Introduction
Clinical manifestation of chronic venous
insufficiency (CVI) in the lower limbs is complex and varies from
edema, venectasia and varicosities to ulcerations. CVI has a
progressive evolution with a well-known etiopathogeny, prognosis
and therapy (1). CVI prevalence
increases with age, is more common in elderly females and has
certain contributing factors (pregnancy, prolonged standing,
menopause and hormone therapy, among others) (2). The reported prevalence of CVI in
Romania has a variable value (68.4% in 2015 and 25% in 2020)
(3,4), which is similar to the incidence rate
in the rest of Europe (20-60%) (2).
Etiopathogenic CVI can be congenital (Klippel-Trenaunay-Weber-Parks
syndrome), primitive (hydrostatic varicose veins) and secondary
(post-thrombotic, as a result of venous compression or external
trauma) (5).
Vascular stenosis and valvular incompetence, as well
as the procoagulant state represent essential factors for the
initiation and evolution of the thrombotic process. The etiology of
venous thrombosis is multifactorial (6). The process usually starts with an
injury of the vascular endothelium, which is subsequently followed
by activation and aggregation of thrombocytes at the lesion,
initiating a procoagulant effect and the constitution of the
thrombi (7).
Although at present antithrombotic prophylaxis is
performed when necessary, an increase in the incidence of
thromboses has been observed. This may be due to numerous
associated thrombotic factors, including advanced age, invasive
investigations, obesity, prolonged immobilization, birth control,
hormonal therapy and neoplasia, which increase pressure on the vein
wall (8). Furthermore, certain
hematological diseases may induce a procoagulant status, such as
leukemia and polycythemia verra, and certain medications (such as
hydroxyurea) may favor the appearance of lower leg ulcers (9).
Over the last 25 years, hyperhomocysteinemia (HH)
has been included as a risk factor for atherosclerosis and
thromboembolisms, as well as for pregnancy and cardiovascular and
cerebrovascular diseases (10-12).
Homocysteine is an amino acid derived from methionine metabolism
and is usually excreted renally. Plasmatic levels of homocysteine
are normally <12 µmol/l. Increased levels can occur in
congenital HH as a result of genetic deficiencies in cystathionine
β synthase or methylenetetrahydrofolate reductase, enzymes which
are responsible for the metabolism of homocysteine. In the acquired
form, higher homocysteine levels can be a result of folate, vitamin
B12 and vitamin B6 deficiencies, senescence, hypothyroidism,
connective tissue diseases, nephropathies, neoplasia and drug
intake (13,14).
Taking into account that venous thromboembolism
(VTE) can appear during CVI and that the etiopathogenic implication
of HH in the thrombotic process is recognized, the present
prospective study investigated 166 patients with CVI and determined
their homocysteine levels, observing their association with factors
involved in CVI and VTE aggravation. The main aim of the present
study was to determine the association between homocysteine
plasmatic levels and the risk of VTE, in the presence or absence of
anticoagulant treatments and venous ulcers in patients with CVI.
The thrombogenic factors, which are more often linked to HH,
including pregnancy, family and personal history of thrombosis,
smoking and certain drug treatments, were also investigated.
Furthermore, the link between the metabolic, inflammatory and
procoagulant status of patients with HH and CVI was explored.
Materials and methods
Patients
Over a period of one year (June 2011 to May 2012),
166 patients with a mean age of 61.59 years (range, 29-89 years)
with CVI who were admitted to the Clinic of Dermatology, County
Emergency Hospital of Sibiu (Sibiu, Romania), were enrolled in the
present study. Patients under the age of 18 were excluded, as well
as patients with chronic venous disease stages,
Clinical-Etiological-Anatomical-Pathophysiological classifications
(CEAP) C0-C2 (C0, no clinical signs but with symptoms; C1, with
telangiectasias; and C2, with varicose veins) (15). The experimental protocol was
approved by the Ethics Committee of the County Emergency Hospital
of Sibiu. All patients provided signed informed consent before
participating in the present study.
The following information was collected for all
patients: Demographic data, general associated pathology, personal
and family history of thrombosis, procoagulant factors and any
previous or current anticoagulant therapies. For every patient, the
inflammatory, metabolic and procoagulant statuses [activated
partial thromboplastin time (APTT) and prothrombin time (PT)] were
analyzed, as well as the levels of homocysteine at the time of
admission.
High-performance liquid chromatography
(HPLC)
Homocysteine levels were determined using HPLC and a
Homocysteine in Serum/Plasma HPLC kit (cat. no. 45000; Chromsystems
Diagnostics). HPLC was carried out with a Homocysteine in
Serum/Plasma kit (cat. no. 45000; Chromsystems Diagnostics) and
column (cat. no. 39100, Chromsystems Diagnostics). Patient blood
samples were drawn following fasting and the plasma obtained was
centrifuged at ambient temperature for 30 min at 10,000 x g 4,000
rpm and then stored at 2-4˚C. A 100-µl sample volume was analyzed,
and the flow rate was 1.5 ml/min. Levels over 12 µmol/l were
considered to represent increased levels of homocysteine.
Statistical analysis
Data were analyzed using SPSS software (version
23.0; IBM Corp.). The nominal variables are expressed as numbers
and percentages and the quantitative data as mean ± SD. A
Shapiro-Wilk test was applied to evaluate the normal distribution.
An ANOVA was used to compare the means of quantitative data with
Gaussian distribution. For non-Gaussian distributed data, the
Kruskal-Wallis test was used. As appropriate, associations between
two categorical variables were analyzed with Pearson's
χ2 test or Fisher's exact test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Demographic profile
The predominant demographic profile of the CVI
patients taking part in the study was women (58.43%) of urban
origin (55.42%), from the age group 46-75 years (73.49%;
P=0.001).
Clinical forms of CVI
Approximately one-half of the patients were stage
CEAP C6 of CVI with active ulcers (42.17%; P=0.118). Almost
one-quarter of the cases (22.29%) presented with thrombophlebitis
at the time of diagnosis (associated with venous ulcer, 16.07%;
without ulcer, 6.22%). In less than one-half of the cases (42.77%)
the disease was older than 3 years.
Post-thrombotic syndrome was present in 24.09% of
patients (superficial thrombophlebitis, 12.04%; profound
thrombophlebitis, 10.25%; and pulmonary thromboembolis, 1.80%) and
family antecedent of thrombosis in 8.43% of cases.
Procoagulant factors
Procoagulant factors identified in the study group
were smoking (21.67%), surgical interventions (16.27%) and
prolonged immobilization (3.61%). For the female subgroup,
abortions (31.93%) and hormonal treatments (5.42%) were also
identified as procoagulant factors.
Blood coagulation, metabolic and
inflammatory profiles
The blood coagulation profile demonstrated low
values of PT in 1.20% of cases and APTT in 5.42% of the cases. In
approximately one-half of the cases the metabolic profile was
altered, with hyperglycemia (22.89%; P=0.176), hypercholesterolemia
(42.77%; P=0.876), hypertriglyceridemia (28.92%; P=0.752) and
hyperlipidemia (14.46%; P=0.05) (Table
I) being present. Less than one-quarter of the patients with
CVI presented a proinflammatory profile, with increased
inflammation marker levels (erythrocyte sedimentation rate, 23.50%;
C-reactive protein, 16.27%; and fibrinogen, 13.86%).
 | Table IAssociations between homocysteine
levels and the different parameters assessed in the study. |
Table I
Associations between homocysteine
levels and the different parameters assessed in the study.
| | Homocysteine
level | |
|---|
| Parameter | Elevated, n (%) | Normal, n (%) | Total, patients n
(%) | P-value |
|---|
| Frequency | 90 (54.22) | 76 (45.78) | 166(100) | - |
| Sex | | | | 0.412 |
|
Male | 40 (44.44) | 29 (38.16) | 69 (41.57) | |
|
Female | 50 (55.56) | 47 (61.84) | 97 (58.43) | |
| Origin | | | | 0.088 |
|
Urban | 45 (50.00) | 48 (63.16) | 92 (55.42) | |
|
Rural | 45 (50.00) | 28 (36.84) | 74 (44.58) | |
| Age, years | | | | 0.001 |
|
≤45 | 8 (8.88) | 12 (15.79) | 20 (12.05) | |
|
46-60 | 24 (26.67) | 39 (51.32) | 63 (37.95) | |
|
61-75 | 42 (46.67) | 19 (25.00) | 61 (36.75) | |
|
>75 | 16 (17.78) | 6 (7.89) | 22 (13.25) | |
| CVI clinical
stages | | | | 0.118 |
|
CEAP
C3-5 | 47 (52.22) | 49 (64.47) | 96 (57.83) | |
|
CEAP C6 | 43 (47.78) | 27 (35.53) | 70 (42.17) | |
| CVI age, years | | | | 0.217 |
|
<1 | 26 (28.89) | 15 (19.74) | 41 (24.70) | |
|
1-3 | 31 (34.44) | 23 (30.26) | 54 (32.53) | |
|
3-10 | 22 (24.44) | 28 (36.84) | 50 (30.12) | |
|
10-20 | 9 (10.00) | 10 (13.16) | 19 (11.45) | |
|
>20 | 2 (2.23) | 0 (0.00) | 2 (1.20) | |
| Personal thrombosis
history | | | | 0.260 |
|
Superficial
thrombophlebitis | 10 (11.11) | 10 (13.16) | 20 (12.04) | |
|
Profound
thrombophlebitis | 10 (11.11) | 7 (9.21) | 17 (10.25) | |
|
Pulmonary
thromboembolism | 3 (3.33) | 0 (0.00) | 3 (1.80) | |
| Family antecedent
of thrombosis | 4 (2.41) | 10 (6.02) | 14 (8.43) | |
| Procoagulant
factors | | | | |
|
Study
group | | | | |
|
Smoking | 15 (16.67) | 21 (27.63) | 36 (21.67) | 0.088 |
|
Surgical
interventions | 14 (15.56) | 13 (17.11) | 27 (16.27) | 0.788 |
|
Prolonged
immobilization | 3 (3.33) | 3 (3.95) | 6 (3.61) | 0.833 |
| Female study
group | | | | |
|
Abortions,
n | | | | 0.218 |
|
1 | 16 (17.77) | 14 (18.42) | 30 (18.07) | |
|
2 | 1 (1.11) | 7 (9.21) | 8 (4.82) | |
|
3 | 3 (3.33) | 4 (5.26) | 7 (4.22) | |
|
4 | 3 (3.33) | 5 (6.58) | 8 (4.82) | |
|
Hormone
treatment | 6 (6.66) | 3 (3.95) | 9 (5.42) | 0.697 |
| Blood coagulation
profile | | | | |
|
Low PT | 0 (0.00) | 2 (2.63) | 2 (1.20) | 0.189 |
|
Low
APTT | 4 (4.44) | 5 (6.58) | 9 (5.42) | 0.752 |
| Inflammatory
profile | | | | |
|
Elevated
CRP | 12 (13.33) | 15 (19.74) | 27 (16.27) | 0.790 |
|
Elevated
ESR | 16 (17.77) | 23 (30.26) | 39 (23.50) | 0.820 |
|
Elevated
fibrinogen | 10 (11.11) | 13 (17.11) | 23 (13.86) | 0.898 |
| Metabolic
profile | | | | |
|
High
cholesterol | 38 (42.22) | 33 (43.42) | 71 (42.77) | 0.876 |
|
High
triglycerides | 24 (26.67) | 24 (31.58) | 48 (28.92) | 0.752 |
|
High
lipids | 15 (16.67) | 9 (11.84) | 24 (14.46) | 0.050 |
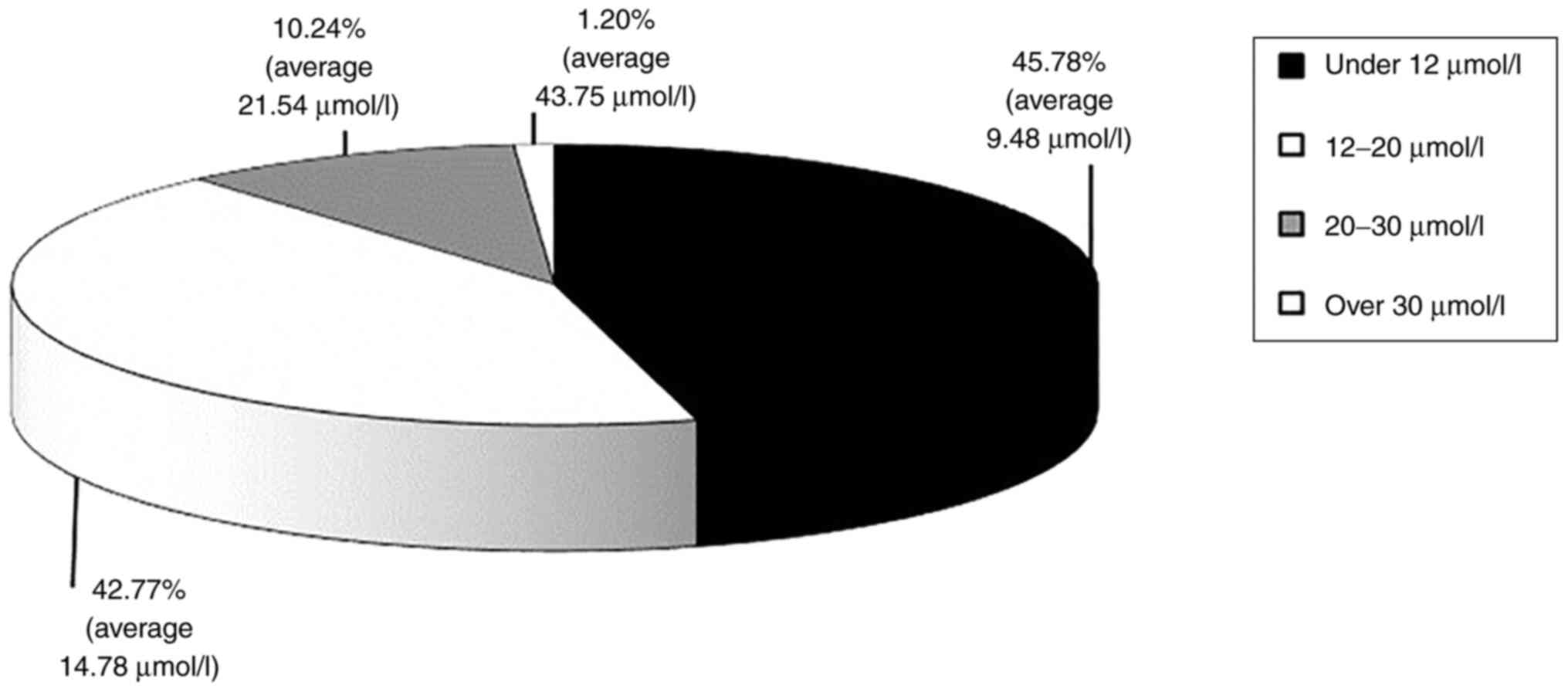
Homocysteine levels
In 54.22% of cases (90 patients), increased
plasmatic levels of homocysteine were identified. In patients with
HH, the average homocysteine level was 17.33±5.75 µmol/l. Moderate
forms of HH, with values of 12-20 µmol/l (42.77%) and 20-30 µmol/l
(10.24%) and intermediary forms, with values over 30 µmol/l but
lower than 100 µmol/l (1.20%) (Fig.
1) were mainly identified. Comparative analysis between average
homocysteine levels in patients with CVI and anticoagulant therapy
(17.31±4.89 µmol/l) compared with patients without anticoagulant
therapy displayed no significant differences.
Homocysteine levels were linked to a CVI diagnosis
with ulcers (15.24±7.02 µmol/l) and a CVI diagnosis with ulcers and
thrombophlebitis (14.13±4.08 µmol/l), compared with 13.12±5.81
µmol/l for patients with CVI CEAP C3-5 (P=0.138) (Table II). Homocysteine levels were also
linked to pulmonary thromboembolism (15.33±4.07 µmol/l) and deep
vein thrombosis (DVT; 14.08±5.97 µmol/l) (P=0.819) (Table II). The average homocysteine levels
presented the highest levels for patients >75 years of age
(15.03 µmol/l) and the age group 61-75 years (14.53 µmol/l).
 | Table IIMean ± SD homocysteine levels in the
study group, associated with the diagnosis of CVI, disease age,
personal thrombosis history and obesity. |
Table II
Mean ± SD homocysteine levels in the
study group, associated with the diagnosis of CVI, disease age,
personal thrombosis history and obesity.
| | Homocysteine | |
|---|
| Parameter | Total patients,
n | Mean | Standard
deviation | P-value |
| Diagnosis | | | | |
|
CVI with
ulcer and thrombophlebitis | 26 | 14.1319 | 4.0896 | 0.138 |
|
CVI with
thrombophlebitis | 11 | 11.4745 | 4.2995 | |
|
CVI with
ulcer | 44 | 15.2450 | 7.0275 | |
|
CVI without
ulcer | 85 | 13.1275 | 5.8134 | |
|
Total | 166 | 13.7366 | 5.9071 | |
| Disease age,
years | | | | |
|
<1 | 4 | 11.4450 | 3.2300 | 0.148 |
|
1-3 | 17 | 12.4618 | 4.1013 | |
|
3-10 | 50 | 12.4368 | 4.7084 | |
|
10-20 | 54 | 14.5261 | 6.7933 | |
|
>20 | 41 | 15.0339 | 6.4982 | |
|
Total | 166 | 13.7366 | 5.9071 | |
| Obesity | | | | |
|
Without | 37 | 13.3373 | 7.1972 | 0.931 |
|
With | 129 | 13.7792 | 4.0765 | |
|
Total | 166 | 13.7366 | 5.9071 | |
| Personal thrombosis
history | | | | |
|
Superficial
thrombophlebitis | 20 | 12.6835 | 2.8670 | 0.819 |
|
Profound
thrombophlebitis | 17 | 14.0847 | 5.9785 | |
|
Pulmonary
thromboembolism | 3 | 15.3333 | 4.0723 | |
|
Without | 126 | 13.8187 | 6.3014 | |
|
Total | 166 | 13.7366 | 5.9071 | |
The age of CVI was linked to HH (P=0.217). Average
homocysteine levels varied between 11.44±3.23 µmol/l in patients
with a CVI disease age of <1 year and 15.03±6.49 µmol/l in
patients with CVI >20 years (P=0.148) (Table II). The differential analysis of
homocysteine on subgroups of patients presenting with CVI without
anticoagulant therapies, compared with patients with anticoagulant
therapies, demonstrated that for a disease age ≤10 years, 56.40%
compared with 35.70% of the cases presented with normal
homocysteine levels, and for a disease age of >30 years, 87.50%
of the cases compared with 71.40% of cases presented with increased
homocysteine levels (P=0.050). The average homocysteine levels in
patients with obesity compared with patients of normal weight was
similar (13.77±4.07 µmol/l vs. 13.33±7.19 µmol/l; P=0.931)
(Table II).
For patients with HH, no statistically significant
associations were demonstrated between homocysteine levels and the
inflammatory, metabolic and blood coagulation profiles, as well as
certain thrombogenic factors, including prolonged immobilization,
recent surgical interventions and hormone therapies (Table I). Among the procoagulant factors,
smoking was statistically linked to HH in patients with CVI without
anticoagulant therapy (P=0.088) compared with patients with CVI
with chronic anticoagulant therapy.
Discussion
In CVI of the lower limbs, an increase in the
intraluminal pressure determines alterations in endothelial
structures with secondary cutaneous lesions. The dysfunction of the
endothelium results in endothelial adhesion molecule expression,
producing a chemotactic effect for leukocytes. The activated
leukocytes release cytokines, including transforming growth
factor-β, vascular endothelial growth factor, interleukin-1 (IL-1),
tumor necrosis factor α and proteinases, which consequently
initiate and maintain the inflammatory signaling cascade resulting
in a procoagulant and proatherosclerotic state (16). The procoagulant state is a key
etio-pathogenic factor of VTE and indirectly of venous and
post-thrombotic ulcers. The interaction between chronic
inflammation and thrombosis serves an essential role in
post-thrombotic chronic ulceration (16). Matrix metalloproteinases (MMPs),
whose activity is stimulated by chronic inflammation, serve an
important role in the occurrence of ulcers (17,18).
Excess MMP results in changes in fibroblast function alongside
dermal fibrosis, proliferation of dermal capillaries and
deterioration of extracellular matrix and glycocalyx (19).
Deficiencies in anticoagulant factors (such as
anti-thrombin 3, S protein, C protein, factor V) or mutations of
certain antithrombotic genes (such as prothrombin gene G20210A),
activated C protein resistance and HH, can induce a congenital
procoagulant state. Numerous diseases have also previously been
determined to induce acquired hypercoagulant states, including
neoplasia, cardiovascular diseases, cerebrovascular diseases,
venous and hepatic insufficiency, diabetes mellitus, dyslipidemia,
connective tissue diseases and HH, as well as tobacco smoking,
prolonged immobilization and the long use of oral
contraceptives.
As a factor with procoagulant value, HH can occur
via congenital deficiencies, including cystathionine β synthase
deficiency, methylenetetrahydrofolate reductase deficiency or
innate deficiencies in the metabolism of cobalamin. However, HH can
also be acquired, such as in folate, vitamin B12 and vitamin B6
metabolism disorders, chronic renal insufficiency, hypothyroidism
and neoplasia (breast, ovarian and pancreatic neoplasms, and
leukemia), as well as a result of certain treatments (for example,
methotrexate, phenytoin, theophylline and phosphodiesterase
inhibitors), advanced age and smoking (13,14,20-24).
In the present study, a hyper-coagulant state induced by CVI,
mainly via HH (54.22%), and the general associated pathology
(86.14% of cases presented with associated cardiovascular
pathology) were identified.
Advanced age is a risk factor for
hypercoagulability. This is a consequence of endothelial
modifications, which occur during young adulthood and middle age as
a result of cardiovascular risk factors (25). Increases in the concentrations of
certain procoagulant factors, such as fibrinogen, factor VII, VIII
and X and the reduction of certain anticoagulant factors, such as
plasminogen, antithrombin III and tissular activator of
plasminogen, also contribute towards endothelial modifications
(16). In the present study, HH
occurred more often in individuals >60 years old (64.45%), with
an average homocysteine level of 17.93 µmol/l, compared with that
in individuals <60 years old (35.55%), with an average
homocysteine level of 15.55 µmol/l (P=0.024).
Blood coagulation and fibrinogen profiles have also
been demonstrated to be linked to thrombosis risk. Therefore, low
APTT and elevated fibrinogen levels are associated with an
increased risk of DVT and pulmonary thromboembolism. Low PT and
international normalized ratio also serve a minor role in
determining the procoagulant state (16). In the present study, a small number
of patients displayed elevated fibrinogen (13.86%) and low APTT
(5.42%) levels.
HH is a risk factor in arteriosclerotic
cardiovascular and cerebrovascular diseases, as it is a promoter of
endothelial oxidative stress and stimulates the production of
reactive oxygen species. Furthermore, HH favors leukocyte inflow
mediated by endothelial adhesion molecules, with the infiltration
of leukocytes in the vascular wall and the release of chemokines
inducing a proinflammatory response (26). In the present study group, in
patients with CVI, HH was associated with pulmonary thromboembolism
(100%), the presence of ulcers (61.50%), ulcers associated with
thrombophlebitis (61.40%; P=0.003) and profound thrombophlebitis
(58.80%; P=0.260). Alterations of lipid status (with the
implication of adipokines and adiponectin) results in a
proinflammatory effect over time. Chronic inflammation of the
vascular wall associated with HH can influence the hypercoagulant
state (27). In the present study,
the analysis of the metabolic profile (glucidic and lipid) in
patients with HH compared with patients with normal levels of
homocysteine, was not significant. HH also contributes to venous
and arterial thrombotic diseases by increasing the expression of
tissular factors, alleviating anticoagulant processes, improving
the thrombocyte activity, increasing thrombin production,
intensifying the activity of factor V, altering the fibrinolytic
potential and favoring vascular injuries (28). HH is a recognized thrombotic risk
factor and also serves a role in certain complex diseases, such as
Klippel-Trenaunay-Weber syndrome (29,30).
Homocysteine treatments are useful, as HH is one of
the few post-thrombotic risk factors that can be corrected by
administering folic acid and vitamins B6 and B12. However, although
treatment using folic acid and vitamin B supplements can lower the
homocysteine level by 25%, it does not prevent venous thrombosis
from reappearing (31). The
prevalence of HH in patients with CVI in the present study was
21.18%. In a meta-analysis published by Ray (32), the prevalence of HH in patients with
VTE varied between 5.7 and 34.8% compared with 0-7.1% in healthy
individuals. This study also reported that an increase of 5 µmol/l
homocysteine can lead to 2-3-fold increase in the risk of VTE.
Compared with the study by Ray (32), the present study determined that HH
prevalence is relatively similar for patients with CVI and VTE.
HH can be moderate (<30 µmol/l), intermediary
(30-100 µmol/l) and severe (>100 µmol/l) (33). In the present study, 54.22% of
patients with CVI presented with HH. Almost all patients with HH
presented with moderate HH (97.79%) and 2.22% presented with
intermediary HH, with no cases of severe HH. These results
indicated that moderate and intermediary HH may be an aggravating
factor in CVI evolution with DVT and venous ulcer risk.
Increases in plasmatic levels of homocysteine were
also demonstrated in the present patients with CVI with ulcers,
thrombophlebitis and pulmonary thromboembolic episodes, in elderly
patients, in patients with advanced disease age and in smokers.
In conclusion, HH is a well-known risk factor in
arterial and venous thrombotic diseases and also serves an
important role in CVI, which increases the thrombogenic risk in
these patients, especially in the elderly and those with an
advanced venous disease age. Treatment of moderate HH in CVI by
administration of folic acid, vitamin B6 and vitamin B12 can lead
to the alleviation of symptoms, reducing the risk of VTE and their
complications and implicitly slowing down the progression of
CVI.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported in part by a grant (no.
221) from ‘Lucian Blaga’ University of Sibiu and Advanced Medical
Services.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MR and GMI created and designed the present study,
analyzed and interpreted the patient data, and drafted and revised
the manuscript for important intellectual content. IB was
responsible for data acquisition, analysis and interpretation of
patient data, manuscript drafting and design. All authors read and
approved the final manuscript. All authors confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the County Emergency Hospital of Sibiu (Sibiu, Romania; approval
no. 356) and all patients provided written inform consent regarding
participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
IOANA BALDOVIN is Ioana Baldovin née Rădulescu.
References
|
1
|
Nicolaides AN, Allegra C, Bergan J,
Bradbury A, Cairols M, Carpentier P, Comerota A, Delis C, Eklof B,
Fassiadis N, et al: Management of chronic venous disorders of the
lower limbs guidelines according to scientific evidence. Int
Angiol. 27:1–59. 2008.PubMed/NCBI
|
|
2
|
Zolotukhin IA, Seliverstov EI, Shevtsov
YN, Avakiants IP, Nikishkov AS, Tatarintsev AM and Kirienko AI:
Prevalence and risk factors for chronic venous disease in the
general Russian population. Eur J Vasc Endovasc Surg. 54:752–758.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Feodor T, Baila S, Mitea I, Branisteanu D
and Vittos O: Epidemiology and clinical characteristics of chronic
venous disease in Romania. Exp Ther Med. 17:1097–1105.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rabe E, Regnier C, Goron F, Salmat G and
Pannier F: The prevalence, disease characteristics and treatment of
chronic venous disease: An international web-based study. J Comp
Eff Res. 9:1205–1218. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Patel SK and Surowiec SM: Venous
Insufficiency. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2021.
|
|
6
|
Ashorobi D, Ameer MA and Fernandez R:
Thrombosis. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2021.
|
|
7
|
Yau JW, Teoh H and Verma S: Endothelial
cell control of thrombosis. BMC Cardiovasc Disord.
15(130)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hotoleanu C: Association between obesity
and venous thromboembolism. Med Pharm Rep. 93:162–168.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Iancu GM, Ocneanu A and Rotaru M:
Hydroxyurea-induced superinfected ulcerations: Two case reports and
review of the literature. Exp Ther Med. 20(191)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fortin LJ and Genest J Jr: Measurement of
homocyst(e)ine in the prediction of arteriosclerosis. Clin Biochem.
28:155–162. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tinelli C, Di Pino A, Ficulle E, Marcelli
S and Feligioni M: Hyperhomocysteinemia as a risk factor and
potential nutraceutical target for certain pathologies. Front Nutr.
6(49)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cattaneo M: Hyperhomocysteinemia and
venous thromboembolism. Semin Thromb Hemost. 32:716–723.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brustolin S, Giugliani R and Felix TM:
Genetics of homocysteine metabolism and associated disorders. Bra J
Med Biol Res. 4:1–7. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Son P and Lewis L: Hyperhomocysteinemia.
In: StatPearls [Internet]. StatPearls Publishing, Treasure Island,
FL, 2020.
|
|
15
|
Zegarra TI and Tadi P: CEAP Classification
of Venous Disorders. In: StatPearls [Internet]. StatPearls
Publishing, Treasure Island, FL, 2021.
|
|
16
|
Hantusch B: Morphological and functional
characteristics of blood and lymphatic vessels. In: Fundamentals of
Vascular Biology. Learning Materials in Biosciences. Geiger M (ed).
Springer, Cham, pp1-43, 2019.
|
|
17
|
Herouy Y: The role of matrix
metalloproteinases (MMPs) and their inhibitors in venous leg ulcer
healing. Phebolymphology. 44:31–267. 2004.
|
|
18
|
Barbu A, Neamțu B, Zăhan M, Iancu GM,
Bacila C and Mireșan V: Current trends in advanced alginate-based
wound dressings for chronic Wounds. J Pers Med.
11(890)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ali MM, Mahmoud AM, Le Master E, Levitan I
and Phillips SA: Role of matrix metalloproteinases and histone
deacetylase in oxidative stress-induced degradation of the
endothelial glycocalyx. Am J Physiol Heart Circ Physiol.
316:H647–H663. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brattström L and Wilcken DE: Homocysteine
and cardiovascular disease: Cause or effect? Am J Clin Nutr.
72:315–323. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sule AA, Chin TJ and Khien LH: Recurrent
unprovoked venous thrombembolism in a young female patient with
high levels of homocysteine. Int J Angiol. 21:95–98.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bolal M, Ates I, Demir BF, Altay M, Turhan
T and Yilmaz N: The relationship between homocysteine and
autoimmune subclinical hypothyroidism. Int J Med Biochem. 3:1–7.
2020.
|
|
23
|
Hasan T, Arora R, Bansal AK, Bhattacharya
R, Sharma GS and Sigh LR: Disturbed homocysteine metabolism in
associated with cancer. Exp Mol Med. 51:1–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lovčić V, Klobučić M, Bašić-Jukić N and
Lovčić P: Is hyperhomocysteinemia approaching traditional risk
factors for cardiovascular diseases? Acta Clin Croat. 45 (Suppl
1):S65–S72. 2006.
|
|
25
|
Elian V, Cioca G, Pantea-Stoian A,
Dobjanschi C and Serafinceanu C: Metabolic syndrome and chronic
kidney disease: Pathogenic, clinical, and therapeutic correlations.
In: Proceedings of the 1st International Conference on
Interdisciplinary Management of Diabetes Mellitus and its
Complications (INTERDIAB), Bucharest, 2015.
|
|
26
|
Papatheodorou L and Weiss N: Vascular
oxidant stress and inflammation in hyperhomocysteinemia. Antioxid
Redox Signal. 9:1941–1958. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Esfahani M, Movahedian A, Baranchi M and
Goodarzi MT: Adiponectin: An adipokine with protective features
against metabolic syndrome. Iran J Basic Med Sci. 18:430–442.
2015.PubMed/NCBI
|
|
28
|
Undas A, Brozek J and Szczeklik A:
Homocysteine and thrombosis: From basic science to clinical
evidence. Thromb Haemost. 94:907–915. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Samonakis DN, Oustamanolakis P, Manousou
P, Kouroumalis EA and Burroughs AK: Klippel-Trenaunay syndrome,
pregnancy and the liver: An unusual interplay. Ann Gastroenterol.
25:365–367. 2012.PubMed/NCBI
|
|
30
|
Rotaru M and Iancu GM:
Klippel-Trenaunay-Weber syndrome. Acta Medica Transilvanica.
2:265–267. 2010.
|
|
31
|
den Heijer M, Willems HPJ, Blom HJ,
Gerrits WBJ, Cattaneo M, Eichinger S, Rosendaal FR and Bos GMJ:
Homocysteine lowering by B vitamins and the secondary prevention of
deep vein thrombosis and pulmonary embolism: A randomized,
placebo-controlled, double-blind trial. Blood. 109:139–144.
2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ray JG: Meta-analysis of
hyperhomocysteinemia as a risk factor for venous thromboembolic
disease. Arch Intern Med. 158:2101–2106. 1998.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ganguly P and Alam SF: Role of
homocysteine in the development of cardiovascular disease. Nutr J.
14(6)2015.PubMed/NCBI View Article : Google Scholar
|