Introduction
Diaphragmatic hernia is determined by a defect in
the diaphragm that allows the abdominal viscera to protrude into
the thoracic cavity. Most of the cases are congenital diaphragmatic
hernia (CDH) interfering with the normal development of the lungs,
while acquired diaphragmatic hernias are most commonly of traumatic
origin (1,2). According to the European Surveillance
of Congenital Anomalies, the reported incidence of CDH in 2019 for
all pregnancies from 20 weeks onwards was 3.11 per 10,000 and 2.15
per 10,000 for live-born infants (3). This study focuses on neonatal CDH
cases as this is one of the most complicated conditions that could
be surgically operated on-site.
The Newborn Intensive Care Unit at ‘Maria S. Curie’
Emergency Clinical Hospital for Children is one of the largest
units of this type in Eastern Europe. Built in 2013, inspired by
the Iowa Neonatal Intensive Care Unit, the NICU provides
specialized intensive care treatment for surgical neonatal
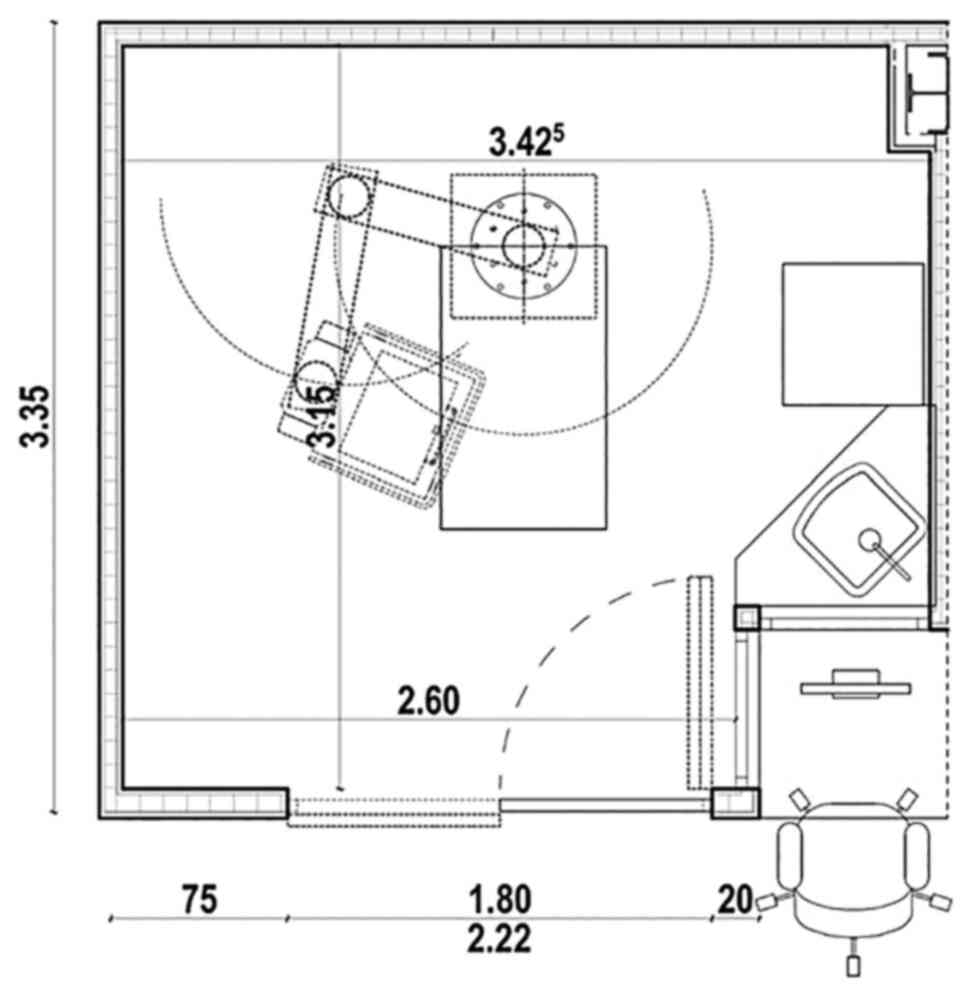
patients. There are 27 beds in individual rooms with areas of
approximately 12 square meters (Fig.
1). Each room is equipped with central air flow system (6 air
change cycles per hour), different types of light (natural light, 2
ceiling projectors for procedures, and 1 indirect light), pendants
with outlets for O2, medical air, vacuum, iNO,
CO2, helium and areas for hand washing and disinfection.
In addition, every room is equipped with one open incubator, one
ventilator, one or two docking stations with 10 syringe pumps each,
one transcutaneous monitor, central monitoring, alarming equipment
software, electronical data sheet for the patients/ICCA, individual
medical cart for medication and consumables with extendable table
for procedures.
The ‘Maria S. Curie’ Hospital provides medical care
to children of all ages, but it presents no delivery room. As such,
all patients admitted to the NICU are transferred from referral
hospital either by neonatal ambulance transport or helicopter.
There are indications that the stress endured during the transport
worsens the medical condition of the patients at the admission
time. Transferring unstable neonates over long distances is
frequently associated with notable deterioration of the general
condition. Similar risks, albeit with lower incidence and severity,
can occur during the patient's transfer from the NICU to the
operating room (OP) (4).
Performing the intervention in the NICU can thus avoid the
complications associated with the discontinuation of monitoring,
dislocation of artificial airways, accidental removal of vascular
access, hypothermia or variations in heart rate and blood pressure
(5,6).
Bedside surgery is a well-established practice at
‘Maria S. Curie’ NICU as both the technical equipment and medical
expertise allow for this type of intervention (Fig. 2). Since 2011, a series of onsite
interventions have been performed, including surgery for
retinopathy (laser therapy and avastin injections), ligation of the
patent ductus arteriosus, emergency atrial septostomy, placement of
extracorporeal membrane oxygenation (ECMO) or continuous renal
replacement therapy (CRRT) hemodialysis catheter, external
ventricular drainage for hydrocephalus, tracheostomy, pleural
drainage, peritoneal dialysis catheter placement and procedure.
Patients and methods
The present study is based on the retrospective
analysis of 22 patients with CDH that were admitted to ‘Maria S.
Curie’ NICU between January 2011 and December 2020. The COVID-19
pandemic did not affect the provision and the quality of the
medical care in our unit. Specifically, the study was not
influenced as there were no positive patients in our data sample as
well as in the NICU during this time. The dataset supporting this
study consists of 10 patients that were surgically operated on-site
in the NICU. In addition, a control group of 12 patients that could
not be operated on even on-site due to severe respiratory and
hemodynamically instability was used to provide a reference to the
survival rate for non-operated patients. Two patients were excluded
from the control group due to very severe status at the time of the
admission in the NICU followed by death within 12 h.
For all analyzed cases, the decision to surgically
operate on-site or not to operate at all, was made following a
multidisciplinary discussion involving medical doctors from the
neonatology, cardiology, anesthesiology, and surgical team, with
the outcome documented in a medical report. The criteria to operate
on-site, as well as the decision regarding proper surgery time, was
a team decision based on clinical and hemodynamical stability of
each patient, dependent on the inotropic medication, high-frequency
oscillatory ventilation (HFOV), iNO and ECMO procedures. An
additional consideration was the operatory infrastructure as it is
not possible to administer NO and apply HVOF in the OR. The
arguments supporting this type of intervention were presented to
the parents and in all cases the decision to operate was supported
by parental consent (except for one emergency operation for
pneumoperitoneum). For the cases admitted during the COVID-19
pandemic, strict regulations to prevent in-hospital dissemination
of Sars-cov-2 infection were observed consisting of RT-PCR testing
at admission, mask wearing by hospital personnel, and frequent
disinfection of the surfaces (7).
Upon admission into the NICU, a series of
investigations were performed according to the internal evaluation
protocol for CDH. These investigations included microbiological
studies (microbial skin culture, blood culture), complete blood
count, kidney function, coagulation screening and inflammatory
tests, genetic tests, and serial blood gas tests. In addition,
echocardiography was performed to exclude associated malformations,
to measure the right-to-left shunt, to estimate the severity of
pulmonary hypertension and to evaluate the anatomy and velocities
at the pulmonary veins and pulmonary arterial branches. The
protocol also included brain and kidney ultrasound and chest X-ray
to evaluate the mediastinal shifting, pneumothorax, severity of
atelectasis and the presence of fluid at the side of the
herniation. In the past few years, since it has become possible to
have patients on ECMO; the levels of B-type natriuretic peptide
(BNP) are used as a prognostic marker for the development of
pulmonary hypertension as well as the need for ECMO (8-11).
In addition, a series of personal general data was collected and
analyzed (e.g. antenatal diagnosis, sex, gestational age, type of
birth, Apgar score) as well as details of the care procedure
immediately after delivery (e.g. intubation, type of ventilation
and peripheral capillary oxygen saturation (SpO2) with
minimal fraction of inspired oxygen (FiO2) on the first
day of life). Since all newborns were transferred to our clinic
mainly on the first day of life, this general data was collected in
the hospitals where the children were born.
Vital parameters were recorded at admission time for
all patients. The data recorded prior and after the intervention
constituted the basis for the analysis of the surgically operated
patients. These parameters included the following metrics: heart
rate (HR) (continuously measured by electrocardiography), blood
pressure (BP) as systolic blood pressure, diastolic blood pressure
and mean arterial pressure (MAP), either intermittently measured
peripheral or via central preductal arterial line, preductal oxygen
saturation (SpO2), blood gas (capillary or arterial
stemple), glycemia, urine output, ventilation parameters, iNO, and
the doses of the continuous intravenous medication as individual
and as the Vasoactive-inotropic score (VIS). The VIS was calculated
as: dopamine dose (µg/kg/min) + dobutamine dose (µg/kg/min) + 100 x
adrenaline dose (µg/kg/min) +100 x noradrenaline dose (µg/kg/min) +
10 x milrinone dose (µg/kg/min) (12).
In addition, the patient age at the day of the
surgery, ASA anesthesiology risk, intraoperative events and
modifications, the length of the intervention and surgery
particularities (herniated organs, need for patch suture),
immediate complication, long term complication and mortality were
also recorded. To evaluate infection, samples of tracheal aspirate
and blood cultures were collected regularly. The results were
correlated with the inflammatory and hematological markers,
clinical signs of pneumonia or sepsis, and radiographic findings.
C-reactive protein (CRP) is not collected in our unit in the first
days after surgery as it has been shown that an elevated value is
most likely a reaction due to post-operatory inflammation given
that the patients can have drainage tubes or be on ECMO.
In terms of organization for the surgery time, there
were no major changes in patient care; medication was left in
place, ventilation was mainly maintained in HFOV mode, the radiant
warmer was switched to matrass manual mode and the temperature was
monitored via a transcutaneous sensor. Although the risk of
hypothermia was reduced by not transporting the patient to the OR,
it was considered necessary to evaluate the changes in temperature
during the surgery time. As is known, general anesthesia inhibits
thermoregulation, and this effect is compounded by the fact that
newborns (especially premature) have less brown fat and the skin
surface exposed during surgery is proportionally larger relative to
older children (13).
Our surgeons are able to perform this type of
surgery with minimally invasive methods (laparoscopy,
thoracoscopy), but it was considered that these unstable patients
could not tolerate CO2 insufflation, so they were all
operated on via an open abdominal or thoracic approach (14). The anesthesiology team used total
intravenous anesthesia, mainly midazolam (0.05-0.1 mg/kg/h) and
fentanyl (2-10 µg/kg/h), while muscle relaxation was provided
during the surgery time using atracrium or vecuronium. Pre- and
post-surgery sedation was maintained by intermittent or continuous
doses of narcotics and benzodiazepines.
The literature regarding on-site neonatal surgery is
limited. While our dataset is affected by the same shortcomings as
other studies (e.g., small sample size), our sample is homogenous
in terms of weight, gestational age and sex distribution, which
further supports the relevance of the results. The control group
shares the same characteristics as the operable group with the
notable difference that the respective patients could not be
operated on.
The data were summarized and analyzed using Excel as
part of the Microsoft Office 365 Suite and the results are
presented using the relevant descriptive statistics.
The study was approved by the Ethics Committees of
the ‘M. S. Curie’ Emergency Clinical Hospital for Children and
‘Carol Davila’ University of Medicine and Pharmacy (Bucharest,
Romania). Written informed consent was obtained from the patients
in order to use their data for academic purposes.
Results
The study covers a period of 10 years and is based
on data collected from 10 newborn patients with CDH surgically
operated on-site in the NICU. This represents 12.8% of the total of
78 CDH patients operated on in our clinic during the observation
period.
In our test group, both sexes were equally
represented, chronologically term newborns, one small and nine
appropriate for gestational age (median birth weight 2,691 g; range
2,300-3,300). The type of birth was Cesarean section in 8 cases and
spontaneous vaginal birth for 2 cases. Apgar scores ranged between
3 and 8. Six newborns were diagnosed antenatal with CDH [at a main
gestational age of 26.5 weeks of gestation (WG)] and 4 of them were
uninvestigated pregnancies, and for this reason the common protocol
of intubation immediately after birth was not applied. Two patients
were reanimated via positive pressure ventilation (PPV) and
intubated in the delivery room for respiratory distress and two of
them were intubated after a few hours when the general status had
deteriorated.
Relative to the test group, the control sample
shared the same characteristics in terms of antenatal diagnostic,
gestational age and birth weight. The patients included in the
control sample had a relatively lower Apgar score, a higher
intubation rate at birth and a higher need for iNO and inotropic
medication. This indicated a worse medical condition from the first
day of life which, in all cases, rendered the surgical operation
not feasible. Table I presents the
summarized statistics of both groups of patients included in this
study.
 | Table ISummary statistics of the groups
included in the study. |
Table I
Summary statistics of the groups
included in the study.
| Variables | Operated | Unoperated |
|---|
| Number of cases
included in the study, n | 10 | 12 |
| Antenatal diagnosis
of CDH (%) | 60% | 58.3% |
| Gestational age at
birth (weeks), mean (range) | 37.6 (37-39) | 37.0 (33-39) |
| Birth weight (g),
mean (range) | 2,691
(2,300-3,200) | 2,970
(2,470-3,500) |
| Apgar score, mean
(range) | 5.8 (3-8) | 4.5 (1-8) |
| Intubated at birth
(%) | 60% | 91.6% |
| HFOV on the first day
of life (%) | 100% | 100% |
| iNO on the first day
of life (%) | 60% | 83% |
| Inotropes on the
first day of life (%) | 60% | 100% |
Despite receiving adequate treatment for their
condition, all patients were respiratory and hemodynamically
unstable and received inotropic and vasoactive agents in
association of one, two or three drugs. As such, by the time of the
surgery, half of the operated patients received adrenaline (main
dose 0.08 µg/kg/min), 60% received noradrenaline (main dose 0.8
µg/kg/min), 60% received milrinone (main dose 0.5 µg/kg/min), 70%
received dopamine (mean dose 15 µg/kg/min) and 60% were
administered dobutamine (mean dose 12 µg/kg/min). Table II presents the simultaneously
administered inotropic medication by the time of the surgery for
patients that were operated on.
 | Table IISimultaneously administered inotropic
medication. |
Table II
Simultaneously administered inotropic
medication.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|
| Dopamine | X | X | X | X | | X | X | X | | X |
| Dobutamine | X | X | | X | X | X | | | X | X |
| Adrenaline | | X | X | | X | X | X | | | |
| Noradrenaline | X | X | | | | X | X | X | | |
| Milrinone | X | | | | | X | X | X | | X |
In order to have a more accurate assessment, the
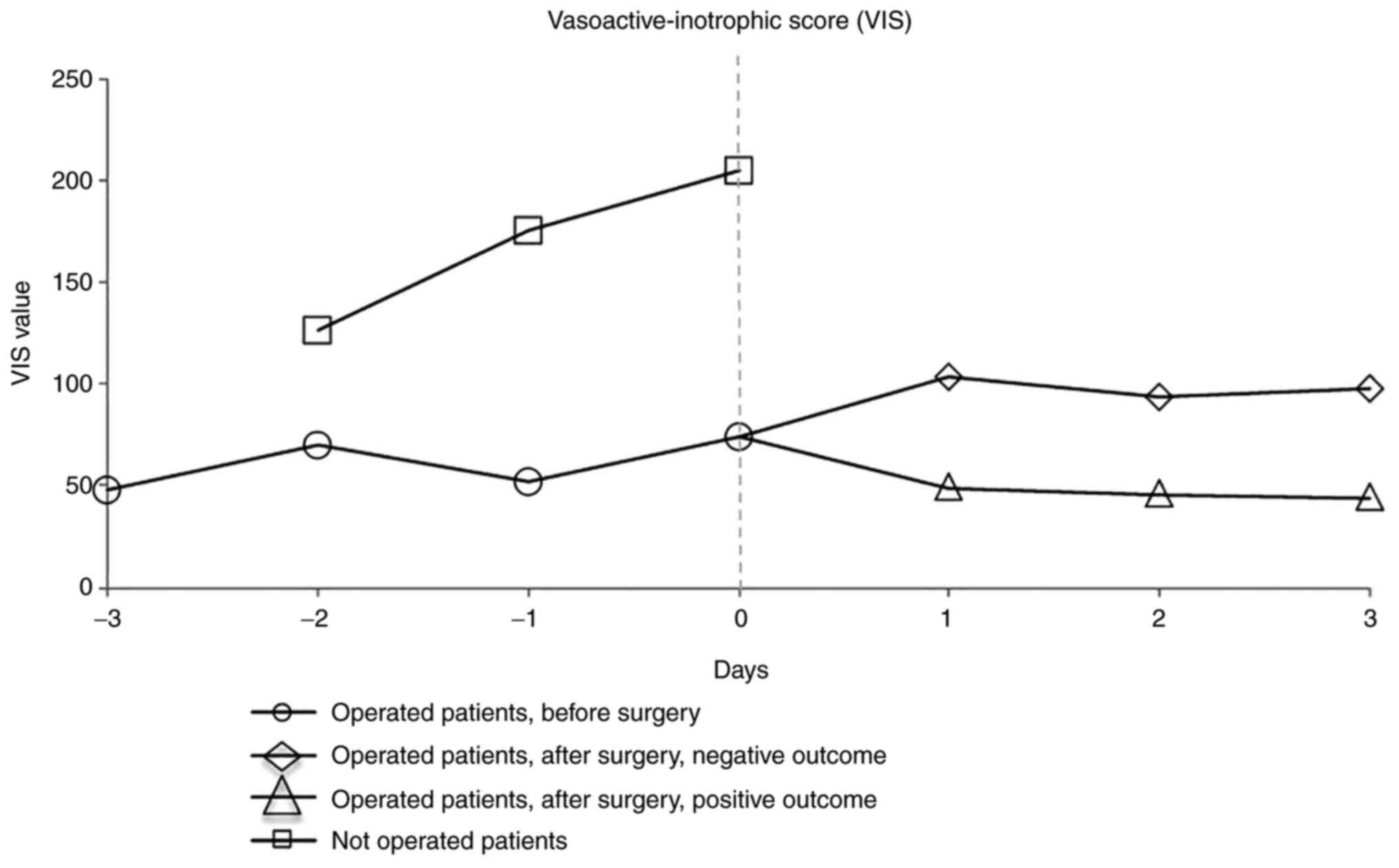
Vasoactive-inotropic score (VIS) was calculated for each group. The
VIS is usually used in ICUs to scale the amount of vasoactive and
inotropic support and to evaluate the outcome after infant cardiac
surgery (15,16) or in pediatric sepsis (17).
We centered the 2 samples for day ‘0’ representing
the day of operation for the test group (main day 5) and the last
day of survival for the control group (main day 4) to highlight the
fact that the patients in the control group were not in the
condition to be operated on (the patient with a 28-day survival due
to prolonged ECMO was not considered representative of the
group).
The dynamics of the VIS values for the previous 3
days and for the post-surgery 3 days for the operations are
presented in Fig. 3.
Based on this result, in regards to the control
group (unoperated patients), not only the absolute value of VIS per
day was visibly higher, but also the upward trend, directly
correlated with the poor clinical condition. For the group of
operated patients, although the VIS value was also high (>40
estimates reserved prognosis) (15), the trend was constant, indicating
relative clinical stability that allowed surgery. After the day of
surgery, the separation of the two subgroups was observed, with a
natural decreasing tendency for the surviving infants.
All operated patients had been on HFOV ventilation
before surgery and needed continuous iNO (mean dose was 20 ppm).
One patient had been on ECMO for 4 days prior to surgery, out of
ECMO and operated on a few hours later when the coagulation values
were at accepted limits. After surgery, the patient was put back on
ECMO for another 3 days. Another patient was operated on due to
emergency for pneumothorax and pneumoperitoneum; for another
patient, the surgery took place under special safety conditions as
needed when involving the care of a newborn of a human
immunodeficiency virus (HIV)-positive mother.
In the present study, the average age at time of
surgery was approximately 5 days (ranging from 2 to 10 days). The
one case operated on in the second day of life (28 h of life) was
an emergency surgery for pneumothorax and pneumomediastinum, and
the one operated on in the 10th day of life was due to a prolonged
unstable status.
All operated patients underwent laparotomy, and the
operative time ranged from 65 to 140 min (mean 94.5 min). There was
no difference with regards to the duration of the surgery compared
to a usual surgery performed in the OR, taking into consideration
the severity of the cases. In 7 cases, the diaphragmatic defect was
on the left side, and on the right side for the remaining 3
patients. The survivors were 3 left side CDH and 1 right side CDH
newborns, confirming the previous findings in literature that the
mortality is higher when CDH is on the right side (18). Regarding the type of surgery,
primary repair was possible for 8 patients while the other 2 needed
synthetical patch repair due to agenesis of the diaphragm. Both
were associated with severe lung hypoplasia and did not
survive.
In terms of the CDH anatomy, all our operated cases
presented with the small intestine in the thorax. All left side CDH
cases presented with herniated stomach and 85% had herniated large
intestine and spleen.
The liver was herniated in the thorax for 8 out of
10 cases. The other 2 cases (one left and one right side hernias)
were also the specific cases exhibiting good outcomes, as the
literature also suggests a positive prognosis for non-herniated
liver (19,20).
Regarding the associated pathology of the operated
patients, 8 cases presented congenital cardiac anomalies, 3 had
hypoxic-ischemic encephalopathy and 1 patient was an infant exposed
to an HIV-positive mother. All operated patients had persistent
pulmonary hypertension and 9 of them associated cardiac
failure.
In terms of the non-surgery-related complications, 1
patient had severe pulmonary hemorrhage, 2 patients had sepsis and
1 patient with chylothorax did not survive, while another patient
with chylothorax and 1 patient with right jugular thrombosis and
hydropericardium correlated with ECMO had good outcomes (Table III).
 | Table IIIGeneral characteristics of the
surgically operated patient population including associated
pathologies, complications and outcomes. |
Table III
General characteristics of the
surgically operated patient population including associated
pathologies, complications and outcomes.
| Case | Age when operated
on (days) | Side of the
diaphragmatic defect | Herniated
liver | Surgery duration
(min) | Associated
pathologies/pre-surgery complications | Complications | Outcome |
|---|
| 1 | 3 | Left | Yes | 100 | Mild left ventricle
hypoplasia; Atrial septal defect; Congestive heart failure | Persistent
pulmonary hypertension; Cardiac failure | Negative |
| 2 | 4 | Left | Yes | 80 | Mitral
regurgitation; Congestive heart failure | Persistent
pulmonary hypertension; Cardiac failure | Negative |
| 3 | 3 | Right | Yes | 120 | Atrial septal
defect | Persistent
pulmonary hypertension; Cardiac failure; Pulmonary hemorrhage | Negative |
| 4 | 10 | Left | Yes | 90 | Hypoxic-ischemic
encephalopathy | Persistent
pulmonary hypertension; Cardiac failure; Chylothorax | Negative |
| 5 | 7 | Left | Yes | 100 | Mild
hypoxic-ischemic encephalopathy | Persistent
pulmonary hypertension; Cardiac failure; Sepsis | Negative |
| 6 | 3 | Right | Yes | 110 | Right pulmonary
vein hypoplasia | Persistent
pulmonary hypertension; Cardiac failure; Sepsis | Negative |
| 7 | 7 | Left | No | 140 | Atrial septal
defect; Congestive heart failure-ECMO | Persistent
pulmonary hypertension; Cardiac failure; Right jugular thrombosis;
Hydropericardium | Positive |
| 8 | 10 | Left | No | 70 | Hypoxic-ischemic
encephalopathy; HIV exposed | Persistent
pulmonary hypertension; Cardiac failure; Chylothorax | Positive |
| 9 | 2 | Right | Yes | 65 | Preoperatory
pneumothorax and pneumoperitoneum | Persistent
pulmonary hypertension; Cardiac failure; Inferior vena cava
thrombosis | Positive |
| 10 | 8 | Left | Yes | 70 | Atrial septal
defect | Persistent
pulmonary hypertension | Positive |
During surgery, the ventilator settings were
maintained with constant maximal parameters for each patient in
order to maintain preductal SpO2 values above 85%.
FiO2 ranged from 65 to 100% (mean 95.5%) and mean airway
pressure ranged from 6 to 20 cm H2O (mean value 12.5 cm
H2O).
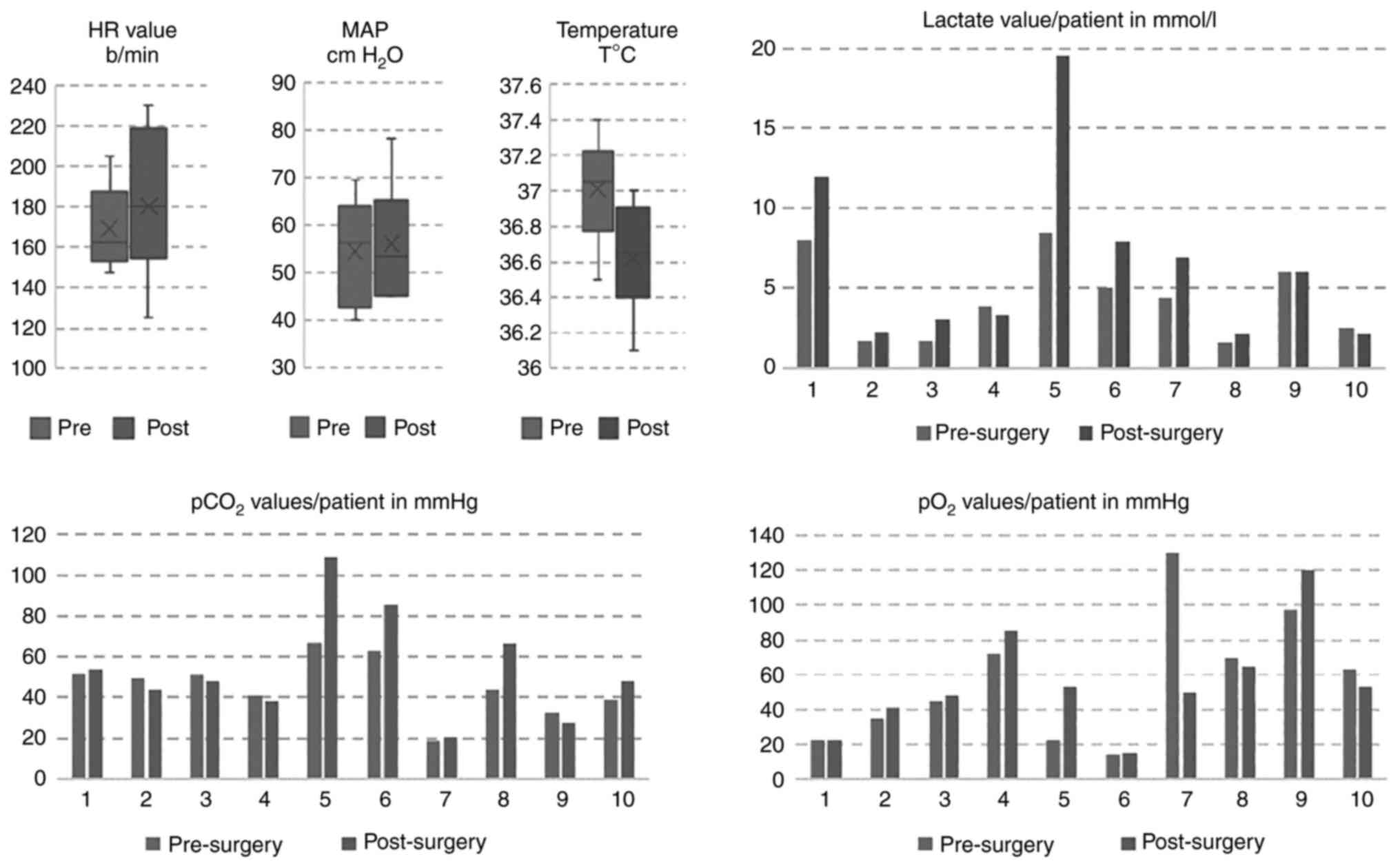
Regarding blood gas analyses, the pH values ranged
from 7.17 to 7.5 (mean value 7.33). As shown in Fig. 4, 7 patients had a higher
post-operative level of lactate, one presented a constant value and
2 had minimally decreased levels. Correlated with the pH level, 6
patients had a mild to severe increase in pCO2 levels
and 4 had a mild pCO2 improvement. When SpO2
was analyzed, before and immediately after surgery, 5 patients had
an improved level, 2 had a constant level and another 2 had a
slight decreasing trend. Since the precision of the capillary
samples is insufficient when compared to arterial samples, the gas
analyses were conducted in terms of relative improvement or
deterioration and not necessarily in absolute value, in line with
similar practices reported in the literature (21). A direct correlation between the gas
analyses and the outcome was not established except for the 2
patients with the highest lactate levels who died days after the
surgery. Several studies have shown that the NLRP3 inflammasome is
critically involved in the pathogenesis of inflammation and sepsis
in newborns, as well as in bronchopulmonary hypoplasia (22-24).
Targeting this pathological pathway may be a future therapeutical
target.
The body temperature was maintained within the
standard range for all of the patients. The mean HR increased from
169.1 beats/min to 180.3 beats/min (Fig. 4).
Historically, the ECMO procedure could be provided
in our clinic since 2016 and for this reason, most patients with
severe CDH underwent surgical repair despite the ECMO criteria
being met. For 2 patients (both included in the control group) who
were on veno-venous ECMO, the surgery was not possible due to
severe, unstable respiratory and hemodynamic status. However, the
only patient under ECMO included in the test group was also the
first patient in our clinic who was put on ECMO (veno-venous
initially, then veno-arterial due to cardiac failure) and operated
on with a good outcome.
The overall mortality rate for the test group (i.e.,
where on-site surgery could be performed) was 60% due to the
severity of the cases. However, the negative outcomes were not
determined by perioperative events. In contrast, none of the
patients in the control group (i.e., not operated patients)
survived more than a mean age of 4 days (range from 1 to 9 days of
life, with an extreme of 28 days of life for one patient on ECMO).
Other characteristics such as associated comorbidities or
complications were not relevant when comparing the groups.
Discussion
The present study demonstrated that bedside surgery
increases the chances of survival for the most severe congenital
diaphragmatic hernia (CDH) cases that cannot be transferred to the
operating room (OR). Nevertheless, the OR still provides the most
appropriate facilities for surgical procedures. An increasing
number of clinics around the world are applying this method and it
can therefore be considered a lifesaving practice for unstable
patients that cannot be transported to the OR (25-28).
We found that eliminating the stress of transporting
newborns to the OR reduces the risk of hypothermia and maintains
the hemodynamic balance. Keeping the same ventilation mode together
with continuous NO are important factors in keeping our patients
stabile, as nitric oxide (NO) and high-frequency oscillatory
ventilation (HFOV), cannot be administered during transport or in
the OR.
Performing procedures on-site with continuity of
care by neonatal medical staff is an additional advantage not only
to the patients but also to the anesthetist who may be unfamiliar
with the patient's ventilation type mode and medication.
One of the concerns when performing surgery outside
the OR is the risk of infection. None of the patients analyzed in
our sample suffered from post-operatory infection. This can be
partially explained by the fact that our NICU is equipped with all
the necessary equipment to provide the sterile setup necessary for
surgery, as demonstrated also by other authors (29).
The medical literature indicates that there is no
ideal time for repairing CDH (30,31).
In our case the decision to operate on-site, rather than not to
operate at all, was primarily based on expert opinion. The
operations were conducted in the neonatal transition period, as
soon as the infants were considered physiologically stable for
surgery. This approach has a subjective component as no objective
method of determining the proper timing has been published to
date.
We consider the Vasoactive-inotropic score (VIS) as
a reliable marker that can be used to quantify cardiovascular and
hemodynamic support, allowing comparison of patient populations
across studies.
There are several limitations to this study. The
limited case number may have contributed to the nonsignificant
difference and heterogeneous characteristics between the operated
and unoperated patients. The data were collected retrospectively
from patient records and electronic health records and was not
designed as a study from the beginning. It was also a single-center
study and may not be generalizable to all institutions that care
for CDH patients. Further studies should be designed to compare the
performing of surgery in the NICU or in the main OR.
In conclusion, our experience indicates that bedside
surgery increases the chances of survival for the very severe
cases, non-transferable to OR. This conclusion is also confirmed by
the comparative analysis with a representative sample of patients
for which surgery was not possible to be performed. The possibility
to operate on-site is one of the most relevant characteristics that
differentiates the test from the control sample, further supporting
the conclusion that the intervention seriously influences the
survival rate.
Neonatal surgery in the NICU is a safe procedure
that can be utilized in cases of neonates with severe respiratory
failure, severe pulmonary hypertension and hemodynamically
unstable. As such, it is recommended for ICU planners to consider
creating the infrastructure needed to conduct on-site surgery in
the NICU.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AEG, CGC, BS, AMB, CMH, MD, FJA, MC and CF were
responsible for conceptualization of the research concept. CGC, FDM
and RIS performed validation of the data and formal analysis. AEG,
CGC, BS, MD, FJA and MC searched the literature for similar work
and articles and had major contributions in writing the manuscript.
CGC, BS, MD, FJA and MC critically reviewed the manuscript for
important intellectual content, edited the manuscript and
supervized the research. AEG, CGC and RIS confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committees of
the ‘Maria S. Curie’ Emergency Clinical Hospital for Children and
‘Carol Davila’ University of Medicine and Pharmacy (Bucharest,
Romania) (nr 6/2021).
Patient consent for publication
Written informed consent was obtained from the
patients in order to use their data for academic purposes.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chandrasekharan PK, Rawat M, Madappa R,
Rothstein DH and Lakshminrusimha S: Congenital diaphragmatic
hernia-a review. Matern Health Neonatol Perinatol.
3(6)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dumitrescu D, Savlovschi C, Borcan R,
Pantu H, Serban D, Gradinaru S, Smarandache G, Trotea T, Branescu
C, Musat L, et al: Clinical case-voluminous diaphragmatic
hernia-surgically acute abdomen: Diagnostic and therapeutical
challenges. Chirurgia (Bucur). 106:657–660. 2011.PubMed/NCBI(In Romanian).
|
|
3
|
McGivern MR, Best KE, Rankin J, Wellesley
D, Greenlees R, Addor MC, Arriola L, de Walle H, Barisic I, Beres
J, et al: Epidemiology of congenital diaphragmatic hernia in
Europe: a register-based study. Arch Dis Child Fetal Neonatal Ed.
100:F137–F144. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mallick MS, Jado AM and Al-Bassam AR:
Surgical procedures performed in the neonatal intensive care unit
on critically ill neonates: Feasibility and safety. Ann Saudi Med.
28:105–108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fanning NF, Casey W and Corbally MT:
In-situ emergency paediatric surgery in the intensive care unit.
Pediatr Surg Int. 13:587–589. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Beckmann U, Gillies DM, Berenholtz SM, Wu
AW and Pronovost P: Incidents relating to the intra-hospital
transfer of critically ill patients. An analysis of the reports
submitted to the Australian incident monitoring study in intensive
care. Intensive Care Med. 30:1579–1585. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Serban D, Socea B, Badiu CD, Tudor C,
Balasescu SA, Dumitrescu D, Trotea AM, Spataru RI, Vancea G,
Dascalu AM and Tanasescu C: Acute surgical abdomen during the
COVID-19 pandemic: Clinical and therapeutic challenges. Exp Ther
Med. 21(519)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Snoek KG, Reiss IK, Greenough A, Capolupo
I, Urlesberger B, Wessel L, Storme L, Deprest J, Schaible T, van
Heijst A, et al: CDH EURO Consortium. Standardized postnatal
management of infants with congenital diaphragmatic hernia in
Europe: The CDH EURO Consortium Consensus-2015 Update. Neonatology.
110:66–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Partridge EA, Hanna BD, Rintoul NE,
Herkert L, Flake AW and Adzick NS: Partrige, Brain-type natriuretic
peptide levels correlate with pulmonary hypertension and
requirement for extracorporeal membrane oxygenation in congenital
diaphragmatic hernia. J Pediatr Surg. 50:263–266. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Steurer MA, Moon-Grady AJ, Fineman JR, Sun
CE, Lusk LA, Wai KC and Keller RL: B-type natriuretic peptide:
Prognostic marker in congenital diaphragmatic hernia. Pediatr Res.
76:549–554. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Heindel K, Holdenrieder S, Patel N,
Bartmann P, Schroeder L, Berg C, Merz WM, Mueller A and Kipfmueller
F: Early postnatal changes of circulating N-terminal-pro-B-type
natriuretic peptide in neonates with congenital diaphragmatic
hernia. Early Hum Dev. 146(105049)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Davidson J, Tong S, Hancock H, Hauck A, da
Cruz E and Kaufman J: Prospective validation of the
vasoactive-inotropic score and correlation to short-term outcomes
in neonates and infants after cardiothoracic surgery. Intensive
Care Med. 38:1184–1190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Boyer TJ and Kritzmire SM: Neonatal
Anesthesia. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2021.
|
|
14
|
Yang EY, Allmendinger N, Johnson SM, Chen
C, Wilson JM and Fishman SJ: Neonatal thoracoscopic repair of
congenital diaphragmatic hernia: Selection criteria for successful
outcome. J Pediatr Surg. 40:1369–1375. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gaies MG, Jeffries HE, Niebler RA,
Pasquali SK, Donohue JE, Yu S, Gall C, Rice TB and Thiagarajan RR:
Vasoactive-inotropic score is associated with outcome after infant
cardiac surgery: An analysis from the Pediatric Cardiac Critical
Care Consortium and Virtual PICU System Registries. Pediatr Crit
Care Med. 15:529–537. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dilli D, Akduman H, Orun UA, Tasar M,
Tasoglu I, Aydogan S, Citli R and Tak S: Predictive value of
vasoactive-inotropic score for mortality in newborns undergoing
cardiac surgery. Indian Pediatr. 56:735–740. 2019.PubMed/NCBI
|
|
17
|
McIntosh AM, Tong S, Deakyne SJ, Davidson
JA and Scott HF: Validation of the Vasoactive-inotropic score in
pediatric sepsis. Pediatr Crit Care Med. 18:750–757.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Duess JW, Zani-Ruttenstock EM, Garriboli
M, Puri P, Pierro A and Hoellwarth ME: Outcome of right-sided
diaphragmatic hernia repair: A multicentre study. Pediatr Surg Int.
31:465–471. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Le LD, Keswani SG, Biesiada J, Lim FY,
Kingma PS, Haberman BE, Frischer J, Habli M and Crombleholme TM:
The congenital diaphragmatic hernia composite prognostic index
correlates with survival in left-sided congenital diaphragmatic
hernia. J Pediatr Surg. 47:57–62. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mullassery D, Ba'ath ME, Jesudason EC and
Losty PD: Value of liver herniation in prediction of outcome in
fetal congenital diaphragmatic hernia: A systematic review and
meta-analysis. Ultrasound Obstet Gynecol. 35:609–614.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Collot V, Malinverni S, Haltout J,
Schweitzer E, Mols P and Bartiaux M: Agreement between arterial and
capillary pH, pCO2 and lactate in patients in the
emergency department. Emerg Med Int. 2021(7820041)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Omer M, Melo AM, Kelly L, Mac Dermott EJ,
Leahy TR, Killeen O, Saugstad OD, Savani RC and Molloy EJ: Emerging
Role of the NLRP3 inflammasome and interleukin-1β in neonates.
Neonatology. 117:545–554. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Suceveanu AI, Mazilu L, Katsiki N, Parepa
I, Voinea F, Pantea-Stoian A, Rizzo M, Botea F, Herlea V, Serban D
and Suceveanu AP: NLRP3 Inflammasome Biomarker-Could Be the new
tool for improved cardiometabolic syndrome outcome. Metabolites.
10(448)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liao J, Kapadia VS, Brown LS, Cheong N,
Longoria C, Mija D, Ramgopal M, Mirpuri J, McCurnin DC and Savani
RC: The NLRP3 inflammasome is critically involved in the
development of bronchopulmonary dysplasia. Nat Commun.
6(8977)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hall NJ, Stanton MP, Kitteringham LJ,
Wheeler RA, Griffiths DM, Drewett M and Burge DM: Scope and
feasibility of operating on the neonatal intensive care unit: 312
cases in 10 years. Pediatr Surg Int. 28:1001–1005. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pelizzo G, Bagolan P, Morini F, Aceti M,
Alberti D, Andermarcher M, Avolio L, Bartoli F, Briganti V,
Cacciaguerra S, et al: Bedside surgery in the newborn infants:
Survey of the Italian society of pediatric surgery. Ital J Pediatr.
46(134)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kumar Sinha S and Neogi S: Bedside
neonatal intensive care unit surgery-myth or reality! J Neonatal
Surg. 2(20)2013.PubMed/NCBI
|
|
28
|
Wang YL, Jeng SF, Tsao PN, Chou HC, Chen
CY and Hsieh WS: Operating room within the neonatal intensive care
unit-experience of a medical Center in Taiwan. Pediatr Neonatol.
56:220–225. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
He ZR, Lin TI, Ko PJ, Tey SL, Yeh ML, Wu
HY, Wu CY, Yang YSH, Yang SN and Yang YN: The beneficial effect of
air cleanliness with ISO 14644-1 class 7 for surgical intervention
in a neonatal intensive care unit: A 10-year experience. Medicine
(Baltimore). 97(e12257)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kardon G, Ackerman KG, McCulley DJ, Shen
Y, Wynn J, Shang L, Bogenschutz E, Sun X and Chung WK: Congenital
diaphragmatic hernias: from genes to mechanisms to therapies. Dis
Model Mech. 10:955–970. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Okuyama H, Usui N, Hayakawa M and Taguchi
T: Japanese CDH study group. Appropriate timing of surgery for
neonates with congenital diaphragmatic hernia: Early or delayed
repair? Pediatr Surg Int. 33:133–138. 2017.PubMed/NCBI View Article : Google Scholar
|