPS-induced
hPDLSCs.
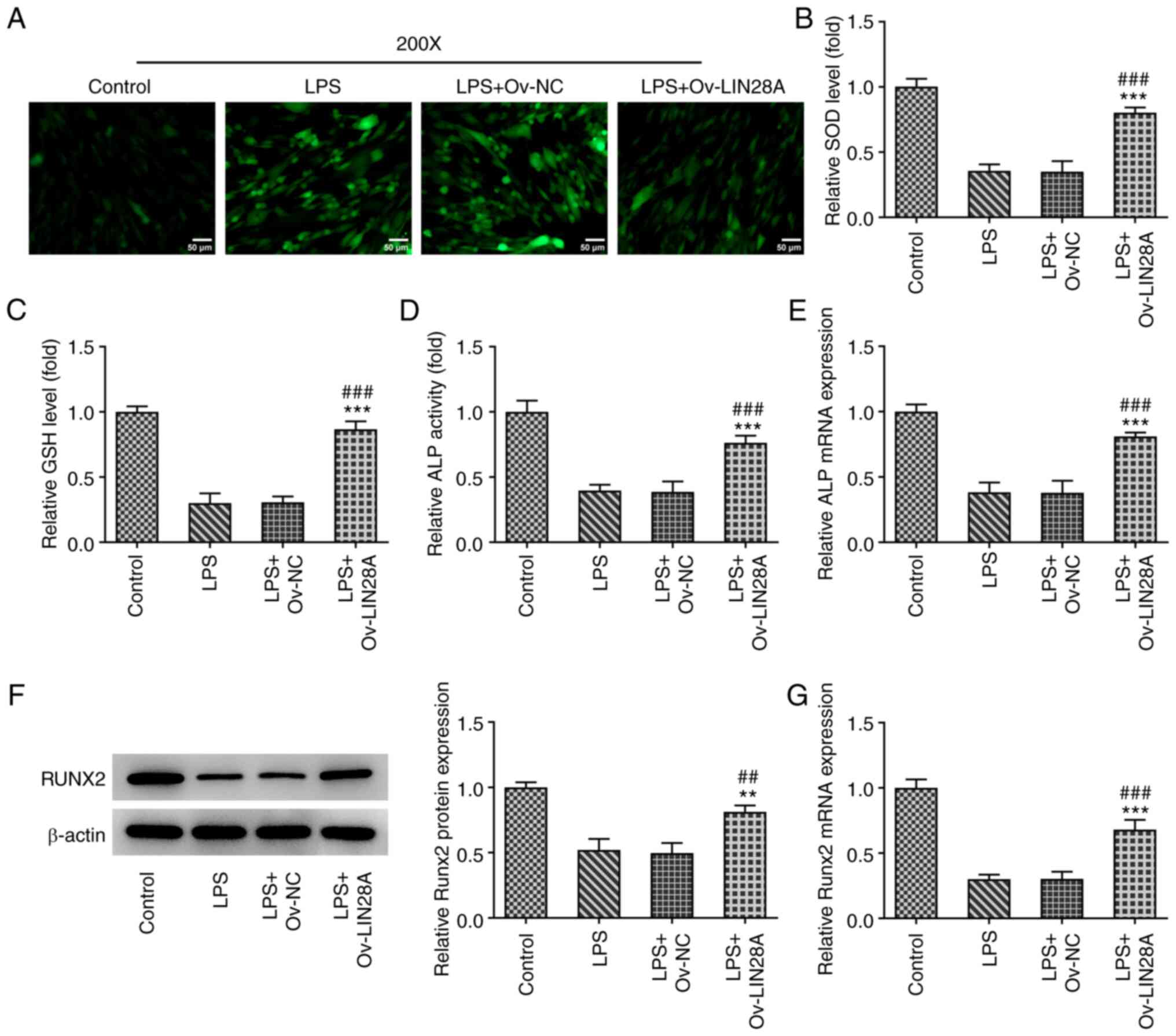 | Figure 3LIN28A overexpression alleviates
oxidative stress damage and upregulates RUNX2 expression in
LPS-induced hPDLSCs. (A) Reactive oxygen species levels in
transfected hPDLSCs induced by LPS were measured using a
2',7'-dichlorofluorescin diacetate measurement kit. Scale bars, 50
µm. The levels of (B) SOD and (C) GSH in transfected hPDLSCs
induced by LPS were determined using their corresponding kits. (D)
An ALP kit was used for detecting the activity of ALP in
transfected hPDLSCs induced by LPS. (E) RT-qPCR was used to measure
ALP expression in transfected hPDLSCs induced by LPS. RUNX2
expression in transfected hPDLSCs induced by LPS was tested by (F)
western blotting and (G) RT-qPCR. **P<0.01 and
***P<0.001 vs. control; ##P<0.01 and
###P<0.001 vs. LPS + Ov-NC. LIN28A, Lin-28 homeobox
A; RUNX2, Runt-related transcription factor 2; LPS,
lipopolysaccharide; hPDSCs, human periodontal ligament stem cells;
Ov, overexpression; NC, negative control; SOD, superoxide
dismutase; GSH, glutathione; ALP, alkaline phosphatase. |
LIN28A reverses ALP activity
impairment and restores RUNX2 expression in LPS-induced
hPDLSCs
To elucidate the effects of LIN28A on hPDLSCs,
detection of ALP activity was performed using corresponding kits
whereas the expression of ALP and RUNX2 was examined using RT-qPCR
and western blotting. As a catalytic enzyme for osteoblast
differentiation, the activity and expression of ALP were found to
be markedly decreased following the treatment of LPS, but
significantly increased following LIN28A overexpression relative to
the LPS+Ov-NC group (Fig. 3D and
E). Additionally, LPS treatment
reduced the expression of the osteoblast transcription factor RUNX2
and there was a significant increase in the expression of RUNX2
after the hPDLSCs were transfected with the Ov-LIN28A plasmid
(Fig. 3F and G). As a consequence, increased RUNX2
expression, osteoblast differentiation and the restoration of ALP
activity all suggest an ameliorative effect of LIN28A
overexpression on LPS-induced hPDLSCs.
LIN28A facilitates osteoblast
mineralization in LPS-induced hPDLSCs
To assess the role of LIN28A on osteoblast
mineralization, Alizarin red staining was used to evaluate the
mineralization capacity of osteoblasts cultured for 21 days.
RT-qPCR and western blotting were used for the measurement of the
expression of osteogenic-specific matrix proteins OPN and OCN, in
addition to the osteoblast-specific transcription factor OSX. As
shown in Fig. 4A, the degree of
Alizarin red staining was lower following LPS induction compared
with that in the control group, but was restored following LIN28A
overexpression. In addition, LPS treatment reduced the expression
of OPN, OSX and OCN while a significant elevation in OPN, OSX and
OCN expression was observed compared with that in LPS+Ov-NC group
(Fig. 4B-E). These results suggest
that LIN28A facilitated osteoblast mineralization in hPDLSCs
induced by LPS.
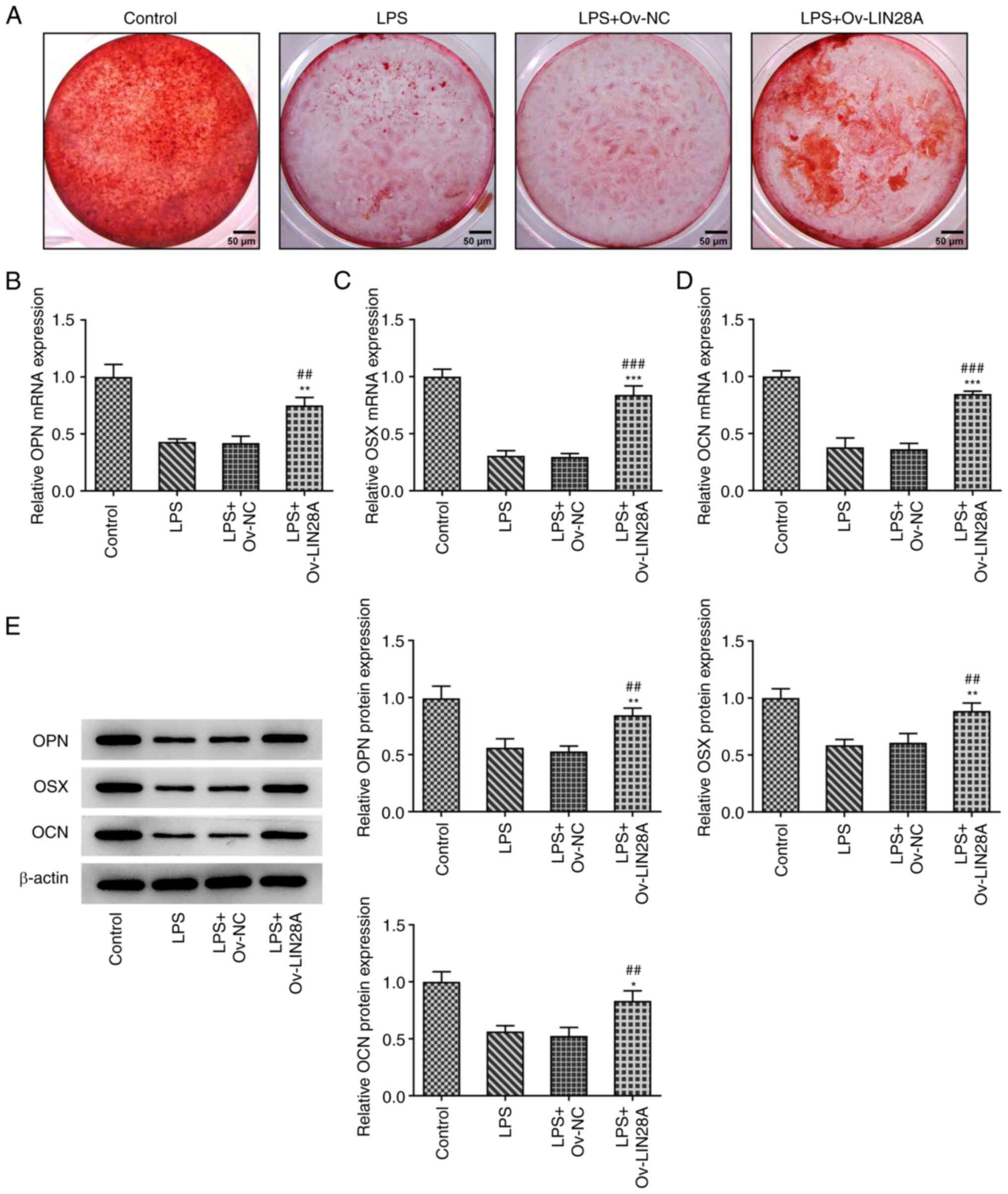 | Figure 4LIN28A facilitates osteoblast
mineralization in LPS-induced hPDLSCs. (A) Alizarin red staining
was used to evaluate the mineralization capacity of osteoblasts.
Scale bars, 50 µm. The expression of (B) OPN, (C) OSX and (D) OCN
in transfected hPDLSCs induced by LPS were assessed by reverse
transcription-quantitative PCR. (E) The protein levels of OPN, OSX
and OCN in transfected hPDLSCs induced by LPS were measured by
western blotting. *P<0.05, **P<0.01 and
***P<0.001 vs. Control; ##P<0.01 and
###P<0.001 vs. LPS + Ov-NC. Lin-28 homeobox A; LPS,
lipopolysaccharide; hPDSCs, human periodontal ligament stem cells;
OPN, osteopontin; OSX, osterix; OCN, osteocalcin; Ov,
overexpression; NC, negative control. |
Discussion
A number of recent studies have demonstrated that
LIN28A is a highly conserved RNA-binding protein that is closely
associated with the differentiation of keratinocytes (21) and glial lineage cells (22). LIN28A has been previously found to
regulate neuronal differentiation and regulate miR-9 expression
(23). In addition, LIN28A
expression was revealed to be downregulated during human and murine
embryonic stem cell differentiation, where the balance between the
expression of LIN28A and let-7 miRNAs was proposed to be important
for embryonic stem cell self-renewal and differentiation (24,25).
However, the effects of LIN28A on osteogenic differentiation and
mineralization in hPDLSCs remain poorly understood.
RUNX2 is a transcription factor that is essential
for osteoblast differentiation (26), as it directs pluripotent
mesenchymal cells towards an osteoblast lineage (27). ALP is a byproduct of osteoblast
activity, where its elevation indicates the possible presence of
active bone formation (28). The
present study found that LIN28A expression was decreased in
periodontal tissues effected by periodontitis, whereas a positive
correlation was observed between RUNX2 and LIN28A expression.
Consequently, these results suggest that LIN28A can improve
osteoblast differentiation under LPS treatment. Downstream, RUNX2
can induce the expression of OPN, OCN and OSX (29), which have been revealed to regulate
bone formation (30). The present
study also demonstrated that LIN28A overexpression promoted the
expression of RUNX2, ALP, OPN, OSX and OCN. In addition, Alizarin
red staining also demonstrated that LIN28A enhanced the
mineralization ability of osteoblasts. These results suggest that
LIN28A may promote osteoblast differentiation and mineralization in
LPS-induced hPDLSCs.
Previous studies have indicated that LIN28A is
associated with the inflammatory response (31). LIN28 has been revealed to
participate in the LPS-induced astrocyte inflammatory response
through the NF-κB signaling pathway (32). Additionally, an inflammatory signal
may initiate an epigenetic switch into the oncogenic gene
expression program through a positive feedback loop involving
NF-κB, LIN28, let-7 and IL-6 in cancer cells (33). It has been reported that NF-κB is a
sequence-specific transcription factor that serves an important
role in the inflammatory response (34,35).
Activation of NF-κB leads to the production of proinflammatory
cytokines and chemokines, such as IL-1, IL-6, IL-8 and TNF-α
(3). In the present study,
proinflammatory cytokines IL-8, IL-1β and IL-6, the levels of NF-κB
p65 in the nucleus and p-NF-κB p65 in hPDLSCs induced by LPS were
all significantly decreased following the overexpression of LIN28A.
By contrast, the levels of NF-κB p65 was increased in the
cytoplasm. Therefore, results from the present study suggest that
LIN28A can attenuate inflammatory damage in LPS-induced
hPDLSCs.
Oxidative stress refers to the imbalance between the
oxidative and antioxidative systems within cells and tissues,
resulting in the excessive accumulation of oxidative free radicals
and associated with ROS (36). SOD
and GSH are important antioxidant enzymes and antioxidants in the
body that protect an organism from oxidative damage (37). A previous study has indicated that
LIN28A overexpression can reduce ROS production in
hypoxia/reoxygenation induced cardiomyocytes under high
glucose/high fat conditions (38).
In the present study, LIN28A overexpression reduced ROS levels
whilst inducing an increase in SOD and GSH levels in hPDLSCs
treated with LPS, suggesting that LIN28A can alleviate oxidative
stress damage in LPS-induced hPDLSCs.
In conclusion, results of the present study revealed
that LIN28A expression was decreased in periodontal biopsy tissues
and LPS-induced hPDLSCs, which was also positively correlated with
RUNX2 expression. Furthermore, LIN28A was found to attenuate
inflammatory damage and oxidative stress, whilst improving
osteoblast differentiation and mineralization in LPS-induced
hPDLSCs. Therefore, LIN28A may serve as a potential therapeutic
target in patients with periodontitis, which is potentially a novel
mechanism underlying this disease.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by General projects of
Natural Science Foundation of Heilongjiang Province (grant no.
H2017041). Scientific research projects of traditional Chinese
medicine in Heilongjiang Province (grant no. 2HY2020-068) and
scientific Research and Cultivation Project of Meizhou People's
Hospital (grant no. PY-C20210018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG and LL designed the study and analyzed the data.
LL performed the experiments. LG and LL drafted the manuscript and
interpreted the data. LG and LL confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Meizhou People's Hospital, Meizhou Academy of Medical
Sciences (Meizhou, China). All participants and their guardians
signed written informed consent to take part in the study.
Patient consent for participation
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sanz M, Marco Del Castillo A, Jepsen S,
Gonzalez-Juanatey JR, D'Aiuto F, Bouchard P, Chapple I, Dietrich T,
Gotsman I, Graziani F, et al: Periodontitis and cardiovascular
diseases: Consensus report. J Clin Periodontol. 47:268–288.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Slots J: Periodontitis: Facts, fallacies
and the future. Periodontology 2000. 75:7–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Venugopal P, Koshy T, Lavu V, Ranga Rao S,
Ramasamy S, Hariharan S and Venkatesan V: Differential expression
of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction
with NF-kB pathway genes in periodontitis pathogenesis. J Cell
Physiol. 233:5877–5884. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Papapanou PN, Sanz M, Buduneli N, Dietrich
T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani
F, et al: Periodontitis: Consensus report of workgroup 2 of the
2017 world workshop on the classification of periodontal and
peri-implant diseases and conditions. J Clin Periodontol. 45 (Suppl
20):S162–S170. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sanz M, Herrera D, Kebschull M, Chapple I,
Jepsen S, Beglundh T, Sculean A and Tonetti MS: EFP Workshop
Participants and Methodological Consultants. Treatment of stage
I-III periodontitis-The EFP S3 level clinical practice guideline. J
Clin Periodontol. 47 (Suppl 22):S4–S60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Righolt AJ, Jevdjevic M, Marcenes W and
Listl S: Global-, regional-, and country-level economic impacts of
dental diseases in 2015. J Dent Res. 97:501–507. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shyh-Chang N and Daley GQ: Lin28: Primal
regulator of growth and metabolism in stem cells. Cell Stem Cell.
12:395–406. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ma F, Du X, Wei Y, Zhou Z, Clotaire DZJ,
Li N, Peng S, Li G and Hua J: LIN28A activates the transcription of
NANOG in dairy goat male germline stem cells. J Cell Physiol.
234:8113–8121. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhong Y, Cao L, Ma H, Wang Q, Wei P, Yang
J, Mo Y, Cao L, Shuai C and Peng S: Lin28A regulates stem-like
properties of ovarian cancer cells by enriching RAN and HSBP1 mRNA
and up-regulating its protein expression. Int J Biol Sci.
16:1941–1953. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rhee YH, Kim TH, Jo AY, Chang MY, Park CH,
Kim SM, Song JJ, Oh SM, Yi SH, Kim HH, et al: LIN28A enhances the
therapeutic potential of cultured neural stem cells in a
Parkinson's disease model. Brain. 139:2722–2739. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park JH, Park BW, Kang YH, Byun SH, Hwang
SC, Kim DR, Woo DK and Byun JH: Lin28a enhances in vitro
osteoblastic differentiation of human periosteum-derived cells.
Cell Biochem Funct. 35:497–509. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Li X, Liang T, Chen SS, Wang M, Wang R, Li
K, Wang JC, Xu CW, Du N, Qin S and Ren H: Matrine suppression of
self-renewal was dependent on regulation of LIN28A/Let-7 pathway in
breast cancer stem cells. J Cell Biochem. 121:2139–2149.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
McDaniel K, Huang L, Sato K, Wu N, Annable
T, Zhou T, Ramos-Lorenzo S, Wan Y, Huang Q, Francis H, et al: The
let-7/Lin28 axis regulates activation of hepatic stellate cells in
alcoholic liver injury. J Biol Chem. 292:11336–11347.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lam JS, Anderson EM and Hao Y: LPS
quantitation procedures. Methods Mol Biol. 1149:375–402.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cao Y, Chen J, Ren G, Zhang Y, Tan X and
Yang L: Punicalagin prevents inflammation in LPS-induced RAW264.7
macrophages by inhibiting FoxO3a/autophagy signaling pathway.
Nutrients. 11(2794)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shah SA, Khan M, Jo MH, Jo MG, Amin FU and
Kim MO: Melatonin stimulates the SIRT1/Nrf2 signaling pathway
counteracting lipopolysaccharide (LPS)-induced oxidative stress to
rescue postnatal rat brain. CNS Neurosci Ther. 23:33–44.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cai P, Cai T, Li X, Fan L, Chen G, Yu B
and Liu T: Herbacetin treatment remitted LPS induced inhibition of
osteoblast differentiation through blocking AKT/NF-κB signaling
pathway. Am J Transl Res. 11:865–874. 2019.PubMed/NCBI
|
|
18
|
Ning T, Shao J, Zhang X, Luo X, Huang X,
Wu H, Xu S, Wu B and Ma D: Ageing affects the proliferation and
mineralization of rat dental pulp stem cells under inflammatory
conditions. Int Endod J. 53:72–83. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
He Q, Yang S, Gu X, Li M, Wang C and Wei
F: Long noncoding RNA TUG1 facilitates osteogenic differentiation
of periodontal ligament stem cells via interacting with Lin28A.
Cell Death Dis. 9(455)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jang S, Jang S, Kim SY, Ko J, Kim E, Park
JY, Hyung H, Lee JH, Lim SG, Park S, et al: Overexpression of
Lin28a aggravates psoriasis-like phenotype by regulating the
proliferation and differentiation of keratinocytes. J Inflamm Res.
14:4299–4312. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Luo J, Zou H, Deng L, Sun X, Yuan P and Li
P: Lin28 inhibits the differentiation from mouse embryonic stem
cells to glial lineage cells through upregulation of Yap1. Stem
Cells Int. 2021(6674283)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nowak JS, Choudhury NR, de Lima Alves F,
Rappsilber J and Michlewski G: Lin28a regulates neuronal
differentiation and controls miR-9 production. Nat Commun.
5(3687)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Faas L, Warrander FC, Maguire R,
Ramsbottom SA, Quinn D, Genever P and Isaacs HV: Lin28 proteins are
required for germ layer specification in xenopus. Development.
140:976–986. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Viswanathan SR and Daley GQ: Lin28: A
microRNA regulator with a macro role. Cell. 140:445–449.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Komori T: Roles of Runx2 in skeletal
development. Adv Exp Med Biol. 962:83–93. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Komori T: Regulation of osteoblast
differentiation by transcription factors. J Cell Biochem.
99:1233–1239. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sharma U, Pal D and Prasad R: Alkaline
phosphatase: An overview. Indian J Clin Biochem. 29:269–278.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yin N, Zhu L, Ding L, Yuan J, Du L, Pan M,
Xue F and Xiao H: MiR-135-5p promotes osteoblast differentiation by
targeting HIF1AN in MC3T3-E1 cells. Cell Mol Biol Lett.
24(51)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ni SY, Xu WT, Liao GY, Wang YL and Li J:
LncRNA HOTAIR promotes LPS-induced inflammation and apoptosis of
cardiomyocytes via Lin28-mediated PDCD4 stability. Inflammation.
44:1452–1463. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yue Y, Zhang D, Jiang S, Li A, Guo A, Wu
X, Xia X, Cheng H, Tao T and Gu X: LIN28 expression in rat spinal
cord after injury. Neurochem Res. 39:862–874. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Iliopoulos D, Hirsch HA and Struhl K: An
epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and
IL6 links inflammation to cell transformation. Cell. 139:693–706.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chawla M, Roy P and Basak S: Role of the
NF-κB system in context-specific tuning of the inflammatory gene
response. Curr Opin Immunol. 68:21–27. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Barnabei L, Laplantine E, Mbongo W,
Rieux-Laucat F and Weil R: NF-κB: At the borders of autoimmunity
and inflammation. Front Immunol. 12(716469)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Newsholme P, Cruzat VF, Keane KN, Carlessi
R and de Bittencourt PI Jr: Molecular mechanisms of ROS production
and oxidative stress in diabetes. Biochem J. 473:4527–4550.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Djordjevic A, Spasic S, Jovanovic-Galovic
A, Djordjevic R and Grubor-Lajsic G: Oxidative stress in diabetic
pregnancy: SOD, CAT and GSH-Px activity and lipid peroxidation
products. J Matern Fetal Neonatal Med. 16:367–372. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang M, Niu X, Hu J, Yuan Y, Sun S, Wang
J, Yu W, Wang C, Sun D and Wang H: Lin28a protects against
hypoxia/reoxygenation induced cardiomyocytes apoptosis by
alleviating mitochondrial dysfunction under high glucose/high fat
conditions. PLoS One. 9(e110580)2014.PubMed/NCBI View Article : Google Scholar
|