Introduction
Diabetes mellitus is a type of metabolic disease
characterized by hyperglycemia (1). Long-standing hyperglycemia may lead
to multiple types of tissue injury, such as eye, kidney and heart
dysfunction and chronic damage of blood vessels and nerves
(2-4).
Diabetic retinopathy (DR) is characterized by retinal lesions and
is often accompanied by abnormal angiogenesis (5). DR involves pathological
characteristics, including loss of pericytes, thickening of the
basement membrane and adhesion of white blood cells (6,7).
Endothelial cell (EC) dysfunction serves a key role in the
structure and pathophysiology of the retina (8). Therefore, novel studies and the
development of drugs for improving retinal (R)EC dysfunction may
promote the effective treatment of DR.
Naringenin (4',5,7-trihydroxyflavanone) is a natural
flavonoid compound that is found in grapefruit, tomato and citrus
fruits of the Rutaceae family (9,10).
Compared with other flavonoids, naringenin is easily absorbed by
the gastrointestinal tract and is characterized by its high
bioavailability and low toxicity (11). Naringenin exhibits biological
effects, such as antibacterial, anti-inflammatory, antioxidant,
immune regulation and anti-tumor activity (12-15).
Naringenin is effective in treating obesity (16), atherosclerosis (17) and diabetes (18). Zeng et al (19) demonstrated that naringenin improves
high glucose (HG)-induced injury of vascular ECs. Another study
revealed that naringenin exerts a protective effect against
alkali-induced corneal burn by attenuating secretion of
inflammatory cytokines and resisting oxidation (20). To the best of our knowledge,
however, the effect of naringenin on REC injury has not been
previously investigated.
Guanosine triphosphate cyclohydrolase 1 (GTPCH1), a
key enzyme that catalyzes production of tetrahydrobiopterin (BH4),
is involved in the synthesis of numerous hormones and
neurotransmitters and serves a vital role in a series of
pathophysiological processes in the body (21,22).
For instance, inhibition of GTPCH1 reduces the inflammation of
microglia (23). GTPCH1
participates in endothelial dysfunction in atherosclerosis
(24). Nitric oxide (NO) produced
by endothelial NO synthase (eNOS) serves a key role in maintaining
EC homeostasis due to its anti-inflammatory and antioxidant
effects. Furthermore, BH4 is a key factor involved in maintaining
eNOS activity and determining the balance of NO and eNOS-produced
superoxide (25). eNOS should be
fully saturated with BH4 to be fully coupled with reduced
nicotinamide adenine dinucleotide phosphate to be oxidized into NO.
Under BH4 deficiency, eNOS functions in an ‘uncoupled’ form,
resulting in generation of superoxide and
H2O2 and aggravating oxidative stress
responses in organisms (26,27).
Previous studies showed that BH4 supplementation decreases
endothelial dysfunction in patients with atherosclerosis and
diabetes mellitus (28,29). Furthermore, GTPCH1 is downregulated
in ECs isolated from diabetic rats with decreased BH4 levels and
uncoupled eNOS (30). Multiple
studies have demonstrated that GTPCH1 upregulation improves
different types of EC injury, such as brain microvascular (31), palmitic acid-induced islet
(32) and HG-induced aortic EC
injury (22).
Therefore, the present study aimed to investigate
the effect of naringenin on HG-induced REC injury and whether the
effects of naringenin are associated with regulation of the
GTPCH1/eNOS axis.
Materials and methods
Cell culture and treatment
Human (H)RECs were purchased from Ningbo Mingzhou
Biotechnology Co., Ltd. (cat. no. MZ-1174). Cells were cultured in
Endothelial Cell Medium (cat. no. 1001; HyClone; Cytiva)
supplemented with 5% FBS (cat. no. 10091141; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C in an
atmosphere containing 5% CO2. Cells in the logarithmic
growth phase were firstly treated with naringenin (1, 10, 20, 50
and 100 µM). Naringenin at concentrations of 1 and 10 µM were
selected for subsequent experiments as naringenin was cytotoxic at
≥20 µM. Subsequently, cells were treated with 30 mM HG (cat. no.
50-99-7; MilliporeSigma), HG + 1 µM naringenin (cat. no.
67604-48-2; MilliporeSigma) or HG + 10 µM naringenin at 37˚C for
24, 48 and 72 h. After GTPCH1 was silenced, cells were further
divided into the following five groups: i) Control; ii) 30 mM HG;
iii) HG + 10 µM naringenin; iv) HG + 10 µM naringenin + siRNA-NC;
and v) HG + 10 µM naringenin + siRNA-GTPCH1. Untreated cells served
as the control group.
Cell Counting Kit-8 (CCK-8) assay
HRECs were seeded into a 96-well cell culture plate
at a density of 1x103 cells/well and incubated overnight
at 37˚C with 5% CO2. Following treatment as
aforementioned, HRECs in each well were supplemented with 10 µl
CCK-8 solution (cat. no. A311-01/02; Vazyme Biotech Co., Ltd.) and
incubated at 37˚C with 5% CO2 for 4 h. Finally, the
absorbance in each well was measured at a wavelength of 450 nm
using the Varioskan™ LUX Multi-function microplate reader (Thermo
Fisher Scientific, Inc.). Relative cell viability (%) was
calculated as follows: [Treated optical density
(OD)A450-blank ODA450]/(control
ODA450-blank ODA450) x100%.
TUNEL assay
The effect of naringenin on HG-induced HREC
apoptosis was assessed using a TUNEL assay. Briefly,
1x105 cells/well, pretreated as aforementioned, were
collected and washed three times with PBS. Following fixing with 4%
paraformaldehyde at room temperature for 5 min, cells were gently
washed twice with PBS for 2 min each. Subsequently, cells were
treated with DAPI staining solution (cat. no. C1005; Beyotime
Institute of Biotechnology) at room temperature for 3-5 min,
followed by washing with PBS 2-3 times for 3-5 min each. The cells
were treated with 0.3% Triton-X-100 at room temperature for 5 min.
Subsequently, cells were supplemented with 50 µl TUNEL assay
solution (cat. no. C1086; Beyotime Institute of Biotechnology) and
incubated at 37˚C in the dark for 1 h according to the
manufacturer's instructions. Cell nuclei were stained with DAPI (1
mg/ml) at room temperature for 10 min in the dark. Following
incubation, the detection solution was discarded and cells were
washed three times with PBS. Finally, cells were sealed with
anti-fluorescence quenched sealing solution and observed in three
randomly selected fields of view with a total of 300-500 cells
under a fluorescence microscope (Zeiss GmbH; magnification, x200).
The excitation wavelength range used was 450-500 nm and the
emission wavelength range was 515-565 nm (green fluorescence).
Reactive oxygen species (ROS)
detection
ROS levels in HG-induced HRECs treated in the
presence or absence of naringenin (as aforementioned) were measured
using the Reactive Oxygen Species Assay Kit (cat. no. S0033S;
Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Cells were randomly selected from 5
fields of view and an inverted fluorescence microscope (Olympus
Corporation; magnification, x200) was used to observe excitation
and emission wavelengths within the range of 450-500 and 515-565 nm
(green fluorescence). A BH4 ELISA Kit (cat. no. EK-H12416; EK
Biosciences GmbH) was used to assess levels of BH4 in the cells,
according to the manufacturer's instructions.
Cell transfection
HRECs were seeded into 6-well plates at a density of
1x105 cells/well and cultured for 24 h at 37˚C with 5%
CO2. Subsequently, cells were transfected with small
interfering (si)RNA clones (50 nM) against GTPCH1
(5'-TAGATTTCTACAATCCTCG-3') or empty vector for the negative
control (NC) group (5'-ACGTGACACGTTCGGAGAATT-3') using
Lipofectamine 2000® (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C for 24 h, according to the manufacturer's
instructions. Untreated cells served as the blank control group
(control). All plasmids were obtained from Shanghai GenePharma Co.,
Ltd. At 48 h post-transfection, transfection efficiency was
assessed via reverse transcription-quantitative (RT-q)PCR.
Western blot analysis
HRECs were washed three times with pre-cooled PBS
and lysed with RIPA lysis buffer (cat. no. P0013C; Beyotime
Institute of Biotechnology) for 30 min on ice. Subsequently, the
cell lysate was collected and centrifuged at 400 x g for 15-20 min
at 4˚C and the protein supernatant from each group was transferred
to Eppendorf tubes. The total protein concentration was measured
using the compat-Able™ BCA protein assay kit (cat. no. 23229;
Thermo Fisher Scientific, Inc). The protein samples from each group
(30 µg per lane) were separated by 10% SDS-PAGE and transferred
onto a PVDF membrane (cat. no. FFP24; Beyotime Institute of
Biotechnology). Following blocking with 5% skimmed milk powder at
room temperature for 4 h, the membranes were washed three times
with 1X TBS-0.1% Tween-20 followed by incubation with primary
antibodies (all 1:1,000; all Abcam) against Bcl-2 (cat. no.
ab194583), Bax (cat. no. ab32503), cleaved-caspase 3 (cat. no.
ab32042), caspase 3 (cat. no. ab32351), eNOS (cat. no. ab252439),
GTPCH1 (cat. no. ab236387), Ki67 (cat. no. ab15580), PCNA (cat. no.
ab92552) and β-actin (cat. no. ab8226) at 4˚C overnight.
Subsequently, the membranes were incubated with goat anti-rabbit
horseradish peroxidase-conjugated IgG secondary antibody (1:1,000;
cat. no. ab288151; Abcam) for 4 h at room temperature and the
protein bands were visualized using ECL reagent (Thermo Fisher
Scientific, Inc.). The protein expression levels were
semi-quantified using ImageJ (version 1.8.0; National Institutes of
Health).
RT-qPCR
Total RNA was extracted from HRECs using RNAzol RT
reagent (MilliporeSigma), according to the manufacturer's
instructions. RNA concentration was measured using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.). Following
digestion with DNase I, total RNA was reverse transcribed into cDNA
using the QuantiTect Reverse Transcription kit (Qiagen GmbH),
according to the manufacturer's protocol. qPCR was performed using
the QuantiTect SYBR Green PCR kit (Qiagen GmbH), according to the
manufacturer's instructions. The thermocycling conditions were as
follows: 95˚C for 10 min, followed by 40 cycles of 95˚C for 10 sec
and 60˚C for 1 min. The primer sequences used (all GenScript) were
as follows: GTPCH1 forward, 5'-CGAGCTGAACCTCCCTAACC-3' and reverse,
5'-AGCATCGTTTAGGACATCTGAG-3'; TNF-α forward,
5'-GAGGCCAAGCCCTGGTATG-3' and reverse, 5'-CGGGCCGATTGATCTCAGC-3';
IL-1β forward, 5'-GGATATGGAGCAACAAGTGG-3' and reverse,
5'-GAAGTCAGTTATATCCTGGC-3'; IL-6 forward,
5'-CATCCTCGACGGCATCTCAG-3' and reverse, 5'-TCACCAGGCAAGTCTCCTCA-3'
and β-actin forward, 5'-GTTGCTATCCAGGCTGTG-3' and reverse,
5'-TGATCTTGATCTTCATTGTG-3'. The mRNA expression levels were
quantified using the 2-ΔΔCq method (33); β-actin served as the internal
reference gene.
Statistical analysis
Data are presented as the mean ± SD from ≥3
independent experiments. All statistical analysis was performed
using GraphPad Prism 8.0 software (GraphPad Software, Inc.). The
differences between multiple groups were compared using one-way
ANOVA followed by post hoc Tukey's test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Naringenin ameliorates HG-induced HREC
damage
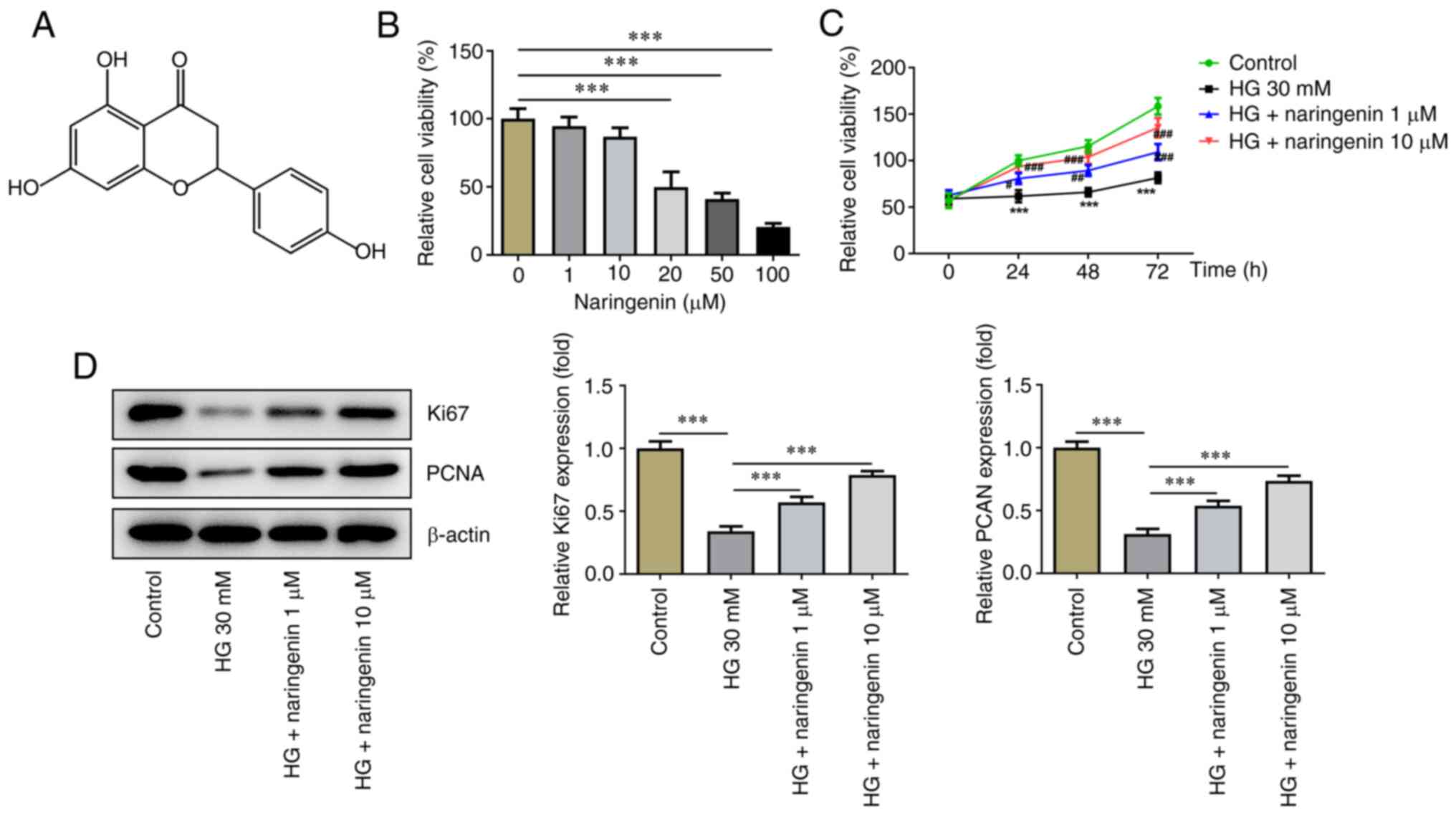
The molecular structure of naringenin is presented
in Fig. 1A. To evaluate the effect
of different concentrations of naringenin (1, 10, 20, 50 and 100
µM) on HREC viability, a CCK-8 assay was performed. The results
showed that high concentrations of naringenin (20, 50 and 100 µM)
exerted an inhibitory effect on HREC viability, while low
concentrations of naringenin (1 and 10 µM) had no significant
effect on HREC viability. Therefore, final concentrations of 1 and
10 µM naringenin were selected to assess the protective effect of
naringenin on HRECs for the reason that naringenin was cytotoxic at
≥20 µM (Fig. 1B). Compared with
that of the control, the viability of HRECs treated with HG for 24,
48 and 72 h significantly decreased, while co-treatment with
naringenin increased HREC viability under HG conditions, which
implied that naringenin attenuated HG-induced cell injury in a
concentration-dependent manner (Fig.
1C). To evaluate the proliferative ability of HRECs, protein
expression levels of intracellular proliferation markers Ki67 and
PCNA were determined (Fig. 1D). HG
significantly inhibited protein expression levels of Ki67 and PCNA
in HRECs, while co-treatment with 1 or 10 µM naringenin improved
HG-reduced intracellular proliferation-associated protein
expression to varying degrees. Overall, naringenin attenuated
HG-elicited HREC viability injury.
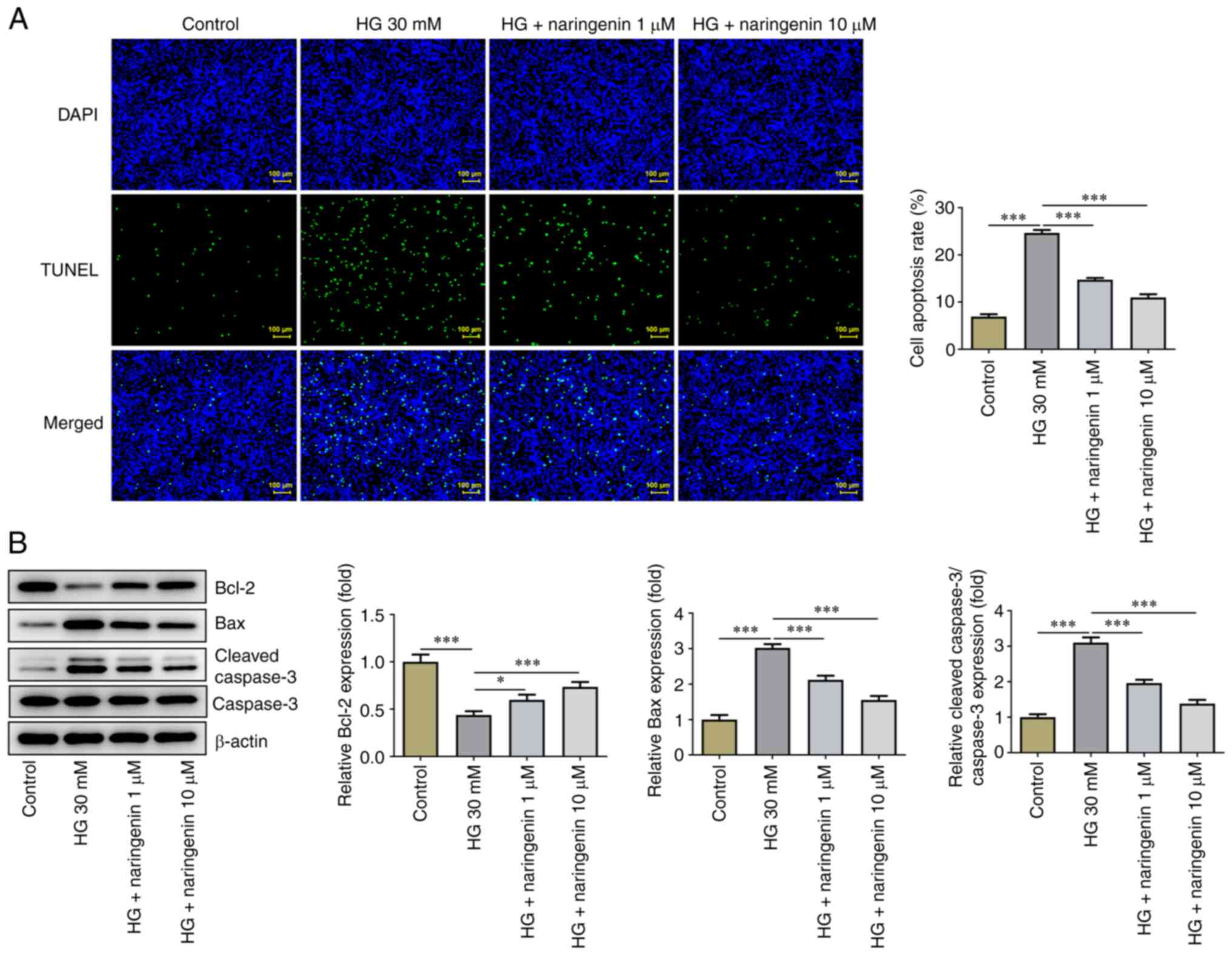
Naringenin inhibits HG-induced HREC
apoptosis
HREC apoptosis was assessed using TUNEL assay. Cell
apoptosis was significantly increased in the HG compared with the
control group, while treatment with 1 or 10 µM naringenin
significantly inhibited HG-induced HREC apoptosis (Fig. 2A). Expression levels of
apoptosis-associated proteins were detected by western blot
analysis (Fig. 2B). The expression
levels of anti-apoptotic protein Bcl-2 were significantly
decreased, whereas those of pro-apoptotic proteins Bax and
cleaved-caspase 3 were significantly increased in the HG compared
with the control group. Treatment with 1 or 10 µM naringenin
partially reversed HG-induced HREC apoptosis. In summary,
naringenin suppressed HG-enhanced HREC apoptosis.
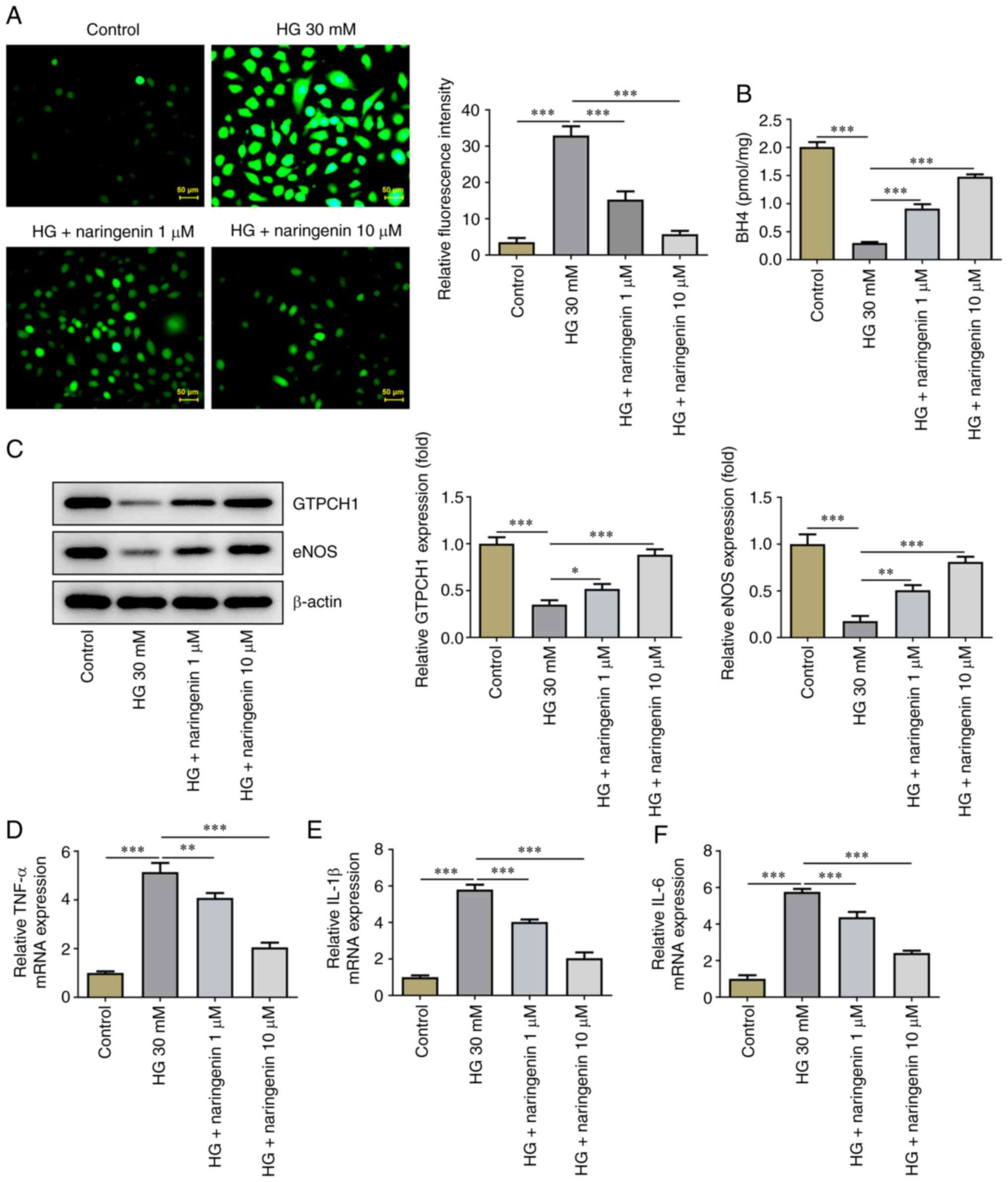
Naringenin upregulates eNOS and GTPCH1
in HG-induced HRECs
Subsequently, the effect of naringenin on ROS
overproduction in HG-induced HRECs was investigated. Treatment with
naringenin reversed HG-induced ROS overproduction in a
dose-dependent manner (Fig. 3A).
The effect of naringenin on BH4 (Fig.
3B), GTPCH1 and eNOS levels (Fig.
3C) in HRECs was assessed. Compared with the control, HG
significantly decreased BH4 contents and protein expression levels
of GTPCH1 and eNOS in HRECs; this was partially reversed by
naringenin. In addition, RT-qPCR showed that naringenin also
reversed the HG-induced increase in inflammatory factors (TNF-α,
IL-1β and IL-6) in a concentration-dependent manner (Fig. 3D-F). These results indicated that
naringenin ameliorated HG-induced HREC oxidative stress and
inflammatory response by enhancing GTPCH1/eNOS signaling.
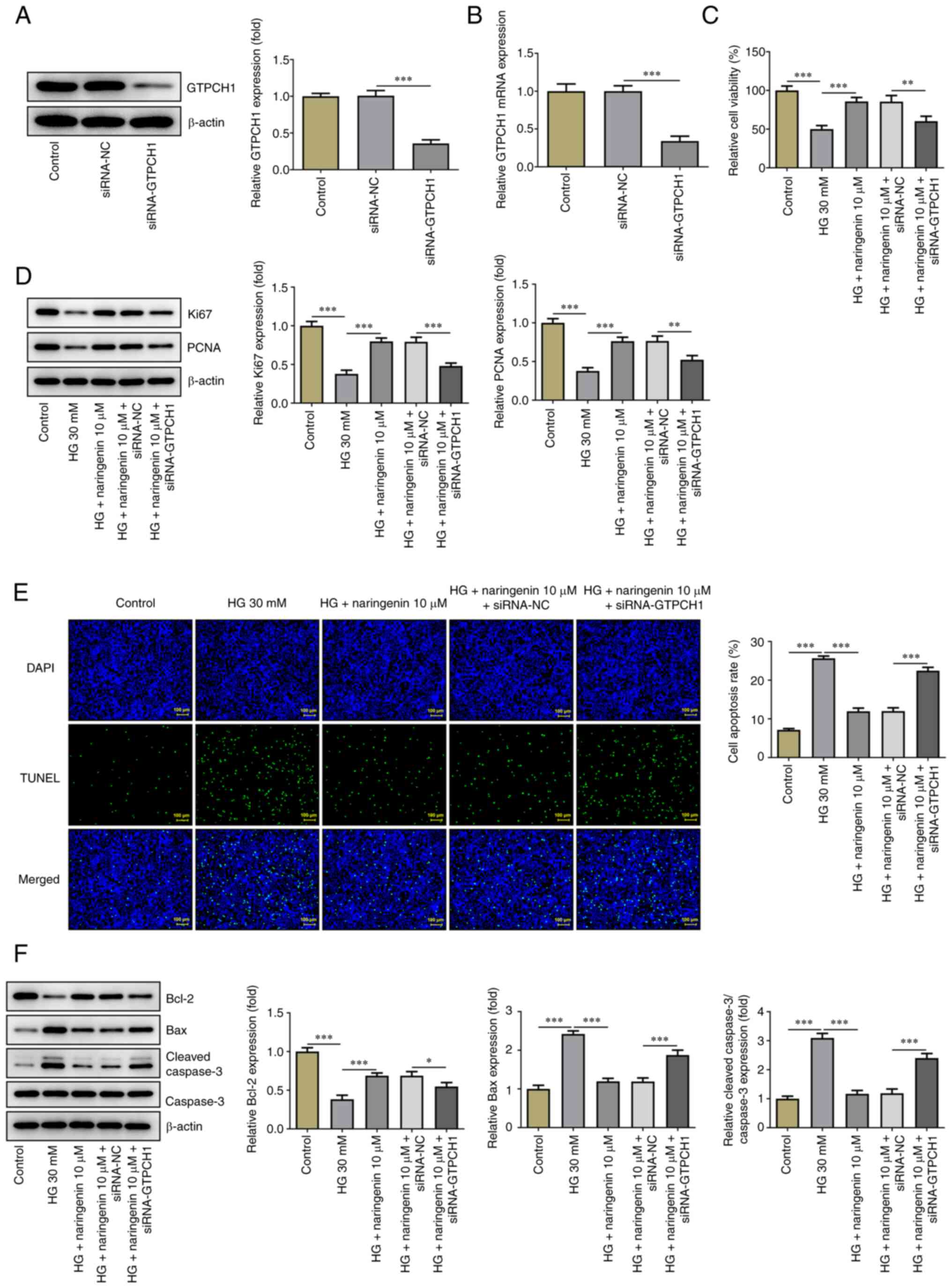
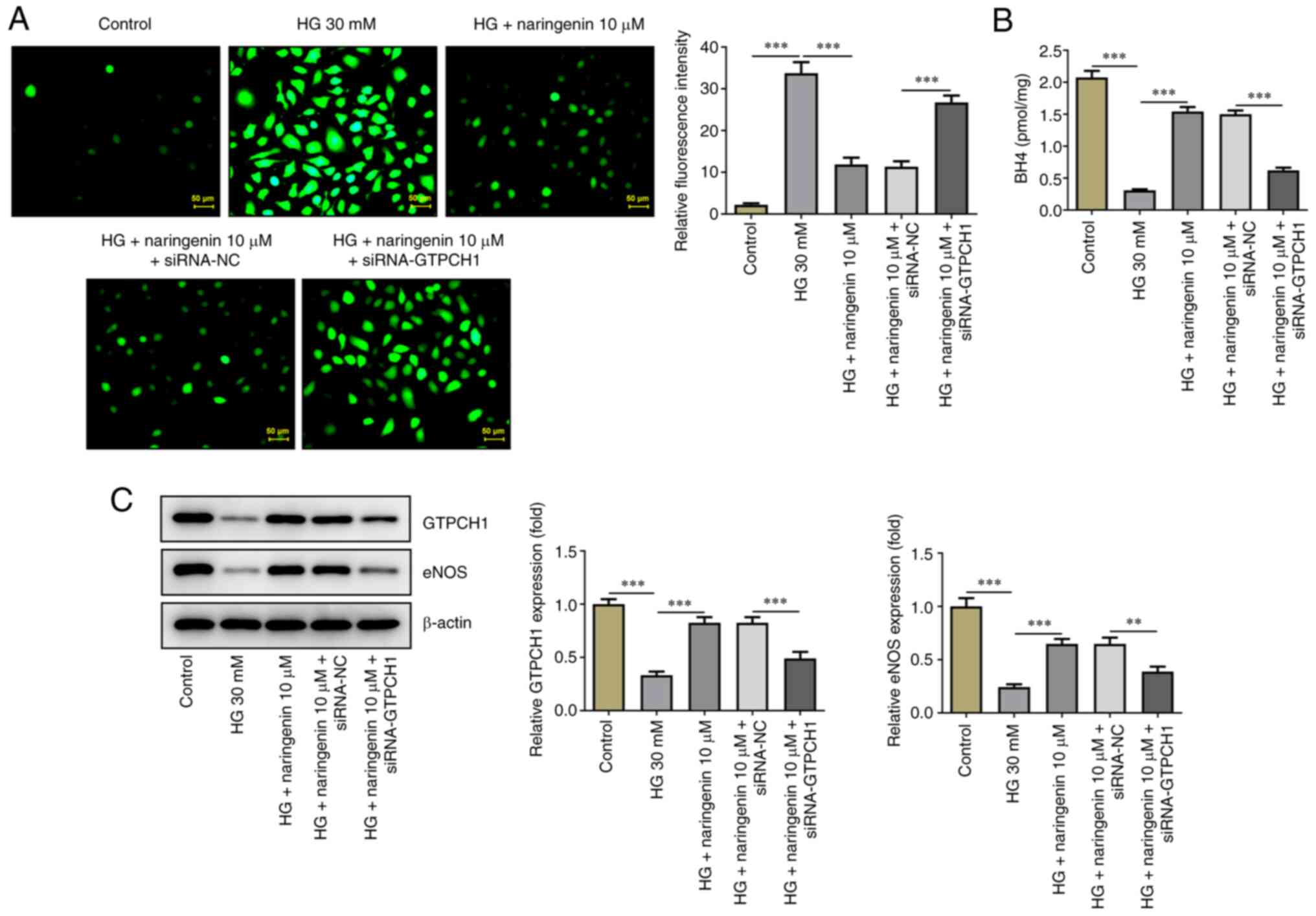
GTPCH1 knockdown reverses the
inhibitory effect of naringenin on HG-induced HREC injury
To uncover the mechanism underlying the effect of
naringenin on improving HG-induced HREC injury via upregulation of
GTPCH1, its role in GTPCH1-knockdown HRECs was investigated.
Western blotting and RT-qPCR showed that expression of GTPCH1 was
significantly decreased in the siRNA-GTPCH1 compared with the
siRNA-NC group (Fig. 4A and
B). HREC viability, proliferative
ability and apoptosis were then assessed. HREC viability and
expression levels of Ki67 and PCNA (Fig. 4C and D) were significantly decreased in the HG
+ 10 µM naringenin + siRNA-GTPCH1 compared with the HG + 10 µM
naringenin + siRNA-NC group. Additionally, the inhibitory effect of
naringenin on HG-induced HREC apoptosis was reversed in the HG + 10
µM naringenin + siRNA-GTPCH1 group compared with the HG + 10 µM
naringenin + siRNA-NC group (Fig.
4E and F). Naringenin-reduced
ROS generation in HG-insulted HRECs was improved again after
silencing of GTPCH1 (Fig. 5A), and
GTPCH1 depletion reversed the elevated BH4 content, and GTPCH1 and
eNOS expression imposed by naringenin administration in HG-treated
HRECs (Fig. 5B and C).
Discussion
It has been reported that exposure to HG promotes
overproduction of intracellular ROS, leading to oxidative stress,
apoptosis and dysfunction of ECs (34). Levels of BH4, a key co-factor of
eNOS, are regulated by GTPCH1(35). In the absence of BH4, eNOS produces
ROS instead of NO, which is also referred to as eNOS uncoupling
(27). ROS are partially derived
from eNOS uncoupling. Studies have shown that naringenin exerts
different cytotoxic effects on different types of cells, including
polymorphonuclear leukocytes and Wilms tumor cells (36,37).
In the present study, naringenin was cytotoxic at 20 µM. Therefore,
concentrations of 1 and 10 µM were selected for subsequent
experiments. The present results demonstrated that HG promoted HREC
apoptosis, increased ROS production, downregulated protein
expression levels of GTPCH1 and eNOS and attenuated BH4 secretion,
suggesting that HG induced oxidative stress and dysfunction of BH4
secretion, thus promoting cell apoptosis. Naringenin inhibited
HG-induced HREC apoptosis, upregulated Ki67 and PCNA expression and
effectively decreased intracellular ROS levels in a dose-dependent
manner. Furthermore, naringenin upregulated GTPCH1/eNOS signaling,
promoted release of BH4 and notably alleviated HREC injury. GTPCH1
knockdown confirmed that the GTPCH1/eNOS signaling pathway was
involved in the protective role of naringenin in HG-induced
HRECs.
DR, a common microvascular complication in patients
with diabetes, is primarily characterized by retinal structure and
functional abnormality, which causes blindness in severe cases
(38). Retinal endothelial
dysfunction is the primary pathological process of DR (39). Previous studies have suggested that
long-term hyperglycemia causes multiple types of EC injury,
including brain microvascular (40), aortic (41), human umbilical vein (42) and HREC injury (43). Here, a retinal injury model was
established by treating HRECs with 30 mM glucose as an inducer.
Previous studies suggested that this dose of glucose significantly
enhances levels of intracellular inflammatory factors in HRECs and
promotes EC dysfunction (43,44).
Another study demonstrated that naringenin effectively decreases
diabetes-induced oxidative stress response caused by impaired NO
synthesis in rat ECs (45). In
addition, naringenin protects the eye by inhibiting corneal
angiogenesis (20) and improving
macular degeneration (46). To the
best for our knowledge, the present study is the first to
demonstrate that naringenin attenuates generation of ROS and
oxidative injury in HG-induced HRECs.
Steady-state imbalance of NO and ROS may lead to
endothelial-dependent impaired vasodilation and enhanced
inflammatory responses, oxidative stress and EC injury (47). eNOS is the key rate-limiting enzyme
for NO synthesis (48) and
catalyzes conversion of L-arginine into NO. However, under
pathological conditions, eNOS promotes conversion to superoxide
instead of NO (eNOS uncoupling) (49). eNOS uncoupling is partially
promoted by GTPCH1 downregulation, which leads to dysfunction of
BH4 secretion and impaired NO synthesis (50). An et al (51) showed that enhanced GTPCH1-mediated
eNOS recirculation alleviates HG-induced endothelial dysfunction.
In addition, exogenous zinc supplementation restores diabetic
endothelial dysfunction via upregulating GTPCH1(22). In the present study, HG-mediated
induction of HRECs decreased protein expression levels of GTPCH1
and eNOS, thus supporting the abnormal increase in ROS levels in
HRECs. Naringenin significantly increased protein expression levels
of GTPCH1 and eNOS and BH4 secretion in HRECs, thus attenuating ROS
generation and cell apoptosis. However, GTPCH1 knockdown partially
restored the protective effects of naringenin on HG-treated HRECs,
suggesting that naringenin improved oxidative stress, cell
apoptosis and impaired BH4 secretion via the GTPCH1/eNOS signaling
pathway.
To the best of our knowledge, the present study is
the first to report the positive effects and underlying mechanism
of naringenin on diabetic REC injury; however, these findings were
only supported by in vitro experiments. Therefore, in
vivo studies are needed to verify the aforementioned results.
The role of the GTPCH1/eNOS signaling pathway in
naringenin-mediated protection of RECs was verified only in
GTPCH1-knockdown HRECs. Therefore, experiments using GTPCH1
antagonists or inhibitors should be performed to confirm the
results of the present study. Bai et al (52) showed that naringenin metabolism in
different species is a complex multi-pathway process, therefore the
specific metabolic pathway of naringenin and its toxicity in
vivo need to be studied. Additionally, the involvement of
pathways other than the GTPCH1/eNOS pathway in the protective
effect of naringenin cannot be ruled out. To verify the accuracy of
the present study, it is necessary to evaluate the potential role
of GTPCH1 siRNA in control cells in future. In addition, Annexin
V-PI staining should be used for detection of apoptosis in future
studies.
In summary, the present study suggested that
naringenin improved oxidative stress, cell apoptosis and
dysfunction of BH4 secretion in HG-treated HRECs by upregulating
the GTPCH1/eNOS signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BX conceptualized and designed the study. YW
acquired, analyzed and interpreted data. BX and YW drafted the
manuscript and revised it critically for important intellectual
content. All authors agreed to be held accountable for the current
study in ensuring questions related to the integrity of any part of
the work are appropriately investigated and resolved. All authors
have read and approved the final manuscript. BX and YW confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wojciechowska J, Krajewski W, Bolanowski
M, Kręcicki T and Zatoński T: Diabetes and cancer: A review of
current knowledge. Exp Clin Endocrinol Diabetes. 124:263–275.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li Y and Ren K: The mechanism of
contrast-induced acute kidney injury and its association with
diabetes mellitus. Contrast Media Mol Imaging.
2020(3295176)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Basu P and Basu A: In vitro and in vivo
effects of flavonoids on peripheral neuropathic pain. Molecules.
25(1171)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Piano I, Di Paolo M, Corsi F, Piragine E,
Bisti S, Gargini C and Di Marco S: Retinal neurodegeneration:
Correlation between nutraceutical treatment and animal model.
Nutrients. 13(770)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lechner J, O'Leary OE and Stitt AW: The
pathology associated with diabetic retinopathy. Vision Res.
139:7–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hammes HP: Diabetic retinopathy:
Hyperglycaemia, oxidative stress and beyond. Diabetologia.
61:29–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang Y, Liu Y, Li Y, Chen Z, Xiong Y, Zhou
T, Tao W, Xu F, Yang H, Ylä-Herttuala S, et al: MicroRNA-15b
targets VEGF and inhibits angiogenesis in proliferative diabetic
retinopathy. J Clin Endocrinol Metab. 105:3404–3415.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fu D, Yu JY, Yang S, Wu M, Hammad SM,
Connell AR, Du M, Chen J and Lyons TJ: Survival or death: A dual
role for autophagy in stress-induced pericyte loss in diabetic
retinopathy. Diabetologia. 59:2251–2261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hua YQ, Zeng Y, Xu J and Xu XL: Naringenin
alleviates nonalcoholic steatohepatitis in middle-aged
Apoe-/-mice: Role of SIRT1. Phytomedicine.
81(153412)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fuster MG, Carissimi G, Montalbán MG and
Víllora G: Improving anticancer therapy with naringenin-loaded silk
fibroin nanoparticles. Nanomaterials (Basel).
10(718)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hernández-Aquino E and Muriel P:
Beneficial effects of naringenin in liver diseases: Molecular
mechanisms. World J Gastroenterol. 24:1679–1707. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Naraki K, Rezaee R and Karimi G: A review
on the protective effects of naringenin against natural and
chemical toxic agents. Phytother Res. 35:4075–4091. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Patel K, Singh GK and Patel DK: A review
on pharmacological and analytical aspects of naringenin. Chin J
Integr Med. 24:551–560. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tutunchi H, Naeini F, Ostadrahimi A and
Hosseinzadeh-Attar MJ: Naringenin, a flavanone with antiviral and
anti-inflammatory effects: A promising treatment strategy against
COVID-19. Phytother Res. 34:3137–3147. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Al-Dosari DI, Ahmed MM, Al-Rejaie SS,
Alhomida AS and Ola MS: Flavonoid naringenin attenuates oxidative
stress, apoptosis and improves neurotrophic effects in the diabetic
rat retina. Nutrients. 9(1161)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Heidary Moghaddam R, Samimi Z, Moradi SZ,
Little PJ, Xu S and Farzaei MH: Naringenin and naringin in
cardiovascular disease prevention: A preclinical review. Eur J
Pharmacol. 887(173535)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Burke AC, Sutherland BG, Telford DE,
Morrow MR, Sawyez CG, Edwards JY and Huff MW: Naringenin enhances
the regression of atherosclerosis induced by a chow diet in
Ldlr-/- mice. Atherosclerosis. 286:60–70.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qurtam AA, Mechchate H, Es-Safi I,
Al-Zharani M, Nasr FA, Noman OM, Aleissa M, Imtara H, Aleissa AM,
Bouhrim M and Alqahtani AS: Citrus flavanone narirutin, in vitro
and in silico mechanistic antidiabetic potential. Pharmaceutics.
13(1818)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zeng B, Chen K, Du P, Wang SS, Ren B, Ren
YL, Yan HS, Liang Y and Wu FH: Phenolic compounds from clinopodium
chinense (Benth.) O. Kuntze and their inhibitory effects on
α-glucosidase and vascular endothelial cells injury. Chem
Biodivers. 13:596–601. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oguido APMT, Hohmann MSN, Pinho-Ribeiro
FA, Crespigio J, Domiciano TP, Verri WA Jr and Casella AMB:
Naringenin eye drops inhibit corneal neovascularization by
anti-inflammatory and antioxidant mechanisms. Invest Ophthalmol Vis
Sci. 58:5764–5776. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nasser A, Møller AT, Hellmund V, Thorborg
SS, Jespersgaard C, Bjerrum OJ, Dupont E, Nachman G, Lykkesfeldt J,
Jensen TS and Møller LB: Heterozygous mutations in
GTP-cyclohydrolase-1 reduce BH4 biosynthesis but not pain
sensitivity. Pain. 159:1012–1024. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu P, Liu J, Wu Y, Xi W, Wei Y, Yuan Z
and Zhuo X: Zinc supplementation protects against diabetic
endothelial dysfunction via GTP cyclohydrolase 1 restoration.
Biochem Biophys Res Commun. 521:1049–1054. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liang YH, Chen GW, Li XS, Jia S and Meng
CY: Guanosine-5'-triphosphate cyclohydrolase 1 regulated long
noncoding RNAs are potential targets for microglial activation in
neuropathic pain. Neural Regen Res. 16:596–600. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li J, Liu S, Cao G, Sun Y, Chen W, Dong F,
Xu J, Zhang C and Zhang W: Nicotine induces endothelial dysfunction
and promotes atherosclerosis via GTPCH1. J Cell Mol Med.
22:5406–5417. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sandrim VC, Yugar-Toledo JC, Desta Z,
Flockhart DA, Moreno H Jr and Tanus-Santos JE: Endothelial nitric
oxide synthase haplotypes are related to blood pressure elevation,
but not to resistance to antihypertensive drug therapy. J
Hypertens. 24:2393–2397. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Satoh M, Fujimoto S, Haruna Y, Arakawa S,
Horike H, Komai N, Sasaki T, Tsujioka K, Makino H and Kashihara N:
NAD(P)H oxidase and uncoupled nitric oxide synthase are major
sources of glomerular superoxide in rats with experimental diabetic
nephropathy. Am J Physiol Renal Physiol. 288:F1144–F1152.
2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang S, Xu J, Song P, Wu Y, Zhang J, Chul
Choi H and Zou MH: Acute inhibition of guanosine triphosphate
cyclohydrolase 1 uncouples endothelial nitric oxide synthase and
elevates blood pressure. Hypertension. 52:484–490. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Alp NJ, McAteer MA, Khoo J, Choudhury RP
and Channon KM: Increased endothelial tetrahydrobiopterin synthesis
by targeted transgenic GTP-cyclohydrolase I overexpression reduces
endothelial dysfunction and atherosclerosis in ApoE-knockout mice.
Arterioscler Thromb Vasc Biol. 24:445–450. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pannirselvam M, Simon V, Verma S, Anderson
T and Triggle CR: Chronic oral supplementation with sepiapterin
prevents endothelial dysfunction and oxidative stress in small
mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol.
140:701–706. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Meininger CJ, Marinos RS, Hatakeyama K,
Martinez-Zaguilan R, Rojas JD, Kelly KA and Wu G: Impaired nitric
oxide production in coronary endothelial cells of the spontaneously
diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem
J. 349:353–356. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu L, Zhang H, Shi Y and Pan L:
Prostaglandin E1 improves cerebral microcirculation through
activation of endothelial NOS and GRPCH1. J Mol Neurosci.
70:2041–2048. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Le Y, Wei R, Yang K, Lang S, Gu L, Liu J,
Hong T and Yang J: Liraglutide ameliorates palmitate-induced
oxidative injury in islet microvascular endothelial cells through
GLP-1 receptor/PKA and GTPCH1/eNOS signaling pathways. Peptides.
124(170212)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rizwan H, Pal S, Sabnam S and Pal A: High
glucose augments ROS generation regulates mitochondrial dysfunction
and apoptosis via stress signalling cascades in keratinocytes. Life
Sci. 241(117148)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang S, Xu J, Song P, Viollet B and Zou
MH: In vivo activation of AMP-activated protein kinase attenuates
diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes.
58:1893–1901. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li RF, Feng YQ, Chen JH, Ge LT, Xiao SY
and Zuo XL: Naringenin suppresses K562 human leukemia cell
proliferation and ameliorates Adriamycin-induced oxidative damage
in polymorphonuclear leukocytes. Exp Ther Med. 9:697–706.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li H, Chen P, Chen L and Wang X: The
natural flavonoid naringenin inhibits the cell growth of wilms
tumor in children by suppressing TLR4/NF-κB signaling. Anticancer
Agents Med Chem. 21:1120–1126. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ahsan H: Diabetic retinopathy-biomolecules
and multiple pathophysiology. Diabetes Metab Syndr. 9:51–54.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Simó R, Stitt AW and Gardner TW:
Neurodegeneration in diabetic retinopathy: Does it really matter?
Diabetologia. 61:1902–1912. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jin H, Zhu Y, Li Y, Ding X, Ma W, Han X
and Wang B: BDNF-mediated mitophagy alleviates high-glucose-induced
brain microvascular endothelial cell injury. Apoptosis. 24:511–528.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cao Y, Yuan G, Zhang Y and Lu R: High
glucose-induced circHIPK3 downregulation mediates endothelial cell
injury. Biochem Biophys Res Commun. 507:362–368. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ding X, Yao W, Zhu J, Mu K, Zhang J and
Zhang JA: Resveratrol attenuates high glucose-induced vascular
endothelial cell injury by activating the E2F3 pathway. Biomed Res
Int. 2020(6173618)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang Y, Lv X, Hu Z, Ye X, Zheng X, Ding
Y, Xie P and Liu Q: Protection of Mcc950 against
high-glucose-induced human retinal endothelial cell dysfunction.
Cell Death Dis. 8(e2941)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Long L, Li Y, Yu S, Li X, Hu Y, Long T,
Wang L, Li W, Ye X, Ke Z and Xiao H: Scutellarin prevents
angiogenesis in diabetic retinopathy by downregulating
VEGF/ERK/FAK/Src pathway signaling. J Diabetes Res.
2019(4875421)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wojnar W, Zych M and Kaczmarczyk-Sedlak I:
Antioxidative effect of flavonoid naringenin in the lenses of type
1 diabetic rats. Biomed Pharmacother. 108:974–984. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen W and Lin B, Xie S, Yang W, Lin J, Li
Z, Zhan Y, Gui S and Lin B: Naringenin protects RPE cells from
NaIO3-induced oxidative damage in vivo and in vitro
through up-regulation of SIRT1. Phytomedicine.
80(153375)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Meza CA, La Favor JD, Kim DH and Hickner
RC: Endothelial dysfunction: Is there a hyperglycemia-induced
imbalance of NOX and NOS? Int J Mol Sci. 20(3775)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kim DH, Meza CA, Clarke H, Kim JS and
Hickner RC: Vitamin D and endothelial function. Nutrients.
12(575)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gielis JF, Lin JY, Wingler K, Van Schil
PE, Schmidt HH and Moens AL: Pathogenetic role of eNOS uncoupling
in cardiopulmonary disorders. Free Radic Biol Med. 50:765–776.
2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wu Y, Ding Y, Ramprasath T and Zou MH:
Oxidative stress, GTPCH1, and endothelial nitric oxide synthase
uncoupling in hypertension. Antioxid Redox Signal. 34:750–764.
2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
An H, Wei R, Ke J, Yang J, Liu Y, Wang X,
Wang G and Hong T: Metformin attenuates fluctuating glucose-induced
endothelial dysfunction through enhancing GTPCH1-mediated eNOS
recoupling and inhibiting NADPH oxidase. J Diabetes Complications.
30:1017–1024. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bai Y, Peng W, Yang C, Zou W, Liu M, Wu H,
Fan L, Li P, Zeng X and Su W: Pharmacokinetics and metabolism of
naringin and active metabolite naringenin in rats, dogs, humans,
and the differences between species. Front Pharmacol.
11(364)2020.PubMed/NCBI View Article : Google Scholar
|