Introduction
Hepatic fibrosis (HF) is characterized by the
abnormal secretion and degradation of the extracellular matrix
(ECM) in the liver that may be caused by a number of different
factors, including viral infection, alcohol consumption and drug
abuse (1). HF is considered to be a
healing reaction to liver injury, which if occurs in excess, can
eventually develop into cirrhosis (2). The majority of pharmacological agents
that are currently being investigated have been suggested to exert
weak antifibrotic and also certain hepatoprotective effects
(3). In particular, inhibition of
hepatic stellate cell (HSC) proliferation is considered to be an
important component for both preventing and subsequently treating
HF, as in chronic liver injury HSCs are activated, proliferate and
produce abundant ECM, thereby differentiating into myofibroblasts
(4).
Ionotus obliquus, commonly known as Chaga, is
a medicinal fungus (order, Hymenochaetales; division,
Basidiomycota) that primarily grows on birch in the temperate
regions or cold northern climates (5). Ionotus obliquus has been
previously reported to exhibit anticancer, antiaging and
hepatoprotective effects (6). In
recent years, there has been an increasing number of studies
exploring the clinical application of Inonotus obliquus and
determining its biologically active ingredients (7). Inonotsuoxide B is considered to serve
a role in mediating the effects of Ionotus obliquus and can
be isolated by silica, octadecylsilane and Sephadex LH-20 column
chromatography as white, needle-like crystals (8). The structure and composition of
inonotsuoxide B have been previously confirmed by mass
spectrometry, 1H- and 13C-nuclear magnetic
resonance spectral data, where inonotsuoxide B has been referenced
previously in published literature as a tetracyclic triterpenoid
(8). Although tetracyclic
triterpenoids have been previously suggested to potentially inhibit
the proliferation of hepatocytes and gastric cancer cells, the
potential antifibrotic effects of this compound remain poorly
understood. Therefore, the present study examined the in
vitro effects of inonotsuoxide B isolated from Inonotus
obliquus on platelet-derived growth factor (PDGF)-BB-induced
HSC proliferation and activation.
Materials and methods
Cells and reagents
Inonotsuoxide B was provided by Dr. Wenming Cheng,
Department of Natural Medicine and Chemistry, School of Pharmacy,
Anhui Medical University (Hefei, China). The rat hepatic stellate
cell line HSC-T6 was purchased from Jiangsu KeyGEN BioTECH Corp.,
Ltd. PDGF-BB was obtained from PeproTech China. TRIzol®
reagent was purchased from Thermo Fisher Scientific, Inc. (cat. no.
14050) and TB Green™ Premix Ex Taq™ II kit was purchased from
Takara Biotechnology Co., Ltd. (cat. no. AH80340A). The following
antibodies were used: Anti-α-smooth-muscle actin (α-SMA; cat. no.
bs-10196R; lot. no. AG0734630), anti-collagen I (COL-I; cat. no.
bs-10423R; lot no. AE120941; both from BIOSS), anti-AKT (cat. no.
ESAP12208; Elabscience, Inc.), anti-phosphorylated (p)-AKT (cat.
no. AF0016; lot no. 17q2826; Affinity Biosciences), anti-PI3K (cat.
no. bsm-33219M; lot no. AH01181961; BIOSS), anti-phosphorylated
(p)-PI3K (cat. no. ab138364; lot no. GR154304-8; Abcam), anti-p-ERK
(cat. no. WL2201; lot no. I09051512), anti-ERK (cat. no. WL02371;
lot no. I04102371; both from Wanlei Biological Technology Co.,
Ltd.), anti-β-actin (cat. no. TA-09; lot no. 19C10511; OriGene
Technologies, Inc.), horseradish peroxidase (HRP)-conjugated
anti-rabbit (cat. no. S0001; lot no. 56j9958; Affinity Biosciences)
and HRP-conjugated anti-mouse (cat. no. ZB-2305; lot no. 193701224,
OriGene Technologies, Inc.).
Cell culture and drug preparation
The purchased frozen HSC-T6 cells were transferred
to DMEM (Nanjing Wisent Biological Co., Ltd.) containing 5% FBS
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.) and
cultured at 37˚C with 5% CO2. The culture medium was
replaced every 2 days and cells were sub-cultured every 2-3 days.
Following three passages, cells in a logarithmic growth phase were
used for subsequent experiments.
Solid inonotsuoxide B crystals were dissolved in
DMSO at a concentration of 1x105 µg/ml.
Cell treatment
HSC-T6 cells were collected and seeded into a
96-well culture plate at a concentration of 1x104
cells/well or HSC-T6 cells were seeded at a concentration of
1x105/ml into a six-well culture plate and incubated in
DMEM containing 5% FBS and cultured at 37˚C with 5% CO2
(9). The cells were then divided
into the following five groups: Control, PDGF-BB (10 ng/ml) and
PDGF-BB + inonotsuoxide B (5, 10 and 20 µg/ml). Following culture
for 24 h at 37˚C, the medium was changed to serum-free DMEM and
appropriate concentrations of inonotsuoxide B were added to the
PDGF-BB + inonotsuoxide B groups. After 30 min, apart from the
control group that did not receive any treatment, all other groups
were treated with PDGF-BB.
The experimental groups used for treatment with the
PI3K inhibitor LY294002 were as follows: Control, PDGF-BB (10
ng/ml), PDGF-BB + inonotsuoxide B (20 µg/ml) and PDGF-BB + LY294002
(20 nmol/l). HSC-T6 cells were seeded at a concentration of
1x105/ml into a six-well culture plate and incubated at
37˚C with 5% CO2. Following culture for 24 h at 37˚C,
the medium was changed to serum-free DMEM and innotsuoxide B (20
µg/ml) and LY294002 (20 nmol/l) were added to PDGF-BB +
inootsuoxide B group and PDGF-BB + LY294002 group, respectively.
Following incubation at 37˚C for 30 min, all groups received
PDGF-BB treatment except for the control group that did not receive
any treatment. Following incubation for 24 h at 37˚C, the cells
were lysed in RIPA lysis buffer. The experimental groups used for
treatment with the ERK inhibitor UO126 were as follows: Control,
PDGF-BB (10 ng/ml), PDGF-BB + inonotsuoxide B (20 µg/ml) and
PDGF-BB + UO126 (20 nmol/l). The aforementioned experimental method
was followed.
Cell cytotoxicity measurement
HSC-T6 cells were collected and seeded into a
96-well culture plate at a concentration of 1x104
cells/well (8) and divided into
seven groups: Control, inonotsuoxide B (5, 10, 20, 40 and 80 µg/ml)
and DMSO (0.1 µl). A total of four duplicate wells were used for
each group, with 100 µl cell suspension per well. The outer wells
were filled with 100 µl PBS and the plate was incubated in an
incubator at 37˚C with 5% CO2 for 8 h. Following
attachment of the cells, the medium was replaced with serum-free
DMEM. DMSO was added to the DMSO groups, whilst solutions of
inonotsuoxide B at the stated concentrations were added to the
cells designated to the inonotsuoxide B groups. Following 24 h, the
culture medium was discarded and 20 µl MTT solution (5 mg/ml) were
added to each well. Following incubation at 37˚C for 4 h, the
supernatant was discarded and 150 µl DMSO were added to each well
and incubated on a shaker at 37˚C for 10 min. The absorbance of
each well was measured at 570 nm (10).
Cell viability assay
Before the effect of inonotsuoxide B on cell
viability in the presence of PDGF-BB was determined using the MTT
assay, HSC-T6 cells were collected. The cells were then divided
into the following five groups: Control, PDGF-BB (10 ng/ml) and
PDGF-BB + inonotsuoxide B (5, 10 and 20 µg/ml). The PDGF-BB +
inonotsuoxide B groups were treated with corresponding
concentrations of inonotsuoxide B for 30 min at 37˚C. Thereafter,
all groups except for the control were treated with 10 ng/ml
PDGF-BB at 37˚C for 24 h. The MTT assay experimental procedure was
performed as aforementioned. Cell viability or cytotoxicity
calculation formula: Cell viability=[(mean absorbance sample-mean
absorbance blank)/(mean absorbance control-mean absorbance
blank)]*100.
Western blot analysis
HSC-T6 cells were seeded at a concentration of
1x105/ml into a six-well culture plate and incubated at
37˚C with 5% CO2. The cells were then divided into the
following five groups: Control, PDGF-BB (10 ng/ml) and PDGF-BB +
inonotsuoxide B (5, 10 and 20 µg/ml). The processed cells were
lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology).
Protein concentration was measured using the bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology) and the
samples (50 µg) were subjected to 10% SDS-PAGE (11,12)
and then transferred onto PVDF membranes, which were blocked with
5% skimmed milk at 25˚C for 3 h. Subsequently, rabbit anti-AKT,
rabbit anti-p-ERK, rabbit anti-p-PI3K, rabbit anti-p-AKT, rabbit
anti-ERK, rabbit anti-PI3K, rabbit anti-α-SMA, rabbit anti-COL-Ι
and mouse anti-β-actin (all diluted 1:1,000) were added to the
membranes and incubated at 4˚C overnight. The membranes were then
incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit
or HRP-conjugated anti-mouse (all diluted 1:1,000) the following
day for 1 h at room temperature, before the ECL chromogenic
solution (Pierce; Thermo Fisher Scientific, Inc.) was added to
develop the membrane (13).
Finally, ImageJ software (version 1.8.0_101; National Institutes of
Health) was used for the quantification of densitometry.
Reverse transcription-quantitative PCR
(RT-qPCR)
HSC-T6 cells were divided into the following five
groups: Control, PDGF-BB (10 ng/ml) and PDGF-BB + inonotsuoxide B
(5, 10 and 20 µg/ml). Cells in each group were collected and total
RNA was extracted using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Briefly, 1 ml lysis buffer was
added to the cells and incubated for 5 min at room temperature.
Subsequently, 200 µl CHCl3 was added, mixed with the
cell extract via vigorous shaking and the solution was incubated
for 10 min at room temperature. The samples were centrifuged
(14,800 x g) at 4˚C for 30 min. The supernatant (400 µl) was
collected and added to an equivalent volume of isopropyl alcohol.
The mixture was stored at -20˚C for 2 h and centrifuged (14,800 x
g) at 4˚C for 30 min. Subsequently, the supernatant was discarded
and 1 ml of ethanol was added to each sample, which were
centrifuged (14,800 x g) at 4˚C for 15 min. RNA was collected
following air-drying for 10 min before 20 µl RNase-free water was
added to dissolve the collected RNA, which was stored at -80˚C
until subsequent analysis (14).
According to the manufacturer's protocol, cDNA was synthesized by
reverse transcription using Takara PrimeScript RT Master Mix kit
(Takara Bio, Inc.). The temperature protocol was as follows: 15 min
at 37˚C; 5 sec at 85˚C; and 10 min at 4˚C. qPCR was performed using
SYBR®-Green Master Mix (Takara Bio, Inc.) according to
the manufacturer's protocol. qPCR amplification was performed using
β-actin as an internal reference. The following primer sequences
were used: β-actin forward, 5'-CCGAGATCTCACCGACTACC-3' and reverse,
5'-TCCAGAGCGACATAGCACAG-3' and α-SMA forward,
5'-TCCTCCTGAGCGCAAGTACTCT-3' and reverse,
5'-GCTCAGTAACAGTCCGCCTAGAA-3'. Pre-denaturation was performed at
95˚C for 30 sec, followed by 40 cycles of 95˚C for 5 sec and 60˚C
for 30 sec (15). Quantification of
the mRNA expression was performed using the 2-ΔΔCq
method (16,17).
Statistical analysis
Data are presented as the mean ± SEM. Statistical
analysis was performed via one-way ANOVA followed by Tukey's post
hoc test using GraphPad Prism v5 software (GraphPad Software,
Inc.). Each group of experiments was performed ≥3 times. P<0.05
was considered to indicate a statistically significant
difference.
Results
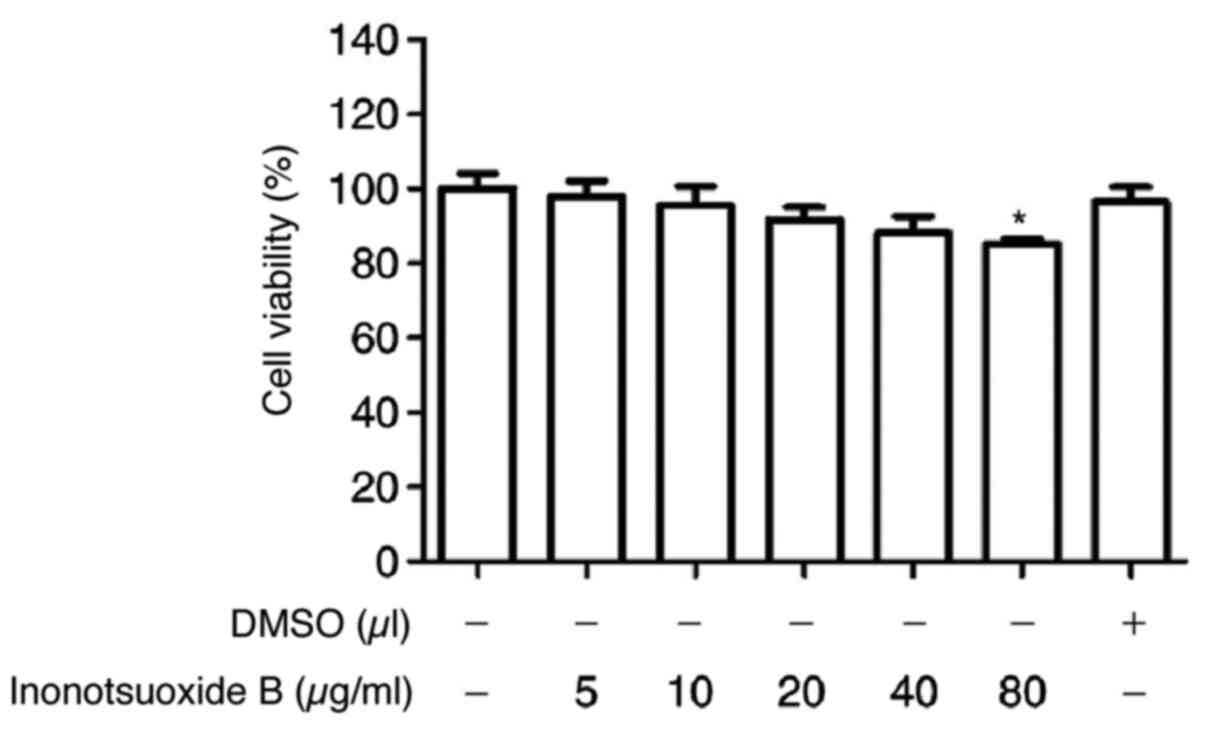
Effect of inonotsuoxide B on cell
cytotoxicity
HSC-T6 cells were cultured with different
concentrations of inonotsuoxide B dissolved in DMSO (5, 10, 20, 40
and 80 µg/ml) for 24 h. DMSO exhibited no effects on cell
viability. Compared with that of the control group, inonotsuoxide B
(5, 10, 20 and 40 µg/ml) exhibited only minor effects on cell
viability (Fig. 1). However, cell
viability was significantly reduced following incubation with 80
µg/ml of inonotsuoxide B. As a result, inonotsuoxide B at
concentrations of 5, 10 and 20 µg/ml were used for subsequent
experiments (Fig. 1).
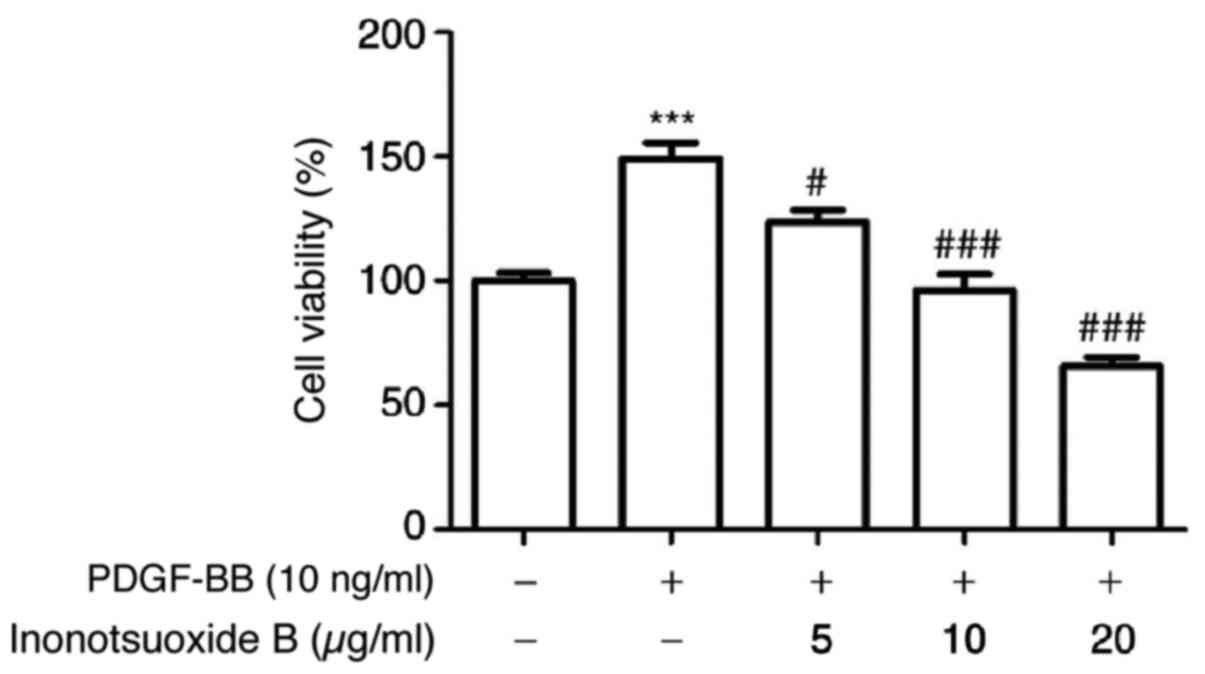
Inonotsuoxide B reverses
PDGF-BB-mediated increases in HSC viability
Compared with the control group, PDGF-BB (10 ng/ml)
significantly increased the viability of HSC-T6 cells, which was in
turn inhibited by inonotsuoxide B (5, 10 and 20 µg/ml) in a
concentration-dependent manner (Fig.
2).
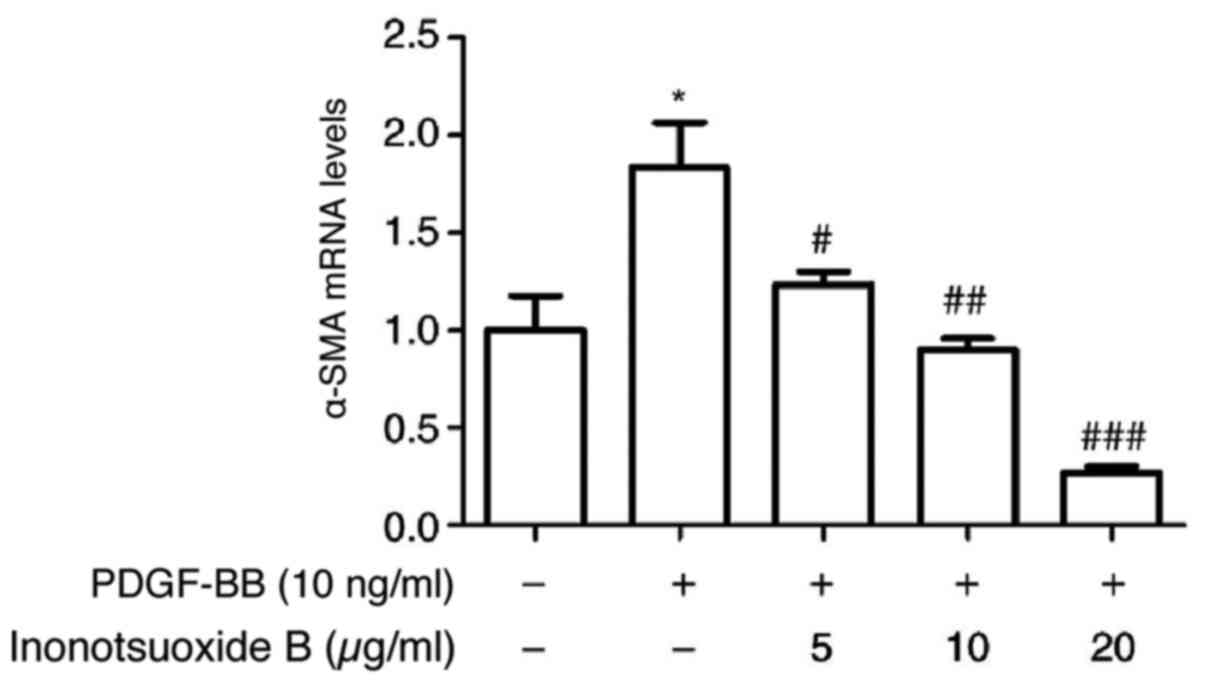
Inonotsuoxide B suppresses
PDGF-BB-induced α-SMA mRNA expression in HSCs
The effect of inonotsuoxide B on the mRNA levels of
α-SMA in HSCs was examined by RT-qPCR. As presented in Fig. 3, α-SMA mRNA expression in the
PDGF-BB group was significantly increased compared with the control
group. At concentrations of 5, 10 and 20 µg/ml, inonotsuoxide B
significantly reduced the mRNA expression of α-SMA compared with
the PDGF-BB group in a dose-dependent manner (Fig. 3).
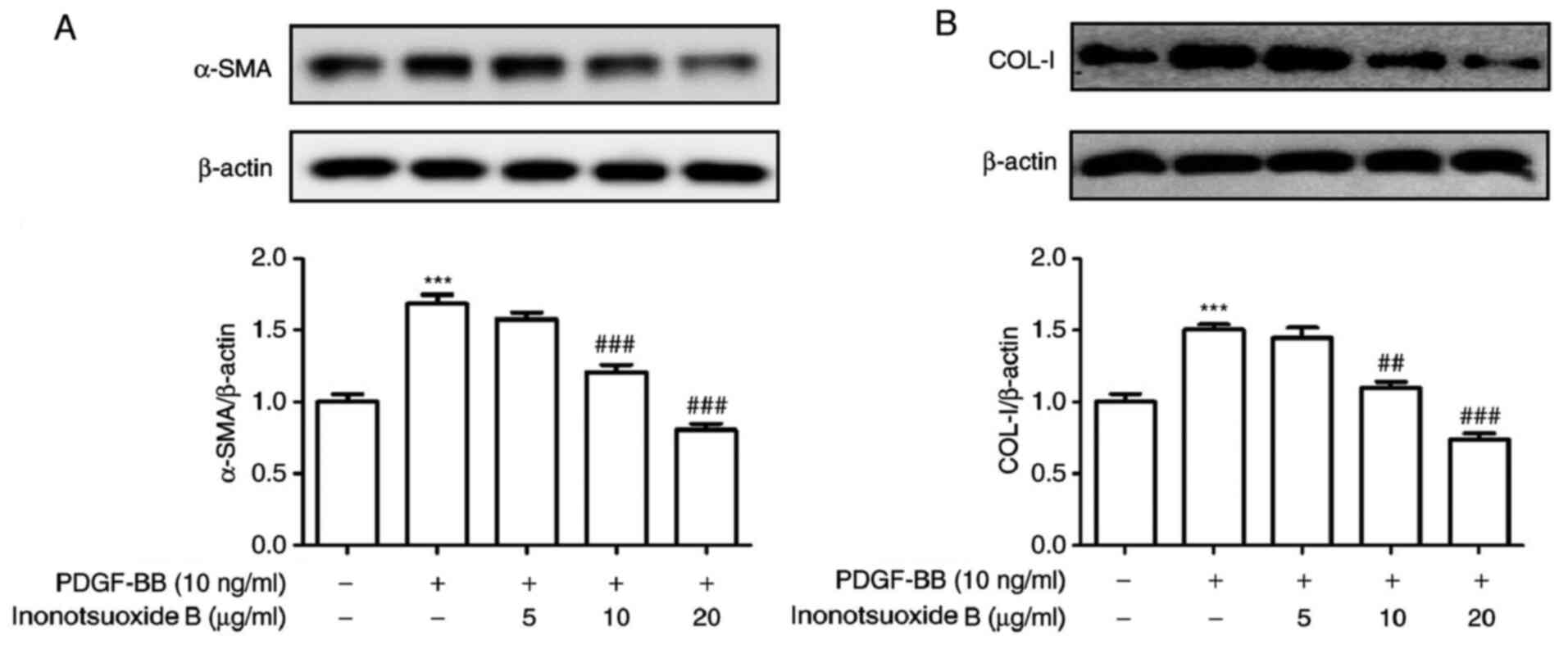
Inonotsuoxide B suppresses the
PDGF-BB-induced increases in α-SMA and COL-Ⅰ protein expression in
HSCs
The protein expression of the HSC activation markers
α-SMA and COL-Ⅰ was measured using western blotting. The protein
expression levels of α-SMA and COL-Ⅰ were significantly increased
in the PDGF-BB group compared the control group, but were
significantly decreased following inonotsuoxide B (10 and 20 µg/ml)
treatment. By contrast, 5 µg/ml inonotsuoxide B did not exert
significant effects on the protein expression levels of α-SMA and
COL-Ⅰ compared with those in the PDGF-BB group (Fig. 4).
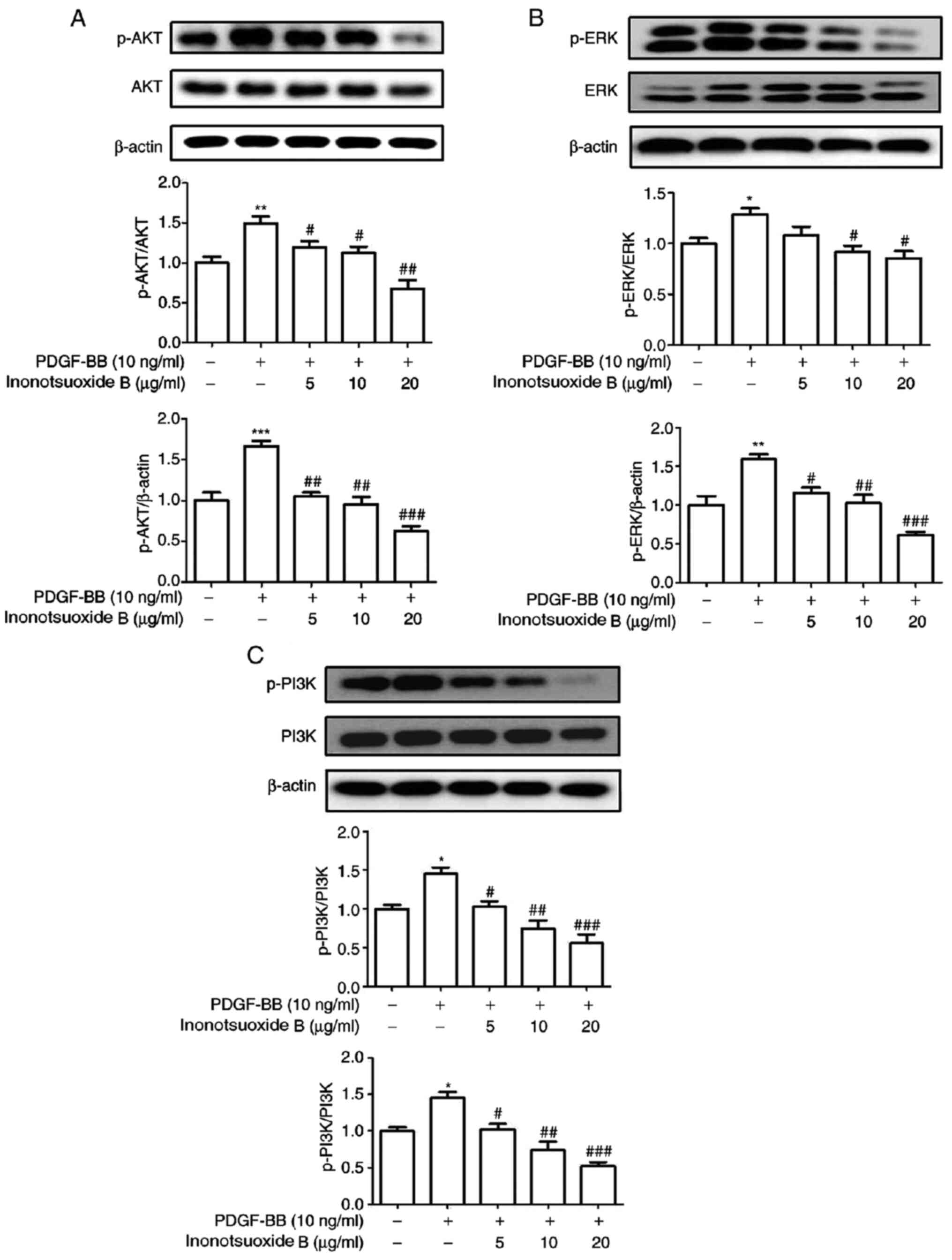
Inonotsuoxide B suppresses
PDGF-BB-induced AKT, ERK1/2 and PI3K phosphorylation in HSCs
The phosphorylation of AKT, ERK1/2 and PI3K in each
treatment group was measured via western blotting. As demonstrated
in Fig. 5, the levels of AKT,
ERK1/2 and PI3K phosphorylation were significantly increased in the
PDGF-BB group compared with the control group. Treatment with
inonotsuoxide B (5, 10 and 20 µg/ml) markedly reduced the levels of
AKT and PI3K phosphorylation compared with those in the PDGF-BB
group in a dose-dependent manner. However, the protein expression
level of p-ERK was not significantly altered following treatment
with 5 µg/ml innotsuoxide B, while p-ERK was significantly
decreased after treatment with innotsuoxide B at 10 and 20
µg/ml.
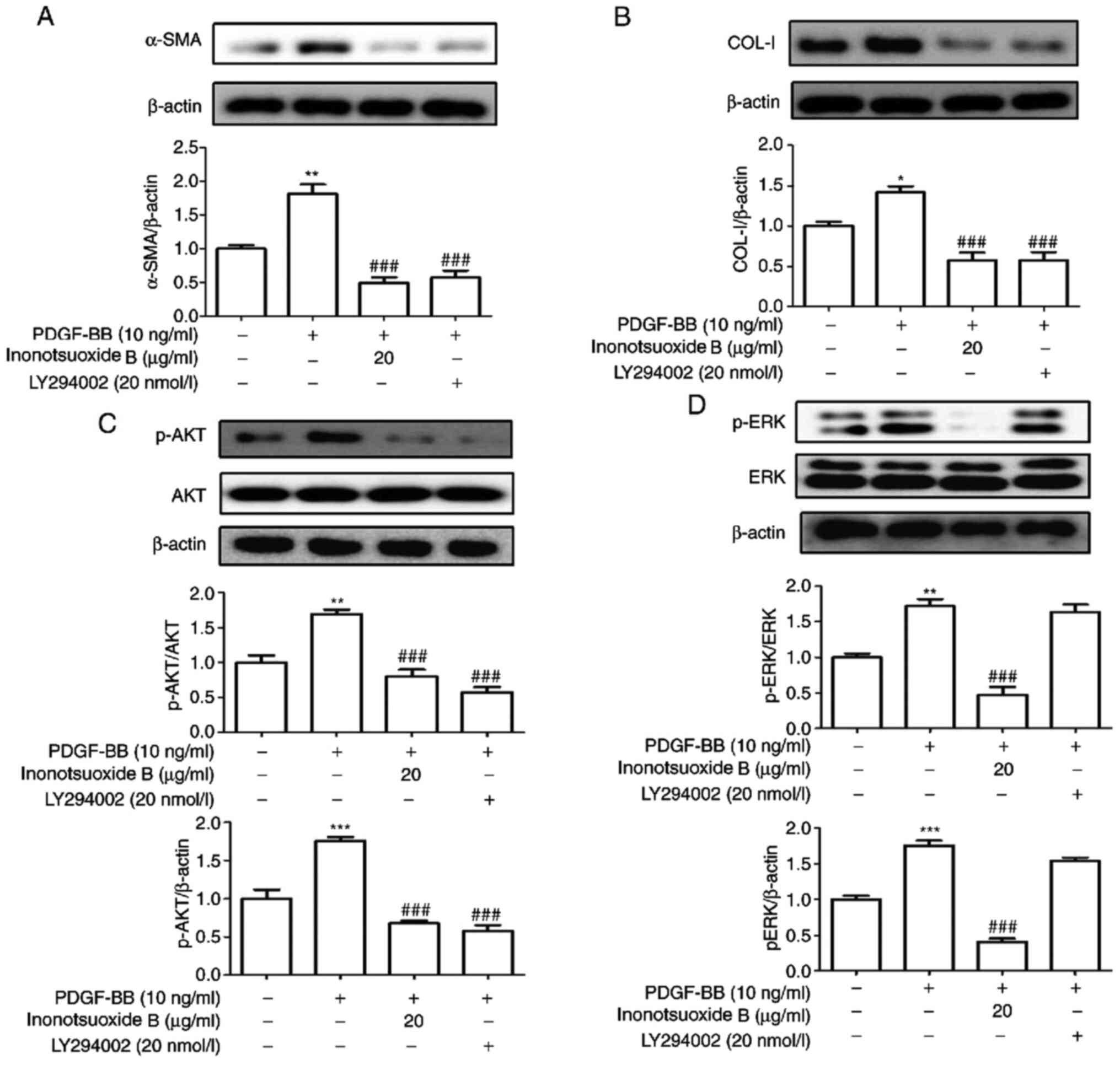
PI3K inhibitor LY294002 suppresses
PDGF-BB-induced α-SMA and COL-I expression in addition to AKT
phosphorylation in HSCs
LY294002 is a specific blocker of the PI3K signaling
pathway (18). The effects of
LY294002 on the expression of the α-SMA, COL-I, AKT and ERK1/2
phosphorylation was examined by western blotting. As indicated in
Fig. 6, the expression levels of
α-SMA and COL-I proteins, in addition to AKT and ERK
phosphorylation, were significantly increased in PDGF-BB-treated
HSC-T6 cells compared with control cells. The expression levels of
α-SMA, COL-I and AKT phosphorylation were also significantly
decreased in the presence of LY294002, whilst LY294002 did not
exert significant effects on ERK1/2 phosphorylation compared with
the PDGF-BB group. Compared with the PDGF-BB group, the protein
expression levels of α-SMA, COL-I, p-AKT and p-ERK1/2 in PDGF-BB +
inonotsuoxide B (20 µg/ml) group were significantly reduced.
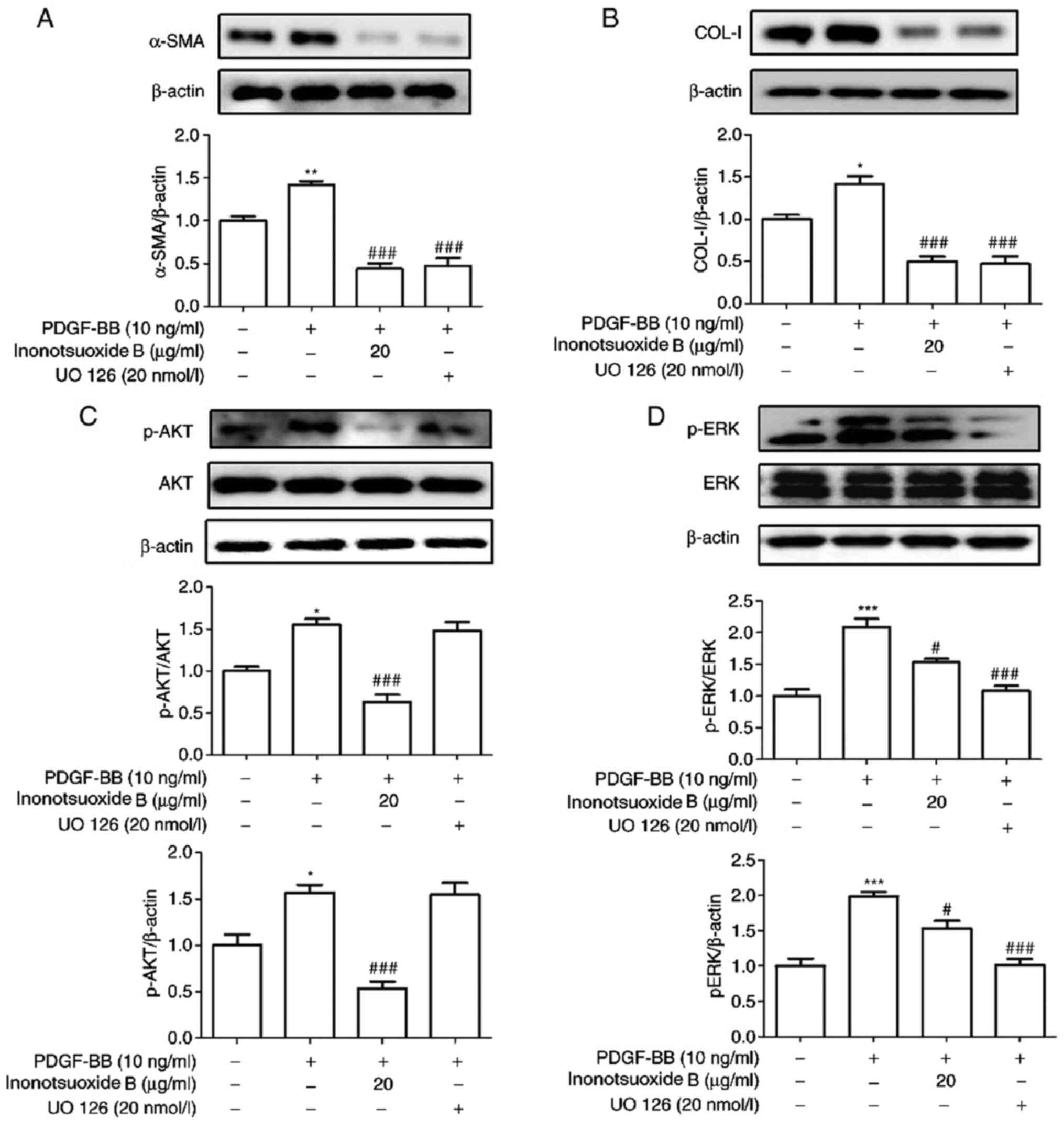
ERK inhibitor UO126 suppresses
PDGF-BB-induced increases in α-SMA and COL-Ⅰ expression in addition
to ERK phosphorylation in HSCs
UO126 is a specific blocker of the ERK signaling
pathway (19). The effect of UO126
on the expression of the α-SMA and COL-I in addition to AKT and
ERK1/2 phosphorylation was examined via western blotting. As
demonstrated in Fig. 7, the
expression levels of α-SMA, COL-I, AKT phosphorylation and ERK
phosphorylation were significantly increased in PDGF-BB-treated
HSC-T6 cells compared with the control cells. The expression levels
of α-SMA, COL-I and ERK phosphorylation were found to be
significantly reduced in the UO126-treated group compared with
those in the PDGF-BB group. By contrast, UO126 treatment did not
result in significant effects on AKT phosphorylation compared with
that in the PDGF-BB group. In addition, compared with the PDGF-BB
group, the protein expression levels of α-SMA, COL-I, p-AKT and
p-ERK1/2 in PDGF-BB + inonotsuoxide B (20 µg/ml) group were
significantly reduced.
Discussion
The mechanism of liver fibrosis is complex (20). The proliferation and activation of
HSCs and associated signaling pathways have been the focus of
studies on liver fibrosis (21).
However, research on potential treatments against liver fibrosis
has primarily focused on singular targets and pathways.
Consequently, treatments that are currently available for liver
fibrosis have not always been effective (22). Previous studies have suggested that
the chemical constituents of Inonotus obliquus
(polysaccharide, inonot and betulin) exhibited anticancer,
antiaging, blood sugar and blood pressure reducing pharmacological
effects (23,24). Inonotsuoxide B is a tetracyclic
triterpenoid that can be extracted from Inonotus obliquus
and has been previously demonstrated to inhibit the growth of liver
and gastric cancer cells (24).
However, its effects on liver fibrosis remain poorly understood.
The present study primarily focused on the effects of inonotsuoxide
B treatment on HSC viability and activation with respect to the
PI3K/AKT and ERK1/2 signaling pathways.
PDGF is an important mitogenic factor in HSC-T6
cells (2). During liver fibrosis,
PDGF has been demonstrated to promote HSC proliferation and
collagen expression (2). A previous
study has reported that 10 ng/ml PDGF-BB stimulated the activation
of HSCs (25). Therefore, the
effect of inonotsuoxide B on HSC-T6 cells following treatment with
PDGF-BB (10 ng/ml) was examined in the present study. Compared with
those in the untreated cell group, PDGF-BB significantly increased
the cell viability and activation of HSC-T6 cells, in addition to
potentiating the expression of α-SMA and COL-Ⅰ. Furthermore, all of
these aforementioned effects were reversed following treatment of
the cells with inonotsuoxide B. These findings indicated that
inonotsuoxide B reduced the viability and activation of
PDGF-BB-stimulated HSC-T6 cells.
A previous study demonstrated that the PI3K/AKT and
ERK signaling pathways primarily mediated PDGF-induced signaling
(26). PI3K regulates the
phosphorylation of AKT, where the subsequent PI3K/AKT downstream
signaling pathway has been suggested to serve a regulatory role in
a number of cell proliferation and activation processes (27). By contrast, the ERK signaling
pathway has been reported to serve a key role in transducing
signals from cell-surface receptors to the nucleus, thereby
regulating the activation and proliferation of cells (28). Therefore, the phosphorylation of
PI3K, AKT and ERK1/2 was examined in the present study. It was
observed that the PI3K/AKT and ERK1/2 signaling pathways were
associated with the inhibitory effects of inonotsuoxide B on the
viability and activation of PDGF-BB-stimulated HSC-T6 cells.
Compared with the untreated cell group, PDGF-BB treatment
significantly upregulated the phosphorylation of PI3K, AKT and ERK
in HSC-T6 cells, all of which were revealed to be reversed by
inonotsuoxide B treatment. UO126 and LY294002 have been
demonstrated to be specific blockers of the ERK and PI3K/AKT
signaling pathways, respectively (29). Previous studies have reported that
UO126 and LY294002 inhibited the proliferation of HSCs at
concentrations <20 nmol/l, in a concentration-dependent manner
(30,31). Therefore, in the present study,
UO126 and LY294002 at 20 nmol/l were used to investigate their
potential effects on PDGF-BB-induced HSC proliferation and
activation. The results indicated that the expression levels of the
α-SMA and COL-Ⅰ proteins in PDGF-BB-stimulated HSCs were decreased
by both UO126 and LY294002 treatments.
Liver fibrosis has been indicated to be reversible,
as effective drug therapy can inhibit the activation and
proliferation of HSCs and reduce the degree of hepatic fibrosis
(32). The results of the present
study demonstrated that inonotsuoxide B inhibited the viability and
activation of PDGF-BB-stimulated HSC-T6 cells. The underlying
mechanism of action of inonotsuoxide B may be associated with the
inhibition of the PI3K/AKT and ERK signaling pathways. These
observations are clinically relevant and may enable the development
of an efficient treatment strategy against liver fibrosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by Research Fund for the
Back-up Candidates of the Academic and Technical Leaders of Anhui,
China (grant no. 2015H040), the Young top talents Program of Anhui
Medical University, Foundation for Distinguished Young Talents in
Higher Education of Anhui, China (grant no. gxyqZD2016049), the
Natural Science Research Project in Higher Education of Anhui,
China (grant no. KJ2017A192), the Hospital Youth Fund of West
Branch of The First Affiliated Hospital of University of Science
and Technology of China (grant no. 2018YJQN016), the Young Talent
Support Plan of Anhui Medical University in 2017-2019 (grant no.
0601037104), 2018 Young Talents Double Training Project Grant
(grant no. 0601037206), Science and Technology Fund of Anhui
Province for Outstanding Youth of China (grant no. 1908085J30) and
2020-2022 Anhui Medical University Basic and Clinical Cooperative
Research Promotion Project (grant no. 2019xkjT015).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and WC designed the current study. JJ and HY
performed the experiments. LH and YW analyzed the data. CH drafted
the manuscript and analyzed data. KW and ZW interpreted data and
revised the final manuscript. WW performed the experiments and
wrote the manuscript. JJ and YH confirm the authenticity of all the
raw data All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ping J, Li JT, Liao ZX, Shang L and Wang
H: Indole-3-carbinol inhibits hepatic stellate cells proliferation
by blocking NADPH oxidase/reactive oxygen species/p38 MAPK pathway.
Eur J Pharmacol. 650:656–662. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tsai MK, Lin YL and Huang YT: Differential
inhibitory effects of salvianolic acids on activation of rat
hepatic stellate cells by platelet-derived growth factor. Planta
Med. 77:1495–1503. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang YH, Sun YC, Zuo LQ, Wang YN and Huang
Y: ASIC1a promotes high glucose and PDGF-induced hepatic stellate
cell activation by inducing autophagy through CaMKKβ/ERK signaling
pathway. Toxicol Lett. 300:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shuping You, Jun Zhao and Long Ma: Effects
of total glycosides of cistanche deserticola on the proliferation
of hepatic stellate cells induced by platelet-derived growth
factor. Chin J Pharmacol. 32:1231–1235. 2016.
|
|
5
|
Choi JH, Hwang YP, Park BH, Choi CY, Chung
YC and Jeong HG: Anthocyanins isolated from the purple-fleshed
sweet potato attenuate the proliferation of hepatic stellate cells
by blocking the PDGF receptor. Environ Toxicol Pharmacol.
31:212–219. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Youn MJ, Kim JK, Park SY, Kim Y, Kim SJ,
Lee JS, Chai KY, Kim HJ, Cui MX, So HS, et al: Chaga mushroom
(Inonotus obliquus) induces G0/G1 arrest and apoptosis in human
hepatoma HepG2 cells. World J Gastroenterol. 14:511–517.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Duru KC, Kovaleva EG, Danilova IG and van
der Bijl P: The pharmacological potential and possible molecular
mechanisms of action of Inonotus obliquus from preclinical studies.
Phytother Res. 33:1966–1980. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Kun WU, Wenming Cheng, Chunru Li, et al:
Screening and chemical constituents of anti-tumor active parts of
inonotus obliquus. J Anhui Medical University. 51:1468–1472.
2016.
|
|
9
|
Huang Y, Li X, Wang Y, Wang H, Huang C and
Li J: Endoplasmic reticulum stress-induced hepatic stellate cell
apoptosis through calcium-mediated JNK/P38 MAPK and
Calpain/Caspase-12 pathways. Mol Cell Biochem. 394:1–12.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Foo NP, Lin SH, Lee YH, Wu MJ and Wang YJ:
α-Lipoic acid inhibits liver fibrosis through the attenuation of
ROS-triggered signaling in hepatic stellate cells activated by PDGF
and TGF-β. Toxicology. 282:39–46. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang Y, Leng TD, Inoue K, Yang T, Liu M,
Horgen FD, Fleig A, Li J and Xiong ZG: TRPM7 channels play a role
in high glucose-induced endoplasmic reticulum stress and neuronal
cell apoptosis. J Biol Chem. 293:14393–14406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang H, Wang YH, Yang F, Li XF, Tian YY,
Ni MM, Zuo LQ, Meng XM and Huang Y: Effect of Acid-sensing ion
Channel 1a on the process of liver fibrosis under hyperglycemia.
Biochem Biophys Res Commun. 468:758–765. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li J, Li X, Xu W, Wang S, Hu Z, Zhang Q,
Deng X, Wang J, Zhang J and Guo C: Antifibrotic effects of luteolin
on hepatic stellate cells and liver fibrosis by targeting
AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways. Liver Int.
35:1222–1233. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tsai TH, Shih SC, Ho TC, Ma HI, Liu MY,
Chen SL and Tsao YP: Pigment epithelium-derived factor 34-mer
peptide prevents liver fibrosis and hepatic stellate cell
activation through down-regulation of the PDGF receptor. PLoS One.
9(e95443)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yujie Zhang, Yutao Hong and Lei Jie:
Effects of melatonin on proliferation of HSC-T6 cells induced by
PDGF-BB and its mechanism. J Anhui Med University. 53:12–17.
2018.
|
|
16
|
You SP, Zhao J, Ma L, Tudimat M, Zhang SL
and Liu T: Preventive effects of phenylethanol glycosides from
Cistanche tubulosa on bovine serum albumin-induced hepatic fibrosis
in rats. Daru. 23(52)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expres-sion data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mazumder AG, Kumari S and Singh D:
Anticonvulsant action of a selective phosphatidylinositol-3-kinase
inhibitor LY294002 in pentylenetetrazole-mediated convulsions in
zebrafish. Epilepsy Res. 157(106207)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cole GW Jr, Alleva AM, Zuo JT, Sehgal SS,
Yeow WS, Schrump DS and Nguyen DM: Suppression of pro-metastasis
phenotypes expression in malignant pleural mesothelioma by the PI3K
inhibitor LY294002 or the MEK inhibitor UO126. Anticancer Res.
26:809–821. 2006.PubMed/NCBI
|
|
20
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bataller R and Brenner DA: Hepatic
stellate cells as a target for the treatment of liver fibrosis.
Semin Liver Dis. 21:437–452. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Y, Gao J, Zhang D, Zhang J, Ma J and
Jiang H: New insights into the antifibrotic effects of sorafenib on
hepatic stellate cells and liver fibrosis. J Hepatol. 53:132–144.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Balandaykin ME and Zmitrovich IV: Review
on chaga medicinal mushroom, inonotus obliquus (Higher
Basidiomycetes): Realm of medicinal applications and approaches on
estimating its resource potential. Int J Med Mushrooms. 17:95–104.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Glamočlija J, Ćirić A, Nikolić M,
Fernandes Â, Barros L, Calhelha RC, Ferreira IC, Soković M and van
Griensven LJ: Chemical characterization and biological activity of
Chaga (Inonotus obliquus), a medicinal ‘mushroom’. J
Ethnopharmacol. 162:323–332. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang GJ, Huang YJ, Chen DH and Lin YL:
Ganoderma lucidum extract attenuates the proliferation of
hepatic stellate cells by blocking the PDGF receptor. Phytother
Res. 23:833–839. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Jiang M, Wu YL, Li X, Zhang Y, Xia KL, Cui
BW, Lian LH and Nan JX: Oligomeric proanthocyanidin derived from
grape seeds inhibited NF-κB signaling in activated HSC: Involvement
of JNK/ERK MAPK and PI3K/Akt pathways. Biomed Pharmacother.
93:674–680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kao YH, Chen PH, Wu TY, Lin YC, Tsai MS,
Lee PH, Tai TS, Chang HR and Sun CK: Lipopolysaccharides induce
Smad2 phosphorylation through PI3K/Akt and MAPK cascades in HSC-T6
hepatic stellate cells. Life Sci. 184:37–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fang L, Zhan S, Huang C, Cheng X, Lv X, Si
H and Li J: TRPM7 channel regulates PDGF-BB-induced proliferation
of hepatic stellate cells via PI3K and ERK pathways. Toxicol Appl
Pharmacol. 27:713–725. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
He W, Shi F, Zhou ZW, Li B, Zhang K, Zhang
X, Ouyang C, Zhou SF and Zhu X: A bioinformatic and mechanistic
study elicits the antifibrotic effect of ursolic acid through the
attenuation of oxidative stress with the involvement of ERK,
PI3K/Akt, and p38 MAPK signaling pathways in human hepatic stellate
cells and rat liver. Drug Des Devel Ther. 9:3989–4104.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Jinxing, Zhu Xianli and Li Yu: Rats
with diffuse brain injury block the ERK pathway and down-regulate
the expression of MMP-9 mRNA in brain tissue. Chin J Clin
Neurosurg. 11:288–291. 2006.
|
|
31
|
You Linlin, Dai Lili, et al: Inhibitory
effect of Danshensu on hepatic stellate cells stimulated by
PDGF-BB. J Third Military Med University. 33:1610–1614. 2011.
|
|
32
|
Xu DD, Li XF, Li YH, Liu YH, Huang C, Meng
XM and Li J: TIPE2 attenuates liver fibrosis by reversing the
activated hepatic stellate cells. Biochem Biophys Res Commun.
498:199–206. 2018.PubMed/NCBI View Article : Google Scholar
|