Introduction
Although the success rate of resuscitation from
cardiac arrest can reach 50% after the implementation of
high-quality cardiopulmonary resuscitation (CPR), only 5-15% of
cases can survive to discharge due to the poor prognosis (1). The syndrome shown by myocardial
dysfunction, cerebral injury and systemic organic
ischemia-reperfusion injury (IRI) which could be illustrated as IRI
and due to the systemic inflammatory responses is the core
characteristic of post-resuscitation syndrome (2). Studies have confirmed that the
intestines may be the most sensitive parts to IRI. Cardiac arrest
leads to a continuous decrease in intestinal blood flow and an
increase in intestinal permeability, subsequently triggering
systemic inflammatory responses, which could be the underlying
mechanism to cause the sepsis (3-5).
Inflammatory cytokines are elevated after CPR on the occurrence of
sepsis. However, it remains unclear whether the post-resuscitation
syndrome will affect intestinal circulatory function. The
microcirculation is important in the treatment for patients in need
of critical care since it plays a pivotal role in the oxygen supply
and the nutritional supplementation of tissues (6).
It has been reported that there is an inconsistency
between systemic blood flow and tissue perfusion in patients with
cardiac arrest (7,8), but the microcirculation is correlated
with the prognosis of patients (7,9-11).
Therefore, microcirculatory dysfunction could be decisive in the
prognosis of circulatory failure (12,13).
Microcirculation varies greatly among different organs,
particularly under low-flow conditions (3,8,14).
It is easy to detect the microcirculation in sublingual region as a
perfect part for evaluating microcirculation (15). However, it is unclear whether it
can fully reflect the visceral microcirculation.
Anisodamine, a commonly used anti-shock drug, is an
anticholinergic drug extracted from the Chinese herbal medicine
Anisodus tonguticus with numerous beneficial effects.
Anisodamine was reported to increase intestinal perfusion during
the electric shock (16), but
there are few studies on its effects on intestinal mucosal blood
flow and metabolism. In the present study, the intestinal and
sublingual microcirculation was evaluated in CPR pig model, and
changes in hemodynamic indicators and inflammatory cytokines were
detected to identify the relationship between microcirculatory
changes and inflammatory responses. Furthermore, anisodamine
hydrobromide (AH) was administered to evaluate its protective
effects.
Materials and methods
Chemicals and reagents
Interleukin 4 (IL-4; cat.no. ELP-IL4-1), IL-2 (cat.
no. ELP-IL2-1), IL-10 (cat.no. KSC0102) and interferon-γ (IFN-γ,
cat. no. KSC4021) assay kits were purchased from Sunbio Biotech Co.
Ltd. AH was purchased from the National Institutes for Food and
Drug Control (Beijing, China) with more than 99% purity. AH was
dissolved in saline for treatment.
Experimental procedures and
treatment
A total of 24 male Beijing white pigs (12-14 months
old, 30±2 kg) were purchased from the Institute of Zoology, Chinese
Academy of Sciences (Beijing, China). All animals were housed in a
specific pathogen-free environment at 23±2˚C and 40-70% humidity
under a 12-h light/dark cycle. Pigs had free access to food and
water during the experimental period. All procedures were performed
following the Animal Care Guidelines of the Institutional Animal
Care and Use Committee of Capital Medical University (Beijing,
China; approval no. 2020-3-18-92). Pigs were fasted overnight but
were allowed free access to water before the experiment.
After an intramuscular injection of midazolam (0.5
mg/kg; Sinopharm Chemical Reagent Co., Ltd.), anesthesia was
induced by the intravenous injection of propofol (1.0 mg/kg,
Sinopharm Chemical Reagent Co. Ltd.) and maintained with
intravenous infusion of pentobarbital (8 mg/kg/h; Sinopharm
Chemical Reagent Co. Ltd.). Heart rate and electrocardiogram
measurements were monitored using a four-channel physical recorder
(BL-420F Data Acquisition & Analysis System; TME Technology Co.
Ltd.). A cuffed 6.5 mm cannula was advanced into the trachea. Pigs
were ventilated with a volume-controlled ventilator (Servo 900C;
Siemens AG) with a fraction of inspiration O2
(FiO2) at 0.35 and a respiratory frequency of 12
breaths/min using a tidal volume of 15 ml/kg. The aortic pressure
was measured by an angiographic catheter inserting from the femoral
artery into the aortic arch. All hemodynamic parameters were
monitored by the M1165 system (Hewlett-Packard).
Prior to the induction of cardiac arrest, pigs were
allowed to equilibrate for 30 min to reach the stable level after
anesthesia. The conductor of temporary pacemaker was inserted into
the right ventricle through the right sheath and connected to an
electrical stimulator (GY-600A; Kaifeng Huanan Equipment Co., Ltd.)
with the S1S2 mode (300/200 ms; 40 V), which provided the
continuous electrical stimulation with a proportion of 8:1 and a
step length of 10 ms, until ventricular fibrillation (VF) occurred
(17) and the mean aortic pressure
suddenly dropped to zero.
Ventilation was withheld for 8 min after the onset
of VF. Manual CPR was then conducted at a frequency of 100
compressions/min with ventilation at FiO2 of 100% and a
compression-to-ventilation ratio of 30:2. The quality of chest
compressions was controlled by a HeartStart MRx
Monitor/Defibrillator with Q-CPR (Philips Medical Systems, The
Netherlands). If the spontaneous circulation was not restored,
defibrillation was attempted with the mode of 150 J.
Restoration of spontaneous circulation (ROSC) was
defined by the systolic blood pressure >50 mm Hg for more than
10 min. If spontaneous circulation was not restored within 30 min,
the pig was considered dead (18).
Immediately after successful CPR, pigs were randomly divided into 2
groups (n=8): Saline and AH. Saline or AH (4 mg/kg) was
administered via central venous injection. The same procedures
without VF initiation were conducted in the Sham group. At the end
of the study, pigs were euthanized with an overdose of
pentobarbital (150 mg/kg) via the femoral artery.
Outcome measurement
Both the real-time mean arterial blood pressure and
central venous pressure were measured. Continuous cardiac output
and global ejection fraction were determined through a pulmonary
artery catheter.
Arterial blood gas
Arterial blood was collected at baseline and 0, 1,
2, 4 and 6 h after ROSC, and arterial blood gas and lactate levels
were measured using the blood gas analyzer (GEM Premier 3000;
Instrumentation Laboratory S.P.A.).
Measurement of cytokines
Heart tissue lysate was harvested with the Mammalian
Cell Lysis Kit (cat. no. MCL1-1KT; MilliporeSigma). IL-2, IL-4,
IL-10 as well as IFN-γ in the serum and heart tissue lysate were
evaluated using enzyme-linked immunosorbent assay in accordance
with the manufacturer's protocols.
Caspase activity measurement
The caspase 3 and caspase 9 activities in the heart
tissue lysate were determined using the colorimetric assay kits
(cat. no. ABIN6965464 for caspase 3 and cat no. ABIN6965484 for
caspase 9; Antibodies-online, Inc.) by the microplate reader,
according to the manufacturer's protocol.
Pathological analysis
Heart tissues were fixed in 2.5% glutaraldehyde for
3 h at room temperature and then rinsed three times with PBS.
Tissues were then fixed in 1% osmium tetroxide for 2 h at room
temperature. After rinsing three time with PBS, tissues were
processed through a graded series of ethanol (50% for 15 min, 70%
for 15 min and 90% for 15 min) at 4˚C and finally processed in 100%
acetone for 15 min at room temperature. After being embedded in
EMBED 812 epoxy (Electron Microscopy Sciences), samples were
polymerized at 60˚C for 24 h. Sections (50-nm thick) were cut
sagittally with LKB ultramicrotomy. The ultrastructural changes
were observed by transmission electron microscopy (JEOL Ltd.).
Western blot analysis
The heart tissues were harvested, washed in PBS
three times and then homogenized with Mammalian Cell Lysis Kit
(cat. no. MCL1-1KT; MilliporeSigma). Protein concentration was
determined by bicinchoninic acid assay. Samples with 50 µg protein
were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel,
and proteins were electro-transferred onto PVDF membranes. The
membranes were blocked with 5% non-fat milk in PBS for 1 h at room
temperature and then incubated with primary antibodies (anti-Bax
rabbit pAb, cat. no. ab104156, 1:500; anti-Bcl-2 rabbit pAb, cat.
no. BS-4563R, 1:1,000; and anti-β-actin rabbit pAb, cat. no.
ab8227; 1:3,000) at 4˚C overnight. After washing, blots were washed
with PBS, and then incubated with the horseradish
peroxidase-conjugated secondary antibody (goat anti-rabbit IgG
H&L HRP, cat. no. ab205718, 1:3,000) for 1 h at room
temperature. Signals were detected using an ECL kit (Bio-Rad
Laboratories, Inc.) according to the manufacturer's protocol. The
densitometric analysis was performed with ImageJ software (Version
1.53n; National Institutes of Health).
Microcirculation
A video microscope, namely the sidestream dark field
imaging (MicroScan; MicroVision Medical) was used for the
observation of the microcirculation. Shortly, a handheld video
microscope emits stroboscopic green light, which is absorbed by the
erythrocytic hemoglobin and released back immediately. The images
of the erythrocytes moving in the micro-vessels are transmitted to
the camera through the microscope, thereby obtaining a non-invasive
real-time image of the microcirculation. The microcirculation of
the experimental animals at 0, 1, 2, 4 and 6 h after ROSC was
recorded by manual operation. In order to observe the intestinal
microcirculation, 2-3 cm segment of jejunum was isolated.
Additionally, it was coated with gauze soaked in warm saline. The
mesentery on the serosal side was used as an observing region to
evaluate intestinal microcirculation. After the observation, the
intestine was back to the peritoneal cavity. The abdominal wall was
sutured, and the skin incision was closed through the mode of a
wound clip. The images were stored and analyzed offline using
AVA-Automated Vascular Analysis 3.1 (Microvision Medical) to obtain
perfused vessel density (PVD) and microvascular flow index (MFI)
parameters. These parameters were calculated for microvessels (≤20
µm in diameter). For PVD, microvessels were classified as either
perfused (hyperdynamic, continuous or slow blood flow in the
vessels) or non-perfused (intermittent or no blood flow) based on
the microcirculation. For the quantification of MFI, the images
were placed into four parts. In addition, the microcirculatory
condition was shown in a scheduled scale.
Statistical analysis
Data were presented as the mean ± SD and analyzed by
SPSS 17.0 (SPSS, Inc.). Data were analyzed using one-way analysis
of variance followed by Tukey's post hoc test. The correlation
analysis was performed with Pearson's analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
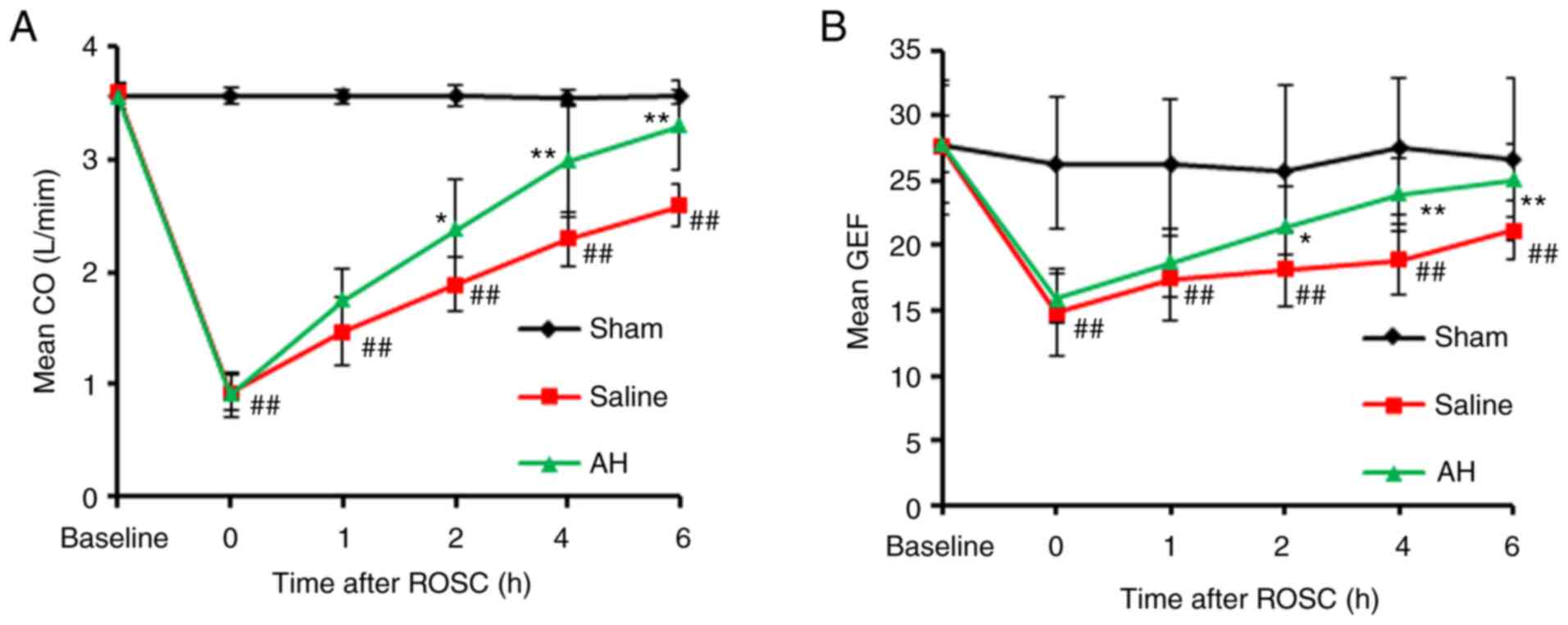
AH Treatment promotes the recovery of
cardiac function
No distinct variation could be observed in body
weight, hemodynamic indexes and blood parameters at baseline
between groups (Table I). All
animals induced by VF had been resuscitated in a favourable
condition. There was no variation of defibrillation and the time of
resuscitation between Saline and AH group. With the comparative
analysis of baseline group, the cardiac function had been seriously
impaired after CPR. The recovery was gradual with the duration, and
the cardiac function in the AH group recovered faster than the SA
group (Fig. 1 and Table II).
 | Table IBaseline characteristics. |
Table I
Baseline characteristics.
| Characteristics | Sham (n=8) | Saline (n=8) | Anisodamine
hydrobromide (n=8) |
|---|
| Weight (kg) | 23.75±0.96 | 24.13±1.96 | 24.0±1.41 |
| Heart rate (bpm) | 101.75±5.85 | 102.30±6.63 | 101.60±5.53 |
| Mean arterial
pressure (mm Hg) | 99.50±11.94 | 100.70±12.38 | 99.45±11.63 |
| Cardiac output
(l/min) | 3.60±0.22 | 3.65±0.28 | 3.68±0.28 |
| pH | 7.41±0.06 | 7.38±0.09 | 7.34±0.09 |
| Lactate (mmol/l) | 2.15±0.44 | 2.17±0.57 | 2.14±0.55 |
 | Table IICharacteristics at 6 h
post-resuscitation. |
Table II
Characteristics at 6 h
post-resuscitation.
| Group | Sham (n=8) | Saline (n=8) | Anisodamine
hydrobromide (n=8) |
|---|
| Heart rate (bpm) | 102.5±6.61 |
128.25±8.83c |
110.0±7.80d |
| Mean arterial
pressure (mm Hg) | 90.25±4.28 |
105.38±6.22a |
97.58±5.91b |
| Cardiac output
(l/min) | 3.64±0.17 |
2.77±0.23a |
3.34±0.33d |
| pH | 7.38±0.03 |
7.05±0.13a |
7.28±0.12b |
| Lactate
(mmol/l) | 1.98±0.31 |
4.79±0.41c |
3.11±0.38b |
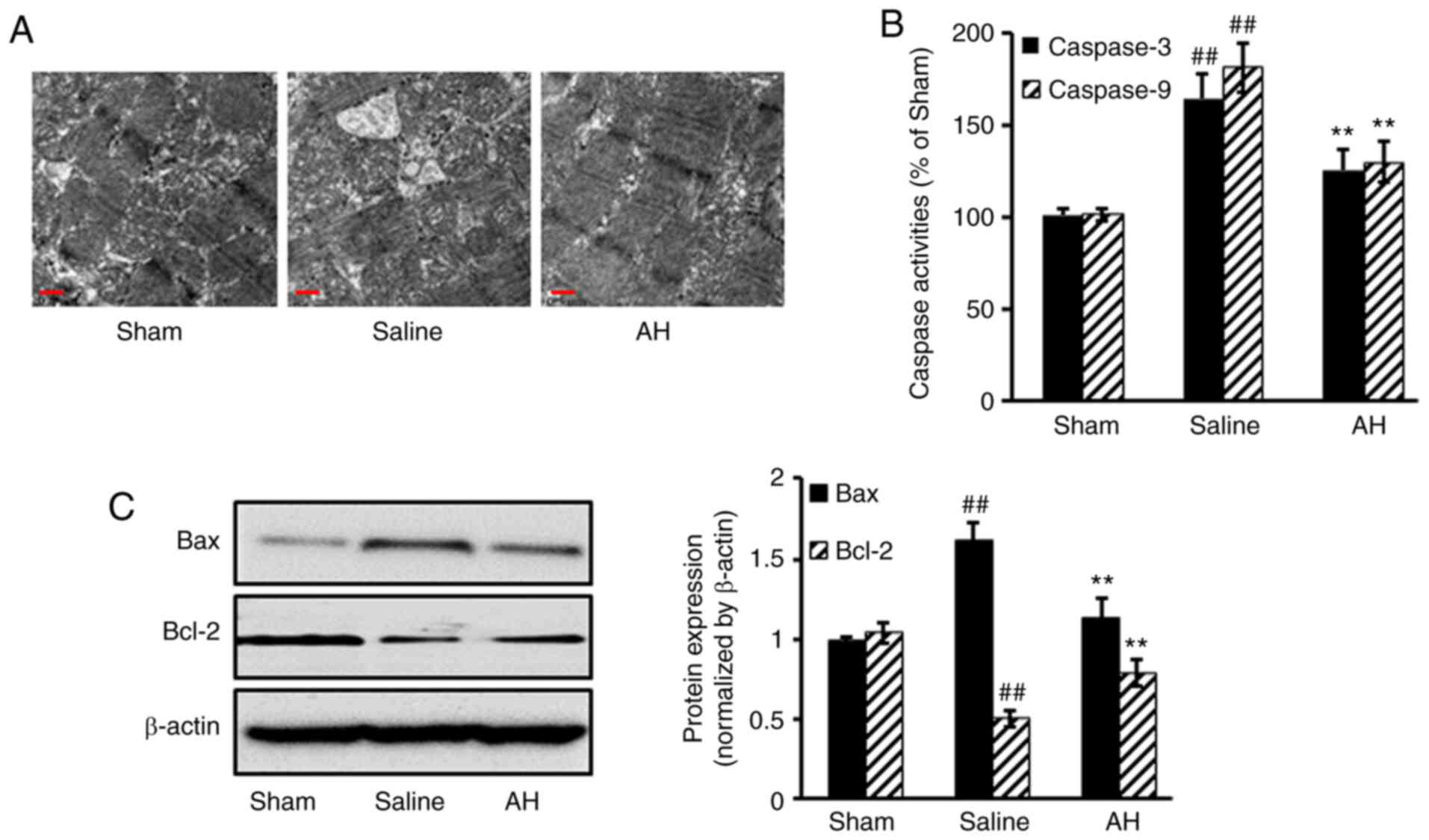
AH treatment decreases myocardial
tissue damage
After treatment, hearts were harvested for
histopathological analysis. Obvious pathological changes were
observed in the saline group. However, myocardial tissue damage was
markedly ameliorated after AH treatment. AH treatment significantly
decreased the activities of caspase 3/9 compared with the saline
group. Moreover, Pro-apoptotic protein Bax expression decreased,
but anti-apoptotic protein Bcl-2 increased after AH treatment
compared with the saline group (Fig.
2).
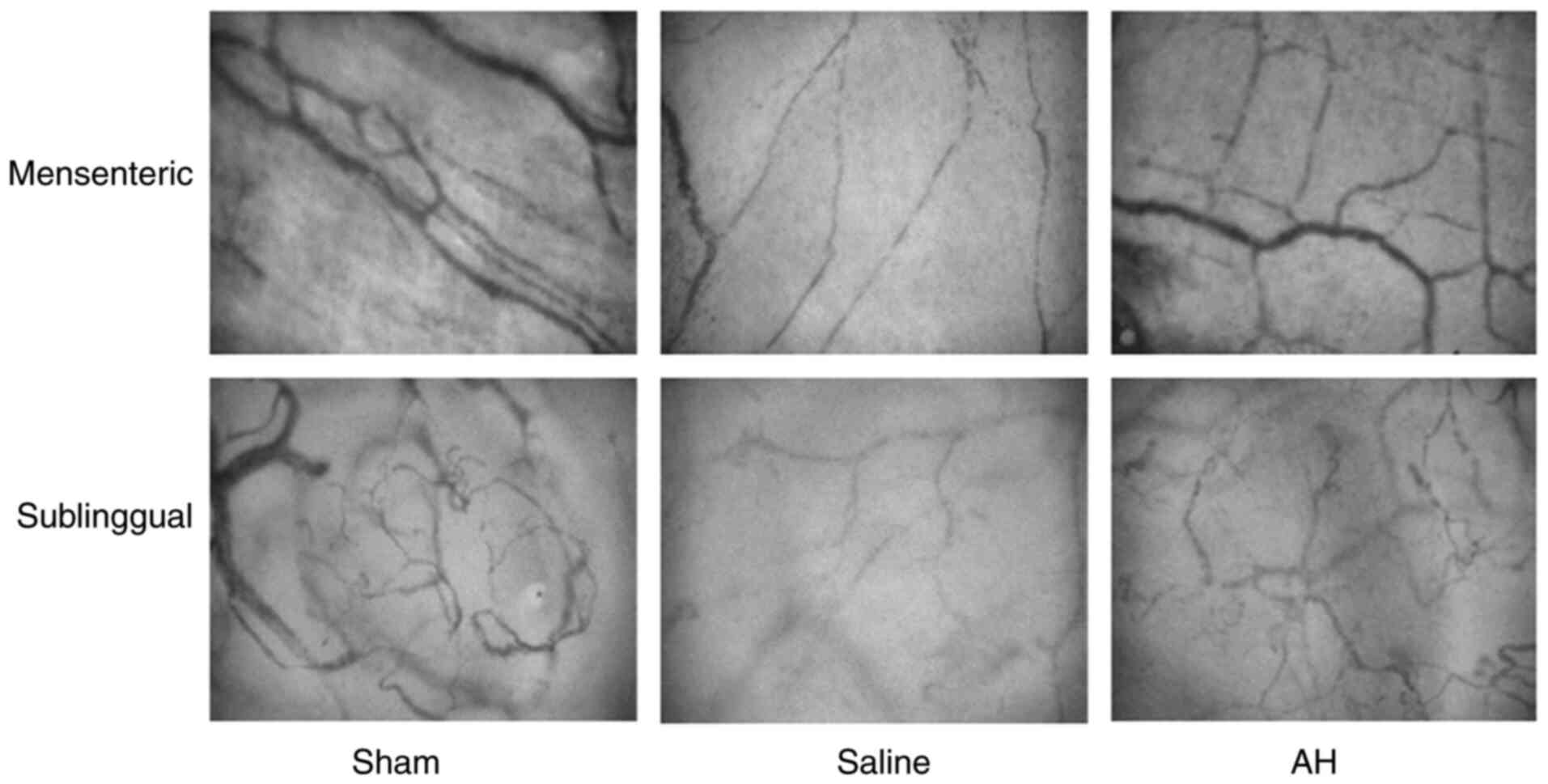
AH treatment improves the
microcirculation
After resuscitation, PVD and MFI decreased
significantly in the intestines and the sublingual region compared
with the Sham group. Afterwards, PVD and MFI gradually recovered
with time. However, these microcirculatory indexes recovered faster
in the AH group than the Saline group. At 2, 4 and 6 h after
resuscitation, there were significant differences of PVD and MFI in
both intestines and the sublingual region between two groups.
Moreover, the microcirculatory indexes (PVD and MFI) had a strong
correlation between the intestines and the sublingual regions
(Figs. 3 and 4).
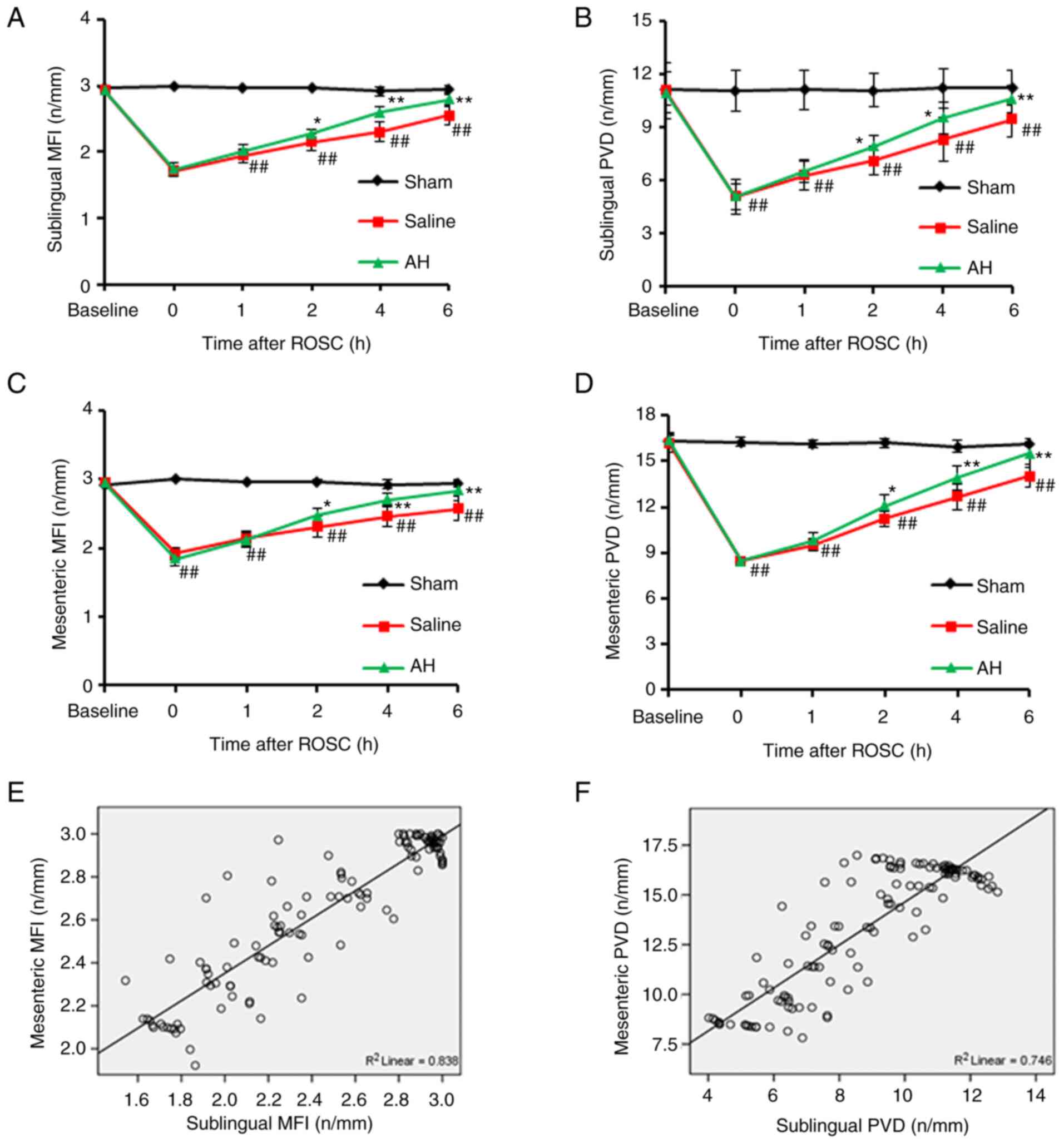 | Figure 3AH treatment improves the
microcirculation. (A-D) PVD and MFI in the intestines and the
sublingual regions were recorded at 0, 1, 2, 4 and 6 h after ROSC.
PVD and MFI decreased significantly compared with the Sham group.
However, these microcirculatory indexes recovered faster in the AH
group than the Saline group. (E and F) Moreover, the
microcirculatory indexes (PVD and MFI) had a strong correlation
between the intestines and the sublingual regions. Data were
expressed as the mean ± SD (n=8). ##P<0.01 vs. Sham
group; *P<0.05, **P<0.01 vs. Saline
group. AH, anisodamine hydrobromide; PVD, Perfused vessel density;
MFI, microvascular flow index; ROSC, restoration of spontaneous
circulation. |
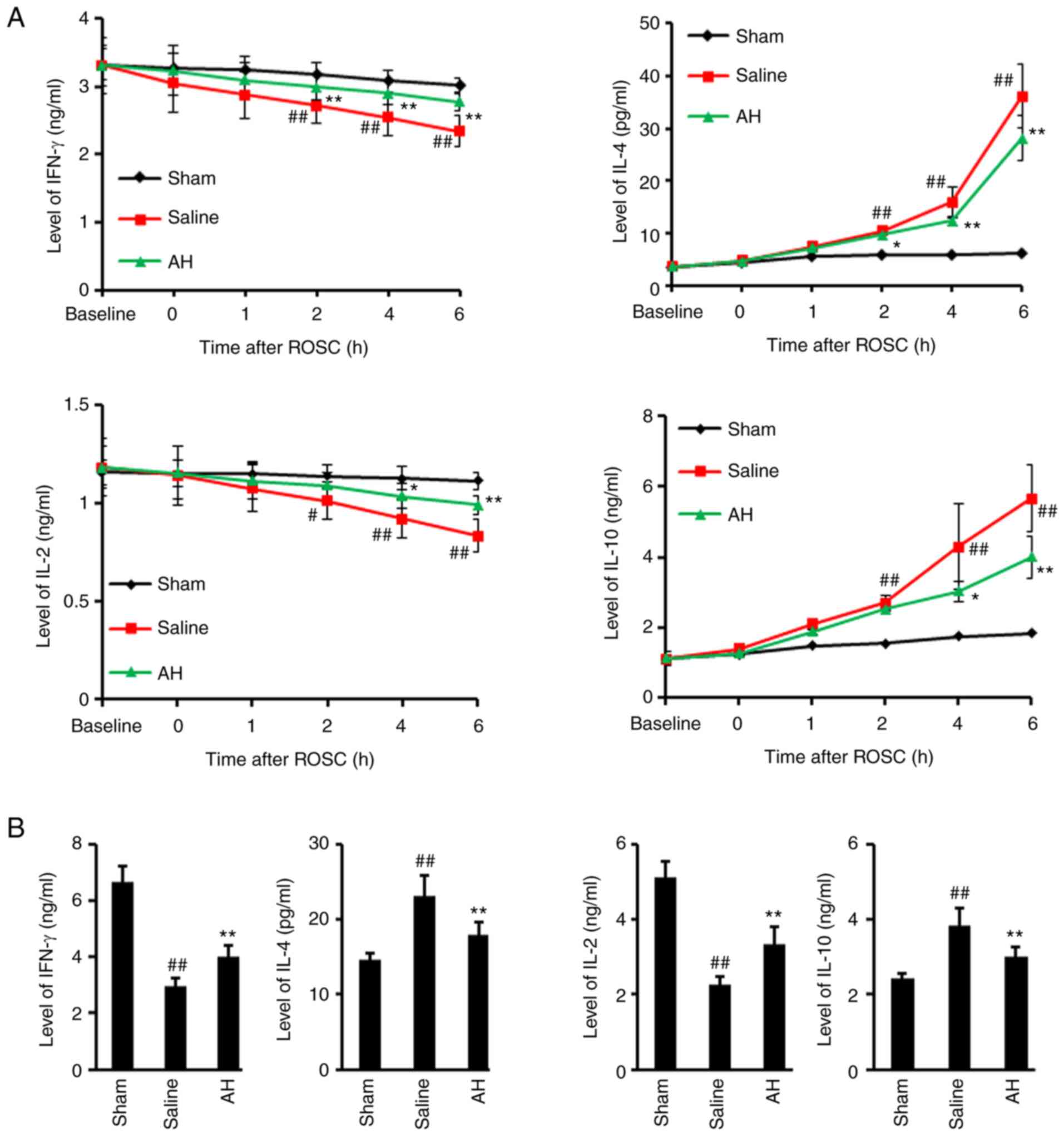
AH treatment inhibits the
transformation of Th1 to Th2
IL-2, IL-4, IL-10 and IFN-γ levels were determined
in serum and heart tissue lysate. The results showed that IL-4 and
IL-10 secretion significantly increased after ROSC, but IFN-γ and
IL-2 levels significantly decreased. However, AH treatment
significantly decreased the IL-4 as well as IL-10 levels and
increased the IFN-γ as well as the IL-2 levels (Fig. 5). These data indicated that ROSC
promoted Th1 to Th2 transformation, but was largely inhibited by AH
treatment.
Discussion
The present study demonstrated that the intestinal
and sublingual microcirculatory blood flow declined dramatically
after cardiac arrest and successful CPR. The correlation analysis
between the microcirculatory indexes and the hemodynamic indexes
showed that intestinal microcirculatory dysfunction has a
favourable relationship with the serious situation of
post-resuscitation syndrome. In addition, variations in sublingual
microcirculation have been key to the changes in the intestinal
microcirculation. AH treatment can help the recovery of hemodynamic
function and the maintenance of immune balance, and had protective
effects after resuscitation.
The sublingual region is the easiest part for the
measurement of microcirculation. Previously, the sublingual
microcirculation has been regarded as a surrogate index for
visceral blood flow. Studies have proposed that non-invasive
sublingual CO2 detection can be used as an alternative
measurement of gastric pressure in case of microcirculatory
disturbance (19-22).
The present study showed that the sublingual microcirculation had a
similar performance to the intestinal microcirculation in the early
post-resuscitation stage by the visualized method. This result was
consistent not only with previous studies on microcirculatory
disturbance, but also with studies on endotoxin and septic shock,
indicating that the microcirculatory changes in severity and
process are similar between sublingual and intestinal region
(23-25).
Nevertheless, some controversies remain. A clinical study proposed
that the microcirculatory changes in the two parts were not
parallel on the first day after the occurrence of sepsis (26). Another animal study showed that the
correlation of the microcirculation in the two parts disappeared
with time (27). It is
hypothesized that certain of the treatment measures given in the
two aforementioned studies may affect the intestinal
microcirculation, leading to different conclusions of these two
studies. In addition, sepsis itself is highly heterogeneous
(28).
The abnormal manifestations after resuscitation
resemble those of sepsis, and cardiac dysfunction shall be one of
the main features. The process of the cardiac function after CPR
causes a decrease in visceral blood flow. However, the changes in
microcirculation are not exactly equivalent to the variations
expressed in the blood flow. In addition, there are various points
between visceral organs, in particular notes, in the situation of
low flow (29). The correlation
between the microcirculation and the cardiac function in the early
post-resuscitation stage was confirmed in the present study. The
parameters of the intestinal microcirculation after resuscitation
showed a tendency to recover, which was considered to have a
certain relationship with the automatic regulation and the
treatment measures of the body. Actually, it could be observed that
the control of microcirculatory blood flow concerning the
intestinal wall was too complex.
In terms of both systemic and local factors could
make contribution to the intestinal microcirculation, including but
not limited to inflammatory cytokines and vasomotor function. In
the present study, the parameters of sublingual and intestinal
microcirculation and the hemodynamic parameters indicated that the
cardiac function of animals was improved in the AH group compared
with the Saline group, suggesting that AH can help improve the
microcirculation and the cardiac function, and has protective
effects after resuscitation.
The integrity of the intestinal mucosal barrier
takes a proactive function in the progress of systemic inflammatory
response syndrome sepsis as well as multiple organ failures. Th1
lymphocytes produce pro-inflammatory cytokines, such as IFN-γ and
IL-2, which mainly contribute to cell-mediated immune responses.
Th2 lymphocytes secrete anti-inflammatory cytokines, such as IL-4
and IL-10, for host defence against invasion by exogenous pathogens
(30,31). The balance between Th1 and Th2
cells plays a vital role in maintaining normal immune function. In
previous ROSC studies on cardiac arrest models, abnormal Th1/Th2
ratios were identified in spleen, lung and myocardium (32,33).
It was revealed in the present study that after resuscitation,
levels of serum IFN-γ and IL-2 continued to decrease, meanwhile,
levels of serum IL-4 and IL-10 increased significantly.
Furthermore, levels of IFN-γ and IL-2 were significantly lower, but
levels of IL-4 and IL-10 were significantly higher in myocardial
tissues after resuscitation. These data were consistent with
previous observations in individuals and animal models of cardiac
arrest undergoing CPR (6). AH
treatment significantly decreased levels of Th2 cytokines IL-4 and
IL-10, but increased levels of Th1 cytokines IFN-γ and IL-2.
Although Th1/Th2 subtypes were not detected at the cellular level,
the present data suggested the relationship between the two
cytokine profiles and indicated that AH alleviated the
transformation of Th1 to Th2, and improved the immune imbalance
induced by IRI. The inflammatory cell infiltration and Th1/Th2
subtypes at the cellular level may be useful to be examined in
future studies.
Clinically, intestinal injury after cardiac arrest
may be underestimated after successful CPR due to non-specific and
delayed manifestations, although it may lead to fatal complications
(34). It is not clinically
feasible to observe the intestinal microcirculation directly in
vivo, thus the early detection is difficult. The present study
demonstrated the variations in the process of sublingual
microcirculation after resuscitation that could indicate the
variations in the process of intestinal microcirculation. In
addition, to certain extent, the changes in the cardiac function,
suggested that the sublingual microcirculation may be a new
alternative for bedside monitoring of post-resuscitation
patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GJD and SBG substantially contributed to the
conception and the design of the study. GJD, JY and XZ were
responsible for the acquisition, analysis and interpretation of the
data. GJD and SBG confirm the authenticity of all the raw data. GJD
contributed to manuscript drafting. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures were performed following the Animal
Care Guidelines of the Institutional Animal Care and Use Committee
of Capital Medical University (Beijing, China; approval no.
2020-3-18-92).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goldberger ZD, Chan PS, Berg RA, Kronick
SL, Cooke CR, Lu M, Banerjee M, Hayward RA, Krumholz HM and
Nallamothu BK: American Heart Association Get With The
Guidelines-Resuscitation (formerly National Registry of
Cardiopulmonary Resuscitation) Investigators. Duration CNCNof
resuscitation efforts and survival after in-hospital cardiac
arrest: An observational study. Lancet. 380:1473–1481.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Neumar RW, Nolan JP, Adrie C, Aibiki M,
Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC,
et al: Post-cardiac arrest syndrome: Epidemiology, pathophysiology,
treatment, and prognostication. A consensus statement from the
International Liaison Committee on Resuscitation (American Heart
Association, Australian and New Zealand Council on Resuscitation,
European Resuscitation Council, Heart and Stroke Foundation of
Canada, InterAmerican Heart Foundation, Resuscitation Council of
Asia, and the Resuscitation Council of Southern Africa); the
American Heart Association Emergency Cardiovascular Care Committee;
the Council on Cardiovascular Surgery and Anesthesia; the Council
on Cardiopulmonary, Perioperative, and Critical Care; the Council
on Clinical Cardiology; and the Stroke Council. Circulation.
118:2452–2483. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Korth U, Krieter H, Denz C, Janke C,
Ellinger K, Bertsch T, Henn C and Klein J: Intestinal ischaemia
during cardiac arrest and resuscitation: Comparative analysis of
extracellular metabolites by microdialysis. Resuscitation.
58:209–217. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stallion A, Kou TD, Latifi SQ, Miller KA,
Dahms BB, Dudgeon DL and Levine AD: Ischemia/reperfusion: A
clinically relevant model of intestinal injury yielding systemic
inflammation. J Pediatr Surg. 40:470–477. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Coopersmith CM, Stromberg PE, Dunne WM,
Davis CG, Amiot DM II, Buchman TG, Karl IE and Hotchkiss RS:
Inhibition of intestinal epithelial apoptosis and survival in a
murine model of pneumonia-induced sepsis. JAMA. 287:1716–1721.
2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Adrie C, Adib-Conquy M, Laurent I, Monchi
M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P,
Spaulding C, et al: Successful cardiopulmonary resuscitation after
cardiac arrest as a ‘sepsis-like’ syndrome. Circulation.
106:562–568. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van Genderen ME, Lima A, Akkerhuis M,
Bakker J and van Bommel J: Persistent peripheral and
microcirculatory perfusion alterations after out-of-hospital
cardiac arrest are associated with poor survival. Crit Care Med.
40:2287–2294. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Donadello K, Favory R, Salgado-Ribeiro D,
Vincent JL, Gottin L, Scolletta S, Creteur J, De Backer D and
Taccone FS: Sublingual and muscular microcirculatory alterations
after cardiac arrest: A pilot study. Resuscitation. 82:690–695.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qian J, Yang Z, Cahoon J, Xu J, Zhu C,
Yang M, Hu X, Sun S and Tang W: Post-resuscitation intestinal
microcirculation: Its relationship with sublingual microcirculation
and the severity of post-resuscitation syndrome. Resuscitation.
85:833–839. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Buijs EA, Verboom EM, Top AP,
Andrinopoulou ER, Buysse CM, Ince C and Tibboel D: Early
microcirculatory impairment during therapeutic hypothermia is
associated with poor outcome in post-cardiac arrest children: A
prospective observational cohort study. Resuscitation. 85:397–404.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Omar YG, Massey M, Andersen LW, Giberson
TA, Berg K, Cocchi MN, Shapiro NI and Donnino MW: Sublingual
microcirculation is impaired in post-cardiac arrest patients.
Resuscitation. 84:1717–1722. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Top AP, Ince C, de Meij N, van Dijk M and
Tibboel D: Persistent low microcirculatory vessel density in
nonsurvivors of sepsis in pediatric intensive care. Crit Care Med.
39:8–13. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Backer D, Donadello K, Sakr Y,
Ospina-Tascon G, Salgado D, Scolletta S and Vincent JL:
Microcirculatory alterations in patients with severe sepsis: Impact
of time of assessment and relationship with outcome. Crit Care Med.
41:791–799. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu J, Ramadeen A, Tsui AK, Hu X, Zou L,
Wilson DF, Esipova TV, Vinogradov SA, Leong-Poi H, Zamiri N, et al:
Quantitative assessment of brain microvascular and tissue
oxygenation during cardiac arrest and resuscitation in pigs.
Anaesthesia. 68:723–735. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Massey MJ and Shapiro NI: A guide to human
in vivo microcirculatory flow image analysis. Crit Care.
20(35)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sheng CY, Gao WY, Guo ZR and He LX:
Anisodamine restores bowel circulation in burn shock. Burns.
23:142–146. 1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang S, Li C, Ji X, Yang L, Su Z and Wu J:
Effect of continuous compressions and 30:2 cardiopulmonary
resuscitation on global ventilation/perfusion values during
resuscitation in a porcine model. Crit Care Med. 38:2024–2030.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Valenzuela TD, Roe DJ, Cretin S, Spaite DW
and Larsen MP: Estimating effectiveness of cardiac arrest
interventions: A logistic regression survival model. Circulation.
96:3308–3313. 1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Goedhart PT, Khalilzada M, Bezemer R,
Merza J and Ince C: Sidestream Dark Field (SDF) imaging: A novel
stroboscopic LED ring-based imaging modality for clinical
assessment of the microcirculation. Opt Express. 15:15101–15114.
2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Weil MH, Nakagawa Y, Tang W, Sato Y,
Ercoli F, Finegan R, Grayman G and Bisera J: Sublingual capnometry:
A new noninvasive measurement for diagnosis and quantitation of
severity of circulatory shock. Crit Care Med. 27:1225–1229.
1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Povoas HP, Weil MH, Tang W, Moran B,
Kamohara T and Bisera J: Comparisons between sublingual and gastric
tonometry during hemorrhagic shock. Chest. 118:1127–1132.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Creteur J, De Backer D, Sakr Y, Koch M and
Vincent JL: Sublingual capnometry tracks microcirculatory changes
in septic patients. Intensive Care Med. 32:516–523. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Verdant CL, De Backer D, Bruhn A, Clausi
CM, Su F, Wang Z, Rodriguez H, Pries AR and Vincent JL: Evaluation
of sublingual and gut mucosal microcirculation in sepsis: A
quantitative analysis. Crit Care Med. 37:2875–2881. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dubin A, Edul VS, Pozo MO, Murias G,
Canullán CM, Martins EF, Ferrara G, Canales HS, Laporte M,
Estenssoro E and Ince C: Persistent villi hypoperfusion explains
intramucosal acidosis in sheep endotoxemia. Crit Care Med.
36:535–542. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fries M, Weil MH, Sun S, Huang L, Fang X,
Cammarata G, Castillo C and Tang W: Increases in tissue Pco2 during
circulatory shock reflect selective decreases in capillary blood
flow. Crit Care Med. 34:446–452. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Boerma EC, van der Voort PH, Spronk PE and
Ince C: Relationship between sublingual and intestinal
microcirculatory perfusion in patients with abdominal sepsis. Crit
Care Med. 35:1055–1060. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pranskunas A, Pilvinis V, Dambrauskas Z,
Rasimaviciute R, Planciuniene R, Dobozinskas P, Veikutis V,
Vaitkaitis D and Boerma EC: Early course of microcirculatory
perfusion in eye and digestive tract during hypodynamic sepsis.
Crit Care. 16(R83)2012.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Nacul FE, Guia IL, Lessa MA and Tibiriçá
E: The effects of vasoactive drugs on intestinal functional
capillary density in endotoxemic rats: Intravital video-microscopy
analysis. Anesth Analg. 110:547–554. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hiltebrand LB, Krejci V, Banic A, Erni D,
Wheatley AM and Sigurdsson GH: Dynamic study of the distribution of
microcirculatory blood flow in multiple splanchnic organs in septic
shock. Crit Care Med. 28:3233–3241. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bretscher PA: On the mechanism determining
the TH1/TH2 phenotype of an immune response, and its pertinence to
strategies for the prevention, and treatment, of certain infectious
diseases. Scand J Immunol. 79:361–376. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Paul WE and Zhu J: How are T(H)2-type
immune responses initiated and amplified? Nat Rev Immunol.
10:225–235. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Gu W, Zhang Q and Li CS: Effect of splenic
regulatory T-cell apoptosis on the postresuscitation immune
dysfunction in a porcine model. Chin Med J (Engl). 129:1577–1583.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gu W, Li C, Yin W and Hou X: Effects of
Shenfu injection on the expression of transcription factors
T-bet/GATA-3 in pigs with post-resuscitation myocardial
dysfunction. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 27:190–196.
2015.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
34
|
Katsoulis IE, Balanika A, Sakalidou M,
Gogoulou I, Stathoulopoulos A and Digalakis MK: Extensive colonic
necrosis following cardiac arrest and successful cardiopulmonary
resuscitation: Report of a case and literature review. World J
Emerg Surg. 7(35)2012.PubMed/NCBI View Article : Google Scholar
|