Introduction
Deep venous thrombosis (DVT) is a major threat to
psychological health (1). It has
high morbidity and its early diagnosis is difficult, as evidence by
~100,000 patients being diagnosed with DVT between 2007-2016 in
China alone (2). The pathogenesis
of DVT is complicated, in which endothelial cells, leukocytes,
platelets, coagulation factors and the fibrinolytic system are
involved (3). The structural
disorder and dysfunction of venous endothelial cells are the
initiating factors of DVT, which impact the development and process
of DVT by regulating the systole and diastole of vessels (4). Structural disorder and dysfunction of
venous endothelial cells modulates the adherence, activation,
recruitment and interaction between platelets and leukocytes and
disrupts the balance of coagulation/anticoagulation and
fibrinolysis/antifibrinolysis (4).
The endothelial cell injury caused by endoplasmic reticulum stress
(ERS) serves an important role in the formation of DVT (5). Endothelial cells protect the vessels
as the first barrier by regulating blood flow, participating in
material exchange, preventing lipid leakage and inhibiting platelet
aggregation and thrombogenesis (6).
In addition, endothelial cells, especially in new vessels, exert a
secretory function as the highly-developed endoplasmic reticulum is
visible under an electron microscope, which makes endothelial cells
highly allergic to the factors that induce ERS, including
Ca2+ metabolism imbalance and oxidative stress
stimulation (7,8). ERS is reported to be involved in
multiple types of vascular diseases, such as atherosclerosis
(9) and Kawasaki disease (10) by inducing the apoptosis of
endothelial cells (11).
Transcriptional factor X-box binding protein 1 (XBP1) is an
important mediator in the process of ERS signal transduction in
mammalian cells (12). With the
development of ERS, inositol-requiring kinase1 (IRE1), an important
transmembrane protein molecule in the endoplasmic reticulum cavity,
disconnects with glucose-regulated protein 78 to be oligomerized
and auto-phosphorylated, hence inducing specific splicing on XBP1
mRNA to combine with ERS reaction components in the nucleus, such
as the unfolded protein reaction target molecule glucose-regulated
protein 78 (GRP78) (13,14). As a result, the relative expression
level of certain ERS-related genes, including activating
transcription factor 6 (ATF6) and eukaryotic translation initiation
factor 2a (eIF2a) (15), is
elevated at the transcriptional level (16).
XBP1 is a novel protein that is closely related to
protein folding and endoplasmic reticulum construction; it is an
important transcriptional factor in the leucine zipper protein
family (17). XBP1 functions as a
significant signal regulator for the ERS reaction by binding with
the X box cis-acting element located in the promoter region of the
major histocompatibility complex gene (18). In the process of ERS, the unspliced
X-box binding protein-1 (XBP1-u) composed of 261 amino acids is
transformed to spliced X-box binding protein-1 (XBP1-s) composed of
376 amino acids via transcriptional activation in the presence of
IRE1. XBP1-s-regulated ERS normally promotes the survival of cells
at the early stage of diseases (19,20).
However, the persistent activation of ERS will finally result in
tissue over-apoptosis with the continuous activation of ERS
(21). In addition, C/EBP
homologous protein (CHOP) is one of the important factors involved
in the ERS-mediated apoptotic pathway (22). Following ERS, the expression level
of CHOP is elevated, which further induces apoptosis (23).
The present study explored the impact of XBP1/CHOP
signaling pathway on the apoptosis of endothelial cells under the
stimulation of hyperglycemia to provide the fundamental basis for
the treatment of DVT. Small interfering (si) RNA technology was
used to downregulate XBP1 in HUVECs, followed by stimulation of
hyperglycemia and measurement of the change of apoptotic rate and
the expression level of downstream proteins of XBP1/CHOP pathway.
In addition, CHOP was also knocked down using siRNA, followed by
stimulation of hyperglycemia and the change in apoptosis rate was
measured. The results of the present study may elucidate a
potential biomarker for the clinical diagnosis and treatment of
DVT.
Materials and methods
Cell culture and treatments
HUVECs (cat. no. iCell-h110) were purchased from
iCell Bioscience Inc. and cultured in DMEM (cat. no. KGM12800S-500;
Nanjing KeyGen Biotech Co., Ltd.) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin at 37°C with 5% CO2.
D-glucose (cat. no. G116307) was purchased from Shanghai Aladdin
Biochemical Technology Co., Ltd. and had a purity >99.5%.
Cell transfection
HUVECs culture medium was changed to DMEM without
serum when the density of cells reached 70%. A total of ~125 µl of
Opti-MEM (Takara Bio Inc.) was added into 2 Eppendorf tubes with
the cells at a density of 1x106 cells/tube. One tube was
filled with 5 µl of Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) and one was filled with 12.5 µl of
siRNA (Takara Bio Inc.). After incubation for 15 min and mixing the
2 tubes, the mixture was added into the 6-well-plate and placed
into a cell incubator after the cell density reached 70%. After 4 h
of transfection at 37°C, 1 ml of complete DMEM
containing 20% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) was added into each well. The sequences of the siRNAs
targeting XBP1 and CHOP are shown in Table I. The negative control (NC) for
siXBP1 and for siCHOP was non-targeting (Takara Bio, Inc.). The
sequences of the NC are presented in Table I. The aforementioned agents were
added to the six-well-plate after transfection for 48 h. The cells
were collected for subsequent experimentation after another 48 h of
incubation at 37°C. Untransfected HUVECs were taken as
the control group.
 | Table ISequences of siRNAs targeting XBP1
and CHOP. |
Table I
Sequences of siRNAs targeting XBP1
and CHOP.
| siRNAs | siRNA sequences
(5'-3') |
|---|
| CHOP (human)
siRNA-1 | S:
AGAAAGAACAGGAGAAUGATT |
| | A:
UCAUUCUCCUGUUCUUUCUTT |
| CHOP (human)
siRNA-2 | S:
GGAGGAAGACCAAGGGAGATT |
| | A:
UCUCCCUUGGUCUUCCUCCTT |
| CHOP (human)
siRNA-3 | S:
AGGAGAAAGAACAGGAGAATT |
| | A:
UUCUCCUGUUCUUUCUCCUTT |
| XBP1 (human)
siRNA-1 | S:
CGAAAGAAGGCUCGAAUGATT |
| | A:
UCAUUCGAGCCUUCUUUCGTT |
| XBP1 (human)
siRNA-2 | S:
AGUGGUAGAUUUAGAAGAATT |
| | A:
UUCUUCUAAAUCUACCACUTT |
| XBP1 (human)
siRNA-3 | S:
GGUAUUGACUCUUCAGAUUTT |
| | A:
AAUCUGAAGAGUCAAUACCTT |
| NC | S:
UUCUCCGAACGUGUCACGUTT |
| | A:
ACGUGACACGUUCGGAGAATT |
Groups of cells
The use of D-glucose to injure endothelial cells has
been previously reported (24), and
5.5 mm is the concentration of D-glucose. The effect of different
concentrations of D-glucose on endothelial cell proliferation was
assessed by conducting a CCK8 assay (25). It was determined that 33.3 mM
D-glucose had the most significant inhibitory effect on cell
proliferation (data not shown). There were 7 groups of cells in the
present study: i) Control (5.5 mM D-glucose); ii) hypertonic
(hypertonic control, 27.8 mM mannitol and 5.5 mM D-glucose); iii)
16.7 mM D-glucose; iv) 33.3 mM D-glucose; v) 33.3 mM + NC (33.3 mM
D-glucose incubated with NC); vi) 33.3 mM + si-XBP1 (33.3 mM
D-glucose incubated with siRNA against XBP1); and vii) 33.3 mM +
si-CHOP (33.3 mM D-glucose incubated with siRNA against CHOP). The
33.3 mM D-glucose group was representative of the hyperglycemic
condition.
Western blotting
RIPA lysis buffer (cat. no. P0013D; Beyotime
Institute of Biotechnology) was used to isolate the proteins from
HUVECs at a density of 1x106 cells/group. Protein (~35
µg/lane) was separated on a 12% SDS-polyacrylamide gel. The gel was
transferred to a polyvinylidene difluoride membrane
(MilliporeSigma). The membrane was blocked with 5% skimmed milk in
Tris buffered saline/0.1% Tween-20 (pH 7.4) for 1 h at room
temperature and incubated overnight at 4°C with the
following primary rabbit anti-human antibodies: XBP1 (1:1,000; cat.
no. AF5110; Affinity Biosciences, Ltd.), CHOP (1:1,000; cat. no.
AF5110, Affinity Biosciences, Ltd.), GRP78 (1:1,000; cat. no.
AF5366; Affinity Biosciences, Ltd.), Puma, (1:1,000; cat. no.
AF5173; Affinity Biosciences, Ltd.), caspase-3 (1:1,000; cat. no.
Ab2302; Abcam), cytochrome C (1:1,000; cat. no. AF0146; Affinity
Biosciences, Ltd.) and GAPDH (1:2,000; cat. no. TA-08; OriGene
Technologies, Inc.). A horseradish peroxidase (HRP)-conjugated
antibody against rabbit IgG (1:5,000; cat. no. ZB-2305; OriGene
Technologies, Inc.) was used as a secondary antibody that was
incubated at room temperature for 1.5 h. Blots were incubated with
the ECL reagents (Beyotime Institute of Biotechnology) and exposed
to Tanon 5200-multi (Tanon Science and Technology Co., Ltd.) to
detect protein expression. Image J software V1.8.0 (National
Institutes of Health) was used to quantify the relative expression
level of target proteins. GAPDH was used as a loading control. A
total of 3 independent experiments were performed.
Flow cytometry for cell apoptosis
HUVECs were collected in 1.5 ml tubes. Each tube was
added with 10 µl fluorescently labeled Annexin V-FITC reagent [cat.
no. AP101-100-kit; Multi Sciences (Lianke) Biotech Co., Ltd.] and 5
µl of propidium iodide (PI) reagent [AP101-100-kit; Multi Sciences
(Lianke) Biotech Co., Ltd.]. Each tube was incubated for 10 min at
room temperature. Cells (~200 µl) were added into flow tubes
containing 2 ml of PBS and tested by flow cytometry (FACSAria III;
BD Biosciences). The data were analyzed using FlowJo V10.8 software
(BD Biosciences). A total of 3 independent experiments were
performed and both early apoptosis and late apoptosis were
detected.
Statistical analysis
All tests were performed using GraphPad Prism 5
software (GraphPad Inc.) and data were presented as the mean ± SD.
Statistically significant differences for continuous variables were
determined by one-way ANOVA with the post hoc Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference. Three statistical replicates were performed for each
experiment.
Results
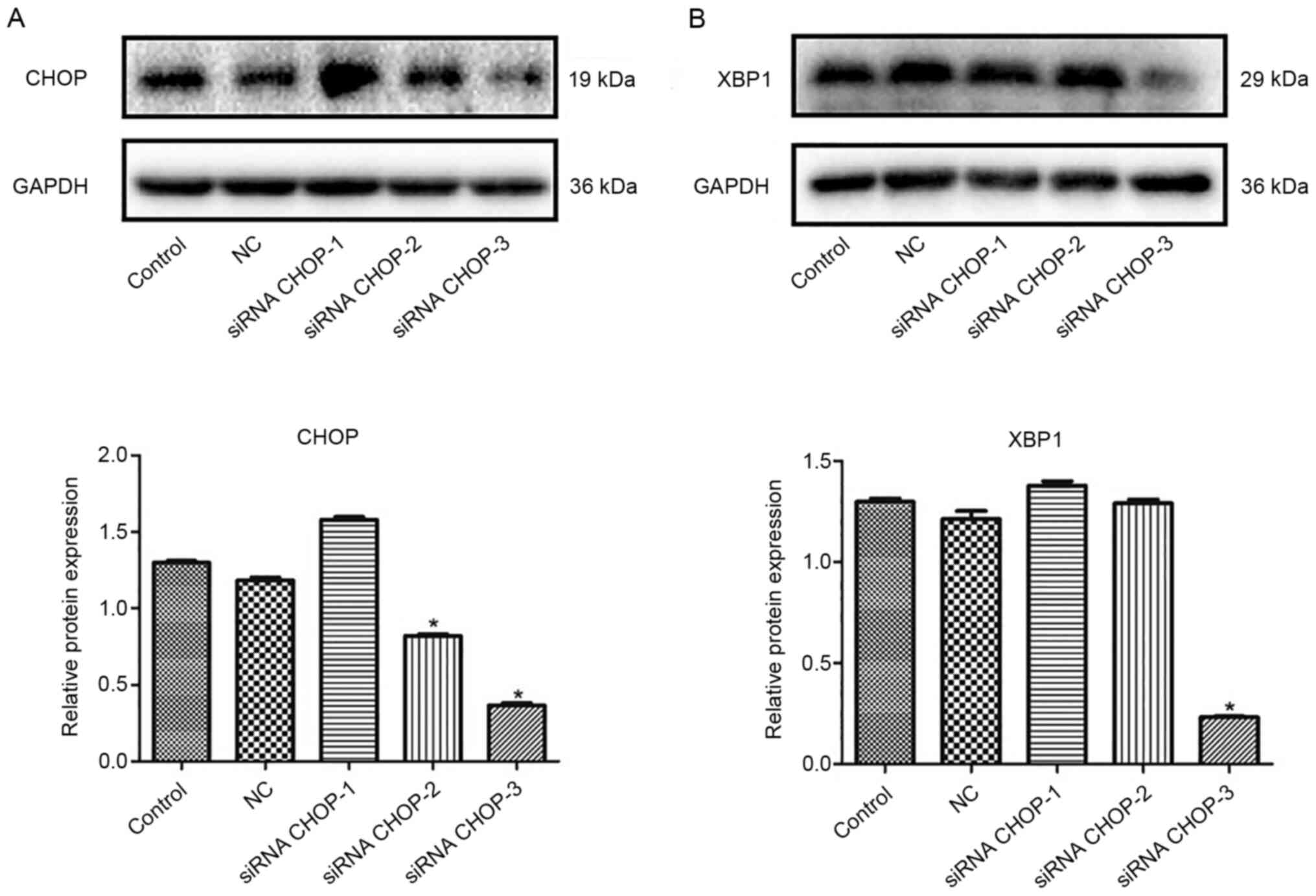
CHOP and XBP1 knockdown in HUVECs
Western blotting was performed to detect the
interference efficiency of CHOP, XBP1 and interference vectors in
endothelial cells. Compared with the control (untransfected
HUVECs), CHOP was significantly downregulated in the siRNA CHOP-2
and siRNA CHOP-3 groups, especially in the siRNA CHOP-3 group
(*P<0.05; Fig. 1A).
The expression of XBP1 deceased greatly in the siRNA XBP1-3 groups
compared with that in the control (*P<0.05; Fig. 1B). Hence, siRNA CHOP-3 and siRNA
XBP1-3 were selected for use in subsequent experiments.
Knockdown of CHOP or XBP1 inhibits the
apoptosis of HUVECs
Apoptosis was detected by performing flow cytometry.
Compared with the control, the apoptosis rate of HUVECs increased
greatly with the increase in the concentration of D-glucose
(*P<0.05; Fig. 2).
Compared with the 33.3 mM D-glucose group, the HUVECs incubated
with 33.3 mM D-glucose and si-XBP1 or 33.3 mM D-glucose and si-CHOP
showed significantly lower apoptosis rates (^P<0.05;
Fig. 2). In addition, compared to
the 33.3 mM+si-XBP1 group, a slightly lower apoptotic rate was
observed in the 33.3 mM+si-CHOP group (Fig. 2). The results revealed that XBP1 and
CHOP knockdown suppressed high glucose-induced endothelial cell
apoptosis.
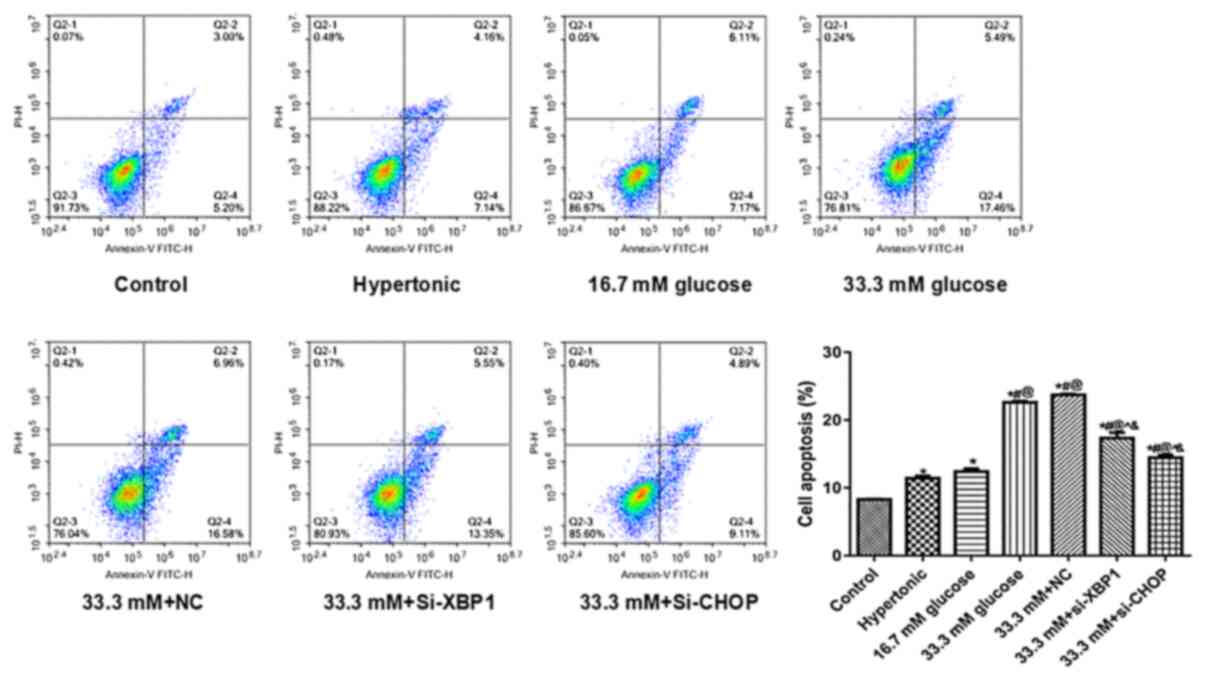 | Figure 2Effects of knocking down XBP1 or CHOP
on apoptotic rate. Flow cytometry was performed to detect the
effects of Si-XBP1 and Si-CHOP on the apoptosis of high glucose
induced endothelial cells. The gating strategies for the flow
cytometry assay were as follows: i) Early stage of HUVECs apoptosis
(Annexin V+PI-); ii) advanced stage of HUVECs
apoptosis (Annexin V+PI+); iii) normal HUVECs
(Annexin V-PI-); and iv) necrotic HUVECs
(Annexin V-PI+). *P<0.05 vs.
Control, #P<0.05 vs. Hypertonic,
@P<0.05 vs. 16.7 mM D-glucose, ^P<0.05
vs. 33.3 mM D-glucose, &P<0.05 vs. 33.3 mM+NC.
Si, small interfering; NC, negative control; CHOP, C/EBP homologous
protein; XBP1, X box binding protein 1; PI, propidium iodide;
control, untransfected and untreated HUVECs. |
Knockdown of CHOP or XBP1 suppresses
the expression of GRP78, Puma, cleaved caspase-3 and cytochrome
c
Western blotting was performed to detect the
expression levels of XBP1, GRP78, CHOP, Puma, cleaved caspase-3 and
cytochrome C. Compared with the control, XBP1, GRP78, CHOP, Puma,
cleaved caspase-3 and cytochrome c were significantly upregulated
in the hypertonic, 16.7 mM D-glucose, 33.3 mM D-glucose, and 33.3
mM + NC groups (*P<0.05; Fig. 3). Compared with the 33.3 mM
D-glucose group, the expression levels of XBP1, GRP78, CHOP, Puma,
cleaved caspase-3, and cytochrome c in the 33.3 mM + si-XBP1 or
33.3 mM + si-CHOP groups significantly decreased
(^P<0.05; Fig. 3). In
addition, compared with the 33.3 mM + NC group, lowest expression
level of Puma was observed in the 33.3 mM+si-XBP1 group, while the
lowest expression level of GRP78 and cytochrome c was observed in
the 33.3 mM + si-CHOP group (&P<0.05; Fig. 3). The results indicated that XBP1
and CHOP knockdown inhibited ER stress and apoptotic gene
expression in high glucose treated endothelial cells.
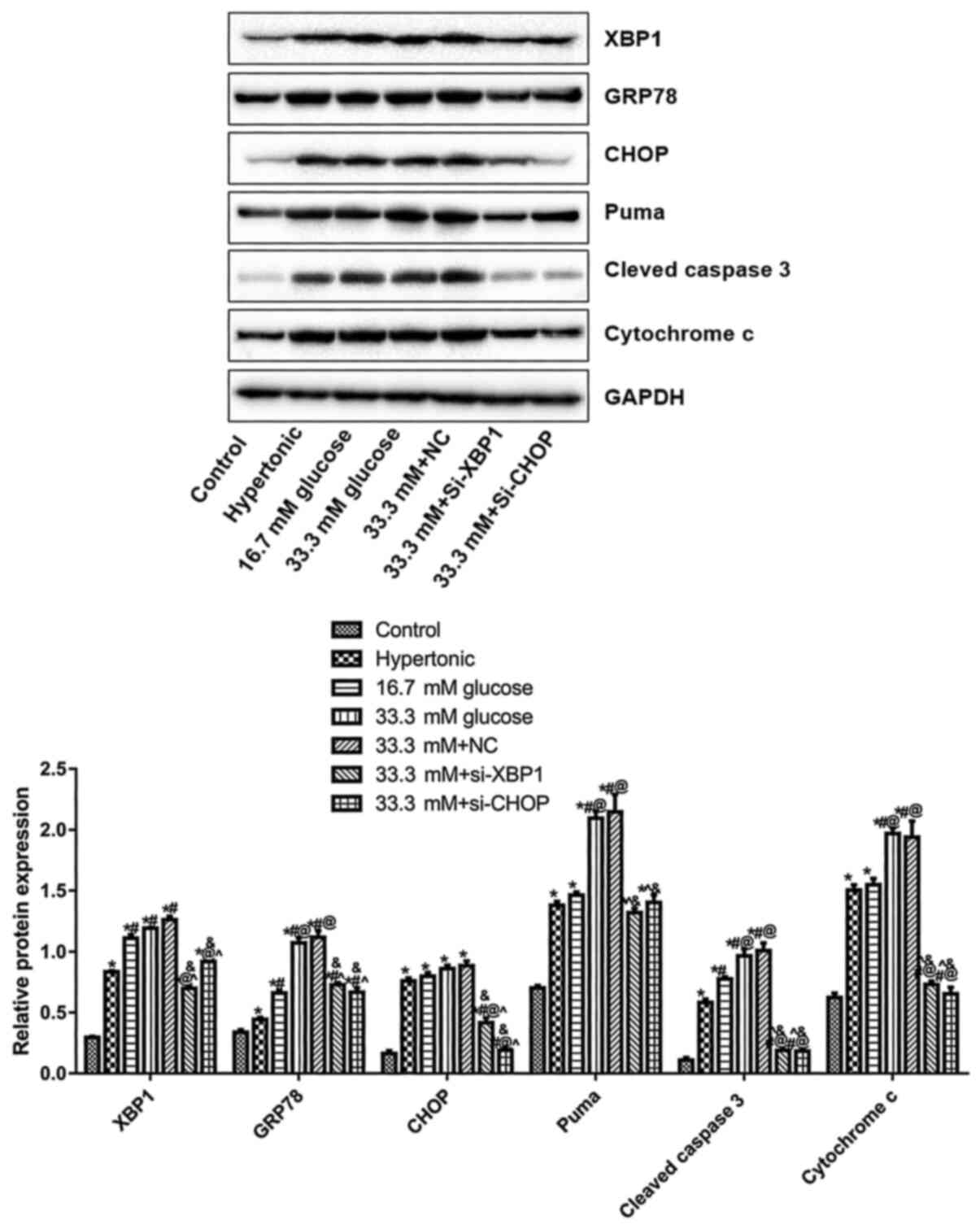 | Figure 3Effects of knocking down XBP1 or CHOP
on the expression level of GRP78, puma, cleaved caspase-3 and
Cytochrome c. The expression levels of XBP1, GRP78, CHOP, puma,
cleaved caspase-3 and Cytochrome c were evaluated by western
blotting. *P<0.05 vs. Control, #P<0.05
vs. Hypertonic, @P<0.05 vs. 16.7 mM D-glucose,
^P<0.0, vs. 33.3 mM D-glucose,
&P<0.05 vs. 33.3 mM+NC. Three independent
experiments were performed. Si, small interfering; NC, negative
control; CHOP, C/EBP homologous protein; XBP1, X box binding
protein 1; control, untransfected and untreated HUVECs. |
Discussion
Normal venous endothelium has antithrombosis effects
and venous wall injury is one of the important factors that can
result in thrombogenesis (26).
Local continuous platelet aggregation adheres to the endothelium
when collagen is exposed due to endothelial cell injury, meanwhile,
the coagulation system is initiated. The permeability of the
endothelium is promoted by the dysfunction of the endothelium,
which results in the adhesion of leucocytes to release certain
inflammatory factors, such as IL-6, TNF-α and IL-1β. Fibrin
deposition is inhibited by the released inflammatory factors to
suppress the fibrinolytic system, which contributes to the
formation of the prethrombotic state (27). High glucose concentration-induced
ERS results in the dysfunction of endothelial cells, the inhibition
of cell proliferation and cell death, thereby contributing to
injury to vessels and various vascular diseases, including diabetic
vascular disease (28,29). Autophagy, apoptosis, inflammation
and senescence of endothelial cells can be induced by high dosage
of glucose (30). XBP1 is a central
regulator in the process of ERS signal transfer in mammals
(19). When ERS arises in the
cells, IRE1, which is located in the endoplasmic reticulum lumen,
is separated from GRP78 to be activated by oligomerization and
autophosphorylation, resulting in the specific splicing of XBP1
mRNA. The expression of ERS-related genes is upregulated when
XBP1-s binds with the ERS reaction components in the nucleus
(16,31,32).
The survival rate of cells is promoted by activating IRE1
artificially under ERS, which indicates that XBP1 serves an
important role in cell survival and apoptosis (33). XBP1 is reported to induce
endothelial cell injury, cell apoptosis and coagulation leading to
thrombogenesis (34). CHOP is an
important signal factor mediating ERS and cell apoptosis; it
induces cell apoptosis through excessive ER stress (35). ERS was initiated in primary neonatal
mouse cardiomyocytes by stimulation with high concentration of
glucose and the expression of XBP1 and CHOP was observed to be
upregulated (36). By inhibition of
the activation of XBP1 or downregulation of the expression of XBP1,
XBP1 splicing was suppressed and CHOP was upregulated, which
indicated that the transcription and expression of CHOP could be
regulated by XBP1 to induce the apoptosis of mouse cardiomyocytes
(36). Consistent with the above
reports, in the present study, elevated expression level of both
XBP1 and CHOP could be induced by high glucose concentration. ERS
in endothelial cells, denoted by upregulated XBP1 and CHOP, was
stimulated by treatment with 16.7 mM glucose, which induced a 1 and
2-fold increase in the expression of XBP1 and CHOP, respectively.
In the present study, a 0.92 and 2.20-fold increase in the
expression level of XBP1 and CHOP, respectively were observed in
33.3 mM glucose treated endothelial cells. In the present study,
the findings related to apoptosis demonstrated that it was induced
by the upregulation of XBP1 and CHOP and high concentrations of
glucose in a dose-dependent manner, which indicated that the
XBP1/CHOP signaling pathway exerted important roles in the
processing of endothelial cell apoptosis. On the contrary,
apoptosis was suppressed by downregulating the expression of XBP1
or CHOP, which further verified the involvement of XBP1 and CHOP in
the process of high glucose induced apoptosis.
GRP78 is a type of molecular chaperone in the
endoplasmic reticulum, and high expression of GRP78 can be regarded
as the symbol of ERS (37). The
increased distribution of GRP78 on the cell membrane exerts a
regulatory function on cell apoptosis and cell proliferation
(38). GRP78 and CHOP were
upregulated in Sertoli cells by a high dosage of glucose, which
indicated that the activation of the CHOP signaling pathway under
ERS is the mechanism underlying the pro-apoptotic effects of high
glucose concentration (39). In the
present study, it was found that the expression of GRP78 was
upregulated by a high dosage of glucose, which was reversed by
downregulating the expression level of XBP1 or CHOP.
Puma is an important pro-apoptotic gene and serves
an important role in the initiation of cell apoptosis and the
induction of numerous other diseases, such as hepatocyte injury and
bone marrow hyperplasia (40). Puma
is reported to be involved in p53-dependent or independent cell
apoptosis and tumor processing (40-42).
Puma is significantly upregulated when apoptosis occurs (43). Caspase is the operator of apoptosis,
which is responsible for the transfer, transduction and integration
of apoptotic signals (44). Tiong
et al (45) reported that
Puma was upregulated under hyperglycemia in Schwann cells and
Cazanave et al (46) claimed
that the mRNA and protein level of CHOP in adipocytes was
positively related to that of Puma. In the present study, the
expression of Puma could be elevated under high glucose
concentrations. Approximately 1.1 and 1.9-fold increases in Puma
expression were observed in 16.7 mM glucose-treated and 33.3 mM
glucose-treated endothelial cells, respectively. In contrast, high
protein expression of Puma induced by high concentration of glucose
was suppressed by downregulating XBP1 or CHOP in the present
study.
Caspase-3 is one of the most important apoptotic
operators in the caspase family and exists in the cytoplasm as an
inactivated proenzyme post synthesis (47). Caspase 3 is activated by stimulating
apoptotic signals that degrades multiple types of protein
substrates, including pro-caspase-3, pro-caspase-6, pro-caspase-9
and DNA-dependent protein kinase (DNA-PK), in the processing of
apoptosis (48). The complex
composed of caspase-9 and Cytochrome c is one of the inducers of
the activation of caspase-3, which is also reported to be involved
in the mitochondrial apoptosis pathway (49). Mitochondrial permeability transition
pores are opened under the stimulation of active oxygen and ATP,
which contribute to the imbalance of the H+
concentration and differing pressure between the inside and outside
of the mitochondria (50).
Cytochrome c, which is located within the mitochondria is released
into the cytoplasm as a result of differing pressure (31). By binding with caspase-9, cell
apoptosis can be induced by Cytochrome c (31). Jiang et al (51) reported that the expression level of
cleaved-caspase-3 and Cytochrome c in rat cartilage endplate cells
was significantly elevated under hyperglycemia. Similarly, in the
present study, the expression of Puma, cleaved caspase-3 and
Cytochrome c was upregulated by the high dosage of glucose (33.3
mM), which was reversed by knocking down XBP1 and CHOP.
To sum up, in the present study the expression level
of proapoptotic proteins, such as GRP78, Puma, caspase-3, and
cytochrome c, were elevated by hyperglycemia in a dose-dependent
manner, which further contributed to the apoptosis of HUVECs.
Following inhibition of the XBP1/CHOP signal pathway, the
expression level of pro-apoptotic proteins was suppressed, which
further inhibited the apoptosis of endothelial cells. However, the
current study has limitations. The present study only investigated
the effect of the XBP1/CHOP pathway on ER stress in high glucose
induced cells, meaning that the effect of ER stress inhibition on
the prevention and treatment of DVT was not investigated in
vivo. Future studies should therefore focus on in vivo
assessment. Taken together, XBP1/CHOP may be a potential target for
the treatment of DVT as one of the key pathways regulating ERS
processing through mediating cell apoptosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MT conceived and designed the current study. JL
acquired, analyzed and interpreted the data. YH performed
statistical analysis. YZ made substantial contributions to
conception and design, drafted the manuscript, and revised it for
important intellectual content. All authors have read and approved
the final manuscript. MT and YZ confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nicholson M, Chan N, Bhagirath V and
Ginsberg J: Prevention of venous thromboembolism in 2020 and
beyond. J Clin Med. 9(2467)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang Z, Lei J, Shao X, Dong F, Wang J,
Wang D, Wu S, Xie W, Wan J, Chen H, et al: Trends in
hospitalization and In-Hospital mortality from VTE, 2007 to. 2016,
in China. Chest. 155:342–353. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao N, Zhang J, Jiang T, Chen X, Wang J,
Ding C, Liu F, Qian K and Jiang R: Risk factors of deep venous
thrombosis associated with peripherally inserted central venous
catheter in upper extremity in ICU. Zhonghua Wei Zhong Bing Ji Jiu
Yi Xue. 29:167–171. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
4
|
Huang Y, Wang C, Zhang Y, Ning Y, Kui L,
Song L, Zhi X, Yan D and Ji X: Incidence of lower limb deep venous
thrombosis and coagulation status in severe patients after thoracic
surgery. Zhongguo Fei Ai Za Zhi. 21:864–867. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
5
|
Chen YL, Shou LH and Zhang ZX: Association
of interleukin-18 gene polymorphism and its protein expression with
the lower extremity deep venous thrombosis in the Chinese Han
population: A case-control study. J Clin Lab Anal.
32(e22345)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fang CH, Song YS, So BI, Kim H, Shin JH
and Kim KS: Concentration-dependent differential effects of
udenafil on viability, proliferation, and apoptosis in vascular
endothelial and smooth muscle cells. Indian J Pharmacol.
46:292–297. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Qi Z and Chen L: Endoplasmic reticulum
stress and autophagy. Adv Exp Med Biol. 1206:167–177.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu D, Wu M, Lu Y, Xian T, Wang Y, Huang
B, Zeng G and Huang Q: Protective effects of 6-Gingerol on vascular
endothelial cell injury induced by high glucose via activation of
PI3K-AKT-eNOS pathway in human umbilical vein endothelial cells.
Biomed Pharmacother. 93:788–795. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dong Y, Fernandes C, Liu Y, Wu Y, Wu H,
Brophy ML, Deng L, Song K, Wen A, Wong S, et al: Role of
endoplasmic reticulum stress signalling in diabetic endothelial
dysfunction and atherosclerosis. Diab Vasc Dis Res. 14:14–23.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu M, Qi Q, Men L, Wang S, Li M, Xiao M,
Chen X, Wang S, Wang G, Jia H and Liu C: Berberine protects
Kawasaki disease-induced human coronary artery endothelial cells
dysfunction by inhibiting of oxidative and endoplasmic reticulum
stress. Vascul Pharmacol. 127(106660)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xiaxia C, Lei B and Ding Y: Effect of
quercetin on vascular endothelial cell injury under glucosamine
treatment. Food Sci. 34:224–228. 2013.
|
|
12
|
Yu X, Ren LP, Wang C, Zhu YJ, Xing HY,
Zhao J and Song GY: Role of X-box binding Protein-1 in
Fructose-induced de novo Lipogenesis in HepG2 cells. Chin Med J
(Engl). 131:2310–2319. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li J, Zhao Y, Zhou N, Li L and Li K:
Dexmedetomidine attenuates myocardial ischemia-reperfusion injury
in diabetes mellitus by inhibiting endoplasmic reticulum stress. J
Diabetes Res. 2019(7869318)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lyu X, Zhang M, Li G, Cai Y, Li G and Qiao
Q: Interleukin-6 production mediated by the IRE1-XBP1 pathway
confers radioresistance in human papillomavirus-negative
oropharyngeal carcinoma. Cancer Sci. 110:2471–2484. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang S, Cao M and Fang F: The role of
epigallocatechin-3-Gallate in autophagy and endoplasmic reticulum
Stress (ERS)-induced apoptosis of human diseases. Med Sci Monit.
26(e924558)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gaballah HH, Zakaria SS, Mwafy SE, Tahoon
NM and Ebeid AM: Mechanistic insights into the effects of quercetin
and/or GLP-1 analogue liraglutide on high-fat
diet/streptozotocin-induced type 2 diabetes in rats. Biomed
Pharmacother. 92:331–339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen S, Chen J, Hua X, Sun Y, Cui R, Sha J
and Zhu X: The emerging role of XBP1 in cancer. Biomed
Pharmacother. 127(110069)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jiang M, Yu S, Yu Z, Sheng H, Li Y, Liu S,
Warner DS, Paschen W and Yang W: XBP1 (X-Box-Binding
Protein-1)-dependent O-GlcNAcylation is neuroprotective in ischemic
stroke in young mice and its impairment in aged mice is rescued by
Thiamet-G. Stroke. 48:1646–1654. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yoshida H, Oku M, Suzuki M and Mori K:
pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded
protein response activator pXBP1(S) in mammalian ER stress
response. J Cell Biol. 172:565–575. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nekrutenko A and He J: Functionality of
unspliced XBP1 is required to explain evolution of overlapping
reading frames. Trends Genet. 22:645–648. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen L, Zhao M, Li J, Wang Y, Bao Q, Wu S,
Deng X, Tang X, Wu W and Liu X: Critical role of X-box binding
protein 1 in NADPH oxidase 4-triggered cardiac hypertrophy is
mediated by receptor interacting protein kinase 1. Cell Cycle.
16:348–359. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Qi AL, Wu Y, Dong N, Chai YF, Zhu XM and
Yao YM: Recombinant human ulinastatin improves immune dysfunction
of dendritic cells in septic mice by inhibiting endoplasmic
reticulum stress-related apoptosis. Int Immunopharmacol.
85(106643)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Y, Guo Y, Tang J, Jiang J and Chen Z:
New insights into the roles of CHOP-induced apoptosis in ER stress.
Acta Biochim Biophys Sin (Shanghai). 47:146–147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao X, Su L, He X, Zhao B and Miao J:
Long noncoding RNA CA7-4 promotes autophagy and apoptosis via
sponging MIR877-3P and MIR5680 in high glucose-induced vascular
endothelial cells. Autophagy. 16:70–85. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou Y, Qi C, Li S, Shao X and Ni Z:
Investigation of the mechanism underlying calcium
dobesilate-mediated improvement of endothelial dysfunction and
inflammation caused by high glucose. Mediators Inflamm.
2019(9893682)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Valeriani E, Riva N, Di Nisio M and Ageno
W: Splanchnic vein thrombosis: Current perspectives. Vasc Health
Risk Manag. 15:449–461. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mukhopadhyay S, Johnson TA, Duru N, Buzza
MS, Pawar NR, Sarkar R and Antalis TM: Fibrinolysis and
inflammation in venous thrombus resolution. Front Immunol.
10(1348)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fan Z, Guo C, Zhang Y, Yao J, Liao L and
Dong J: Hongjingtian injection inhibits proliferation and migration
and promotes apoptosis in high glucose-induced vascular smooth
muscle cells. Drug Des Devel Ther. 13:4115–4126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang Z, Zhang S, Wang Y, Yang M, Zhang N,
Jin Z, Ding L, Jiang W, Yang J, Sun Z, et al: Autophagy inhibits
high glucose induced cardiac microvascular endothelial cells
apoptosis by mTOR signal pathway. Apoptosis. 22:1510–1523.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huang LY, Yen IC, Tsai WC, Ahmetaj-Shala
B, Chang TC, Tsai CS and Lee SY: Rhodiola crenulata attenuates high
glucose induced endothelial dysfunction in human umbilical vein
endothelial cells. Am J Chin Med. 45:1201–1216. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen R, Gao XG and Zhang L: Cannabidiol
attenuates palmitic acid-induced hepatocytes injury through
promoting autophagic flux. Acad J Second Military Med Univ.
38:583–588. 2017.
|
|
32
|
Li LF, Wen Y, Jiang L and Zhu YQ:
Establishment of a model of endoplasmic reticulum stress response
in dental pulp cells induced by tunicamycin. Shanghai Kou Qiang Yi
Xue. 27:135–138. 2018.PubMed/NCBI(In Chinese).
|
|
33
|
Fink SL, Jayewickreme TR, Molony RD,
Iwawaki T, Landis CS, Lindenbach BD and Iwasaki A: IRE1α promotes
viral infection by conferring resistance to apoptosis. Sci Signal.
10(eaai7814)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kelaini S, Caines R, Zeng L and Margariti
A: Chapter 13-X-box-binding Protein 1 splicing induces an
autophagic response in endothelial cells: Molecular mechanisms in
ECs and Atherosclerosis. Autophagy: Cancer, Other Pathologies,
Inflammation, Immunity, Infection, and Aging, 259-268, 2017
doi:10.1016/B978-0-12-805420-8.00013-5.
|
|
35
|
Su Q, Wang Y, Yang X, Li XD, Qi YF, He XJ
and Wang YJ: Inhibition of endoplasmic reticulum stress apoptosis
by estrogen protects human umbilical vein endothelial cells through
the PI3 Kinase-Akt signaling pathway. J Cell Biochem.
118:4568–4574. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Miyazaki Y, Kaikita K, Endo M, Horio E,
Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, et
al: C/EBP homologous protein deficiency attenuates myocardial
reperfusion injury by inhibiting myocardial apoptosis and
inflammation. Arterioscler Thromb Vasc Biol. 31:1124–1132.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Staquicini DI, D'Angelo S, Ferrara F,
Karjalainen K, Sharma G, Smith TL, Tarleton CA, Jaalouk DE,
Kuniyasu A, Baze WB, et al: Therapeutic targeting of
membrane-associated GRP78 in leukemia and lymphoma: Preclinical
efficacy in vitro and formal toxicity study of BMTP-78 in rodents
and primates. Pharmacogenomics J. 18:436–443. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liang G, Fang X, Yang Y and Song Y:
Knockdown of CEMIP suppresses proliferation and induces apoptosis
in colorectal cancer cells: Downregulation of GRP78 and attenuation
of unfolded protein response. Biochem Cell Biol. 96:332–341.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang Y, Huang H, Feng D, Liu W, Cheng X,
Ba Y and Cui L: Effects of N-acetylcysteine on fluoride-induced
endoplasmic reticulum stress in Sertoli cells. Wei Sheng Yan Jiu.
43:805–808, 813. 2014.PubMed/NCBI(In Chinese).
|
|
40
|
Shan Z, Liu Q, Li Y, Wu J, Sun D and Gao
Z: PUMA decreases the growth of prostate cancer PC-3 cells
independent of p53. Oncol Lett. 13:1885–1890. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xu T, Yuan Y and Xiao DJ: The clinical
relationship between the slug-mediated Puma/p53 signaling pathway
and radiotherapy resistance in nasopharyngeal carcinoma. Eur Rev
Med Pharmacol Sci. 21:953–958. 2017.PubMed/NCBI
|
|
42
|
Xia HB, Cui HW, Su L, Zhang ZH, Yang XY,
Ning SQ and Su XL: Clinical significance and expression of PUMA,
MCL-1, and p53 in human renal cell carcinoma and para-carcinoma
tissues. Genet Mol Res. (16)2017.PubMed/NCBI View Article : Google Scholar : doi:
10.4238/gmr16039278.
|
|
43
|
Schubert F, Rapp J, Brauns-Schubert P,
Schlicher L, Stock K, Wissler M, Weiß M, Charvet C, Borner C and
Maurer U: Requirement of GSK-3 for PUMA induction upon loss of
pro-survival PI3K signaling. Cell Death Dis. 9(470)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang J, Yu S, Li J, Li H, Jiang H, Xiao P,
Pan Y, Zheng J, Yu L and Jiang J: Protective role of
N-acetyl-l-tryptophan against hepatic ischemia-reperfusion injury
via the RIP2/caspase-1/IL-1β signaling pathway. Pharma Biol.
57:385–391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tiong YL, Ng KY, Koh RY, Ponnudurai G and
Chye SM: Melatonin prevents oxidative stress-induced mitochondrial
dysfunction and apoptosis in high glucose-treated Schwann cells via
upregulation of Bcl2, NF-κB, mTOR, Wnt signalling pathways.
Antioxidants (Basel). 8(198)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cazanave SC, Elmi NA, Akazawa Y, Bronk SF,
Mott JL and Gores GJ: CHOP and AP-1 cooperatively mediate PUMA
expression during lipoapoptosis. Am J Physiol Gastrointest Liver
Physiol. 299:G236–G243. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Choudhary GS, Al-Harbi S and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 1219:1–9.
2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu H, Zhou Y and Tang L: Caffeine induces
sustained apoptosis of human gastric cancer cells by activating the
caspase9/caspase3 signalling pathway. Mol Med Rep. 16:2445–2454.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang Y, Liu C, Wang J, Zhang Y and Chen L:
Iodine-131 induces apoptosis in human cardiac muscle cells through
the p53/Bax/caspase-3 and PIDD/caspase-2/t-BID/cytochrome
c/caspase-3 signaling pathway. Oncol Rep. 38:1579–1586.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhai KF, Duan H, Chen Y, Khan GJ, Cao WG,
Gao GZ, Shan LL and Wei ZJ: Apoptosis effects of imperatorin on
synoviocytes in rheumatoid arthritis through
mitochondrial/caspase-mediated pathways. Food Funct. 9:2070–2079.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jiang Z, Lu W, Zeng Q, Li D, Ding L and Wu
J: High glucose-induced excessive reactive oxygen species promote
apoptosis through mitochondrial damage in rat cartilage endplate
cells. J Orthop Res. 36:2476–2483. 2018.PubMed/NCBI View Article : Google Scholar
|