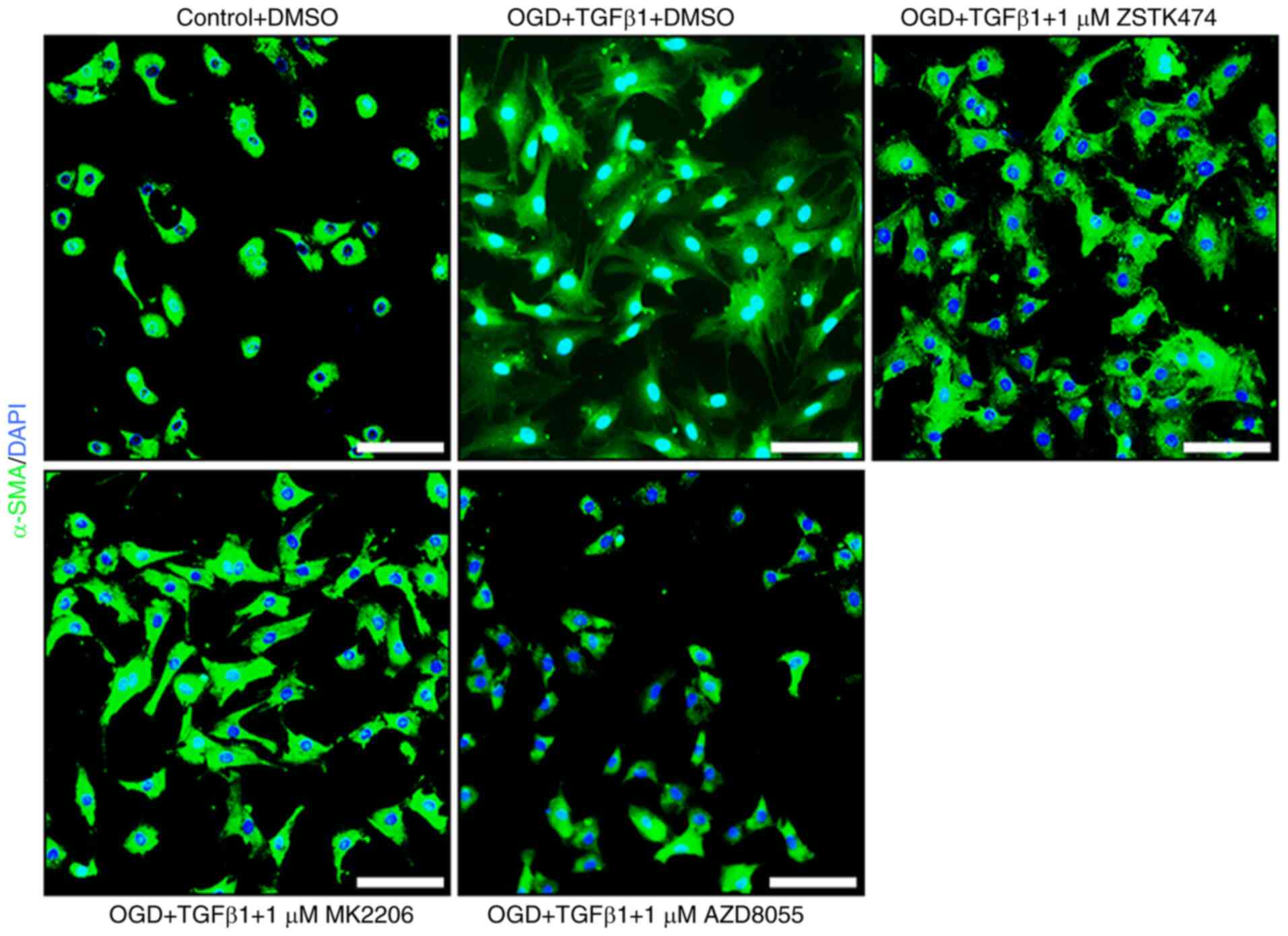
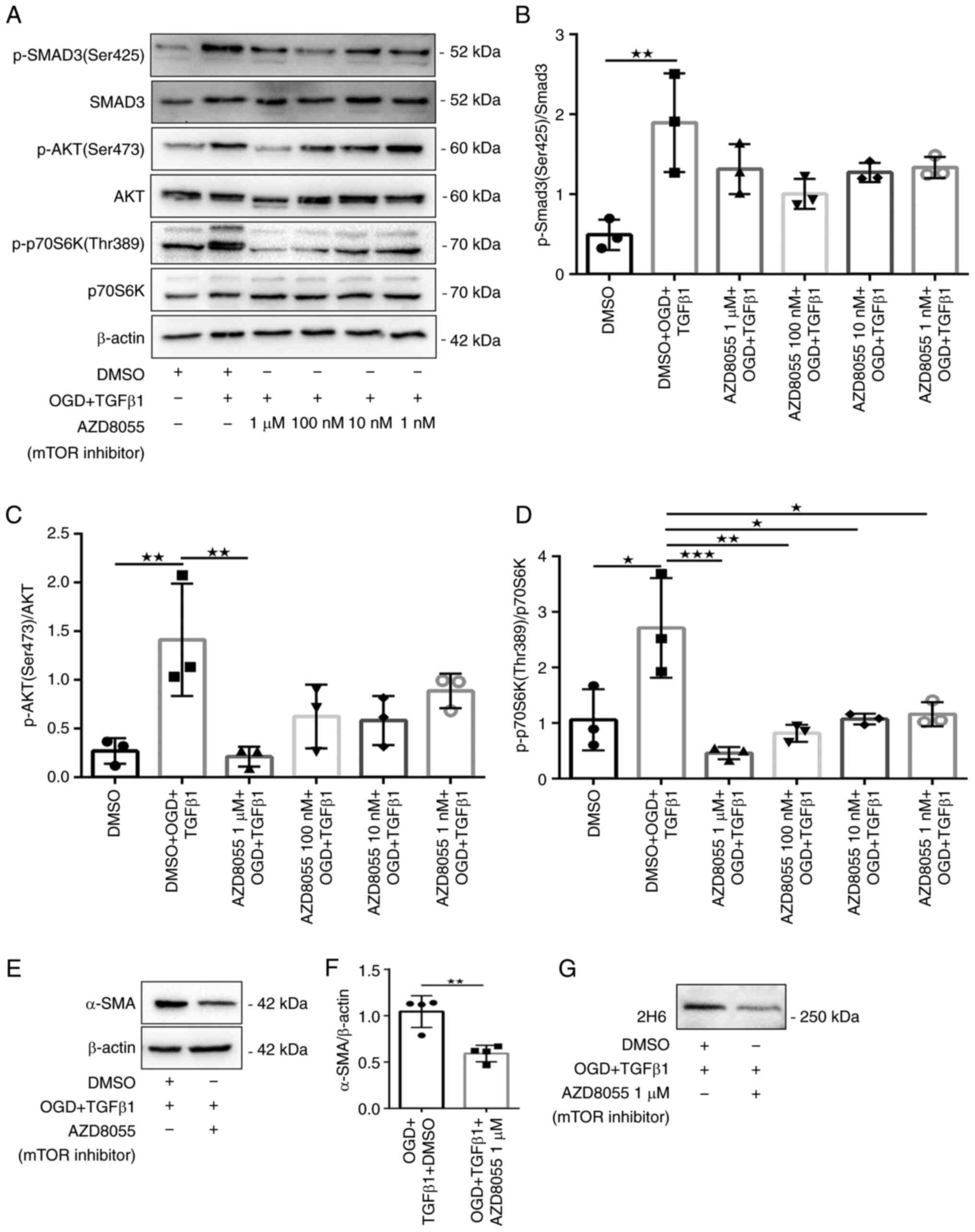
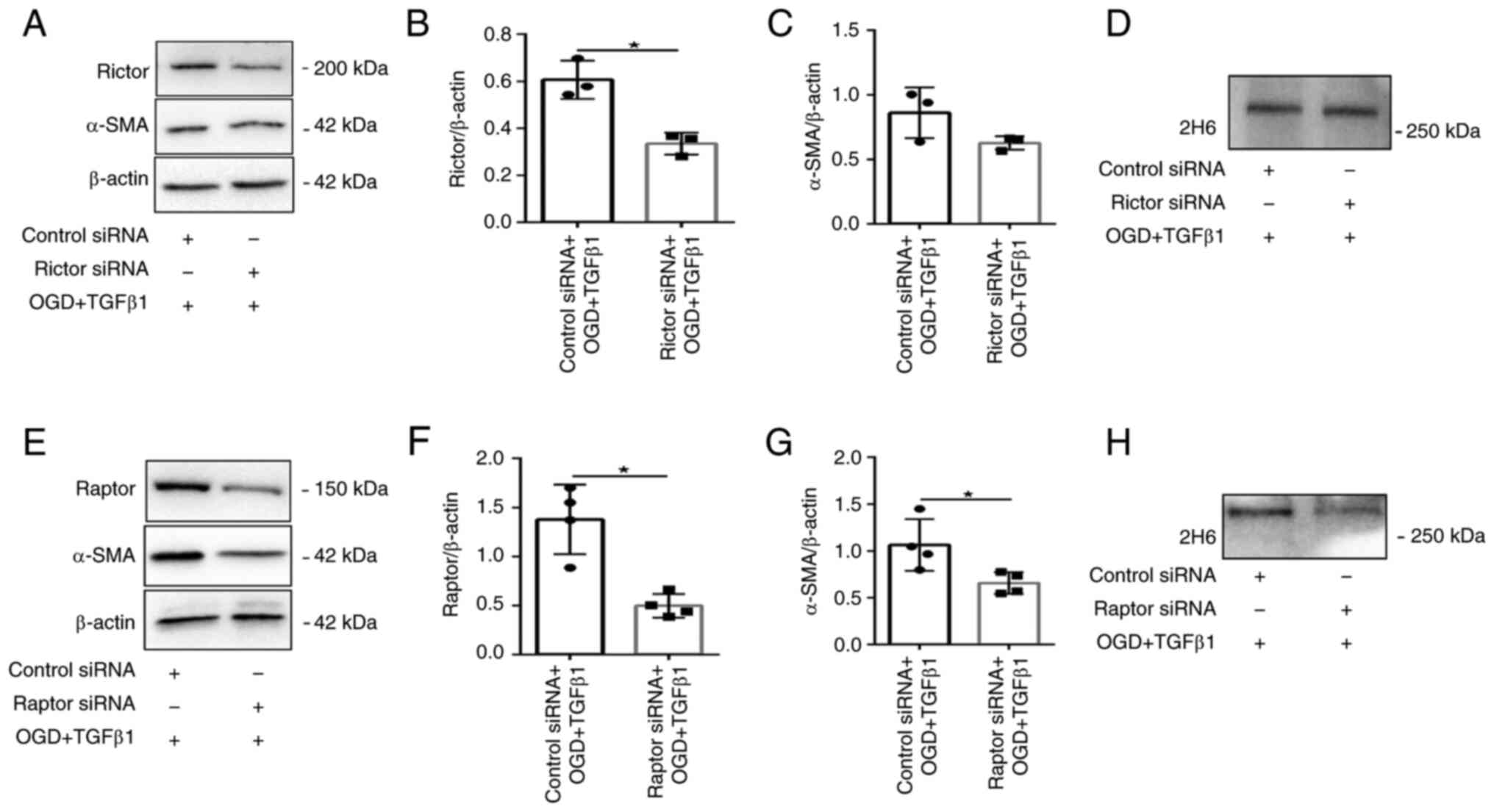
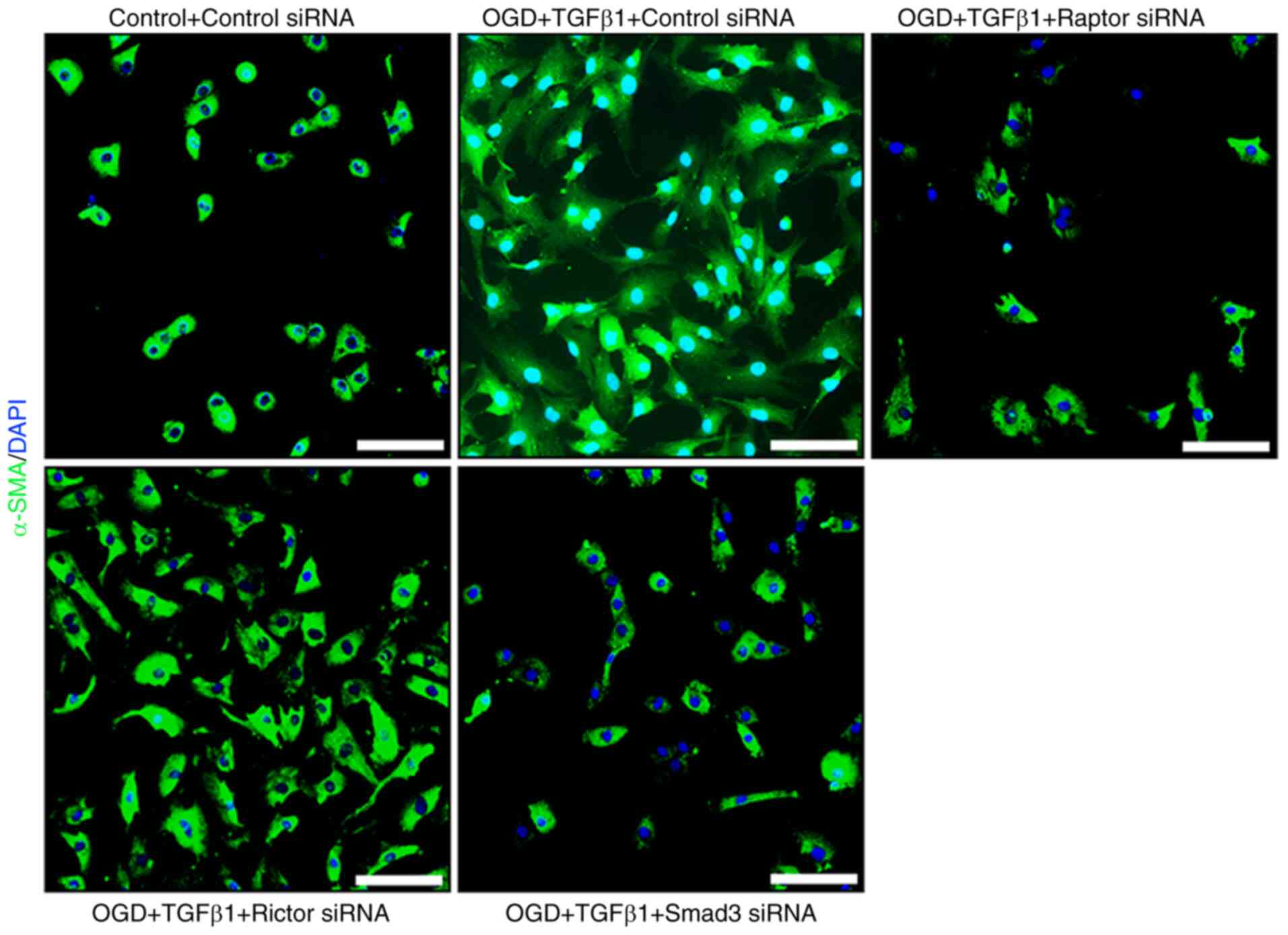
|
1
|
Wu Y, Liu H and Wang X: Cardioprotection
of pharmacological postconditioning on myocardial
ischemia/reperfusion injury. Life Sci. 264(118628)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lai TC, Lee TL, Chang YC, Chen YC, Lin SR,
Lin SW, Pu CM, Tsai JS and Chen YL: MicroRNA-221/222 mediates
ADSC-Exosome-Induced cardioprotection against ischemia/reperfusion
by targeting PUMA and ETS-1. Front Cell Dev Biol.
8(569150)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen W and Frangogiannis NG: Fibroblasts
in post-infarction inflammation and cardiac repair. Biochim Biophys
Acta. 1833:945–953. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Molkentin JD, Bugg D, Ghearing N, Dorn LE,
Kim P, Sargent MA, Gunaje J, Otsu K and Davis J:
Fibroblast-specific genetic manipulation of p38 mitogen-activated
protein kinase in vivo reveals its central regulatory role in
fibrosis. Circulation. 136:549–561. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhao RR, Ackers-Johnson M, Stenzig J, Chen
C, Ding T, Zhou Y, Wang P, Ng SL, Li PY, Teo G, et al: Targeting
chondroitin sulfate glycosaminoglycans to treat cardiac fibrosis in
pathological remodeling. Circulation. 137:2497–2513.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chelini G, Pantazopoulos H, Durning P and
Berretta S: The tetrapartite synapse: A key concept in the
pathophysiology of schizophrenia. Eur Psychiatry. 50:60–69.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hayes AJ and Melrose J: Neural tissue
homeostasis and repair is regulated via CS and DS proteoglycan
motifs. Front Cell Dev Biol. 9(696640)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kai Y, Tomoda K, Yoneyama H, Kitabatake M,
Nakamura A, Ito T, Yoshikawa M and Kimura H: Silencing of
carbohydrate sulfotransferase 15 hinders murine pulmonary fibrosis
development. Mol Ther Nucleic Acids. 6:163–172. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barallobre-Barreiro J, Radovits T, Fava M,
Mayr U, Lin WY, Ermolaeva E, Martínez-López D, Lindberg EL,
Duregotti E, Daróczi L, et al: Extracellular matrix in heart
failure: Role of ADAMTS5 in proteoglycan remodeling. Circulation.
144:2021–2034. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gardner RT and Habecker BA:
Infarct-derived chondroitin sulfate proteoglycans prevent
sympathetic reinnervation after cardiac ischemia-reperfusion
injury. J Neurosci. 33:7175–7183. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gardner RT, Wang L, Lang BT, Cregg JM,
Dunbar CL, Woodward WR, Silver J, Ripplinger CM and Habecker BA:
Targeting protein tyrosine phosphatase sigma after myocardial
infarction restores cardiac sympathetic innervation and prevents
arrhythmias. Nat Commun. 6(6235)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Miyata S and Kitagawa H: Chondroitin
sulfate and neuronal disorders. Front Biosci (Landmark Ed).
21:1330–1340. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Woodcock HV, Eley JD, Guillotin D, Platé
M, Nanthakumar CB, Martufi M, Peace S, Joberty G, Poeckel D, Good
RB, et al: The mTORC1/4E-BP1 axis represents a critical signaling
node during fibrogenesis. Nat Commun. 10(6)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Susarla BT, Laing ED, Yu P, Katagiri Y,
Geller HM and Symes AJ: Smad proteins differentially regulate
transforming growth-β-mediated induction of chondroitin sulfate
proteoglycans. J Neurochem. 119:868–878. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jahan N and Hannila SS: Transforming
growth factor β-induced expression of chondroitin sulfate
proteoglycans is mediated through non-Smad signaling pathways. Exp
Neurol. 263:372–384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tian K, Liu Z, Wang J, Xu S, You T and Liu
P: Sirtuin-6 inhibits cardiac fibroblasts differentiation into
myofibroblasts via inactivation of nuclear factor kappaB signaling.
Transl Res. 165:374–386. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ryou MG and Mallet RT: An in vitro
oxygen-glucose deprivation model for studying ischemia-reperfusion
injury of neuronal cells. Methods Mol Biol. 1717:229–235.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Olivares-Silva F, Espitia-Corredor J,
Letelier A, Vivar R, Parra-Flores P, Olmedo I, Montenegro J,
Pardo-Jiménez V and Díaz-Araya G: TGF-β1 decreases CHOP expression
and prevents cardiac fibroblast apoptosis induced by endoplasmic
reticulum stress. Toxicology In vitro. 70(105041)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vivar R, Humeres C, Ayala P, Olmedo I,
Catalán M, García L, Lavandero S and Díaz-Araya G: TGF-β1 prevents
simulated ischemia/reperfusion-induced cardiac fibroblast apoptosis
by activation of both canonical and non-canonical signaling
pathways. Biochim Biophys Acta. 1832:754–762. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Frangogiannis NG: Cardiac fibrosis: Cell
biological mechanisms, molecular pathways and therapeutic
opportunities. Mol Aspects Med. 65:70–99. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pearson CS, Mencio CP, Barber AC, Martin
KR and Geller HM: Identification of a critical sulfation in
chondroitin that inhibits axonal regeneration. ELife.
7(e37139)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khalil H, Kanisicak O, Prasad V, Correll
RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, et al:
Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac
fibrosis. J Clin Invest. 127:3770–3783. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Huang S, Chen B, Su Y, Alex L, Humeres C,
Shinde AV, Conway SJ and Frangogiannis NG: Distinct roles of
myofibroblast-specific Smad2 and Smad3 signaling in repair and
remodeling of the infarcted heart. J Mol Cell Cardiol. 132:84–97.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang J, Fan G, Zhao H, Wang Z, Li F,
Zhang P, Zhang J, Wang X and Wang W: Targeted inhibition of focal
adhesion kinase attenuates cardiac fibrosis and preserves heart
function in adverse cardiac remodeling. Sci Rep.
7(43146)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bradley JM, Spaletra P, Li Z, Sharp TE
III, Goodchild TT, Corral LG, Fung L, Chan KW, Sullivan RW,
Swindlehurst CA and Lefer DJ: A novel fibroblast activation
inhibitor attenuates left ventricular remodeling and preserves
cardiac function in heart failure. Am J Physiol Heart Circ Physiol.
315:H563–H570. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kwok JC, Heller JP, Zhao RR and Fawcett
JW: Targeting inhibitory chondroitin sulphate proteoglycans to
promote plasticity after injury. Methods Mol Biol. 1162:127–138.
2014.PubMed/NCBI View Article : Google Scholar
|