Introduction
Hematopoietic stem cell transplantation (HSCT) is an
important treatment method for a number of refractory malignant
hematopoietic disorders, such as myelodysplastic syndrome, T-cell
lymphomas, multiple myeloma, and chronic myelomonocytic leukemia
(1-4).
However, successful applications of HSCT are frequently limited by
relapse and graft-versus-host disease (GvHD) (5,6). The
pathogenesis of acute GvHD (aGVHD) is associated with the
activation of donor T cells (5).
Therefore, effective inhibition of activated T cells (effector T
cells) in the donor sample is key for the prophylaxis and treatment
of aGVHD (5).
Activated T cells require a constant source of
energy production to meet the demands caused by rapid proliferation
(7). Aerobic glycolysis has been
previously recognized to be the main metabolic pathway activated in
effector T cells during GVHD (8-11).
However, fatty acid oxidative phosphorylation has also been
reported to be active in activated T cells (8-11).
Similar to activated T cells, cancer cells also depend on
glycolysis for ATP generation, such that glycolysis inhibition has
demonstrated efficacy in the inhibition of leukemia, lymphoma and
testicular tumor cells (12-14).
In a previous study by Nguyen et al (8), it was shown that
allo-antigen-activated T cells required glycolysis for optimal
function in a murine bone marrow transplant model. Therefore,
inhibition of glycolysis can be a feasible intervention option for
aGVHD prevention.
The compound of 3-bromopyruvate (3-BrPA) is an
effective glycolysis inhibitor that can target both
hypoxia-inducible factor (HIF)-1α and GAPDH pathways (15-17).
It has also been reported to exert anti-cancer effects by
inhibiting aerobic glycolysis in cancer cells, such as breast and
colorectal cancer cells (17). In
addition, it has been demonstrated to reverse resistance to
anti-cancer drugs by suppressing the activity of the ATP-dependent
multi-drug resistance transporter (17). However, inhibition of glycolysis
and the subsequently reduced glucose metabolic activity/ATP
concentration will activate the mTOR pathway, producing
compensatory survival signals (12).
Rapamycin (RAPA) was the first mTOR inhibitor to be
generated and has been applied for the treatment of various types
of malignancies, including breast, leukemia, prostate, lung and
skin (18). It has also been used
as an immunosuppressor for GVHD prevention (7). A previous study has revealed that
RAPA treatment can induce the accumulation of regulatory T cells
(Treg) in the skin of mice after bone marrow transplantation
(19). In a recent study, Scheurer
et al (20) found that RAPA
treatment can increase the immunosuppressive potential of
myeloid-derived suppressor cells (MDSCs) whilst maintaining the
anti-tumor cytotoxicity of T cells [Graft vs. tumor (GvT)] without
impairing the induction of Treg in a bone marrow transplantation
mouse model (20). However, other
in vitro studies and clinical findings demonstrated that the
development of RAPA resistance typically occurs (21). In addition, toxicity is another
concern of this drug (8).
Therefore, combination of low doses of RAPA with glycolysis
inhibitors may serve to be an attractive treatment option, since it
may maintain the efficacy of these drugs for the suppression of
GVHD whilst preventing the resistance and toxicity effects of
RAPA.
The effects of combined treatment with RAPA and
3-BrPA for aGVHD remain poorly understood. Therefore, in present
study, the efficacy of combined 3-BrPA and RAPA treatment on the
pathogenesis of aGVHD, on glycolysis and mTOR signaling in
activated T cells was investigated.
Materials and methods
Animals
A total of 94 male C57BL/6 (H-2b) mice (age, 8
weeks; weight, 18.7±0.7 g) and 21 female BALB/c (H-2d) mice (age, 8
weeks; weight, 19.0±0.5 g) were obtained from Southern Medical
University (Guangzhou, China). All mouse studies were approved by
the Ethics Committee of Southern Medical University, performed in
The Experimental Animal Center of Southern Medical University and
fulfilled the regulations of The Institutional Animal Care and Use
Committee (IACUC). In total, four mice were housed in a cage with
free access to food pellets and drinking water. The mice were
maintained in conditions of 21±2˚C, relative humidity of 40-60% and
a 12-h light-dark cycle.
Isolation of splenocytes
In total, three female BALB/c (H-2d) mice and three
male C57BL/6 (H-2b) mice were used for the isolation of splenocytes
by following a previously reported protocol (22). Briefly, the mice were sacrificed by
cervical dislocation before the spleen was collected. Subsequently,
the spleen was cut into small pieces by using an ophthalmic
scissors and then ground with the plunger handle of a syringe on a
plastic plate in a sterile biosafety cabinet at room temperature to
release the splenocytes. The cells were collected in a centrifuge
tube after washing with PBS and then lysed with red blood cell
lysis buffer (Beyotime Institute of Biotechnology). The lysis was
performed followed the manufacturer's instruction. Briefly, the
cells were mixed with the lysis working solution at room
temperature for 2 min with gentle shaking. Afterwards, the cells
were collected by centrifuge at 400 g for 5 min at 4˚C. This step
was repeated until cracking was completed. The isolated splenocytes
were cultured with RPMI-1640 medium (Thermo Fisher Scientific,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS,
Beyotime Institute of Biotechnology) in an incubator under 37˚C and
5% CO2.
RAPA- and 3-BrPA-supplemented one-way
mixed lymphocyte reaction (MLR)
Splenocytes isolated from the male C57BL/6 (H-2b)
mice as designated to be responders, whereas splenocytes isolated
from the female BALB/c (H-2d) mice were inactivated by mitomycin-C
(MilliporeSigma) at room temperature for 2 h (10 µg/ml) before
being designated to be stimulators. A total of 5x105
responder cells (0.1 ml) and an equivalent density of stimulator
cells (0.1 ml) were co-cultured in the 96-well cell culture plate
supplemented with RPMI 1640 medium (Thermo Fisher Scientific,
Waltham, MA, USA), supplemented with 10% FBS (Beyotime Institute of
Biotechnology), 1% Penicillin-streptomycin (MilliporeSigma) and 1%
1% glutamine (MilliporeSigma). RAPA, 3-BrPA or the combination of
both RAPA and 3-BrPA, at concentrations ranging from 0-100 µM, were
added into the mixed lymphocyte culture (MLC). RAPA and 3-BrPA were
obtained from MilliporeSigma. The RAPA and 3-BrPA stock solutions
were prepared by dissolving in DMSO to produce 10 mmol/l and stored
at -20˚C before use. For cell culture, quantities of RAPA and
3-BrPA stock solutions were added into the medium at room
temperature to achieve the required final concentrations. An
equivalent amount of DMSO was added as the control. These cells
were maintained in an incubator under standard cell culture
conditions of 37˚C, 90% humidity and 5% CO2. The cells
were analyzed after 24 and 48 h of culture.
Glucose consumption, cellular
viability and synergistic effect evaluations
Glucose consumption of cells in the MLC was
evaluated using the Glucose (HK) Assay kit (GAHK20, MilliporeSigma)
according to the manufacturer's protocol to test the efficiency of
glycolysis inhibition. Cells without any drug treatment was used as
control. This glucose consumption assay is used to measure the
conversion of glucose into 6-phosphogluconate and reduced NADH to
reflect the activity of glycolysis.
The viability of the cells in the MLC was measured
24 and 48 h after culture by using the Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc.) according to the
manufacturer's protocol. Briefly, 20 µl CCK-8 was added into each
well and incubated under 37˚C, 5% CO2 and 100% humidity
in a cell incubator for 2 h. The absorbance of the cell culture
medium was measured at 450 nm wavelength in a microplate reader
(Wallac 1420 Victor2, Perkin Elmer).
The degree of synergism between 3-BrPA and RAPA was
calculated using the Chou-Talalay method (23). For any inhibition ratios exerted by
the combination treatment of 3-BrPA and RAPA according to the cell
viability data, if the combination index (CI) was calculated to be
<1, this would be considered that this particular combination of
combined treatment in question exhibits a synergistic effect.
Cell apoptosis evaluation
The MLC were harvested after culture for 48 h and
washed once by PBS before the staining. Cells were centrifuge at
400 x g for 5 min, and subsequently resuspended in Annexin V
binding buffer (Cell Apoptosis Analysis Kit, KGA108, Nanjing KeyGen
Biotech Co., Ltd). For the staining, 5 µl FITC-Annexin V and 10 µl
propidium iodide solutions were added into 100 µl cell suspension
(1x105 cells in binding buffer) and the cells were
stained for 15 min at room temperature according to the
manufacturer's protocol. After the staining, the cells were
analyzed by flow cytometry (CytoFLEX, Beckman Coulter, Brea, CA,
USA). The flow cytometric data were analyzed by using the FlowJo
V10 software (BD Life Sciences-FlowJo).
ELISA
The supernatant of the MLC was collected after
treatment with 3-BrPA and/or RAPA for 48 h. The concentrations of
IL-4 and IFN-γ in the supernatant were then measured by using the
mouse IL-4 ELISA kit (Cat: P1612) and mouse IFN-γ ELISA kit (Cat:
1508) according to the manufacturer's protocols (Beyotime Institute
of Biotechnology). The IL-4 and IFN-γ levels in the serum of mice
with aGVHD were also measured by ELISA 7 days after
transplantation, using the same products and protocol.
Establishment of the aGVHD model and
drug administration
The aGVHD model was established by the injection of
bone marrow cells and spleen cells from the donor male C57BL/6
(H-2b) mice, which were sacrificed by cervical dislocation, to the
receiver female BALB/c (H-2d) mice (24,25).
In total, 91 female receiver BALB/c (H-2d) mice and 16 male donor
C57BL/6 (H-2b) mice were used for the aGVHD study. The
C57BL/6(H-2b) mice was used for extraction of the splenocytes and
bone marrow cells for transplantation into the BALB/c (H-2d) mice,
and cells isolated from 16 C57BL/6 (H-2b) mouse were used for
transplantation into 78 BALB/c (H-2d) mice (1 to 5). The study
groups were designated as follows (n=13): i) TBI group, where the
receiver mice only underwent TBI; ii) aGVHD group, which is
identical with the TBI group except for being transplanted with
both bone marrow and spleen cells after TBI; iii) RAPA-2.5 mg
group, which is identical to the aGVHD group but was treated with
RAPA (2.5 mg/kg/day) for 7 days; iv) RAPA-5 mg group, which is the
aGVHD group treated with RAPA (5 mg/kg/day) for 7 days; v) 3-BrPA
group, which is the aGVHD group treated with 3-BrPA (10 mg/kg/day)
for 7 days; vi) combination group, which is the aGVHD group treated
with both RAPA (2.5 mg/kg/day) and 3-BrPA (5 mg/kg/day) for 7 days;
and vii) bone marrow transplantation (BMT) group, which was the TBI
group that was only transplanted with bone marrow cells
(6x105).
The isolation of splenocytes was performed as
aforementioned. For isolation of bone marrow cells, the femurs of
the mice were first separated from the hind legs, and the bone
marrow plug was subsequently flushed out by using PBS after the
epiphyseal head were removed. The cells were filtrated using 70 µl
nylon mesh after pipetting in the 15 ml plastic centrifuge tube.
The cells were lysed with red blood cell lysis buffer (Beyotime
Institute of Biotechnology) using protocol as mentioned above. The
isolated bone marrow cells and splenocytes were suspended in
RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS, Beyotime Institute of Biotechnology)
in an incubator under 37˚C and 5% CO2 before
transplantation. These cells were transplanted within 3 h after
preparation.
The total body irradiation (TBI) was performed to
kill the hemopoietic stem cells of the recipient mice. Each
recipient mice received total 8 Gy (0.5 Gy/min, room temperature)
TBI X-ray irradiation (Varian 2100C/D, Varian Medical Systems, Palo
Alto, CA, USA). For the transplantation, 6x105 bone
marrow cells and/or 6x105 splenocytes from donor mice
were transplanted into each receiver mouse by tail vein injection
≤4 h after 7.5 Gy total body irradiation (TBI) performed for 15 min
at room temperature. Various drugs were then intraperitoneal
injection to each mouse 1 h after the injection of cells. For
animal experiments, the RAPA and 3-BrPA stock solutions were
diluted using 1X PBS for intraperitoneal injection into the mice
(20). The aGVHD and BMT groups
were injected with an equivalent amount of DMSO but without
drugs.
Chimeric rate examination
To determine the chimeric rate in the transplanted
mice, the mice were first anesthetized by injection with
pentobarbital sodium (50 mg/kg) 11 days (4 days without further
treatment) after transplantation, before ~2 mm of the tail was cut
and 50 µl blood was collected from the tail vein of each mouse
using a micropipette. The blood was diluted by addition of 150 µl
EDTA solution. MHC Class I (H-2Kb) Monoclonal Antibody
(AF6-88.5.5.3), FITC (eBioscience, Thermo Fisher Scientific,
Waltham, MA, USA) were added into the diluted blood and incubated
for 20 min at room temperature. Then, the cells were assessed by a
flow cytometer (BD FACSCANTO II, BD Biosciences). The flow
cytometer detected the FITC-positive cells, and the ratio of these
positive cells in each tested aliquot was analyzed by using FlowJo
V10 software (BD Life Sciences-FlowJo, Ashland, OR, USA) and
defined as the allogeneic chimeric rate. The aGVHD, RAPA-2.5 mg,
RAPA-5 mg, 3-BrPA, RAPA and 3-BrPA combination and BMT groups were
analyzed by using cells from three mice/group.
Survival analysis
The cumulative survival curves of the mice in each
group were calculated using the Kaplan-Meier method. For the
calculation of median survival time and comparison of the survival
results among the groups, log-rank test was performed followed by
Bonferroni's correction was applied. P<0.00238 was considered to
indicate a statistically significant difference for the comparison
of the seven groups in this case.
GvHD scoring
The GvHD scores of the mice were calculated by
measurement of five parameters (weight loss, posture, activity, fur
texture and skin integrity) as previously described by Cooke et
al and the grade details were mentioned in Table I (26). In this evaluation system, the
severity of GvHD was quantified by the change percentage of each
parameter and graded from 0 (normal or reduced less than 10%), 1
(reduced 10-30%; mild reduced), and 2 (reduced >30% or serious
change observed) for each criterion. The final GvHD score was
obtained by summation of scores of the five criteria. None of the
mice received any further treatment after 7 days. Scores were
recorded on day 7, 14 and 21. No mouse survived in the aGVHD group
14 days after transplantation.
 | Table IGraft vs. host disease scores in the
transplanted mice. |
Table I
Graft vs. host disease scores in the
transplanted mice.
| Parameter | 0 | 1 | 2 |
|---|
| Weight loss | Reduced
<10% | Reduced 10-25% | Reduced
>25% |
| Posture | Normal | Hunching only at
rest | Serious
hunching |
| Activity | Normal | Mild to moderately
reduced | No activity unless
stimulated |
| Fur texture | Normal | Mild to Moderate
ruffling | Serious
ruffling |
| Skin integrity | Normal | Scaling of
paws/tail | Significantly
denuded skin |
Histological analysis
Tissue response to aGVHD in the main organs of the
mice, specifically the lung and liver, were assessed by H&E
histological staining after euthanasia by cervical dislocation. The
organs were harvested 12 days after transplantation (3 mice/group)
and then fixed in 4% paraformaldehyde under room temperature for 24
h before paraffin embedding. The sections of 4 µm thickness were
obtained by using a rotary microtome (Leica RM2235, Leica
Biosystems). The hematoxylin and Eosin (H&E) staining was
conducted under room temperature. Briefly, the sections were
firstly deparaffinized by in two changes of xylene (10 min each
immersion), subsequently the sections were rehydrated by immersion
in two changes of 100% alcohol with 5 min for each, and then in 95%
alcohol for 5 min, in 70% alcohol for 5 min. After that, the slides
were washed in distilled water and stained in hematoxylin for 1
min, followed by washing in tap water for 30 sec. Following, the
slides were immersed in 1% acid alcohol superfast differentiation
solution for 15 sec (Beyotime Institute of Biotechnology, Shanghai,
China), followed by washing in tap water for 15 sec. Afterwards,
the slides were counterstained in eosin solution for 15 sec. The
slides were then dehydrated by immersion in a series of one 95%
alcohol for 5 min, two changes of 100% alcohol with 5 min for each
and two changes of xylene with 15 sec for each. Then, the slides
were mounted with mounting medium. Images of the slides were taken
at 20X magnification in a Leica DM6B upright fluorescence
microscope (Leica Biosystems, Wetzlar, Germany). The staining
images were then scored by three independent pathologists who were
blinded before scoring, with normal as 1, slight tissue damage as
2, mild tissue damage as 3 and severe tissue damage as 4.
Statistical analysis
Results of cellular viability, cellular apoptosis,
IL-4, IFN-γ and the glucose consumption are presented as mean ±
standard deviation. Median scores were presented in GvHD scores and
histological scores. GraphPad Prism (Version 8.0.2, GraphPad
Software, Inc.) was used for data processing. In cases where there
were two variables (RAPA and 3-BrPA), two-way ANOVA followed by
Sidak's post hoc test was performed. For the analysis of the
Kaplan-Meier's survival results, log-rank test was performed
followed by the Bonferroni's correction. In this case, P<0.00238
was considered to indicate a statistically significant difference
for the comparison of the seven groups. The statistical analysis of
the GvHD and histological scores results was conducted by using
Kruskal-Wallis test followed by the Dunn's post hoc test. For the
analysis of the in vivo IL-4 and IFN-γ results, one-way
ANOVA followed by Tukey's test was performed. All experiments were
repeated twice except aGVHD mouse study was performed once) and ≥
three samples were tested for each test at each timepoint. Apart
from the Kaplan-Meier analysis, P<0.05 was considered to
indicate a statistically significant difference.
Results
Cellular viability and synergistic
effect evaluation
It was found that the 3-BrPA treatment can
effectively inhibit the glucose consumption in a dose-dependent
manner (Fig. S1). For the
3-BrPA-alone treatment groups, increasing the 3-BrPA concentration
from 10 to 100 µM progressively decreased glucose consumption
(Fig. S1). Significant glucose
consumption inhibition effect were observed for RAPA-alone
treatment compared to control (0 µM 3-BrPA). In the combination
treatment groups, the addition of RAPA alongside 3-BrPA induced
significant reductions in glucose consumption at each tested
concentration of 3-BrPA (Fig.
S1). Furthermore, increasing 3-BrPA concentration from 0 to 100
µM induced significant reductions in glucose consumption in the
combination groups in a dose-dependent manner (Fig. S1).
In terms of cell viability (Fig. 1), both 3-BrPA and combined
treatment exerted significant effects on the inhibition of cell
viability at both 24 and 48 h, where there were cumulative effect
between 3-BrPA and RAPA on cell viability at both time points based
on the two-way ANOVA analysis. After 24 h (Fig. 1A), there was no statistical
significance in cell viability between the 0 and 10 µM 3-BrPA
treatment-alone groups. However, treatment with 20, 50 and 100 µM
3-BrPA alone induced decreases in cell viability compared with that
in the 0 or 10 µM 3-BrPA-alone groups (Fig. 1A). Additionally, the cellular
viability decreased as 3-BrPA concentration increased from 20 to
100 µM (Fig. 1A). RAPA
co-treatment exerted significant reduction in cell viability at
each 3-BrPA concentration compared with that in their corresponding
3-BrPA-alone counterparts (Fig.
1A). Among the combined treatment groups, the 0 µM 3-BrPA
combination treatment group showed no significant differences
compared with that in the 10 µM 3-BrPA combination treatment group.
However, cell viability was significantly decreased in the
combination treatment groups of 20, 50 and 100 µM 3-BrPA compared
with that in the 0 µM group (Fig.
1A). In addition, the cellular viability decreased remarkably
when the 3-BrPA concentration increased from 20 to 100 µM in the
combination treatment groups (Fig.
1A).
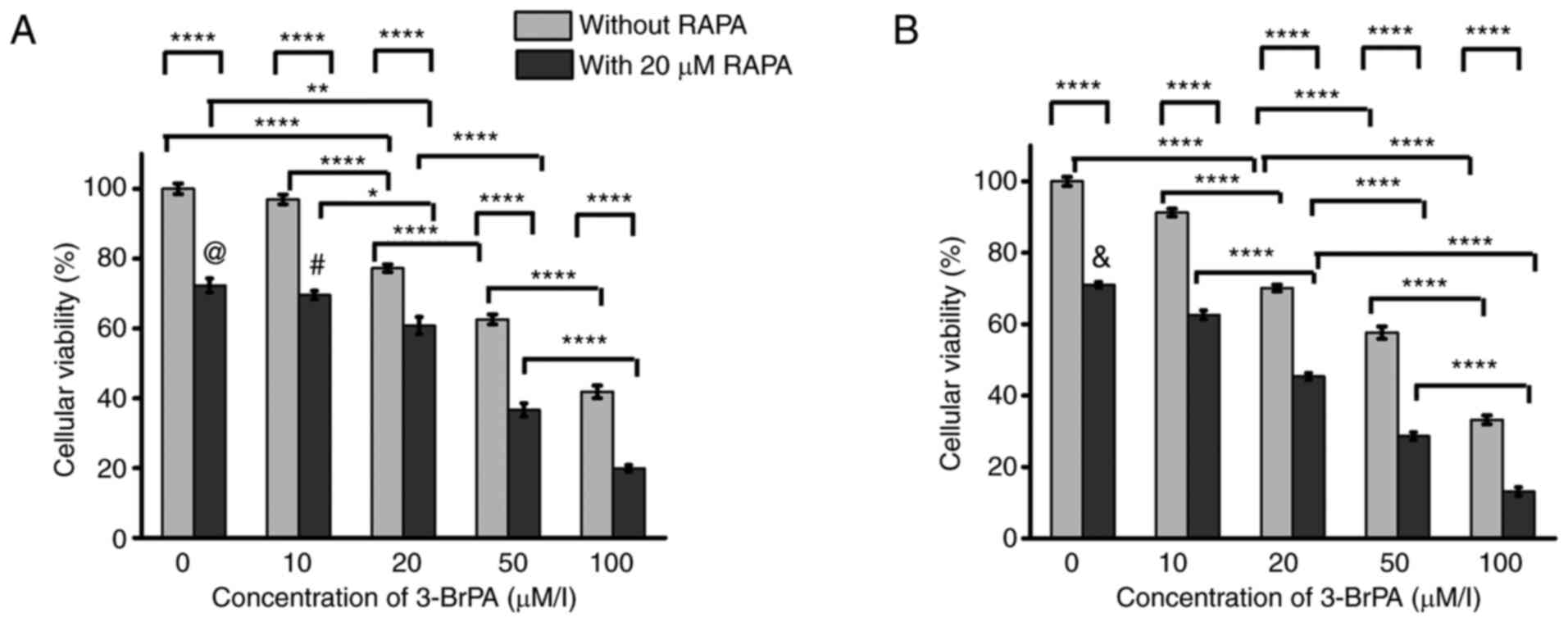 | Figure 1Cellular viability analysis of the
MLC. Cellular viability analysis of the MLC after treatment of
3-BrPA and RAPA for (A) 24 and (B) 48 h. At each time point,
cellular viability was determined using the Cell Counting Kit-8
method and was presented as percentage of the optical value in each
group relative to that in the control group (no drug treatment, set
as 100%). *P<0.05, **P<0.005,
****P<0.0001. In (A), @P<0.0001,
#P<0.0001 compared to combination group with 50 µM
3-BrPA. In (B), &P<0.0001 compared to 10 µM
3-BrPA group. MLC, mixed lymphocyte culture; RAPA, rapamycin;
3-BrPA, 3-bromopyruvate. |
After 48 h of treatment (Fig. 1B), the cellular viability reduced
as increasing the 3-BrPA concentration from 0 to 100 µM (Fig. 1B). Combined treatment with 3-BrPA
and RAPA mediated significant reductions in cell viability compared
with that in their corresponding 3-BrPA-alone groups at each 3-BrPA
concentration tested (Fig. 1B).
Among the combination treatment groups, the cellular viability also
decreased when increasing the 3-BrPA concentration from 0 to 100 µM
(Fig. 1B).
Subsequently, the CI of the combined treatment of
3-BrPA and RAPA were calculated. After the MLC has been treated
with both 20 µM 3-BrPA and 20 µM RAPA for 24 h, the ~39% inhibition
ratio corresponded to a CI value of 0.71. After 48 h of treatment,
the 20 µM 3-BrPA and 20 µM RAPA combined treatment induced ~55%
inhibition ratio, which corresponded to a CI value of 0.44.
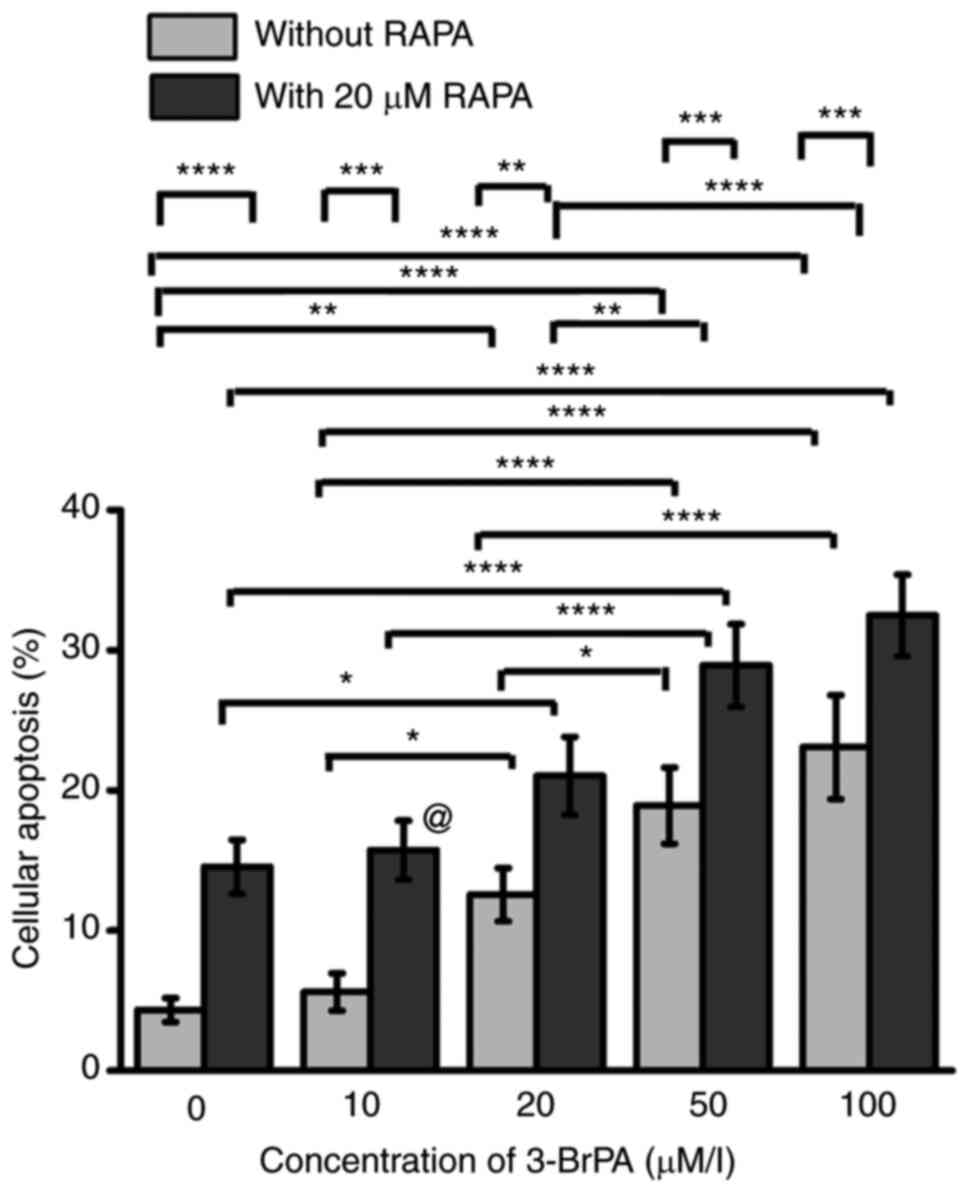
Cell apoptosis in the MLC
As shown in Figs. 2
and S2, both 3-BrPA treatment
alone and when combined with RAPA mediated significant inhibition
effects on apoptosis. After treatment with 3-BrPA alone,
significant increases in apoptosis were found after comparing the 0
µM 3-BrPA-alone group with the 20, 50 or 100 µM 3-BrPA treatment
group (Figs. 2 and S2). Additionally, the cellular apoptosis
improved as increasing the 3-BrPA concentration from 10 to 100 µM
(Figs. 2 and S2). After the cells were treated with
the combination of 3-BrPA and RAPA, apoptosis was significantly
increased compared with that in their corresponding 3-BrPA-alone
groups at each concentration tested (Figs. 2 and S2). Among the combination treatment
groups, there were significant enhancement in cellular apoptosis by
comparing the 0 µM to the 20, 50 µM or 100 µM 3-BrPA group
(Figs. 2 and S2). Moreover, there were remarkable
increase in cellular apoptosis as improving the 3-BrPA
concentration from 20 to 100 µM for the combination groups
(Figs. 2 and S2).
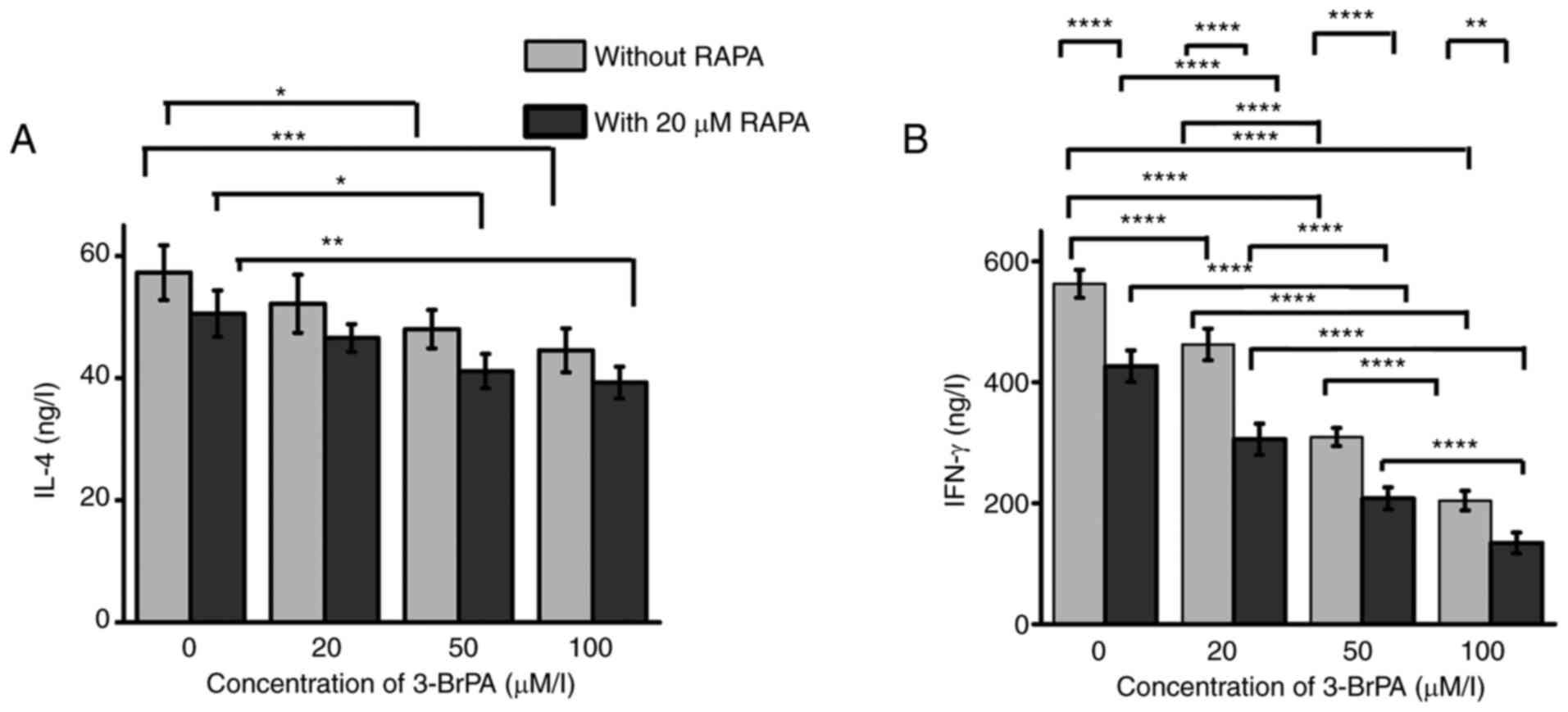
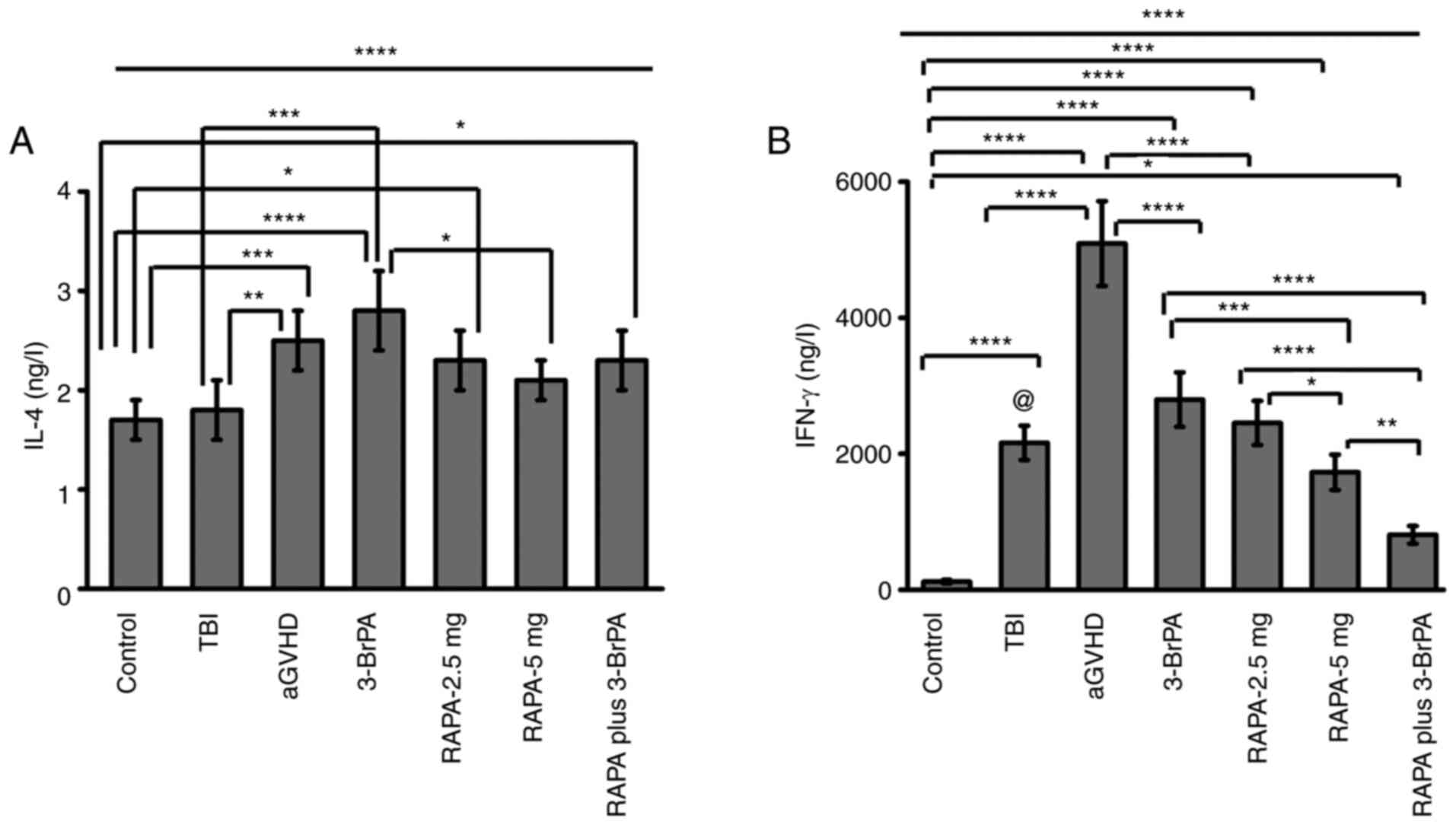
IL-4 and IFN-γ levels in the MLC
In terms of IL-4 production, both 3-BrPA treatment
alone and combination treatment exerted significant inhibition
effects (Fig. 3A). Among the
3-BrPA-alone treatment groups, the 0 µM group showed significantly
lower IL-4 production compared to the 50 or 100 µM groups (Fig. 3A). RAPA co-treatment could not
mediate a significant difference in IL-4 production compared with
that in their corresponding 3-BrPA-alone counterparts at each
3-BrPA concentration tested (Fig.
3A). Among the combined treatment groups, there were
significant reduction in IL-4 production comparing the 0 µM 3-BrPA
group to the 50 or 100 µM 3-BrPA groups (Fig. 3A).
Regarding IFN-γ production (Fig. 3B), both 3-BrPA alone and
combination treatment with RAPA showed significant inhibition
effects. There was a cumulative effect between the RAPA and 3-BrPA
treatments on the inhibition of IFN-γ production. Among the
3-BrPA-alone treatment groups, the IFN-γ production decreased when
increasing the 3-BrPA concentration (Fig. 3B). Combined treatment alongside
RAPA induced significant reductions in IFN-γ production compared
with that in their corresponding 3-BrPA-alone counterparts at each
concentration tested (Fig. 3B).
Among the combination treatment groups, the IFN-γ production
decreased significantly as the concentration of 3-BrPA increased
(Fig. 3B).
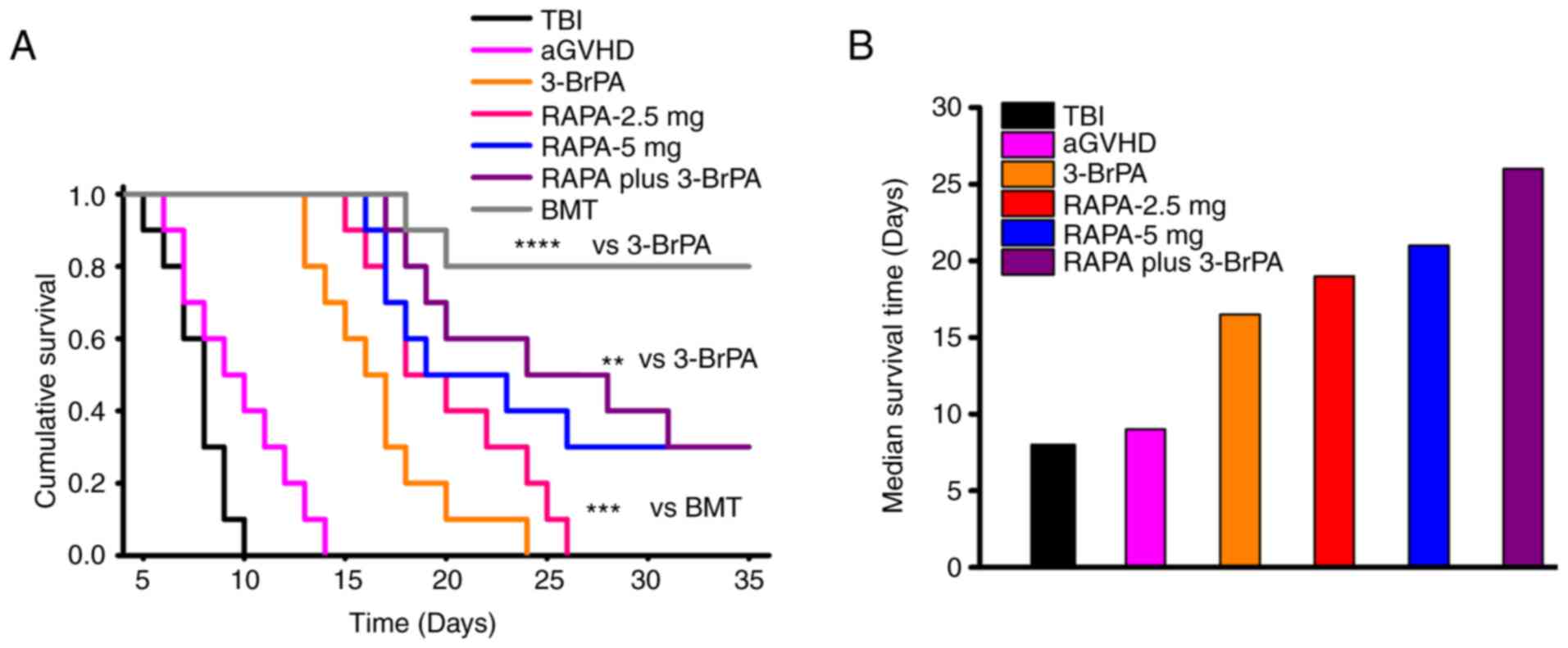
Survival time and GVHD score
analyses
Kaplan-Meier survival analysis revealed that all the
3-BrPA- and RAPA-containing treatment groups displayed longer
survival time compared with either TBI or aGVHD (Fig. 4A). Additionally, the RAPA plus
3-BrPA and BMT groups demonstrated superior survival compared to
the 3-BrPA group, and the BMT group also outperformed the RAPA-2.5
mg group in terms of survival (Fig.
4A). The combination treatment group showed the highest median
survival time among all the treatment groups (Fig. 4B). The RAPA-5 mg group displayed
slightly higher median survival time compared with that in the
RAPA-2.5 mg group. By contrast, the median survival time in the
3-BrPA group was inferior compared with that in the RAPA-2.5 mg
group (Fig. 4B).
The chimeric ratios in the aGVHD, 3-BrPA, 2.5 and 5
mg RAPA groups, the combination treatment group, and BMT group were
found to be 93.1±2.6, 95.1±3.4, 93.7±3.8, 94.2±3.1, 95.6±3.5% and
95.3±2.8%, respectively (Fig.
S3). There were no significant differences among these groups,
suggesting high efficacy of bone marrow transplantation in
hemopoietic system regeneration.
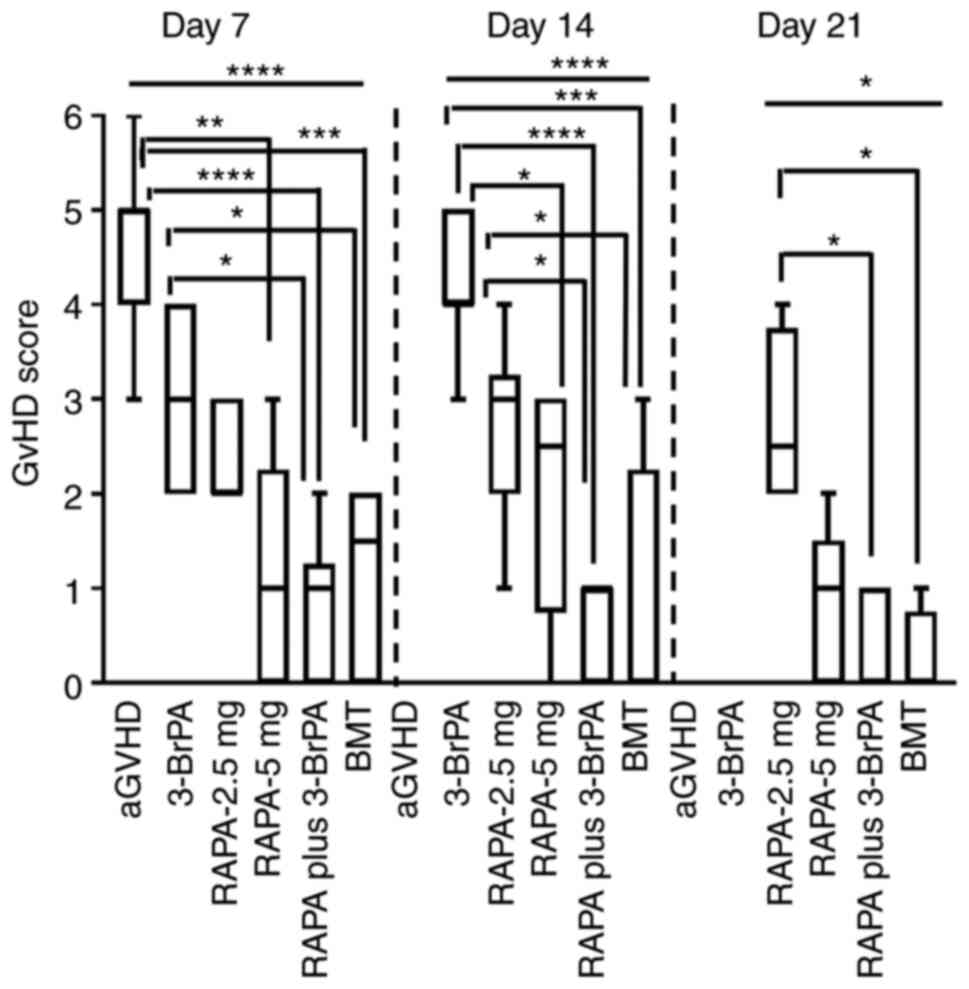
Regarding the GVHD scores, it was found that there
were significant differences among the groups on days 7, 14 and 21
(Fig. 5). On day 7, there were
significant decrease of GVHD score in RAPA-5 mg, RAPA plus 3-BrPA
and BMT compared to aGVHD group (Fig.
5). The RAPA plus 3-BrPA and BMT groups also displayed reduced
GVHD scores than the 3-BrPA group (Fig. 5). On day 14, 3-BrPA showed
significant higher GVHD score than the ones of RAPA-5 mg, RAPA plus
3-BrPA and BMT (Fig. 5). RAPA-2.5
mg also demonstrated superior GVHD score to the RAPA plus 3-BrPA
and BMT (Fig. 5). On day 21,
RAPA-2.5 mg showed remarkably higher GVHD score than RAPA plus
3-BrPA and BMT (Fig. 5).
Histological analysis of lung and
liver tissues from mice with aGVHD mice
In the lung tissues (Fig. 6A), those from the 5-mg RAPA
treatment group showed reduced levels of small blood clots compared
with those in the 3-BrPA and 2.5-mg RAPA treatment groups. The
tissues from the combined treatment group only showed capillary
dilatation without obvious blood clots (Fig. 6A). In the liver tissues (Fig. 6A), fatty deposits could be observed
after 3-BrPA treatment. The 5-mg RAPA treatment group and the
combination treatment group presented features that are also
comparable to those in the control, without clear signs of
inflammation or tissue necrosis.
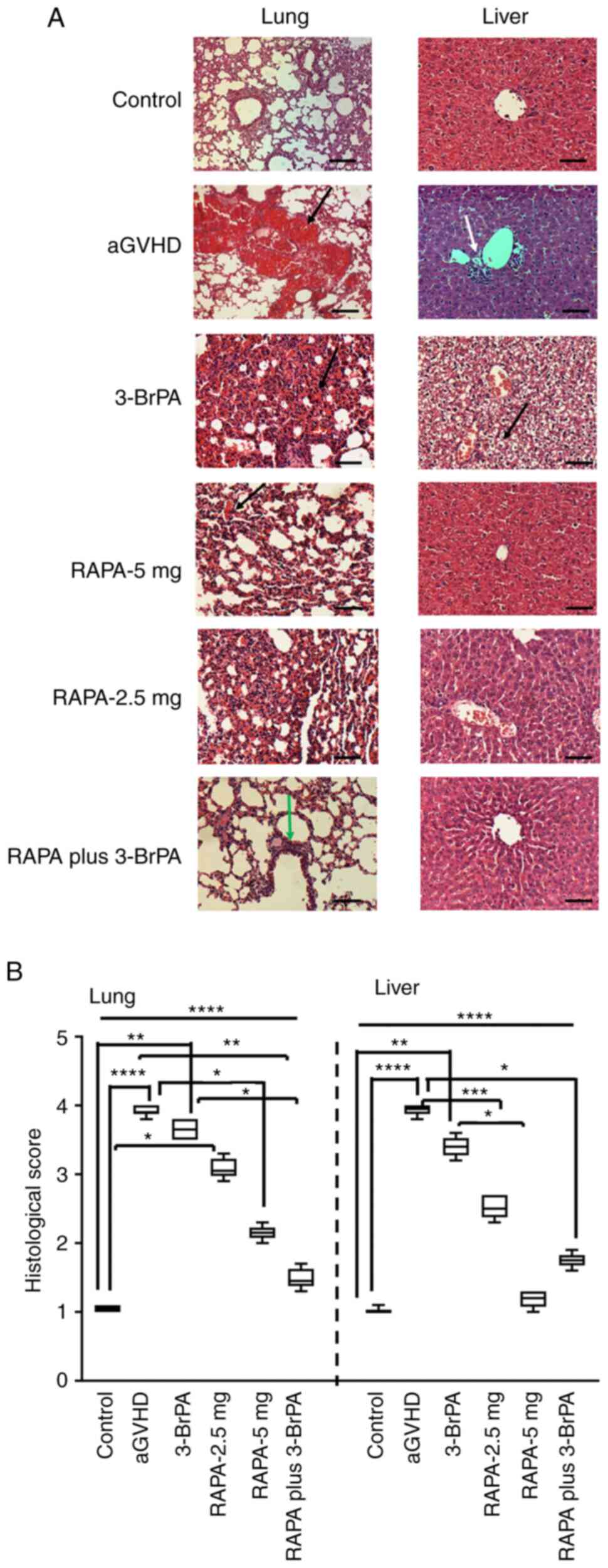 | Figure 6H&E staining and quantitative
evaluation of the lung and liver from the mice after
transplantation. (A) H&E staining images and (B) corresponding
pathological scoring. Black arrows showed the blood clots formed in
the lung tissue during aGVHD, the green arrow indicated capillary
dilatation. In the right panel of (A), white arrow indicated tissue
necrosis and the black arrow indicated the fat granules formed in
the liver. Before drug treatment, all mice underwent total body
radiation before bone marrow and spleen cell transplantation.
*P<0.05, **P<0.005,
***P<0.001, ****P<0.0001. Scale bar,
100 µm. aGVHD, acute graft vs. host disease; TBI, total body
irradiation; BMT, bone marrow transplantation; RAPA, rapamycin;
3-BrPA, 3-bromopyruvate. |
It was subsequently found that there were
significant differences among the groups for the histological
scores in the lung and liver tissues (Fig. 6B). For the histological scores in
the lung, the Control showed significantly lower histological score
than the aGVHD, 3-BrPA or RAPA-2.5 mg groups (Fig. 6B). Treatment with 5 mg RAPA and
RAPA plus 3-BrPA resulted in recovery of the lung, as there were no
significant differences in the scores compared with those in the
control group (Fig. 6B). In
addition, these two treatments induced significant reductions in
the histological score compared with those in the aGVHD group
(Fig. 6B). RAPA plus 3-BrPA
treatment mediated significant reductions in the score compared
with that in the 3-BrPA group (Fig.
6B).
For the histological score of the liver, aGVHD and
3-BrPA groups showed higher histological scores than the Control
(Fig. 6B). But there were no
significant differences between Control and RAPA-2.5 mg, RAPA-5 mg
or RAPA plus 3-BrPA, indicating these treatments were efficient in
prevention of aGVHD (Fig. 6B).
Furthermore, aGVHD showed significant higher histological score
than RAPA-5 mg or RAPA plus 3-BrPA, suggesting that these two
treatments were viable for alleviating liver damage caused by
aGVHD. The RAPA-5 mg group also showed a significant decrease
compared with that in the 3-BrPA group (Fig. 6B).
IL-4 and IFN-γ levels in mice with
aGVHD
Regarding in vivo IL-4 production (Fig. 7A), there were significant
differences among the groups. The Control group showed lower IL-4
production compared to the aGVHD, 3-BrPA, RAPA-2.5 mg and the
combination treatment group (Fig.
7A). The TBI group only showed inferior IL-4 production to the
aGVHD and 3-BrPA group (Fig. 7A).
Additionally, there were no significant differences between aGVHD
and 3-BrPA, RAPA-2.5 mg, RAPA-5 mg or RAPA plus 3-BrPA (Fig. 7A). There were also no significant
differences among the RAPA-containing treatment groups (Fig. 7A). The 3-BrPA group showed a
significantly higher level of IL-4 production compared with that of
the RAPA-5 mg group, but without significant differences by
comparing to the RAPA-2.5 mg or RAPA plus 3-BrPA group.
In terms of IFN-γ (Fig.
7B), there were significant differences among the groups.
Control group showed the least IFN-γ production among the groups
(Fig. 7B). The TBI group showed
inferior IFN-γ production compared to aGVHD group but superior
IFN-γ production to the combination treatment group (Fig. 7B). The aGVHD group showed the
highest IFN-γ production among the groups (Fig. 7B). After 3-BrPA and/or RAPA
treatment, the IFN-γ production was significantly reduced compared
to the aGVHD group (Fig. 7B).
Among the 3-BrPA and/or RAPA treatment groups, the combination
treatment group induced the lowest IFN-γ level (Fig. 7B). The RAPA-5 mg group showed lower
IFN-γ production compared with 3-BrPA and RAPA-2.5 mg groups.
Discussion
To synergistically improve the aGVHD treatment
outcome, the glycolysis inhibitor 3-BrPA was combined with the mTOR
inhibitor RAPA to target activated T cells in the present study.
This strategy revealed desirable synergistic effects in inhibiting
the viability of activated T cells. This combined treatment method
also potentiated the apoptosis of activated T cells. In addition,
decreased production of the proinflammatory cytokine IFN-γ
validated the potential efficacy of this strategy further. This
combination of low-dose RAPA and 3-BrPA treatment also yielded
promising outcomes in the in vivo aGVHD mouse model by
prolonging the survival time. These preclinical results support the
hypothesis that 3-BrPA and RAPA can mediate synergistic effects in
preventing aGVHD, providing a potentially feasible approach to
address this condition.
It has been previously reported that both 3-BrPA and
RAPA possess profound anti-cancer properties (27). However, their mechanisms of action
are distinct. 3-BrPA exerts its function by targeting GAPDH and
hexokinase by alkylating their active sites, with the former
serving as the predominant inhibition pathway (17). Additionally, 3-BrPA can also target
the HIF-1α pathway to regulate GAPDH activity further (15,28).
It has been reported that administration of 3-BrPA led to the
depletion of intracellular ATP in breast cancer cells and thus to
deprive cells of energy, inhibiting cell proliferation whilst
inducing cytotoxicity and cell apoptosis (15). RAPA is also called Sirolimus and
binds to mTOR, which serves key roles in nutrient metabolism
regulation, cell proliferation and survival (26). In a previous aGVHD study, RAPA was
found to induce the apoptotic cell death of conventional
CD4+CD25- T cells by inhibiting the mTOR/PI3K
pathway (29). Another previous
study demonstrated the immunosuppressive effects of RAPA on Treg
and CD8+ T cells in mice model (30). In the present study, the
combination of 3-BrPA and RAPA were tested on activated T cells
before subsequently investigating their effects on an in
vivo aGVHD model.
In the in vitro MLR model, application of
3-BrPA alone significantly inhibited cell viability at doses as low
as 20 µM. This is most likely due to the potent inhibition of the
glycolytic energy production pathway in the rapidly proliferating
and differentiating T cells (18).
Additionally, combined treatment exhibited synergism in inhibiting
cell viability even when the 3-BrPA concentration was 20 µM. This
synergistic effect mediated by low doses of both 3-BrPA and RAPA
could be of clinical importance for the treatment of aGVHD, because
the application of RAPA is highly compromised by adverse side
effects (31).
The reduced cell viability observed in the present
study may be caused by the inhibition of cell proliferation or
reduction of cell number. Therefore, the extent of T cell apoptosis
was next measured. Severe cell apoptosis was observed following
combined drug treatment. This was likely to be due to the vital
energy pathways of HIF-1α, GAPDH and mTOR all being suppressed.
Results from the present study are consistent with those from a
previous study (18), which
reported that RAPA treatment enhanced the anti-tumor capacity of
3-BrPA in lung cancer cell lines H1299 and H23. Treatment with both
3-BrPA and RAPA resulted in the apoptosis of T cells in a
synergistic manner. Therefore, this strategy provides a promising
option for the prevention of GVHD.
T helper 1 (Th1) and T helper 2 (Th2)
CD4+ T cells can regulate aGVHD by producing a variety
of cytokines (32). Th1 cells
mainly produce IFN-γ, TNF-β and IL-2, while Th2 cells mainly
secrete IL-4, IL-5, IL-6 and IL-10(29). Th1 cells are associated with the
development of aGVHD, whilst Th2 cells are able to reduce the
severity of GVHD (32). Therefore,
Th1/Th2 polarization serves a critical role in aGVHD pathogenesis
(33), such that targeting Th1
transcription factors can prevent GvHD (34). In the present study, the relatively
stable IL-4 levels among the treatment groups but markedly reduced
IFN-γ levels in the combined treatment groups suggest that the
inhibitory effects of this treatment were mainly exerted on Th1
cells instead of Th2 cells. In future studies, the populations of
Th1 and Th2 cells after RAPA and 3-BrPA treatment should be
investigated.
In the efferent stage of aGVHD, activated T cells
and inflammatory cytokines coordinate to attack target tissues and
damage the organs (32). These
cells include Th1 cells and cytotoxic CD8+ cells, with
cytokines including IFN-γ and TNF-β (35). In the present aGVHD mouse model, it
was found that the administration of 3-BrPA, RAPA and both
treatments combined mitigated the damage mediated by aGVHD in the
main organs, which was likely to be due to the inhibition of
various different pathways in the activated T cells, such as mTOR
and glycolysis. These findings are consistent with a previous study
by Nguyen et al (8), which
found that glycolysis inhibition can ameliorate aGVHD after
allo-HSCT in mice. In that previous study, another glycolysis
inhibitor 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one was
applied to inhibit glycolysis, by targeting
6-phosphofructo-2-kinase (8). The
staining results in the present study revealed that the combination
treatment group appears to be superior compared with the 3-BrPA and
low-dose RAPA treatment groups whilst being similar to the 5-mg
RAPA treatment group. In the 3-BrPA group, inhibition of glycolysis
in the mice also induced lipid deposition in the liver. However,
this was not observed in the combined treatment group, likely due
to the reduced dose of 3-BrPA.
In vivo cytokine levels are important
indicators of aGVHD (33). IFN-γ
levels in the serum has been previously associated with the
severity of aGVHD (36). Findings
from the present study also support this conclusion, since the
aGVHD group presented higher IFN-γ levels compared with those in
the control. In addition, inhibition of GAPDH, mTOR or both
pathways efficiently reduced the IFN-γ levels, indicating
successful suppression of the activity of Th1 cells. Since there
were no obvious changes in the IL-4 levels after any of the
treatments, the direction of Th1/Th2 polarization was likely to be
biased towards the Th2 subset. Although it would be of interest to
perform western blotting assay to evaluate the key targets of both
mTOR and glycolytic pathways, it was not possible to obtain the
required volume of blood from each mouse for the isolation of
sufficient T cells for western blotting. Therefore, only the
cytokine concentration from the blood was measured using ELISA in
the present study.
Minimizing GVHD whilst maximizing the GvT effect is
the ideal outcome of HSCT (20).
There is a limitation in the present study, only the prevention of
aGVHD by combining the glycolysis inhibitor 3-BrPA and the mTOR
inhibitor RAPA was focused upon. In future experiments, the GvT
effect of this combination treatment can be evaluated on animals
with blood cancer (such as leukemia, lymphoma and myeloma) and
underwent HSCT. This combination is advantageous, since both the
in vitro and in vivo data confirmed the high efficacy
of this strategy. Furthermore, this approach likely exerts
synergistic GvT effects, since the glycolysis inhibitor is able to
suppress cancer cell viability whereas mTOR inhibition can hinder
the proliferation and survival of cancer cells. In future studies,
the influence of this system on the GvT effect, in addition to
other immune cell types, such as Tregs and MDSCs (20), should be studied.
In conclusion, the present study applied both in
vitro MLR and in vivo aGVHD mouse models to reveal that
glycolysis inhibition is an efficient approach for aGVHD
prevention. Furthermore, combination treatment with 3-BrPA plus
low-dose RAPA appears to be advantageous, since it targets multiple
signaling pathways and requires lower doses of RAPA. Therefore, the
present study opened a novel avenue for aGVHD prevention and
treatment. In the future, introducing novel glycolysis inhibitors
and immunosuppressors with minimal side effects and higher efficacy
to target multiple signaling pathways would be highly desirable for
the combination therapy of aGVHD.
Supplementary Material
Glucose consumption of cells in the
mixed lymphocyte culture after 48 h. Glucose consumption was
measured using a Glucose (HK) Assay Kit. Cells without any drug
treatment was used as control and the corresponding obtained value
was set as 100%. *P<0.05, **P<0.005,
***P<0.001, ****P<0.0001.
@P<0.0001 vs. combination treatment group with 20 μM
3-BrPA. aGVHD, acute graft vs. host disease; TBI, total body
irradiation; BMT, bone marrow transplantation; RAPA, rapamycin;
3-BrPA, 3-bromopyruvate.
Representative flow cytometry images
of cell apoptosis after the combination treatment of 3-BrPA and
RAPA on the mixed lymphocyte culture for 48 h. RAPA, rapamycin;
3-BrPA, 3-bromopyruvate.
Representative flow cytometry diagrams
showing the chimeric ratios of aGVHD, 3-BrPA, RAPA-2.5 mg, RAPA-5
mg, RAPA-plus 3-BrPA treatment and BMT groups. aGVHD, acute graft
versus host disease; RAPA, rapamycin; 3-BrPA, 3-bromopyruvate; BMT,
bone marrow transplantation; FSC, forward scatter.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of China (grant no. 81600147), the Zhongshan Science and
Technology Research Major Project (grant no. 2017B002), Shenzhen
Science and Technology Plan Basic Research Project (grant nos.
JCYJ20180307150408596 and JCYJ20190809172403604), Sanming Project
of Medicine in Shenzhen (grant no. SZSM201911004), the Fundamental
Research Funds for the Central (grant no. 2175060), Starting Grant
from The Seventh Affiliated Hospital Sun Yat-sen University (grant
no. ZSQYRSF0008) and The Young Talents Fostering Grant from Sun
Yat-sen University (grant no. 20ykpy18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RQZ and XJX confirm the authenticity of all the raw
data. RQZ analyzed and interpreted data and wrote the manuscript.
XBW and YBY analyzed and interpreted data. BL analyzed in
vitro data. JW, ZWG and PS analyzed in vitro data. WJM
and ZY analyzed in vivo data. LPY supervised the study,
analyzed and interpreted data and wrote and edited the manuscript.
XJX conceived and designed the study and revised the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of Southern Medical University (Guangzhou, China) and
fulfilled the regulations of Institutional Animal Care and Use
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh AK and McGuirk JP: Allogeneic stem
cell transplantation: A historical and scientific overview. Cancer
Res. 76:6445–6451. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Witte T, Bowen D, Robin M, Malcovati L,
Niederwieser D, Yakoub-Agha I, Mufti GJ, Fenaux P, Sanz G, Martino
R, et al: Allogeneic hematopoietic stem cell transplantation for
MDS and CMML: Recommendations from an international expert panel.
Blood. 129:1753–1762. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schmitz N, Lenz G and Stelljes M:
Allogeneic hematopoietic stem cell transplantation for T-cell
lymphomas. Blood. 132:245–253. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gonsalves WI, Buadi FK, Ailawadhi S,
Bergsagel PL, Khan AA, Dingli D, Dispenzieri A, Fonseca R, Hayman
SR, Kapoo P, et al: Utilization of hematopoietic stem cell
transplantation for the treatment of multiple myeloma: A mayo
stratification of myeloma and risk-adapted therapy (mSMART)
consensus statement. Bone Marrow Transplant. 54:353–367.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ghimire S, Weber D, Mavin E, Wang XN,
Dickinson AM and Holler E: Pathophysiology of GvHD and other
HSCT-related major complications. Front Immunol.
8(79)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bhatia S, Armenian SH and Landier W: How I
monitor long-term and late effects after blood or marrow
transplantation. Blood. 130:1302–1314. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zeiser R and Blazar BR: Acute
graft-versus-host disease-biologic process, prevention, and
therapy. N Eng J Med. 377:2167–2179. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nguyen HD, Chatterjee S, Haarberg KM, Wu
Y, Bastian D, Heinrichs J, Fu J, Daenthanasanmak A, Schutt S,
Shrestha S, et al: Metabolic reprogramming of alloantigen-activated
T cells after hematopoietic cell transplantation. J Clin Invest.
126:1337–1352. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pearce EL, Poffenberger MC, Chang CH and
Jones RG: Fueling immunity: Insights into metabolism and lymphocyte
function. Science. 342(1242454)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gatza E, Wahl DR, Opipari AW, Sundberg TB,
Reddy P, Liu C, Glick GD and Ferrara JLM: Manipulating the
bioenergetics of alloreactive T cells causes their selective
apoptosis and arrests graft-versus-host disease. Sci Transl Med.
3(67ra8)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Byersdorfer CA, Tkachev V, Opipari AW,
Goodell S, Swanson J, Sandquist S, Glick GD and Ferrara JLM:
Effector T cells require fatty acid metabolism during murine
graft-versus-host disease. Blood. 122:3230–3237. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Akers LJ, Fang W, Levy AG, Franklin AR,
Huang P and Zweidler-McKay PA: Targeting glycolysis in leukemia: A
novel inhibitor 3-BrOP in combination with rapamycin. Leuk Res.
35:814–820. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu RH, Pelicano H, Zhang H, Giles FJ,
Keating MJ and Huang P: Synergistic effect of targeting mTOR by
rapamycin and depleting ATP by inhibition of glycolysis in lymphoma
and leukemia cells. Leukemia. 19:2153–2158. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou S, Min Z, Sun K, Qu S, Zhou J, Duan
H, Liu H, Liu X, Gong Z and Li D: MiR-199a-3p/Sp1/LDHA axis
controls aerobic glycolysis in testicular tumor cells. Int J Mol
Med. 42:2163–2174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Attia YM, El-Abhar HS, Al Marzabani MM and
Shouman SA: Targeting glycolysis by 3-bromopyruvate improves
tamoxifen cytotoxicity of breast cancer cell lines. BMC Cancer.
15(838)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Del Rey MJ, Valín Á, Usategui A,
García-Herrero CM, Sánchez-Aragó M, Cuezva JM, Galindo M, Bravo B,
Cañete JD, Blanco FJ, et al: Hif-1α knockdown reduces glycolytic
metabolism and induces cell death of human synovial fibroblasts
under normoxic conditions. Sci Rep. 7(3644)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Abdel-Wahab AF, Mahmoud W and Al-Harizy
RM: Targeting glucose metabolism to suppress cancer progression:
Prospective of anti-glycolytic cancer therapy. Pharmacol Res.
150(104511)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang Q, Pan J, Lubet RA, Komas SM,
Kalyanaraman B, Wang Y and You M: Enhanced antitumor activity of
3-bromopyruvate in combination with rapamycin in vivo and in vitro.
Cancer Prev Res (Phila). 8:318–326. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Palmer JM, Chen BJ, DeOliveira D, Le ND
and Chao NJ: Novel mechanism of rapamycin in GVHD: Increase in
interstitial regulatory T cells. Bone Marrow Transplant.
45:379–384. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Scheurer J, Reisser T, Leithäuser F,
Messmann JJ, Holzmann K, Debatin KM and Strauss G: Rapamycin-based
graft-versus-host disease prophylaxis increases the
immunosuppressivity of myeloid-derived suppressor cells without
affecting T cells and anti-tumor cytotoxicity. Clin Exp Immunol.
202:407–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gruppuso PA, Boylan JM and Sanders JA: The
physiology and pathophysiology of rapamycin resistance:
Implications for cancer. Cell Cycle. 10:1050–1058. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fantini MC, Dominitzki S, Rizzo A, Neurath
MF and Becker C: In vitro generation of CD4+ CD25+ regulatory cells
from murine naive T cells. Nat ProtocC. 2:1789–1794.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chou TC: Drug combination studies and
their synergy quantification using the chou-talalay method. Cancer
Res. 70:440–446. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ni X, Xia Y, Zhou S, Peng H, Wu X, Lu H,
Wang H, Liu R, Blazar BR, Gu J and Lu L: Reduction in murine acute
GVHD severity by human gingival tissue-derived mesenchymal stem
cells via the CD39 pathways. Cell Death Dis. 10(13)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Q, Ning J, Zhang Y, Wu X, Luo X and
Fan Z: Idiopathic pneumonia syndrome in mice after allogeneic bone
marrow transplantation: Association between idiopathic pneumonia
syndrome and acute graft-versus-host disease. Transpl Immunol.
23:12–17. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cooke KR, Kobzik L, Martin TR, Brewer J,
Delmonte J Jr, Crawford JM and Ferrara JL: An experimental model of
idiopathic pneumonia syndrome after bone marrow transplantation: I.
The roles of minor H antigens and endotoxin. Blood. 88:3230–3239.
1996.PubMed/NCBI
|
|
27
|
Ju XP, Xu B, Xiao ZP, Li JY, Chen L, Lu SQ
and Huang ZX: Cytokine expression during acute graft-versus-host
disease after allogeneic peripheral stem cell transplantation. Bone
Marrow Transplant. 35:1179–1186. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shi LZ, Wang R, Huang G, Vogel P, Neale G,
Green DR and Chi H: HIF1alpha-dependent glycolytic pathway
orchestrates a metabolic checkpoint for the differentiation of TH17
and Treg cells. J Exp Med. 208:1367–1376. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shin HJ, Baker J, Leveson-Gower DB, Smith
AT, Sega EI and Negrin RS: Rapamycin and IL-2 reduce lethal acute
graft-versus-host disease associated with increased expansion of
donor type CD4+CD25+Foxp3+ regulatory T cells. Blood.
118:2342–2350. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zeiser R, Nguyen VH, Beilhack A, Buess M,
Schulz S, Baker J, Contag CH and Negrin RS: Inhibition of CD4+CD25+
regulatory T-cell function by calcineurin-dependent interleukin-2
production. Blood. 108:390–399. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sánchez-Fructuoso AI, Ruiz JC,
Pérez-Flores I, Alamillo CG, Romero NC and Arias M: Comparative
analysis of adverse events requiring suspension of mTOR inhibitors:
Everolimus versus sirolimus. Transplant Proc. 42:3050–3052.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nikolic B, Lee S, Bronson RT, Grusby MJ
and Sykes M: Th1 and Th2 mediate acute graft-versus-host disease,
each with distinct end-organ targets. J Clin Invest. 105:1289–1298.
2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Guo H, Qiao Z, Zhu L, Wang H, Su L, Lu Y,
Cui Y, Jiang B, Zhu Q and Xu L: Th1/Th2 cytokine profiles and their
relationship to clinical features in patients following
nonmyeloablative allogeneic stem cell transplantation. Am J
Hematol. 75:78–83. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yu Y, Wang D, Liu C, Kaosaard K, Semple K,
Anasetti C and Yu XZ: Prevention of GVHD while sparing GVL effect
by targeting Th1 and Th17 transcription factor T-bet and RORγt in
mice. Blood. 118:5011–5020. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Golubovskaya V and Wu L: Different subsets
of T cells, memory, effector functions, and CAR-T immunotherapy.
Cancers (Basel). 8(36)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Choi J, Ziga ED, Ritchey J, Collins L,
Prior JL, Cooper ML, Piwnica-Worms D and DiPersio JF: IFNγR
signaling mediates alloreactive T-cell trafficking and GVHD. Blood.
120:4093–4103. 2012.PubMed/NCBI View Article : Google Scholar
|