Introduction
Laparoscopic surgery has long been regarded with
concern for malignant disease, especially for colon cancer. The
extent of resection, exploration for staging and trocar site
recurrences are the main causes of concern (1). Since the first laparoscopic assisted
interventions for colon cancer in the 1990s, a decade later two
large multi-institutional trials, COST and COLOR, have established
that laparoscopic surgery is not inferior and is an acceptable
alternative to open surgery for non-metastatic, resectable colon
cancer (2-4).
Since the early years of laparoscopy, several technological
advances have been made, ranging from improved laparoscopes that
provide better visualization to wound protectors, devices for
hemostasis and excellent mechanical suture. The inherent benefits
of laparoscopic surgery should therefore be offered to patients
with malignant disease that otherwise would have large incisions,
longer hospital stays and possibly difficulty in returning to
work.
During the last decades, the increasing numbers of
laparoscopic interventions have led to increased confidence in this
approach. In addition, several advances in understanding patterns
of malignant dissemination and tumor biology have led to
standardization of resection techniques (5,6).
Complete excision of embryologic compartments than contain
malignant tumors as complete mesocolic excision for colon cancer is
now widely accepted, as is total mesorectal excision (TME) for
rectal cancer (7,8). The standardization of techniques
facilitates the shortening of the learning curve for laparoscopic
colon resection for cancer (9).
The oncological clearance for colon cancer following
surgery for non-metastatic, resectable colon cancer is evaluated by
lymph node harvest and confirmation of free circumferential and
axial surgical specimen margins. (10-12).
The present study retrospectively evaluated the
quality of non-metastatic colon cancer resections between two
groups, laparoscopic and open surgery. Of the eight senior surgeons
in the Department of Surgery of Elias University Emergency
Hospital, two practice routinely laparoscopic colon resections.
Materials and methods
Between January 2017 and December 2020, 311 patients
underwent interventions for colon cancer in the Department of
Surgery, Elias Emergency University Hospital. The present study
retrospectively studied the pathology reports and charts of 219
patients that had undergone elective procedures for uncomplicated,
non-metastatic, resectable colon cancer, excluding patients
operated on in an emergency setting. The present study focused on
the quality of the resection. A total of 92 patients with
metastatic colon cancer or complications such as bleeding,
intestinal obstruction, perforation and peritonitis were excluded.
The retrospective observational study was approved by the Elias
University Emergency Hospital Ethics Committee (decision no.
13376/27.11.2021) and all patients provided written informed
consent prior to surgery, both for surgery and for inclusion in any
future research.
All studied patients had preoperative definitive
diagnosis; biopsy-proven colon cancer. Colonoscopy and computerized
tomography scan for complete staging were performed for every
patient. The Elias Hospital Multidisciplinary Tumor Board approved
the surgical management for each case. No in-hospital mortality was
recorded.
All interventions, for both laparoscopic and open
surgery, followed the no-touch isolation technique with primary
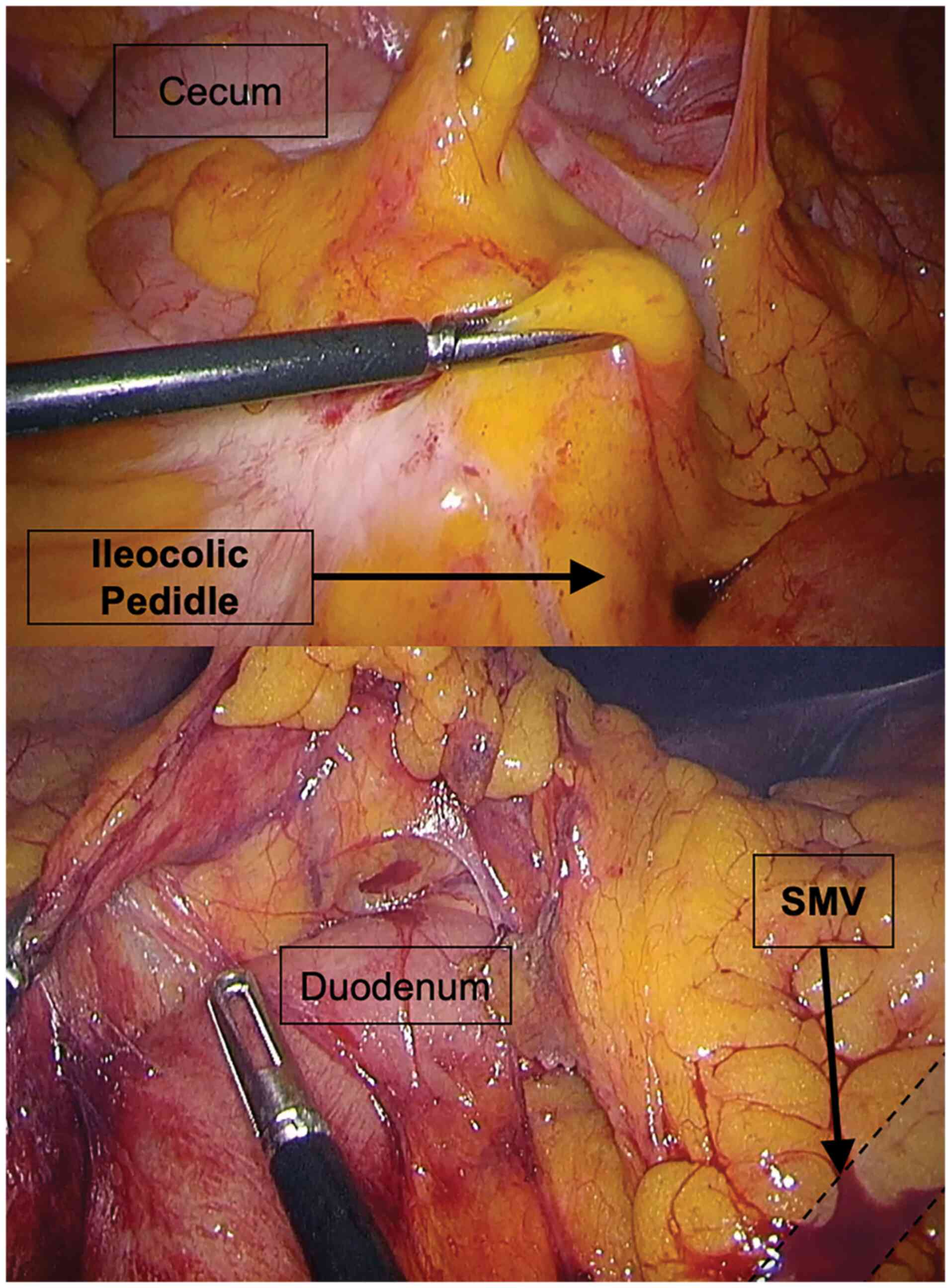
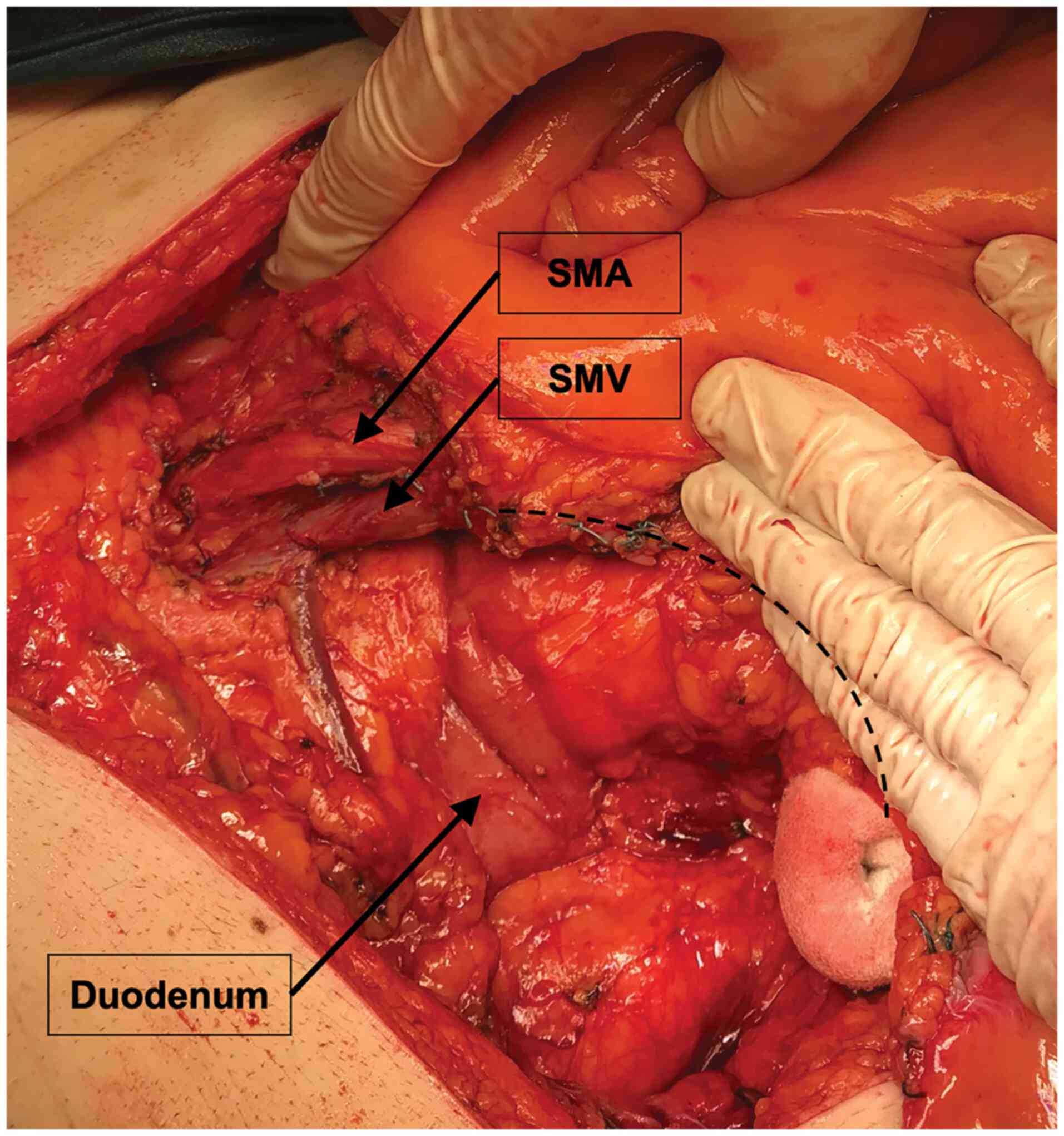
vascular ligation (13) (Fig. 1). The no-touch isolation technique
as a unit standard was used in every case. Complete mesocolic
excision is not used routinely in Elias Emergency University
Hospital, yet a wide excision of mesocolon was encouraged (Fig. 2). For laparoscopic surgery,
standard endobags and small, protected transverse incisions were
used to retrieve the specimen.
All interventions were completed by senior surgeons
leading surgical teams that included general surgery residents. A
total of two surgeons performed the interventions in the
laparoscopic group. Each performed at least 30 laparoscopic
colorectal operations prior to the current study period, thus
having completed their learning curve (14). No conversion was recorded in the
laparoscopic group.
The retrieved specimens were sent uncut to the
pathologist and TNM edition 8 was used for staging (15).
The quality of resection was evaluated in terms of
specimen margins, lymph node harvest and n-ratio defined as the
number of positive (metastatic) lymph nodes divided by the total
number of examined lymph nodes.
The pooled data was studied using ANOVA models and
medians comparison for continuous data and t-test or chi-square
test for variables that were normally distributed, as well as
nonparametric tests for skewed data (Mann-Witney U Test) or
Fisher's exact test. Correlations were tested using the Pearson
method or Spearman's correlation. The results are listed in
Tables I and II. P<0.05 was considered to indicate
a statistically significant difference.
 | Table IPatient demographics, tumor location,
T stage (TNM 8th edition) and grading. |
Table I
Patient demographics, tumor location,
T stage (TNM 8th edition) and grading.
| Characteristic | Laparoscopic Group
(n=52) | Open Group
(n=167) | P-value |
|---|
| Age | | | 0.364 |
|
Mean age
(years) | 66.3 | 68.0 | |
|
Median (±
standard deviation) | 67 (±11.11) | 68 (±11.52) | |
| Sex | | | <0.001 |
|
Female | 18 | 83 | |
|
Male | 34 | 84 | |
| Tumor location | | | <0.001 |
|
Cecum | 7 | 21 | |
|
Ascending
colon | 11 | 31 | |
|
Hepatic
flexure | 3 | 7 | |
|
Transverse
colon | 5 | 16 | |
|
Splenic
flexure | 1 | 15 | |
|
Descending
colon | 4 | 15 | |
|
Sigmoid
colon | 20 | 62 | |
| T Stage | | | 0.003 |
|
Tis | 0 | 1 | |
|
T1 | 5 | 2 | |
|
T2 | 14 | 29 | |
|
T3 | 29 | 89 | |
|
T4a | 2 | 30 | |
|
T4b | 2 | 16 | |
| Grading | | | 0.636 |
|
G1 | 17 (32.69%) | 43 (25.75%) | |
|
G2 | 29 (55.77%) | 95 (56.89%) | |
|
G3 | 5 (9.62%) | 26 (15.57%) | |
|
G4 | 1 (1.92%) | 3 (1.80%) | |
 | Table IIOncologic resection overview. |
Table II
Oncologic resection overview.
| Quality of
resection parameters | | Laparoscopic Group
(n=52) | Open Group
(n=167) | P-value |
|---|
| Harvested lymph
nodes | | | | |
|
Mean | | 16.12 | 17.31 | 0.448 |
|
Median (±
standard deviation) | | 14 (±6.632) | 15 (±8.452) | |
| Invaded lymph
nodes | | | | |
|
Mean | | 1.31 | 2.68 | 0.015 |
|
Median (±
standard deviation) | | 0 (±2.397) | 1 (±5.030) | |
| N-RATIO | | | | |
|
Mean | Mean | 0.089 | 0.157 | 0.003 |
|
Median (±
standard deviation) | Median (± standard
deviation) | 0 (±0.17) | 0.03 (±0.23) | |
| Axial Specimen
Margins | | | | 0.637 |
|
No | No | 3 | 7 | |
|
Percentage | % | 5.77% | 4.19% | |
| Circumferential
Specimen Margins | | | | 0.035 |
|
No | | 0 | 12 | |
|
Percentage | | 0 | 7.19% | |
Results
Demographics, tumor location, T stage and grading
are listed in Table I. The mean
age of the patients in both groups was similar: 68 (±11.52) years
in the open group (OG) and 66 (±11.11) years in the laparoscopic
group (LG). There was a readiness for laparoscopic approach
apparently in men but while this was not a randomized prospective
study we can assume that more advanced disease or difficult
interventions were expected in some female patients. As expected,
fewer cases in the LG were recorded for difficult tumor locations
(the splenic and hepatic flexures and descending colon as well as
fewer advanced tumors) with only four T4 tumors in the LG.
As expected, a statistically significant number of
more advanced tumors were recorded in the OG (r=0.2459,
P=0.0003).
The mean number of harvested lymph nodes was 16.12
for the LG and 17.31 for the OG without statistical significance
between groups (P=0.448). Although statistical significance between
groups regarding total or mean number of harvested lymph nodes
between groups was not found, the open interventions proved to be
more constant in the number of harvested lymph nodes (ANOVA;
P<0.0001).
While the mean number of invaded lymph nodes was
1.31 for the LG and 2.68 for the OG, the n-ratio was significantly
lower in the LG (r=0.1324; P=0.003). N-ratio was corelated with T
stage and as a higher number of advanced tumors were recorded in
the OG, a higher n-ratio had to be expected. A significant
correlation was observed between tumor grading and n-ratio in the
LG that contained more less advanced tumors (r=0.2994, P=0.03),
meaning that less advanced tumors were found in the LG.
No circumferential margins were found to be invaded
in the LG while in the OG 12 specimens had invaded margins (7.19%;
P=0.035). Axial margins were found to be invaded in three cases in
the LG (5.77%) and seven cases in the OG (4.19%; P=0.637).
Circumferential margins proved to be more frequently
free after laparoscopic interventions (r=0.1343; P=0.035). No
significant correlations were found between groups regarding axial
margins.
Discussion
The COST and COLOR trials proved that laparoscopic
resections for colon cancer are by no means inferior to open
resections but the routine of laparoscopic surgery still needs to
be implemented (3,16). The uptake in laparoscopic
resections for colorectal cancer in western countries is very
encouraging, over a ten-fold increase in a period of less than 10
years, the largest increase being seen in high volume private
hospitals (17). Exact figures for
Romania have not been published but laparoscopic resections in the
general surgery department of Elias Emergency University Hospital
have been slowly increasing in the past 10 years from <7% to the
current 31.13%.
As expected, morbidity and mortality for elective
procedures decrease with volume size, facts suggested by numerous
systematic reviews (18-20).
Surgeon volume appears to be more important for interventions with
a shorter length of stay while hospital volume is correlated with
major interventions that require longer length of stay and
intensive care (21). Currently
there is much debate around the case volume per surgeon to define
high and low volumes. The conventionally accepted learning curve of
20 to 50 cases was overtaken by both senior surgeons in Elias
Emergency University Hospital general surgery department that now
routinely employ laparoscopic colorectal resections (22,23).
Attaining advanced laparoscopic skills is mandatory for colon
resections and as laparoscopic surgery is taught early on during
residency a shorter learning curve should be expected. None of the
of the surgeons whose patients are listed in the present study
performed fewer than 7-10 elective colon resections per year,
giving them a medium volume status. As for Elias University
Emergency Hospital that houses our surgery department is fair to
say it is in the same range of medium volume, with 219 elective
colon resections in the last three years and 92 emergency
interventions for complicated colon cancer in the same period
(19).
Despite the fact that complete mesocolic excision
was not routinely used in the surgery department at Elias Emergency
University Hospital, the median number of harvested lymph nodes was
higher than the published COST trial (3). The COLOR trial reported a median of
10 lymph nodes removed for both the laparoscopic and open
resections (4). The number of
recorded lymph nodes depends on the methods used for detection and
in the present study, pathological dissection, palpation and
careful naked eye examination were used; time consuming processes
considering many metastatic lymph nodes are <5 mm in diameter.
The Elias University Emergency Hospital Pathology Department does
not use chemical fat clearance or entire residual mesenteric tissue
examination in order to better define the lymph node yield, but
then again this is not standard practice (24). Complete mesocolic excision promises
an oncologically superior specimen than standard surgery for
resectable colon cancer, with a median number of retrieved lymph
nodes ranging between 18 and 30(10). The number of retrieved lymph nodes
was not linked with survival but lymph node ratio is an independent
prognostic factor for colon cancer and is used to optimize staging
(25,26). In the present study, taking all
tumors into account, n-ratio was positively corelated with T stage
(r=0,2151; P=0,001). In the LG, with less advanced tumors, n-ratio
was corelated with tumor grading (r=0,2994; P=0,03) while in the
OG, n-ratio was correlated with T stage (r=0,2045; P=0,008). The
number of harvested lymph nodes was not significantly correlated
with the type of surgery (P=0.448). However, there may be more
factors that contribute to the number of excised lymph nodes,
especially in laparoscopic procedures, such as previous abdominal
surgery, BMI and tumor size and type, which may be of great
interest for future studies, as is the case for in-depth comparison
of postoperative complications, length of stay, the need for blood
transfusions and disease recurrence.
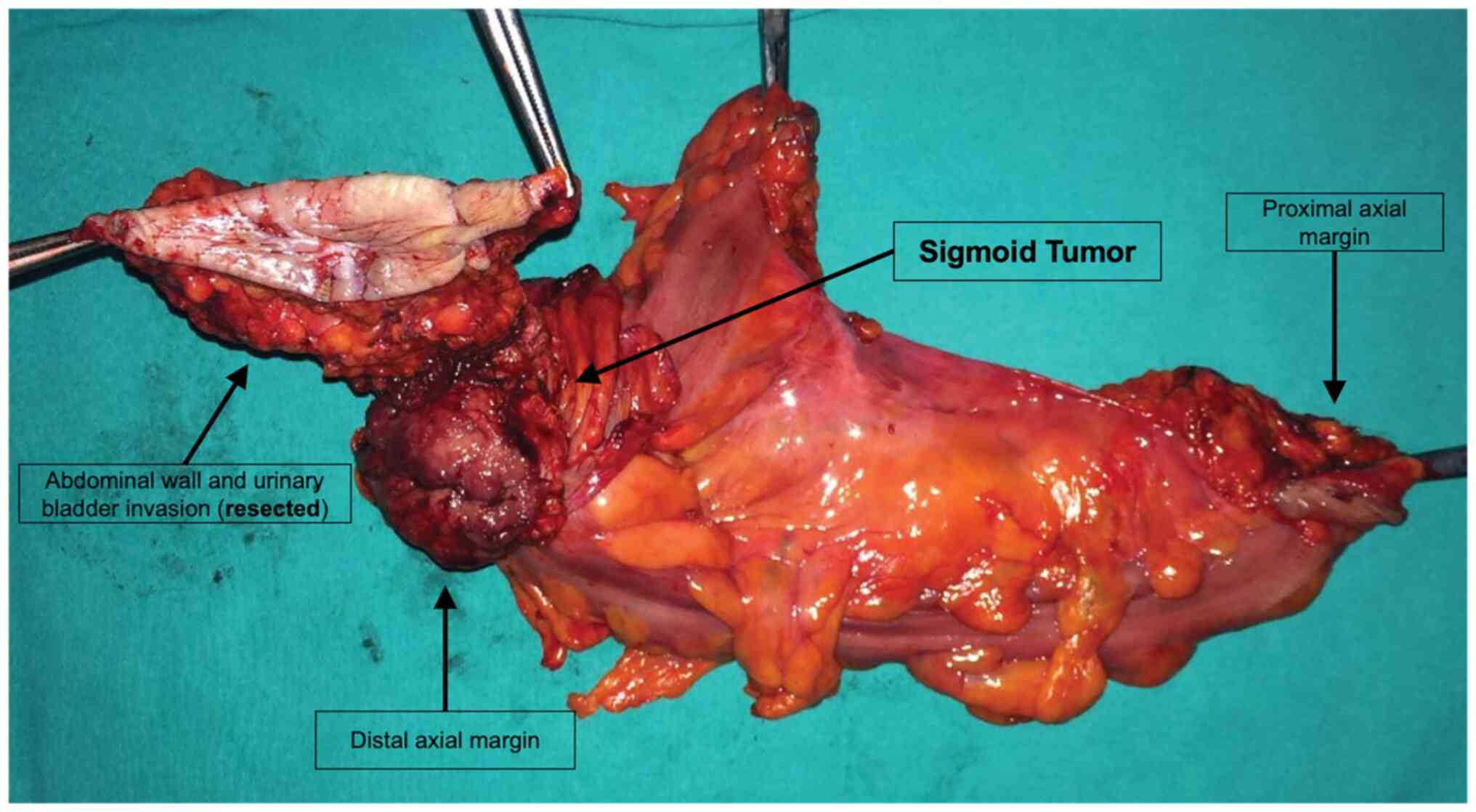
Regarding the resection margins, surgeons in the
general surgery department at Elias Hospital are encouraged to keep
>5 cm distance from the tumor and extensive bowel resections are
indicated when the tumor falls between large feeding vessels. In
the present study, in 12 cases (7.9%) circumferential margins were
microscopically invaded in the open group. To be noted that the OG
contained 46 T4 tumors and additional visceral en-block resections
were demanded (Fig. 3). None of
the cases in the LG had positive circumferential margins, although
four cases had advanced tumors. This could be explained by the
augmented visualization and magnification of the laparoscopic
camera and the employment of less blunt dissection than in open
surgery. Axial resections margins were positive in a reasonable
percentage both for the OG and LG.
The present study suggested that laparoscopic
surgery is not inferior to open surgery for non-metastatic colon
cancer in a medium volume center. With more experience, case load
and other surgeons undertaking laparoscopic surgery for colon
cancer, more patients may be offered the benefits of minimally
invasive surgery.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
OE was responsible for the design of the study,
supervised the collection of the data, and had a decisive
contribution to the discussions. AA, VC, and ET made substantial
contributions to conception, design and interpretation of data, IS,
LT and AM contributed to the analysis and data interpretation. AT
and RT collected and analyzed the data. LR, AB-S and DM were
involved in the statistical data analysis and submission process.
LT and AM supervised the research and contributed to the final
version of the manuscript. All the authors confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study received approval from Elias
University Emergency Hospital Ethics Committee (decision number
13376/27.11.2021).
Patient consent for publication
All patients provided written informed consent for
the use of the data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reilly WT, Nelson H, Schroeder G, Wieand
HS, Bolton J and O'connell MJ: Wound recurrence following
conventional treatment of colorectal cancer. A rare but perhaps
underestimated problem. Dis Colon Rectum. 39:200–207.
1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jacobs M, Verdeja JC and Goldstein HS:
Minimally invasive colon resection (laparoscopic colectomy). Surg
Laparosc Endosc. 1:144–150. 1991.PubMed/NCBI
|
|
3
|
Fleshman J, Sargent DJ, Green E, Anvari M,
Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W and
Nelson H: Clinical Outcomes of Surgical Therapy Study Group.
Laparoscopic colectomy for cancer is not inferior to open surgery
based on 5-year data from the COST Study Group trial. Ann Surg.
246:655–662; discussion 662-4. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
COLOR Study Group. COLOR: A randomized
clinical trial comparing laparoscopic and open resection for colon
cancer. Dig Surg. 17:617–622. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Colon Cancer Laparoscopic or Open
Resection Study Group. Buunen M, Veldkamp R, Hop WC, Kuhry E,
Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, et al: Survival
after laparoscopic surgery versus open surgery for colon cancer:
Long-term outcome of a randomised clinical trial. Lancet Oncol.
10:44–52. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Veldkamp R, Gholghesaei M, Bonjer HJ,
Meijer DW, Buunen M, Jeekel J, Anderberg B, Cuesta MA, Cuschierl A,
Fingerhut A, et al: Laparoscopic resection of colon cancer:
Consensus of the European Association of Endoscopic Surgery (EAES).
Surg Endosc. 18:1163–1185. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hohenberger W, Weber K, Matzel K,
Papadopoulos T and Merkel S: Standardized surgery for colonic
cancer: Complete mesocolic excision and central ligation-technical
notes and outcome. Colorectal Dis. 11:354–364; discussion 364-5.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Heald RJ, Moran BJ, Ryall RD, Sexton R and
MacFarlane JK: Rectal cancer: The Basingstoke experience of total
mesorectal excision, 1978-1997. Arch Surg. 133:894–899.
1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tekkis PP, Senagore AJ, Delaney CP and
Fazio VW: Evaluation of the learning curve in laparoscopic
colorectal surgery: Comparison of right-sided and left-sided
resections. Ann Surg. 242:83–91. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
West NP, Hohenberger W, Weber K, Perrakis
A, Finan PJ and Quirke P: Complete mesocolic excision with central
vascular ligation produces an oncologically superior specimen
compared with standard surgery for carcinoma of the colon. J Clin
Oncol. 28:272–278. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Abraham NS, Young JM and Solomon MJ:
Meta-analysis of short-term outcomes after laparoscopic resection
for colorectal cancer. Br J Surg. 91:1111–1124. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Chang GJ, Rodriguez-Bigas MA, Skibber JM
and Moyer VA: Lymph node evaluation and survival after curative
resection of colon cancer: Systematic review. J Natl Cancer Inst.
99:433–441. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wiggers T, Jeekel J, Arends JW, Brinkhorst
AP, Kluck HM, Luyk CI, Munting JD, Povel JA, Rutten AP, Volovics A,
et al: No-Touch isolation technique in colon cancer: A controlled
prospective trial. Br J Surg. 75:409–415. 1988.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bennett CL, Stryker SJ, Ferreira MR, Adams
J and Beart RW: The learning curve for laparoscopic colorectal
surgery: Preliminary results from a prospective analysis of 1194
laparoscopic-assisted colectomies. Arch Surg. 132:41–44; discussion
45. 1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gospodarowicz MK, Brierley JD and
Wittekind C (eds): TNM classification of malignant tumours. 8th
edition. John Wiley & Sons, Hoboken, NJ, 2017.
|
|
16
|
Veldkamp R, Kuhry E, Hop WC, Jeekel J,
Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, et
al: Laparoscopic surgery versus open surgery for colon cancer:
Short-term outcomes of a randomised trial. Lancet Oncol. 6:477–484.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Thompson BS, Coory MD and Lumley JW:
National trends in the uptake of laparoscopic resection for
colorectal cancer, 2000-2008. Med J Aust. 194:443–447.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gruen RL, Pitt V, Green S, Parkhill A,
Campbell D and Jolley D: The effect of provider case volume on
cancer mortality: Systematic review and meta-analysis. CA Cancer J
Clin. 59:192–211. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huo YR, Phan K, Morris DL and Liauw W:
Systematic review and a meta-analysis of hospital and surgeon
volume/outcome relationships in colorectal cancer surgery. J
Gastrointest Oncol. 8:534–546. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rogers SO Jr, Wolf RE, Zaslavsky AM,
Wright WE and Ayanian JZ: Relation of surgeon and hospital volume
to processes and outcomes of colorectal cancer surgery. Ann Surg.
244:1003–1011. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Birkmeyer JD, Stukel TA, Siewers AE,
Goodney PP, Wennberg DE and Lucas FL: Surgeon volume and operative
mortality in the United States. N Engl J Med. 349:2117–2127.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schlachta CM, Mamazza J, Seshadri PA,
Cadeddu M, Gregoire R and Poulin EC: Defining a learning curve for
laparoscopic colorectal resections. Dis Colon Rectum. 44:217–222.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li JC, Hon SS, Ng SS, Lee JF, Yiu RY and
Leung KL: The learning curve for laparoscopic colectomy: Experience
of a surgical fellow in an university colorectal unit. Surg Endosc.
23:1603–1608. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schofield JB, Mounter NA, Mallett R and
Haboubi NY: The importance of accurate pathological assessment of
lymph node involvement in colorectal cancer. Colorectal Dis.
8:460–470. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vaccaro CA, Im V, Rossi GL, Quintana GO,
Benati ML, de Arenaza DP and Bonadeo FA: Lymph node ratio as
prognosis factor for colon cancer treated by colorectal surgeons.
Dis Colon Rectum. 52:1244–1250. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Moug SJ, Saldanha JD, McGregor JR,
Balsitis M and Diament RH: Positive lymph node retrieval ratio
optimises patient staging in colorectal cancer. Br J Cancer.
100:1530–1533. 2009.PubMed/NCBI View Article : Google Scholar
|