Introduction
Bacterial meningitis (BM) is an impacting infectious
disease of the central nervous system. The annual incidence of BM
in China ranged from 6.95 to 22.30 cases in children under five,
and 1.84 to 2.93 cases per 100,000 population overall between 2006
and 2009(1). BM is commonly caused
by Streptococcus pneumoniae (SP) in 50 to 70% of cases
(2). Pneumococcal meningitis has a
high mortality rate, and almost half of the survivors suffer from
long-term disabling sequelae, such as bilateral hearing loss
cognitive and motor deficit (3).
Therefore, it is of considerable clinical significance to
investigate pathological mechanisms and discover therapeutic
molecular targets.
The process of craniocerebral injury mediated by SP
infection is regulated by multiple pattern recognition receptors
(TLRs, NLRs and G-protein-coupled formyl peptide receptors),
inflammatory factors and inflammatory mediators (4-6).
Cysteinyl leukotrienes (CysLTs; LTC4, LTD4
and LTE4) regulate the inflammatory responses in the
5-lipoxygenase (5-LOX) pathway through the arachidonic acid
metabolism. It is predominantly produced by microglia, astrocytes
and leukocytes. CysLTs modulate central nervous system inflammatory
diseases primarily via CysLT1R and CysLT2R;
these are two G-protein-coupled receptors that have a role in the
development of various inflammatory diseases of the central nervous
system (7-9).
CysLT1R has been revealed-in vivo and in
vitro- to contribute to the HBMEC monolayer invasion and the
blood-brain barrier penetration by bacteria that cause meningitis
such as Escherichia coli (E. coli) and group B
streptococcus (10,11). The CysLT1R antagonist
montelukast effectively inhibits Cryptococcus neoformans
(C. neoformans) penetration of the blood-brain barrier (BBB)
and has neuroprotective effects on C. neoformans
meningoencephalitis (12). A
previous study revealed that CysLTR expression is upregulated with
SP-induced meningitis (13). Taken
together, these findings indicated that CysLTR may be critical in
the pathogenesis of SP meningitis. Pyroptosis is a newly discovered
type of inflammatory programmed cell death that is mediated by
inflammasome and is dependent on the caspase-1 activation (14). The NLR family pyrin
domain-containing 3 (NLRP3) inflammasome mediates the maturation of
caspase-1 and secretion of interleukin (IL)-1β and IL-18 in the
murine meningitis model induced by SP (15). Thus, the NLRP3 inflammasome plays
an essential role in systemic inflammation regulation and brain
injury development in pneumococcal meningitis.
In the present study, the effects of the
CysLT1R antagonist pranlukast and the selective
CysLT2R antagonist HAMI 3379 on the injury and
inflammatory responses in a rat model of bacterial meningitis were
evaluated by injecting SP into the posterior cistern of rats.
Furthermore, the NLRP3 and caspase-1 expression changes in response
to antagonists were determined. The present results provided a
theoretical basis for further exploring the possible inflammatory
regulation mechanism of CysLTR in SP-caused meningitis.
Materials and methods
Chemicals and bacterial strain
Pranlukast (purity 99.89%) and HAMI 3379 (purity
98%) were purchased from Cayman Chemical Company. Cresyl violet was
purchased from Sigma-Aldrich; Merck KGaA. All other reagents used
in the experiment were of analytical grade.
The Chinese Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China) provided
the SP serotype III standard strain. The bacteria were cultured
overnight at 37˚C on sheep blood agar plates under anaerobic
conditions (H2 and CO2; 95:5, v/v). Then,
selected single colonies were cultured overnight to logarithmic
phase in broth at 37˚C and harvested by centrifugation at 2,500 x g
for 10 min at 4˚C. Bacteria suspension was adjusted to
107 colony forming units (CFU)/ml with sterile saline
for intracisternal injection.
Animal preparations
A total of 80 male Sprague-Dawley rats (3 weeks-old,
weighing 50-60 g) were provided by the Experimental Animal Center,
Zhejiang Academy of Medical Sciences. All animals were kept in
regular environmental conditions (20-24˚C, 12/12 h light/dark
cycle) prior to testing, with free access to water and food. All
experimental protocols were approved by the Laboratory Animal Care
and Ethics Committee of Hangzhou Children's Hospital (Hangzhou,
China; approval number 2021-04) and conducted in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals. All efforts were made to minimize suffering of
animals and reduce the number of animals used.
Induction of SP meningitis rat models
and drug administration
The rats were divided into the following 4 groups
(20 animals per each group): i) phosphate-buffered saline control
(PBS group), ii) SP model (SP group), iii) pranlukast (SP + Pran at
0.1 mg/kg) and iv) HAMI 3379 (SP + HAMI 3379 at 0.1 mg/kg)-treated
group. The 80 rats were anesthetized by intraperitoneal injection
of sodium pentobarbital (50 mg/kg), and their heads were fixed on
the brain stereotaxic apparatus. Cerebrospinal fluid (CSF; 10 µl)
was removed from all rats and a direct intracisternal injection of
an equal volume of solution was conducted. PBS group rats were
injected with sterile PBS and sacrificed on the fifth day after
injection. Rats in the SP group were inoculated with SP
(1x107 CFU/ml) and sacrificed at 5 days after
inoculation. Pranlukast and HAMI 3379 were injected
intraperitoneally to the rats 1 h following inoculation with SP on
the first day, then once daily until the time of euthanasia. The
same volume of saline (2 ml/kg) was injected intraperitoneally into
PBS and SP rats at the same time points. To assess clinical disease
status, the animals were monitored daily by measuring weight and
evaluating neurological deficit score. The following scores were
used to evaluate disease severity as previously described (16): 1 represented coma; 2 represented
the rat did not turn upright when positioned on its back; 3
represented the rat turned upright within 30 sec; 4 represented the
spontaneous rat activity decreased, turned upright within 5 sec;
and 5 represented normal. At the designated endpoint, rats were
anesthetized with sodium pentobarbital (50 mg/kg) and then
euthanized by cervical dislocation.
Histopathology and
immunohistochemistry
After neurological examination, rats were
anesthetized as aforementioned, perfused transcardially with
saline, then 4% paraformaldehyde in PBS and euthanized by
decapitation. Brains were post-fixed in 4% paraformaldehyde for 24
h at 4˚C, incubated in 30% sucrose for 3 days at 4˚C and embedded
in paraffin. Subsequently, 10 µm-thick slices of the coronal
tissues were sliced on a CM 1900 cryomicrotomy (Leica Microsystems
GmbH). The sections were stained with 1% cresyl violet for 20 min
at room temperature to assess the neuronal organization of the
brain as previously described (17). The stained specimens were observed
and images were captured using a fluorescence microscope (BX51;
Olympus Corporation). The neurons in hippocampus and cortex were
calculated using ImageJ 2.0 software (National Institutes of
Health).
To investigate the activation of microglial and
astrocyte, immunohistochemistry was performed for Iba-1 (1:200;
cat. no. 10904-1-AP), a biomarker of macrophages/microglia, and
GFAP (1:200; cat. no. 16825-1-AP, both from ProteinTech Group,
Inc.), a biomarker of astrocytes. The brain cryo-sections were
prepared and immunohistochemical assays were performed as
previously described (13). The
numbers of Iba-1 and GFAP positive cells were counted using a light
microscope and analyzed by ImageJ 2.0 software (National Institutes
of Health).
Reverse transcription-quantitative
(RT-q) PCR analyses
Using TRIzol® reagent (Takara Bio, Inc.),
total RNA was extracted from CSF cells following the manufacturer's
protocol. Subsequently, total RNA was reverse transcribed to cDNA
using the Prime Script RT reagent kit (Takara Bio, Inc.), according
to the manufacturer's protocol. The specific primers used for qPCR
are listed in Table I. RT-qPCR was
performed using SYBR Green fluorophore (Thermo Fisher Scientific,
Inc.) on an Applied Biosystems 7500 System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions for
qPCR were as follows: Initial denaturation at 95˚C for 10 min;
then, 40 denaturation cycles at 95˚C for 15 sec; annealing and
elongation at 60˚C for 60 sec. Cytokine mRNA relative expression
level was analyzed using the 2-ΔΔCq method (18) and normalized to the internal
reference gene GAPDH.
 | Table IPrimer sequences for quantitative
PCR. |
Table I
Primer sequences for quantitative
PCR.
| Gene name | Primer sequence
(5'→3') | Length (base
pairs) |
|---|
| IL-1β | F:
TGACCTGTTCTTTGAGGCTGAC | 272 |
| | R:
CATCATCCCACGAGTCACAGAG | |
| TNF-α | F:
CCAGGTTCTCTTCAAGGGACAA | 80 |
| | R:
GGTATGAAATGGCAAATCGGCT | |
| IL-6 | F:
AGGATACCACCCACAACAGACC | 109 |
| | R:
TTGCCATTGCACAACTCTTTTC | |
| IL-10 | F:
CACTGCTATGTTGCCTGCTCTT | 100 |
| | R:
GTCTGGCTGACTGGGAAGTGG | |
| IL-18 | F:
TCAGACCACTTTGGCAGACTTC | 134 |
| | R:
GATTCGTTGGCTGTTCGGTC | |
| IFN-γ | F:
CCAGGCCATCAGCAACAACATAA | 213 |
| | R:
CACCGACTCCTTTTCCGCTTC | |
| GAPDH | F:
CTGGAGAAACCTGCCAAGTATG | 138 |
| | R:
GGTGGAAGAATGGGAGTTGCT | |
Western blot analysis
Homogenized brain tissues were centrifuged at 12,000
x g for 30 min at 4˚C in ice-cold RIPA lysis buffer (cat. no.
G2002; Wuhan Servicebio Technology Co., Ltd.). Protein
concentration was determined using a BCA protein assay kit. Equal
protein quantities (80 µg) were separated on 10% SDS-PAGE gels and
then transferred onto polyvinylidene difluoride membranes. The
membranes were incubated with the following primary antibodies:
NLRP3 (1:1,000; cat. no. 19771-1-AP; ProteinTech Group, Inc.),
caspase-1 (1:300; cat. no. 22915-1-AP; ProteinTech Group, Inc.) and
β-actin (1:2,000; cat. no. GB12001; Beyotime Institute of
Biotechnology) overnight at 4˚C after blocking for 1 h at room
temperature in 5% non-fat dry milk. Following the primary
incubation, membranes were incubated with HRP-conjugated secondary
antibody (13,000; cat. no. GB23302; Beyotime Institute of
Biotechnology) for 1 h at room temperature. Odyssey infrared
imaging system was utilized to detect the immunoblot (LI-COR
Biosciences). Quantity One v4.6.2 analysis software was utilized to
quantify the protein bands (Bio-Rad Laboratories, Inc.).
Statistical analysis
All values are presented as the mean ± SEM. One-way
analysis of variance (ANOVA) followed by Newman-Keuls post hoc
analysis was performed using the Prism version 5 (GraphPad
Software, Inc.) software to determine statistical significance. In
all results, P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of pranlukast and HAMI 3379 on
the body weight and neurological deficit
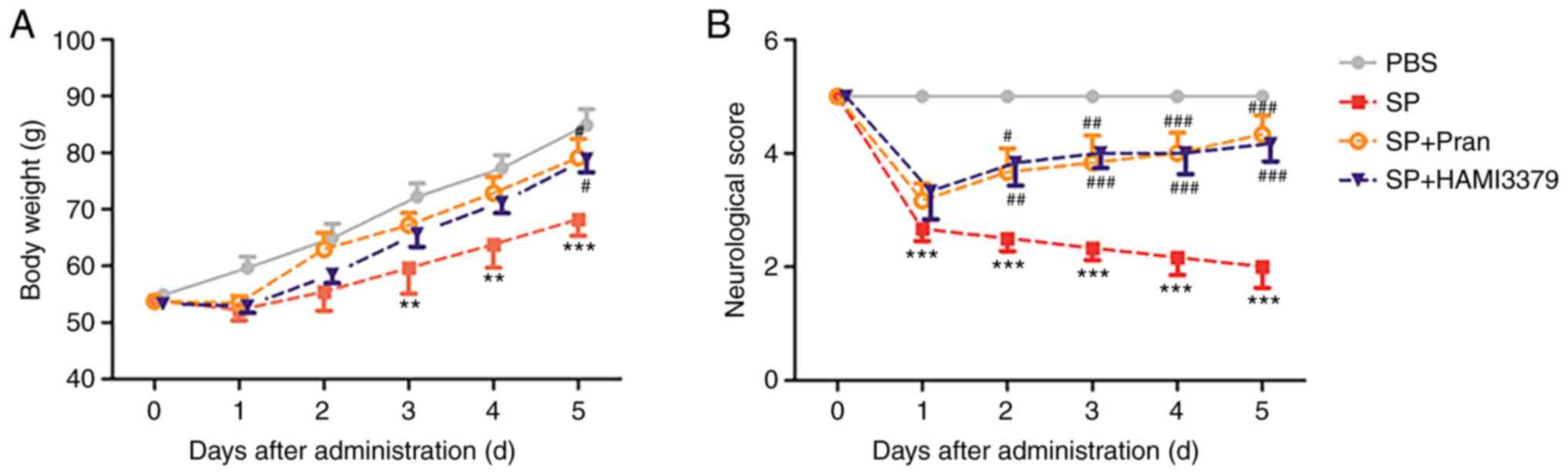
As revealed in Fig.
1A, the body weight of rats in the PBS group grew steadily
while the rate of weight increase in the SP group significantly
decreased from day 3 to day 5 after infection compared with the PBS
group (P<0.01). The body weight loss was significantly improved
after treatment with pranlukast or HAMI 3379 (0.1 mg/kg) on the
fifth day compared with the SP group (P<0.05).
It was demonstrated that the neurological deficit
scores were significantly decreased at 1 day after infection
(Fig. 1B; P<0.001). The
neurological deficit score of the pranlukast-treated group was
significantly increased compared with the SP group from the second
day (day 2, P<0.05; day 3, P<0.01; days 4 and 5, P<0.001).
A significant improvement was also observed in the HAMI
3379-treated group compared with that in the SP group from the
second day (day 2, P<0.01; days 3 to 5, P<0.001). These
results indicated that pranlukast and HAMI 3379 had similar
protective effects against SP-induced brain infection.
Effects of pranlukast and HAMI 3379 on
neuronal damage
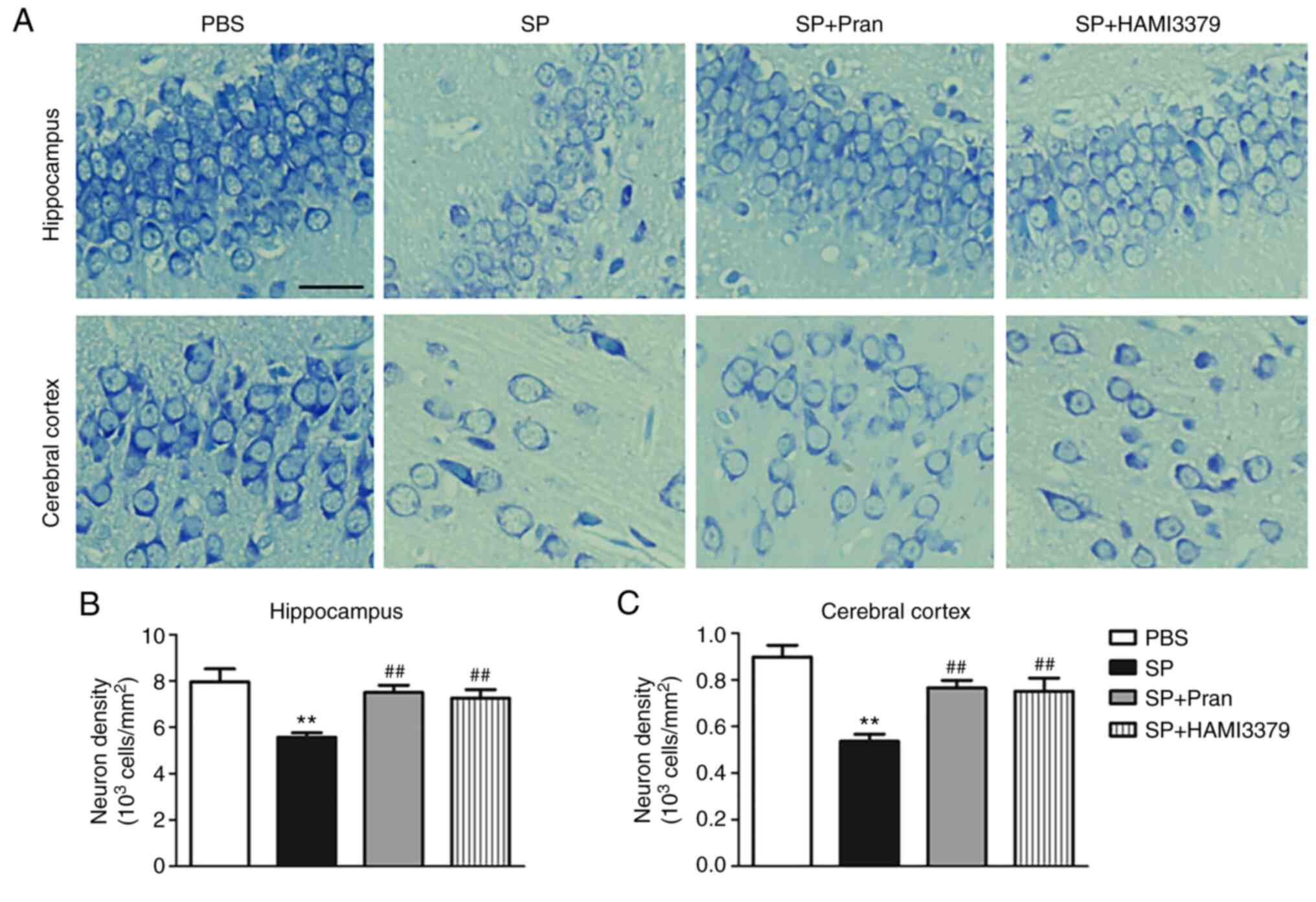
To assess the effect of pranlukast and HAMI 3379 on
neuronal injury, the changes of neuronal damage in the hippocampus
and cortex after treatment were observed (Fig. 2). Nissl staining revealed
significant neuronal damage in the hippocampus and cortex of the SP
group. Representative images showed that the neurons were
disorderly arranged, cell spacing was widened and the Nissl bodies
were shrunken or completely disappeared (Fig. 2A). Pranlukast and HAMI 3379
treatment significantly ameliorated the neuronal loss in the
hippocampus and cortex at 5 days following administration of SP
compared with the SP group (Fig.
2B; both P<0.01).
Effects of pranlukast and HAMI 3379 on
glial cells
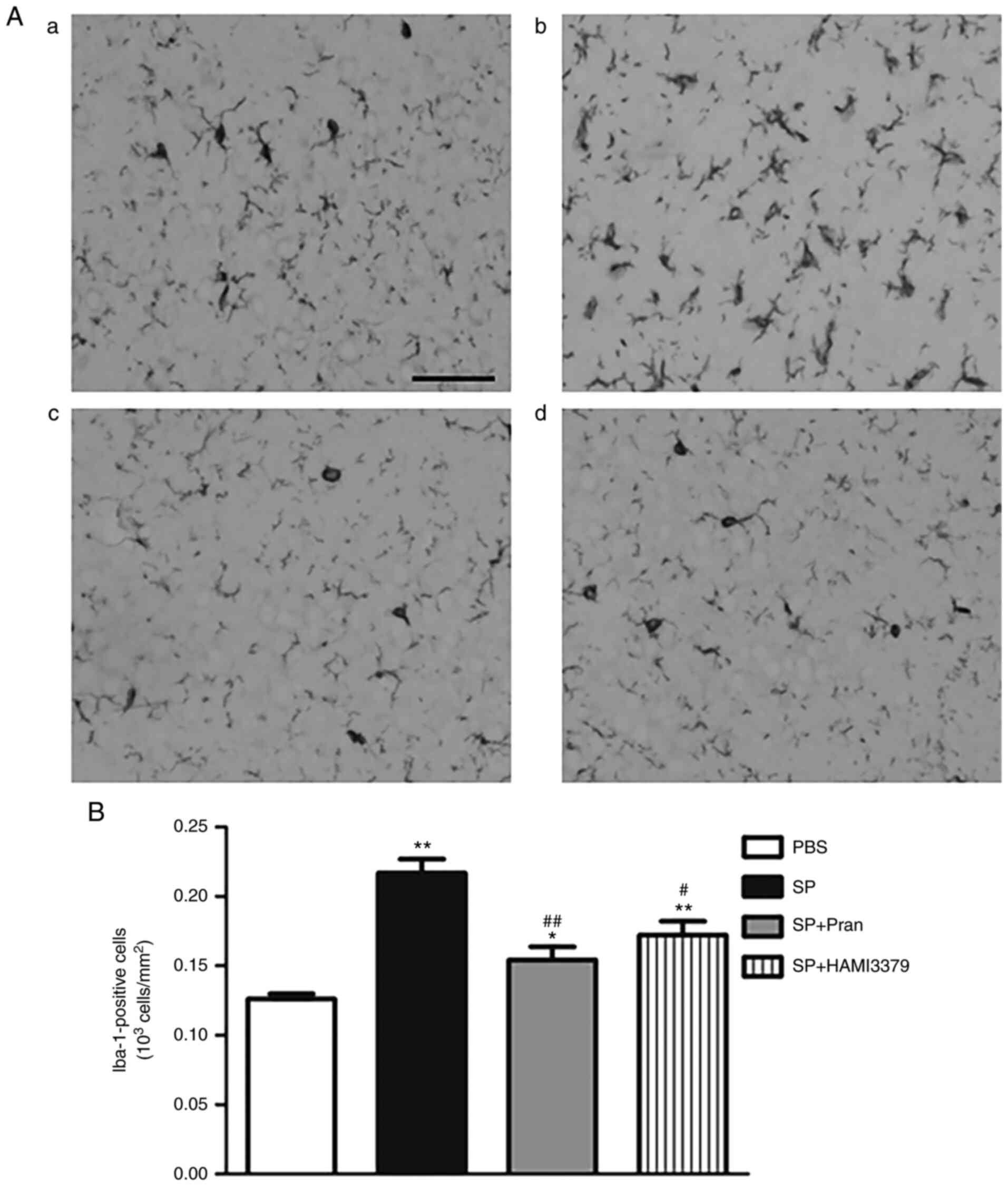
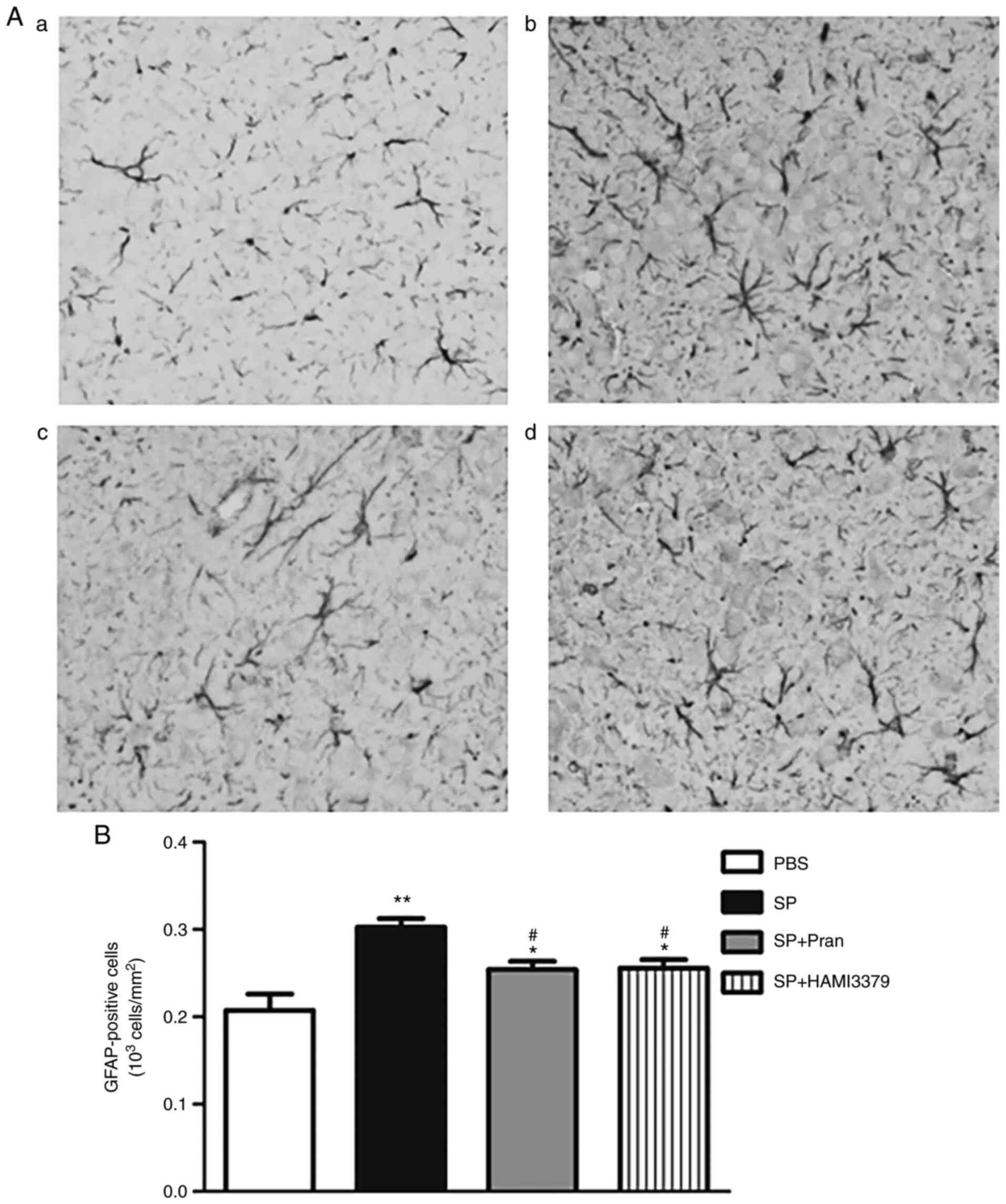
To determine whether pranlukast and HAMI 3379
affected SP-induced neuroinflammation, an analysis of microglia
activation and astrogliosis response in the cerebral cortex was
performed by staining with Iba-1 (Fig.
3) and GFAP (Fig. 4). As
revealed in images captured using a light microscope, there were
remarkable increases in the number of ramified Iba-1-positive
microglial cells in the rats of the SP group in comparison with the
PBS group (Fig. 3B; P<0.01). By
contrast, pranlukast and HAMI 3379 administration caused a notable
decrease in the number of Iba-1-positive cells (P<0.05) compared
with the SP group and indicated a reduction of microglial
activation (Fig. 3A). Meanwhile,
GFAP-positive astrocytes in the SP group were activated with
hyperplasia/hypertrophy (Fig. 4A);
pranlukast and HAMI 3379 significantly inhibited the GFAP-positive
astrocyte number increase in the cortex compared with the SP group
(Fig. 4B; P<0.05).
Effects of pranlukast and HAMI 3379 on
the expression of inflammatory cytokines
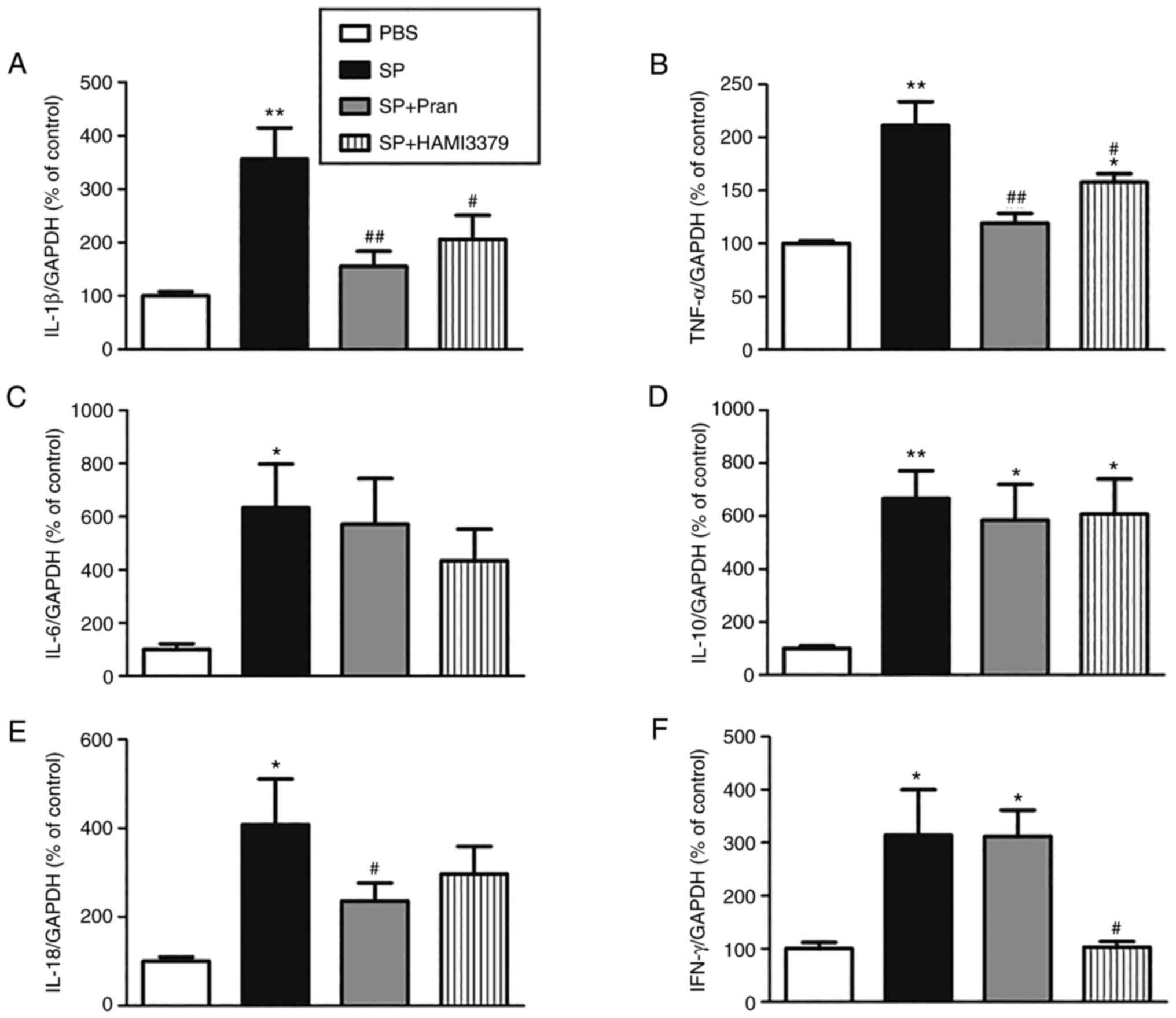
As revealed in Fig.
5, the mRNA expression of inflammatory cytokines (IL-1β, TNF-α,
IL-6, IL-10, IL-18 and IFN-γ) in CSF cells greatly increased after
SP meningitis infection (P<0.05). Compared with the SP group,
pranlukast significantly reduced the upregulation of mRNA
expression of IL-1β and TNF-α, while HAMI 3379 markedly decreased
mRNA expression of IL-1β, TNF-α and IFN-γ (P<0.05). The
expression of IL-6, IL-10 was not affected by neither agent.
Effects of pranlukast and HAMI 3379 on
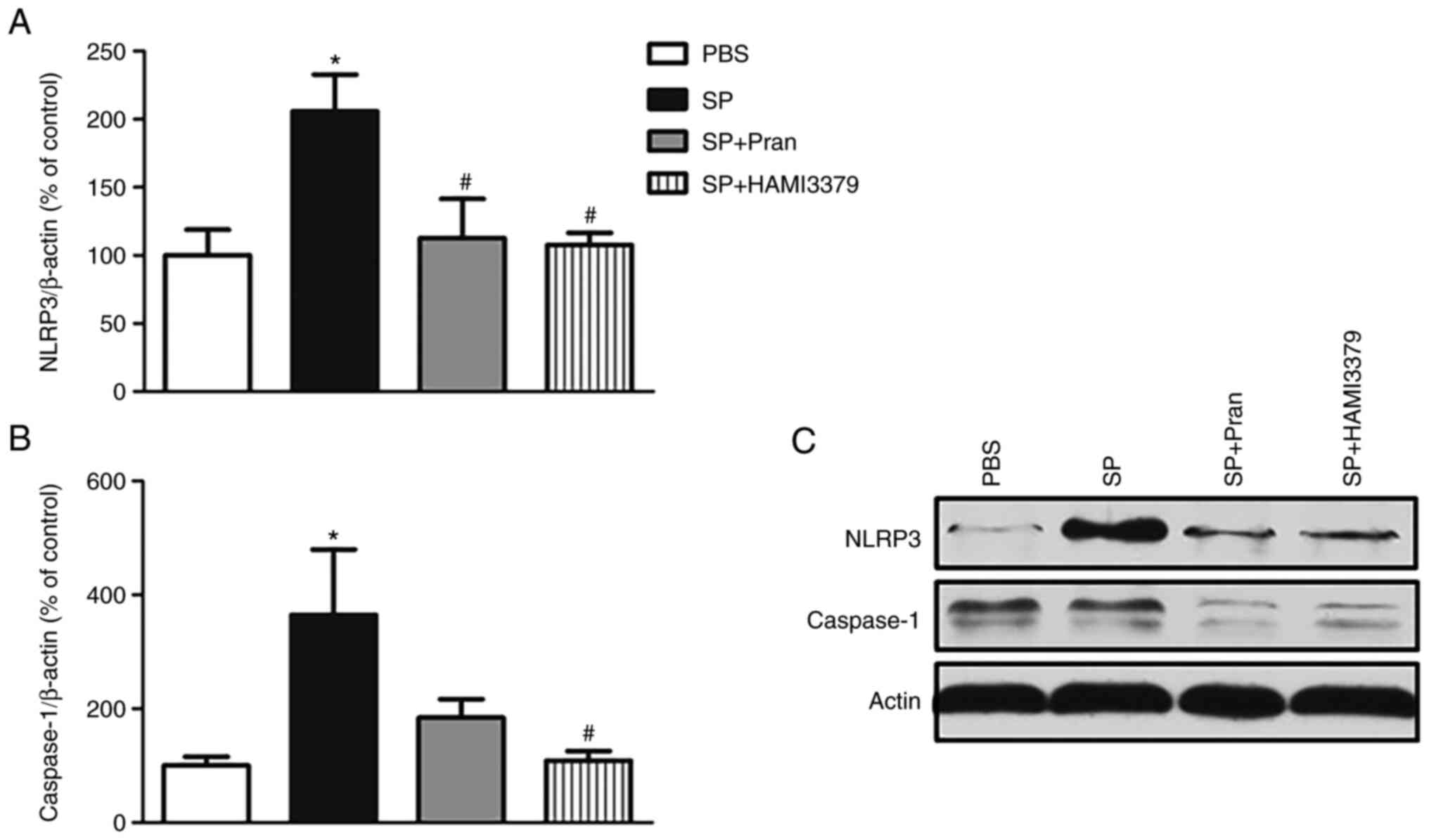
the expression of the NLRP3 inflammasome
Western blot analysis revealed that the protein
expression of NLRP3 and caspase-1 was significantly increased in
the SP group compared with the PBS group (Fig. 6; P<0.05). Pranlukast diminished
the expression of NLRP3. In addition, a significant decrease in the
NLRP3 expression and caspase-1 was observed in HAMI 3379 treatment
group compared with the SP group (P<0.05). These results
suggested that the NLRP3 inflammasome activation in rats was
suppressed by CysLT antagonists.
Discussion
SP meningitis is a common central nervous system
infectious disease in the pediatrics (19). The effects of CysLTR antagonists
were assessed in a rat model of pneumococcal meningitis in the
present study. The results showed that intraperitoneal injection of
pranlukast and HAMI 3379 have neuroprotective effects on brain
injury following SP meningitis in rats, which was manifested by
improvement of neurological deficit function and body weight loss.
Furthermore, these antagonists attenuated neuronal loss and
inhibited microglia activation and astrocyte proliferation.
Numerous studies on human cases and pneumococcal
meningitis model demonstrated that the development of cortical
brain injury was strongly associated with diminished cerebral blood
flow and cerebral blood volume (20-22).
Cerebral ischemia secondary to intracranial infection is closely
correlated with neurological sequelae or death in children
(23). Previous studies showed
that intraperitoneally-injected pranlukast and HAMI 3379 reduced
the brain damage following acute and chronic cerebral ischemia with
an effective dose of 0.1 mg/kg (24,25),
thus 0.1 mg/kg was selected as the effective dosage in the present
experiment. Based on a preliminary study, the pranlukast and HAMI
3379 time-dependent effect was examined. It was identified that the
therapeutic time window was within 1 h after infection (data not
shown). Continuous administration for 5 days after infection is to
ensure the role of pranlukast and HAMI 3379 in the peak
inflammatory phase of the brain. Using this regimen, the
neurological deficit score was used to quantitatively reflect the
degree of nerve damage caused by meningitis. It was observed that
pranlukast and HAMI 3379 could significantly ameliorate
neurological deficits. Additionally, the histopathological
characteristic of experimental pneumococcal meningitis is extensive
neuronal injury, which predominantly manifested as acute neuronal
necrosis in the cortex and apoptosis in the dentate gyrus of the
hippocampus (26,27). It has been identified that the
CysLT1R antagonist montelukast exhibited less neuronal
injury of animals with E. coli meningitis (28). The present results are consistent
with the aforementioned study, as more survival neurons may
directly contribute to the improved neurological function recovery.
In primary cultures of neurons (microglia and mixed cortical
cells), a previous study revealed that CysLTs and their receptors
are not directly involved in ischemic injury of neurons in rats;
HAMI3379 indirectly protects neurons by inhibiting microglia
activation (29). These in
vitro findings indicated that SP-induced neuronal injury is a
comprehensive effect with multicellular interaction and protective
effect of CysLTR antagonists on neurons may be mediated by
microglia or astrocytes.
In the present study, it was also found that
pranlukast and HAMI 3379 attenuate microgliosis and astrogliosis in
response to an inflammatory insult. As it has been previously
reported, gliosis is a hallmark feature of brain injury (30). Glial cells such as microglia and
astrocytes represent the first line of defense against SP and they
participate in the initiation and/or progression of inflammation
(31). Persistent or excessive
activation of microglia and astrocytes may be partially
contributing to cognitive decline in meningitis survivors (32). In the first stage of gliosis,
activated microglia transform from ramified to an amoeboid
morphology, then play pivotal roles in initiation and modulation of
astrogliosis; inhibition of microglia activation can also reduce
the number of ascrocytes (33,34).
Therefore, it can be hypothesized that microglia are an important
regulator of activation of astrocytes. Astrocytes themselves also
secrete certain factors to achieve either self-regulation or
feedback regulation of microglia. Meanwhile, activated microglia
and astrocytes can release inflammatory cytokines such as TNF-α and
IL-1β and mediators such as 5-LOX and CysLTs. In addition to
increasing the susceptibility of neurons, these inflammatory
mediators may cause the impairment of the BBB, which may eventually
lead to focal ischemia and necrosis of brain tissue (30). Previous studies demonstrated that
CysLT1R and CysLT2R mediate neuronal damage
in the acute phase, but microgliosis and astrogliosis in the
chronic/subacute phase following focal cerebral ischemia (35-37).
Both pranlukast and HAMI 3379 inhibited BBB disruption, indicating
that CysLTR is involved in regulating the permeability of BBB.
Compared with pranlukast, the inhibitory effect of HAMI 3379 on
astrogliosis is more dependent on the regulation of microglia
(25). Collectively, it is
hypothesized that CysLT1R is a master regulator of
astrogliosis proliferation and glial scar formation, while
CysLT2R plays a major role in earlier stage of
microglial activation during SP-induced neuroinflammation.
Nevertheless, there are certain limitations to the present study.
It needs to be further clarified how both antagonists act on the
cells and the crosstalk between different cell types in a brain
inflammation model caused by gram-positive pneumococci in
vitro.
In relation to the inflammatory cytokines, the
present results showed that both pranlukast and HAMI 3379
significantly inhibit the increased expression of typical
pro-inflammatory cytokines TNF-α and IL-1β during murine
pneumococcal meningitis, indicating that they exert neuroprotective
effects partly through their general anti-inflammatory properties.
However, they had different effects on the expression of IFN-γ;
HAMI 3379 significantly decreased the expression of IFN-γ. IFN-γ is
produced by natural killer cells and type 1 T helper (TH1) cells,
also involved in the pathology of pneumococcal meningitis by
inhibiting bacterial clearance, as well as modulating myeloid
recruitment and activation (38).
The findings of the present study are in accordance with the
results of others, confirming that pranlukast did not affect the
release of IFN-γ in the asthmatic mice lung (39). Fujii et al (40) found that IFN-γ led to the increased
CysLT2R expression on eosinophils in patients with
asthma. Moreover, inhibiting the expression of CysLT2R
was shown to attenuate the apoptosis of human umbilical vein
endothelial cells by altering the IFN-γ secretion. The mechanisms
underlying the effects of CysLTR antagonists on cytokine expression
after SP meningitis require further investigation.
The NLRP3 inflammasome is a multi-protein complex
composed of NLRP3, ASC adaptor (apoptosis-associated speck-like
protein) and caspase-1. It plays an important role in the immune
defense pathology of SP meningitis by promoting the occurrence of
pyroptosis (15). Meanwhile, the
arachidonic acid anabolic pathway is also closely related to
inflammatory immune response. Therefore, it was wondered if there
was an interrelationship between these two seemingly independent
immune responses. In the present study, the results revealed that
both pranlukast and HAMI 3379 significantly reduced NLRP3
expression; HAMI 3379 could additionally reduce the upregulated
expression of caspase-1. It has been found that blockade of enzymes
involved in arachidonic acid metabolism could inhibit macrophages
M1 polarization and activation of NLRP3 inflammasome in monosodium
urate crystal (MSU)-induced inflammation (41). Upon stimulation with MSU,
LTB4, a metabolite of 5-LOX, was associated with the
assembly of the NLRP3 inflammasome and caspase 1-dependent IL-1β
production in vitro and in vivo (42). Zhang et al further verified
that the selective NLRP3 inhibitor MCC950 can significantly reduce
the level of LTC4 in serum of allergic rhinitis mice
(43). The aforementioned findings
imply a complex interaction between CysLTs and pyroptosis mediated
by the NLRP3 inflammasome. Consequently, it was hypothesized that
CysLTR antagonists may ameliorate detrimental inflammatory
responses in murine pneumococcal meningitis by inhibiting
pyroptosis.
In conclusion, the results of the present study
first revealed that intraperitoneal injection of CysLTR antagonists
could improve neurological deficit, interfering neuronal injury,
microgliosis and astrogliosis, decrease the expression of
inflammatory cytokines and inhibit over-activation of NLRP3
inflammasome, resulting in the amelioration of immune-inflammatory
reaction in a rat model of SP meningitis. CysLTR antagonists may be
a novel therapeutic strategy for SP meningitis. However, the
neuroprotective effects of CysLTR antagonists were only proved in
rats, thus the precise mechanisms underlying the effects of
antagonists in SP meningitis require further elucidation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Commission of Hangzhou (grant nos. 20170533B55 and
20191203B123) and by the Medical Science and Technology Planning
Project in Zhejiang Province (grant no. 2017KY557).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SYY, XJC and JY designed the research. SYY, XJC, XYL
and YYJ performed the experiments and data collection. SYY and JY
analyzed the data. SYY and XJC confirmed the authenticity of all
the raw data and wrote the manuscript. All authors read and
approved the final manuscript, and ensure the integrity of the
work..
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Care and Use Committee of Hangzhou Children's Hospital (Hangzhou,
China) and conducted in accordance with the National Institutes of
Health Guidelines for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Yin Z, Shao Z, Li M, Liang X, Sandhu
HS, Hadler SC, Li J, Sun Y, Li J, et al: Population-based
surveillance for bacterial meningitis in China, September
2006-December 2009. Emerg Infect Dis. 20:61–69. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brouwer MC and van de Beek D: Epidemiology
of community-acquired bacterial meningitis. Curr Opin Infect Dis.
31:78–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Edmond K, Scott S, Korczak V, Ward C,
Sanderson C, Theodoratou E, Clark A, Griffiths U, Rudan I and
Campbell H: Long term sequelae from childhood pneumonia; systematic
review and meta-analysis. PLoS One. 7(e31239)2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Oldekamp S, Pscheidl S, Kress E, Soehnlein
O, Jansen S, Pufe T, Wang JM, Tauber SC and Brandenburg LO: Lack of
formyl peptide receptor 1 and 2 leads to more severe inflammation
and higher mortality in mice with of pneumococcal meningitis.
Immunology. 143:447–461. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Geldhoff M, Mook-Kanamori BB, Brouwer MC,
Troost D, Leemans JC, Flavell RA, Van der Ende A, Van der Poll T
and Van de Beek D: Inflammasome activation mediates inflammation
and outcome in humans and mice with pneumococcal meningitis. BMC
Infect Dis. 13(358)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang G, Fu Y, Ma K, Liu J and Liu X: NOD2
regulates microglial inflammation through the TAK1-NF-κB pathway
and autophagy activation in murine pneumococcal meningitis. Brain
Res Bull. 158:20–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bäck M, Dahlén SE, Drazen JM, Evans JF,
Serhan CN, Shimizu T, Yokomizo T and Rovati GE: International union
of basic and clinical pharmacology. LXXXIV: Leukotriene receptor
nomenclature, distribution, and pathophysiological functions.
Pharmacol Rev. 63:539–584. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Singh R, Gupta S, Dastidar S and Ray A:
Cysteinyl leukotrienes and their receptors: Molecular and
functional characteristics. Pharmacology. 85:336–349.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takahashi Y, Imai K, Ikeda H, Kubota Y,
Yamazaki E and Susa F: Open study of pranlukast add-on therapy in
intractable partial epilepsy. Brain Dev. 35:236–244.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhu L, Maruvada R, Sapirstein A, Malik KU,
Peters-Golden M and Kim KS: Arachidonic acid metabolism regulates
Escherichia coli penetration of the blood-brain barrier. Infect
Immun. 78:4302–4310. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maruvada R, Zhu L, Pearce D, Zheng Y,
Perfect J, Kwon-Chung KJ and Kim KS: Cryptococcus neoformans
phospholipase B1 activates host cell Rac1 for traversal across the
blood-brain barrier. Cell Microbiol. 14:1544–1553. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu L, Maruvada R, Sapirstein A,
Peters-Golden M and Kim K: Cysteinyl leukotrienes as novel host
factors facilitating Cryptococcus neoformans penetration into the
brain. Cell Microbiol 19: 10.1111/cmi.12661, 2017.
|
|
13
|
Yu S, Yan J, Chen X, Zhu X, Li X and Liao
L: Expression of cysteinyl leukotriene receptor in brain tissues of
rats with Streptococcus pneumoniae meningitis. Int J Clin Exp
Pathol. 12:4242–4252. 2019.PubMed/NCBI
|
|
14
|
Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J,
Li M, Zhao T, Yang H, Xu R, et al: Melatonin prevents endothelial
cell pyroptosis via regulation of long noncoding RNA
MEG3/miR-223/NLRP3 axis. J Pineal Res. 64:2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim J, Paton J, Briles D, Rhee D and Pyo
S: Streptococcus pneumoniae induces pyroptosis through the
regulation of autophagy in murine microglia. Oncotarget.
6:44161–44178. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Loeffler JM, Ringer R, Hablutzel M, Tauber
MG and Leib SL: The free radical scavenger alpha-phenyl-tert-butyl
nitrone aggravates hippocampal apoptosis and learning deficits in
experimental pneumococcal meningitis. J Infect Dis. 183:247–252.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Türeyen K, Vemuganti R, Sailor KA and
Dempsey RJ: Infarct volume quantification in mouse focal cerebral
ischemia: A comparison of triphenyltetrazolium chloride and cresyl
violet staining techniques. J Neurosci Methods. 139:203–207.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mostowy R, Croucher NJ, Hanage WP, Harris
SR, Bentley S and Fraser C: Heterogeneity in the frequency and
characteristics of homologous recombination in pneumococcal
evolution. PLoS Genet. 10(e1004300)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pfister LA, Tureen JH, Shaw S, Christen S,
Ferriero DM, Täuber MG and Leib SL: Endothelin inhibition improves
cerebral blood flow and is neuroprotective in pneumococcal
meningitis. Ann Neurol. 47:329–335. 2000.PubMed/NCBI
|
|
21
|
Förderreuther S, Tatsch K, Einhäupl KM and
Pfister HW: Abnormalities of cerebral blood flow in the acute phase
of bacterial meningitis in adults. J Neurol. 239:431–436.
1992.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tureen JH, Täuber MG and Sande MA: Effect
of hydration status on cerebral blood flow and cerebrospinal fluid
lactic acidosis in rabbits with experimental meningitis. J Clin
Invest. 89:947–953. 1992.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pschibul A, Janzarik WG, Franck P,
Hufnagel M, Beck C and Korinthenberg R: Cystic encephalomalacia
following vasculopathy and vasospasm of proximal intracranial
arteries due to pneumococcal meningitis in a infant.
Neuropediatrics. 49:213–216. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu GL, Wei EQ, Wang ML, Zhang WP, Zhang
SH, Weng JQ, Chu LS, Fang SH, Zhou Y, Chen Z, et al: Pranlukast, a
cysteinyl leukotriene receptor-1 antagonist, protects against
chronic ischemic brain injury and inhibits the glial scar formation
in mice. Brain Res. 1053:116–125. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shi QJ, Wang H, Liu ZX, Fang SH, Song XM,
Lu YB, Zhang WP, Sa XY, Ying HZ and Wei EQ: HAMI 3379, a CysLT2R
antagonist, dose- and time-dependently attenuates brain injury and
inhibits microglial inflammation after focal cerebral ischemia in
rats. Neuroscience. 291:53–69. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Leib SL, Kim YS, Chow LL, Sheldon RA and
Täuber MG: Reactive oxygen intermediates contribute to necrotic and
apoptotic neuronal injury in an infant rat model of bacterial
meningitis due to group B streptococci. J Clin Invest.
98:2632–2639. 1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wellmer A, Noeske C, Gerber J, Munzel U
and Nau R: Spatial memory and learning deficits after experimental
pneumococcal meningitis in mice. Neurosci Lett. 296:137–140.
2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu N, Liu W, Prakash A, Zhang C and Kim
KS: Targeting E. coli invasion of the blood-brain barrier for
investigating the pathogenesis and therapeutic development of E.
coli meningitis. Cell Microbiol. 22(e13231)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang XY, Wang XR, Xu DM, Yu SY, Shi QJ,
Zhang LH, Chen L, Fang SH, Lu YB, Zhang WP and Wei EQ: HAMI 3379, a
CysLT2 receptor antagonist, attenuates ischemia-like neuronal
injury by inhibiting microglial activation. J Pharmacol Exp Ther.
346:328–341. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ramesh G, MacLean A and Philipp M:
Cytokines and chemokines at the crossroads of neuroinflammation,
neurodegeneration, and neuropathic pain. Mediators Inflamm.
2013(480739)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu X, Chauhan VS, Young AB and Marriott
I: NOD2 mediates inflammatory responses of primary murine glia to
Streptococcus pneumoniae. Glia. 58:839–847. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Giridharan VV, Collodel A, Generoso JS,
Scaini G, Wassather R, Selvaraj S, Hasbun R, Dal-Pizzol F,
Petronilho F and Barichello T: Neuroinflammation trajectories
precede cognitive impairment after experimental meningitis-evidence
from an in vivo PET study. J Neuroinflammation.
17(5)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang D, Hu X, Qian L, O'Callaghan JP and
Hong JS: Astrogliosis in CNS pathologies: Is there a role for
microglia? Mol Neurobiol. 41:232–241. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rohl C, Lucius R and Sievers J: The effect
of activated microglia on astrogliosis parameters in astrocyte
cultures. Brain Res. 1129:43–52. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fang SH, Wei EQ, Zhou Y, Wang ML, Zhang
WP, Yu GL, Chu LS and Chen Z: Increased expression of cysteinyl
leukotriene receptor-1 in the brain mediates neuronal damage and
astrogliosis after focal cerebral ischemia in rats. Neuroscience.
140:969–979. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fang SH, Zhou Y, Chu LS, Zhang WP, Wang
ML, Yu GL, Peng F and Wei EQ: Spatio-temporal expression of
cysteinyl leukotriene receptor-2 mRNA in rat brain after focal
cerebral ischemia. Neurosci Lett. 412:78–83. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhao CZ, Zhao B, Zhang XY, Huang XQ, Shi
WZ, Liu HL, Fang SH, Lu YB, Zhang WP, Tang FD and Wei EQ: Cysteinyl
leukotriene receptor 2 is spatiotemporally involved in neuron
injury, astrocytosis and microgliosis after focal cerebral ischemia
in rats. Neuroscience. 189:1–11. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mitchell AJ, Yau B, McQuillan JA, Ball HJ,
Too LK, Abtin A, Hertzog P, Leib SL, Jones CA, Gerega SK, et al:
Inflammasome-dependent IFN-γ drives pathogenesis in Streptococcus
pneumoniae meningitis. J Immunol. 189:4970–4980. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matsuse H, Kondo Y, Machida I, Kawano T,
Saeki S, Tomari S, Obase Y, Fukushima C, Mizuta Y and Kohno S:
Effects of anti-inflammatory therapies on recurrent and low-grade
respiratory syncytial virus infections in a murine model of asthma.
Ann Allergy Asthma Immunol. 97:55–60. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fujii M, Tanaka H and Abe S:
Interferon-gamma up-regulates expression of cysteinyl leukotriene
type 2 receptors on eosinophils in asthmatic patients. Chest.
128:3148–3155. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu Y, Duan C, Chen H, Wang C, Liu X, Qiu
M, Tang H, Zhang F, Zhou X and Yang J: Inhibition of COX-2/mPGES-1
and 5-LOX in macrophages by leonurine ameliorates monosodium urate
crystal-induced inflammation. Toxicol Appl Pharmacol. 351:1–11.
2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Amaral FA, Costa VV, Tavares LD, Sachs D,
Coelho FM, Fagundes CT, Soriani FM, Silveira TN, Cunha LD, Zamboni
DS, et al: NLRP3 inflammasome-mediated neutrophil recruitment and
hypernociception depend on leukotriene B(4) in a murine model of
gout. Arthritis Rheum. 64:474–484. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang W, Ba G, Tang R, Li M and Lin H:
Ameliorative effect of selective NLRP3 inflammasome inhibitor
MCC950 in an ovalbumin-induced allergic rhinitis murine model. Int
Immunopharmacol. 83(106394)2020.PubMed/NCBI View Article : Google Scholar
|